User login
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Infectious disease pop quiz: Clinical challenge #5 for the ObGyn
What are the major manifestations of congenital rubella syndrome?
Continue to the answer...
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the major manifestations of congenital rubella syndrome?
Continue to the answer...
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
What are the major manifestations of congenital rubella syndrome?
Continue to the answer...
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Fever following cesarean delivery: What are your steps for management?
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (
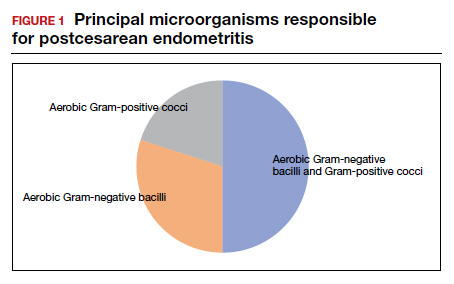
The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
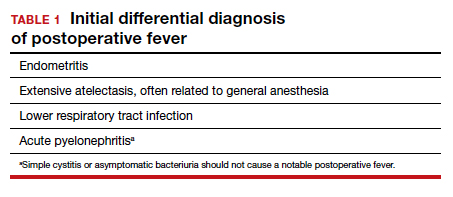
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1
Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
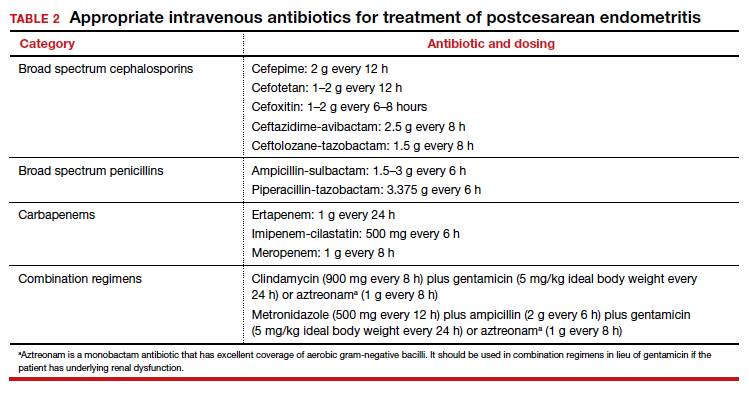
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.
Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
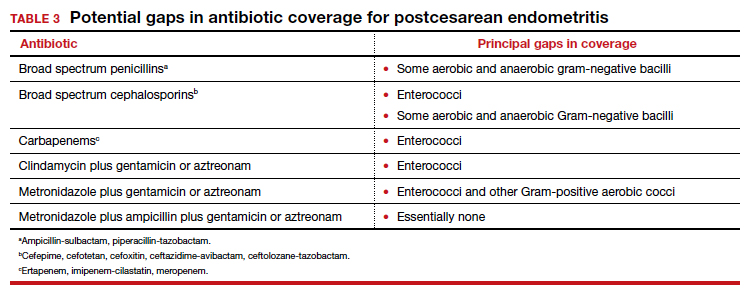
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4
Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
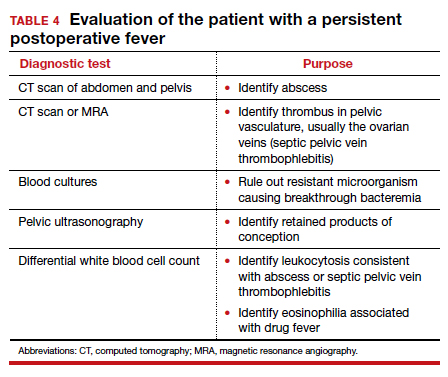
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.
Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
Infectious disease pop quiz: Clinical challenge #4 for the ObGyn
What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Continue to the answer...
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Continue to the answer...
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Continue to the answer...
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Infectious disease pop quiz: Clinical challenge #3 for the ObGyn
What are the major complications of pyelonephritis in pregnancy?
Continue to the answer...
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the major complications of pyelonephritis in pregnancy?
Continue to the answer...
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
What are the major complications of pyelonephritis in pregnancy?
Continue to the answer...
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Infectious disease pop quiz: Clinical challenge #2 for the ObGyn
Which major organisms cause urinary tract infections (UTIs) in women?
