User login
2023 Update on cervical disease
Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
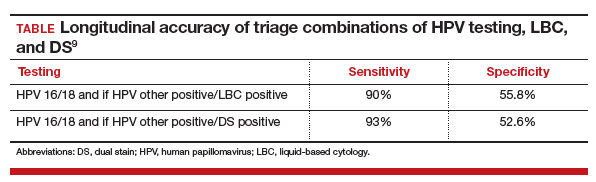
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9
DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.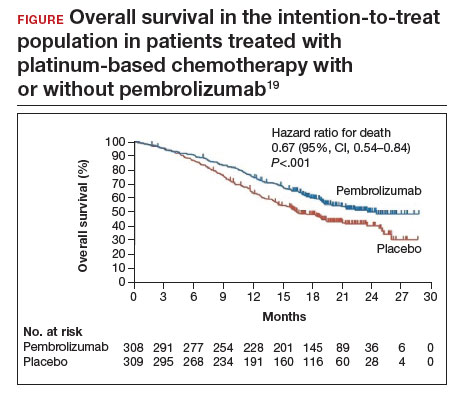
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Case Q: How soon after taking emergency contraception can a patient begin hormonal contraception?
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
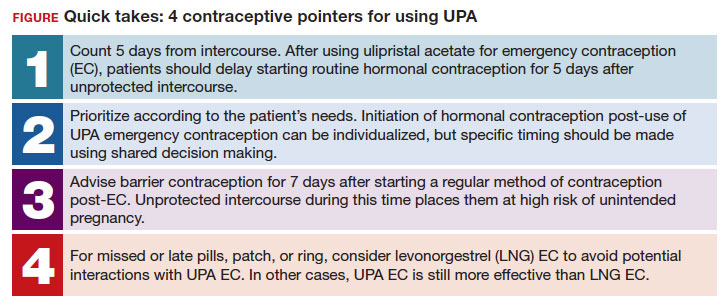
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●

Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●

- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
Potential Uses of Nonthermal Atmospheric Pressure Technology for Dermatologic Conditions in Children
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Practice Points
- Nonthermal atmospheric plasma (NTAP)(also known as cold atmospheric plasma) has been shown to cause minimal damage to skin when application time is minimized.
- Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or pharmacologic adverse effects.
- Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders.
The Clinical Diversity of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory disorder that affects individuals worldwide.1 Although AD previously was commonly described as a skin-limited disease of childhood characterized by eczema in the flexural folds and pruritus, our current understanding supports a more heterogeneous condition.2 We review the wide range of cutaneous presentations of AD with a focus on clinical and morphological presentations across diverse skin types—commonly referred to as skin of color (SOC).
Defining SOC in Relation to AD
The terms SOC, race, and ethnicity are used interchangeably, but their true meanings are distinct. Traditionally, race has been defined as a biological concept, grouping cohorts of individuals with a large degree of shared ancestry and genetic similarities,3 and ethnicity as a social construct, grouping individuals with common racial, national, tribal, religious, linguistic, or cultural backgrounds.4 In practice, both concepts can broadly be envisioned as mixed social, political, and economic constructs, as no one gene or biologic characteristic distinguishes one racial or ethnic group from another.5
The US Census Bureau recognizes 5 racial groupings: White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.6 Hispanic or Latinx origin is considered an ethnicity. It is important to note the limitations of these labels, as they do not completely encapsulate the heterogeneity of the US population. Overgeneralization of racial and ethnic categories may dull or obscure true differences among populations.7
From an evolutionary perspective, skin pigmentation represents the product of 2 opposing clines produced by natural selection in response to both need for and protection from UV radiation across lattitudes.8 Defining SOC is not quite as simple. Skin of color often is equated with certain racial/ethnic groups, or even binary categories of Black vs non-Black or White vs non-White. Others may use the Fitzpatrick scale to discuss SOC, though this scale was originally created to measure the response of skin to UVA radiation exposure.9 The reality is that SOC is a complex term that cannot simply be defined by a certain group of skin tones, races, ethnicities, and/or Fitzpatrick skin types. With this in mind, SOC in the context of this article will often refer to non-White individuals based on the investigators’ terminology, but this definition is not all-encompassing.
Historically in medicine, racial/ethnic differences in outcomes have been equated to differences in biology/genetics without consideration of many external factors.10 The effects of racism, economic stability, health care access, environment, and education quality rarely are discussed, though they have a major impact on health and may better define associations with race or an SOC population. A discussion of the structural and social determinants of health contributing to disease outcomes should accompany any race-based guidelines to prevent inaccurately pathologizing race or SOC.10
Within the scope of AD, social determinants of health play an important role in contributing to disease morbidity. Environmental factors, including tobacco smoke, climate, pollutants, water hardness, und urban living, are related to AD prevalence and severity.11 Higher socioeconomic status is associated with increased AD rates,12 yet lower socioeconomic status is associated with more severe disease.13 Barriers to health care access and suboptimal care drive worse AD outcomes.14 Underrepresentation in clinical trials prevents the generalizability and safety of AD treatments.15 Disparities in these health determinants associated with AD likely are among the most important drivers of observed differences in disease presentation, severity, burden, and even prevalence—more so than genetics or ancestry alone16—yet this relationship is poorly understood and often presented as a consequence of race. It is critical to redefine the narrative when considering the heterogeneous presentations of AD in patients with SOC and acknowledge the limitations of current terminology when attempting to capture clinical diversity in AD, including in this review, where published findings often are limited by race-based analysis.
Epidemiology
The prevalence of AD has been increasing over the last few decades, and rates vary by region. In the United States, the prevalence of childhood and adult AD is 13% and 7%, respectively.17,18 Globally, higher rates of pediatric AD are seen in Africa, Oceania, Southeast Asia (SEA), and Latin America compared to South Asia, Northern Europe, and Eastern Europe.19 The prevalence of AD varies widely within the same continent and country; for example, throughout Africa, prevalence was found to be anywhere between 4.7% and 23.3%.20
Lesion Morphology
Although AD lesions often are described as pruritic erythematous papules and plaques, other common morphologies in SOC populations include prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions (Table). Instead of applying normative terms such as classic vs atypical to AD morphology, we urge clinicians to be familiar with the full spectrum of AD skin signs.
Prurigo Nodules—Prurigo nodules are hyperkeratotic or erosive nodules with severe pruritus, often grouped symmetrically on the extensor surfaces of the arms, legs, and trunk (Figure 1).14,21 The skin between lesions usually is unaffected but can be dry or lichenified or display postinflammatory pigmentary changes.14 Prurigo nodules are common. In a study of a cohort of patients with prurigo nodularis (N=108), nearly half (46.3%) were determined to have either an atopic predisposition or underlying AD as a contributing cause of the lesions.21
Prurigo nodules as a phenotype of AD may be more common in certain SOC populations. Studies from SEA have reported a higher prevalence of prurigo nodules among patients with AD.28 Although there are limited formal studies assessing the true prevalence of this lesion type in African American AD patients in the United States, clinical evidence supports more frequent appearance of prurigo nodules in non-White patients.29 Contributing factors include suboptimal care for AD in SOC populations and/or barriers to health care access, resulting in more severe disease that increases the risk for this lesion type.14
Lichenoid Papules—Papular lichenoid lesions often present on the extensor surfaces of the arms and legs in AD (Figure 2).22 In a study of Nigerian patients with AD (N=1019), 54.1% had lichenoid papules.24 A systematic review of AD characteristics by region similarly reported an increased prevalence of this lesion type in African studies.28 Lichenoid variants of AD have been well described in SOC patients in the United States.23 In contrast to the lesions of lichen planus, the lichenoid papules of AD usually are round, rarely display koebnerization, do not have Wickham striae, and predominantly are located on extensor surfaces.
Perifollicular Papules—Perifollicular accentuation—dermatitis enhanced around hair follicles—is a well-described lesional morphology of AD that is noted in all racial/ethnic groups (Figure 3).22 In fact, perifollicular accentuation is included as one of the Hanifin and Rajka minor criteria for AD.30 Studies performed in Nigeria and India showed perifollicular accentuation in up to 70% of AD patients.24,31 In a study of adult Thai patients (N=56), follicular lesions were found more frequently in intrinsic AD (29%) compared with extrinsic AD (12%).32
Nummular and Psoriasiform Lesions—Nummular lesions may be red, oozing, excoriated, studded with pustules and/or present on the extensor extremities (Figure 4). In SOC patients, these lesions often occur in areas where hyperpigmentation is noted.22 Studies in the United States and Mexico demonstrated that 15% to 17% of AD patients displayed nummular lesions.23,33 Similar to follicular papules, nummular lesions were linked to intrinsic AD in a study of adult Thai patients.32
Psoriasiform lesions show prominent scaling, lichenification, and clear demarcation.25 It has been reported that the psoriasiform phenotype of AD is more common in Asian patients,25 though this is likely an oversimplification. The participants in these studies were of Japanese and Korean ancestry, which covers a broad geographic region, and the grouping of individuals under a heterogeneous Asian category is unlikely to convey generalizable biologic or clinical information. Unsurprisingly, a systematic review of AD characteristics by region noted considerable phenotypical differences among patients in SEA, East Asia, Iran, and India.28
Disease Severity
Several factors contribute to AD disease severity,34 including objective assessments of inflammation, such as erythema and lichenification (Table), as well as subjective measures of symptoms, such as itch. The severity of AD is exacerbated by the social determinants of health, and a lower socioeconomic status, lower household income, lower parental education level and health, dilapidated housing, and presence of garbage on the street are among factors linked to worse AD disease severity.13,17 Although non-White individuals with AD often are reported to have more severe disease than their White counterparts,35 these types of health determinants may be the most relevant causes of observed differences.
Erythema—Erythema is a feature of inflammation used in the AD severity assessment. Erythema may appear in shades beyond red, including maroon, violaceous, or brown, in patients with darker pigmented skin, which may contribute to diagnosis of AD at a later disease stage.26 Multiple AD severity scoring tools, such as the SCORing Atopic Dermatitis and Eczema Area and Severity Index, include erythema as a measure, which can lead to underestimation of AD severity in SOC populations. After adjusting for erythema score, one study found that Black children with AD had a risk for severe disease that was 6-times higher than White children.36 Dermatological training must adequately teach physicians to recognize erythema across all skin tones.37
Erythroderma (also known as exfoliative dermatitis) is rapidly spreading erythema on at least 90% of the total body surface area, often sparing the palms and soles.32 Erythroderma is a potentially life-threatening manifestation of severe AD. Although erythroderma may have many underlying causes, AD has been reported to be the cause in 5% to 24% of cases,38 and compared to studies in Europe, the prevalence of erythroderma was higher in East Asian studies of AD.28
Excoriation and Pruritus—Pruritus is a defining characteristic of AD, and the resulting excoriations often are predominant on physical examination, which is a key part of severity scores. Itch is the most prevalent symptom among patients with AD, and a greater itch severity has been linked to decreased health-related quality of life, increased mental health symptoms, impaired sleep, and decreased daily function.39,40 The burden of itch may be greater in SOC populations. The impact of itch on quality of life among US military veterans was significantly higher in those who identified as non-White (P=.05).41 In another study of US military veterans, African American individuals reported a significantly higher emotional impact from itch (P<.05).42
Lichenification—Lichenification is thickening of the skin due to chronic rubbing and scratching that causes a leathery elevated appearance with exaggerated skin lines.27 Lichenification is included as a factor in common clinical scoring tools, with greater lichenification indicating greater disease severity. Studies from SEA and Africa suggested a higher prevalence of lichenification in AD patients.28 A greater itch burden and thus increased rubbing/scratching in these populations may contribute to some of these findings.42,43
Xerosis—Xerosis (or dry skin) is a common finding in AD that results from increased transepidermal water loss due to a dysfunctional epidermal barrier.44 In a systematic review of AD characteristics by region, xerosis was among the top 5 most reported AD features globally in all regions except SEA.28 Xerosis may be more stigmatizing in SOC populations because of the greater visibility of scaling and dryness on darker skin tones.1
Postinflammatory Dyspigmentation—Postinflammatory pigment alteration may be a consequence of AD lesions, resulting in hyperpigmented and hypopigmented macules and patches. Patients with AD with darker skin tones are more likely to develop postinflammatory dyspigmentation.26 A study of AD patients in Nigeria found that 63% displayed postinflammatory dyspigmentation.45 Dyschromia, including postinflammatory hyperpigmentation, is one of the most common reasons for SOC patients to seek dermatologic care.46 Postinflammatory pigment alteration can cause severe distress in patients, even more so than the cutaneous findings of AD. Although altered skin pigmentation usually returns to normal over weeks to months, skin depigmentation from chronic excoriation may be permanent.26 Appropriately treating hyperpigmentation and hypopigmentation in SOC populations can greatly improve quality of life.47
Conclusion
Atopic dermatitis is a cutaneous inflammatory disease that presents with many clinical phenotypes. Dermatologists should be trained to recognize the heterogeneous signs of AD present across the diverse skin types in SOC patients. Future research should move away from race-based analyses and focus on the complex interplay of environmental factors, social determinants of health, and skin pigmentation, as well as how these factors drive variations in AD lesional morphology and inflammation.
- Alexis A, Woolery-Lloyd H, Andriessen A, et al. Insights in skin of color patients with atopic dermatitis and the role of skincare in improving outcomes. J Drugs Dermatol. 2022;21:462-470. doi:10.36849/jdd.6609
- Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172-176. doi:10.36849/JDD.6408
- Taylor SC, Cook-Bolden F. Defining skin of color. Cutis. 2002;69:435-437.
- Georgetown University Center for Child and Human Development. Bridging the cultural divide in health care settings: the essential role of cultural broker programs. Accessed October 6, 2023. https://nccc.georgetown.edu/culturalbroker/8_Definitions/2_Definitions.html#:~:text=ethnic%3A%20Of%20or%20relating%20to,or%20cultural%20origin%20or%20background
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96-102. doi:10.1016/j.sder.2009.04.005
- US Census Bureau. About the topic of race. Revised March 1, 2022. Accessed October 5, 2023. https://www.census.gov/topics/population/race/about.html
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol. 2002;138:673-674. doi:10.1001/archderm.138.5.673
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871. doi:10.1001/archderm.124.6.869
- Amutah C, Greenidge K, Mante A, et al. Misrepresenting race—the role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872-878. doi:10.1056/NEJMms2025768
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26. doi:10.1080/1744666x.2016.1212660
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146. doi:10.1111/pde.14058
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31:489-492. doi:10.1111/pde.12355
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114. doi:10.1097/DER.0000000000000034
- Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol. 2018;121:622-624. doi:10.1016/j.anai.2018.07.019
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-8.e23. doi:10.1016/j.jaci.2009.10.009
- Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247-258. doi:10.1111/j.1398-9995.2007.01325.x
- Iking A, Grundmann S, Chatzigeorgakidis E, et al. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550-557. doi:10.1111/j.1468-3083.2012.04481.x
- Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35:354-359. doi:10.1016/j.clindermatol.2017.03.007
- Allen HB, Jones NP, Bowen SE. Lichenoid and other clinical presentations of atopic dermatitis in an inner city practice. J Am Acad Dermatol. 2008;58:503-504. doi:10.1016/j.jaad.2007.03.033
- Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739-744. doi:10.1111/j.1365-4632.2004.02360.x
- Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-1264. doi:10.1016/j.jaci.2015.08.015
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979. doi:10.1177/20406223211002979
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401. doi:10.1016/j.jaad.2018.09.035
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402. doi:10.1111/j.1525-1470.2012.01740.x
- Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44-47.
- Dutta A, De A, Das S, et al. A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-590. doi:10.4103/ijd.ijd_1046_20
- Kulthanan K, Boochangkool K, Tuchinda P, et al. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011;1:80-86. doi:10.5415/apallergy.2011.1.2.80
- Julián-Gónzalez RE, Orozco-Covarrubias L, Durán-McKinster C, et al. Less common clinical manifestations of atopic dermatitis: prevalence by age. Pediatr Dermatol. 2012;29:580-583. doi:10.1111/j.1525-1470.2012.01739.x
- Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: a review of the literature. J Am Acad Dermatol. 2022;87:688-689. doi:10.1016/j.jaad.2022.02.005
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- McKenzie S, Brown-Korsah JB, Syder NC, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87:1261-1270. doi:10.1016/j.jaad.2022.03.067
- Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. A practical approach to the diagnosis and treatment of adult erythroderma [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2018;109:777-790. doi:10.1016/j.ad.2018.05.011
- Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183:875-882. doi:10.1111/bjd.18920
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340-347. doi:10.1016/j.anai.2018.07.006
- Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150:613-620. doi:10.1001/jamadermatol.2013.7696
- Shaw FM, Luk KMH, Chen KH, et al. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63-69. doi:10.1016/j.jaad.2017.01.016
- Oh CC, Li H, Lee W, et al. Biopsychosocial factors associated with prurigo nodularis in endogenous eczema. Indian J Dermatol. 2015;60:525. doi:10.4103/0019-5154.164451
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, et al. Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci. 2018;40:549-554. doi:10.1111/ics.12496
- George AO. Atopic dermatitis in Nigeria. Int J Dermatol. 1989;28:237-239. doi:10.1111/j.1365-4362.1989.tb04811.x
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and post-inflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/jdd.2020.4937
Atopic dermatitis (AD) is a chronic inflammatory disorder that affects individuals worldwide.1 Although AD previously was commonly described as a skin-limited disease of childhood characterized by eczema in the flexural folds and pruritus, our current understanding supports a more heterogeneous condition.2 We review the wide range of cutaneous presentations of AD with a focus on clinical and morphological presentations across diverse skin types—commonly referred to as skin of color (SOC).
Defining SOC in Relation to AD
The terms SOC, race, and ethnicity are used interchangeably, but their true meanings are distinct. Traditionally, race has been defined as a biological concept, grouping cohorts of individuals with a large degree of shared ancestry and genetic similarities,3 and ethnicity as a social construct, grouping individuals with common racial, national, tribal, religious, linguistic, or cultural backgrounds.4 In practice, both concepts can broadly be envisioned as mixed social, political, and economic constructs, as no one gene or biologic characteristic distinguishes one racial or ethnic group from another.5
The US Census Bureau recognizes 5 racial groupings: White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.6 Hispanic or Latinx origin is considered an ethnicity. It is important to note the limitations of these labels, as they do not completely encapsulate the heterogeneity of the US population. Overgeneralization of racial and ethnic categories may dull or obscure true differences among populations.7
From an evolutionary perspective, skin pigmentation represents the product of 2 opposing clines produced by natural selection in response to both need for and protection from UV radiation across lattitudes.8 Defining SOC is not quite as simple. Skin of color often is equated with certain racial/ethnic groups, or even binary categories of Black vs non-Black or White vs non-White. Others may use the Fitzpatrick scale to discuss SOC, though this scale was originally created to measure the response of skin to UVA radiation exposure.9 The reality is that SOC is a complex term that cannot simply be defined by a certain group of skin tones, races, ethnicities, and/or Fitzpatrick skin types. With this in mind, SOC in the context of this article will often refer to non-White individuals based on the investigators’ terminology, but this definition is not all-encompassing.
Historically in medicine, racial/ethnic differences in outcomes have been equated to differences in biology/genetics without consideration of many external factors.10 The effects of racism, economic stability, health care access, environment, and education quality rarely are discussed, though they have a major impact on health and may better define associations with race or an SOC population. A discussion of the structural and social determinants of health contributing to disease outcomes should accompany any race-based guidelines to prevent inaccurately pathologizing race or SOC.10
Within the scope of AD, social determinants of health play an important role in contributing to disease morbidity. Environmental factors, including tobacco smoke, climate, pollutants, water hardness, und urban living, are related to AD prevalence and severity.11 Higher socioeconomic status is associated with increased AD rates,12 yet lower socioeconomic status is associated with more severe disease.13 Barriers to health care access and suboptimal care drive worse AD outcomes.14 Underrepresentation in clinical trials prevents the generalizability and safety of AD treatments.15 Disparities in these health determinants associated with AD likely are among the most important drivers of observed differences in disease presentation, severity, burden, and even prevalence—more so than genetics or ancestry alone16—yet this relationship is poorly understood and often presented as a consequence of race. It is critical to redefine the narrative when considering the heterogeneous presentations of AD in patients with SOC and acknowledge the limitations of current terminology when attempting to capture clinical diversity in AD, including in this review, where published findings often are limited by race-based analysis.
Epidemiology
The prevalence of AD has been increasing over the last few decades, and rates vary by region. In the United States, the prevalence of childhood and adult AD is 13% and 7%, respectively.17,18 Globally, higher rates of pediatric AD are seen in Africa, Oceania, Southeast Asia (SEA), and Latin America compared to South Asia, Northern Europe, and Eastern Europe.19 The prevalence of AD varies widely within the same continent and country; for example, throughout Africa, prevalence was found to be anywhere between 4.7% and 23.3%.20
Lesion Morphology
Although AD lesions often are described as pruritic erythematous papules and plaques, other common morphologies in SOC populations include prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions (Table). Instead of applying normative terms such as classic vs atypical to AD morphology, we urge clinicians to be familiar with the full spectrum of AD skin signs.

