User login
Female Runner, 47, with Inguinal Lump
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
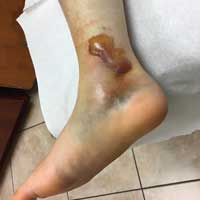
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
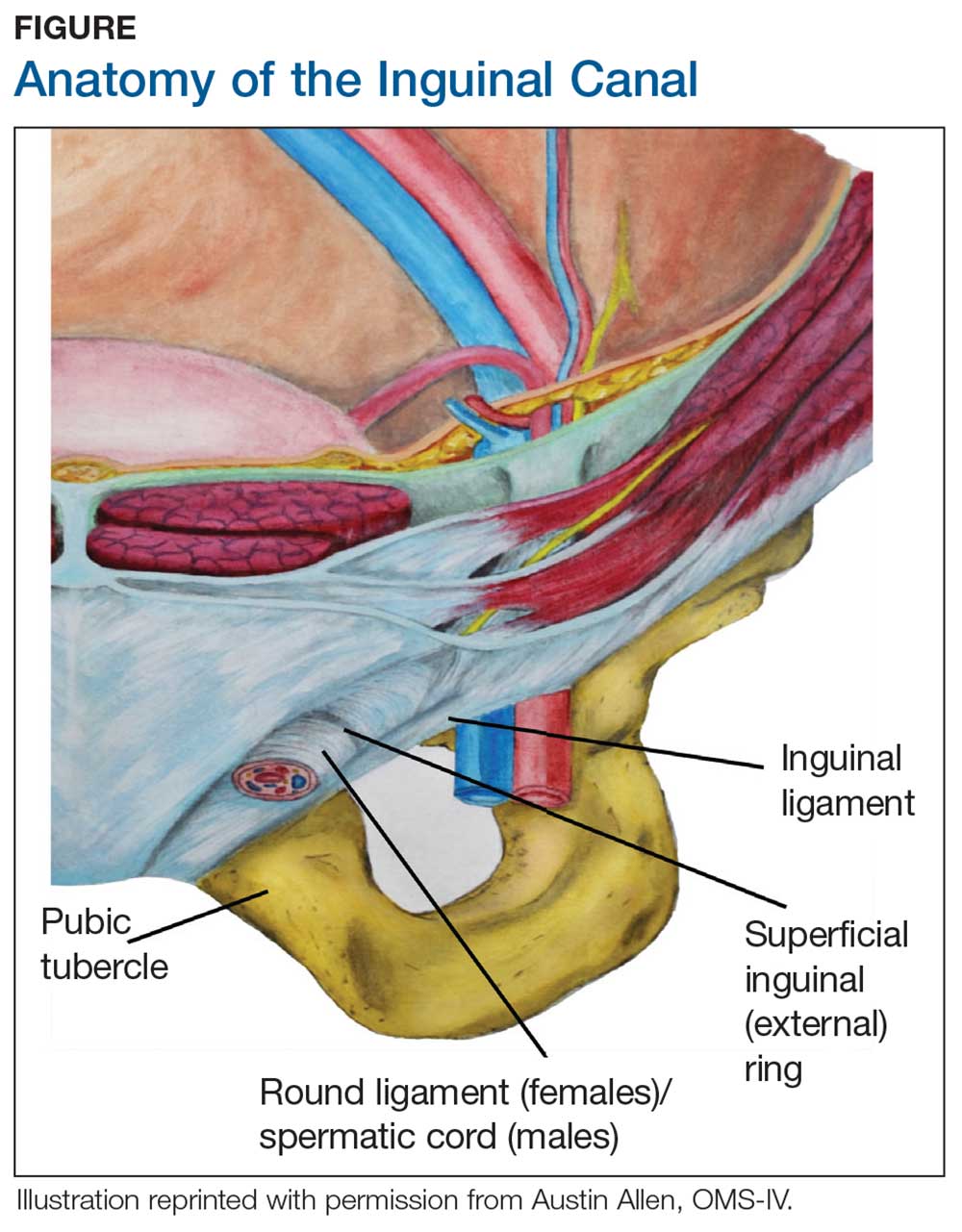
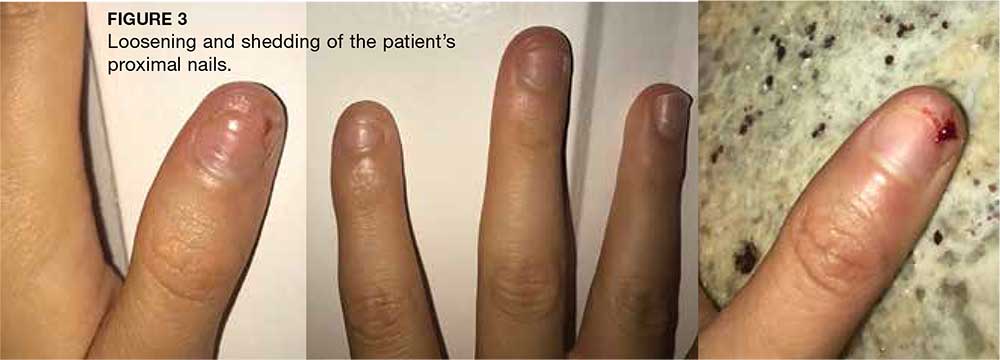
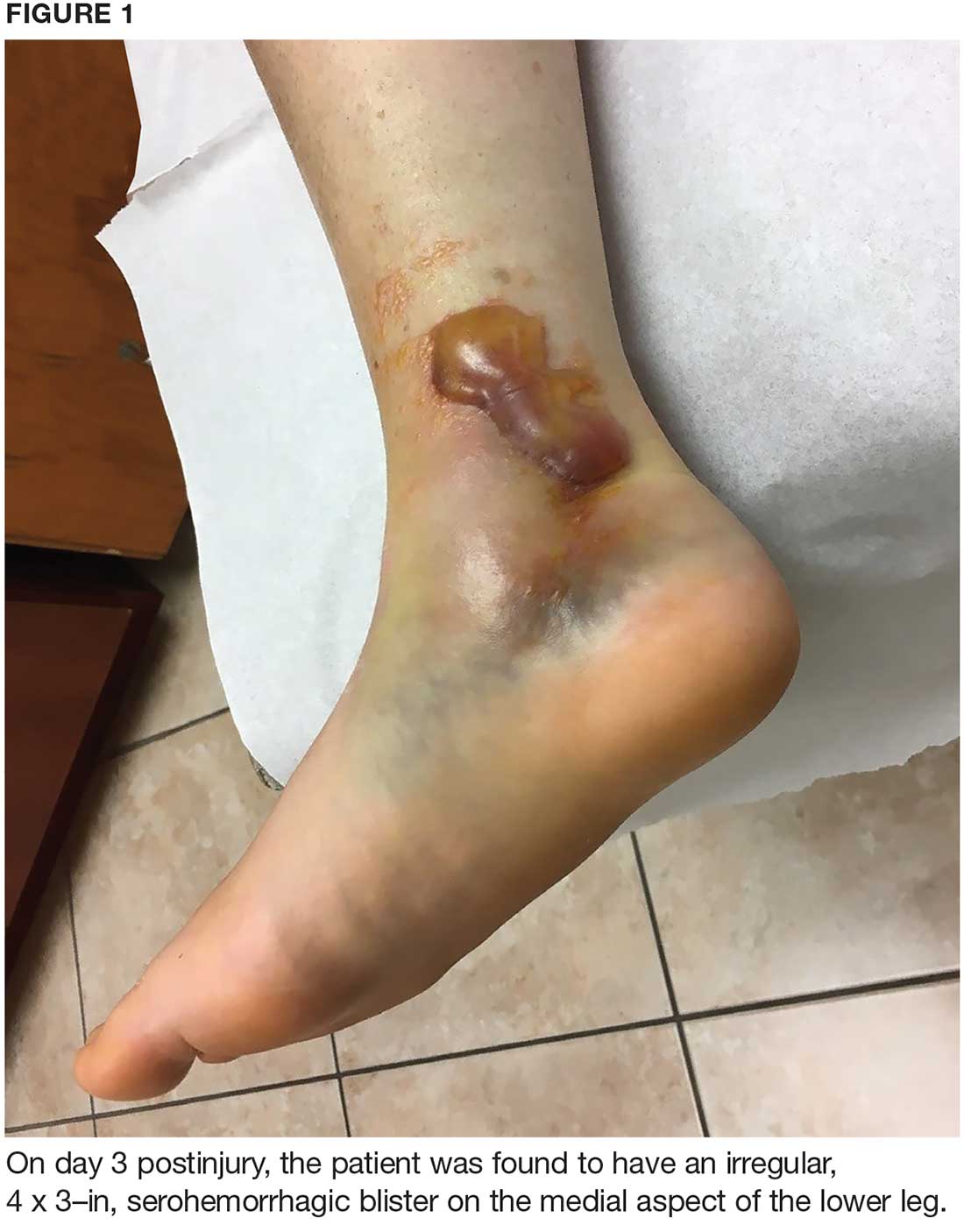
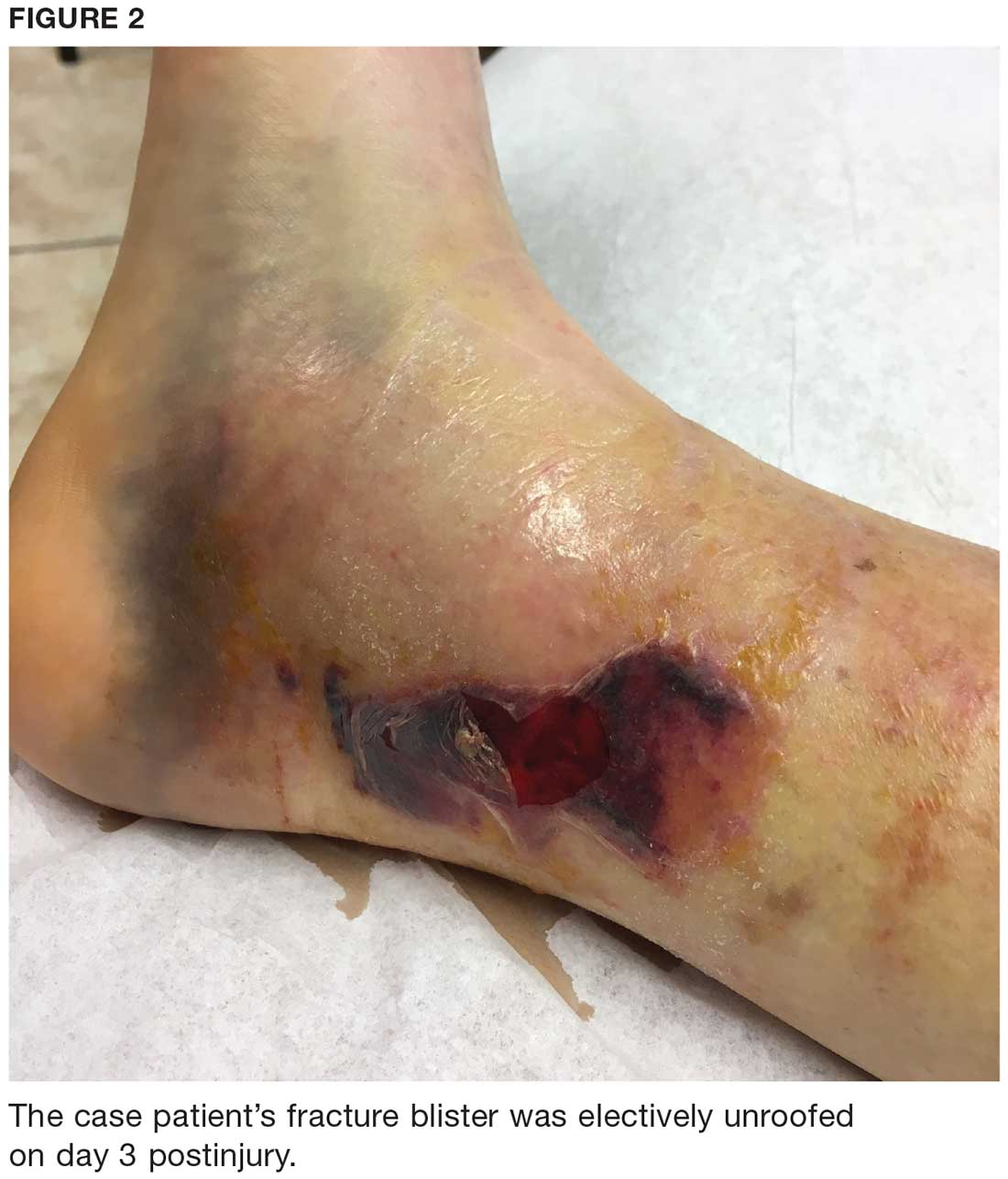
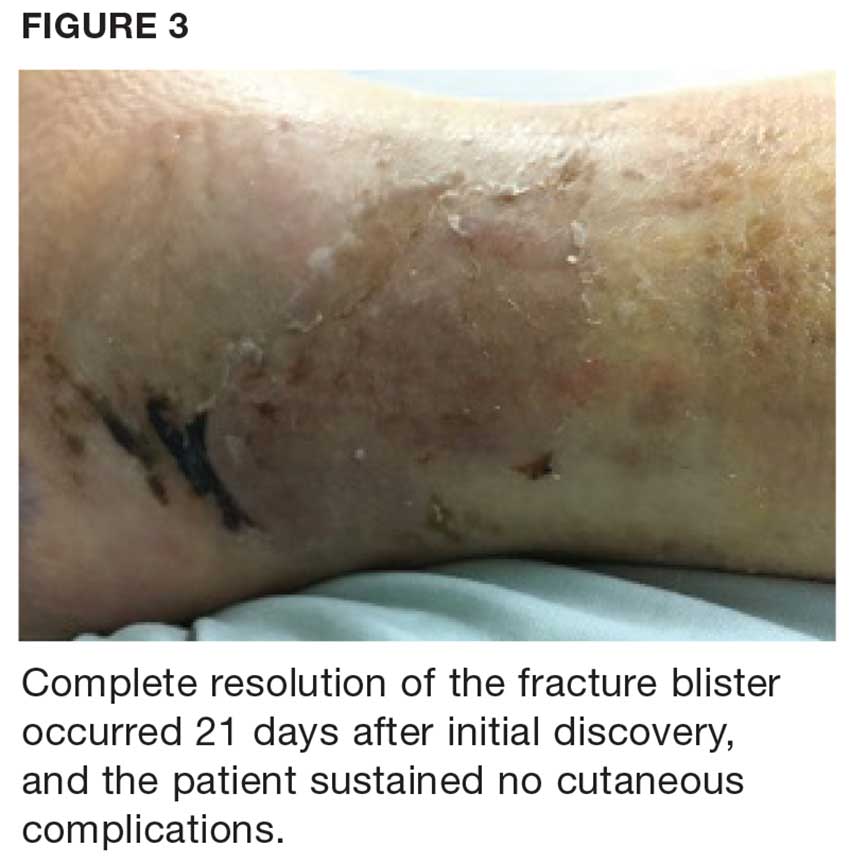
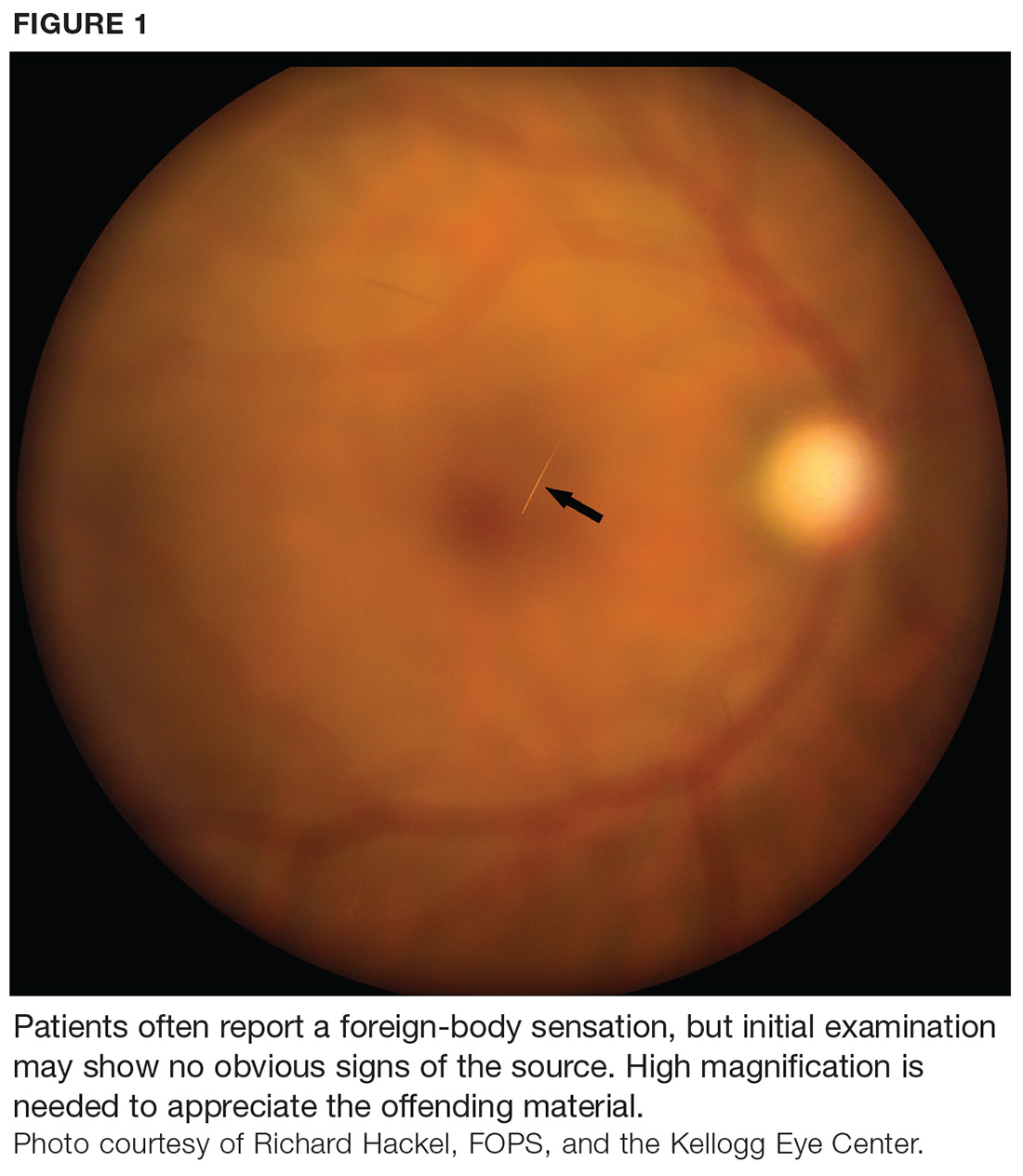
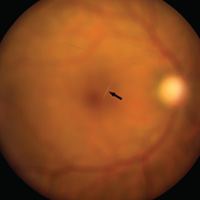
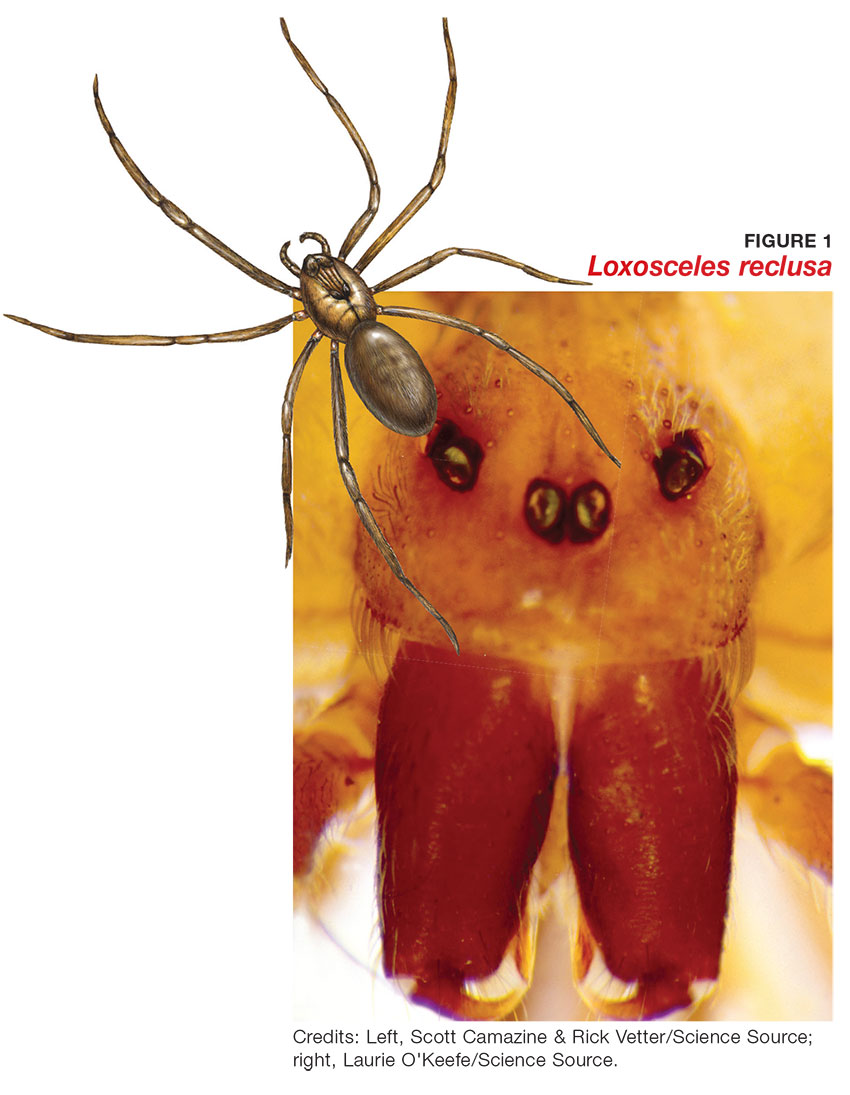
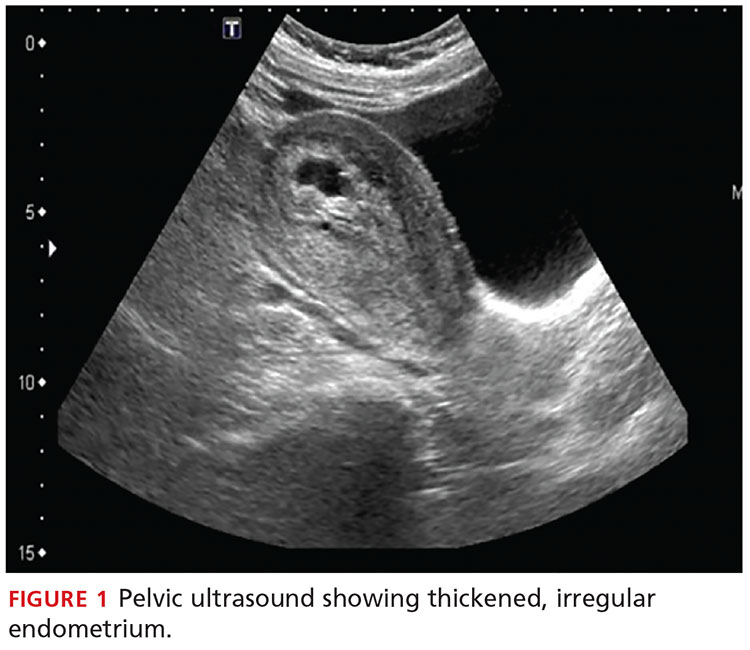
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
Acute Palmar and Plantar Rash in a 52-Year-Old Woman
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
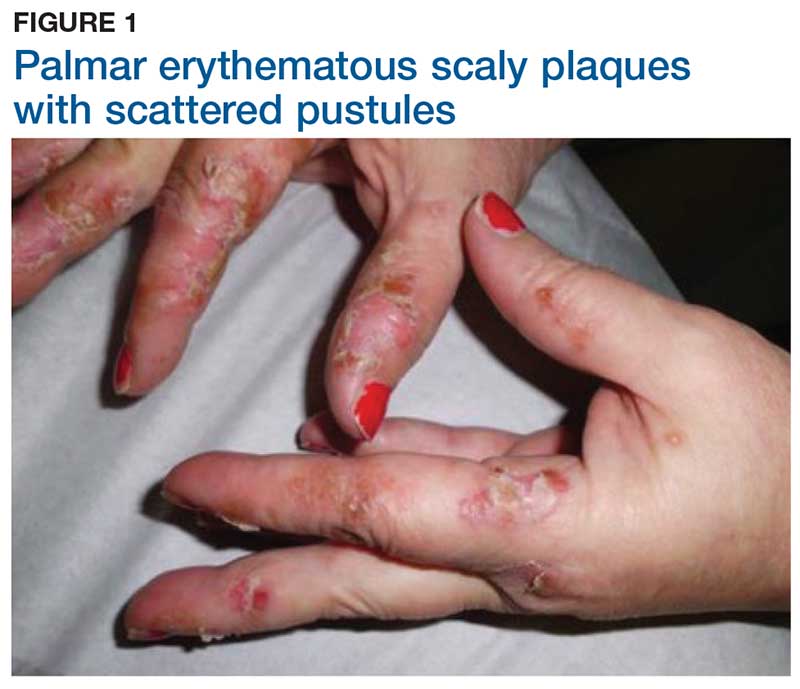
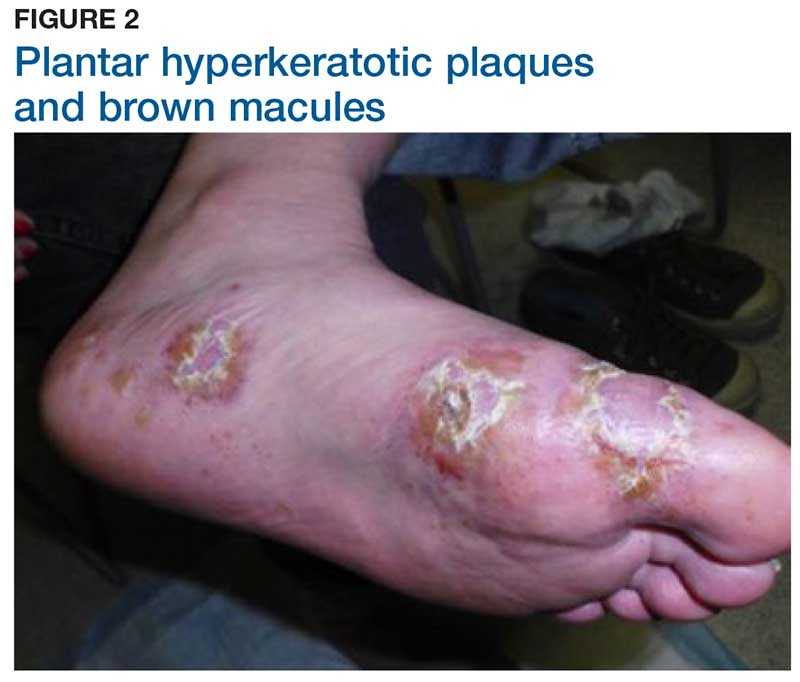
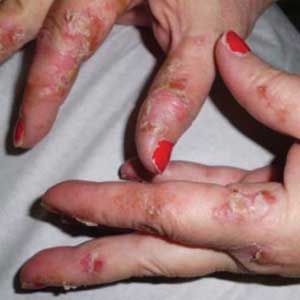
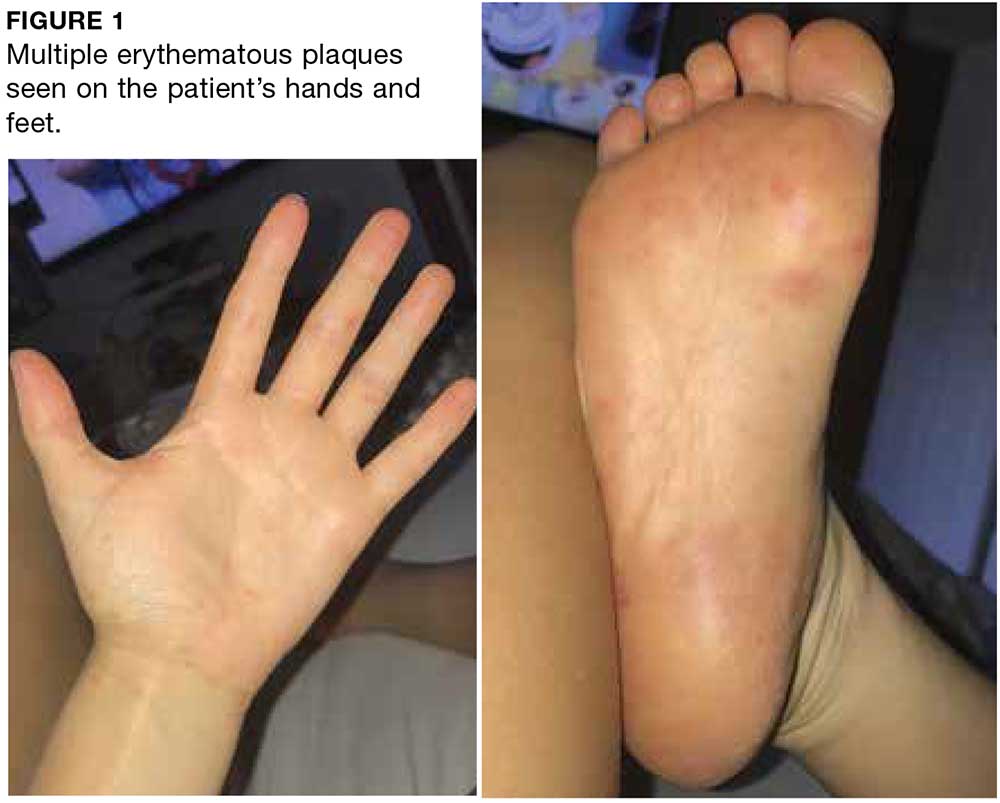
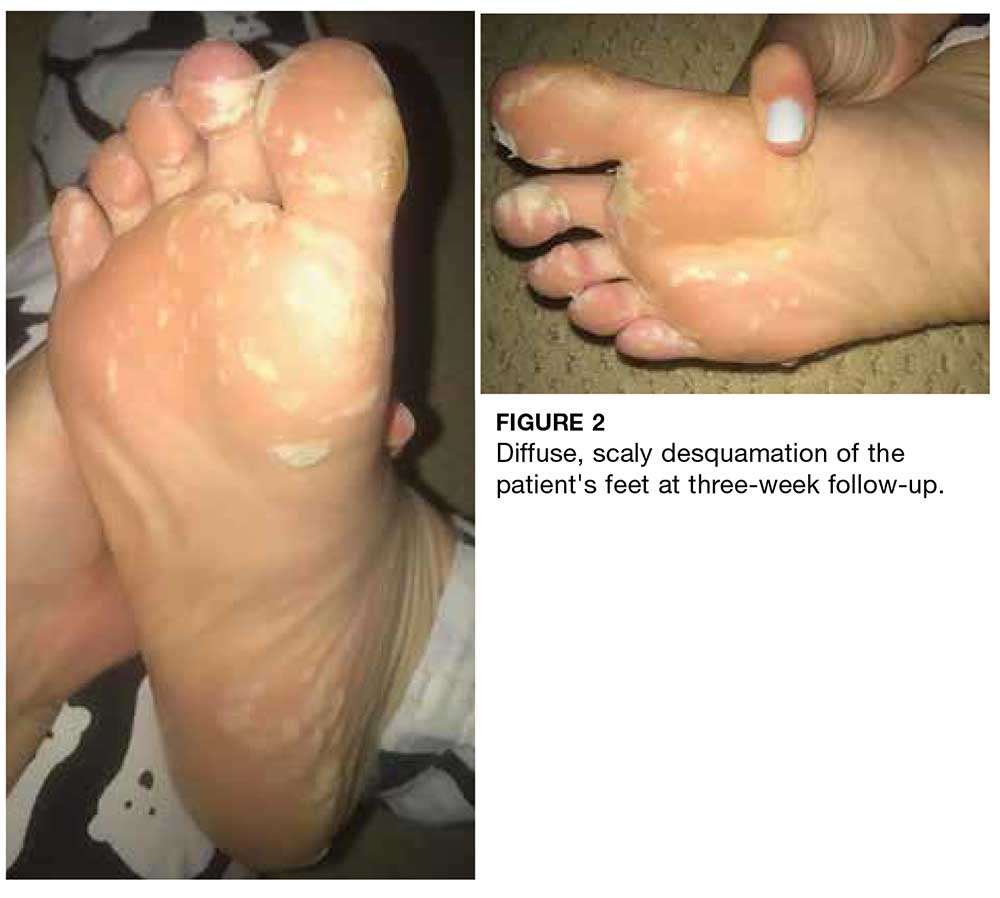
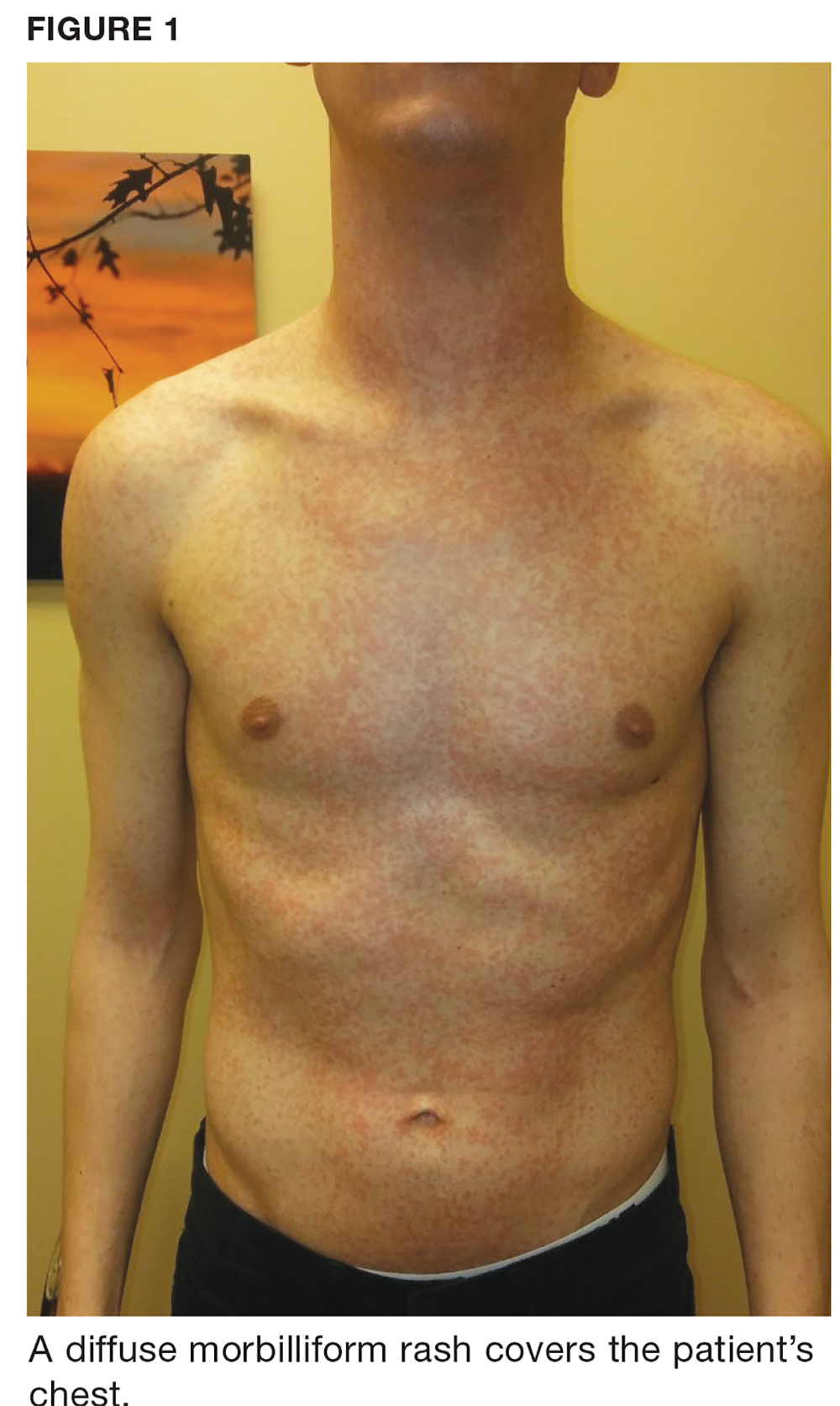
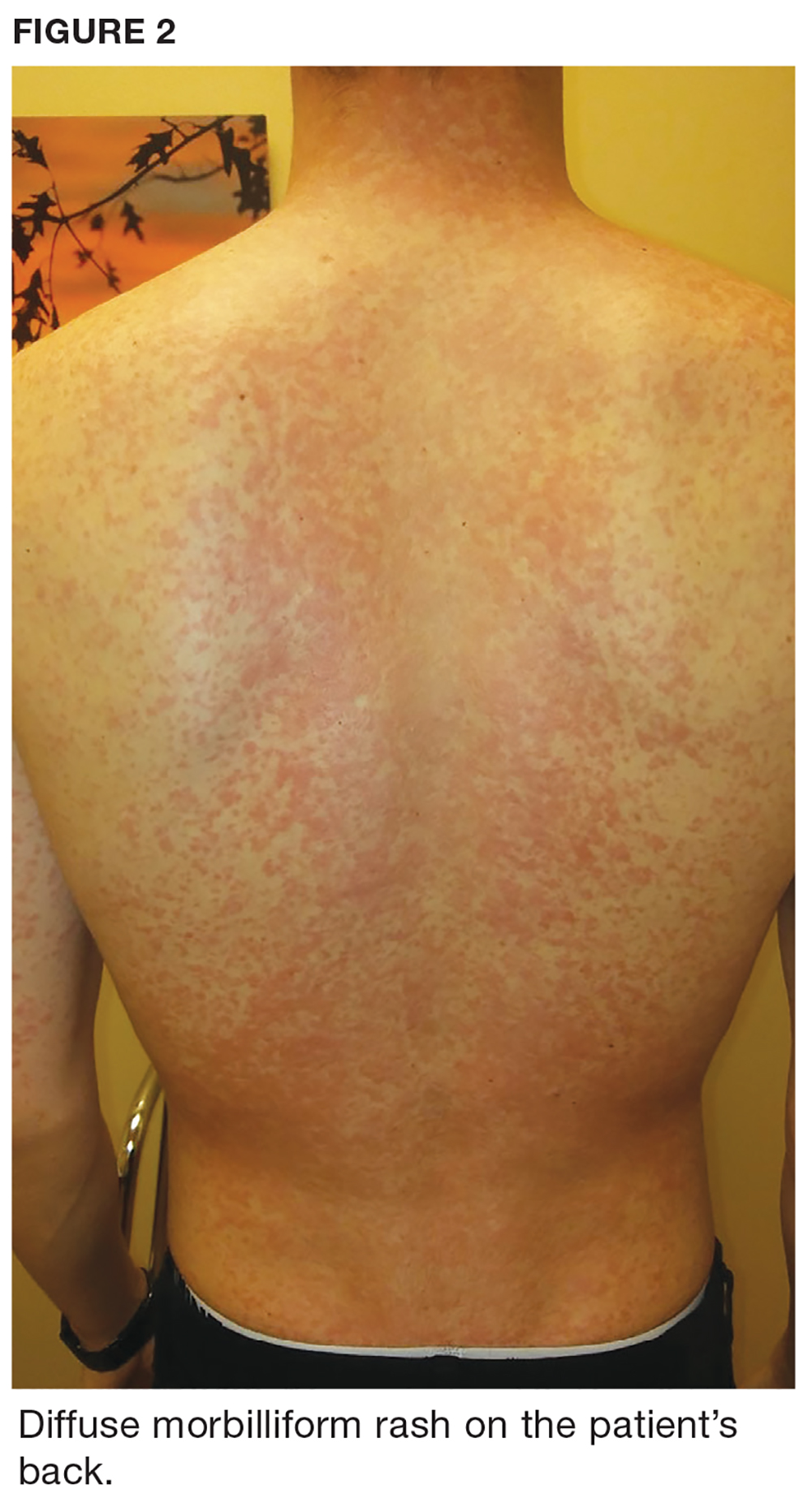
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
Man, 46, With Wrist Laceration