Continue to the answer...
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Which major organisms cause urinary tract infections (UTIs) in women?
Continue to the answer...
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
Which major organisms cause urinary tract infections (UTIs) in women?
Continue to the answer...
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Infectious disease pop quiz: Clinical challenge #1 for the ObGyn
What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
Continue to the answer...
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
Continue to the answer...
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
Continue to the answer...
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Hepatitis in pregnancy: Sorting through the alphabet
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).
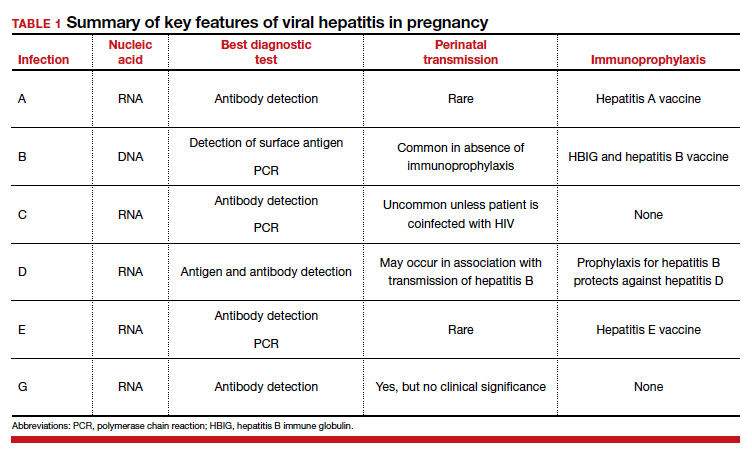
Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).
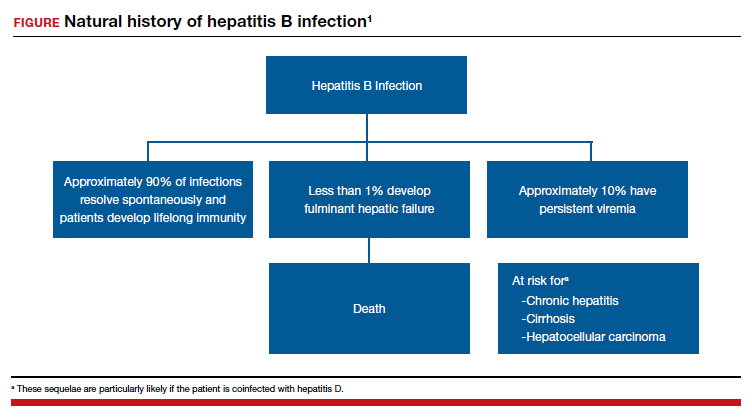

All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
Patient-facing mobile apps: Are ObGyns uniquely positioned to integrate them into practice?
Incorporating mobile apps into patient care programs can add immense value. Mobile apps enable data collection when a patient is beyond the walls of a doctor’s office and equip clinicians with new and relevant patient data. Patient engagement apps also provide a mechanism to “nudge” patients to encourage adherence to their care programs. These additional data and increased patient adherence can enable more personalized care,1 and ultimately can lead to improved outcomes. For example, a meta-analysis of 1,657 patients with diabetes showed a 5% reduction in hemoglobin A1c (HbA1c) values for those who used a diabetes-related app.2 The literature also shows positive results for heart failure, weight management, smoking reduction, and lifestyle improvement.3-5 Given their value, why aren’t patient engagement apps more routinely integrated into patient care programs?
From a software development perspective, mobile apps are fairly easy to create. However, from a user retention standpoint, creating an app with a large, sustainable userbase is challenging.5 User retention is measured in monthly active users. We are familiar with unused apps collecting digital dust on our smartphone home screens. A 2019 study showed that 25% of people typically use an application only once,6 and health care apps are not an exception. There are hundreds of thousands of mobile health apps on the market, but only 7% of these applications have more than 50,000 monthly active users. Further, 62% of digital health apps have less than 1,000 monthly active users.7
Using a new app daily or several times weekly requires new habit formation. Anyone who has tried to incorporate a new routine into their daily life knows how difficult habit formation can be. However, ObGyn patients may be particularly well suited to incorporate ObGyn-related apps into their care, given how many women already use mobile applications to track their menstrual cycles. A recent survey found that across all age groups, 47% of women use a mobile phone app to track their menstrual cycle,8 compared with 8% of US adults who regularly use an app to measure general health metrics.7 This removes one of the largest obstacles of market penetration since the habit of using an app for ObGyn purposes has already been established. As such, it presents an exciting opportunity to capitalize on the userbase already leveraging mobile apps to track their cycles and expand the patient engagement footprint into additional features that can broaden care to create a seamless, holistic patient application for ObGyn patient care.