Prurigo Nodules—Prurigo nodules are hyperkeratotic or erosive nodules with severe pruritus, often grouped symmetrically on the extensor surfaces of the arms, legs, and trunk (Figure 1).14,21 The skin between lesions usually is unaffected but can be dry or lichenified or display postinflammatory pigmentary changes.14 Prurigo nodules are common. In a study of a cohort of patients with prurigo nodularis (N=108), nearly half (46.3%) were determined to have either an atopic predisposition or underlying AD as a contributing cause of the lesions.21

Prurigo nodules as a phenotype of AD may be more common in certain SOC populations. Studies from SEA have reported a higher prevalence of prurigo nodules among patients with AD.28 Although there are limited formal studies assessing the true prevalence of this lesion type in African American AD patients in the United States, clinical evidence supports more frequent appearance of prurigo nodules in non-White patients.29 Contributing factors include suboptimal care for AD in SOC populations and/or barriers to health care access, resulting in more severe disease that increases the risk for this lesion type.14
Lichenoid Papules—Papular lichenoid lesions often present on the extensor surfaces of the arms and legs in AD (Figure 2).22 In a study of Nigerian patients with AD (N=1019), 54.1% had lichenoid papules.24 A systematic review of AD characteristics by region similarly reported an increased prevalence of this lesion type in African studies.28 Lichenoid variants of AD have been well described in SOC patients in the United States.23 In contrast to the lesions of lichen planus, the lichenoid papules of AD usually are round, rarely display koebnerization, do not have Wickham striae, and predominantly are located on extensor surfaces.

Perifollicular Papules—Perifollicular accentuation—dermatitis enhanced around hair follicles—is a well-described lesional morphology of AD that is noted in all racial/ethnic groups (Figure 3).22 In fact, perifollicular accentuation is included as one of the Hanifin and Rajka minor criteria for AD.30 Studies performed in Nigeria and India showed perifollicular accentuation in up to 70% of AD patients.24,31 In a study of adult Thai patients (N=56), follicular lesions were found more frequently in intrinsic AD (29%) compared with extrinsic AD (12%).32
Nummular and Psoriasiform Lesions—Nummular lesions may be red, oozing, excoriated, studded with pustules and/or present on the extensor extremities (Figure 4). In SOC patients, these lesions often occur in areas where hyperpigmentation is noted.22 Studies in the United States and Mexico demonstrated that 15% to 17% of AD patients displayed nummular lesions.23,33 Similar to follicular papules, nummular lesions were linked to intrinsic AD in a study of adult Thai patients.32

Psoriasiform lesions show prominent scaling, lichenification, and clear demarcation.25 It has been reported that the psoriasiform phenotype of AD is more common in Asian patients,25 though this is likely an oversimplification. The participants in these studies were of Japanese and Korean ancestry, which covers a broad geographic region, and the grouping of individuals under a heterogeneous Asian category is unlikely to convey generalizable biologic or clinical information. Unsurprisingly, a systematic review of AD characteristics by region noted considerable phenotypical differences among patients in SEA, East Asia, Iran, and India.28
Disease Severity
Several factors contribute to AD disease severity,34 including objective assessments of inflammation, such as erythema and lichenification (Table), as well as subjective measures of symptoms, such as itch. The severity of AD is exacerbated by the social determinants of health, and a lower socioeconomic status, lower household income, lower parental education level and health, dilapidated housing, and presence of garbage on the street are among factors linked to worse AD disease severity.13,17 Although non-White individuals with AD often are reported to have more severe disease than their White counterparts,35 these types of health determinants may be the most relevant causes of observed differences.
Erythema—Erythema is a feature of inflammation used in the AD severity assessment. Erythema may appear in shades beyond red, including maroon, violaceous, or brown, in patients with darker pigmented skin, which may contribute to diagnosis of AD at a later disease stage.26 Multiple AD severity scoring tools, such as the SCORing Atopic Dermatitis and Eczema Area and Severity Index, include erythema as a measure, which can lead to underestimation of AD severity in SOC populations. After adjusting for erythema score, one study found that Black children with AD had a risk for severe disease that was 6-times higher than White children.36 Dermatological training must adequately teach physicians to recognize erythema across all skin tones.37
Erythroderma (also known as exfoliative dermatitis) is rapidly spreading erythema on at least 90% of the total body surface area, often sparing the palms and soles.32 Erythroderma is a potentially life-threatening manifestation of severe AD. Although erythroderma may have many underlying causes, AD has been reported to be the cause in 5% to 24% of cases,38 and compared to studies in Europe, the prevalence of erythroderma was higher in East Asian studies of AD.28
Excoriation and Pruritus—Pruritus is a defining characteristic of AD, and the resulting excoriations often are predominant on physical examination, which is a key part of severity scores. Itch is the most prevalent symptom among patients with AD, and a greater itch severity has been linked to decreased health-related quality of life, increased mental health symptoms, impaired sleep, and decreased daily function.39,40 The burden of itch may be greater in SOC populations. The impact of itch on quality of life among US military veterans was significantly higher in those who identified as non-White (P=.05).41 In another study of US military veterans, African American individuals reported a significantly higher emotional impact from itch (P<.05).42
Lichenification—Lichenification is thickening of the skin due to chronic rubbing and scratching that causes a leathery elevated appearance with exaggerated skin lines.27 Lichenification is included as a factor in common clinical scoring tools, with greater lichenification indicating greater disease severity. Studies from SEA and Africa suggested a higher prevalence of lichenification in AD patients.28 A greater itch burden and thus increased rubbing/scratching in these populations may contribute to some of these findings.42,43
Xerosis—Xerosis (or dry skin) is a common finding in AD that results from increased transepidermal water loss due to a dysfunctional epidermal barrier.44 In a systematic review of AD characteristics by region, xerosis was among the top 5 most reported AD features globally in all regions except SEA.28 Xerosis may be more stigmatizing in SOC populations because of the greater visibility of scaling and dryness on darker skin tones.1
Postinflammatory Dyspigmentation—Postinflammatory pigment alteration may be a consequence of AD lesions, resulting in hyperpigmented and hypopigmented macules and patches. Patients with AD with darker skin tones are more likely to develop postinflammatory dyspigmentation.26 A study of AD patients in Nigeria found that 63% displayed postinflammatory dyspigmentation.45 Dyschromia, including postinflammatory hyperpigmentation, is one of the most common reasons for SOC patients to seek dermatologic care.46 Postinflammatory pigment alteration can cause severe distress in patients, even more so than the cutaneous findings of AD. Although altered skin pigmentation usually returns to normal over weeks to months, skin depigmentation from chronic excoriation may be permanent.26 Appropriately treating hyperpigmentation and hypopigmentation in SOC populations can greatly improve quality of life.47
Conclusion
Atopic dermatitis is a cutaneous inflammatory disease that presents with many clinical phenotypes. Dermatologists should be trained to recognize the heterogeneous signs of AD present across the diverse skin types in SOC patients. Future research should move away from race-based analyses and focus on the complex interplay of environmental factors, social determinants of health, and skin pigmentation, as well as how these factors drive variations in AD lesional morphology and inflammation.
Atopic dermatitis (AD) is a chronic inflammatory disorder that affects individuals worldwide.1 Although AD previously was commonly described as a skin-limited disease of childhood characterized by eczema in the flexural folds and pruritus, our current understanding supports a more heterogeneous condition.2 We review the wide range of cutaneous presentations of AD with a focus on clinical and morphological presentations across diverse skin types—commonly referred to as skin of color (SOC).
Defining SOC in Relation to AD
The terms SOC, race, and ethnicity are used interchangeably, but their true meanings are distinct. Traditionally, race has been defined as a biological concept, grouping cohorts of individuals with a large degree of shared ancestry and genetic similarities,3 and ethnicity as a social construct, grouping individuals with common racial, national, tribal, religious, linguistic, or cultural backgrounds.4 In practice, both concepts can broadly be envisioned as mixed social, political, and economic constructs, as no one gene or biologic characteristic distinguishes one racial or ethnic group from another.5
The US Census Bureau recognizes 5 racial groupings: White, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.6 Hispanic or Latinx origin is considered an ethnicity. It is important to note the limitations of these labels, as they do not completely encapsulate the heterogeneity of the US population. Overgeneralization of racial and ethnic categories may dull or obscure true differences among populations.7
From an evolutionary perspective, skin pigmentation represents the product of 2 opposing clines produced by natural selection in response to both need for and protection from UV radiation across lattitudes.8 Defining SOC is not quite as simple. Skin of color often is equated with certain racial/ethnic groups, or even binary categories of Black vs non-Black or White vs non-White. Others may use the Fitzpatrick scale to discuss SOC, though this scale was originally created to measure the response of skin to UVA radiation exposure.9 The reality is that SOC is a complex term that cannot simply be defined by a certain group of skin tones, races, ethnicities, and/or Fitzpatrick skin types. With this in mind, SOC in the context of this article will often refer to non-White individuals based on the investigators’ terminology, but this definition is not all-encompassing.
Historically in medicine, racial/ethnic differences in outcomes have been equated to differences in biology/genetics without consideration of many external factors.10 The effects of racism, economic stability, health care access, environment, and education quality rarely are discussed, though they have a major impact on health and may better define associations with race or an SOC population. A discussion of the structural and social determinants of health contributing to disease outcomes should accompany any race-based guidelines to prevent inaccurately pathologizing race or SOC.10
Within the scope of AD, social determinants of health play an important role in contributing to disease morbidity. Environmental factors, including tobacco smoke, climate, pollutants, water hardness, und urban living, are related to AD prevalence and severity.11 Higher socioeconomic status is associated with increased AD rates,12 yet lower socioeconomic status is associated with more severe disease.13 Barriers to health care access and suboptimal care drive worse AD outcomes.14 Underrepresentation in clinical trials prevents the generalizability and safety of AD treatments.15 Disparities in these health determinants associated with AD likely are among the most important drivers of observed differences in disease presentation, severity, burden, and even prevalence—more so than genetics or ancestry alone16—yet this relationship is poorly understood and often presented as a consequence of race. It is critical to redefine the narrative when considering the heterogeneous presentations of AD in patients with SOC and acknowledge the limitations of current terminology when attempting to capture clinical diversity in AD, including in this review, where published findings often are limited by race-based analysis.
Epidemiology
The prevalence of AD has been increasing over the last few decades, and rates vary by region. In the United States, the prevalence of childhood and adult AD is 13% and 7%, respectively.17,18 Globally, higher rates of pediatric AD are seen in Africa, Oceania, Southeast Asia (SEA), and Latin America compared to South Asia, Northern Europe, and Eastern Europe.19 The prevalence of AD varies widely within the same continent and country; for example, throughout Africa, prevalence was found to be anywhere between 4.7% and 23.3%.20
Lesion Morphology
Although AD lesions often are described as pruritic erythematous papules and plaques, other common morphologies in SOC populations include prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions (Table). Instead of applying normative terms such as classic vs atypical to AD morphology, we urge clinicians to be familiar with the full spectrum of AD skin signs.

Prurigo Nodules—Prurigo nodules are hyperkeratotic or erosive nodules with severe pruritus, often grouped symmetrically on the extensor surfaces of the arms, legs, and trunk (Figure 1).14,21 The skin between lesions usually is unaffected but can be dry or lichenified or display postinflammatory pigmentary changes.14 Prurigo nodules are common. In a study of a cohort of patients with prurigo nodularis (N=108), nearly half (46.3%) were determined to have either an atopic predisposition or underlying AD as a contributing cause of the lesions.21

Prurigo nodules as a phenotype of AD may be more common in certain SOC populations. Studies from SEA have reported a higher prevalence of prurigo nodules among patients with AD.28 Although there are limited formal studies assessing the true prevalence of this lesion type in African American AD patients in the United States, clinical evidence supports more frequent appearance of prurigo nodules in non-White patients.29 Contributing factors include suboptimal care for AD in SOC populations and/or barriers to health care access, resulting in more severe disease that increases the risk for this lesion type.14
Lichenoid Papules—Papular lichenoid lesions often present on the extensor surfaces of the arms and legs in AD (Figure 2).22 In a study of Nigerian patients with AD (N=1019), 54.1% had lichenoid papules.24 A systematic review of AD characteristics by region similarly reported an increased prevalence of this lesion type in African studies.28 Lichenoid variants of AD have been well described in SOC patients in the United States.23 In contrast to the lesions of lichen planus, the lichenoid papules of AD usually are round, rarely display koebnerization, do not have Wickham striae, and predominantly are located on extensor surfaces.

Perifollicular Papules—Perifollicular accentuation—dermatitis enhanced around hair follicles—is a well-described lesional morphology of AD that is noted in all racial/ethnic groups (Figure 3).22 In fact, perifollicular accentuation is included as one of the Hanifin and Rajka minor criteria for AD.30 Studies performed in Nigeria and India showed perifollicular accentuation in up to 70% of AD patients.24,31 In a study of adult Thai patients (N=56), follicular lesions were found more frequently in intrinsic AD (29%) compared with extrinsic AD (12%).32
Nummular and Psoriasiform Lesions—Nummular lesions may be red, oozing, excoriated, studded with pustules and/or present on the extensor extremities (Figure 4). In SOC patients, these lesions often occur in areas where hyperpigmentation is noted.22 Studies in the United States and Mexico demonstrated that 15% to 17% of AD patients displayed nummular lesions.23,33 Similar to follicular papules, nummular lesions were linked to intrinsic AD in a study of adult Thai patients.32