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
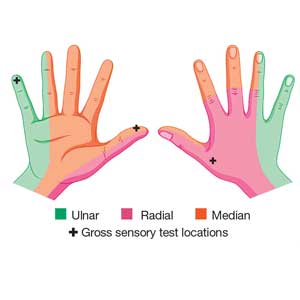
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
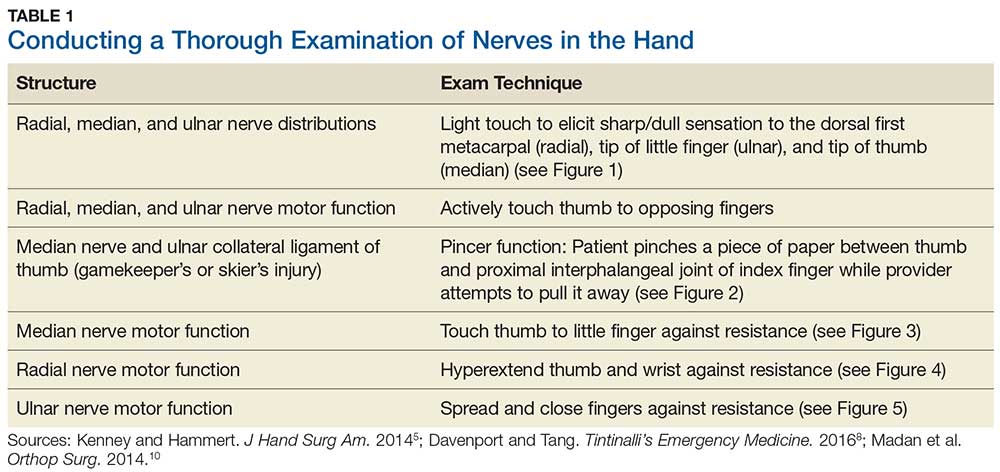
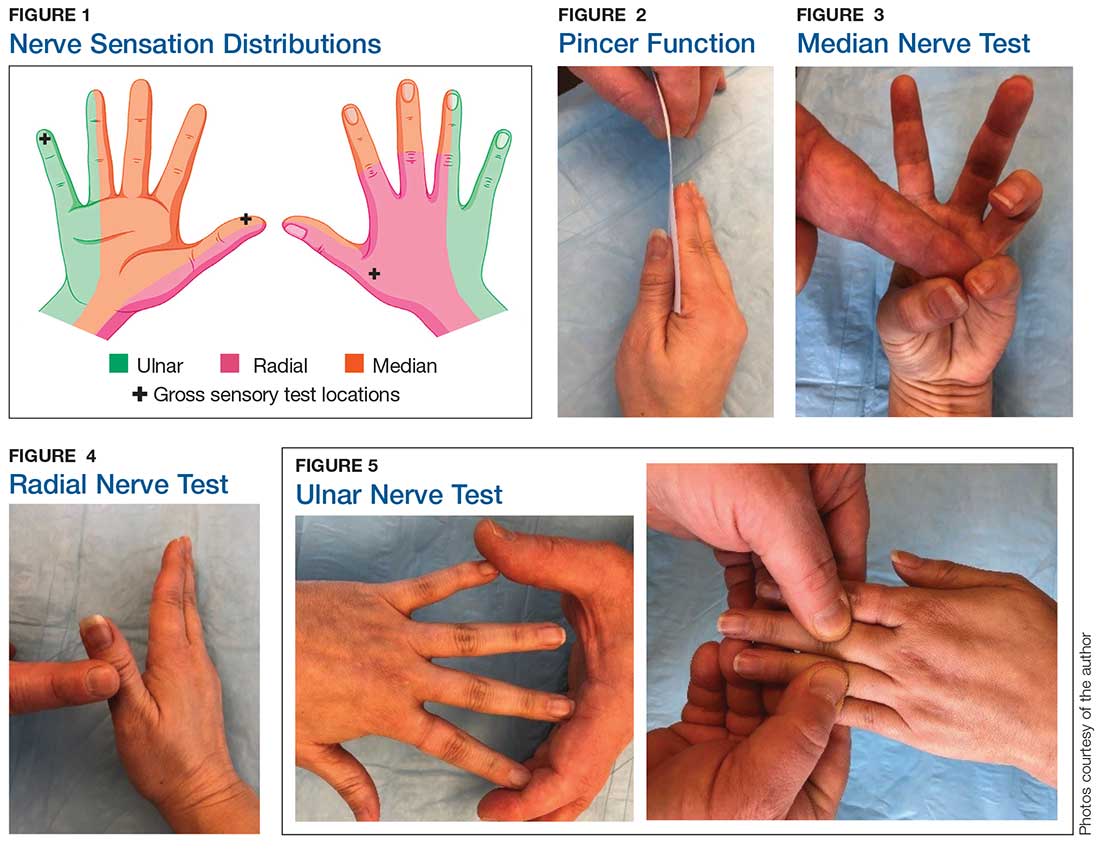
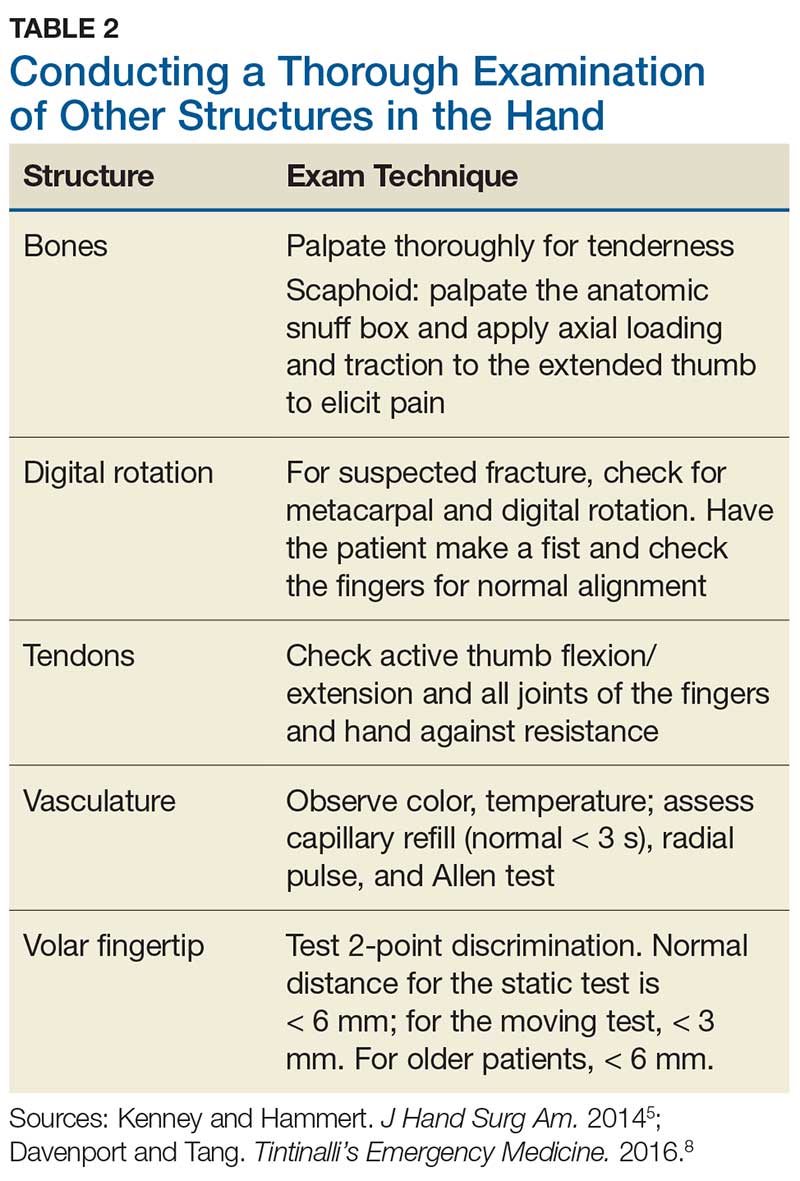
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
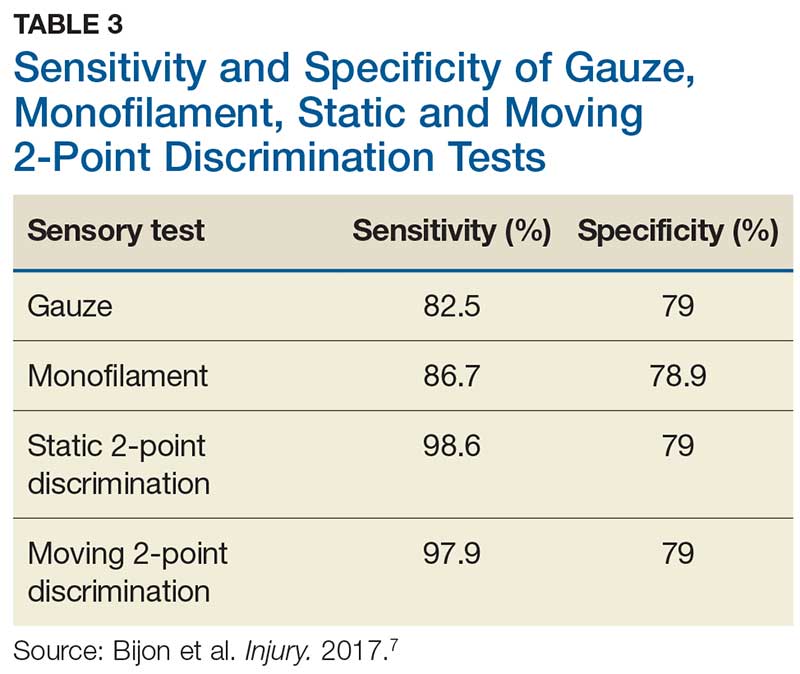
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
Woman, 18, With Sore Throat, Fever, and Painful Rash
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
Man, 25, With Sinus Pain, Sore Throat, and Rash
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
Woman, 57, With Painful, Swollen Ankle
IN THIS ARTICLE
- Diagnosis
- Treatment
- Care outcome
A 57-year-old horticulturist is working on a ladder leaned up against a tree trunk when the ladder slips, causing her to fall six feet onto concrete. Her right foot and ankle sustain the force of the fall; she is in excruciating pain and unable to bear weight on the foot. She is immediately transported to a local emergency department for evaluation.