One can envision a future in which a patient is “prescribed” an ObGyn app during their ObGyn appointment. Within the app, the patient can track their health data, engage with health providers, and gain access to educational materials. The clinician would be able to access data captured in the patient app at an aggregate level to analyze trends over time.
Current, patient-facing ObGyn mobile apps available for download on smartphones are for targeted aspects of ObGyn health. For example, there are separate apps for menstrual cycle tracking, contraception education, and medication adherence tracking. In the future, it would be ideal for clinicians and patients to have access to a single, holistic ObGyn mobile app that supports the end-to-end ObGyn patient journey, one in which clinicians could turn modules on or off given specific patient concerns. The technology for this type of holistic patient engagement platform exists, but unfortunately it is not as simple as downloading a mobile app. Standing up an end-to-end patient engagement platform requires enterprise-wide buy-in, tailoring user workflows, and building out integrations (eg, integrating provider dashboards into the existing electronic health record system). Full-scale solutions are powerful, but they can be expensive and time consuming to stand up. Until there is a more streamlined, outside-the-box ObGyn-tailored solution, there are patientfacing mobile apps available to support your patients for specific concerns.
Continue to: The top 3 recommended menstrual cycle tracking apps...
The top 3 recommended menstrual cycle tracking apps from Moglia and colleagues9 are listed in Dr. Chen’s article, “Top free menstrual cycle tracking apps for your patients.”10 The top 3 recommended contraception education mobile apps from Lunde and colleagues11 are listed in TABLE 1 and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, important special features).12 The top 3 recommended patient medication adherence mobile apps from the study by Santo and colleagues13 are listed in TABLE 2. The apps in the Stoyanov et al14 study were evaluated using the 23-item Mobile App Rating System scale. ●
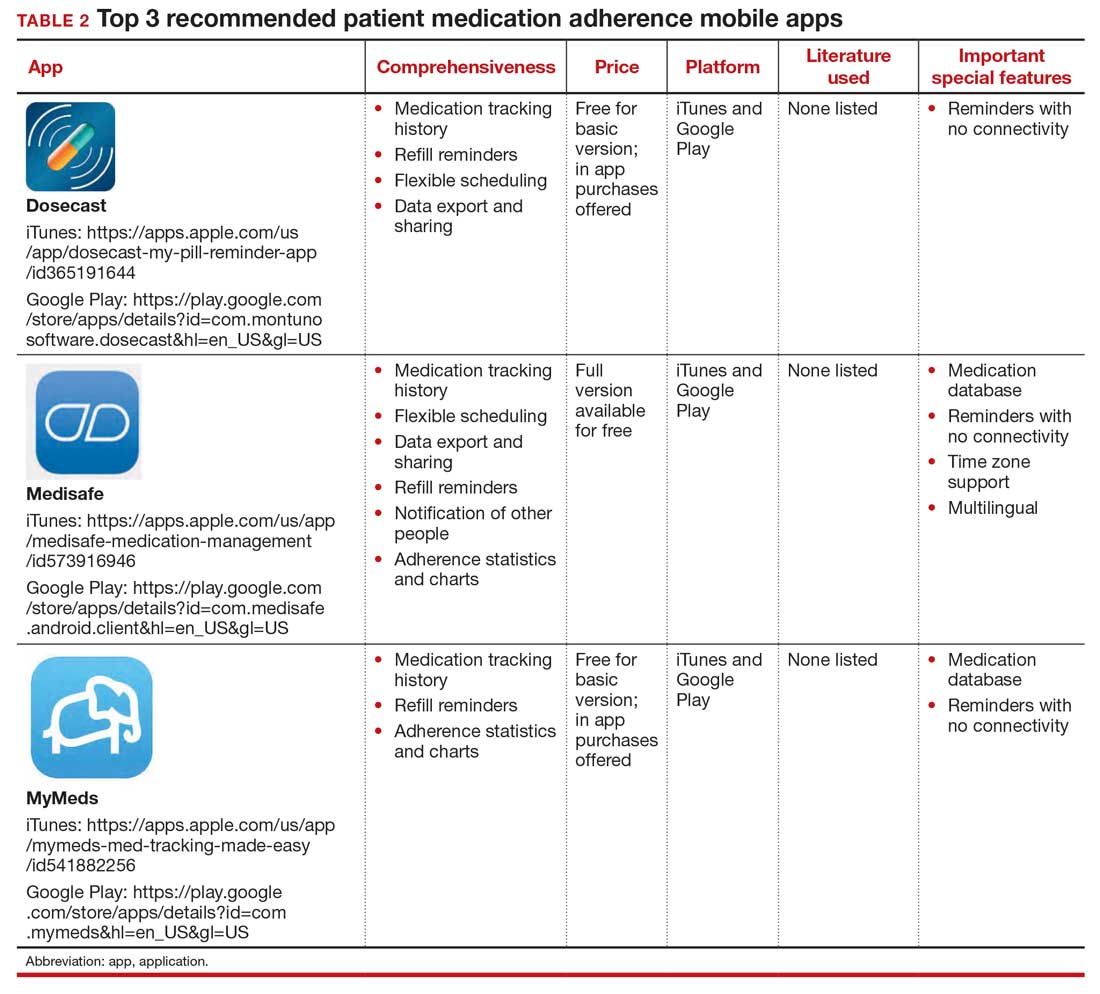
- van Dijk MR, Koster MPH, et al. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod BioMed Online. 2017;35:453-460. doi:10.1016/j. rbmo.2017.06.014.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a metaanalysis. Diabet Med. 2011;28:455-463. doi:10.1111/j.1464- 5491.2010.03180.x.