Psoriasiform lesions show prominent scaling, lichenification, and clear demarcation.25 It has been reported that the psoriasiform phenotype of AD is more common in Asian patients,25 though this is likely an oversimplification. The participants in these studies were of Japanese and Korean ancestry, which covers a broad geographic region, and the grouping of individuals under a heterogeneous Asian category is unlikely to convey generalizable biologic or clinical information. Unsurprisingly, a systematic review of AD characteristics by region noted considerable phenotypical differences among patients in SEA, East Asia, Iran, and India.28
Disease Severity
Several factors contribute to AD disease severity,34 including objective assessments of inflammation, such as erythema and lichenification (Table), as well as subjective measures of symptoms, such as itch. The severity of AD is exacerbated by the social determinants of health, and a lower socioeconomic status, lower household income, lower parental education level and health, dilapidated housing, and presence of garbage on the street are among factors linked to worse AD disease severity.13,17 Although non-White individuals with AD often are reported to have more severe disease than their White counterparts,35 these types of health determinants may be the most relevant causes of observed differences.
Erythema—Erythema is a feature of inflammation used in the AD severity assessment. Erythema may appear in shades beyond red, including maroon, violaceous, or brown, in patients with darker pigmented skin, which may contribute to diagnosis of AD at a later disease stage.26 Multiple AD severity scoring tools, such as the SCORing Atopic Dermatitis and Eczema Area and Severity Index, include erythema as a measure, which can lead to underestimation of AD severity in SOC populations. After adjusting for erythema score, one study found that Black children with AD had a risk for severe disease that was 6-times higher than White children.36 Dermatological training must adequately teach physicians to recognize erythema across all skin tones.37
Erythroderma (also known as exfoliative dermatitis) is rapidly spreading erythema on at least 90% of the total body surface area, often sparing the palms and soles.32 Erythroderma is a potentially life-threatening manifestation of severe AD. Although erythroderma may have many underlying causes, AD has been reported to be the cause in 5% to 24% of cases,38 and compared to studies in Europe, the prevalence of erythroderma was higher in East Asian studies of AD.28
Excoriation and Pruritus—Pruritus is a defining characteristic of AD, and the resulting excoriations often are predominant on physical examination, which is a key part of severity scores. Itch is the most prevalent symptom among patients with AD, and a greater itch severity has been linked to decreased health-related quality of life, increased mental health symptoms, impaired sleep, and decreased daily function.39,40 The burden of itch may be greater in SOC populations. The impact of itch on quality of life among US military veterans was significantly higher in those who identified as non-White (P=.05).41 In another study of US military veterans, African American individuals reported a significantly higher emotional impact from itch (P<.05).42
Lichenification—Lichenification is thickening of the skin due to chronic rubbing and scratching that causes a leathery elevated appearance with exaggerated skin lines.27 Lichenification is included as a factor in common clinical scoring tools, with greater lichenification indicating greater disease severity. Studies from SEA and Africa suggested a higher prevalence of lichenification in AD patients.28 A greater itch burden and thus increased rubbing/scratching in these populations may contribute to some of these findings.42,43
Xerosis—Xerosis (or dry skin) is a common finding in AD that results from increased transepidermal water loss due to a dysfunctional epidermal barrier.44 In a systematic review of AD characteristics by region, xerosis was among the top 5 most reported AD features globally in all regions except SEA.28 Xerosis may be more stigmatizing in SOC populations because of the greater visibility of scaling and dryness on darker skin tones.1
Postinflammatory Dyspigmentation—Postinflammatory pigment alteration may be a consequence of AD lesions, resulting in hyperpigmented and hypopigmented macules and patches. Patients with AD with darker skin tones are more likely to develop postinflammatory dyspigmentation.26 A study of AD patients in Nigeria found that 63% displayed postinflammatory dyspigmentation.45 Dyschromia, including postinflammatory hyperpigmentation, is one of the most common reasons for SOC patients to seek dermatologic care.46 Postinflammatory pigment alteration can cause severe distress in patients, even more so than the cutaneous findings of AD. Although altered skin pigmentation usually returns to normal over weeks to months, skin depigmentation from chronic excoriation may be permanent.26 Appropriately treating hyperpigmentation and hypopigmentation in SOC populations can greatly improve quality of life.47
Conclusion
Atopic dermatitis is a cutaneous inflammatory disease that presents with many clinical phenotypes. Dermatologists should be trained to recognize the heterogeneous signs of AD present across the diverse skin types in SOC patients. Future research should move away from race-based analyses and focus on the complex interplay of environmental factors, social determinants of health, and skin pigmentation, as well as how these factors drive variations in AD lesional morphology and inflammation.
- Alexis A, Woolery-Lloyd H, Andriessen A, et al. Insights in skin of color patients with atopic dermatitis and the role of skincare in improving outcomes. J Drugs Dermatol. 2022;21:462-470. doi:10.36849/jdd.6609
- Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172-176. doi:10.36849/JDD.6408
- Taylor SC, Cook-Bolden F. Defining skin of color. Cutis. 2002;69:435-437.
- Georgetown University Center for Child and Human Development. Bridging the cultural divide in health care settings: the essential role of cultural broker programs. Accessed October 6, 2023. https://nccc.georgetown.edu/culturalbroker/8_Definitions/2_Definitions.html#:~:text=ethnic%3A%20Of%20or%20relating%20to,or%20cultural%20origin%20or%20background
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96-102. doi:10.1016/j.sder.2009.04.005
- US Census Bureau. About the topic of race. Revised March 1, 2022. Accessed October 5, 2023. https://www.census.gov/topics/population/race/about.html
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol. 2002;138:673-674. doi:10.1001/archderm.138.5.673
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871. doi:10.1001/archderm.124.6.869
- Amutah C, Greenidge K, Mante A, et al. Misrepresenting race—the role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872-878. doi:10.1056/NEJMms2025768
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26. doi:10.1080/1744666x.2016.1212660
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146. doi:10.1111/pde.14058
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31:489-492. doi:10.1111/pde.12355
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114. doi:10.1097/DER.0000000000000034
- Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol. 2018;121:622-624. doi:10.1016/j.anai.2018.07.019
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-8.e23. doi:10.1016/j.jaci.2009.10.009
- Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247-258. doi:10.1111/j.1398-9995.2007.01325.x
- Iking A, Grundmann S, Chatzigeorgakidis E, et al. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550-557. doi:10.1111/j.1468-3083.2012.04481.x
- Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35:354-359. doi:10.1016/j.clindermatol.2017.03.007
- Allen HB, Jones NP, Bowen SE. Lichenoid and other clinical presentations of atopic dermatitis in an inner city practice. J Am Acad Dermatol. 2008;58:503-504. doi:10.1016/j.jaad.2007.03.033
- Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739-744. doi:10.1111/j.1365-4632.2004.02360.x
- Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-1264. doi:10.1016/j.jaci.2015.08.015
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979. doi:10.1177/20406223211002979
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401. doi:10.1016/j.jaad.2018.09.035
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402. doi:10.1111/j.1525-1470.2012.01740.x
- Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44-47.
- Dutta A, De A, Das S, et al. A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-590. doi:10.4103/ijd.ijd_1046_20
- Kulthanan K, Boochangkool K, Tuchinda P, et al. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011;1:80-86. doi:10.5415/apallergy.2011.1.2.80
- Julián-Gónzalez RE, Orozco-Covarrubias L, Durán-McKinster C, et al. Less common clinical manifestations of atopic dermatitis: prevalence by age. Pediatr Dermatol. 2012;29:580-583. doi:10.1111/j.1525-1470.2012.01739.x
- Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: a review of the literature. J Am Acad Dermatol. 2022;87:688-689. doi:10.1016/j.jaad.2022.02.005
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- McKenzie S, Brown-Korsah JB, Syder NC, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87:1261-1270. doi:10.1016/j.jaad.2022.03.067
- Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. A practical approach to the diagnosis and treatment of adult erythroderma [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2018;109:777-790. doi:10.1016/j.ad.2018.05.011
- Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183:875-882. doi:10.1111/bjd.18920
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340-347. doi:10.1016/j.anai.2018.07.006
- Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150:613-620. doi:10.1001/jamadermatol.2013.7696
- Shaw FM, Luk KMH, Chen KH, et al. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63-69. doi:10.1016/j.jaad.2017.01.016
- Oh CC, Li H, Lee W, et al. Biopsychosocial factors associated with prurigo nodularis in endogenous eczema. Indian J Dermatol. 2015;60:525. doi:10.4103/0019-5154.164451
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, et al. Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci. 2018;40:549-554. doi:10.1111/ics.12496
- George AO. Atopic dermatitis in Nigeria. Int J Dermatol. 1989;28:237-239. doi:10.1111/j.1365-4362.1989.tb04811.x
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and post-inflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/jdd.2020.4937
- Alexis A, Woolery-Lloyd H, Andriessen A, et al. Insights in skin of color patients with atopic dermatitis and the role of skincare in improving outcomes. J Drugs Dermatol. 2022;21:462-470. doi:10.36849/jdd.6609
- Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21:172-176. doi:10.36849/JDD.6408
- Taylor SC, Cook-Bolden F. Defining skin of color. Cutis. 2002;69:435-437.
- Georgetown University Center for Child and Human Development. Bridging the cultural divide in health care settings: the essential role of cultural broker programs. Accessed October 6, 2023. https://nccc.georgetown.edu/culturalbroker/8_Definitions/2_Definitions.html#:~:text=ethnic%3A%20Of%20or%20relating%20to,or%20cultural%20origin%20or%20background
- Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96-102. doi:10.1016/j.sder.2009.04.005
- US Census Bureau. About the topic of race. Revised March 1, 2022. Accessed October 5, 2023. https://www.census.gov/topics/population/race/about.html
- Williams HC. Have you ever seen an Asian/Pacific Islander? Arch Dermatol. 2002;138:673-674. doi:10.1001/archderm.138.5.673
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871. doi:10.1001/archderm.124.6.869
- Amutah C, Greenidge K, Mante A, et al. Misrepresenting race—the role of medical schools in propagating physician bias. N Engl J Med. 2021;384:872-878. doi:10.1056/NEJMms2025768
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15-26. doi:10.1080/1744666x.2016.1212660
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146. doi:10.1111/pde.14058
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31:489-492. doi:10.1111/pde.12355
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114. doi:10.1097/DER.0000000000000034
- Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol. 2018;121:622-624. doi:10.1016/j.anai.2018.07.019
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-8.e23. doi:10.1016/j.jaci.2009.10.009
- Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247-258. doi:10.1111/j.1398-9995.2007.01325.x
- Iking A, Grundmann S, Chatzigeorgakidis E, et al. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27:550-557. doi:10.1111/j.1468-3083.2012.04481.x
- Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol. 2017;35:354-359. doi:10.1016/j.clindermatol.2017.03.007
- Allen HB, Jones NP, Bowen SE. Lichenoid and other clinical presentations of atopic dermatitis in an inner city practice. J Am Acad Dermatol. 2008;58:503-504. doi:10.1016/j.jaad.2007.03.033
- Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739-744. doi:10.1111/j.1365-4632.2004.02360.x
- Noda S, Suárez-Fariñas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-1264. doi:10.1016/j.jaci.2015.08.015
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Girolomoni G, de Bruin-Weller M, Aoki V, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979. doi:10.1177/20406223211002979
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401. doi:10.1016/j.jaad.2018.09.035
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402. doi:10.1111/j.1525-1470.2012.01740.x
- Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44-47.
- Dutta A, De A, Das S, et al. A cross-sectional evaluation of the usefulness of the minor features of Hanifin and Rajka diagnostic criteria for the diagnosis of atopic dermatitis in the pediatric population. Indian J Dermatol. 2021;66:583-590. doi:10.4103/ijd.ijd_1046_20
- Kulthanan K, Boochangkool K, Tuchinda P, et al. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011;1:80-86. doi:10.5415/apallergy.2011.1.2.80
- Julián-Gónzalez RE, Orozco-Covarrubias L, Durán-McKinster C, et al. Less common clinical manifestations of atopic dermatitis: prevalence by age. Pediatr Dermatol. 2012;29:580-583. doi:10.1111/j.1525-1470.2012.01739.x
- Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: a review of the literature. J Am Acad Dermatol. 2022;87:688-689. doi:10.1016/j.jaad.2022.02.005
- Kim Y, Blomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- McKenzie S, Brown-Korsah JB, Syder NC, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87:1261-1270. doi:10.1016/j.jaad.2022.03.067
- Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. A practical approach to the diagnosis and treatment of adult erythroderma [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2018;109:777-790. doi:10.1016/j.ad.2018.05.011
- Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183:875-882. doi:10.1111/bjd.18920
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121:340-347. doi:10.1016/j.anai.2018.07.006
- Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150:613-620. doi:10.1001/jamadermatol.2013.7696
- Shaw FM, Luk KMH, Chen KH, et al. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77:63-69. doi:10.1016/j.jaad.2017.01.016
- Oh CC, Li H, Lee W, et al. Biopsychosocial factors associated with prurigo nodularis in endogenous eczema. Indian J Dermatol. 2015;60:525. doi:10.4103/0019-5154.164451
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, et al. Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci. 2018;40:549-554. doi:10.1111/ics.12496
- George AO. Atopic dermatitis in Nigeria. Int J Dermatol. 1989;28:237-239. doi:10.1111/j.1365-4362.1989.tb04811.x
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and post-inflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/jdd.2020.4937
Practice Points
- Social determinants of health play a central role in observed racial and ethnic differences in studies of atopic dermatitis (AD) in patients with skin of color.
- Prurigo nodules, lichenoid papules, perifollicular papules, nummular lesions, and psoriasiform lesions are among the diverse lesion morphologies seen with AD.
- Key signs of cutaneous inflammation and lesional severity, including erythema, may present differently in darker skin tones and contribute to underestimation of severity.
- Postinflammatory dyspigmentation is common among patients with skin of color, and treatment can substantially improve quality of life.
Implementing shared decision making in labor and delivery: TeamBirth is a model for person-centered birthing care
CASE The TeamBirth experience: Making a difference
“At a community hospital in Washington where we had implemented TeamBirth (a labor and delivery shared decision making model), a patient, her partner, a labor and delivery nurse, and myself (an ObGyn) were making a plan for the patient’s induction of labor admission. I asked the patient, a 29-year-old (G2P1001), how we could improve her care in relation to her first birth. Her answer was simple: I want to be treated with respect. Her partner went on to describe their past experience in which the provider was inappropriately texting while in between the patient’s knees during delivery. Our team had the opportunity to undo some of the trauma from her first birth. That’s what I like about TeamBirth. It gives every patient the opportunity, regardless of their background, to define safety and participate in their care experience.”
–Angela Chien, MD, Obstetrician and Quality Improvement leader, Washington
Unfortunately, disrespect and mistreatment are far from an anomaly in the obstetrics setting. In a systematic review of respectful maternity care, the World Health Organization delineated 7 dimensions of maternal mistreatment: physical abuse, sexual abuse, verbal abuse, stigma and discrimination, failure to meet professional standards of care, poor rapport between women and providers, and poor conditions and constraints presented by the health system.1 In 2019, the Giving Voice to Mothers study showed that 17% of birthing people in the United States reported experiencing 1 or more types of maternal mistreatment.2 Rates of mistreatment were disproportionately greater in populations of color, hospital-based births, and among those with social, economic, or health challenges.2 It is well known that Black and African American and American Indian and Alaska Native populations experience the rare events of severe maternal morbidity and mortality more frequently than their White counterparts; the disproportionate burden of mistreatment is lesser known and far more common.
Overlooking the longitudinal harm of a negative birth experience has cascading impact. While an empowering perinatal experience can foster preventive screening and management of chronic disease, a poor experience conversely can seed mistrust at an individual, generational, and community level.
The patient quality enterprise is beginning to shift attention toward maternal experience with the development of PREMs (patient-reported experience measures), PROMs (patient-reported outcome measures), and novel validated scales that assess autonomy and trust.3 Development of a maternal Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey on childbirth is forthcoming.4 Of course, continuing to prioritize physical safety through initiatives on blood pressure monitoring and severe maternal morbidity and mortality remains paramount. Yet emotional and psychological safety also must be recognized as essential pillars of patient safety. Transgressions related to autonomy and dignity, as well as racism, sexism, classicism, and ableism, should be treated as “adverse and never events.”5
How the TeamBirth model works
Shared decision making (SDM) is cited in medical pedagogy as the solution to respectfullyrecognizing social context, integrating subjective experience, and honoring patient autonomy.6 The onus has always been on individual clinicians to exercise SDM. A new practice model, TeamBirth, embeds SDM into the culture and workflow. It offers a behavioral framework to mitigate implicit bias and operationalizes SDM tools, such that every patient is an empowered participant in their care.
TeamBirth was created through Ariadne Labs’ Delivery Decisions Initiative, a research and social impact program that designs, tests, and scales transformative, systems-level solutions that promote quality, equity, and dignity in childbirth. By the end of 2023, TeamBirth will be implemented in more than 100 hospitals across the United States, cumulatively touching over 200,000 lives. (For more information on the TeamBirth model, view the “Why TeamBirth” video at: https://www.youtube.com/watch?v=EoVrSaGk7gc.)
The tenets of TeamBirth are enacted through a patient-facing, shared whiteboard or dry-erase planning board in the labor room (FIGURE 1). Research has demonstrated how dry-erase boards in clinical settings can support safety and dignity in care, especially to improve patient-provider communication, teamwork, and patient satisfaction.7,8 The planning board is initially filled out by a clinical team member and is updated during team “huddles” throughout labor.

Huddles are care plan discussions with the full care team (the patient, nurse, doula and/or other support person(s), delivering provider, and interpreter or social worker as needed). At a minimum, huddles occur on admission, with changes to the clinical course and care plan, and at the request of any team member. Huddles can transpire through in-person, virtual, or phone communication.9 The concept builds on interdisciplinary and patient-centered rounding and establishes a communication system that is suited to the dynamic environment and amplified patient autonomy unique to labor and delivery. Dr. Bob Barbieri, a steadfast leader and champion of TeamBirth implementation at Brigham and Women’s Hospital in Boston (and the Editor in Chief of OBG M
Continue to: Patient response to TeamBirth is positive...
Patient response to TeamBirth is positive
Patients and providers alike have endorsed TeamBirth. In initial pilot testing across 4 sites, 99% of all patients surveyed “definitely” or “somewhat” had the role they wanted in making decisions about their labor.9
In partnership with the Oklahoma Perinatal Quality Improvement Collaborative (OPQIC), the impact of TeamBirth was assessed in a statewide patient cohort (n = 3,121) using the validated Mothers Autonomy in Decision Making (MADM) scale created by the Birth Place Lab at the University of British Columbia. The percentage of patients who scored in the highest MADM quartile was 31.3% higher for patients who indicated participation in a huddle during labor compared with those who did not participate in a huddle. This trend held across all racial and ethnic groups: For example, 93% of non-Hispanic Black/African American patients who had a TeamBirth huddle reported high autonomy, a nearly 20 percentage point increase from those without a huddle (FIGURE 2). Similarly, a higher percentage of agreement was observed across all 7 items in the MADM scale for patients who reported a TeamBirth huddle (FIGURE 3). TeamBirth’s effect has been observed across surveys and multiple validated metrics.
Data collection related to TeamBirth continues to be ongoing, with reported values retrieved on July 14, 2023. Rigorous review of patient-reported outcomes is forthcoming, and assessing impact on clinical outcomes, such as NTSV (nulliparous, term, singleton vertex) cesarean delivery rates and severe maternal morbidity, is on the horizon.
Qualitative survey responses reinforce how patients value TeamBirth and appreciate huddles and whiteboards.
Continue to: Patient testimonials...
Patient testimonials
The following testimonials were obtained from a TeamBirth survey that patients in participating Massachusetts hospitals completed in the postpartum unit prior to discharge.
According to one patient, “TeamBirth is great, feels like all obstacles are covered by multiple people with many talents, expertise. Feels like mom is part of the process, much different than my delivery 2 years ago when I felt like things were decided for me/I was ‘told’ what we were doing and questioned if I felt uneasy about it…. We felt safe and like all things were covered no matter what may happen.”
Another patient, also at a Massachusetts hospital, offered these comments about TeamBirth: “The entire staff was very genuine and my experience the best it could be. They deserve updated whiteboards in every room. I found them to be very useful.”
The clinician perspective
To be certain, clinician workflow must be a consideration for any practice change. The feasibility, acceptability, and safety of the TeamBirth model to clinicians was validated through a study at 4 community hospitals across the United States in which TeamBirth had been implemented in the 8 months prior.9
The clinician response rate was an impressive 78%. Ninety percent of clinicians, including physicians, midwives, and nurses, indicated that they would “definitely” (68%) or “probably” (22%) recommend TeamBirth for use in other labor and delivery units. None of the clinicians surveyed (n = 375) reported that TeamBirth negatively impacted care delivery.9
Obstetricians also provided qualitative commentary, noting that, while at times huddling infringed on efficiency, it also enhanced staff fulfillment. An obstetrician at a Massachusetts hospital observed, “Overall I think [TeamBirth is] helpful in slowing us down a little bit to really make sure that we’re providing the human part of the care, like the communication, and not just the medical care. And I think most providers value the human part and the communication. You know, we all think most providers value good communication with the patients, but when you’re in the middle of running around doing a bunch of stuff, you don’t always remember to prioritize it. And I think that at the end of the day…when you know you’ve communicated well with your patients, you end up feeling better about what you’re doing.”
As with most cross-sectional survey studies, selection bias remains an important caveat; patients and providers may decide to complete or not complete voluntary surveys based on particularly positive or negative experiences.
Metrics aside, obstetricians have an ethical duty to provide dignified and safe care, both physically and psychologically. Collectively, as a specialty, we share the responsibility to mitigate maternal mistreatment. As individuals, we can prevent perpetuation of birth trauma and foster healing and empowerment, one patient at a time, by employing tenets of TeamBirth.
To connect with Delivery Decisions Initiative, visit our website: https://www.ariadnelabs.org/deliverydecisions-initiative/ or contact: deliverydecisions@ ariadnelabs.org
Steps for implementing the TeamBirth model
To incorporate TeamBirth into your practice:
- Make patients the “team captain” and center them as the primary decision maker.
- Elicit patient preferences and subjective experiences to develop a collaborative plan on admission and when changes occur in clinical status.
- Round with and utilize the expertise of the full care team—nurse and midwife or obstetrician, as well as support person(s) and/or doula, learners, interpreter, and social worker as applicable.
- Ensure that the patient knows the names and roles of the care team members and provide updates at shift change.
- If your birthing rooms have a whiteboard, use it to keep the patient and team informed of the plan.
- Delineate status updates by maternal condition, fetal condition, and labor progress.
- Provide explicit permission for patients to call for a team huddle at any time and encourage support from their support people and/or doula. ●
This project is supported by:
- The Oklahoma Department of Health as part of the State Maternal Health Innovation Program Grant, Maternal and Child Health Bureau, Health Resources and Services Administration, Department of Health and Human Services.
- The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of an award to the Oklahoma State Department of Health. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U.S. Government. For more information, please visit HRSA.gov.
- The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant T76MC00001 and entitled Training Grant in Maternal and Child Health.
- Point32 Health’s Clinical Innovation Fund.
Data included in this article was collected and analyzed in partnership with the Oklahoma Perinatal Quality Improvement Collaborative, Department of OB/GYN, University of Oklahoma Health Sciences Center, Oklahoma City.
- Bohren MA, Vogel JP, Hunter EC, et al. The mistreatment of women during childbirth in health facilities globally: a mixedmethods systematic review. PLoS Med. 2015;12:e100184. doi:10.1371/journal.pmed.1001847
- Vedam S, Stoll K, Taiwo TK, et al. The Giving Voice to Mothers study: inequity and mistreatment during pregnancy and childbirth in the United States. Reprod Health. 2019;16. doi:10.1186/s12978-019-0729-2
- Kemmerer A, Alteras T. Evolving the maternal health quality measurement enterprise to support the communitybased maternity model. Maternal Health Hub. April 25, 2023. Accessed September 13, 2023. https:/www .maternalhealthhub.org
- Potential CAHPS survey to assess patients’ prenatal and childbirth care experiences. Agency for Healthcare Research and Quality. March 2023. Accessed September 13, 2023. https://www.ahrq.gov/news/cahps-comments-sought.html
- Lyndon A, Davis DA, Sharma AE, et al. Emotional safety is patient safety. BMJ Qual Saf. 2023;32:369-372. doi:10.1136 /bmjqs-2022-015573
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 819. Informed consent and shared decision making in obstetrics and gynecology. Obstet Gynecol. 2021;137:e34-e41. Accessed September 13, 2023. https://www.acog.org/clinical/clinical-guidance /committee-opinion/articles/2021/02/informed -consent-and-shared-decision-making-in-obstetrics-and -gynecology
- Goyal AA, Tur K, Mann J, et al. Do bedside visual tools improve patient and caregiver satisfaction? A systematic review of the literature. J Hosp Med. 2017;12:930-936. doi:10.12788 /jhm.2871
- Sehgal NL, Green A, Vidyarthi AR, et al. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5:234-239. doi:10.1002/jhm.638
- Weiseth A, Plough A, Aggarwal R, et al. Improving communication and teamwork during labor: a feasibility, acceptability, and safety study. Birth. 2022:49:637-647. doi:10.1111/birt.12630
CASE The TeamBirth experience: Making a difference
“At a community hospital in Washington where we had implemented TeamBirth (a labor and delivery shared decision making model), a patient, her partner, a labor and delivery nurse, and myself (an ObGyn) were making a plan for the patient’s induction of labor admission. I asked the patient, a 29-year-old (G2P1001), how we could improve her care in relation to her first birth. Her answer was simple: I want to be treated with respect. Her partner went on to describe their past experience in which the provider was inappropriately texting while in between the patient’s knees during delivery. Our team had the opportunity to undo some of the trauma from her first birth. That’s what I like about TeamBirth. It gives every patient the opportunity, regardless of their background, to define safety and participate in their care experience.”
–Angela Chien, MD, Obstetrician and Quality Improvement leader, Washington
Unfortunately, disrespect and mistreatment are far from an anomaly in the obstetrics setting. In a systematic review of respectful maternity care, the World Health Organization delineated 7 dimensions of maternal mistreatment: physical abuse, sexual abuse, verbal abuse, stigma and discrimination, failure to meet professional standards of care, poor rapport between women and providers, and poor conditions and constraints presented by the health system.1 In 2019, the Giving Voice to Mothers study showed that 17% of birthing people in the United States reported experiencing 1 or more types of maternal mistreatment.2 Rates of mistreatment were disproportionately greater in populations of color, hospital-based births, and among those with social, economic, or health challenges.2 It is well known that Black and African American and American Indian and Alaska Native populations experience the rare events of severe maternal morbidity and mortality more frequently than their White counterparts; the disproportionate burden of mistreatment is lesser known and far more common.
Overlooking the longitudinal harm of a negative birth experience has cascading impact. While an empowering perinatal experience can foster preventive screening and management of chronic disease, a poor experience conversely can seed mistrust at an individual, generational, and community level.
The patient quality enterprise is beginning to shift attention toward maternal experience with the development of PREMs (patient-reported experience measures), PROMs (patient-reported outcome measures), and novel validated scales that assess autonomy and trust.3 Development of a maternal Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey on childbirth is forthcoming.4 Of course, continuing to prioritize physical safety through initiatives on blood pressure monitoring and severe maternal morbidity and mortality remains paramount. Yet emotional and psychological safety also must be recognized as essential pillars of patient safety. Transgressions related to autonomy and dignity, as well as racism, sexism, classicism, and ableism, should be treated as “adverse and never events.”5
How the TeamBirth model works
Shared decision making (SDM) is cited in medical pedagogy as the solution to respectfullyrecognizing social context, integrating subjective experience, and honoring patient autonomy.6 The onus has always been on individual clinicians to exercise SDM. A new practice model, TeamBirth, embeds SDM into the culture and workflow. It offers a behavioral framework to mitigate implicit bias and operationalizes SDM tools, such that every patient is an empowered participant in their care.
TeamBirth was created through Ariadne Labs’ Delivery Decisions Initiative, a research and social impact program that designs, tests, and scales transformative, systems-level solutions that promote quality, equity, and dignity in childbirth. By the end of 2023, TeamBirth will be implemented in more than 100 hospitals across the United States, cumulatively touching over 200,000 lives. (For more information on the TeamBirth model, view the “Why TeamBirth” video at: https://www.youtube.com/watch?v=EoVrSaGk7gc.)
The tenets of TeamBirth are enacted through a patient-facing, shared whiteboard or dry-erase planning board in the labor room (FIGURE 1). Research has demonstrated how dry-erase boards in clinical settings can support safety and dignity in care, especially to improve patient-provider communication, teamwork, and patient satisfaction.7,8 The planning board is initially filled out by a clinical team member and is updated during team “huddles” throughout labor.