Physical exam reveals a tearful middle-aged female in moderate distress and acute pain. There is moderate swelling of the right medial and lateral malleolus, as well as the midfoot, with blue and purple discoloration on the medial and lateral malleolus. Radiographs of the right ankle identify nondisplaced fractures of the distal fibula and tibia. Foot x-rays are unremarkable. A splint is ordered. The patient is given crutches (non-weight-bearing status), pain medication, and a referral to orthopedics.
On day 3, the patient presents to orthopedics, where the splint is removed. An irregular, 4 × 3–in (at largest diameter), serohemorrhagic blister is discovered on the medial aspect of the lower leg, above the right malleolus (see Figure 1). Multiple 1- to 3-mm vesicles surround much of the anterior border. Moderate edema is noted from the top of the lesion to the midfoot, concentrated around the lateral and medial malleolus. Extensive blue, purple, and black discoloration is seen below the malleolus. The patient is diagnosed with a fracture blister.
DISCUSSION
Fracture blisters are taut, bullous, subepidermal vesicles that can accompany fractures or severe twisting injuries. They overlie markedly edematous soft tissue and histologically resemble a second-degree burn.1,2
Physiologically, blisters are caused by increased interstitial pressure due to swelling, with subsequent increased filtration pressure and colloid osmotic pressure in the epidermal gap.3 This causes a disruption that allows fluid to move into the weakened area.3 Areas most at risk for fracture blister formation are those with tight, closely adhered skin without muscle or enveloping fascia, where there is less soft tissue between the skin and bone prominences (eg, ankle, elbow, foot, distal tibia).2-4
Approximately 3% of all patients with acute fractures requiring hospitalization develop a fracture blister.4 Any condition that predisposes a patient to poor wound healing (eg, peripheral vascular disease, diabetes, hypertension) increases risk for a fracture blister.2 Recognizing which patients are at greatest risk is vital, as implementing prevention strategies and intervening when fracture blisters do form can help decrease complications—including infection and delayed surgery—and improve fracture resolution. In this patient’s case, the extent of the injury and force of the fall caused the fracture blister to form.
Diagnosis
Diagnosis of a fracture blister is based on clinical presentation. There are two types: hemorrhagic blisters and clear fluid-filled blisters. Hemorrhagic blisters indicate more severe injury and longer healing time (approximately 16 d), while clear fluid-filled blisters demonstrate minimal injury and therefore are quicker to heal.2,4
The differential diagnosis for fracture blisters includes friction blisters and disorders such as epidermolysis bullosa and bullous pemphigoid. Friction blisters form when the epidermis is subjected to repeated friction or shear forces (eg, from a cast or splint).5,6 These forces mechanically separate epidermal cells at the stratum spinosum layer.7 The pressure that moves across the skin forces fluid into the deeper open spaces, filling them but leaving the surface layer intact.1
Epidermolysis bullosa (EB) is a group of rare inherited cutaneous and mucus membrane disorders. EB involves fragility and detachment of subepithelial tissues, which results in blistering and erosions.8,9 The blisters tend to develop in areas subject to minor trauma, such as the extensor aspects of the elbows and the dorsal aspects of the hands and feet.9 They can also be triggered by exposure to heat, friction, scratching, and adhesive tape.10
Bullous pemphigoid, a chronic autoimmune skin disorder, is characterized by pruritic, bullous lesions. When IgG autoantibodies bind to certain hemidesmosomal antigens, complement activation causes a subepidermal blister.11While bullous pemphigoid most commonly affects those older than 60, it can also occur in children. Diagnosis is confirmed by skin biopsy and immunofluorescence testing.11
Treatment and management
Although several recommendations have been published, there is no gold standard and treatment of fracture blisters remains controversial. Early surgical intervention for fractures could decrease the incidence of fracture blisters.1,3
The goal of treatment is to achieve re-epithelialization of the dermis.3,12,13 Once a blister forms, management techniques vary. Some recommend keeping closed blisters covered with a dry dressing to protect them from damage.3 Strauss et al recommend unroofing to avoid traumatic rupture; however, this does increase risk for infection.12 Recommendations differ depending on provider preference and each patient’s individual situation.
Elective unroofing of a blister is typically followed with one of several treatment options. These include covering the open blister with a topical antibiotic cream (eg, silver sulfadiazine 2%); applying a nonadherent, occlusive bismuth-tribromophenate-petroleum gauze dressing; or elevating and immobilizing the affected extremity.12,13
Treatment of spontaneously ruptured fracture blisters entails
- Unroofing the blister completely and applying a topical antimicrobial (eg, silver sulfadiazine, polymyxin B, neomycin, bacitracin).
- Applying a hydrocolloid dressing to keep the environment moist.
- Using a first-aid gel containing melaleuca (tea tree) oil.
- Initiating prophylactic oral antibiotics.
- Using whirlpool treatments.
- Elevating and immobilizing the affected extremity.3,12,14
OUTCOME FOR THE CASE PATIENT
The fracture blister was electively unroofed (see Figure 2) based on provider preference. The patient was instructed to clean the wound daily and apply topical cream (silver sulfadiazine 2% bid) to the wound and cover it with gauze. The patient was made non-weight-bearing to the right lower extremity. Continuous elevation was highly encouraged except for bathing and restroom use, and an NSAID was recommended as needed for pain. She was reassessed the following day and, due to partial refilling, the blister required additional unroofing. The patient was instructed to resume previous wound care orders.
No surgical intervention was required. CT of the right foot and ankle without contrast (performed on day 4 postinjury) confirmed a nondisplaced transverse fracture of the medial malleolus and a sagittal avulsion fracture of the anterior-inferior lateral malleolus. Multiple smaller fracture fragments were noted posterior and medial to the medial malleolus as well as inferiorly along the course of the deltoid ligament. There was a small, nondisplaced avulsion fracture of the medial malleolus at the anterolateral and posterolateral tibial plafond.
Due to the extent of the swelling, multiple fractures, and blister formation, the patient was essentially bed bound for the first three weeks; complete resolution of the fracture blister occurred 21 days after initial discovery (see Figure 3). The patient did not experience cutaneous complications. Her lower extremity was then casted in a short-leg removable cast for 10 weeks. She underwent physical therapy, and after 12 weeks, the patient was weight-bearing and was discharged from orthopedics. The patient reported refractory pain and swelling for an additional eight weeks following injury, warranting daily ibuprofen.
CONCLUSION
Fracture blisters are rare, and experience and knowledge about them in primary care is lacking. But clinicians need to be able to identify, diagnose, and refer at-risk patients to orthopedics in a timely manner.
Current management and treatment recommendations are inconsistent. Treatment varies depending on the site, severity, type, and status of the blister and the overall health of the patient. Fracture blisters may be left intact, electively unroofed, or treated after spontaneous rupture. More research is needed to clarify management recommendations, specifically regarding the decision to unroof a blister or leave it intact. Early surgical intervention may prevent the development of a fracture blister.
1. Wallace GF, Sullivan J. Fracture blisters. Clin Podiatr Med Surg. 1995;12(4):801-811.
2. Halawi MJ. Fracture blisters after primary total knee arthroplasty. Am J Orthop. 2015; 44(8):E291-E293.
3. McCann S, Gruen G. Fracture blisters: a review of the literature. Orthop Nurs. 1997; 16(2):17-24.
4. Uebbing CM, Walsh M, Miller JB, et al. Fracture blister. West J Emerg Med. 2011; 12(1):131-133.
5. Kirkham S, Lam S, Nester C, Hashmi F. The effect of hydration on the risk of friction blister formation on the heel of the foot. Skin Res Tech. 2014;20:246-253.
6. Boyd A, Benjamin H, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-24.
7. Knapik J, Reynolds K, Duplantis K, Jones B. Friction blisters. Pathophysiology, prevention and treatment. Sports Med. 1995; 20(3):136-147.
8. Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022-1030.
9. Peraza DM. Epidermolysis bullosa acquisita. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/epidermolysis-bullosa-acquisita. Accessed January 26, 2018.
10. Lyons F, Ousley L. Dermatology for the Advanced Practice Nurse. New York, NY: Springer; 2015.
11. Peraza D. Bullous pemphigoid. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/bullous-pemphigoid. Accessed January 26, 2018.
12. Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9): 618-622.
13. Tolpinrud WL, Rebolledo BJ, Lorich DG, Grossman ME. A case of extensive fracture bullae: a multidisciplinary approach for acute management. JAAD Case Rep. 2015;1(3):132-135.
14. Cox H, Nealon L. Case report: the use of Burnaid Gel on fracture blisters. Wound Practice and Research. 2008;16(1):32-36.
IN THIS ARTICLE
- Diagnosis
- Treatment
- Care outcome
A 57-year-old horticulturist is working on a ladder leaned up against a tree trunk when the ladder slips, causing her to fall six feet onto concrete. Her right foot and ankle sustain the force of the fall; she is in excruciating pain and unable to bear weight on the foot. She is immediately transported to a local emergency department for evaluation.
Physical exam reveals a tearful middle-aged female in moderate distress and acute pain. There is moderate swelling of the right medial and lateral malleolus, as well as the midfoot, with blue and purple discoloration on the medial and lateral malleolus. Radiographs of the right ankle identify nondisplaced fractures of the distal fibula and tibia. Foot x-rays are unremarkable. A splint is ordered. The patient is given crutches (non-weight-bearing status), pain medication, and a referral to orthopedics.
On day 3, the patient presents to orthopedics, where the splint is removed. An irregular, 4 × 3–in (at largest diameter), serohemorrhagic blister is discovered on the medial aspect of the lower leg, above the right malleolus (see Figure 1). Multiple 1- to 3-mm vesicles surround much of the anterior border. Moderate edema is noted from the top of the lesion to the midfoot, concentrated around the lateral and medial malleolus. Extensive blue, purple, and black discoloration is seen below the malleolus. The patient is diagnosed with a fracture blister.
DISCUSSION
Fracture blisters are taut, bullous, subepidermal vesicles that can accompany fractures or severe twisting injuries. They overlie markedly edematous soft tissue and histologically resemble a second-degree burn.1,2
Physiologically, blisters are caused by increased interstitial pressure due to swelling, with subsequent increased filtration pressure and colloid osmotic pressure in the epidermal gap.3 This causes a disruption that allows fluid to move into the weakened area.3 Areas most at risk for fracture blister formation are those with tight, closely adhered skin without muscle or enveloping fascia, where there is less soft tissue between the skin and bone prominences (eg, ankle, elbow, foot, distal tibia).2-4
Approximately 3% of all patients with acute fractures requiring hospitalization develop a fracture blister.4 Any condition that predisposes a patient to poor wound healing (eg, peripheral vascular disease, diabetes, hypertension) increases risk for a fracture blister.2 Recognizing which patients are at greatest risk is vital, as implementing prevention strategies and intervening when fracture blisters do form can help decrease complications—including infection and delayed surgery—and improve fracture resolution. In this patient’s case, the extent of the injury and force of the fall caused the fracture blister to form.
Diagnosis
Diagnosis of a fracture blister is based on clinical presentation. There are two types: hemorrhagic blisters and clear fluid-filled blisters. Hemorrhagic blisters indicate more severe injury and longer healing time (approximately 16 d), while clear fluid-filled blisters demonstrate minimal injury and therefore are quicker to heal.2,4
The differential diagnosis for fracture blisters includes friction blisters and disorders such as epidermolysis bullosa and bullous pemphigoid. Friction blisters form when the epidermis is subjected to repeated friction or shear forces (eg, from a cast or splint).5,6 These forces mechanically separate epidermal cells at the stratum spinosum layer.7 The pressure that moves across the skin forces fluid into the deeper open spaces, filling them but leaving the surface layer intact.1
Epidermolysis bullosa (EB) is a group of rare inherited cutaneous and mucus membrane disorders. EB involves fragility and detachment of subepithelial tissues, which results in blistering and erosions.8,9 The blisters tend to develop in areas subject to minor trauma, such as the extensor aspects of the elbows and the dorsal aspects of the hands and feet.9 They can also be triggered by exposure to heat, friction, scratching, and adhesive tape.10
Bullous pemphigoid, a chronic autoimmune skin disorder, is characterized by pruritic, bullous lesions. When IgG autoantibodies bind to certain hemidesmosomal antigens, complement activation causes a subepidermal blister.11While bullous pemphigoid most commonly affects those older than 60, it can also occur in children. Diagnosis is confirmed by skin biopsy and immunofluorescence testing.11
Treatment and management
Although several recommendations have been published, there is no gold standard and treatment of fracture blisters remains controversial. Early surgical intervention for fractures could decrease the incidence of fracture blisters.1,3
The goal of treatment is to achieve re-epithelialization of the dermis.3,12,13 Once a blister forms, management techniques vary. Some recommend keeping closed blisters covered with a dry dressing to protect them from damage.3 Strauss et al recommend unroofing to avoid traumatic rupture; however, this does increase risk for infection.12 Recommendations differ depending on provider preference and each patient’s individual situation.
Elective unroofing of a blister is typically followed with one of several treatment options. These include covering the open blister with a topical antibiotic cream (eg, silver sulfadiazine 2%); applying a nonadherent, occlusive bismuth-tribromophenate-petroleum gauze dressing; or elevating and immobilizing the affected extremity.12,13
Treatment of spontaneously ruptured fracture blisters entails
- Unroofing the blister completely and applying a topical antimicrobial (eg, silver sulfadiazine, polymyxin B, neomycin, bacitracin).
- Applying a hydrocolloid dressing to keep the environment moist.
- Using a first-aid gel containing melaleuca (tea tree) oil.
- Initiating prophylactic oral antibiotics.
- Using whirlpool treatments.
- Elevating and immobilizing the affected extremity.3,12,14
OUTCOME FOR THE CASE PATIENT
The fracture blister was electively unroofed (see Figure 2) based on provider preference. The patient was instructed to clean the wound daily and apply topical cream (silver sulfadiazine 2% bid) to the wound and cover it with gauze. The patient was made non-weight-bearing to the right lower extremity. Continuous elevation was highly encouraged except for bathing and restroom use, and an NSAID was recommended as needed for pain. She was reassessed the following day and, due to partial refilling, the blister required additional unroofing. The patient was instructed to resume previous wound care orders.
No surgical intervention was required. CT of the right foot and ankle without contrast (performed on day 4 postinjury) confirmed a nondisplaced transverse fracture of the medial malleolus and a sagittal avulsion fracture of the anterior-inferior lateral malleolus. Multiple smaller fracture fragments were noted posterior and medial to the medial malleolus as well as inferiorly along the course of the deltoid ligament. There was a small, nondisplaced avulsion fracture of the medial malleolus at the anterolateral and posterolateral tibial plafond.
Due to the extent of the swelling, multiple fractures, and blister formation, the patient was essentially bed bound for the first three weeks; complete resolution of the fracture blister occurred 21 days after initial discovery (see Figure 3). The patient did not experience cutaneous complications. Her lower extremity was then casted in a short-leg removable cast for 10 weeks. She underwent physical therapy, and after 12 weeks, the patient was weight-bearing and was discharged from orthopedics. The patient reported refractory pain and swelling for an additional eight weeks following injury, warranting daily ibuprofen.
CONCLUSION
Fracture blisters are rare, and experience and knowledge about them in primary care is lacking. But clinicians need to be able to identify, diagnose, and refer at-risk patients to orthopedics in a timely manner.
Current management and treatment recommendations are inconsistent. Treatment varies depending on the site, severity, type, and status of the blister and the overall health of the patient. Fracture blisters may be left intact, electively unroofed, or treated after spontaneous rupture. More research is needed to clarify management recommendations, specifically regarding the decision to unroof a blister or leave it intact. Early surgical intervention may prevent the development of a fracture blister.
IN THIS ARTICLE
- Diagnosis
- Treatment
- Care outcome
A 57-year-old horticulturist is working on a ladder leaned up against a tree trunk when the ladder slips, causing her to fall six feet onto concrete. Her right foot and ankle sustain the force of the fall; she is in excruciating pain and unable to bear weight on the foot. She is immediately transported to a local emergency department for evaluation.
Physical exam reveals a tearful middle-aged female in moderate distress and acute pain. There is moderate swelling of the right medial and lateral malleolus, as well as the midfoot, with blue and purple discoloration on the medial and lateral malleolus. Radiographs of the right ankle identify nondisplaced fractures of the distal fibula and tibia. Foot x-rays are unremarkable. A splint is ordered. The patient is given crutches (non-weight-bearing status), pain medication, and a referral to orthopedics.
On day 3, the patient presents to orthopedics, where the splint is removed. An irregular, 4 × 3–in (at largest diameter), serohemorrhagic blister is discovered on the medial aspect of the lower leg, above the right malleolus (see Figure 1). Multiple 1- to 3-mm vesicles surround much of the anterior border. Moderate edema is noted from the top of the lesion to the midfoot, concentrated around the lateral and medial malleolus. Extensive blue, purple, and black discoloration is seen below the malleolus. The patient is diagnosed with a fracture blister.
DISCUSSION
Fracture blisters are taut, bullous, subepidermal vesicles that can accompany fractures or severe twisting injuries. They overlie markedly edematous soft tissue and histologically resemble a second-degree burn.1,2
Physiologically, blisters are caused by increased interstitial pressure due to swelling, with subsequent increased filtration pressure and colloid osmotic pressure in the epidermal gap.3 This causes a disruption that allows fluid to move into the weakened area.3 Areas most at risk for fracture blister formation are those with tight, closely adhered skin without muscle or enveloping fascia, where there is less soft tissue between the skin and bone prominences (eg, ankle, elbow, foot, distal tibia).2-4
Approximately 3% of all patients with acute fractures requiring hospitalization develop a fracture blister.4 Any condition that predisposes a patient to poor wound healing (eg, peripheral vascular disease, diabetes, hypertension) increases risk for a fracture blister.2 Recognizing which patients are at greatest risk is vital, as implementing prevention strategies and intervening when fracture blisters do form can help decrease complications—including infection and delayed surgery—and improve fracture resolution. In this patient’s case, the extent of the injury and force of the fall caused the fracture blister to form.
