User login
Norgestrel for nonprescription contraception: What you and your patients need to know
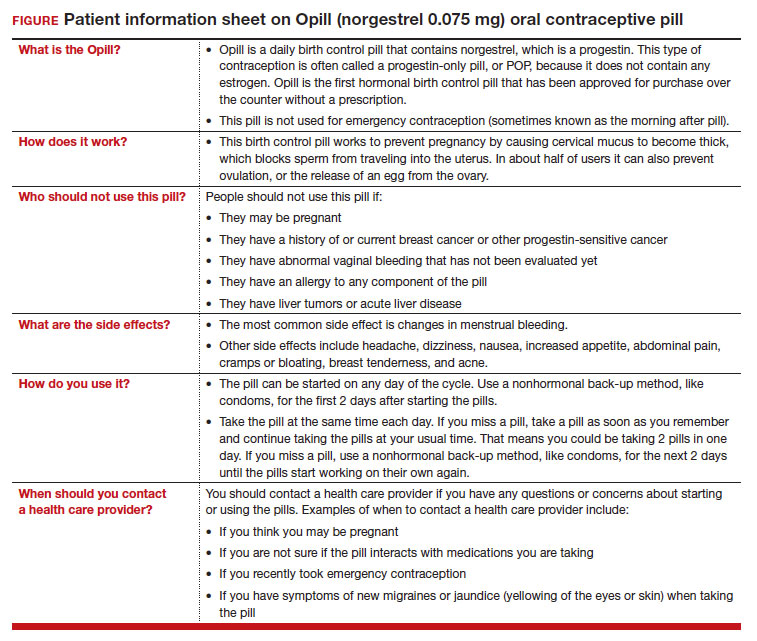
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.
How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8
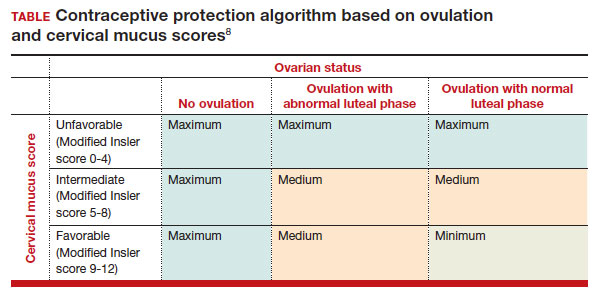
In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.

How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8

In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.

How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8

In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
Recruiting ObGyns: Starting salary considerations
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
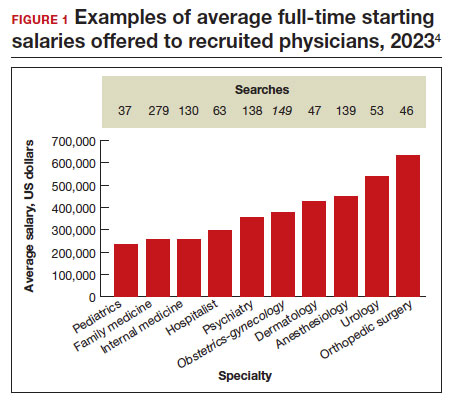
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
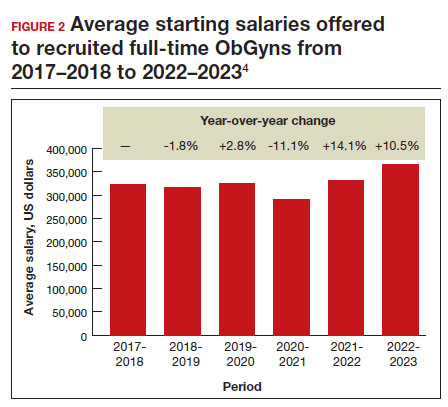
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4
Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4


Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4


Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
Patient counseling for breast cancer screening: Taking changes to USPSTF recommendations into account
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.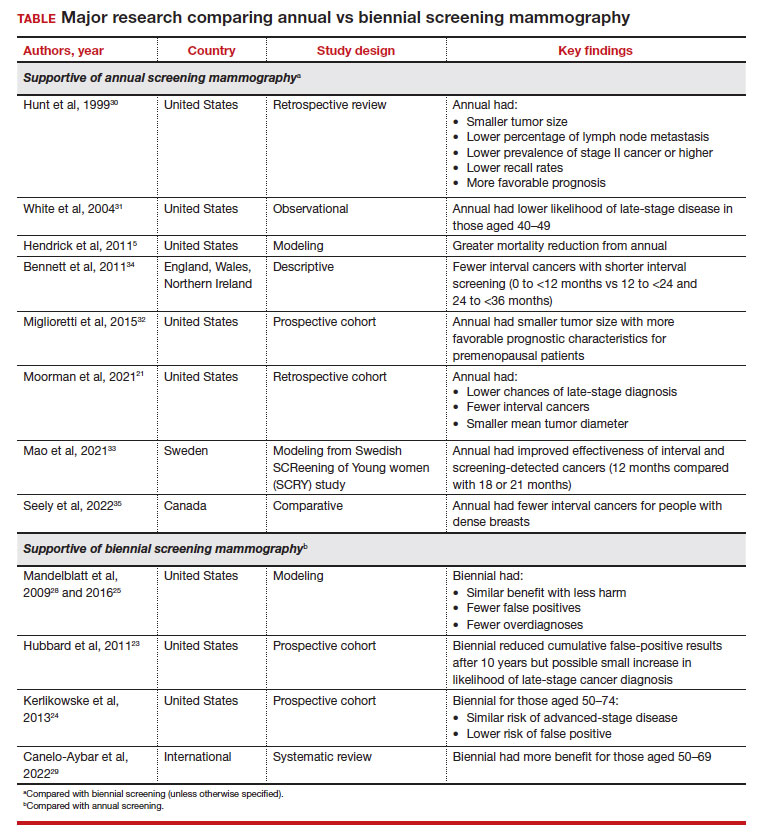
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Focus on long-COVID: Perimenopause and post-COVID chronic fatigue
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.
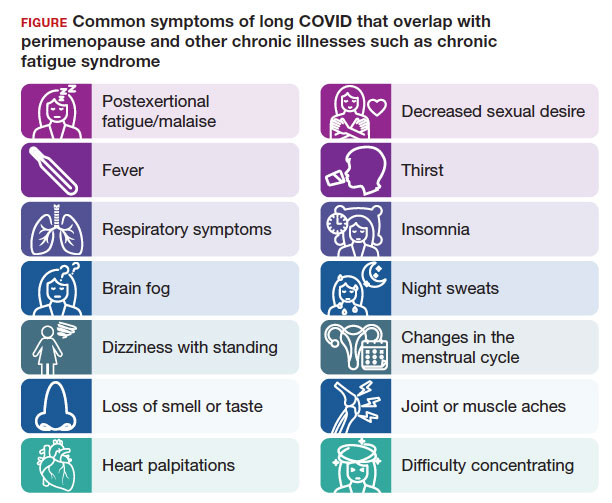
Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Time to rethink endometrial ablation: A gyn oncology perspective on the sequelae of an overused procedure
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.
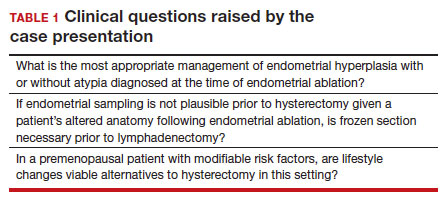
Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.
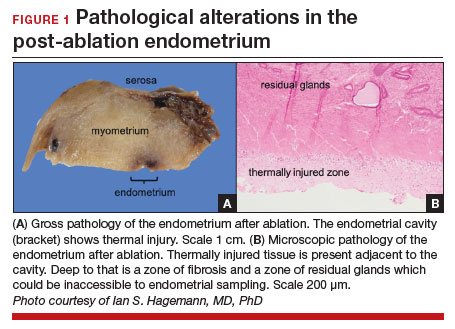
Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.
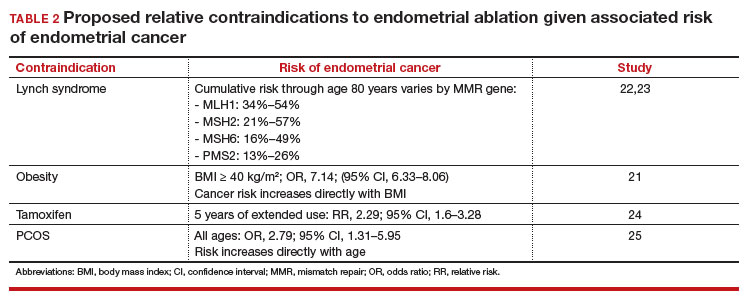
Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.