Huddles are care plan discussions with the full care team (the patient, nurse, doula and/or other support person(s), delivering provider, and interpreter or social worker as needed). At a minimum, huddles occur on admission, with changes to the clinical course and care plan, and at the request of any team member. Huddles can transpire through in-person, virtual, or phone communication.9 The concept builds on interdisciplinary and patient-centered rounding and establishes a communication system that is suited to the dynamic environment and amplified patient autonomy unique to labor and delivery. Dr. Bob Barbieri, a steadfast leader and champion of TeamBirth implementation at Brigham and Women’s Hospital in Boston (and the Editor in Chief of OBG M
Continue to: Patient response to TeamBirth is positive...
Patient response to TeamBirth is positive
Patients and providers alike have endorsed TeamBirth. In initial pilot testing across 4 sites, 99% of all patients surveyed “definitely” or “somewhat” had the role they wanted in making decisions about their labor.9
In partnership with the Oklahoma Perinatal Quality Improvement Collaborative (OPQIC), the impact of TeamBirth was assessed in a statewide patient cohort (n = 3,121) using the validated Mothers Autonomy in Decision Making (MADM) scale created by the Birth Place Lab at the University of British Columbia. The percentage of patients who scored in the highest MADM quartile was 31.3% higher for patients who indicated participation in a huddle during labor compared with those who did not participate in a huddle. This trend held across all racial and ethnic groups: For example, 93% of non-Hispanic Black/African American patients who had a TeamBirth huddle reported high autonomy, a nearly 20 percentage point increase from those without a huddle (FIGURE 2). Similarly, a higher percentage of agreement was observed across all 7 items in the MADM scale for patients who reported a TeamBirth huddle (FIGURE 3). TeamBirth’s effect has been observed across surveys and multiple validated metrics.


Data collection related to TeamBirth continues to be ongoing, with reported values retrieved on July 14, 2023. Rigorous review of patient-reported outcomes is forthcoming, and assessing impact on clinical outcomes, such as NTSV (nulliparous, term, singleton vertex) cesarean delivery rates and severe maternal morbidity, is on the horizon.
Qualitative survey responses reinforce how patients value TeamBirth and appreciate huddles and whiteboards.
Continue to: Patient testimonials...
Patient testimonials
The following testimonials were obtained from a TeamBirth survey that patients in participating Massachusetts hospitals completed in the postpartum unit prior to discharge.
According to one patient, “TeamBirth is great, feels like all obstacles are covered by multiple people with many talents, expertise. Feels like mom is part of the process, much different than my delivery 2 years ago when I felt like things were decided for me/I was ‘told’ what we were doing and questioned if I felt uneasy about it…. We felt safe and like all things were covered no matter what may happen.”
Another patient, also at a Massachusetts hospital, offered these comments about TeamBirth: “The entire staff was very genuine and my experience the best it could be. They deserve updated whiteboards in every room. I found them to be very useful.”
The clinician perspective
To be certain, clinician workflow must be a consideration for any practice change. The feasibility, acceptability, and safety of the TeamBirth model to clinicians was validated through a study at 4 community hospitals across the United States in which TeamBirth had been implemented in the 8 months prior.9
The clinician response rate was an impressive 78%. Ninety percent of clinicians, including physicians, midwives, and nurses, indicated that they would “definitely” (68%) or “probably” (22%) recommend TeamBirth for use in other labor and delivery units. None of the clinicians surveyed (n = 375) reported that TeamBirth negatively impacted care delivery.9
Obstetricians also provided qualitative commentary, noting that, while at times huddling infringed on efficiency, it also enhanced staff fulfillment. An obstetrician at a Massachusetts hospital observed, “Overall I think [TeamBirth is] helpful in slowing us down a little bit to really make sure that we’re providing the human part of the care, like the communication, and not just the medical care. And I think most providers value the human part and the communication. You know, we all think most providers value good communication with the patients, but when you’re in the middle of running around doing a bunch of stuff, you don’t always remember to prioritize it. And I think that at the end of the day…when you know you’ve communicated well with your patients, you end up feeling better about what you’re doing.”
As with most cross-sectional survey studies, selection bias remains an important caveat; patients and providers may decide to complete or not complete voluntary surveys based on particularly positive or negative experiences.
Metrics aside, obstetricians have an ethical duty to provide dignified and safe care, both physically and psychologically. Collectively, as a specialty, we share the responsibility to mitigate maternal mistreatment. As individuals, we can prevent perpetuation of birth trauma and foster healing and empowerment, one patient at a time, by employing tenets of TeamBirth.
To connect with Delivery Decisions Initiative, visit our website: https://www.ariadnelabs.org/deliverydecisions-initiative/ or contact: deliverydecisions@ ariadnelabs.org
Steps for implementing the TeamBirth model
To incorporate TeamBirth into your practice:
- Make patients the “team captain” and center them as the primary decision maker.
- Elicit patient preferences and subjective experiences to develop a collaborative plan on admission and when changes occur in clinical status.
- Round with and utilize the expertise of the full care team—nurse and midwife or obstetrician, as well as support person(s) and/or doula, learners, interpreter, and social worker as applicable.
- Ensure that the patient knows the names and roles of the care team members and provide updates at shift change.
- If your birthing rooms have a whiteboard, use it to keep the patient and team informed of the plan.
- Delineate status updates by maternal condition, fetal condition, and labor progress.
- Provide explicit permission for patients to call for a team huddle at any time and encourage support from their support people and/or doula. ●
This project is supported by:
- The Oklahoma Department of Health as part of the State Maternal Health Innovation Program Grant, Maternal and Child Health Bureau, Health Resources and Services Administration, Department of Health and Human Services.
- The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of an award to the Oklahoma State Department of Health. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U.S. Government. For more information, please visit HRSA.gov.
- The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant T76MC00001 and entitled Training Grant in Maternal and Child Health.
- Point32 Health’s Clinical Innovation Fund.
Data included in this article was collected and analyzed in partnership with the Oklahoma Perinatal Quality Improvement Collaborative, Department of OB/GYN, University of Oklahoma Health Sciences Center, Oklahoma City.
CASE The TeamBirth experience: Making a difference
“At a community hospital in Washington where we had implemented TeamBirth (a labor and delivery shared decision making model), a patient, her partner, a labor and delivery nurse, and myself (an ObGyn) were making a plan for the patient’s induction of labor admission. I asked the patient, a 29-year-old (G2P1001), how we could improve her care in relation to her first birth. Her answer was simple: I want to be treated with respect. Her partner went on to describe their past experience in which the provider was inappropriately texting while in between the patient’s knees during delivery. Our team had the opportunity to undo some of the trauma from her first birth. That’s what I like about TeamBirth. It gives every patient the opportunity, regardless of their background, to define safety and participate in their care experience.”
–Angela Chien, MD, Obstetrician and Quality Improvement leader, Washington
Unfortunately, disrespect and mistreatment are far from an anomaly in the obstetrics setting. In a systematic review of respectful maternity care, the World Health Organization delineated 7 dimensions of maternal mistreatment: physical abuse, sexual abuse, verbal abuse, stigma and discrimination, failure to meet professional standards of care, poor rapport between women and providers, and poor conditions and constraints presented by the health system.1 In 2019, the Giving Voice to Mothers study showed that 17% of birthing people in the United States reported experiencing 1 or more types of maternal mistreatment.2 Rates of mistreatment were disproportionately greater in populations of color, hospital-based births, and among those with social, economic, or health challenges.2 It is well known that Black and African American and American Indian and Alaska Native populations experience the rare events of severe maternal morbidity and mortality more frequently than their White counterparts; the disproportionate burden of mistreatment is lesser known and far more common.
Overlooking the longitudinal harm of a negative birth experience has cascading impact. While an empowering perinatal experience can foster preventive screening and management of chronic disease, a poor experience conversely can seed mistrust at an individual, generational, and community level.
The patient quality enterprise is beginning to shift attention toward maternal experience with the development of PREMs (patient-reported experience measures), PROMs (patient-reported outcome measures), and novel validated scales that assess autonomy and trust.3 Development of a maternal Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey on childbirth is forthcoming.4 Of course, continuing to prioritize physical safety through initiatives on blood pressure monitoring and severe maternal morbidity and mortality remains paramount. Yet emotional and psychological safety also must be recognized as essential pillars of patient safety. Transgressions related to autonomy and dignity, as well as racism, sexism, classicism, and ableism, should be treated as “adverse and never events.”5
How the TeamBirth model works
Shared decision making (SDM) is cited in medical pedagogy as the solution to respectfullyrecognizing social context, integrating subjective experience, and honoring patient autonomy.6 The onus has always been on individual clinicians to exercise SDM. A new practice model, TeamBirth, embeds SDM into the culture and workflow. It offers a behavioral framework to mitigate implicit bias and operationalizes SDM tools, such that every patient is an empowered participant in their care.
TeamBirth was created through Ariadne Labs’ Delivery Decisions Initiative, a research and social impact program that designs, tests, and scales transformative, systems-level solutions that promote quality, equity, and dignity in childbirth. By the end of 2023, TeamBirth will be implemented in more than 100 hospitals across the United States, cumulatively touching over 200,000 lives. (For more information on the TeamBirth model, view the “Why TeamBirth” video at: https://www.youtube.com/watch?v=EoVrSaGk7gc.)
The tenets of TeamBirth are enacted through a patient-facing, shared whiteboard or dry-erase planning board in the labor room (FIGURE 1). Research has demonstrated how dry-erase boards in clinical settings can support safety and dignity in care, especially to improve patient-provider communication, teamwork, and patient satisfaction.7,8 The planning board is initially filled out by a clinical team member and is updated during team “huddles” throughout labor.

Huddles are care plan discussions with the full care team (the patient, nurse, doula and/or other support person(s), delivering provider, and interpreter or social worker as needed). At a minimum, huddles occur on admission, with changes to the clinical course and care plan, and at the request of any team member. Huddles can transpire through in-person, virtual, or phone communication.9 The concept builds on interdisciplinary and patient-centered rounding and establishes a communication system that is suited to the dynamic environment and amplified patient autonomy unique to labor and delivery. Dr. Bob Barbieri, a steadfast leader and champion of TeamBirth implementation at Brigham and Women’s Hospital in Boston (and the Editor in Chief of OBG M
Continue to: Patient response to TeamBirth is positive...
Patient response to TeamBirth is positive
Patients and providers alike have endorsed TeamBirth. In initial pilot testing across 4 sites, 99% of all patients surveyed “definitely” or “somewhat” had the role they wanted in making decisions about their labor.9
In partnership with the Oklahoma Perinatal Quality Improvement Collaborative (OPQIC), the impact of TeamBirth was assessed in a statewide patient cohort (n = 3,121) using the validated Mothers Autonomy in Decision Making (MADM) scale created by the Birth Place Lab at the University of British Columbia. The percentage of patients who scored in the highest MADM quartile was 31.3% higher for patients who indicated participation in a huddle during labor compared with those who did not participate in a huddle. This trend held across all racial and ethnic groups: For example, 93% of non-Hispanic Black/African American patients who had a TeamBirth huddle reported high autonomy, a nearly 20 percentage point increase from those without a huddle (FIGURE 2). Similarly, a higher percentage of agreement was observed across all 7 items in the MADM scale for patients who reported a TeamBirth huddle (FIGURE 3). TeamBirth’s effect has been observed across surveys and multiple validated metrics.