Diagnosis
Diagnosis of a fracture blister is based on clinical presentation. There are two types: hemorrhagic blisters and clear fluid-filled blisters. Hemorrhagic blisters indicate more severe injury and longer healing time (approximately 16 d), while clear fluid-filled blisters demonstrate minimal injury and therefore are quicker to heal.2,4
The differential diagnosis for fracture blisters includes friction blisters and disorders such as epidermolysis bullosa and bullous pemphigoid. Friction blisters form when the epidermis is subjected to repeated friction or shear forces (eg, from a cast or splint).5,6 These forces mechanically separate epidermal cells at the stratum spinosum layer.7 The pressure that moves across the skin forces fluid into the deeper open spaces, filling them but leaving the surface layer intact.1
Epidermolysis bullosa (EB) is a group of rare inherited cutaneous and mucus membrane disorders. EB involves fragility and detachment of subepithelial tissues, which results in blistering and erosions.8,9 The blisters tend to develop in areas subject to minor trauma, such as the extensor aspects of the elbows and the dorsal aspects of the hands and feet.9 They can also be triggered by exposure to heat, friction, scratching, and adhesive tape.10
Bullous pemphigoid, a chronic autoimmune skin disorder, is characterized by pruritic, bullous lesions. When IgG autoantibodies bind to certain hemidesmosomal antigens, complement activation causes a subepidermal blister.11While bullous pemphigoid most commonly affects those older than 60, it can also occur in children. Diagnosis is confirmed by skin biopsy and immunofluorescence testing.11
Treatment and management
Although several recommendations have been published, there is no gold standard and treatment of fracture blisters remains controversial. Early surgical intervention for fractures could decrease the incidence of fracture blisters.1,3
The goal of treatment is to achieve re-epithelialization of the dermis.3,12,13 Once a blister forms, management techniques vary. Some recommend keeping closed blisters covered with a dry dressing to protect them from damage.3 Strauss et al recommend unroofing to avoid traumatic rupture; however, this does increase risk for infection.12 Recommendations differ depending on provider preference and each patient’s individual situation.
Elective unroofing of a blister is typically followed with one of several treatment options. These include covering the open blister with a topical antibiotic cream (eg, silver sulfadiazine 2%); applying a nonadherent, occlusive bismuth-tribromophenate-petroleum gauze dressing; or elevating and immobilizing the affected extremity.12,13
Treatment of spontaneously ruptured fracture blisters entails
- Unroofing the blister completely and applying a topical antimicrobial (eg, silver sulfadiazine, polymyxin B, neomycin, bacitracin).
- Applying a hydrocolloid dressing to keep the environment moist.
- Using a first-aid gel containing melaleuca (tea tree) oil.
- Initiating prophylactic oral antibiotics.
- Using whirlpool treatments.
- Elevating and immobilizing the affected extremity.3,12,14
OUTCOME FOR THE CASE PATIENT
The fracture blister was electively unroofed (see Figure 2) based on provider preference. The patient was instructed to clean the wound daily and apply topical cream (silver sulfadiazine 2% bid) to the wound and cover it with gauze. The patient was made non-weight-bearing to the right lower extremity. Continuous elevation was highly encouraged except for bathing and restroom use, and an NSAID was recommended as needed for pain. She was reassessed the following day and, due to partial refilling, the blister required additional unroofing. The patient was instructed to resume previous wound care orders.
No surgical intervention was required. CT of the right foot and ankle without contrast (performed on day 4 postinjury) confirmed a nondisplaced transverse fracture of the medial malleolus and a sagittal avulsion fracture of the anterior-inferior lateral malleolus. Multiple smaller fracture fragments were noted posterior and medial to the medial malleolus as well as inferiorly along the course of the deltoid ligament. There was a small, nondisplaced avulsion fracture of the medial malleolus at the anterolateral and posterolateral tibial plafond.
Due to the extent of the swelling, multiple fractures, and blister formation, the patient was essentially bed bound for the first three weeks; complete resolution of the fracture blister occurred 21 days after initial discovery (see Figure 3). The patient did not experience cutaneous complications. Her lower extremity was then casted in a short-leg removable cast for 10 weeks. She underwent physical therapy, and after 12 weeks, the patient was weight-bearing and was discharged from orthopedics. The patient reported refractory pain and swelling for an additional eight weeks following injury, warranting daily ibuprofen.
CONCLUSION
Fracture blisters are rare, and experience and knowledge about them in primary care is lacking. But clinicians need to be able to identify, diagnose, and refer at-risk patients to orthopedics in a timely manner.
Current management and treatment recommendations are inconsistent. Treatment varies depending on the site, severity, type, and status of the blister and the overall health of the patient. Fracture blisters may be left intact, electively unroofed, or treated after spontaneous rupture. More research is needed to clarify management recommendations, specifically regarding the decision to unroof a blister or leave it intact. Early surgical intervention may prevent the development of a fracture blister.
1. Wallace GF, Sullivan J. Fracture blisters. Clin Podiatr Med Surg. 1995;12(4):801-811.
2. Halawi MJ. Fracture blisters after primary total knee arthroplasty. Am J Orthop. 2015; 44(8):E291-E293.
3. McCann S, Gruen G. Fracture blisters: a review of the literature. Orthop Nurs. 1997; 16(2):17-24.
4. Uebbing CM, Walsh M, Miller JB, et al. Fracture blister. West J Emerg Med. 2011; 12(1):131-133.
5. Kirkham S, Lam S, Nester C, Hashmi F. The effect of hydration on the risk of friction blister formation on the heel of the foot. Skin Res Tech. 2014;20:246-253.
6. Boyd A, Benjamin H, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-24.
7. Knapik J, Reynolds K, Duplantis K, Jones B. Friction blisters. Pathophysiology, prevention and treatment. Sports Med. 1995; 20(3):136-147.
8. Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022-1030.
9. Peraza DM. Epidermolysis bullosa acquisita. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/epidermolysis-bullosa-acquisita. Accessed January 26, 2018.
10. Lyons F, Ousley L. Dermatology for the Advanced Practice Nurse. New York, NY: Springer; 2015.
11. Peraza D. Bullous pemphigoid. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/bullous-pemphigoid. Accessed January 26, 2018.
12. Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9): 618-622.
13. Tolpinrud WL, Rebolledo BJ, Lorich DG, Grossman ME. A case of extensive fracture bullae: a multidisciplinary approach for acute management. JAAD Case Rep. 2015;1(3):132-135.
14. Cox H, Nealon L. Case report: the use of Burnaid Gel on fracture blisters. Wound Practice and Research. 2008;16(1):32-36.
1. Wallace GF, Sullivan J. Fracture blisters. Clin Podiatr Med Surg. 1995;12(4):801-811.
2. Halawi MJ. Fracture blisters after primary total knee arthroplasty. Am J Orthop. 2015; 44(8):E291-E293.
3. McCann S, Gruen G. Fracture blisters: a review of the literature. Orthop Nurs. 1997; 16(2):17-24.
4. Uebbing CM, Walsh M, Miller JB, et al. Fracture blister. West J Emerg Med. 2011; 12(1):131-133.
5. Kirkham S, Lam S, Nester C, Hashmi F. The effect of hydration on the risk of friction blister formation on the heel of the foot. Skin Res Tech. 2014;20:246-253.
6. Boyd A, Benjamin H, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-24.
7. Knapik J, Reynolds K, Duplantis K, Jones B. Friction blisters. Pathophysiology, prevention and treatment. Sports Med. 1995; 20(3):136-147.
8. Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022-1030.
9. Peraza DM. Epidermolysis bullosa acquisita. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/epidermolysis-bullosa-acquisita. Accessed January 26, 2018.
10. Lyons F, Ousley L. Dermatology for the Advanced Practice Nurse. New York, NY: Springer; 2015.
11. Peraza D. Bullous pemphigoid. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/bullous-pemphigoid. Accessed January 26, 2018.
12. Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9): 618-622.
13. Tolpinrud WL, Rebolledo BJ, Lorich DG, Grossman ME. A case of extensive fracture bullae: a multidisciplinary approach for acute management. JAAD Case Rep. 2015;1(3):132-135.
14. Cox H, Nealon L. Case report: the use of Burnaid Gel on fracture blisters. Wound Practice and Research. 2008;16(1):32-36.
Boy, 9, With Eye Pain, Blurred Vision, and Tearing
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An otherwise healthy 9-year-old boy is brought to the emergency department (ED) by his father for evaluation of severe pain, blurry vision, and four hours of tearing in his right eye. The patient was in school when he experienced sudden-onset irritation and scratching pain that caused him to rub his eye. He says it “feels like there is something in my eye,” but he denies any known substance or foreign body. He has no medical or surgical history, does not wear contact lenses or eyeglasses, and denies loss of vision. There is no history of recent illness or travel.
On evaluation, the patient is in no acute distress but is holding his right eye closed due to foreign-body sensation and increased photosensitivity and tearing. There is no obvious erythema or swelling in the upper or lower eyelids bilaterally. A visual acuity test with a Snellen eye chart shows 20/20 vision in the left eye and 20/50 in the right, secondary to pain, photophobia, and excessive tearing. The patient’s right sclera is significantly injected. Intraocular pressure, measured with a tonometer, is 12 to 14 mm Hg. A fluorescein stain of the eye yields no significant findings. The globe is intact.
At first glance, a slit-lamp exam shows no obvious signs of a foreign body. But much higher magnification reveals substantial conjunctival injection and numerous intracorneal linear foreign bodies in the right eye (see Figure 1 for example [not the case patient]). The anterior chamber shows no inflammatory reaction, and findings in the posterior segment are unremarkable.
The initial diagnosis is simple conjunctivitis—but closer examination reveals multiple fine, barbed hairs embedded in the patient’s right cornea. Upon further questioning, the patient reports that prior to symptom onset, he had been holding the classroom pet, a Chilean Rose tarantula, in the palm of his hands.
DISCUSSION
Foreign body injury is a common cause of ocular pain and corneal damage, which can lead to challenging complications. Ophthalmic emergencies account for 2% of ED visits in the US annually and are a major cause of visual impairment.1 But when a painful eye is the chief complaint, contact with insects, plants, or spiders is rarely included in the differential. Tarantulas are popular classroom and household pets, however, and ocular injury should be suspected in anyone who has been holding a tarantula prior to onset of pain.
Ophthalmia nodosa
Tarantulas are one of the most common arachnids known to cause ophthalmia nodosa—a granulomatous reaction of the conjunctiva or cornea to an implanted plant, insect, or spider hair that typically manifests with photophobia, irritation, and chemosis.2,3 Tarantulas, when scared or defending their eggs, shoot urticating setae at the threat—a defensive mechanism largely unknown to parents, tarantula owners, and medical professionals.
Urticating setae are found in roughly 90% of tarantula species throughout tropical and subtropical regions.4 Depending on the species, setae can be located on the distal prolateral surface of the palpal femur or the dorsum of the abdomen. They can be released when the tarantula scratches its legs against the abdominal urticating setae patch or scratches the palps against the chelicerae (appendages in front of the mouth), or when direct exterior contact is made with the abdominal setae.4
There are six types of urticating hairs. Each is attached to the spider’s cuticle by either a stalk (which represents the break-off region) or a socket.4 Tarantula hairs range in size from 0.1 mm to 0.3 mm and have a sharp, pointed head and numerous barbs, which help embed them in the target.5 They are long and thin, to facilitate deep tissue penetration, and can enter the eyes, lungs, or other body parts (see Figure 2).
Ocular injury from tarantula hairs commonly involves conjunctival injection, foreign body sensation, periorbital facial rash, photophobia, and tearing.3 When a tarantula’s cloud of barbed hairs is flicked into the eye and pierces the cornea, it can cause infection, irritation, scarring on the cornea, or vision loss. Eye movement or rubbing can cause the hairs—and their toxins—to migrate over time, traveling like an arrow (the tip and barbs resist backward movement) to the anterior chamber, lens, vitreous, and retina.6,7 This can cause corneal scars, cataracts, vitritis, or macular edema, and creates the possibility for acute or chronic conjunctivitis.7
Diagnosis and management
Ophthalmic emergencies can affect the visual system and, if left untreated, can lead to permanent vision loss. Affected patients require immediate medical attention and should be referred to an ophthalmologist for follow-up care.
Diagnosis. A thorough history and physical exam are of utmost importance; tiny setae can be easily overlooked if the examiner is not diligent, and the similar symptomatology can lead to misdiagnosis as simple conjunctivitis.3 A visual acuity test and slit-lamp exam are useful for confirmation.
Treatment. Once the diagnosis is confirmed, treatment should consist of mild topical antibiotics and steroids to effectively control infection and inflammation. While topical steroids may be appropriate, local adverse events associated with their use (eg, glaucoma, cataracts) can be problematic. Gentle eye irrigation has been noted by some researchers as contraindicated, while others find it useful to flush out some of the hairs.5,8,9
Most of the visible protruding tarantula hairs can and should be removed under microscopy during slit-lamp exam. Hairs that are buried in the cornea, however, are nearly impossible to remove and pose a threat of further complications, as described. Conservative management with careful observation is therefore recommended. If the patient develops a granuloma, excision—along with a course of systemic steroids and setae removal via vitrectomy—may be needed.9
The good news is that, in many cases, deeper hairs are absorbed without complication, making their removal unnecessary.5 Factors that encourage leaving the setae untouched include a large number of hairs, deep corneal penetration, lack of patient tolerance for the procedure, and risk for perforation.3
More invasive treatments (eg, laser photocoagulation, intraocular surgery) to remove offending hairs are possible, but literature on the outcome of these interventions is limited. One report to date used argon laser photocoagulation to treat endophthalmitis from vitreous hairs.10 The laser can fragment the hairs so that they lose their barbed characteristic and cannot penetrate deeper.6
Follow-up. Close follow-up is advised, and patients should be educated on the importance of medication compliance and return visits for reevaluation. Given the potential dangers of handling these spiders, tarantula owners should be advised to use protective gloving and goggles.2,5,8,9
OUTCOME FOR THE CASE PATIENT
The case patient was sent to an ophthalmologist on day 1. Proparacaine was placed in his right eye, and all of the superficial tarantula hairs were removed using 25- and 30-gauge needles with jeweler forceps under slit-lamp microscopy. Most of the hairs were removed from the superior cornea; fewer were found in the paracentral and inferior regions of the cornea. Approximately five hairs in the paracentral area of the cornea were embedded in the midstromal depth and could not be removed. One drop of ciprofloxacin was administered.
The patient was sent home with an eye shield and instructions to use tobramycin/dexamethasone eye drops (qid in his right eye) and avoid rubbing the eye. (The eye shield, though not technically necessary, was deemed beneficial to help the patient avoid touching the eye.) He was scheduled to return to the clinic one week later.
On follow-up, a careful exam performed under microscopy showed that the five tarantula hairs were still embedded, and an additional six hairs were found in the deep stroma. Superficial punctate keratitis—an eye disorder caused by epithelial cell death on the surface of the cornea—was noted, but no anterior chamber cells were seen. The patient was instructed to continue using the eye drops as prescribed until finished, then start using loteprednol (tid) and artificial lubricating tears (every 2 h).
He returned to the clinic every two weeks for a total of 10 visits. At the end of the treatment course, the remaining tarantula hairs were unable to be removed. The patient used tapering doses of topical eye steroids and antibiotic drops secondary to flare-up.
CONCLUSION
Determining the etiology of ophthalmic emergencies is essential to timely and appropriate management. In this case, a recognized but often overlooked cause, tarantula hairs, made the diagnosis more complicated than simple conjunctivitis. When ocular injury is suspected, the provider must obtain an accurate and detailed history along with a thorough physical exam. Since patients must comply with medication regimens to prevent acute and chronic infection, a clear treatment and follow-up plan should be established. With these in place, ophthalmia nodosa caused by urticating setae can be effectively managed.
1. Fitzpatrick J, Hickman R, Alfes CM. A Guide to Mastery in Clinical Nursing: The Comprehensive Reference. New York, NY: Springer; 2018:114.
2. Lambert SR, Lyons CJ. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus. 5th ed. New York, NY: Elsevier; 2017:138.
3. Stagg BC, Ambati BK. Tarantula hairs as corneal foreign bodies. Case Rep Ophthalmol. 2011;2(3):323-326.
4. Bertani R, Guadanucci JPL. Morphology, evolution, and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba). 2013;30(4):403-418.
5. McAnena L, Murphy C, O’Connor J. Tarantula keratitis: a case report. Ir J Med Sci. 2013;182(3):349-350.
6. Yang Y, Christakis T, Mireskandari K. Acute conjunctivitis and corneal foreign bodies secondary to tarantula hairs. CMAJ. 2016;183(3):212-214.
7. Jain N, Soong HK, Gardner TW. Ophthalmia nodosa. EyeNet Magazine. November 2013. www.aao.org/eyenet/article/blink-mystery-image-17. Accessed January 24, 2018.
8. Choi JTL, Rauf A. Ophthalmia nodosa secondary to tarantula hairs. Eye (Lond). 2003;17(3):433-434.
9. Comez AT, Tufan HA, Gencer B. Ophthalmia nodosa as an occupational disease: is it unusual or is it casual? Ocul Immunol Inflamm. 2013;21(2):144-147.
10. Marti-Huguet T, Pujol O, Cabiro I, et al. Endophthalmos caused by intravitreal caterpillar hairs. Treatment by direct photocoagulation with argon laser [article in French]. J Fr Ophthalmol. 1987;10(10):559-564.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An otherwise healthy 9-year-old boy is brought to the emergency department (ED) by his father for evaluation of severe pain, blurry vision, and four hours of tearing in his right eye. The patient was in school when he experienced sudden-onset irritation and scratching pain that caused him to rub his eye. He says it “feels like there is something in my eye,” but he denies any known substance or foreign body. He has no medical or surgical history, does not wear contact lenses or eyeglasses, and denies loss of vision. There is no history of recent illness or travel.
On evaluation, the patient is in no acute distress but is holding his right eye closed due to foreign-body sensation and increased photosensitivity and tearing. There is no obvious erythema or swelling in the upper or lower eyelids bilaterally. A visual acuity test with a Snellen eye chart shows 20/20 vision in the left eye and 20/50 in the right, secondary to pain, photophobia, and excessive tearing. The patient’s right sclera is significantly injected. Intraocular pressure, measured with a tonometer, is 12 to 14 mm Hg. A fluorescein stain of the eye yields no significant findings. The globe is intact.
At first glance, a slit-lamp exam shows no obvious signs of a foreign body. But much higher magnification reveals substantial conjunctival injection and numerous intracorneal linear foreign bodies in the right eye (see Figure 1 for example [not the case patient]). The anterior chamber shows no inflammatory reaction, and findings in the posterior segment are unremarkable.
The initial diagnosis is simple conjunctivitis—but closer examination reveals multiple fine, barbed hairs embedded in the patient’s right cornea. Upon further questioning, the patient reports that prior to symptom onset, he had been holding the classroom pet, a Chilean Rose tarantula, in the palm of his hands.
DISCUSSION
Foreign body injury is a common cause of ocular pain and corneal damage, which can lead to challenging complications. Ophthalmic emergencies account for 2% of ED visits in the US annually and are a major cause of visual impairment.1 But when a painful eye is the chief complaint, contact with insects, plants, or spiders is rarely included in the differential. Tarantulas are popular classroom and household pets, however, and ocular injury should be suspected in anyone who has been holding a tarantula prior to onset of pain.
Ophthalmia nodosa
Tarantulas are one of the most common arachnids known to cause ophthalmia nodosa—a granulomatous reaction of the conjunctiva or cornea to an implanted plant, insect, or spider hair that typically manifests with photophobia, irritation, and chemosis.2,3 Tarantulas, when scared or defending their eggs, shoot urticating setae at the threat—a defensive mechanism largely unknown to parents, tarantula owners, and medical professionals.
Urticating setae are found in roughly 90% of tarantula species throughout tropical and subtropical regions.4 Depending on the species, setae can be located on the distal prolateral surface of the palpal femur or the dorsum of the abdomen. They can be released when the tarantula scratches its legs against the abdominal urticating setae patch or scratches the palps against the chelicerae (appendages in front of the mouth), or when direct exterior contact is made with the abdominal setae.4
There are six types of urticating hairs. Each is attached to the spider’s cuticle by either a stalk (which represents the break-off region) or a socket.4 Tarantula hairs range in size from 0.1 mm to 0.3 mm and have a sharp, pointed head and numerous barbs, which help embed them in the target.5 They are long and thin, to facilitate deep tissue penetration, and can enter the eyes, lungs, or other body parts (see Figure 2).
Ocular injury from tarantula hairs commonly involves conjunctival injection, foreign body sensation, periorbital facial rash, photophobia, and tearing.3 When a tarantula’s cloud of barbed hairs is flicked into the eye and pierces the cornea, it can cause infection, irritation, scarring on the cornea, or vision loss. Eye movement or rubbing can cause the hairs—and their toxins—to migrate over time, traveling like an arrow (the tip and barbs resist backward movement) to the anterior chamber, lens, vitreous, and retina.6,7 This can cause corneal scars, cataracts, vitritis, or macular edema, and creates the possibility for acute or chronic conjunctivitis.7
Diagnosis and management
Ophthalmic emergencies can affect the visual system and, if left untreated, can lead to permanent vision loss. Affected patients require immediate medical attention and should be referred to an ophthalmologist for follow-up care.
Diagnosis. A thorough history and physical exam are of utmost importance; tiny setae can be easily overlooked if the examiner is not diligent, and the similar symptomatology can lead to misdiagnosis as simple conjunctivitis.3 A visual acuity test and slit-lamp exam are useful for confirmation.
Treatment. Once the diagnosis is confirmed, treatment should consist of mild topical antibiotics and steroids to effectively control infection and inflammation. While topical steroids may be appropriate, local adverse events associated with their use (eg, glaucoma, cataracts) can be problematic. Gentle eye irrigation has been noted by some researchers as contraindicated, while others find it useful to flush out some of the hairs.5,8,9
Most of the visible protruding tarantula hairs can and should be removed under microscopy during slit-lamp exam. Hairs that are buried in the cornea, however, are nearly impossible to remove and pose a threat of further complications, as described. Conservative management with careful observation is therefore recommended. If the patient develops a granuloma, excision—along with a course of systemic steroids and setae removal via vitrectomy—may be needed.9
The good news is that, in many cases, deeper hairs are absorbed without complication, making their removal unnecessary.5 Factors that encourage leaving the setae untouched include a large number of hairs, deep corneal penetration, lack of patient tolerance for the procedure, and risk for perforation.3
More invasive treatments (eg, laser photocoagulation, intraocular surgery) to remove offending hairs are possible, but literature on the outcome of these interventions is limited. One report to date used argon laser photocoagulation to treat endophthalmitis from vitreous hairs.10 The laser can fragment the hairs so that they lose their barbed characteristic and cannot penetrate deeper.6
Follow-up. Close follow-up is advised, and patients should be educated on the importance of medication compliance and return visits for reevaluation. Given the potential dangers of handling these spiders, tarantula owners should be advised to use protective gloving and goggles.2,5,8,9
OUTCOME FOR THE CASE PATIENT
The case patient was sent to an ophthalmologist on day 1. Proparacaine was placed in his right eye, and all of the superficial tarantula hairs were removed using 25- and 30-gauge needles with jeweler forceps under slit-lamp microscopy. Most of the hairs were removed from the superior cornea; fewer were found in the paracentral and inferior regions of the cornea. Approximately five hairs in the paracentral area of the cornea were embedded in the midstromal depth and could not be removed. One drop of ciprofloxacin was administered.