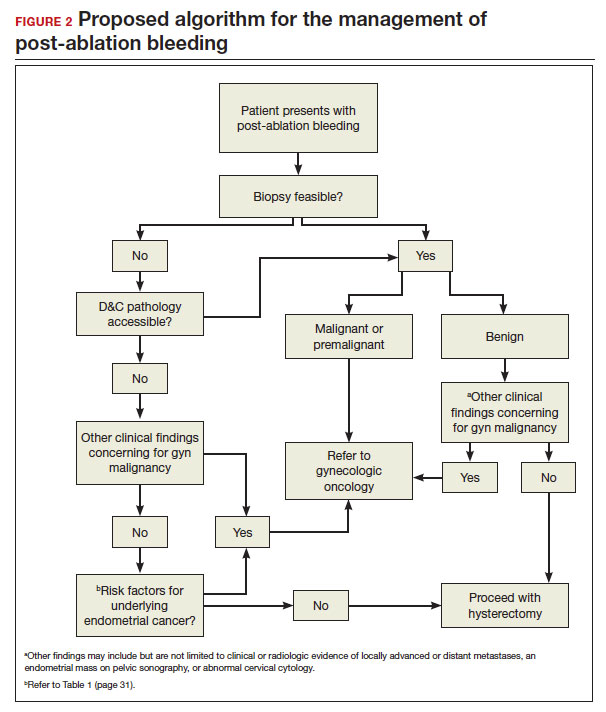
CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
LGBTQI+: Special considerations for reproductive health care
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
Announcement from the publisher
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Case Q: How can I best remove my patient’s difficult-to-find implant?
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).
Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).
Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
New Therapies in Melanoma: Current Trends, Evolving Paradigms, and Future Perspectives