Data collection related to TeamBirth continues to be ongoing, with reported values retrieved on July 14, 2023. Rigorous review of patient-reported outcomes is forthcoming, and assessing impact on clinical outcomes, such as NTSV (nulliparous, term, singleton vertex) cesarean delivery rates and severe maternal morbidity, is on the horizon.
Qualitative survey responses reinforce how patients value TeamBirth and appreciate huddles and whiteboards.
Continue to: Patient testimonials...
Patient testimonials
The following testimonials were obtained from a TeamBirth survey that patients in participating Massachusetts hospitals completed in the postpartum unit prior to discharge.
According to one patient, “TeamBirth is great, feels like all obstacles are covered by multiple people with many talents, expertise. Feels like mom is part of the process, much different than my delivery 2 years ago when I felt like things were decided for me/I was ‘told’ what we were doing and questioned if I felt uneasy about it…. We felt safe and like all things were covered no matter what may happen.”
Another patient, also at a Massachusetts hospital, offered these comments about TeamBirth: “The entire staff was very genuine and my experience the best it could be. They deserve updated whiteboards in every room. I found them to be very useful.”
The clinician perspective
To be certain, clinician workflow must be a consideration for any practice change. The feasibility, acceptability, and safety of the TeamBirth model to clinicians was validated through a study at 4 community hospitals across the United States in which TeamBirth had been implemented in the 8 months prior.9
The clinician response rate was an impressive 78%. Ninety percent of clinicians, including physicians, midwives, and nurses, indicated that they would “definitely” (68%) or “probably” (22%) recommend TeamBirth for use in other labor and delivery units. None of the clinicians surveyed (n = 375) reported that TeamBirth negatively impacted care delivery.9
Obstetricians also provided qualitative commentary, noting that, while at times huddling infringed on efficiency, it also enhanced staff fulfillment. An obstetrician at a Massachusetts hospital observed, “Overall I think [TeamBirth is] helpful in slowing us down a little bit to really make sure that we’re providing the human part of the care, like the communication, and not just the medical care. And I think most providers value the human part and the communication. You know, we all think most providers value good communication with the patients, but when you’re in the middle of running around doing a bunch of stuff, you don’t always remember to prioritize it. And I think that at the end of the day…when you know you’ve communicated well with your patients, you end up feeling better about what you’re doing.”
As with most cross-sectional survey studies, selection bias remains an important caveat; patients and providers may decide to complete or not complete voluntary surveys based on particularly positive or negative experiences.
Metrics aside, obstetricians have an ethical duty to provide dignified and safe care, both physically and psychologically. Collectively, as a specialty, we share the responsibility to mitigate maternal mistreatment. As individuals, we can prevent perpetuation of birth trauma and foster healing and empowerment, one patient at a time, by employing tenets of TeamBirth.
To connect with Delivery Decisions Initiative, visit our website: https://www.ariadnelabs.org/deliverydecisions-initiative/ or contact: deliverydecisions@ ariadnelabs.org
Steps for implementing the TeamBirth model
To incorporate TeamBirth into your practice:
- Make patients the “team captain” and center them as the primary decision maker.
- Elicit patient preferences and subjective experiences to develop a collaborative plan on admission and when changes occur in clinical status.
- Round with and utilize the expertise of the full care team—nurse and midwife or obstetrician, as well as support person(s) and/or doula, learners, interpreter, and social worker as applicable.
- Ensure that the patient knows the names and roles of the care team members and provide updates at shift change.
- If your birthing rooms have a whiteboard, use it to keep the patient and team informed of the plan.
- Delineate status updates by maternal condition, fetal condition, and labor progress.
- Provide explicit permission for patients to call for a team huddle at any time and encourage support from their support people and/or doula. ●
This project is supported by:
- The Oklahoma Department of Health as part of the State Maternal Health Innovation Program Grant, Maternal and Child Health Bureau, Health Resources and Services Administration, Department of Health and Human Services.
- The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of an award to the Oklahoma State Department of Health. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U.S. Government. For more information, please visit HRSA.gov.
- The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant T76MC00001 and entitled Training Grant in Maternal and Child Health.
- Point32 Health’s Clinical Innovation Fund.
Data included in this article was collected and analyzed in partnership with the Oklahoma Perinatal Quality Improvement Collaborative, Department of OB/GYN, University of Oklahoma Health Sciences Center, Oklahoma City.
- Bohren MA, Vogel JP, Hunter EC, et al. The mistreatment of women during childbirth in health facilities globally: a mixedmethods systematic review. PLoS Med. 2015;12:e100184. doi:10.1371/journal.pmed.1001847
- Vedam S, Stoll K, Taiwo TK, et al. The Giving Voice to Mothers study: inequity and mistreatment during pregnancy and childbirth in the United States. Reprod Health. 2019;16. doi:10.1186/s12978-019-0729-2
- Kemmerer A, Alteras T. Evolving the maternal health quality measurement enterprise to support the communitybased maternity model. Maternal Health Hub. April 25, 2023. Accessed September 13, 2023. https:/www .maternalhealthhub.org
- Potential CAHPS survey to assess patients’ prenatal and childbirth care experiences. Agency for Healthcare Research and Quality. March 2023. Accessed September 13, 2023. https://www.ahrq.gov/news/cahps-comments-sought.html
- Lyndon A, Davis DA, Sharma AE, et al. Emotional safety is patient safety. BMJ Qual Saf. 2023;32:369-372. doi:10.1136 /bmjqs-2022-015573
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 819. Informed consent and shared decision making in obstetrics and gynecology. Obstet Gynecol. 2021;137:e34-e41. Accessed September 13, 2023. https://www.acog.org/clinical/clinical-guidance /committee-opinion/articles/2021/02/informed -consent-and-shared-decision-making-in-obstetrics-and -gynecology
- Goyal AA, Tur K, Mann J, et al. Do bedside visual tools improve patient and caregiver satisfaction? A systematic review of the literature. J Hosp Med. 2017;12:930-936. doi:10.12788 /jhm.2871
- Sehgal NL, Green A, Vidyarthi AR, et al. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5:234-239. doi:10.1002/jhm.638
- Weiseth A, Plough A, Aggarwal R, et al. Improving communication and teamwork during labor: a feasibility, acceptability, and safety study. Birth. 2022:49:637-647. doi:10.1111/birt.12630
- Bohren MA, Vogel JP, Hunter EC, et al. The mistreatment of women during childbirth in health facilities globally: a mixedmethods systematic review. PLoS Med. 2015;12:e100184. doi:10.1371/journal.pmed.1001847
- Vedam S, Stoll K, Taiwo TK, et al. The Giving Voice to Mothers study: inequity and mistreatment during pregnancy and childbirth in the United States. Reprod Health. 2019;16. doi:10.1186/s12978-019-0729-2
- Kemmerer A, Alteras T. Evolving the maternal health quality measurement enterprise to support the communitybased maternity model. Maternal Health Hub. April 25, 2023. Accessed September 13, 2023. https:/www .maternalhealthhub.org
- Potential CAHPS survey to assess patients’ prenatal and childbirth care experiences. Agency for Healthcare Research and Quality. March 2023. Accessed September 13, 2023. https://www.ahrq.gov/news/cahps-comments-sought.html
- Lyndon A, Davis DA, Sharma AE, et al. Emotional safety is patient safety. BMJ Qual Saf. 2023;32:369-372. doi:10.1136 /bmjqs-2022-015573
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 819. Informed consent and shared decision making in obstetrics and gynecology. Obstet Gynecol. 2021;137:e34-e41. Accessed September 13, 2023. https://www.acog.org/clinical/clinical-guidance /committee-opinion/articles/2021/02/informed -consent-and-shared-decision-making-in-obstetrics-and -gynecology
- Goyal AA, Tur K, Mann J, et al. Do bedside visual tools improve patient and caregiver satisfaction? A systematic review of the literature. J Hosp Med. 2017;12:930-936. doi:10.12788 /jhm.2871
- Sehgal NL, Green A, Vidyarthi AR, et al. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5:234-239. doi:10.1002/jhm.638
- Weiseth A, Plough A, Aggarwal R, et al. Improving communication and teamwork during labor: a feasibility, acceptability, and safety study. Birth. 2022:49:637-647. doi:10.1111/birt.12630
2023 Update on contraception
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.
As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).
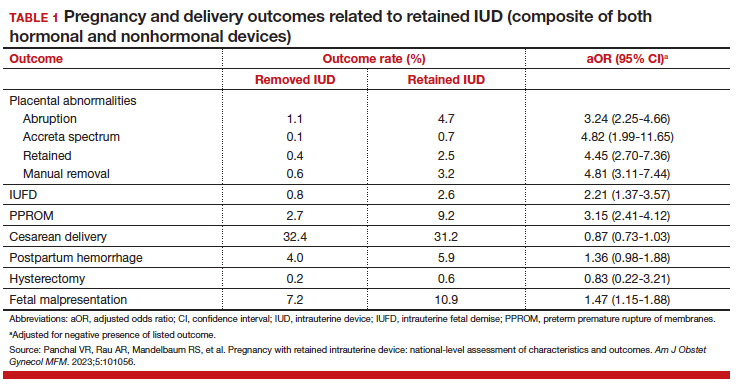
Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.
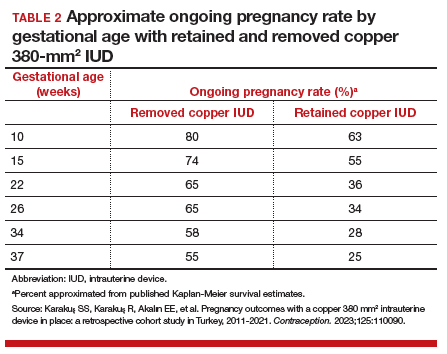
These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).
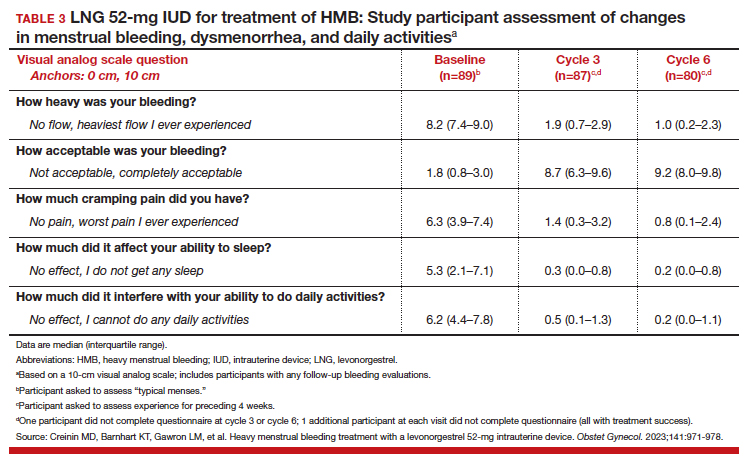
IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
Have you asked your patients: What is your ideal outpatient gynecology experience?
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.
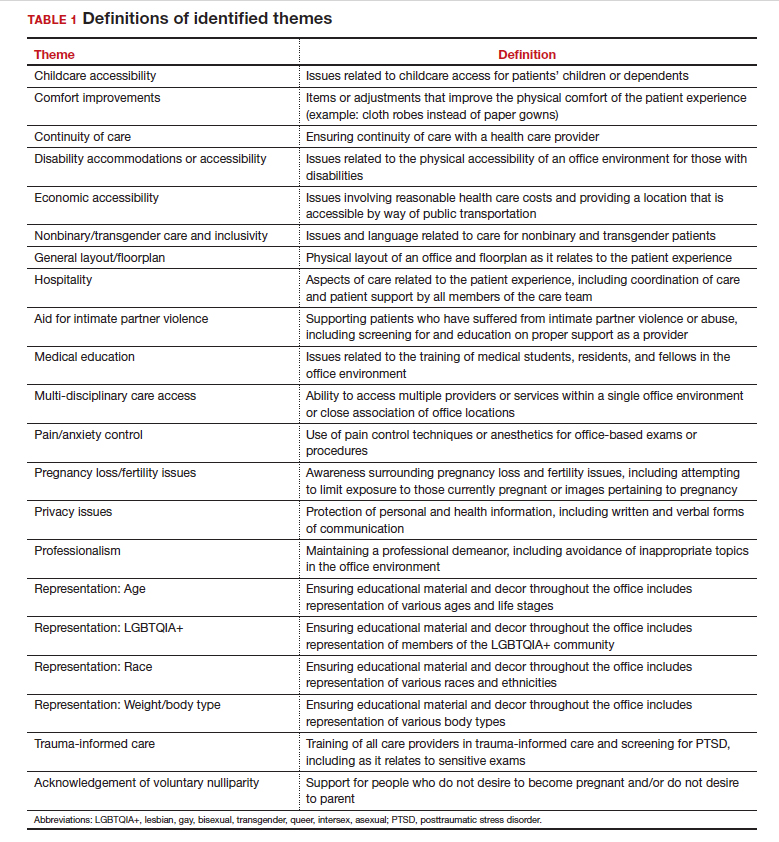
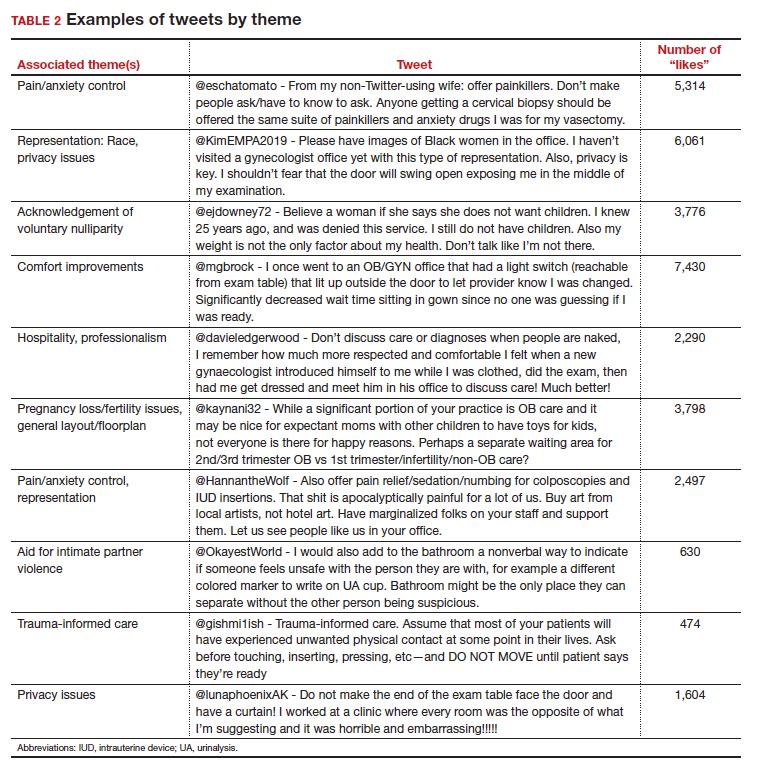
Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).
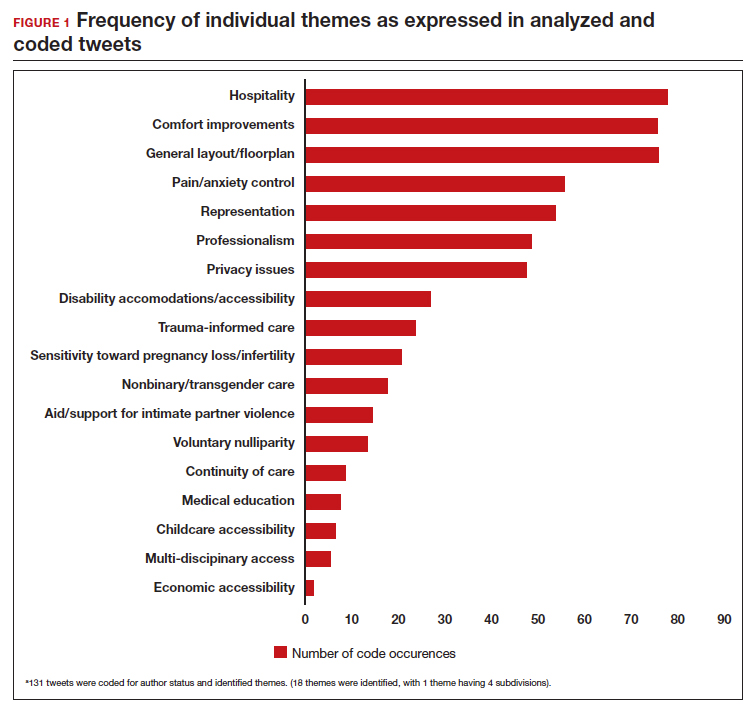
Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).
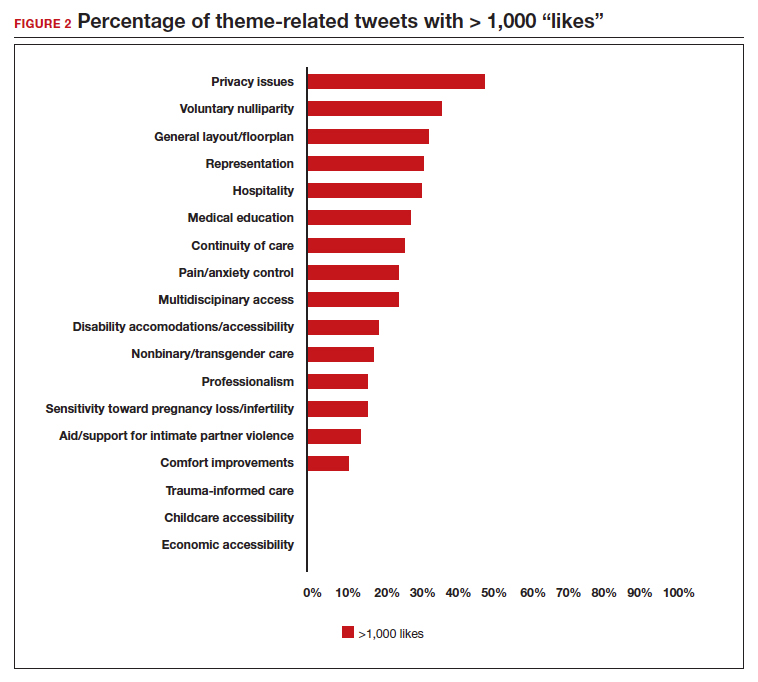
A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).
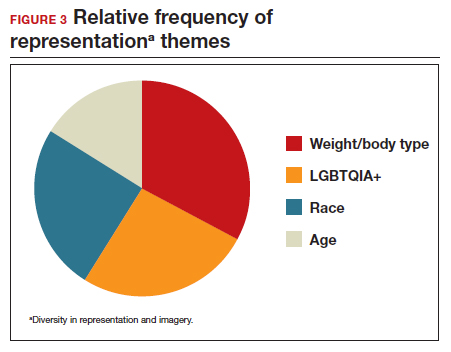
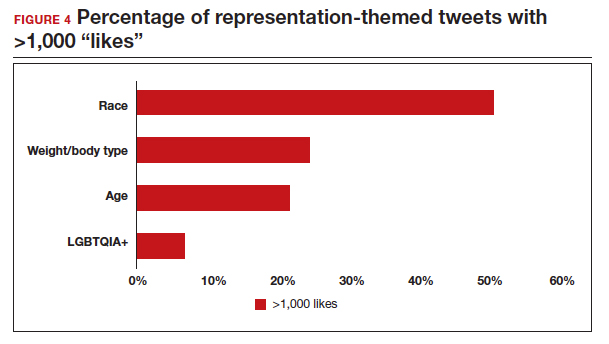
Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.


Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).

Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).

A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).


Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.


Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).

Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).

A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).


Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
2023 Update on abnormal uterine bleeding
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
The medical profession and the 2022 ̶ 2023 Term of the Supreme Court