The patient was sent home with an eye shield and instructions to use tobramycin/dexamethasone eye drops (qid in his right eye) and avoid rubbing the eye. (The eye shield, though not technically necessary, was deemed beneficial to help the patient avoid touching the eye.) He was scheduled to return to the clinic one week later.
On follow-up, a careful exam performed under microscopy showed that the five tarantula hairs were still embedded, and an additional six hairs were found in the deep stroma. Superficial punctate keratitis—an eye disorder caused by epithelial cell death on the surface of the cornea—was noted, but no anterior chamber cells were seen. The patient was instructed to continue using the eye drops as prescribed until finished, then start using loteprednol (tid) and artificial lubricating tears (every 2 h).
He returned to the clinic every two weeks for a total of 10 visits. At the end of the treatment course, the remaining tarantula hairs were unable to be removed. The patient used tapering doses of topical eye steroids and antibiotic drops secondary to flare-up.
CONCLUSION
Determining the etiology of ophthalmic emergencies is essential to timely and appropriate management. In this case, a recognized but often overlooked cause, tarantula hairs, made the diagnosis more complicated than simple conjunctivitis. When ocular injury is suspected, the provider must obtain an accurate and detailed history along with a thorough physical exam. Since patients must comply with medication regimens to prevent acute and chronic infection, a clear treatment and follow-up plan should be established. With these in place, ophthalmia nodosa caused by urticating setae can be effectively managed.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An otherwise healthy 9-year-old boy is brought to the emergency department (ED) by his father for evaluation of severe pain, blurry vision, and four hours of tearing in his right eye. The patient was in school when he experienced sudden-onset irritation and scratching pain that caused him to rub his eye. He says it “feels like there is something in my eye,” but he denies any known substance or foreign body. He has no medical or surgical history, does not wear contact lenses or eyeglasses, and denies loss of vision. There is no history of recent illness or travel.
On evaluation, the patient is in no acute distress but is holding his right eye closed due to foreign-body sensation and increased photosensitivity and tearing. There is no obvious erythema or swelling in the upper or lower eyelids bilaterally. A visual acuity test with a Snellen eye chart shows 20/20 vision in the left eye and 20/50 in the right, secondary to pain, photophobia, and excessive tearing. The patient’s right sclera is significantly injected. Intraocular pressure, measured with a tonometer, is 12 to 14 mm Hg. A fluorescein stain of the eye yields no significant findings. The globe is intact.
At first glance, a slit-lamp exam shows no obvious signs of a foreign body. But much higher magnification reveals substantial conjunctival injection and numerous intracorneal linear foreign bodies in the right eye (see Figure 1 for example [not the case patient]). The anterior chamber shows no inflammatory reaction, and findings in the posterior segment are unremarkable.
The initial diagnosis is simple conjunctivitis—but closer examination reveals multiple fine, barbed hairs embedded in the patient’s right cornea. Upon further questioning, the patient reports that prior to symptom onset, he had been holding the classroom pet, a Chilean Rose tarantula, in the palm of his hands.
DISCUSSION
Foreign body injury is a common cause of ocular pain and corneal damage, which can lead to challenging complications. Ophthalmic emergencies account for 2% of ED visits in the US annually and are a major cause of visual impairment.1 But when a painful eye is the chief complaint, contact with insects, plants, or spiders is rarely included in the differential. Tarantulas are popular classroom and household pets, however, and ocular injury should be suspected in anyone who has been holding a tarantula prior to onset of pain.
Ophthalmia nodosa
Tarantulas are one of the most common arachnids known to cause ophthalmia nodosa—a granulomatous reaction of the conjunctiva or cornea to an implanted plant, insect, or spider hair that typically manifests with photophobia, irritation, and chemosis.2,3 Tarantulas, when scared or defending their eggs, shoot urticating setae at the threat—a defensive mechanism largely unknown to parents, tarantula owners, and medical professionals.
Urticating setae are found in roughly 90% of tarantula species throughout tropical and subtropical regions.4 Depending on the species, setae can be located on the distal prolateral surface of the palpal femur or the dorsum of the abdomen. They can be released when the tarantula scratches its legs against the abdominal urticating setae patch or scratches the palps against the chelicerae (appendages in front of the mouth), or when direct exterior contact is made with the abdominal setae.4
There are six types of urticating hairs. Each is attached to the spider’s cuticle by either a stalk (which represents the break-off region) or a socket.4 Tarantula hairs range in size from 0.1 mm to 0.3 mm and have a sharp, pointed head and numerous barbs, which help embed them in the target.5 They are long and thin, to facilitate deep tissue penetration, and can enter the eyes, lungs, or other body parts (see Figure 2).
Ocular injury from tarantula hairs commonly involves conjunctival injection, foreign body sensation, periorbital facial rash, photophobia, and tearing.3 When a tarantula’s cloud of barbed hairs is flicked into the eye and pierces the cornea, it can cause infection, irritation, scarring on the cornea, or vision loss. Eye movement or rubbing can cause the hairs—and their toxins—to migrate over time, traveling like an arrow (the tip and barbs resist backward movement) to the anterior chamber, lens, vitreous, and retina.6,7 This can cause corneal scars, cataracts, vitritis, or macular edema, and creates the possibility for acute or chronic conjunctivitis.7
Diagnosis and management
Ophthalmic emergencies can affect the visual system and, if left untreated, can lead to permanent vision loss. Affected patients require immediate medical attention and should be referred to an ophthalmologist for follow-up care.
Diagnosis. A thorough history and physical exam are of utmost importance; tiny setae can be easily overlooked if the examiner is not diligent, and the similar symptomatology can lead to misdiagnosis as simple conjunctivitis.3 A visual acuity test and slit-lamp exam are useful for confirmation.
Treatment. Once the diagnosis is confirmed, treatment should consist of mild topical antibiotics and steroids to effectively control infection and inflammation. While topical steroids may be appropriate, local adverse events associated with their use (eg, glaucoma, cataracts) can be problematic. Gentle eye irrigation has been noted by some researchers as contraindicated, while others find it useful to flush out some of the hairs.5,8,9
Most of the visible protruding tarantula hairs can and should be removed under microscopy during slit-lamp exam. Hairs that are buried in the cornea, however, are nearly impossible to remove and pose a threat of further complications, as described. Conservative management with careful observation is therefore recommended. If the patient develops a granuloma, excision—along with a course of systemic steroids and setae removal via vitrectomy—may be needed.9
The good news is that, in many cases, deeper hairs are absorbed without complication, making their removal unnecessary.5 Factors that encourage leaving the setae untouched include a large number of hairs, deep corneal penetration, lack of patient tolerance for the procedure, and risk for perforation.3
More invasive treatments (eg, laser photocoagulation, intraocular surgery) to remove offending hairs are possible, but literature on the outcome of these interventions is limited. One report to date used argon laser photocoagulation to treat endophthalmitis from vitreous hairs.10 The laser can fragment the hairs so that they lose their barbed characteristic and cannot penetrate deeper.6
Follow-up. Close follow-up is advised, and patients should be educated on the importance of medication compliance and return visits for reevaluation. Given the potential dangers of handling these spiders, tarantula owners should be advised to use protective gloving and goggles.2,5,8,9
OUTCOME FOR THE CASE PATIENT
The case patient was sent to an ophthalmologist on day 1. Proparacaine was placed in his right eye, and all of the superficial tarantula hairs were removed using 25- and 30-gauge needles with jeweler forceps under slit-lamp microscopy. Most of the hairs were removed from the superior cornea; fewer were found in the paracentral and inferior regions of the cornea. Approximately five hairs in the paracentral area of the cornea were embedded in the midstromal depth and could not be removed. One drop of ciprofloxacin was administered.
The patient was sent home with an eye shield and instructions to use tobramycin/dexamethasone eye drops (qid in his right eye) and avoid rubbing the eye. (The eye shield, though not technically necessary, was deemed beneficial to help the patient avoid touching the eye.) He was scheduled to return to the clinic one week later.
On follow-up, a careful exam performed under microscopy showed that the five tarantula hairs were still embedded, and an additional six hairs were found in the deep stroma. Superficial punctate keratitis—an eye disorder caused by epithelial cell death on the surface of the cornea—was noted, but no anterior chamber cells were seen. The patient was instructed to continue using the eye drops as prescribed until finished, then start using loteprednol (tid) and artificial lubricating tears (every 2 h).
He returned to the clinic every two weeks for a total of 10 visits. At the end of the treatment course, the remaining tarantula hairs were unable to be removed. The patient used tapering doses of topical eye steroids and antibiotic drops secondary to flare-up.
CONCLUSION
Determining the etiology of ophthalmic emergencies is essential to timely and appropriate management. In this case, a recognized but often overlooked cause, tarantula hairs, made the diagnosis more complicated than simple conjunctivitis. When ocular injury is suspected, the provider must obtain an accurate and detailed history along with a thorough physical exam. Since patients must comply with medication regimens to prevent acute and chronic infection, a clear treatment and follow-up plan should be established. With these in place, ophthalmia nodosa caused by urticating setae can be effectively managed.
1. Fitzpatrick J, Hickman R, Alfes CM. A Guide to Mastery in Clinical Nursing: The Comprehensive Reference. New York, NY: Springer; 2018:114.
2. Lambert SR, Lyons CJ. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus. 5th ed. New York, NY: Elsevier; 2017:138.
3. Stagg BC, Ambati BK. Tarantula hairs as corneal foreign bodies. Case Rep Ophthalmol. 2011;2(3):323-326.
4. Bertani R, Guadanucci JPL. Morphology, evolution, and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba). 2013;30(4):403-418.
5. McAnena L, Murphy C, O’Connor J. Tarantula keratitis: a case report. Ir J Med Sci. 2013;182(3):349-350.
6. Yang Y, Christakis T, Mireskandari K. Acute conjunctivitis and corneal foreign bodies secondary to tarantula hairs. CMAJ. 2016;183(3):212-214.
7. Jain N, Soong HK, Gardner TW. Ophthalmia nodosa. EyeNet Magazine. November 2013. www.aao.org/eyenet/article/blink-mystery-image-17. Accessed January 24, 2018.
8. Choi JTL, Rauf A. Ophthalmia nodosa secondary to tarantula hairs. Eye (Lond). 2003;17(3):433-434.
9. Comez AT, Tufan HA, Gencer B. Ophthalmia nodosa as an occupational disease: is it unusual or is it casual? Ocul Immunol Inflamm. 2013;21(2):144-147.
10. Marti-Huguet T, Pujol O, Cabiro I, et al. Endophthalmos caused by intravitreal caterpillar hairs. Treatment by direct photocoagulation with argon laser [article in French]. J Fr Ophthalmol. 1987;10(10):559-564.
1. Fitzpatrick J, Hickman R, Alfes CM. A Guide to Mastery in Clinical Nursing: The Comprehensive Reference. New York, NY: Springer; 2018:114.
2. Lambert SR, Lyons CJ. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus. 5th ed. New York, NY: Elsevier; 2017:138.
3. Stagg BC, Ambati BK. Tarantula hairs as corneal foreign bodies. Case Rep Ophthalmol. 2011;2(3):323-326.
4. Bertani R, Guadanucci JPL. Morphology, evolution, and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba). 2013;30(4):403-418.
5. McAnena L, Murphy C, O’Connor J. Tarantula keratitis: a case report. Ir J Med Sci. 2013;182(3):349-350.
6. Yang Y, Christakis T, Mireskandari K. Acute conjunctivitis and corneal foreign bodies secondary to tarantula hairs. CMAJ. 2016;183(3):212-214.
7. Jain N, Soong HK, Gardner TW. Ophthalmia nodosa. EyeNet Magazine. November 2013. www.aao.org/eyenet/article/blink-mystery-image-17. Accessed January 24, 2018.
8. Choi JTL, Rauf A. Ophthalmia nodosa secondary to tarantula hairs. Eye (Lond). 2003;17(3):433-434.
9. Comez AT, Tufan HA, Gencer B. Ophthalmia nodosa as an occupational disease: is it unusual or is it casual? Ocul Immunol Inflamm. 2013;21(2):144-147.
10. Marti-Huguet T, Pujol O, Cabiro I, et al. Endophthalmos caused by intravitreal caterpillar hairs. Treatment by direct photocoagulation with argon laser [article in French]. J Fr Ophthalmol. 1987;10(10):559-564.
Man, 32, With Severe Scrotal Pain and Swelling
IN THIS ARTICLE
- Lab values for case patient
- Differential diagnoses
- Case outcome
A 32-year-old man presents to the urgent care center at a community hospital with severe scrotal pain and swelling of five days’ duration. What began as mild left scrotal discomfort is now causing increasing pain, swelling, hematuria, dysuria, low-grade fever, and nausea, prompting him to seek medical attention.
The patient, who is a pipefitter in a hospital, was at work when his symptoms began. He denies any history of scrotal trauma, and his review of systems is otherwise unremarkable. His medical history is significant for mild hypertension and morbid obesity, but he is not immunocompromised. Two months ago, he had an excision and repair of a left ureterocele, for which he was treated prophylactically with ciprofloxacin for one week. He has a 3–pack-year history of smoking and consumes three alcoholic beverages per week. He denies illicit drug use and has no report of sexually transmitted infection.
Upon arrival to urgent care, the patient appears to be in moderate distress, with a blood pressure (BP) of 111/79 mm Hg; pulse, 104 beats/min; respiratory rate, 18 breaths/min-1; temperature, 100.1°F; and SpO2, 94%. Physical exam reveals left scrotal erythema, severe tenderness upon palpation, marked scrotal edema, and a slight amount of foul-smelling discharge seeping from a pinpoint opening in the left perineum (see Figure 1a). Given his scrotal presentation, he is quickly transferred to a regional emergency department (ED) for a urology consult.
In the ED, lab testing yields significant findings (see Table 1). His ECG demonstrates sinus tachycardia at 126 beats/min without rhythm or ST changes. His urinalysis reveals a cloudy appearance, a protein level of 100 mg/dL, and trace leukocyte esterase.
Urgent CT with contrast is obtained; it shows significant soft-tissue inflammatory changes in the left groin and scrotum that extend into the left thigh. In addition, a collection of fluid is seen in the inferior aspect of the left scrotal wall, indicating a probable abscess. There is no free air or lymphadenopathy.
Given the patient’s worsening condition and his apparent advancement to a systemic inflammatory response syndrome, surgical consult is obtained. He is diagnosed with a scrotal abscess and cellulitis; two blood and two scrotal cultures are obtained, and the patient is empirically started on IV ampicillin and gentamicin.
Two hours later, he has a BP of 122/74 mm Hg; pulse, 112 beats/min; respiratory rate, 20 breaths/min-1; and temperature, 103.1°F. His genital inflammation has advanced to the perineum and the left lower abdomen. The purulent, bloody, foul-smelling drainage from the opening in the left perineum is increasingly apparent. The patient is taken emergently to surgery for an incision and drainage, along with exploration of the scrotal abscess. During surgery, the patient is discovered to have Fournier’s gangrene.
DISCUSSION
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineal, perianal, and/or genital areas involving the superficial and deep fascial planes while sparing the deep muscular structures and overlying skin.1 A rare but potentially fatal disease, FG spreads at a rate of up to 3 cm/h.2,3
Mortality rates range from 7.5% to 88%, with the highest mortality occurring within the first 96 hours of hospitalization.1,4-7 Mortality is often related to the onset of sepsis.4,5 Survival requires early recognition; immediate, aggressive surgical debridement of all necrotic tissue; and concomitant, early administration of appropriate antibiotics.1,4,5,8 Mortality risk and prognosis are improved in patients younger than 60 with localized disease and no toxicity, along with sterile blood cultures.1
Risk Factors
FG is most commonly seen in males between the ages of 50 and 70, with a 10:1 male-to-female ratio.3,9 Impaired immunity typically increases a patient’s susceptibility to FG, with type 2 diabetes having the highest incidence (85% of patients).1,4,6,8,10 Other conditions that can increase the risk for FG include obesity, alcoholism, cirrhosis, cardiac disease, tobacco use, peripheral vascular disease, malignancy, chronic steroid use, renal insufficiency, IV drug abuse, and HIV.1,4,6,8,9,11
Trauma frequently initiates the infectious process,with urogenital trauma (eg, placement of urethral instrumentation, surgery, and urinary tract infection) being the main cause of bacterial introduction.1,3 Localized infection causes the development of an obliterative endarteritis, resulting in subcutaneous vascular ischemia, necrosis, and bacterial proliferation.3,7,9
Presentation and Diagnosis
Presenting symptoms of FG include intense, abrupt genital pain that is disproportionate to the physical exam findings.9 This rapidly escalates to include extreme swelling, erythema, bullae, discolored skin, and tissue crepitus with eventual necrosis.2,10 Lab results typically show leukocytosis > 18.0 × 109/L.4 The testicle and spermatic cord are generally unaffected (as in this patient), due to the anatomic relationship between the various layers of fascia within the scrotum and the anterior abdominal wall, as well as the independent blood supply of the compartmentalized testicular tissue.1-3
During an exam of the acute scrotum, the differential diagnosis includes cellulitis, scrotal abscess, acute epididymitis, and testicular torsion, with scrotal abscess being most frequently diagnosed (57% of patients).9,11,12 The distinguishing features of these diagnoses can be found in Table 2. Necrotizing fasciitis in the form of FG tends to be an unexpected, rare finding usually only diagnosed during the surgical draining of an abscess.12
CT is the test of choice to detect FG and determine the extent of its spread by identifying subcutaneous air/gas within the involved fascial planes.10,13 However, an incisional biopsy with culture is needed to confirm the diagnosis.3,9 Most patients with FG require an average of four surgeries (eg, reconstruction, skin grafting, and possibly colostomy if the infection has entered the peritoneal cavity) in order to eradicate the disease and achieve the best functional and cosmetic outcome.4
Etiology
About 83% of FG cases are polymicrobial infections comprised of enterobacter, enterococci, Escherichia coli, group A streptococci, pseudomonas, and clostridium, with symptoms evolving two to four days following the initial insult.4,7,11,14,15 Monomicrobial infections are much less common, but the symptoms progress even more rapidly.15 Methicillin-resistant Staphylococcus aureus (MRSA) necrotizing fasciitis infections occur in about 3% of monomicrobial cases.12 MRSA emerged in the early 2000s as an additional causative pathogen for polymicrobial necrotizing fasciitis infections.12,14,15 Prior to that time, S aureus strains were almost uniformly susceptible to penicillinase-resistant ß lactams.12
A distinction should be made between health care-associated (HA) MRSA and community-acquired (CA) MRSA due to treatment considerations. HA-MRSA infections are contracted through previous health care exposure (within the past year) and are less resistant to treatment.16,17 In contrast, CA-MRSA, which comprises 29% of MRSA cases, causes infections in previously healthy young patients without prior health care contact within the past year.16 CA-MRSA strains are more robust than HA-MRSA strains and can cause sepsis and other invasive, rapidly progressive, and possibly life-threatening infections due to the amount of tissue destruction and necrosis.16,18 Transmission of CA-MRSA is often associated with crowded environments, frequent skin-to-skin contact, compromised skin integrity, contaminated items or surfaces, and lack of cleanliness.16 Over the years, CA-MRSA has developed resistance to multiple antimicrobials; providers should therefore consider CA-MRSA on initial evaluation of necrotizing infections, to ensure appropriate initiation of treatment.12,16
CASE CONTINUED
Extensive debridement was completed down to healthy tissue in all affected areas (see Figure 1b). The necrotizing fasciitis had spared the left testicle and spermatic cord, and a colostomy was not required.
The patient’s initial postoperative vital signs were unremarkable, except for his BP (86/54 mm Hg). The patient was taken postoperatively to the surgical intensive care unit (SICU) with the diagnosis of FG. Aggressive IV fluids were administered for resuscitation, and he was closely monitored for increasing sepsis. Metronidazole was added for anaerobic and gram-positive coverage. His postoperative lab results can also be found in Table 1.
His ECG showed a normal sinus rhythm without ST changes, and he denied any cardiac symptoms. His physical exam was significant for mild pallor, dry mucus membranes, and a left scrotal and pelvic packed dressing. He was given two units of packed red blood cells for acute postoperative blood-loss anemia. The preliminary tissue culture results showed gram-positive cocci consistent with a staphylococcal infection; his antibiotics were then changed to IV ampicillin/sulbactam and clindamycin.
Approximately five hours postoperatively, an ECG suddenly showed acute ST elevation in leads II, II, and aVF, with reciprocal changes. The patient was diagnosed with an acute myocardial infarction (AMI). He denied any chest pain, shortness of breath, or diaphoresis. The SICU team initiated aspirin therapy and immediately contacted cardiology for an emergent coronary angiogram.
The angiogram and cardiac catheterization revealed an elevated left ventricular end diastolic (LVED) volume, inferior wall hypokinesis, a low-normal ejection fraction, and a 30% lesion in the first diagonal of his left anterior descending artery. A postprocedure echocardiogram demonstrated left ventricular (LV) ejection fraction of 50%, with LV hypokinesis in the inferior base and mild left atrial enlargement. The patient was started on metoprolol for myocardial protection and recovery.