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2
In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.
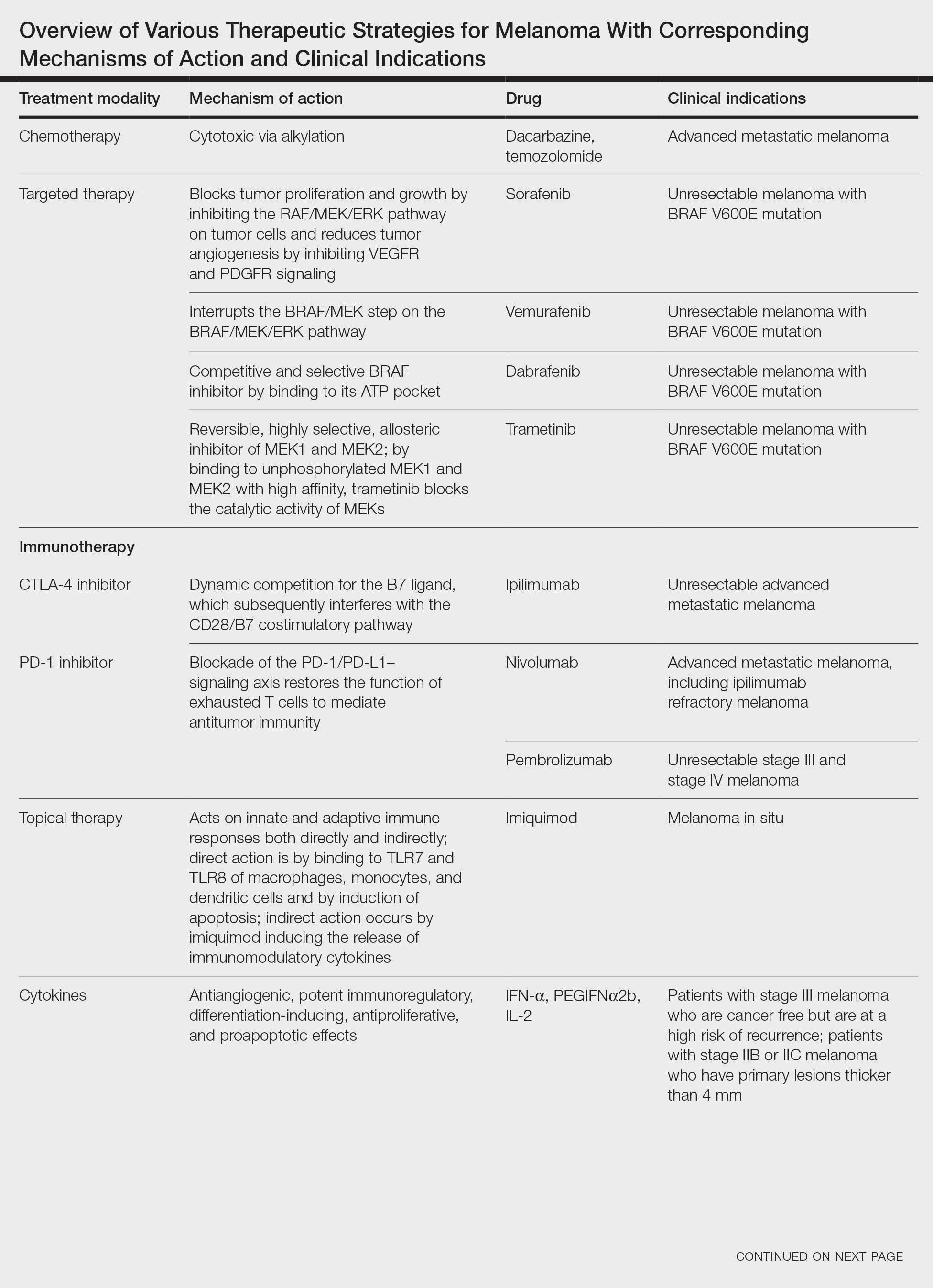
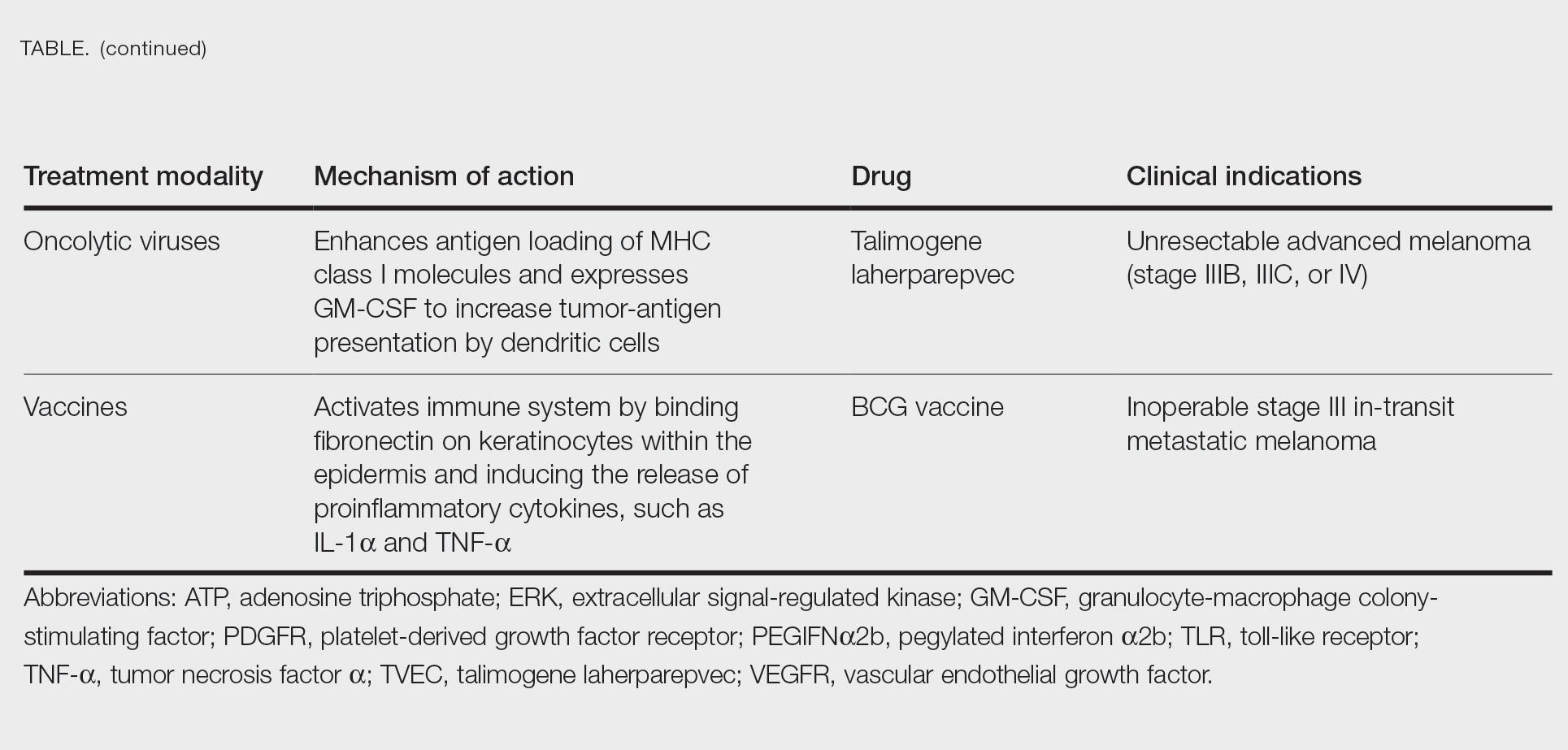
Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8
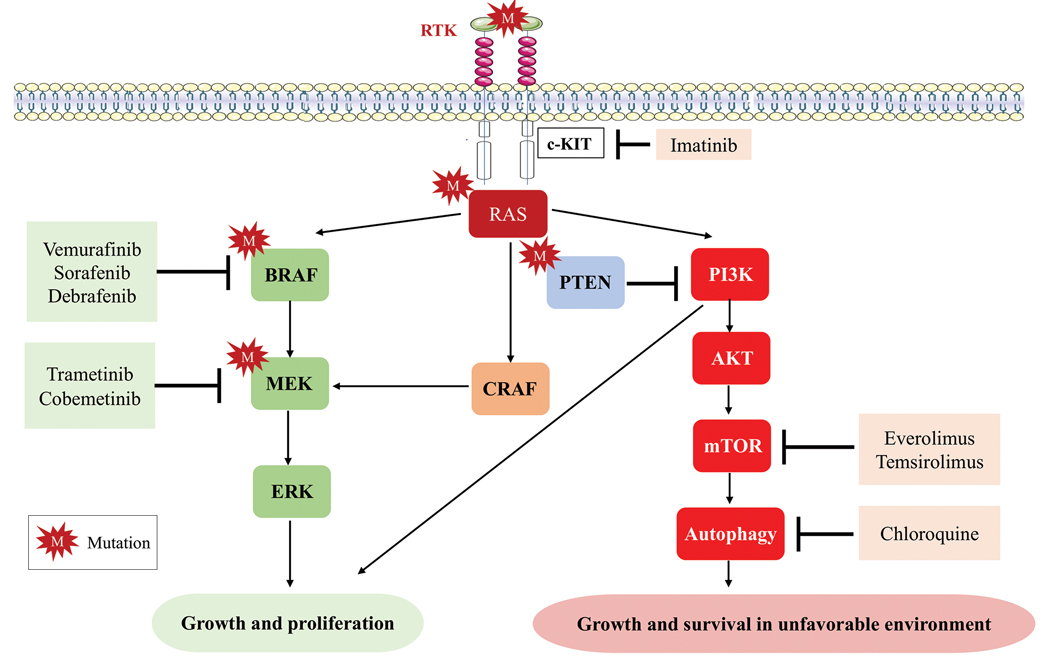
Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Practice Points
- Immune checkpoint inhibition has resulted in a paradigm shift for the treatment of metastatic melanoma.
- Alternative therapies with novel targets such as lymphocyte-activated gene 3 aim to overcome resistance to the usual immune targets such asPD-1/PD-L1 and CTLA-4.