- Laing BY, Mangione CM, Tseng C-H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients. Ann Intern Med. 2014;161(10 suppl):S5-S12. doi:10.7326/m13-3005.
- Dennison L, Morrison L, Conway G, et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15:E86. doi:10.2196/jmir.2583.
- Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi:10.1186/s12966- 016-0454-y.
- 25% of users abandon apps after one use. Upland Software website. Accessed May 6, 2021. https://uplandsoftware.com /localytics/resources/blog/25-of-users-abandon-apps-afterone-use/.
- mHealth economics 2017/2018 – connectivity in digital health. Published March 5, 2019. Accessed May 6, 2021. https:// research2guidance.com/product/connectivity-in-digitalhealth/.
- Epstein DA, Lee NB, Kang JH, et al. Examining menstrual tracking to inform the design of personal informatics tools. Proc SIGCHI Conf Hum Factor Comput Syst. 2017;2017:6876- 6888. doi:10.1145/3025453.3025635.
- Moglia M, Nguyen H, Chyjek K, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127:1153-1160. doi:10.1097/AOG.0000000000001444.
- Chen KT. Top free menstrual cycle tracking apps for your patients. OBG Manag. 2017;27:44-45.
- Lunde B, Perry R, Sridhar A, et al. An evaluation of contraception education and health promotion applications for patients. Womens Health Issues. 2017;27:29-35. doi:10.1016/j.whi.2016.09.012.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483. doi:10.1097 /AOG.0000000000000842.
- Santo K, Richtering SS, Chalmers J, et al. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4:E132. doi:10.2196/mhealth.6742.
- Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:E27. doi:10.2196 /mhealth.3422.
Incorporating mobile apps into patient care programs can add immense value. Mobile apps enable data collection when a patient is beyond the walls of a doctor’s office and equip clinicians with new and relevant patient data. Patient engagement apps also provide a mechanism to “nudge” patients to encourage adherence to their care programs. These additional data and increased patient adherence can enable more personalized care,1 and ultimately can lead to improved outcomes. For example, a meta-analysis of 1,657 patients with diabetes showed a 5% reduction in hemoglobin A1c (HbA1c) values for those who used a diabetes-related app.2 The literature also shows positive results for heart failure, weight management, smoking reduction, and lifestyle improvement.3-5 Given their value, why aren’t patient engagement apps more routinely integrated into patient care programs?