Complications
Perioperative complications of FG, including AMI, must be considered due to the physiologic stress on the body.19 Most patients with perioperative AMI after noncardiac surgery do not experience ischemic symptoms.20
Growing evidence suggests the pivotal role of acute inflammation (postoperatively or from infection) as a precipitating event in AMI.20,21 Chemical mediators, such as inflammatory cytokines, endotoxins, and nitric oxide, may play a role in the development of an AMI.22
If cardiovascular disease and/or significant cardiovascular risk factors (ie, older age, male, cigarette smoking, cardiac family history, acute kidney injury) are present, the risk for AMI increases in the first two days following surgery.21,23 Acute infections and sepsis also initiate or increase systemic inflammatory activity via these same chemical mediators.21
Most suspected infectious agents also produce coronary artery sheer stress and destabilization of vulnerable plaques, leading to plaque rupture and thrombosis.19,24 Proinflammatory cytokines promote enhanced platelet activation and contribute to this thrombotic environment.21,23 Thrombus leads to obstructed coronary blood flow, myocardial ischemia, and finally, infarction.21
A reversible myocardial depression, cardiomyopathy, or myocardial ischemia may occur in patients with acute systemic infection or sepsis when the myocardium is functionally and structurally injured by these inflammatory chemical mediators.19,22-24 Characteristics of such a cardiomyopathy include left ventricle dilation with a low filling pressure, an abnormal increase in LVED volume, and a depressed ejection fraction.22
An acute infectious or septic process can raise troponin levels in 43% to 85% of patients.22,24 Troponin biomarkers can assist in predicting myocardial injury and events after surgery with nearly absolute myocardial tissue specificity.20 Cardiovascular involvement caused by myocardial injury–related sepsis is observed in up to 70% of patients in the ICU for these reasons.23 Therefore, providers should consider measuring troponin biomarkers during such infectious and septic processes, as this team did for the case patient. The providers were able to diagnose his AMI early and institute appropriate treatment measures to avoid extensive myocardial tissue damage.
Several studies have already demonstrated a correlation between pneumococcal pneumonia and an increased risk for AMI, and the same mechanisms are presumed responsible for any severe acute infectious state.21 More research is needed to understand the pathophysiology of AMI in sepsis and acute systemic infections.23
OUTCOME FOR THE CASE PATIENT
On postoperative day 2, the patient’s vital signs and lab results were normal. Additional lab results included an A1C of 5.2%. His ECG showed a resolving ST-elevation myocardial infarction (STEMI). The surgical wound had initiation of early granulation tissue without any further signs of necrosis.
A postoperative acute STEMI was unexpected in this patient, as his only risk factors included being male, mild hypertension, obesity, and tobacco use. At the time of his initial elevated troponin level, he had no cardiac symptoms or ECG changes. This initial high troponin level may have been stress-induced from the acute infectious process, and his acute inferior wall STEMI may have been secondary to a transient thrombotic event. The STEMI may then have resolved on its own during the cardiac catheterization with the administration of heparin, IV fluids, blood products, aspirin, or dye infiltration, thus enhancing reperfusion of the coronary artery system.
The final tissue culture showed MRSA. Given his job and his history of a genitourinary procedure, as well as the less fulminant form of disease and relatively quick recovery, it was likely HA-MRSA (rather than CA-MRSA). Only clindamycin was used for treatment.
The wound continued to have decreasing erythema, a reduction in tenderness, and evidence of viable, pink granulation tissue. HIV testing was not completed during his admission. The remainder of the patient’s hospital course was unremarkable, and he was discharged home with wound care, urology, and cardiology follow-up services.
CONCLUSION
Multiple factors contribute to a delayed or mistaken diagnosis of FG; it may be overlooked in the initial working diagnoses because of its low incidence and manifestations similar to those of other soft-tissue infections (eg, cellulitis, scrotal abscess). The cutaneous signs of FG often lag behind the disease manifestation, with minimal or no external presence while extensive internal tissue destruction is occurring. Constant review of symptoms is required when treating patients with soft-tissue infections, and early signs—such as pain out of proportion to physical findings—should prompt a clinician to include FG in the differential.
Early diagnosis with prompt debridement and antibiotic therapy are crucial to patient survival. Detecting FG within the first 24 hours is critical. Further differentiation between CA-MRSA and HA-MRSA can assist in patient recovery and survival by guiding appropriate antibiotic therapy. Perioperative risk assessment and serial troponin biomarkers may identify patients in need of intensive monitoring and management postoperatively to avoid an AMI, since patients may not experience ischemic symptoms.
1. Norton KS, Johnson LW, Perry T, et al. Management of Fournier’s gangrene: an eleven-year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68(8):709-713.
2. Agostini T, Mori F, Perello R, et al. Successful combined approach to a severe Fournier’s gangrene. Indian J Plast Surg. 2014;47(1):132-136.
3. Cabrera G, March P. Fournier’s gangrene. Glendale, CA: Cinahl Information Systems; 2016.
4. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15(12):1529-1536.
5. Sugihara T, Yasunaga H, Horiguchi H, et al. Impact of surgical intervention timing on the case fatality rate for Fournier’s gangrene: an analysis of 379 cases. BJU Int. 2012;110(11c):E1096-1100.
6. Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier’s gangrene: experience with 50 patients. Scand J Urol. 2014;48(4):393-399.
7. Taken K, Oncu MR, Ergun M, et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32(3):746-750.
8. Palvolgyi R, Kaji AH, Valeriano J, et al. Fournier’s gangrene: a model for early prediction. Am Surg. 2014;80(10):926-931.
9. Pais V, Santora T. Fournier gangrene. http://emedicine.medscape.com/article/2028899-overview. Accessed August 16, 2017.
10. Cottrill RR. A demonstration of clinical reasoning through a case of scrotal infection. Urol Nurs. 2013;33(1):33-37.
11. Summers A. Fournier’s gangrene. J Nurse Pract. 2014;10(8):582-587.
12. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445-1453.
13. Gupta N, Zinn K, Bansal I, Weinstein R. Fournier’s gangrene: ultrasound or computed tomography? A letter to the editor. Med Ultrason. 2014;16(4):389-390.
14. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urol. 2013;81(4):752-758.
15. Goh T, Goh LG. Pitfalls in diagnosing necrotizing fasciitis. https://psnet.ahrq.gov/webmm/case/329/pitfalls-in-diagnos ing-necrotizing-fasciitis. Accessed August 16, 2017.
16. Kale P, Dhawan B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Indian J Med Microbiol. 2016;34(3):275-285.
17. CDC. Necrotizing fasciitis. www.cdc.gov/Features/NecrotizingFasciitis/index.html. Accessed August 16, 2017.
18. Barnes BE, Sampson DA. A literature review on community-acquired methicillin-resistant Staphylococcus aureus in the United States: clinical information for primary care nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):23-32.
19. Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11-18.
20. Devereaux PJ, Chan MTV, Alonso-Coello PA, et al; VISION Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
21. Corrales-Medina VF, Fatemi O, Serpa J, et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis. 2009;41(6-7):511-514.
22. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163-183.
23. Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
24. Mattson M. Sepsis and cardiac disease: improving outcomes through recognition and management. Prog Cardiovasc Nurs. 2009;24(4):199-201.
25. Papadakis MA, McPhee SJ. Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Education; 2015:137-138, 151-152, 937.
26. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed August 16, 2017.
IN THIS ARTICLE
- Lab values for case patient
- Differential diagnoses
- Case outcome
A 32-year-old man presents to the urgent care center at a community hospital with severe scrotal pain and swelling of five days’ duration. What began as mild left scrotal discomfort is now causing increasing pain, swelling, hematuria, dysuria, low-grade fever, and nausea, prompting him to seek medical attention.
The patient, who is a pipefitter in a hospital, was at work when his symptoms began. He denies any history of scrotal trauma, and his review of systems is otherwise unremarkable. His medical history is significant for mild hypertension and morbid obesity, but he is not immunocompromised. Two months ago, he had an excision and repair of a left ureterocele, for which he was treated prophylactically with ciprofloxacin for one week. He has a 3–pack-year history of smoking and consumes three alcoholic beverages per week. He denies illicit drug use and has no report of sexually transmitted infection.
Upon arrival to urgent care, the patient appears to be in moderate distress, with a blood pressure (BP) of 111/79 mm Hg; pulse, 104 beats/min; respiratory rate, 18 breaths/min-1; temperature, 100.1°F; and SpO2, 94%. Physical exam reveals left scrotal erythema, severe tenderness upon palpation, marked scrotal edema, and a slight amount of foul-smelling discharge seeping from a pinpoint opening in the left perineum (see Figure 1a). Given his scrotal presentation, he is quickly transferred to a regional emergency department (ED) for a urology consult.
In the ED, lab testing yields significant findings (see Table 1). His ECG demonstrates sinus tachycardia at 126 beats/min without rhythm or ST changes. His urinalysis reveals a cloudy appearance, a protein level of 100 mg/dL, and trace leukocyte esterase.
Urgent CT with contrast is obtained; it shows significant soft-tissue inflammatory changes in the left groin and scrotum that extend into the left thigh. In addition, a collection of fluid is seen in the inferior aspect of the left scrotal wall, indicating a probable abscess. There is no free air or lymphadenopathy.
Given the patient’s worsening condition and his apparent advancement to a systemic inflammatory response syndrome, surgical consult is obtained. He is diagnosed with a scrotal abscess and cellulitis; two blood and two scrotal cultures are obtained, and the patient is empirically started on IV ampicillin and gentamicin.
Two hours later, he has a BP of 122/74 mm Hg; pulse, 112 beats/min; respiratory rate, 20 breaths/min-1; and temperature, 103.1°F. His genital inflammation has advanced to the perineum and the left lower abdomen. The purulent, bloody, foul-smelling drainage from the opening in the left perineum is increasingly apparent. The patient is taken emergently to surgery for an incision and drainage, along with exploration of the scrotal abscess. During surgery, the patient is discovered to have Fournier’s gangrene.
DISCUSSION
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineal, perianal, and/or genital areas involving the superficial and deep fascial planes while sparing the deep muscular structures and overlying skin.1 A rare but potentially fatal disease, FG spreads at a rate of up to 3 cm/h.2,3
Mortality rates range from 7.5% to 88%, with the highest mortality occurring within the first 96 hours of hospitalization.1,4-7 Mortality is often related to the onset of sepsis.4,5 Survival requires early recognition; immediate, aggressive surgical debridement of all necrotic tissue; and concomitant, early administration of appropriate antibiotics.1,4,5,8 Mortality risk and prognosis are improved in patients younger than 60 with localized disease and no toxicity, along with sterile blood cultures.1
Risk Factors
FG is most commonly seen in males between the ages of 50 and 70, with a 10:1 male-to-female ratio.3,9 Impaired immunity typically increases a patient’s susceptibility to FG, with type 2 diabetes having the highest incidence (85% of patients).1,4,6,8,10 Other conditions that can increase the risk for FG include obesity, alcoholism, cirrhosis, cardiac disease, tobacco use, peripheral vascular disease, malignancy, chronic steroid use, renal insufficiency, IV drug abuse, and HIV.1,4,6,8,9,11
Trauma frequently initiates the infectious process,with urogenital trauma (eg, placement of urethral instrumentation, surgery, and urinary tract infection) being the main cause of bacterial introduction.1,3 Localized infection causes the development of an obliterative endarteritis, resulting in subcutaneous vascular ischemia, necrosis, and bacterial proliferation.3,7,9
Presentation and Diagnosis
Presenting symptoms of FG include intense, abrupt genital pain that is disproportionate to the physical exam findings.9 This rapidly escalates to include extreme swelling, erythema, bullae, discolored skin, and tissue crepitus with eventual necrosis.2,10 Lab results typically show leukocytosis > 18.0 × 109/L.4 The testicle and spermatic cord are generally unaffected (as in this patient), due to the anatomic relationship between the various layers of fascia within the scrotum and the anterior abdominal wall, as well as the independent blood supply of the compartmentalized testicular tissue.1-3
During an exam of the acute scrotum, the differential diagnosis includes cellulitis, scrotal abscess, acute epididymitis, and testicular torsion, with scrotal abscess being most frequently diagnosed (57% of patients).9,11,12 The distinguishing features of these diagnoses can be found in Table 2. Necrotizing fasciitis in the form of FG tends to be an unexpected, rare finding usually only diagnosed during the surgical draining of an abscess.12
CT is the test of choice to detect FG and determine the extent of its spread by identifying subcutaneous air/gas within the involved fascial planes.10,13 However, an incisional biopsy with culture is needed to confirm the diagnosis.3,9 Most patients with FG require an average of four surgeries (eg, reconstruction, skin grafting, and possibly colostomy if the infection has entered the peritoneal cavity) in order to eradicate the disease and achieve the best functional and cosmetic outcome.4
Etiology
About 83% of FG cases are polymicrobial infections comprised of enterobacter, enterococci, Escherichia coli, group A streptococci, pseudomonas, and clostridium, with symptoms evolving two to four days following the initial insult.4,7,11,14,15 Monomicrobial infections are much less common, but the symptoms progress even more rapidly.15 Methicillin-resistant Staphylococcus aureus (MRSA) necrotizing fasciitis infections occur in about 3% of monomicrobial cases.12 MRSA emerged in the early 2000s as an additional causative pathogen for polymicrobial necrotizing fasciitis infections.12,14,15 Prior to that time, S aureus strains were almost uniformly susceptible to penicillinase-resistant ß lactams.12
A distinction should be made between health care-associated (HA) MRSA and community-acquired (CA) MRSA due to treatment considerations. HA-MRSA infections are contracted through previous health care exposure (within the past year) and are less resistant to treatment.16,17 In contrast, CA-MRSA, which comprises 29% of MRSA cases, causes infections in previously healthy young patients without prior health care contact within the past year.16 CA-MRSA strains are more robust than HA-MRSA strains and can cause sepsis and other invasive, rapidly progressive, and possibly life-threatening infections due to the amount of tissue destruction and necrosis.16,18 Transmission of CA-MRSA is often associated with crowded environments, frequent skin-to-skin contact, compromised skin integrity, contaminated items or surfaces, and lack of cleanliness.16 Over the years, CA-MRSA has developed resistance to multiple antimicrobials; providers should therefore consider CA-MRSA on initial evaluation of necrotizing infections, to ensure appropriate initiation of treatment.12,16
CASE CONTINUED
Extensive debridement was completed down to healthy tissue in all affected areas (see Figure 1b). The necrotizing fasciitis had spared the left testicle and spermatic cord, and a colostomy was not required.
The patient’s initial postoperative vital signs were unremarkable, except for his BP (86/54 mm Hg). The patient was taken postoperatively to the surgical intensive care unit (SICU) with the diagnosis of FG. Aggressive IV fluids were administered for resuscitation, and he was closely monitored for increasing sepsis. Metronidazole was added for anaerobic and gram-positive coverage. His postoperative lab results can also be found in Table 1.
His ECG showed a normal sinus rhythm without ST changes, and he denied any cardiac symptoms. His physical exam was significant for mild pallor, dry mucus membranes, and a left scrotal and pelvic packed dressing. He was given two units of packed red blood cells for acute postoperative blood-loss anemia. The preliminary tissue culture results showed gram-positive cocci consistent with a staphylococcal infection; his antibiotics were then changed to IV ampicillin/sulbactam and clindamycin.
Approximately five hours postoperatively, an ECG suddenly showed acute ST elevation in leads II, II, and aVF, with reciprocal changes. The patient was diagnosed with an acute myocardial infarction (AMI). He denied any chest pain, shortness of breath, or diaphoresis. The SICU team initiated aspirin therapy and immediately contacted cardiology for an emergent coronary angiogram.
The angiogram and cardiac catheterization revealed an elevated left ventricular end diastolic (LVED) volume, inferior wall hypokinesis, a low-normal ejection fraction, and a 30% lesion in the first diagonal of his left anterior descending artery. A postprocedure echocardiogram demonstrated left ventricular (LV) ejection fraction of 50%, with LV hypokinesis in the inferior base and mild left atrial enlargement. The patient was started on metoprolol for myocardial protection and recovery.
Complications
Perioperative complications of FG, including AMI, must be considered due to the physiologic stress on the body.19 Most patients with perioperative AMI after noncardiac surgery do not experience ischemic symptoms.20
Growing evidence suggests the pivotal role of acute inflammation (postoperatively or from infection) as a precipitating event in AMI.20,21 Chemical mediators, such as inflammatory cytokines, endotoxins, and nitric oxide, may play a role in the development of an AMI.22
If cardiovascular disease and/or significant cardiovascular risk factors (ie, older age, male, cigarette smoking, cardiac family history, acute kidney injury) are present, the risk for AMI increases in the first two days following surgery.21,23 Acute infections and sepsis also initiate or increase systemic inflammatory activity via these same chemical mediators.21
Most suspected infectious agents also produce coronary artery sheer stress and destabilization of vulnerable plaques, leading to plaque rupture and thrombosis.19,24 Proinflammatory cytokines promote enhanced platelet activation and contribute to this thrombotic environment.21,23 Thrombus leads to obstructed coronary blood flow, myocardial ischemia, and finally, infarction.21
A reversible myocardial depression, cardiomyopathy, or myocardial ischemia may occur in patients with acute systemic infection or sepsis when the myocardium is functionally and structurally injured by these inflammatory chemical mediators.19,22-24 Characteristics of such a cardiomyopathy include left ventricle dilation with a low filling pressure, an abnormal increase in LVED volume, and a depressed ejection fraction.22
An acute infectious or septic process can raise troponin levels in 43% to 85% of patients.22,24 Troponin biomarkers can assist in predicting myocardial injury and events after surgery with nearly absolute myocardial tissue specificity.20 Cardiovascular involvement caused by myocardial injury–related sepsis is observed in up to 70% of patients in the ICU for these reasons.23 Therefore, providers should consider measuring troponin biomarkers during such infectious and septic processes, as this team did for the case patient. The providers were able to diagnose his AMI early and institute appropriate treatment measures to avoid extensive myocardial tissue damage.
Several studies have already demonstrated a correlation between pneumococcal pneumonia and an increased risk for AMI, and the same mechanisms are presumed responsible for any severe acute infectious state.21 More research is needed to understand the pathophysiology of AMI in sepsis and acute systemic infections.23
OUTCOME FOR THE CASE PATIENT
On postoperative day 2, the patient’s vital signs and lab results were normal. Additional lab results included an A1C of 5.2%. His ECG showed a resolving ST-elevation myocardial infarction (STEMI). The surgical wound had initiation of early granulation tissue without any further signs of necrosis.
A postoperative acute STEMI was unexpected in this patient, as his only risk factors included being male, mild hypertension, obesity, and tobacco use. At the time of his initial elevated troponin level, he had no cardiac symptoms or ECG changes. This initial high troponin level may have been stress-induced from the acute infectious process, and his acute inferior wall STEMI may have been secondary to a transient thrombotic event. The STEMI may then have resolved on its own during the cardiac catheterization with the administration of heparin, IV fluids, blood products, aspirin, or dye infiltration, thus enhancing reperfusion of the coronary artery system.
The final tissue culture showed MRSA. Given his job and his history of a genitourinary procedure, as well as the less fulminant form of disease and relatively quick recovery, it was likely HA-MRSA (rather than CA-MRSA). Only clindamycin was used for treatment.
The wound continued to have decreasing erythema, a reduction in tenderness, and evidence of viable, pink granulation tissue. HIV testing was not completed during his admission. The remainder of the patient’s hospital course was unremarkable, and he was discharged home with wound care, urology, and cardiology follow-up services.
CONCLUSION
Multiple factors contribute to a delayed or mistaken diagnosis of FG; it may be overlooked in the initial working diagnoses because of its low incidence and manifestations similar to those of other soft-tissue infections (eg, cellulitis, scrotal abscess). The cutaneous signs of FG often lag behind the disease manifestation, with minimal or no external presence while extensive internal tissue destruction is occurring. Constant review of symptoms is required when treating patients with soft-tissue infections, and early signs—such as pain out of proportion to physical findings—should prompt a clinician to include FG in the differential.
Early diagnosis with prompt debridement and antibiotic therapy are crucial to patient survival. Detecting FG within the first 24 hours is critical. Further differentiation between CA-MRSA and HA-MRSA can assist in patient recovery and survival by guiding appropriate antibiotic therapy. Perioperative risk assessment and serial troponin biomarkers may identify patients in need of intensive monitoring and management postoperatively to avoid an AMI, since patients may not experience ischemic symptoms.
IN THIS ARTICLE
- Lab values for case patient
- Differential diagnoses
- Case outcome
A 32-year-old man presents to the urgent care center at a community hospital with severe scrotal pain and swelling of five days’ duration. What began as mild left scrotal discomfort is now causing increasing pain, swelling, hematuria, dysuria, low-grade fever, and nausea, prompting him to seek medical attention.
The patient, who is a pipefitter in a hospital, was at work when his symptoms began. He denies any history of scrotal trauma, and his review of systems is otherwise unremarkable. His medical history is significant for mild hypertension and morbid obesity, but he is not immunocompromised. Two months ago, he had an excision and repair of a left ureterocele, for which he was treated prophylactically with ciprofloxacin for one week. He has a 3–pack-year history of smoking and consumes three alcoholic beverages per week. He denies illicit drug use and has no report of sexually transmitted infection.
Upon arrival to urgent care, the patient appears to be in moderate distress, with a blood pressure (BP) of 111/79 mm Hg; pulse, 104 beats/min; respiratory rate, 18 breaths/min-1; temperature, 100.1°F; and SpO2, 94%. Physical exam reveals left scrotal erythema, severe tenderness upon palpation, marked scrotal edema, and a slight amount of foul-smelling discharge seeping from a pinpoint opening in the left perineum (see Figure 1a). Given his scrotal presentation, he is quickly transferred to a regional emergency department (ED) for a urology consult.
In the ED, lab testing yields significant findings (see Table 1). His ECG demonstrates sinus tachycardia at 126 beats/min without rhythm or ST changes. His urinalysis reveals a cloudy appearance, a protein level of 100 mg/dL, and trace leukocyte esterase.
Urgent CT with contrast is obtained; it shows significant soft-tissue inflammatory changes in the left groin and scrotum that extend into the left thigh. In addition, a collection of fluid is seen in the inferior aspect of the left scrotal wall, indicating a probable abscess. There is no free air or lymphadenopathy.
Given the patient’s worsening condition and his apparent advancement to a systemic inflammatory response syndrome, surgical consult is obtained. He is diagnosed with a scrotal abscess and cellulitis; two blood and two scrotal cultures are obtained, and the patient is empirically started on IV ampicillin and gentamicin.
Two hours later, he has a BP of 122/74 mm Hg; pulse, 112 beats/min; respiratory rate, 20 breaths/min-1; and temperature, 103.1°F. His genital inflammation has advanced to the perineum and the left lower abdomen. The purulent, bloody, foul-smelling drainage from the opening in the left perineum is increasingly apparent. The patient is taken emergently to surgery for an incision and drainage, along with exploration of the scrotal abscess. During surgery, the patient is discovered to have Fournier’s gangrene.