- Newer promising tools such as nanotechnology are being added to the growing armamentarium of melanoma treatment strategies.
2023 USPSTF mammography age to start screening in average-risk patients: What’s new is old again
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.
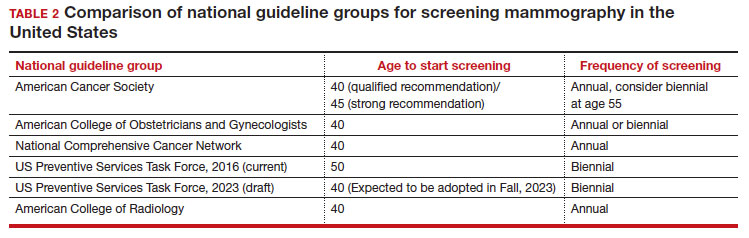
In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14
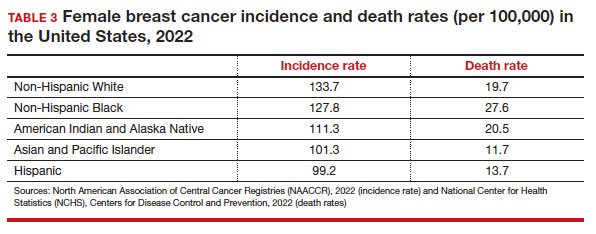
While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.
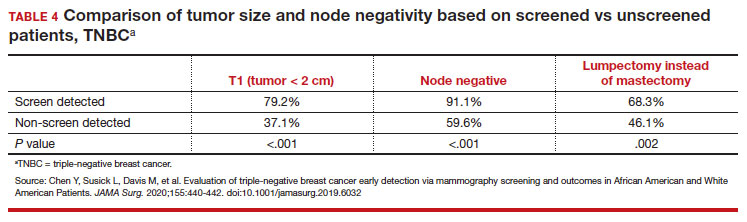
Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.