The 2022-2023 Term of the Supreme Court illustrates how important the Court has become to health-related matters, including decisions regarding the selection and training of new professionals, the daily practice of medicine, and the future availability of new drugs. The importance of several cases is reinforced by the fact that major medical organizations filed amicus curiae (“friend of the court”) briefs in those cases.
Amicus briefs are filed by individuals or organizations with something significant to say about a case to the court—most often to present a point of view, make an argument, or provide information that the parties to the case may not have communicated. Amicus briefs are burdensome in terms of the time, energy, and cost of preparing and filing. Thus, they are not undertaken lightly. Medical organizations submitted amicus briefs in the first 3 cases we consider.
Admissions, race, and diversity
The case: Students for Fair Admissions v President and Fellows of Harvard College
The American College of Obstetricians and Gynecologists (ACOG) joined an amici curiae brief in Students for Fair Admissions v President and Fellows of Harvard College (and the University of North Carolina [UNC]).1 This case challenged the use of racial preferences in college admissions. The Association of American Medical Colleges (AAMC) was the lead organization; nearly 40 other health-related organizations joined the brief.
The legal claim. Those filing the suits asserted that racial preferences by public colleges violate the 14th Amendment’s Equal Protection Clause (“no state shall deny to any person … the equal protection of the law”). That is, if a state university gives racial preferences in selective admissions, it denies some other applicant the equal protection of the law. As for private schools (in this case, Harvard), Title VI of the Civil Rights Act of 1964 has the same standards as the Equal Protection Clause. Thus, the Court consolidated the cases and used the same legal standard in considering public and private colleges (with “colleges” including professional and graduate programs as well as undergraduate institutions).
Background. For nearly 50 years, the Supreme Court has allowed limited racial preferences in college admissions. Those preferences could only operate as a plus, however, and not a negative for applicants and be narrowly tailored. The measure was instituted temporarily; in a 2003 case, the Court said, “We expect that 25 years from now, the use of racial preferences will no longer be necessary.”2
Decision. In a 6-3 decision, the Court held (in the UNC case) that racial preferences generally violate the Constitution, and by a 6-2 decision (in the Harvard case) these preferences violate the Civil Rights Act of 1964. (Justice Jackson was recused in the Harvard case because of a conflict.) The opinion covered 237 pages in the US Reports, so any summary is incomplete.
The majority concluded, “The Harvard and UNC admissions programs cannot be reconciled with the guarantees of the Equal Protection Clause. Both programs lack sufficiently focused and measurable objectives warranting the use of race, unavoidably employ race in a negative manner, involve racial stereotyping, and lack meaningful end points. We have never permitted admissions programs to work in that way, and we will not do so today.”3
There were 3 concurring opinions and 2 dissents in the case. The concurrences reviewed the history of the Equal Protection Clause and the Civil Rights Act, the damage racial preferences can do, and the explicit limits the Court said there must be on racial preferences in higher education. The dissents had a different view of the legal history of the 14th Amendment. They said the majority was turning a blind eye to segregation in society and the race-based gap in America.
As a practical matter, this case means that colleges, including professional schools, cannot use racial preferences. The Court said that universities may consider essays and the like in which applicants describe how their own experiences as an individual (including race) have affected their own lives. However, the Court cautioned that “universities may not simply establish through application essays or other means the regime we hold unlawful today.”3
Continue to: The amici brief...
The amici brief
ACOG joined 40 other health-related organizations in filing an amici brief (multiple “friends”) in Students for Fair Admission. The AAMC led the brief, with the others signing as amici.4 The brief made 3 essential points: diversity in medical education “markedly improves health outcomes,” and a loss of diversity “threaten[s] patients’ health; medical schools engage in an intense “holistic” review of applicants for admission; and medical schools must consider applicants’ “full background” (including race) to achieve their educational and professional goals.4
A powerful part of the brief described the medical school admissions process, particularly the very “holistic” review that is not entirely dependent on admissions scores. The brief effectively weaves the consideration of race into this process, mentioning race (on page 22) only after discussing many other admissions factors.
Child custody decisions related to the Indian Child Welfare Act
The case: Haaland v Brackeen
The American Medical Association (AMA) and the American Academy of Pediatrics filed a brief in Haaland v Brackeen5 involving the constitutionality of the 1978 Indian Child Welfare Act (ICWA). The statute followed a terrible history of Indian children being removed from their families inappropriately, as detailed in a concurring opinion by Justice Gorsuch.5 The two purposes of the act were to promote raising Native American children in their culture and stem the downward trend in tribal membership.
The legal claim. The Court consolidated several cases. Essentially, a 10-month-old child (A.L.M.) was placed in foster care with the Brackeens in Texas. After more than 1 year, the Brackeens sought adoption; the biological father, mother, and grandparents all supported it. The Navajo and Cherokee Nations objected and informed the Texas court that they had found alternative placement with (nonrelative) tribal members in New Mexico. The “court-appointed guardian and a psychological expert … described the strong emotional bond between A.L.M. and his foster parents.” The court denied the adoption petition based on ICWA’s preference for tribe custody, and the Brackeens filed a lawsuit.
Decision. The constitutional claim in the case was that Congress lacked the authority to impose these substantial rules on states in making child custody decisions. The Supreme Court, in a 7-2 decision, upheld the constitutionality of the ICWA.
The amici brief
The joint amici brief of the American Academy of Pediatrics (AAP) and the AMA argued that tribes are “extended families” of Native American children.6 It noted the destructive history of removing Native American children from their families and suggested that kinship care improves children’s health. To its credit, the brief also honestly noted the serious mental health and suicide rates in some tribes, which suggest issues that might arise in child custody and adoption cases.
The Court did not, in this case, take up another constitutional issue that the parties raised—whether the strong preference for Native American over non ̶ Native American custody violates the Equal Protection Clause of the 14th Amendment. The Court said the parties to this case did not have standing to raise the issue. Justice Kavanaugh, concurring, said it was a “serious” issue and invited it to be raised in another case.5
False Claims Act cases
The case: Costs for SuperValu prescriptions
The legal claim. One FCA case this Term involved billings SuperValu made for outpatient prescriptions in Medicare-Medicaid programs. As its “usual and customary” costs, it essentially reported a list price that did not include the substantial discounts it commonly gave.
Background. Subjective knowledge (what the defendant actually knows) may seem impossible to prove—the defendant could just say, “I did not know I was doing wrong.” Over time the law has developed several ways of demonstrating “knowing.” Justice Thomas, writing for a unanimous Court, held that whistleblowers or the government might prove “knowing” in 3 ways:
1. defendants “actually knew that their reported prices were not their ‘usual and customary’ prices when they reported them”
2. were aware of a substantial risk that their higher, retail prices were not their “usual and customary” prices and intentionally avoided learning whether their reports were accurate
3. were aware of such a substantial and unjustifiable risk but submitted the claims anyway.9
Of course, records of the company, information from the whistleblower, and circumstantial evidence may be used to prove any of these; it does not require the company’s admission.
The Court said that if the government or whistleblowers make a showing of any of these 3 things, it is enough.
Decision. The case was returned to the lower court to apply these rules.
The amici brief
The American Hospital Association and America’s Health Insurance Plans filed an amici brief.10 It reminded the Court that many reimbursement regulations are unclear. Therefore, it is inappropriate to impose FCA liability for guessing incorrectly what the regulations mean. Having to check on every possible ambiguity was unworkable. The Court declined, however, the suggestion that defendants should be able to use any one of many “objectively” reasonable interpretations of regulations.
Continue to: The case: Polansky v Executive Health Resources...
The case: Polansky v Executive Health Resources
Health care providers who dislike the FCA may find solace this Term in this second FCA case.11
The legal claim. Polansky, a physician employed by a medical billing company, became an “intervenor” in a suit claiming the company assisted hospitals in false billing (inpatient claims for outpatient services). The government sought to dismiss the case, but Polansky refused.
Decision. The case eventually reached the Supreme Court, which held that the government may enter an FCA case at any time and move to dismiss the case even over the objection of a whistleblower. The government does not seek to enter a case in order to file dismissal motions often. When it does so, whistleblowers are protected by the fact that the dismissal motion requires a hearing before the federal court.
An important part of this case has escaped much attention. Justices Thomas, Kavanaugh, and Barrett invited litigation to determine if allowing private whistleblowers to represent the government’s interest is consistent with Article II of the Constitution.11 The invitation will likely be accepted. We expect to see cases challenging the place of “intervenors” pursuing claims when the government has declined to take up the case. The private intervenor is a crucial provision of the current FCA, and if such a challenge were successful, it could substantially reduce FCA cases.
Criminal false claims
Dubin overbilled Medicaid for psychological testing by saying the testing was done by a licensed psychologist rather than an assistant. The government claimed the “identity theft” was using the patient’s (actual) Medicaid number in submitting the bill.12 The Court unanimously held the overbilling was not aggravated identity theft as defined in federal law. Dubin could be convicted of fraudulent billing but not aggravated identity theft, thereby avoiding the mandatory prison term.
Patents of “genus” targets
The case: Amgen v Sanofi
Background. “Genus” patents allow a single pharmaceutical company to patent every antibody that binds to a specific amino acid on a naturally occurring protein. In this case, the patent office had granted a “genus” patent on “all antibodies” that bind to the naturally occurring protein PCSK9 and block it from hindering the body’s mechanism for removing low-density lipoprotein (LDL) cholesterol from the bloodstream,13 helping to reduce LDL cholesterol levels. These patents could involve millions of antibodies—and Amgen was claiming a patent on all of them. Amgen and Sanofi marketed their products, each with their own unique amino acid sequence.13 Amgen sued Sanofi for violating its patent rights.
Decision. The Court unanimously held that Amgen did not have a valid patent on all antibodies targeting PCSK9, only those that it had explicitly described in its patent application—a ruling based on a 150-year-old technical requirement for receiving a patent. An applicant for a patent must include “a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art…to make and use the same.”13 Amgen’s patent provided the description for only a few of the antibodies, but from the description in its application others could not “make and use” all of the antibodies targeting PCSK9.
While the decision was vital for future pharmaceuticals, the patent principle on which it was based has an interesting history. The Court noted that it affected the telegraph (Morse lost part of his patent), electric lights (Edison won his case against other inventors), and the glue for wood veneering (Perkins Glue Company lost).13
Continue to: Other notable decisions...
Other notable decisions
Student loans
The Court struck down the Biden Administration’s student loan forgiveness program, which would have cost approximately $430 billion.14 The central issue was whether the administration had the authority for such massive loan forgiveness; that is, whether Congress had authorized the broad loan forgiveness. The administration claimed authority from the post ̶ 9/11 HEROES Act, which allows the Secretary of Education to “waive or modify” loan provisions during national emergencies.
Free speech and the wedding web designer
303 Creative v Elenis involved a creative website designer who did not want to be required to create a website for a gay wedding.15 The designer had strong beliefs against same-sex marriages, but Colorado sought to force her to do so under the state “public accommodations” law. In a 6-3 decision, the Court held that the designer had a “free speech” right. That is, the state could not compel her to undertake speech expressing things she did not believe. This was because the website design was an expressive, creative activity and therefore was “speech” under the First Amendment.
Wetlands and the Clean Water Act
The essential issue in Sackett v Environmental Protection Agency (EPA) was the definitions of waters of the United States and related wetlands. The broad definition the EPA used meant it had jurisdiction to regulate an extraordinary amount of territory. It had, for example, prevented the Sacketts from building a modest house claiming it was part of the “waters of the United States because they were near a ditch that fed into a creek, which fed into Priest Lake, a navigable, intrastate lake.” The Court held that the EPA exceeded its statutory authority to define “wetlands.”16
The Court held that under the Clean Water Act, for the EPA to establish jurisdiction over adjacent wetlands, it must demonstrate that16:
1. “the adjacent body of water constitutes waters of the United States (ie, a relatively permanent body of water connected to traditional interstate navigable waters)…”
2. “…the wetland has a continuous surface connection with that water, making it difficult to determine where the water ends, and the wetland begins.”
Under this definition, the Sacketts could build their house. This was a statutory interpretation case. Therefore, Congress can expand or otherwise change the EPA’s authority under the Clean Water Act and other legislation.
Conclusions: A new justice, “shadow docket,” and ethics rules
SCOTUS’ newest member. When the Marshall called the Court into session on October 3, 2022, it had a new member, Justice Ketanji Brown Jackson. She was sworn in on June 30, 2022, when her predecessor (Justice Breyer) officially retired. She had been a law clerk for Justice Breyer in 1999, as well as a district court judge and court of appeals judge. Those who count such things described her as the “chattiest justice.”17 She spoke more than any other justice—by one count, a total of 75,632 words (an average of 1,300 words in each of the 58 arguments).
A more balanced Court? Most commentators view the Court as more balanced or less conservative than the previous Term. For example, Justice Sotomayor was in the majority 40% last Term but 65% this Term. Justice Thomas was in the majority 75% last Term but 55% this Term. Put another way, this Term in the divided cases, the liberal justices were in the majority 64% of the time, compared with the conservative justices 73%.18 Of course, these differences may reflect a different set of cases rather than a change in the direction of the Court. There were 11 (or 12, depending on how 1 case is counted) 6-3 cases, but only 5 were considered ideological. That suggests that, in many cases, the coalitions were somewhat fluid.
“Shadow docket” controversy continues.19 Shadow docket refers to orders the Court makes that do not follow oral arguments and often do not have written opinions. The orders are all publicly available. This Term a close examination of the approximately 30 shadow docket opinions shows that the overwhelming majority were dissents or explanations about denials of certiorari. The Court ordered only a few stays or injunctions via the shadow docket. One shadow docket stay (that prevented a lower court order from going into effect) is particularly noteworthy. A federal judge had ordered the suspension of the distribution of mifepristone while courts considered claims that the US Food and Drug Administration (FDA) had improperly approved the drug. In a shadow docket order, the Court issued the stay to allow mifepristone to be sold while the case challenging its approval was heard.20 The only opinion was a dissent from Justice Alito. But it also demonstrates the importance of the shadow docket. Without this intervention, in at least part of the country, the distribution of mifepristone would have been interrupted pending the outcome of the FDA cases.
In August, the Court delayed a settlement in the Purdue Pharma liability bankruptcy case.21 It also stayed an injunction of a lower court, thereby permitting federal “ghost guns” regulations to go into effect at least temporarily.22
More ethics rules to come? Another area in which the Court faced criticism was formal ethics rules. The justices make financial disclosures, but these are somewhat ambiguous. There is likely to be increasing pressure for a more complete disclosure of non-financial relationships and more formal ethics rules. ●
The Court had, by September 1, 2023, accepted 22 cases for hearing next Term.1 The cases include a challenge to the extraordinary funding provision for the Consumer Financial Protection Bureau, another racial challenge to congressional districts (South Carolina), the status of Americans with Disability Act “testers” who look for violations without ever intending to use the facilities, the level of deference courts should give to interpreting federal statutes (so-called “Chevron” deference), the opioid (OxyContin ) bankruptcy, and limitations on gun ownership. This represents less than half of the cases the Court will likely hear next Term, so the Court will add many more cases to the docket. It promises to be an appealing Term.
Reference
1. October Term 2023. SCOTUSblog website. Accessed August 29, 2023. https://www.scotusblog.com/case-files/terms/ot2023/
When the Court adjourned on June 30, 2023, it had considered 60 cases, plus hundreds of petitions asking it to hear cases. Most commentators count 55 cases decided after briefing and oral argument and where there was a signed opinion. The information below uses 55 cases unless otherwise noted. During the 2022-2023 Term, the Court:
- upheld liability for the involuntary administration of psychotropic drugs in nursing home1
- permitted disabled students, in some instances, both to make Individuals with Disabilities Education Act (IDEA) claims for services and to file Americans with Disabilities Act (ADA) lawsuits against their schools2
- upheld a statute that makes it illegal to “encourage or induce an alien to come to, enter, or reside in the United States, knowing or in reckless disregard of the fact that such coming to, entry, or residence is or will be in violation of law.” The defendant had used a scam promising noncitizens “adult adoptions” (of which there is no such thing) making it legal for them to come to and stay in the United States.3
- narrowed the “fair use” of copyrighted works. It held that Andy Warhol’s use of a copyrighted photograph in his famous Prince prints was not “transformative” in a legal sense largely because the photo and prints “share the same use”—magazine illustrations.4
- in another intellectual property case, held that Jack Daniel’s might sue a dog toy maker for a rubber dog toy that looked like a Jack Daniel’s bottle5
- further expanded the Federal Arbitration Act by holding that a federal district court must immediately stay court proceedings if one party is appealing a decision not to require arbitration6
- held that two social media companies were not responsible for terrorists using their platforms to recruit others to their cause. It did not, however, decide whether §230 of the Communication Decency Act protects companies from liability.7
- made it easier for employees to receive accommodation for their religious practices and beliefs. Employers must make religious accommodations unless the employer can show that “the burden of granting an accommodation would result in substantial increased [financial and other] costs in relation to the conduct of its particular business.”8
- declined to hear an appeal from Johnson & Johnson (through a subsidiary, Ethicon) about pelvic mesh. In this case, the California Attorney General filed a lawsuit against Ethicon for false advertising by failing to detail the risks of pelvic mesh. The lower courts estimated 240,000 written violations of the law by Ethicon between 2008 and 2017. The trial and appeal to California courts resulted in a judgment of $302 million against Johnson & Johnson. The company asked the Court to review that judgment, but the Court denied certiorari. That likely means the $302 million is final.
References
1. Health and Hospital Corporation of Marion Cty. v Talevski, Docket no. 21-806; June 8, 2023.
2. Luna Perez v Sturgis Public Schools, Docket no. 21-887; March 21, 2023.
3. United States v Hansen, Docket no. 22-179; June 23, 2023.
4. Andy Warhol Foundation for Visual Arts, Inc. v Goldsmith, Docket no. 21-869; May 18, 2023.
5. Jack Daniel’s Properties, Inc. v VIP Products LLC, Docket no. 22-148; June 8, 2023.
6. Coinbase, Inc. v Bielski, Docket no. 22-105; June 23, 2023.
7. Gonzalez v Google LLC, Docket no. 21-1333; May 18, 2023.
8. Groff v DeJoy, Docket no. 22-273; June 29, 2023.
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306, 326 (2003).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___, 39 (2023).
- Brief for Amici Curiae Association of American Medical Colleges et al. in Support of Respondents, Students for Fair Admissions v University of North Carolina (July 28, 2022). Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-707/232120/20220728171307159_20 -1199%20and%2021-707%20Amicus%20Brief%20for%20 Association%20of%20American%20Medical%20Colleges%20 et%20al.pdf
- Haaland v Brackeen, Docket no. 21-376; June 15, 2023.
- Brief of American Academy of Pediatrics and American Medical Association as Amici Curiae in Support of Respondents, in Haaland v Brackeen. August 19, 2022. Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-376/234042/20220819140750948_21-376 .amics.brief.FINAL.pdf
- Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021. US Department of Justice website. February 1, 2022. Accessed August 18, 2023. https://www.justice.gov/opa/pr/justice -department-s-false-claims-act-settlements-and-judgments -exceed-56-billion-fiscal-year
- False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022. US Department of Justice website. February 7, 2023. Accessed August 18, 2023. https://www .justice.gov/opa/pr/false-claims-act-settlements-and -judgments-exceed-2-billion-fiscal-year-2022
- United States ex rel. Schutte v Supervalu Inc., Docket no. 21-1326; June 1, 2023.
- Brief of American Hospital Association and America’s Health Insurance Plans as Amici Curiae in Support of Respondents, in Schutte v Supervalu. March 2023. Accessed August 18, 2023. https://www.supremecourt.gov/DocketPDF/21/21-1326 /262428/20230331113854936_3-31-23%20AHA_AHIP _Amicus_Brief.pdf
- United States ex rel. Polansky v Executive Health Resources, Inc., Docket no. 21-1052; June 16, 2023.
- Dubin v United States, Docket no. 22-10; June 8, 2023.
- Amgen v Sanofi, 598 US ___ (2023).
- Biden v Nebraska, 600 US ___ (2023).
- 303 Creative LLC v Elenis, 600 US ___ (2023).
- Sackett v Environmental Protection Agency, Docket no. 21454; May 25, 2023.
- Krochtengel J. Jackson debuts as chattiest Justice. Law360. July 3, 2023. https://www.law360.com/articles/1692839 /jackson-debuts-as-chattiest-justice
- Feldman A. Another One Bites the Dust: End of 2022/2023 Supreme Court Term Statistics. EmpiricalScotus website. 30, 2023. Accessed August 18, 2023. https://empiricalscotus .com/2023/06/30/another-one-bites-2022/
- Vladeck S. The Shadow Docket: How the Supreme Court Uses Stealth Rulings to Amass Power and Undermine the Republic. New York, New York; Basic Books; 2023.
- Danco Laboratories, LLC v Alliance for Hippocratic Medicine. Docket no. 22A902; April 21, 2023.
- Harrington v Purdue Pharma, 23-124 (23A87).
- Garland v Vanderstok, 23-10718 (August 8, 2023).