DISCUSSION
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineal, perianal, and/or genital areas involving the superficial and deep fascial planes while sparing the deep muscular structures and overlying skin.1 A rare but potentially fatal disease, FG spreads at a rate of up to 3 cm/h.2,3
Mortality rates range from 7.5% to 88%, with the highest mortality occurring within the first 96 hours of hospitalization.1,4-7 Mortality is often related to the onset of sepsis.4,5 Survival requires early recognition; immediate, aggressive surgical debridement of all necrotic tissue; and concomitant, early administration of appropriate antibiotics.1,4,5,8 Mortality risk and prognosis are improved in patients younger than 60 with localized disease and no toxicity, along with sterile blood cultures.1
Risk Factors
FG is most commonly seen in males between the ages of 50 and 70, with a 10:1 male-to-female ratio.3,9 Impaired immunity typically increases a patient’s susceptibility to FG, with type 2 diabetes having the highest incidence (85% of patients).1,4,6,8,10 Other conditions that can increase the risk for FG include obesity, alcoholism, cirrhosis, cardiac disease, tobacco use, peripheral vascular disease, malignancy, chronic steroid use, renal insufficiency, IV drug abuse, and HIV.1,4,6,8,9,11
Trauma frequently initiates the infectious process,with urogenital trauma (eg, placement of urethral instrumentation, surgery, and urinary tract infection) being the main cause of bacterial introduction.1,3 Localized infection causes the development of an obliterative endarteritis, resulting in subcutaneous vascular ischemia, necrosis, and bacterial proliferation.3,7,9
Presentation and Diagnosis
Presenting symptoms of FG include intense, abrupt genital pain that is disproportionate to the physical exam findings.9 This rapidly escalates to include extreme swelling, erythema, bullae, discolored skin, and tissue crepitus with eventual necrosis.2,10 Lab results typically show leukocytosis > 18.0 × 109/L.4 The testicle and spermatic cord are generally unaffected (as in this patient), due to the anatomic relationship between the various layers of fascia within the scrotum and the anterior abdominal wall, as well as the independent blood supply of the compartmentalized testicular tissue.1-3
During an exam of the acute scrotum, the differential diagnosis includes cellulitis, scrotal abscess, acute epididymitis, and testicular torsion, with scrotal abscess being most frequently diagnosed (57% of patients).9,11,12 The distinguishing features of these diagnoses can be found in Table 2. Necrotizing fasciitis in the form of FG tends to be an unexpected, rare finding usually only diagnosed during the surgical draining of an abscess.12
CT is the test of choice to detect FG and determine the extent of its spread by identifying subcutaneous air/gas within the involved fascial planes.10,13 However, an incisional biopsy with culture is needed to confirm the diagnosis.3,9 Most patients with FG require an average of four surgeries (eg, reconstruction, skin grafting, and possibly colostomy if the infection has entered the peritoneal cavity) in order to eradicate the disease and achieve the best functional and cosmetic outcome.4
Etiology
About 83% of FG cases are polymicrobial infections comprised of enterobacter, enterococci, Escherichia coli, group A streptococci, pseudomonas, and clostridium, with symptoms evolving two to four days following the initial insult.4,7,11,14,15 Monomicrobial infections are much less common, but the symptoms progress even more rapidly.15 Methicillin-resistant Staphylococcus aureus (MRSA) necrotizing fasciitis infections occur in about 3% of monomicrobial cases.12 MRSA emerged in the early 2000s as an additional causative pathogen for polymicrobial necrotizing fasciitis infections.12,14,15 Prior to that time, S aureus strains were almost uniformly susceptible to penicillinase-resistant ß lactams.12
A distinction should be made between health care-associated (HA) MRSA and community-acquired (CA) MRSA due to treatment considerations. HA-MRSA infections are contracted through previous health care exposure (within the past year) and are less resistant to treatment.16,17 In contrast, CA-MRSA, which comprises 29% of MRSA cases, causes infections in previously healthy young patients without prior health care contact within the past year.16 CA-MRSA strains are more robust than HA-MRSA strains and can cause sepsis and other invasive, rapidly progressive, and possibly life-threatening infections due to the amount of tissue destruction and necrosis.16,18 Transmission of CA-MRSA is often associated with crowded environments, frequent skin-to-skin contact, compromised skin integrity, contaminated items or surfaces, and lack of cleanliness.16 Over the years, CA-MRSA has developed resistance to multiple antimicrobials; providers should therefore consider CA-MRSA on initial evaluation of necrotizing infections, to ensure appropriate initiation of treatment.12,16
CASE CONTINUED
Extensive debridement was completed down to healthy tissue in all affected areas (see Figure 1b). The necrotizing fasciitis had spared the left testicle and spermatic cord, and a colostomy was not required.
The patient’s initial postoperative vital signs were unremarkable, except for his BP (86/54 mm Hg). The patient was taken postoperatively to the surgical intensive care unit (SICU) with the diagnosis of FG. Aggressive IV fluids were administered for resuscitation, and he was closely monitored for increasing sepsis. Metronidazole was added for anaerobic and gram-positive coverage. His postoperative lab results can also be found in Table 1.
His ECG showed a normal sinus rhythm without ST changes, and he denied any cardiac symptoms. His physical exam was significant for mild pallor, dry mucus membranes, and a left scrotal and pelvic packed dressing. He was given two units of packed red blood cells for acute postoperative blood-loss anemia. The preliminary tissue culture results showed gram-positive cocci consistent with a staphylococcal infection; his antibiotics were then changed to IV ampicillin/sulbactam and clindamycin.
Approximately five hours postoperatively, an ECG suddenly showed acute ST elevation in leads II, II, and aVF, with reciprocal changes. The patient was diagnosed with an acute myocardial infarction (AMI). He denied any chest pain, shortness of breath, or diaphoresis. The SICU team initiated aspirin therapy and immediately contacted cardiology for an emergent coronary angiogram.
The angiogram and cardiac catheterization revealed an elevated left ventricular end diastolic (LVED) volume, inferior wall hypokinesis, a low-normal ejection fraction, and a 30% lesion in the first diagonal of his left anterior descending artery. A postprocedure echocardiogram demonstrated left ventricular (LV) ejection fraction of 50%, with LV hypokinesis in the inferior base and mild left atrial enlargement. The patient was started on metoprolol for myocardial protection and recovery.
Complications
Perioperative complications of FG, including AMI, must be considered due to the physiologic stress on the body.19 Most patients with perioperative AMI after noncardiac surgery do not experience ischemic symptoms.20
Growing evidence suggests the pivotal role of acute inflammation (postoperatively or from infection) as a precipitating event in AMI.20,21 Chemical mediators, such as inflammatory cytokines, endotoxins, and nitric oxide, may play a role in the development of an AMI.22
If cardiovascular disease and/or significant cardiovascular risk factors (ie, older age, male, cigarette smoking, cardiac family history, acute kidney injury) are present, the risk for AMI increases in the first two days following surgery.21,23 Acute infections and sepsis also initiate or increase systemic inflammatory activity via these same chemical mediators.21
Most suspected infectious agents also produce coronary artery sheer stress and destabilization of vulnerable plaques, leading to plaque rupture and thrombosis.19,24 Proinflammatory cytokines promote enhanced platelet activation and contribute to this thrombotic environment.21,23 Thrombus leads to obstructed coronary blood flow, myocardial ischemia, and finally, infarction.21
A reversible myocardial depression, cardiomyopathy, or myocardial ischemia may occur in patients with acute systemic infection or sepsis when the myocardium is functionally and structurally injured by these inflammatory chemical mediators.19,22-24 Characteristics of such a cardiomyopathy include left ventricle dilation with a low filling pressure, an abnormal increase in LVED volume, and a depressed ejection fraction.22
An acute infectious or septic process can raise troponin levels in 43% to 85% of patients.22,24 Troponin biomarkers can assist in predicting myocardial injury and events after surgery with nearly absolute myocardial tissue specificity.20 Cardiovascular involvement caused by myocardial injury–related sepsis is observed in up to 70% of patients in the ICU for these reasons.23 Therefore, providers should consider measuring troponin biomarkers during such infectious and septic processes, as this team did for the case patient. The providers were able to diagnose his AMI early and institute appropriate treatment measures to avoid extensive myocardial tissue damage.
Several studies have already demonstrated a correlation between pneumococcal pneumonia and an increased risk for AMI, and the same mechanisms are presumed responsible for any severe acute infectious state.21 More research is needed to understand the pathophysiology of AMI in sepsis and acute systemic infections.23
OUTCOME FOR THE CASE PATIENT
On postoperative day 2, the patient’s vital signs and lab results were normal. Additional lab results included an A1C of 5.2%. His ECG showed a resolving ST-elevation myocardial infarction (STEMI). The surgical wound had initiation of early granulation tissue without any further signs of necrosis.
A postoperative acute STEMI was unexpected in this patient, as his only risk factors included being male, mild hypertension, obesity, and tobacco use. At the time of his initial elevated troponin level, he had no cardiac symptoms or ECG changes. This initial high troponin level may have been stress-induced from the acute infectious process, and his acute inferior wall STEMI may have been secondary to a transient thrombotic event. The STEMI may then have resolved on its own during the cardiac catheterization with the administration of heparin, IV fluids, blood products, aspirin, or dye infiltration, thus enhancing reperfusion of the coronary artery system.
The final tissue culture showed MRSA. Given his job and his history of a genitourinary procedure, as well as the less fulminant form of disease and relatively quick recovery, it was likely HA-MRSA (rather than CA-MRSA). Only clindamycin was used for treatment.
The wound continued to have decreasing erythema, a reduction in tenderness, and evidence of viable, pink granulation tissue. HIV testing was not completed during his admission. The remainder of the patient’s hospital course was unremarkable, and he was discharged home with wound care, urology, and cardiology follow-up services.
CONCLUSION
Multiple factors contribute to a delayed or mistaken diagnosis of FG; it may be overlooked in the initial working diagnoses because of its low incidence and manifestations similar to those of other soft-tissue infections (eg, cellulitis, scrotal abscess). The cutaneous signs of FG often lag behind the disease manifestation, with minimal or no external presence while extensive internal tissue destruction is occurring. Constant review of symptoms is required when treating patients with soft-tissue infections, and early signs—such as pain out of proportion to physical findings—should prompt a clinician to include FG in the differential.
Early diagnosis with prompt debridement and antibiotic therapy are crucial to patient survival. Detecting FG within the first 24 hours is critical. Further differentiation between CA-MRSA and HA-MRSA can assist in patient recovery and survival by guiding appropriate antibiotic therapy. Perioperative risk assessment and serial troponin biomarkers may identify patients in need of intensive monitoring and management postoperatively to avoid an AMI, since patients may not experience ischemic symptoms.
1. Norton KS, Johnson LW, Perry T, et al. Management of Fournier’s gangrene: an eleven-year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68(8):709-713.
2. Agostini T, Mori F, Perello R, et al. Successful combined approach to a severe Fournier’s gangrene. Indian J Plast Surg. 2014;47(1):132-136.
3. Cabrera G, March P. Fournier’s gangrene. Glendale, CA: Cinahl Information Systems; 2016.
4. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15(12):1529-1536.
5. Sugihara T, Yasunaga H, Horiguchi H, et al. Impact of surgical intervention timing on the case fatality rate for Fournier’s gangrene: an analysis of 379 cases. BJU Int. 2012;110(11c):E1096-1100.
6. Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier’s gangrene: experience with 50 patients. Scand J Urol. 2014;48(4):393-399.
7. Taken K, Oncu MR, Ergun M, et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32(3):746-750.
8. Palvolgyi R, Kaji AH, Valeriano J, et al. Fournier’s gangrene: a model for early prediction. Am Surg. 2014;80(10):926-931.
9. Pais V, Santora T. Fournier gangrene. http://emedicine.medscape.com/article/2028899-overview. Accessed August 16, 2017.
10. Cottrill RR. A demonstration of clinical reasoning through a case of scrotal infection. Urol Nurs. 2013;33(1):33-37.
11. Summers A. Fournier’s gangrene. J Nurse Pract. 2014;10(8):582-587.
12. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445-1453.
13. Gupta N, Zinn K, Bansal I, Weinstein R. Fournier’s gangrene: ultrasound or computed tomography? A letter to the editor. Med Ultrason. 2014;16(4):389-390.
14. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urol. 2013;81(4):752-758.
15. Goh T, Goh LG. Pitfalls in diagnosing necrotizing fasciitis. https://psnet.ahrq.gov/webmm/case/329/pitfalls-in-diagnos ing-necrotizing-fasciitis. Accessed August 16, 2017.
16. Kale P, Dhawan B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Indian J Med Microbiol. 2016;34(3):275-285.
17. CDC. Necrotizing fasciitis. www.cdc.gov/Features/NecrotizingFasciitis/index.html. Accessed August 16, 2017.
18. Barnes BE, Sampson DA. A literature review on community-acquired methicillin-resistant Staphylococcus aureus in the United States: clinical information for primary care nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):23-32.
19. Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11-18.
20. Devereaux PJ, Chan MTV, Alonso-Coello PA, et al; VISION Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
21. Corrales-Medina VF, Fatemi O, Serpa J, et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis. 2009;41(6-7):511-514.
22. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163-183.
23. Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
24. Mattson M. Sepsis and cardiac disease: improving outcomes through recognition and management. Prog Cardiovasc Nurs. 2009;24(4):199-201.
25. Papadakis MA, McPhee SJ. Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Education; 2015:137-138, 151-152, 937.
26. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed August 16, 2017.
1. Norton KS, Johnson LW, Perry T, et al. Management of Fournier’s gangrene: an eleven-year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68(8):709-713.
2. Agostini T, Mori F, Perello R, et al. Successful combined approach to a severe Fournier’s gangrene. Indian J Plast Surg. 2014;47(1):132-136.
3. Cabrera G, March P. Fournier’s gangrene. Glendale, CA: Cinahl Information Systems; 2016.
4. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15(12):1529-1536.
5. Sugihara T, Yasunaga H, Horiguchi H, et al. Impact of surgical intervention timing on the case fatality rate for Fournier’s gangrene: an analysis of 379 cases. BJU Int. 2012;110(11c):E1096-1100.
6. Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier’s gangrene: experience with 50 patients. Scand J Urol. 2014;48(4):393-399.
7. Taken K, Oncu MR, Ergun M, et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32(3):746-750.
8. Palvolgyi R, Kaji AH, Valeriano J, et al. Fournier’s gangrene: a model for early prediction. Am Surg. 2014;80(10):926-931.
9. Pais V, Santora T. Fournier gangrene. http://emedicine.medscape.com/article/2028899-overview. Accessed August 16, 2017.
10. Cottrill RR. A demonstration of clinical reasoning through a case of scrotal infection. Urol Nurs. 2013;33(1):33-37.
11. Summers A. Fournier’s gangrene. J Nurse Pract. 2014;10(8):582-587.
12. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445-1453.
13. Gupta N, Zinn K, Bansal I, Weinstein R. Fournier’s gangrene: ultrasound or computed tomography? A letter to the editor. Med Ultrason. 2014;16(4):389-390.
14. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urol. 2013;81(4):752-758.
15. Goh T, Goh LG. Pitfalls in diagnosing necrotizing fasciitis. https://psnet.ahrq.gov/webmm/case/329/pitfalls-in-diagnos ing-necrotizing-fasciitis. Accessed August 16, 2017.
16. Kale P, Dhawan B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Indian J Med Microbiol. 2016;34(3):275-285.
17. CDC. Necrotizing fasciitis. www.cdc.gov/Features/NecrotizingFasciitis/index.html. Accessed August 16, 2017.
18. Barnes BE, Sampson DA. A literature review on community-acquired methicillin-resistant Staphylococcus aureus in the United States: clinical information for primary care nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):23-32.
19. Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11-18.
20. Devereaux PJ, Chan MTV, Alonso-Coello PA, et al; VISION Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
21. Corrales-Medina VF, Fatemi O, Serpa J, et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis. 2009;41(6-7):511-514.
22. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163-183.
23. Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
24. Mattson M. Sepsis and cardiac disease: improving outcomes through recognition and management. Prog Cardiovasc Nurs. 2009;24(4):199-201.
25. Papadakis MA, McPhee SJ. Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Education; 2015:137-138, 151-152, 937.
26. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed August 16, 2017.
Obese Man With Severe Pain and Swollen Hand
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).
Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7
Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).
Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7
Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).
Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7
Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
Woman, 36, With Fever and Malaise
IN THIS ARTICLE
- Clinical presentation and evaluation
- Terminology table
- Outcome for the case patient
A 36-year-old Bengali woman with a history of well-controlled diabetes presents to the emergency department with complaints of feeling “unwell” for about two weeks. She does not speak English, and a hospital-provided phone translator is used to obtain history and explain hospital course. The patient is vague regarding symptomatology, describing general malaise and tiredness. She says she became “much worse” two days ago and has shaking chills, sore throat, headache, and nonproductive cough, but she denies shortness of breath or chest pain. She also developed nausea and vomiting, stating, “I can’t keep anything down.”
She has not recently traveled out of the country and has no known sick contacts. Influenza activity is high in the area, and the patient has not received immunization. She had a “normal” menstrual period two weeks ago and firmly states, “There is no way I can be pregnant.” She admits to vaginal “spotting” off and on for the past two weeks without abdominal pain. She is married with six children and has no history of miscarriage, ectopic pregnancy, or induced abortion; she is not taking any form of birth control.
On exam, the patient is tachycardic, with a heart rate of 127 beats/min, and has a fever of 103.3°F. Blood pressure, respiratory rate, and pulse oximetry are normal. She appears unwell and dehydrated. Her mucous membranes are dry, but no skin rash is noted. There is no tonsillar swelling or exudate and no meningismus; the lung exam is clear, with no adventitious sounds. Abdominal exam demonstrates mild, generalized tenderness in the lower abdomen without peritoneal signs. No costovertebral angle tenderness is noted. Initial diagnostic considerations include sources of fever (eg, influenza, pneumonia, urinary tract infection, viral illness), or abdominal sources, such as appendicitis.
An upright anteroposterior chest x-ray shows no infiltrate, pleural effusion, or cardiomegaly. Laboratory results include a high white blood cell (WBC) count (16.9 k/mm3) with bandemia and normal electrolytes without anion gap. Rapid influenza A and B testing is negative. A urine pregnancy test is positive, and the urinalysis shows no infection but +2 ketones. Rh factor is positive. A serum quantitative β-hCG is 130,581 mIU/mL. Blood cultures are obtained, but results are not available.
Due to cultural differences, the patient is very reluctant to consent to a pelvic exam. After extensive counseling, she agrees to a bimanual exam only. The uterus is boggy and enlarged to about 12 weeks. There is exquisite uterine tenderness and purulent discharge on the gloved finger. The cervical os is closed, and there is scant bleeding.
A transvaginal ultrasound is obtained; it reveals a thickened endometrium with echogenicity, without increased vascularity, and no identifiable intrauterine pregnancy. The adnexa have no masses, and there is no free fluid in the endometrium (see Figures 1 and 2).
The patient is given broad-spectrum antibiotics and urgently transported to the operating room by Ob-Gyn for uterine evacuation. She is found to have a septic abortion due to retained products of conception (RPOC) from an incomplete miscarriage.
Continue for discussion >>
DISCUSSIONIt is not uncommon for a woman to miscarry a very early pregnancy and not realize she had been pregnant.1 Many attribute it to a “heavy” or unusual period. In one study, 11% of patients who denied the possibility of pregnancy were, in fact, pregnant.2
Miscarriage is a frequent outcome of early pregnancy; it is estimated that 11% to 20% of early pregnancies result in a spontaneous miscarriage.3-5 Most resolve without complications, but risk increases with gestational age. When they do occur, complications include RPOC, heavy prolonged bleeding, and endometritis. RPOC refers to placental or fetal tissue that remains in the uterus after a miscarriage, surgical abortion, or preterm/term delivery (see Table for additional terminology related to miscarriage and abortion). Because of increased morbidity, it is important to suspect RPOC after a known miscarriage or an induced abortion, or in a pregnant patient with bleeding.
Incidence and pathophysiologySeptic abortion is a relatively rare complication of miscarriage. It can refer to a spontaneous miscarriage complicated by a subsequent intrauterine infection, often caused by RPOC. Septic abortion is much more common after an induced abortion, in which there is instrumentation of the uterus.
The infection after a spontaneous miscarriage usually begins as endometritis. It involves the necrotic RPOC, which are prone to infection by the cervical and vaginal flora. It may spread further into the parametrium/myometrium and the peritoneal cavity. The infection may then progress to bacteremia and sepsis. Typical causative organisms include Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, hemolytic streptococci, staphylococci, and some anaerobic organisms, including Clostridium perfringens.3
Death, although rare in developed countries, is usually secondary to the sequela of sepsis, including septic shock, renal failure, adult respiratory distress syndrome, and disseminated intravascular coagulation.3,6,7 Pelvic adhesions and hysterectomy are also possible outcomes of a septic abortion.
Continue for clinical presentation and evaluation >>
Clinical presentation and evaluationMany findings suggestive of septic abortion are nonspecific, such as bleeding, pain, uterine tenderness, and fever. A combination of historical risk, physical exam, and laboratory and ultrasound findings will often be needed to confirm the diagnosis.
Fever is never to be expected in an uncomplicated miscarriage. Vaginal bleeding and some cramping are common after miscarriage; women will bleed, on average, between eight and 11 days afterward.5 Women who fall outside the normal range and experience prolonged bleeding, heavy bleeding, or severe abdominal pain should be evaluated.
A workup for patients with a possible septic abortion should include a complete blood count, blood culture with additional laboratory investigation if there is concern for bacteremia/sepsis, and type and screen for Rh factor and for possible blood transfusion, if needed.
All patients with postabortion complications should be screened for Rh factor; Rho(D) immune globulin (RhoGAM) should be administered if results indicate that the patient is Rh-negative and unsensitized. A quantitative β-hCG level can be obtained to confirm pregnancy. A single measurement will not be helpful; β-hCG can remain positive for weeks after an uncomplicated miscarriage. On the other hand, a low level does not exclude RPOC—the RPOC, if necrotic, may remain in the uterus without secreting hormone. The trend of β-hCG over time can be helpful if the diagnosis is unclear.
A careful physical exam, including a pelvic exam, should be performed. Assess for uterine tenderness, peritoneal signs, and purulent discharge from the cervix. An open cervical os is suggestive of RPOC, as the cervix closes quickly after a complete miscarriage, but a closed cervical os does not exclude the possibility of RPOC or septic abortion. The amount of bleeding should be noted, along with any tissue or clots within the vaginal vault or cervix.
A pelvic ultrasound should be obtained in all patients concerning for a septic incomplete miscarriage. Ultrasound findings can be nonspecific, because small amounts of retained tissue can look like blood (a common finding after miscarriage). Ultrasound findings of heterogeneous, echogenic material within the uterus or a thick, irregular endometrium support a diagnosis of RPOC in patients considered at risk.8,9 Increased color Doppler flow is often seen with RPOC, but there may be decreased flow in the case of necrotic RPOC. Ultrasound findings consistent with RPOC in a febrile, ill patient suggest a septic abortion.
Continue for treatment and prognosis >>
Treatment and prognosisPatients with a septic abortion require immediate evacuation of the uterus to prevent deadly complications; antibiotics may not be able to perfuse to the necrotic source of infection.10 Suction curettage is less likely than sharp curettage to cause perforation.