The 2022-2023 Term of the Supreme Court illustrates how important the Court has become to health-related matters, including decisions regarding the selection and training of new professionals, the daily practice of medicine, and the future availability of new drugs. The importance of several cases is reinforced by the fact that major medical organizations filed amicus curiae (“friend of the court”) briefs in those cases.
Amicus briefs are filed by individuals or organizations with something significant to say about a case to the court—most often to present a point of view, make an argument, or provide information that the parties to the case may not have communicated. Amicus briefs are burdensome in terms of the time, energy, and cost of preparing and filing. Thus, they are not undertaken lightly. Medical organizations submitted amicus briefs in the first 3 cases we consider.
Admissions, race, and diversity
The case: Students for Fair Admissions v President and Fellows of Harvard College
The American College of Obstetricians and Gynecologists (ACOG) joined an amici curiae brief in Students for Fair Admissions v President and Fellows of Harvard College (and the University of North Carolina [UNC]).1 This case challenged the use of racial preferences in college admissions. The Association of American Medical Colleges (AAMC) was the lead organization; nearly 40 other health-related organizations joined the brief.
The legal claim. Those filing the suits asserted that racial preferences by public colleges violate the 14th Amendment’s Equal Protection Clause (“no state shall deny to any person … the equal protection of the law”). That is, if a state university gives racial preferences in selective admissions, it denies some other applicant the equal protection of the law. As for private schools (in this case, Harvard), Title VI of the Civil Rights Act of 1964 has the same standards as the Equal Protection Clause. Thus, the Court consolidated the cases and used the same legal standard in considering public and private colleges (with “colleges” including professional and graduate programs as well as undergraduate institutions).
Background. For nearly 50 years, the Supreme Court has allowed limited racial preferences in college admissions. Those preferences could only operate as a plus, however, and not a negative for applicants and be narrowly tailored. The measure was instituted temporarily; in a 2003 case, the Court said, “We expect that 25 years from now, the use of racial preferences will no longer be necessary.”2
Decision. In a 6-3 decision, the Court held (in the UNC case) that racial preferences generally violate the Constitution, and by a 6-2 decision (in the Harvard case) these preferences violate the Civil Rights Act of 1964. (Justice Jackson was recused in the Harvard case because of a conflict.) The opinion covered 237 pages in the US Reports, so any summary is incomplete.
The majority concluded, “The Harvard and UNC admissions programs cannot be reconciled with the guarantees of the Equal Protection Clause. Both programs lack sufficiently focused and measurable objectives warranting the use of race, unavoidably employ race in a negative manner, involve racial stereotyping, and lack meaningful end points. We have never permitted admissions programs to work in that way, and we will not do so today.”3
There were 3 concurring opinions and 2 dissents in the case. The concurrences reviewed the history of the Equal Protection Clause and the Civil Rights Act, the damage racial preferences can do, and the explicit limits the Court said there must be on racial preferences in higher education. The dissents had a different view of the legal history of the 14th Amendment. They said the majority was turning a blind eye to segregation in society and the race-based gap in America.
As a practical matter, this case means that colleges, including professional schools, cannot use racial preferences. The Court said that universities may consider essays and the like in which applicants describe how their own experiences as an individual (including race) have affected their own lives. However, the Court cautioned that “universities may not simply establish through application essays or other means the regime we hold unlawful today.”3
Continue to: The amici brief...
The amici brief
ACOG joined 40 other health-related organizations in filing an amici brief (multiple “friends”) in Students for Fair Admission. The AAMC led the brief, with the others signing as amici.4 The brief made 3 essential points: diversity in medical education “markedly improves health outcomes,” and a loss of diversity “threaten[s] patients’ health; medical schools engage in an intense “holistic” review of applicants for admission; and medical schools must consider applicants’ “full background” (including race) to achieve their educational and professional goals.4
A powerful part of the brief described the medical school admissions process, particularly the very “holistic” review that is not entirely dependent on admissions scores. The brief effectively weaves the consideration of race into this process, mentioning race (on page 22) only after discussing many other admissions factors.
Child custody decisions related to the Indian Child Welfare Act
The case: Haaland v Brackeen
The American Medical Association (AMA) and the American Academy of Pediatrics filed a brief in Haaland v Brackeen5 involving the constitutionality of the 1978 Indian Child Welfare Act (ICWA). The statute followed a terrible history of Indian children being removed from their families inappropriately, as detailed in a concurring opinion by Justice Gorsuch.5 The two purposes of the act were to promote raising Native American children in their culture and stem the downward trend in tribal membership.
The legal claim. The Court consolidated several cases. Essentially, a 10-month-old child (A.L.M.) was placed in foster care with the Brackeens in Texas. After more than 1 year, the Brackeens sought adoption; the biological father, mother, and grandparents all supported it. The Navajo and Cherokee Nations objected and informed the Texas court that they had found alternative placement with (nonrelative) tribal members in New Mexico. The “court-appointed guardian and a psychological expert … described the strong emotional bond between A.L.M. and his foster parents.” The court denied the adoption petition based on ICWA’s preference for tribe custody, and the Brackeens filed a lawsuit.
Decision. The constitutional claim in the case was that Congress lacked the authority to impose these substantial rules on states in making child custody decisions. The Supreme Court, in a 7-2 decision, upheld the constitutionality of the ICWA.
The amici brief
The joint amici brief of the American Academy of Pediatrics (AAP) and the AMA argued that tribes are “extended families” of Native American children.6 It noted the destructive history of removing Native American children from their families and suggested that kinship care improves children’s health. To its credit, the brief also honestly noted the serious mental health and suicide rates in some tribes, which suggest issues that might arise in child custody and adoption cases.
The Court did not, in this case, take up another constitutional issue that the parties raised—whether the strong preference for Native American over non ̶ Native American custody violates the Equal Protection Clause of the 14th Amendment. The Court said the parties to this case did not have standing to raise the issue. Justice Kavanaugh, concurring, said it was a “serious” issue and invited it to be raised in another case.5
False Claims Act cases
The case: Costs for SuperValu prescriptions
The legal claim. One FCA case this Term involved billings SuperValu made for outpatient prescriptions in Medicare-Medicaid programs. As its “usual and customary” costs, it essentially reported a list price that did not include the substantial discounts it commonly gave.
Background. Subjective knowledge (what the defendant actually knows) may seem impossible to prove—the defendant could just say, “I did not know I was doing wrong.” Over time the law has developed several ways of demonstrating “knowing.” Justice Thomas, writing for a unanimous Court, held that whistleblowers or the government might prove “knowing” in 3 ways:
1. defendants “actually knew that their reported prices were not their ‘usual and customary’ prices when they reported them”
2. were aware of a substantial risk that their higher, retail prices were not their “usual and customary” prices and intentionally avoided learning whether their reports were accurate
3. were aware of such a substantial and unjustifiable risk but submitted the claims anyway.9
Of course, records of the company, information from the whistleblower, and circumstantial evidence may be used to prove any of these; it does not require the company’s admission.
The Court said that if the government or whistleblowers make a showing of any of these 3 things, it is enough.
Decision. The case was returned to the lower court to apply these rules.
The amici brief
The American Hospital Association and America’s Health Insurance Plans filed an amici brief.10 It reminded the Court that many reimbursement regulations are unclear. Therefore, it is inappropriate to impose FCA liability for guessing incorrectly what the regulations mean. Having to check on every possible ambiguity was unworkable. The Court declined, however, the suggestion that defendants should be able to use any one of many “objectively” reasonable interpretations of regulations.
Continue to: The case: Polansky v Executive Health Resources...
The case: Polansky v Executive Health Resources
Health care providers who dislike the FCA may find solace this Term in this second FCA case.11
The legal claim. Polansky, a physician employed by a medical billing company, became an “intervenor” in a suit claiming the company assisted hospitals in false billing (inpatient claims for outpatient services). The government sought to dismiss the case, but Polansky refused.
Decision. The case eventually reached the Supreme Court, which held that the government may enter an FCA case at any time and move to dismiss the case even over the objection of a whistleblower. The government does not seek to enter a case in order to file dismissal motions often. When it does so, whistleblowers are protected by the fact that the dismissal motion requires a hearing before the federal court.
An important part of this case has escaped much attention. Justices Thomas, Kavanaugh, and Barrett invited litigation to determine if allowing private whistleblowers to represent the government’s interest is consistent with Article II of the Constitution.11 The invitation will likely be accepted. We expect to see cases challenging the place of “intervenors” pursuing claims when the government has declined to take up the case. The private intervenor is a crucial provision of the current FCA, and if such a challenge were successful, it could substantially reduce FCA cases.
Criminal false claims
Dubin overbilled Medicaid for psychological testing by saying the testing was done by a licensed psychologist rather than an assistant. The government claimed the “identity theft” was using the patient’s (actual) Medicaid number in submitting the bill.12 The Court unanimously held the overbilling was not aggravated identity theft as defined in federal law. Dubin could be convicted of fraudulent billing but not aggravated identity theft, thereby avoiding the mandatory prison term.
Patents of “genus” targets
The case: Amgen v Sanofi
Background. “Genus” patents allow a single pharmaceutical company to patent every antibody that binds to a specific amino acid on a naturally occurring protein. In this case, the patent office had granted a “genus” patent on “all antibodies” that bind to the naturally occurring protein PCSK9 and block it from hindering the body’s mechanism for removing low-density lipoprotein (LDL) cholesterol from the bloodstream,13 helping to reduce LDL cholesterol levels. These patents could involve millions of antibodies—and Amgen was claiming a patent on all of them. Amgen and Sanofi marketed their products, each with their own unique amino acid sequence.13 Amgen sued Sanofi for violating its patent rights.
Decision. The Court unanimously held that Amgen did not have a valid patent on all antibodies targeting PCSK9, only those that it had explicitly described in its patent application—a ruling based on a 150-year-old technical requirement for receiving a patent. An applicant for a patent must include “a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art…to make and use the same.”13 Amgen’s patent provided the description for only a few of the antibodies, but from the description in its application others could not “make and use” all of the antibodies targeting PCSK9.
While the decision was vital for future pharmaceuticals, the patent principle on which it was based has an interesting history. The Court noted that it affected the telegraph (Morse lost part of his patent), electric lights (Edison won his case against other inventors), and the glue for wood veneering (Perkins Glue Company lost).13
Continue to: Other notable decisions...
Other notable decisions
Student loans
The Court struck down the Biden Administration’s student loan forgiveness program, which would have cost approximately $430 billion.14 The central issue was whether the administration had the authority for such massive loan forgiveness; that is, whether Congress had authorized the broad loan forgiveness. The administration claimed authority from the post ̶ 9/11 HEROES Act, which allows the Secretary of Education to “waive or modify” loan provisions during national emergencies.
Free speech and the wedding web designer
303 Creative v Elenis involved a creative website designer who did not want to be required to create a website for a gay wedding.15 The designer had strong beliefs against same-sex marriages, but Colorado sought to force her to do so under the state “public accommodations” law. In a 6-3 decision, the Court held that the designer had a “free speech” right. That is, the state could not compel her to undertake speech expressing things she did not believe. This was because the website design was an expressive, creative activity and therefore was “speech” under the First Amendment.
Wetlands and the Clean Water Act
The essential issue in Sackett v Environmental Protection Agency (EPA) was the definitions of waters of the United States and related wetlands. The broad definition the EPA used meant it had jurisdiction to regulate an extraordinary amount of territory. It had, for example, prevented the Sacketts from building a modest house claiming it was part of the “waters of the United States because they were near a ditch that fed into a creek, which fed into Priest Lake, a navigable, intrastate lake.” The Court held that the EPA exceeded its statutory authority to define “wetlands.”16
The Court held that under the Clean Water Act, for the EPA to establish jurisdiction over adjacent wetlands, it must demonstrate that16:
1. “the adjacent body of water constitutes waters of the United States (ie, a relatively permanent body of water connected to traditional interstate navigable waters)…”
2. “…the wetland has a continuous surface connection with that water, making it difficult to determine where the water ends, and the wetland begins.”
Under this definition, the Sacketts could build their house. This was a statutory interpretation case. Therefore, Congress can expand or otherwise change the EPA’s authority under the Clean Water Act and other legislation.
Conclusions: A new justice, “shadow docket,” and ethics rules
SCOTUS’ newest member. When the Marshall called the Court into session on October 3, 2022, it had a new member, Justice Ketanji Brown Jackson. She was sworn in on June 30, 2022, when her predecessor (Justice Breyer) officially retired. She had been a law clerk for Justice Breyer in 1999, as well as a district court judge and court of appeals judge. Those who count such things described her as the “chattiest justice.”17 She spoke more than any other justice—by one count, a total of 75,632 words (an average of 1,300 words in each of the 58 arguments).
A more balanced Court? Most commentators view the Court as more balanced or less conservative than the previous Term. For example, Justice Sotomayor was in the majority 40% last Term but 65% this Term. Justice Thomas was in the majority 75% last Term but 55% this Term. Put another way, this Term in the divided cases, the liberal justices were in the majority 64% of the time, compared with the conservative justices 73%.18 Of course, these differences may reflect a different set of cases rather than a change in the direction of the Court. There were 11 (or 12, depending on how 1 case is counted) 6-3 cases, but only 5 were considered ideological. That suggests that, in many cases, the coalitions were somewhat fluid.
“Shadow docket” controversy continues.19 Shadow docket refers to orders the Court makes that do not follow oral arguments and often do not have written opinions. The orders are all publicly available. This Term a close examination of the approximately 30 shadow docket opinions shows that the overwhelming majority were dissents or explanations about denials of certiorari. The Court ordered only a few stays or injunctions via the shadow docket. One shadow docket stay (that prevented a lower court order from going into effect) is particularly noteworthy. A federal judge had ordered the suspension of the distribution of mifepristone while courts considered claims that the US Food and Drug Administration (FDA) had improperly approved the drug. In a shadow docket order, the Court issued the stay to allow mifepristone to be sold while the case challenging its approval was heard.20 The only opinion was a dissent from Justice Alito. But it also demonstrates the importance of the shadow docket. Without this intervention, in at least part of the country, the distribution of mifepristone would have been interrupted pending the outcome of the FDA cases.
In August, the Court delayed a settlement in the Purdue Pharma liability bankruptcy case.21 It also stayed an injunction of a lower court, thereby permitting federal “ghost guns” regulations to go into effect at least temporarily.22
More ethics rules to come? Another area in which the Court faced criticism was formal ethics rules. The justices make financial disclosures, but these are somewhat ambiguous. There is likely to be increasing pressure for a more complete disclosure of non-financial relationships and more formal ethics rules. ●
The Court had, by September 1, 2023, accepted 22 cases for hearing next Term.1 The cases include a challenge to the extraordinary funding provision for the Consumer Financial Protection Bureau, another racial challenge to congressional districts (South Carolina), the status of Americans with Disability Act “testers” who look for violations without ever intending to use the facilities, the level of deference courts should give to interpreting federal statutes (so-called “Chevron” deference), the opioid (OxyContin ) bankruptcy, and limitations on gun ownership. This represents less than half of the cases the Court will likely hear next Term, so the Court will add many more cases to the docket. It promises to be an appealing Term.
Reference
1. October Term 2023. SCOTUSblog website. Accessed August 29, 2023. https://www.scotusblog.com/case-files/terms/ot2023/
When the Court adjourned on June 30, 2023, it had considered 60 cases, plus hundreds of petitions asking it to hear cases. Most commentators count 55 cases decided after briefing and oral argument and where there was a signed opinion. The information below uses 55 cases unless otherwise noted. During the 2022-2023 Term, the Court:
- upheld liability for the involuntary administration of psychotropic drugs in nursing home1
- permitted disabled students, in some instances, both to make Individuals with Disabilities Education Act (IDEA) claims for services and to file Americans with Disabilities Act (ADA) lawsuits against their schools2
- upheld a statute that makes it illegal to “encourage or induce an alien to come to, enter, or reside in the United States, knowing or in reckless disregard of the fact that such coming to, entry, or residence is or will be in violation of law.” The defendant had used a scam promising noncitizens “adult adoptions” (of which there is no such thing) making it legal for them to come to and stay in the United States.3
- narrowed the “fair use” of copyrighted works. It held that Andy Warhol’s use of a copyrighted photograph in his famous Prince prints was not “transformative” in a legal sense largely because the photo and prints “share the same use”—magazine illustrations.4
- in another intellectual property case, held that Jack Daniel’s might sue a dog toy maker for a rubber dog toy that looked like a Jack Daniel’s bottle5
- further expanded the Federal Arbitration Act by holding that a federal district court must immediately stay court proceedings if one party is appealing a decision not to require arbitration6
- held that two social media companies were not responsible for terrorists using their platforms to recruit others to their cause. It did not, however, decide whether §230 of the Communication Decency Act protects companies from liability.7
- made it easier for employees to receive accommodation for their religious practices and beliefs. Employers must make religious accommodations unless the employer can show that “the burden of granting an accommodation would result in substantial increased [financial and other] costs in relation to the conduct of its particular business.”8
- declined to hear an appeal from Johnson & Johnson (through a subsidiary, Ethicon) about pelvic mesh. In this case, the California Attorney General filed a lawsuit against Ethicon for false advertising by failing to detail the risks of pelvic mesh. The lower courts estimated 240,000 written violations of the law by Ethicon between 2008 and 2017. The trial and appeal to California courts resulted in a judgment of $302 million against Johnson & Johnson. The company asked the Court to review that judgment, but the Court denied certiorari. That likely means the $302 million is final.
References
1. Health and Hospital Corporation of Marion Cty. v Talevski, Docket no. 21-806; June 8, 2023.
2. Luna Perez v Sturgis Public Schools, Docket no. 21-887; March 21, 2023.
3. United States v Hansen, Docket no. 22-179; June 23, 2023.
4. Andy Warhol Foundation for Visual Arts, Inc. v Goldsmith, Docket no. 21-869; May 18, 2023.
5. Jack Daniel’s Properties, Inc. v VIP Products LLC, Docket no. 22-148; June 8, 2023.
6. Coinbase, Inc. v Bielski, Docket no. 22-105; June 23, 2023.
7. Gonzalez v Google LLC, Docket no. 21-1333; May 18, 2023.
8. Groff v DeJoy, Docket no. 22-273; June 29, 2023.