Broad-spectrum antibiotics should be administered. The bacteria associated with a septic incomplete miscarriage are usually polymicrobial and represent the normal flora of the vagina and cervix. The choice of agents recommended is usually the same as for pelvic inflammatory disease.11
The treatment regimen typically includes clindamycin (900 mg IV q8h), plus gentamicin (5 mg/kg IV once a day), with or without ampicillin (2 g IV q4h).11,12 Alternatively, a combination of ampicillin, gentamicin, and metronidazole (500 mg IV q8h) can be used.
Further surgery, including laparotomy and possible hysterectomy, is indicated in patients who do not respond to uterine evacuation and parenteral antibiotics. Other possible complications requiring surgery include pelvic abscess, necrotizing Clostridium infections in the myometrium, and uterine perforation.
OUTCOME FOR THE CASE PATIENTThe patient was started on IV ampicillin, gentamicin, and clindamycin and taken promptly for a suction dilation and curettage. Pathology later showed a gestational sac with severe acute necrotizing chorioamnionitis and extensive bacterial growth. This confirmed the diagnosis of a septic, incomplete miscarriage.
Blood cultures remained without any growth, and the patient was afebrile on the second postop day. The WBC count and β-hCG level trended downward.
The patient was discharged on a 14-day course of oral doxycycline and metronidazole. She was then lost to further follow-up.
CONCLUSIONThe differential diagnosis in this ill, febrile patient was initially very broad. The importance of suspecting pregnancy in all women of childbearing age, especially those not using contraception, cannot be underestimated. The accuracy of patient history and recall of last menstrual period in determining the possibility of pregnancy is not sufficiently reliable.
1. Promislow JH, Baird DD, Wilcox AJ, et al. Bleeding following pregnancy loss prior to six weeks gestation. Hum Reprod. 2007;22(3):853-857.
2. Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18(1):48-50.
3. Osazuwa H, Aziken M. Septic abortion: a review of social and demographic characteristics. Arch Gynecol Obstet. 2007;275(2):117-119.
4. Hure AJ, Powers JR, Mishra GD, et al. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One. 2012;7(5):e37109.
5. Nielsen S, Hahlin M. Expectant management of first-trimester spontaneous abortion. Lancet. 1995;345(8942):84-86.
6. Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125(5):1042-1048.
7. Rana A, Pradhan N, Gurung G, Singh M. Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res. 2004;30(1):3-8.
8. Abbasi S, Jamal A, Eslamian L, Marsousi V. Role of clinical and ultrasound findings in the diagnosis of retained products of conception. Ultrasound Obstet Gynecol. 2008;32(5):704-707.
9. Esmaeillou H, Jamal A, Eslamian L, et al. Accurate detection of retained products of conception after first- and second-trimester abortion by color doppler sonography. J Med Ultrasound. 2015;23(7):34-38.
10. Finkielman JD, De Feo FD, Heller PG, Afessa B. The clinical course of patients with septic abortion admitted to an intensive care unit. Intensive Care Med. 2004;30(6):1097-1102.
11. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
12. Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;2:CD001067.
IN THIS ARTICLE
- Clinical presentation and evaluation
- Terminology table
- Outcome for the case patient
A 36-year-old Bengali woman with a history of well-controlled diabetes presents to the emergency department with complaints of feeling “unwell” for about two weeks. She does not speak English, and a hospital-provided phone translator is used to obtain history and explain hospital course. The patient is vague regarding symptomatology, describing general malaise and tiredness. She says she became “much worse” two days ago and has shaking chills, sore throat, headache, and nonproductive cough, but she denies shortness of breath or chest pain. She also developed nausea and vomiting, stating, “I can’t keep anything down.”
She has not recently traveled out of the country and has no known sick contacts. Influenza activity is high in the area, and the patient has not received immunization. She had a “normal” menstrual period two weeks ago and firmly states, “There is no way I can be pregnant.” She admits to vaginal “spotting” off and on for the past two weeks without abdominal pain. She is married with six children and has no history of miscarriage, ectopic pregnancy, or induced abortion; she is not taking any form of birth control.
On exam, the patient is tachycardic, with a heart rate of 127 beats/min, and has a fever of 103.3°F. Blood pressure, respiratory rate, and pulse oximetry are normal. She appears unwell and dehydrated. Her mucous membranes are dry, but no skin rash is noted. There is no tonsillar swelling or exudate and no meningismus; the lung exam is clear, with no adventitious sounds. Abdominal exam demonstrates mild, generalized tenderness in the lower abdomen without peritoneal signs. No costovertebral angle tenderness is noted. Initial diagnostic considerations include sources of fever (eg, influenza, pneumonia, urinary tract infection, viral illness), or abdominal sources, such as appendicitis.
An upright anteroposterior chest x-ray shows no infiltrate, pleural effusion, or cardiomegaly. Laboratory results include a high white blood cell (WBC) count (16.9 k/mm3) with bandemia and normal electrolytes without anion gap. Rapid influenza A and B testing is negative. A urine pregnancy test is positive, and the urinalysis shows no infection but +2 ketones. Rh factor is positive. A serum quantitative β-hCG is 130,581 mIU/mL. Blood cultures are obtained, but results are not available.
Due to cultural differences, the patient is very reluctant to consent to a pelvic exam. After extensive counseling, she agrees to a bimanual exam only. The uterus is boggy and enlarged to about 12 weeks. There is exquisite uterine tenderness and purulent discharge on the gloved finger. The cervical os is closed, and there is scant bleeding.
A transvaginal ultrasound is obtained; it reveals a thickened endometrium with echogenicity, without increased vascularity, and no identifiable intrauterine pregnancy. The adnexa have no masses, and there is no free fluid in the endometrium (see Figures 1 and 2).
The patient is given broad-spectrum antibiotics and urgently transported to the operating room by Ob-Gyn for uterine evacuation. She is found to have a septic abortion due to retained products of conception (RPOC) from an incomplete miscarriage.
Continue for discussion >>
DISCUSSIONIt is not uncommon for a woman to miscarry a very early pregnancy and not realize she had been pregnant.1 Many attribute it to a “heavy” or unusual period. In one study, 11% of patients who denied the possibility of pregnancy were, in fact, pregnant.2
Miscarriage is a frequent outcome of early pregnancy; it is estimated that 11% to 20% of early pregnancies result in a spontaneous miscarriage.3-5 Most resolve without complications, but risk increases with gestational age. When they do occur, complications include RPOC, heavy prolonged bleeding, and endometritis. RPOC refers to placental or fetal tissue that remains in the uterus after a miscarriage, surgical abortion, or preterm/term delivery (see Table for additional terminology related to miscarriage and abortion). Because of increased morbidity, it is important to suspect RPOC after a known miscarriage or an induced abortion, or in a pregnant patient with bleeding.
Incidence and pathophysiologySeptic abortion is a relatively rare complication of miscarriage. It can refer to a spontaneous miscarriage complicated by a subsequent intrauterine infection, often caused by RPOC. Septic abortion is much more common after an induced abortion, in which there is instrumentation of the uterus.
The infection after a spontaneous miscarriage usually begins as endometritis. It involves the necrotic RPOC, which are prone to infection by the cervical and vaginal flora. It may spread further into the parametrium/myometrium and the peritoneal cavity. The infection may then progress to bacteremia and sepsis. Typical causative organisms include Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, hemolytic streptococci, staphylococci, and some anaerobic organisms, including Clostridium perfringens.3
Death, although rare in developed countries, is usually secondary to the sequela of sepsis, including septic shock, renal failure, adult respiratory distress syndrome, and disseminated intravascular coagulation.3,6,7 Pelvic adhesions and hysterectomy are also possible outcomes of a septic abortion.
Continue for clinical presentation and evaluation >>
Clinical presentation and evaluationMany findings suggestive of septic abortion are nonspecific, such as bleeding, pain, uterine tenderness, and fever. A combination of historical risk, physical exam, and laboratory and ultrasound findings will often be needed to confirm the diagnosis.
Fever is never to be expected in an uncomplicated miscarriage. Vaginal bleeding and some cramping are common after miscarriage; women will bleed, on average, between eight and 11 days afterward.5 Women who fall outside the normal range and experience prolonged bleeding, heavy bleeding, or severe abdominal pain should be evaluated.
A workup for patients with a possible septic abortion should include a complete blood count, blood culture with additional laboratory investigation if there is concern for bacteremia/sepsis, and type and screen for Rh factor and for possible blood transfusion, if needed.
All patients with postabortion complications should be screened for Rh factor; Rho(D) immune globulin (RhoGAM) should be administered if results indicate that the patient is Rh-negative and unsensitized. A quantitative β-hCG level can be obtained to confirm pregnancy. A single measurement will not be helpful; β-hCG can remain positive for weeks after an uncomplicated miscarriage. On the other hand, a low level does not exclude RPOC—the RPOC, if necrotic, may remain in the uterus without secreting hormone. The trend of β-hCG over time can be helpful if the diagnosis is unclear.
A careful physical exam, including a pelvic exam, should be performed. Assess for uterine tenderness, peritoneal signs, and purulent discharge from the cervix. An open cervical os is suggestive of RPOC, as the cervix closes quickly after a complete miscarriage, but a closed cervical os does not exclude the possibility of RPOC or septic abortion. The amount of bleeding should be noted, along with any tissue or clots within the vaginal vault or cervix.
A pelvic ultrasound should be obtained in all patients concerning for a septic incomplete miscarriage. Ultrasound findings can be nonspecific, because small amounts of retained tissue can look like blood (a common finding after miscarriage). Ultrasound findings of heterogeneous, echogenic material within the uterus or a thick, irregular endometrium support a diagnosis of RPOC in patients considered at risk.8,9 Increased color Doppler flow is often seen with RPOC, but there may be decreased flow in the case of necrotic RPOC. Ultrasound findings consistent with RPOC in a febrile, ill patient suggest a septic abortion.
Continue for treatment and prognosis >>
Treatment and prognosisPatients with a septic abortion require immediate evacuation of the uterus to prevent deadly complications; antibiotics may not be able to perfuse to the necrotic source of infection.10 Suction curettage is less likely than sharp curettage to cause perforation.
Broad-spectrum antibiotics should be administered. The bacteria associated with a septic incomplete miscarriage are usually polymicrobial and represent the normal flora of the vagina and cervix. The choice of agents recommended is usually the same as for pelvic inflammatory disease.11
The treatment regimen typically includes clindamycin (900 mg IV q8h), plus gentamicin (5 mg/kg IV once a day), with or without ampicillin (2 g IV q4h).11,12 Alternatively, a combination of ampicillin, gentamicin, and metronidazole (500 mg IV q8h) can be used.
Further surgery, including laparotomy and possible hysterectomy, is indicated in patients who do not respond to uterine evacuation and parenteral antibiotics. Other possible complications requiring surgery include pelvic abscess, necrotizing Clostridium infections in the myometrium, and uterine perforation.
OUTCOME FOR THE CASE PATIENTThe patient was started on IV ampicillin, gentamicin, and clindamycin and taken promptly for a suction dilation and curettage. Pathology later showed a gestational sac with severe acute necrotizing chorioamnionitis and extensive bacterial growth. This confirmed the diagnosis of a septic, incomplete miscarriage.
Blood cultures remained without any growth, and the patient was afebrile on the second postop day. The WBC count and β-hCG level trended downward.
The patient was discharged on a 14-day course of oral doxycycline and metronidazole. She was then lost to further follow-up.
CONCLUSIONThe differential diagnosis in this ill, febrile patient was initially very broad. The importance of suspecting pregnancy in all women of childbearing age, especially those not using contraception, cannot be underestimated. The accuracy of patient history and recall of last menstrual period in determining the possibility of pregnancy is not sufficiently reliable.
IN THIS ARTICLE
- Clinical presentation and evaluation
- Terminology table
- Outcome for the case patient
A 36-year-old Bengali woman with a history of well-controlled diabetes presents to the emergency department with complaints of feeling “unwell” for about two weeks. She does not speak English, and a hospital-provided phone translator is used to obtain history and explain hospital course. The patient is vague regarding symptomatology, describing general malaise and tiredness. She says she became “much worse” two days ago and has shaking chills, sore throat, headache, and nonproductive cough, but she denies shortness of breath or chest pain. She also developed nausea and vomiting, stating, “I can’t keep anything down.”
She has not recently traveled out of the country and has no known sick contacts. Influenza activity is high in the area, and the patient has not received immunization. She had a “normal” menstrual period two weeks ago and firmly states, “There is no way I can be pregnant.” She admits to vaginal “spotting” off and on for the past two weeks without abdominal pain. She is married with six children and has no history of miscarriage, ectopic pregnancy, or induced abortion; she is not taking any form of birth control.
On exam, the patient is tachycardic, with a heart rate of 127 beats/min, and has a fever of 103.3°F. Blood pressure, respiratory rate, and pulse oximetry are normal. She appears unwell and dehydrated. Her mucous membranes are dry, but no skin rash is noted. There is no tonsillar swelling or exudate and no meningismus; the lung exam is clear, with no adventitious sounds. Abdominal exam demonstrates mild, generalized tenderness in the lower abdomen without peritoneal signs. No costovertebral angle tenderness is noted. Initial diagnostic considerations include sources of fever (eg, influenza, pneumonia, urinary tract infection, viral illness), or abdominal sources, such as appendicitis.
An upright anteroposterior chest x-ray shows no infiltrate, pleural effusion, or cardiomegaly. Laboratory results include a high white blood cell (WBC) count (16.9 k/mm3) with bandemia and normal electrolytes without anion gap. Rapid influenza A and B testing is negative. A urine pregnancy test is positive, and the urinalysis shows no infection but +2 ketones. Rh factor is positive. A serum quantitative β-hCG is 130,581 mIU/mL. Blood cultures are obtained, but results are not available.
Due to cultural differences, the patient is very reluctant to consent to a pelvic exam. After extensive counseling, she agrees to a bimanual exam only. The uterus is boggy and enlarged to about 12 weeks. There is exquisite uterine tenderness and purulent discharge on the gloved finger. The cervical os is closed, and there is scant bleeding.
A transvaginal ultrasound is obtained; it reveals a thickened endometrium with echogenicity, without increased vascularity, and no identifiable intrauterine pregnancy. The adnexa have no masses, and there is no free fluid in the endometrium (see Figures 1 and 2).
The patient is given broad-spectrum antibiotics and urgently transported to the operating room by Ob-Gyn for uterine evacuation. She is found to have a septic abortion due to retained products of conception (RPOC) from an incomplete miscarriage.
Continue for discussion >>
DISCUSSIONIt is not uncommon for a woman to miscarry a very early pregnancy and not realize she had been pregnant.1 Many attribute it to a “heavy” or unusual period. In one study, 11% of patients who denied the possibility of pregnancy were, in fact, pregnant.2
Miscarriage is a frequent outcome of early pregnancy; it is estimated that 11% to 20% of early pregnancies result in a spontaneous miscarriage.3-5 Most resolve without complications, but risk increases with gestational age. When they do occur, complications include RPOC, heavy prolonged bleeding, and endometritis. RPOC refers to placental or fetal tissue that remains in the uterus after a miscarriage, surgical abortion, or preterm/term delivery (see Table for additional terminology related to miscarriage and abortion). Because of increased morbidity, it is important to suspect RPOC after a known miscarriage or an induced abortion, or in a pregnant patient with bleeding.
Incidence and pathophysiologySeptic abortion is a relatively rare complication of miscarriage. It can refer to a spontaneous miscarriage complicated by a subsequent intrauterine infection, often caused by RPOC. Septic abortion is much more common after an induced abortion, in which there is instrumentation of the uterus.
The infection after a spontaneous miscarriage usually begins as endometritis. It involves the necrotic RPOC, which are prone to infection by the cervical and vaginal flora. It may spread further into the parametrium/myometrium and the peritoneal cavity. The infection may then progress to bacteremia and sepsis. Typical causative organisms include Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, hemolytic streptococci, staphylococci, and some anaerobic organisms, including Clostridium perfringens.3
Death, although rare in developed countries, is usually secondary to the sequela of sepsis, including septic shock, renal failure, adult respiratory distress syndrome, and disseminated intravascular coagulation.3,6,7 Pelvic adhesions and hysterectomy are also possible outcomes of a septic abortion.
Continue for clinical presentation and evaluation >>
Clinical presentation and evaluationMany findings suggestive of septic abortion are nonspecific, such as bleeding, pain, uterine tenderness, and fever. A combination of historical risk, physical exam, and laboratory and ultrasound findings will often be needed to confirm the diagnosis.
Fever is never to be expected in an uncomplicated miscarriage. Vaginal bleeding and some cramping are common after miscarriage; women will bleed, on average, between eight and 11 days afterward.5 Women who fall outside the normal range and experience prolonged bleeding, heavy bleeding, or severe abdominal pain should be evaluated.
A workup for patients with a possible septic abortion should include a complete blood count, blood culture with additional laboratory investigation if there is concern for bacteremia/sepsis, and type and screen for Rh factor and for possible blood transfusion, if needed.
All patients with postabortion complications should be screened for Rh factor; Rho(D) immune globulin (RhoGAM) should be administered if results indicate that the patient is Rh-negative and unsensitized. A quantitative β-hCG level can be obtained to confirm pregnancy. A single measurement will not be helpful; β-hCG can remain positive for weeks after an uncomplicated miscarriage. On the other hand, a low level does not exclude RPOC—the RPOC, if necrotic, may remain in the uterus without secreting hormone. The trend of β-hCG over time can be helpful if the diagnosis is unclear.
A careful physical exam, including a pelvic exam, should be performed. Assess for uterine tenderness, peritoneal signs, and purulent discharge from the cervix. An open cervical os is suggestive of RPOC, as the cervix closes quickly after a complete miscarriage, but a closed cervical os does not exclude the possibility of RPOC or septic abortion. The amount of bleeding should be noted, along with any tissue or clots within the vaginal vault or cervix.
A pelvic ultrasound should be obtained in all patients concerning for a septic incomplete miscarriage. Ultrasound findings can be nonspecific, because small amounts of retained tissue can look like blood (a common finding after miscarriage). Ultrasound findings of heterogeneous, echogenic material within the uterus or a thick, irregular endometrium support a diagnosis of RPOC in patients considered at risk.8,9 Increased color Doppler flow is often seen with RPOC, but there may be decreased flow in the case of necrotic RPOC. Ultrasound findings consistent with RPOC in a febrile, ill patient suggest a septic abortion.
Continue for treatment and prognosis >>
Treatment and prognosisPatients with a septic abortion require immediate evacuation of the uterus to prevent deadly complications; antibiotics may not be able to perfuse to the necrotic source of infection.10 Suction curettage is less likely than sharp curettage to cause perforation.
Broad-spectrum antibiotics should be administered. The bacteria associated with a septic incomplete miscarriage are usually polymicrobial and represent the normal flora of the vagina and cervix. The choice of agents recommended is usually the same as for pelvic inflammatory disease.11
The treatment regimen typically includes clindamycin (900 mg IV q8h), plus gentamicin (5 mg/kg IV once a day), with or without ampicillin (2 g IV q4h).11,12 Alternatively, a combination of ampicillin, gentamicin, and metronidazole (500 mg IV q8h) can be used.
Further surgery, including laparotomy and possible hysterectomy, is indicated in patients who do not respond to uterine evacuation and parenteral antibiotics. Other possible complications requiring surgery include pelvic abscess, necrotizing Clostridium infections in the myometrium, and uterine perforation.
OUTCOME FOR THE CASE PATIENTThe patient was started on IV ampicillin, gentamicin, and clindamycin and taken promptly for a suction dilation and curettage. Pathology later showed a gestational sac with severe acute necrotizing chorioamnionitis and extensive bacterial growth. This confirmed the diagnosis of a septic, incomplete miscarriage.
Blood cultures remained without any growth, and the patient was afebrile on the second postop day. The WBC count and β-hCG level trended downward.
The patient was discharged on a 14-day course of oral doxycycline and metronidazole. She was then lost to further follow-up.
CONCLUSIONThe differential diagnosis in this ill, febrile patient was initially very broad. The importance of suspecting pregnancy in all women of childbearing age, especially those not using contraception, cannot be underestimated. The accuracy of patient history and recall of last menstrual period in determining the possibility of pregnancy is not sufficiently reliable.
1. Promislow JH, Baird DD, Wilcox AJ, et al. Bleeding following pregnancy loss prior to six weeks gestation. Hum Reprod. 2007;22(3):853-857.
2. Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18(1):48-50.
3. Osazuwa H, Aziken M. Septic abortion: a review of social and demographic characteristics. Arch Gynecol Obstet. 2007;275(2):117-119.
4. Hure AJ, Powers JR, Mishra GD, et al. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One. 2012;7(5):e37109.
5. Nielsen S, Hahlin M. Expectant management of first-trimester spontaneous abortion. Lancet. 1995;345(8942):84-86.
6. Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125(5):1042-1048.
7. Rana A, Pradhan N, Gurung G, Singh M. Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res. 2004;30(1):3-8.
8. Abbasi S, Jamal A, Eslamian L, Marsousi V. Role of clinical and ultrasound findings in the diagnosis of retained products of conception. Ultrasound Obstet Gynecol. 2008;32(5):704-707.
9. Esmaeillou H, Jamal A, Eslamian L, et al. Accurate detection of retained products of conception after first- and second-trimester abortion by color doppler sonography. J Med Ultrasound. 2015;23(7):34-38.
10. Finkielman JD, De Feo FD, Heller PG, Afessa B. The clinical course of patients with septic abortion admitted to an intensive care unit. Intensive Care Med. 2004;30(6):1097-1102.
11. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
12. Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;2:CD001067.
1. Promislow JH, Baird DD, Wilcox AJ, et al. Bleeding following pregnancy loss prior to six weeks gestation. Hum Reprod. 2007;22(3):853-857.
2. Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18(1):48-50.
3. Osazuwa H, Aziken M. Septic abortion: a review of social and demographic characteristics. Arch Gynecol Obstet. 2007;275(2):117-119.
4. Hure AJ, Powers JR, Mishra GD, et al. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One. 2012;7(5):e37109.
5. Nielsen S, Hahlin M. Expectant management of first-trimester spontaneous abortion. Lancet. 1995;345(8942):84-86.
6. Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015;125(5):1042-1048.
7. Rana A, Pradhan N, Gurung G, Singh M. Induced septic abortion: a major factor in maternal mortality and morbidity. J Obstet Gynaecol Res. 2004;30(1):3-8.
8. Abbasi S, Jamal A, Eslamian L, Marsousi V. Role of clinical and ultrasound findings in the diagnosis of retained products of conception. Ultrasound Obstet Gynecol. 2008;32(5):704-707.
9. Esmaeillou H, Jamal A, Eslamian L, et al. Accurate detection of retained products of conception after first- and second-trimester abortion by color doppler sonography. J Med Ultrasound. 2015;23(7):34-38.
10. Finkielman JD, De Feo FD, Heller PG, Afessa B. The clinical course of patients with septic abortion admitted to an intensive care unit. Intensive Care Med. 2004;30(6):1097-1102.
11. CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
12. Mackeen AD, Packard RE, Ota E, Speer L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;2:CD001067.