The 2022-2023 Term of the Supreme Court illustrates how important the Court has become to health-related matters, including decisions regarding the selection and training of new professionals, the daily practice of medicine, and the future availability of new drugs. The importance of several cases is reinforced by the fact that major medical organizations filed amicus curiae (“friend of the court”) briefs in those cases.
Amicus briefs are filed by individuals or organizations with something significant to say about a case to the court—most often to present a point of view, make an argument, or provide information that the parties to the case may not have communicated. Amicus briefs are burdensome in terms of the time, energy, and cost of preparing and filing. Thus, they are not undertaken lightly. Medical organizations submitted amicus briefs in the first 3 cases we consider.
Admissions, race, and diversity
The case: Students for Fair Admissions v President and Fellows of Harvard College
The American College of Obstetricians and Gynecologists (ACOG) joined an amici curiae brief in Students for Fair Admissions v President and Fellows of Harvard College (and the University of North Carolina [UNC]).1 This case challenged the use of racial preferences in college admissions. The Association of American Medical Colleges (AAMC) was the lead organization; nearly 40 other health-related organizations joined the brief.
The legal claim. Those filing the suits asserted that racial preferences by public colleges violate the 14th Amendment’s Equal Protection Clause (“no state shall deny to any person … the equal protection of the law”). That is, if a state university gives racial preferences in selective admissions, it denies some other applicant the equal protection of the law. As for private schools (in this case, Harvard), Title VI of the Civil Rights Act of 1964 has the same standards as the Equal Protection Clause. Thus, the Court consolidated the cases and used the same legal standard in considering public and private colleges (with “colleges” including professional and graduate programs as well as undergraduate institutions).
Background. For nearly 50 years, the Supreme Court has allowed limited racial preferences in college admissions. Those preferences could only operate as a plus, however, and not a negative for applicants and be narrowly tailored. The measure was instituted temporarily; in a 2003 case, the Court said, “We expect that 25 years from now, the use of racial preferences will no longer be necessary.”2
Decision. In a 6-3 decision, the Court held (in the UNC case) that racial preferences generally violate the Constitution, and by a 6-2 decision (in the Harvard case) these preferences violate the Civil Rights Act of 1964. (Justice Jackson was recused in the Harvard case because of a conflict.) The opinion covered 237 pages in the US Reports, so any summary is incomplete.
The majority concluded, “The Harvard and UNC admissions programs cannot be reconciled with the guarantees of the Equal Protection Clause. Both programs lack sufficiently focused and measurable objectives warranting the use of race, unavoidably employ race in a negative manner, involve racial stereotyping, and lack meaningful end points. We have never permitted admissions programs to work in that way, and we will not do so today.”3
There were 3 concurring opinions and 2 dissents in the case. The concurrences reviewed the history of the Equal Protection Clause and the Civil Rights Act, the damage racial preferences can do, and the explicit limits the Court said there must be on racial preferences in higher education. The dissents had a different view of the legal history of the 14th Amendment. They said the majority was turning a blind eye to segregation in society and the race-based gap in America.
As a practical matter, this case means that colleges, including professional schools, cannot use racial preferences. The Court said that universities may consider essays and the like in which applicants describe how their own experiences as an individual (including race) have affected their own lives. However, the Court cautioned that “universities may not simply establish through application essays or other means the regime we hold unlawful today.”3
Continue to: The amici brief...
The amici brief
ACOG joined 40 other health-related organizations in filing an amici brief (multiple “friends”) in Students for Fair Admission. The AAMC led the brief, with the others signing as amici.4 The brief made 3 essential points: diversity in medical education “markedly improves health outcomes,” and a loss of diversity “threaten[s] patients’ health; medical schools engage in an intense “holistic” review of applicants for admission; and medical schools must consider applicants’ “full background” (including race) to achieve their educational and professional goals.4
A powerful part of the brief described the medical school admissions process, particularly the very “holistic” review that is not entirely dependent on admissions scores. The brief effectively weaves the consideration of race into this process, mentioning race (on page 22) only after discussing many other admissions factors.
Child custody decisions related to the Indian Child Welfare Act
The case: Haaland v Brackeen
The American Medical Association (AMA) and the American Academy of Pediatrics filed a brief in Haaland v Brackeen5 involving the constitutionality of the 1978 Indian Child Welfare Act (ICWA). The statute followed a terrible history of Indian children being removed from their families inappropriately, as detailed in a concurring opinion by Justice Gorsuch.5 The two purposes of the act were to promote raising Native American children in their culture and stem the downward trend in tribal membership.
The legal claim. The Court consolidated several cases. Essentially, a 10-month-old child (A.L.M.) was placed in foster care with the Brackeens in Texas. After more than 1 year, the Brackeens sought adoption; the biological father, mother, and grandparents all supported it. The Navajo and Cherokee Nations objected and informed the Texas court that they had found alternative placement with (nonrelative) tribal members in New Mexico. The “court-appointed guardian and a psychological expert … described the strong emotional bond between A.L.M. and his foster parents.” The court denied the adoption petition based on ICWA’s preference for tribe custody, and the Brackeens filed a lawsuit.
Decision. The constitutional claim in the case was that Congress lacked the authority to impose these substantial rules on states in making child custody decisions. The Supreme Court, in a 7-2 decision, upheld the constitutionality of the ICWA.
The amici brief
The joint amici brief of the American Academy of Pediatrics (AAP) and the AMA argued that tribes are “extended families” of Native American children.6 It noted the destructive history of removing Native American children from their families and suggested that kinship care improves children’s health. To its credit, the brief also honestly noted the serious mental health and suicide rates in some tribes, which suggest issues that might arise in child custody and adoption cases.
The Court did not, in this case, take up another constitutional issue that the parties raised—whether the strong preference for Native American over non ̶ Native American custody violates the Equal Protection Clause of the 14th Amendment. The Court said the parties to this case did not have standing to raise the issue. Justice Kavanaugh, concurring, said it was a “serious” issue and invited it to be raised in another case.5
False Claims Act cases
The case: Costs for SuperValu prescriptions
The legal claim. One FCA case this Term involved billings SuperValu made for outpatient prescriptions in Medicare-Medicaid programs. As its “usual and customary” costs, it essentially reported a list price that did not include the substantial discounts it commonly gave.
Background. Subjective knowledge (what the defendant actually knows) may seem impossible to prove—the defendant could just say, “I did not know I was doing wrong.” Over time the law has developed several ways of demonstrating “knowing.” Justice Thomas, writing for a unanimous Court, held that whistleblowers or the government might prove “knowing” in 3 ways:
1. defendants “actually knew that their reported prices were not their ‘usual and customary’ prices when they reported them”
2. were aware of a substantial risk that their higher, retail prices were not their “usual and customary” prices and intentionally avoided learning whether their reports were accurate
3. were aware of such a substantial and unjustifiable risk but submitted the claims anyway.9
Of course, records of the company, information from the whistleblower, and circumstantial evidence may be used to prove any of these; it does not require the company’s admission.
The Court said that if the government or whistleblowers make a showing of any of these 3 things, it is enough.
Decision. The case was returned to the lower court to apply these rules.
The amici brief
The American Hospital Association and America’s Health Insurance Plans filed an amici brief.10 It reminded the Court that many reimbursement regulations are unclear. Therefore, it is inappropriate to impose FCA liability for guessing incorrectly what the regulations mean. Having to check on every possible ambiguity was unworkable. The Court declined, however, the suggestion that defendants should be able to use any one of many “objectively” reasonable interpretations of regulations.
Continue to: The case: Polansky v Executive Health Resources...
The case: Polansky v Executive Health Resources
Health care providers who dislike the FCA may find solace this Term in this second FCA case.11
The legal claim. Polansky, a physician employed by a medical billing company, became an “intervenor” in a suit claiming the company assisted hospitals in false billing (inpatient claims for outpatient services). The government sought to dismiss the case, but Polansky refused.
Decision. The case eventually reached the Supreme Court, which held that the government may enter an FCA case at any time and move to dismiss the case even over the objection of a whistleblower. The government does not seek to enter a case in order to file dismissal motions often. When it does so, whistleblowers are protected by the fact that the dismissal motion requires a hearing before the federal court.
An important part of this case has escaped much attention. Justices Thomas, Kavanaugh, and Barrett invited litigation to determine if allowing private whistleblowers to represent the government’s interest is consistent with Article II of the Constitution.11 The invitation will likely be accepted. We expect to see cases challenging the place of “intervenors” pursuing claims when the government has declined to take up the case. The private intervenor is a crucial provision of the current FCA, and if such a challenge were successful, it could substantially reduce FCA cases.
Criminal false claims
Dubin overbilled Medicaid for psychological testing by saying the testing was done by a licensed psychologist rather than an assistant. The government claimed the “identity theft” was using the patient’s (actual) Medicaid number in submitting the bill.12 The Court unanimously held the overbilling was not aggravated identity theft as defined in federal law. Dubin could be convicted of fraudulent billing but not aggravated identity theft, thereby avoiding the mandatory prison term.
Patents of “genus” targets
The case: Amgen v Sanofi
Background. “Genus” patents allow a single pharmaceutical company to patent every antibody that binds to a specific amino acid on a naturally occurring protein. In this case, the patent office had granted a “genus” patent on “all antibodies” that bind to the naturally occurring protein PCSK9 and block it from hindering the body’s mechanism for removing low-density lipoprotein (LDL) cholesterol from the bloodstream,13 helping to reduce LDL cholesterol levels. These patents could involve millions of antibodies—and Amgen was claiming a patent on all of them. Amgen and Sanofi marketed their products, each with their own unique amino acid sequence.13 Amgen sued Sanofi for violating its patent rights.
Decision. The Court unanimously held that Amgen did not have a valid patent on all antibodies targeting PCSK9, only those that it had explicitly described in its patent application—a ruling based on a 150-year-old technical requirement for receiving a patent. An applicant for a patent must include “a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art…to make and use the same.”13 Amgen’s patent provided the description for only a few of the antibodies, but from the description in its application others could not “make and use” all of the antibodies targeting PCSK9.
While the decision was vital for future pharmaceuticals, the patent principle on which it was based has an interesting history. The Court noted that it affected the telegraph (Morse lost part of his patent), electric lights (Edison won his case against other inventors), and the glue for wood veneering (Perkins Glue Company lost).13
Continue to: Other notable decisions...
Other notable decisions
Student loans
The Court struck down the Biden Administration’s student loan forgiveness program, which would have cost approximately $430 billion.14 The central issue was whether the administration had the authority for such massive loan forgiveness; that is, whether Congress had authorized the broad loan forgiveness. The administration claimed authority from the post ̶ 9/11 HEROES Act, which allows the Secretary of Education to “waive or modify” loan provisions during national emergencies.
Free speech and the wedding web designer
303 Creative v Elenis involved a creative website designer who did not want to be required to create a website for a gay wedding.15 The designer had strong beliefs against same-sex marriages, but Colorado sought to force her to do so under the state “public accommodations” law. In a 6-3 decision, the Court held that the designer had a “free speech” right. That is, the state could not compel her to undertake speech expressing things she did not believe. This was because the website design was an expressive, creative activity and therefore was “speech” under the First Amendment.
Wetlands and the Clean Water Act
The essential issue in Sackett v Environmental Protection Agency (EPA) was the definitions of waters of the United States and related wetlands. The broad definition the EPA used meant it had jurisdiction to regulate an extraordinary amount of territory. It had, for example, prevented the Sacketts from building a modest house claiming it was part of the “waters of the United States because they were near a ditch that fed into a creek, which fed into Priest Lake, a navigable, intrastate lake.” The Court held that the EPA exceeded its statutory authority to define “wetlands.”16
The Court held that under the Clean Water Act, for the EPA to establish jurisdiction over adjacent wetlands, it must demonstrate that16:
1. “the adjacent body of water constitutes waters of the United States (ie, a relatively permanent body of water connected to traditional interstate navigable waters)…”
2. “…the wetland has a continuous surface connection with that water, making it difficult to determine where the water ends, and the wetland begins.”
Under this definition, the Sacketts could build their house. This was a statutory interpretation case. Therefore, Congress can expand or otherwise change the EPA’s authority under the Clean Water Act and other legislation.
Conclusions: A new justice, “shadow docket,” and ethics rules
SCOTUS’ newest member. When the Marshall called the Court into session on October 3, 2022, it had a new member, Justice Ketanji Brown Jackson. She was sworn in on June 30, 2022, when her predecessor (Justice Breyer) officially retired. She had been a law clerk for Justice Breyer in 1999, as well as a district court judge and court of appeals judge. Those who count such things described her as the “chattiest justice.”17 She spoke more than any other justice—by one count, a total of 75,632 words (an average of 1,300 words in each of the 58 arguments).
A more balanced Court? Most commentators view the Court as more balanced or less conservative than the previous Term. For example, Justice Sotomayor was in the majority 40% last Term but 65% this Term. Justice Thomas was in the majority 75% last Term but 55% this Term. Put another way, this Term in the divided cases, the liberal justices were in the majority 64% of the time, compared with the conservative justices 73%.18 Of course, these differences may reflect a different set of cases rather than a change in the direction of the Court. There were 11 (or 12, depending on how 1 case is counted) 6-3 cases, but only 5 were considered ideological. That suggests that, in many cases, the coalitions were somewhat fluid.
“Shadow docket” controversy continues.19 Shadow docket refers to orders the Court makes that do not follow oral arguments and often do not have written opinions. The orders are all publicly available. This Term a close examination of the approximately 30 shadow docket opinions shows that the overwhelming majority were dissents or explanations about denials of certiorari. The Court ordered only a few stays or injunctions via the shadow docket. One shadow docket stay (that prevented a lower court order from going into effect) is particularly noteworthy. A federal judge had ordered the suspension of the distribution of mifepristone while courts considered claims that the US Food and Drug Administration (FDA) had improperly approved the drug. In a shadow docket order, the Court issued the stay to allow mifepristone to be sold while the case challenging its approval was heard.20 The only opinion was a dissent from Justice Alito. But it also demonstrates the importance of the shadow docket. Without this intervention, in at least part of the country, the distribution of mifepristone would have been interrupted pending the outcome of the FDA cases.
In August, the Court delayed a settlement in the Purdue Pharma liability bankruptcy case.21 It also stayed an injunction of a lower court, thereby permitting federal “ghost guns” regulations to go into effect at least temporarily.22
More ethics rules to come? Another area in which the Court faced criticism was formal ethics rules. The justices make financial disclosures, but these are somewhat ambiguous. There is likely to be increasing pressure for a more complete disclosure of non-financial relationships and more formal ethics rules. ●
The Court had, by September 1, 2023, accepted 22 cases for hearing next Term.1 The cases include a challenge to the extraordinary funding provision for the Consumer Financial Protection Bureau, another racial challenge to congressional districts (South Carolina), the status of Americans with Disability Act “testers” who look for violations without ever intending to use the facilities, the level of deference courts should give to interpreting federal statutes (so-called “Chevron” deference), the opioid (OxyContin ) bankruptcy, and limitations on gun ownership. This represents less than half of the cases the Court will likely hear next Term, so the Court will add many more cases to the docket. It promises to be an appealing Term.
Reference
1. October Term 2023. SCOTUSblog website. Accessed August 29, 2023. https://www.scotusblog.com/case-files/terms/ot2023/
When the Court adjourned on June 30, 2023, it had considered 60 cases, plus hundreds of petitions asking it to hear cases. Most commentators count 55 cases decided after briefing and oral argument and where there was a signed opinion. The information below uses 55 cases unless otherwise noted. During the 2022-2023 Term, the Court:
- upheld liability for the involuntary administration of psychotropic drugs in nursing home1
- permitted disabled students, in some instances, both to make Individuals with Disabilities Education Act (IDEA) claims for services and to file Americans with Disabilities Act (ADA) lawsuits against their schools2
- upheld a statute that makes it illegal to “encourage or induce an alien to come to, enter, or reside in the United States, knowing or in reckless disregard of the fact that such coming to, entry, or residence is or will be in violation of law.” The defendant had used a scam promising noncitizens “adult adoptions” (of which there is no such thing) making it legal for them to come to and stay in the United States.3
- narrowed the “fair use” of copyrighted works. It held that Andy Warhol’s use of a copyrighted photograph in his famous Prince prints was not “transformative” in a legal sense largely because the photo and prints “share the same use”—magazine illustrations.4
- in another intellectual property case, held that Jack Daniel’s might sue a dog toy maker for a rubber dog toy that looked like a Jack Daniel’s bottle5
- further expanded the Federal Arbitration Act by holding that a federal district court must immediately stay court proceedings if one party is appealing a decision not to require arbitration6
- held that two social media companies were not responsible for terrorists using their platforms to recruit others to their cause. It did not, however, decide whether §230 of the Communication Decency Act protects companies from liability.7
- made it easier for employees to receive accommodation for their religious practices and beliefs. Employers must make religious accommodations unless the employer can show that “the burden of granting an accommodation would result in substantial increased [financial and other] costs in relation to the conduct of its particular business.”8
- declined to hear an appeal from Johnson & Johnson (through a subsidiary, Ethicon) about pelvic mesh. In this case, the California Attorney General filed a lawsuit against Ethicon for false advertising by failing to detail the risks of pelvic mesh. The lower courts estimated 240,000 written violations of the law by Ethicon between 2008 and 2017. The trial and appeal to California courts resulted in a judgment of $302 million against Johnson & Johnson. The company asked the Court to review that judgment, but the Court denied certiorari. That likely means the $302 million is final.
References
1. Health and Hospital Corporation of Marion Cty. v Talevski, Docket no. 21-806; June 8, 2023.
2. Luna Perez v Sturgis Public Schools, Docket no. 21-887; March 21, 2023.
3. United States v Hansen, Docket no. 22-179; June 23, 2023.
4. Andy Warhol Foundation for Visual Arts, Inc. v Goldsmith, Docket no. 21-869; May 18, 2023.
5. Jack Daniel’s Properties, Inc. v VIP Products LLC, Docket no. 22-148; June 8, 2023.
6. Coinbase, Inc. v Bielski, Docket no. 22-105; June 23, 2023.
7. Gonzalez v Google LLC, Docket no. 21-1333; May 18, 2023.
8. Groff v DeJoy, Docket no. 22-273; June 29, 2023.
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306, 326 (2003).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___, 39 (2023).
- Brief for Amici Curiae Association of American Medical Colleges et al. in Support of Respondents, Students for Fair Admissions v University of North Carolina (July 28, 2022). Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-707/232120/20220728171307159_20 -1199%20and%2021-707%20Amicus%20Brief%20for%20 Association%20of%20American%20Medical%20Colleges%20 et%20al.pdf
- Haaland v Brackeen, Docket no. 21-376; June 15, 2023.
- Brief of American Academy of Pediatrics and American Medical Association as Amici Curiae in Support of Respondents, in Haaland v Brackeen. August 19, 2022. Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-376/234042/20220819140750948_21-376 .amics.brief.FINAL.pdf
- Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021. US Department of Justice website. February 1, 2022. Accessed August 18, 2023. https://www.justice.gov/opa/pr/justice -department-s-false-claims-act-settlements-and-judgments -exceed-56-billion-fiscal-year
- False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022. US Department of Justice website. February 7, 2023. Accessed August 18, 2023. https://www .justice.gov/opa/pr/false-claims-act-settlements-and -judgments-exceed-2-billion-fiscal-year-2022
- United States ex rel. Schutte v Supervalu Inc., Docket no. 21-1326; June 1, 2023.
- Brief of American Hospital Association and America’s Health Insurance Plans as Amici Curiae in Support of Respondents, in Schutte v Supervalu. March 2023. Accessed August 18, 2023. https://www.supremecourt.gov/DocketPDF/21/21-1326 /262428/20230331113854936_3-31-23%20AHA_AHIP _Amicus_Brief.pdf
- United States ex rel. Polansky v Executive Health Resources, Inc., Docket no. 21-1052; June 16, 2023.
- Dubin v United States, Docket no. 22-10; June 8, 2023.
- Amgen v Sanofi, 598 US ___ (2023).
- Biden v Nebraska, 600 US ___ (2023).
- 303 Creative LLC v Elenis, 600 US ___ (2023).
- Sackett v Environmental Protection Agency, Docket no. 21454; May 25, 2023.
- Krochtengel J. Jackson debuts as chattiest Justice. Law360. July 3, 2023. https://www.law360.com/articles/1692839 /jackson-debuts-as-chattiest-justice
- Feldman A. Another One Bites the Dust: End of 2022/2023 Supreme Court Term Statistics. EmpiricalScotus website. 30, 2023. Accessed August 18, 2023. https://empiricalscotus .com/2023/06/30/another-one-bites-2022/
- Vladeck S. The Shadow Docket: How the Supreme Court Uses Stealth Rulings to Amass Power and Undermine the Republic. New York, New York; Basic Books; 2023.
- Danco Laboratories, LLC v Alliance for Hippocratic Medicine. Docket no. 22A902; April 21, 2023.
- Harrington v Purdue Pharma, 23-124 (23A87).
- Garland v Vanderstok, 23-10718 (August 8, 2023).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306, 326 (2003).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___, 39 (2023).
- Brief for Amici Curiae Association of American Medical Colleges et al. in Support of Respondents, Students for Fair Admissions v University of North Carolina (July 28, 2022). Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-707/232120/20220728171307159_20 -1199%20and%2021-707%20Amicus%20Brief%20for%20 Association%20of%20American%20Medical%20Colleges%20 et%20al.pdf
- Haaland v Brackeen, Docket no. 21-376; June 15, 2023.
- Brief of American Academy of Pediatrics and American Medical Association as Amici Curiae in Support of Respondents, in Haaland v Brackeen. August 19, 2022. Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-376/234042/20220819140750948_21-376 .amics.brief.FINAL.pdf
- Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021. US Department of Justice website. February 1, 2022. Accessed August 18, 2023. https://www.justice.gov/opa/pr/justice -department-s-false-claims-act-settlements-and-judgments -exceed-56-billion-fiscal-year
- False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022. US Department of Justice website. February 7, 2023. Accessed August 18, 2023. https://www .justice.gov/opa/pr/false-claims-act-settlements-and -judgments-exceed-2-billion-fiscal-year-2022
- United States ex rel. Schutte v Supervalu Inc., Docket no. 21-1326; June 1, 2023.
- Brief of American Hospital Association and America’s Health Insurance Plans as Amici Curiae in Support of Respondents, in Schutte v Supervalu. March 2023. Accessed August 18, 2023. https://www.supremecourt.gov/DocketPDF/21/21-1326 /262428/20230331113854936_3-31-23%20AHA_AHIP _Amicus_Brief.pdf
- United States ex rel. Polansky v Executive Health Resources, Inc., Docket no. 21-1052; June 16, 2023.
- Dubin v United States, Docket no. 22-10; June 8, 2023.
- Amgen v Sanofi, 598 US ___ (2023).
- Biden v Nebraska, 600 US ___ (2023).
- 303 Creative LLC v Elenis, 600 US ___ (2023).
- Sackett v Environmental Protection Agency, Docket no. 21454; May 25, 2023.
- Krochtengel J. Jackson debuts as chattiest Justice. Law360. July 3, 2023. https://www.law360.com/articles/1692839 /jackson-debuts-as-chattiest-justice
- Feldman A. Another One Bites the Dust: End of 2022/2023 Supreme Court Term Statistics. EmpiricalScotus website. 30, 2023. Accessed August 18, 2023. https://empiricalscotus .com/2023/06/30/another-one-bites-2022/
- Vladeck S. The Shadow Docket: How the Supreme Court Uses Stealth Rulings to Amass Power and Undermine the Republic. New York, New York; Basic Books; 2023.
- Danco Laboratories, LLC v Alliance for Hippocratic Medicine. Docket no. 22A902; April 21, 2023.
- Harrington v Purdue Pharma, 23-124 (23A87).
- Garland v Vanderstok, 23-10718 (August 8, 2023).
Hepatitis B infection in pregnancy: Essentials of antiviral therapy and immunoprophylaxis
Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.

Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●

Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.