From a software development perspective, mobile apps are fairly easy to create. However, from a user retention standpoint, creating an app with a large, sustainable userbase is challenging.5 User retention is measured in monthly active users. We are familiar with unused apps collecting digital dust on our smartphone home screens. A 2019 study showed that 25% of people typically use an application only once,6 and health care apps are not an exception. There are hundreds of thousands of mobile health apps on the market, but only 7% of these applications have more than 50,000 monthly active users. Further, 62% of digital health apps have less than 1,000 monthly active users.7
Using a new app daily or several times weekly requires new habit formation. Anyone who has tried to incorporate a new routine into their daily life knows how difficult habit formation can be. However, ObGyn patients may be particularly well suited to incorporate ObGyn-related apps into their care, given how many women already use mobile applications to track their menstrual cycles. A recent survey found that across all age groups, 47% of women use a mobile phone app to track their menstrual cycle,8 compared with 8% of US adults who regularly use an app to measure general health metrics.7 This removes one of the largest obstacles of market penetration since the habit of using an app for ObGyn purposes has already been established. As such, it presents an exciting opportunity to capitalize on the userbase already leveraging mobile apps to track their cycles and expand the patient engagement footprint into additional features that can broaden care to create a seamless, holistic patient application for ObGyn patient care.
One can envision a future in which a patient is “prescribed” an ObGyn app during their ObGyn appointment. Within the app, the patient can track their health data, engage with health providers, and gain access to educational materials. The clinician would be able to access data captured in the patient app at an aggregate level to analyze trends over time.
Current, patient-facing ObGyn mobile apps available for download on smartphones are for targeted aspects of ObGyn health. For example, there are separate apps for menstrual cycle tracking, contraception education, and medication adherence tracking. In the future, it would be ideal for clinicians and patients to have access to a single, holistic ObGyn mobile app that supports the end-to-end ObGyn patient journey, one in which clinicians could turn modules on or off given specific patient concerns. The technology for this type of holistic patient engagement platform exists, but unfortunately it is not as simple as downloading a mobile app. Standing up an end-to-end patient engagement platform requires enterprise-wide buy-in, tailoring user workflows, and building out integrations (eg, integrating provider dashboards into the existing electronic health record system). Full-scale solutions are powerful, but they can be expensive and time consuming to stand up. Until there is a more streamlined, outside-the-box ObGyn-tailored solution, there are patientfacing mobile apps available to support your patients for specific concerns.
Continue to: The top 3 recommended menstrual cycle tracking apps...
The top 3 recommended menstrual cycle tracking apps from Moglia and colleagues9 are listed in Dr. Chen’s article, “Top free menstrual cycle tracking apps for your patients.”10 The top 3 recommended contraception education mobile apps from Lunde and colleagues11 are listed in TABLE 1 and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, important special features).12 The top 3 recommended patient medication adherence mobile apps from the study by Santo and colleagues13 are listed in TABLE 2. The apps in the Stoyanov et al14 study were evaluated using the 23-item Mobile App Rating System scale. ●


Incorporating mobile apps into patient care programs can add immense value. Mobile apps enable data collection when a patient is beyond the walls of a doctor’s office and equip clinicians with new and relevant patient data. Patient engagement apps also provide a mechanism to “nudge” patients to encourage adherence to their care programs. These additional data and increased patient adherence can enable more personalized care,1 and ultimately can lead to improved outcomes. For example, a meta-analysis of 1,657 patients with diabetes showed a 5% reduction in hemoglobin A1c (HbA1c) values for those who used a diabetes-related app.2 The literature also shows positive results for heart failure, weight management, smoking reduction, and lifestyle improvement.3-5 Given their value, why aren’t patient engagement apps more routinely integrated into patient care programs?
From a software development perspective, mobile apps are fairly easy to create. However, from a user retention standpoint, creating an app with a large, sustainable userbase is challenging.5 User retention is measured in monthly active users. We are familiar with unused apps collecting digital dust on our smartphone home screens. A 2019 study showed that 25% of people typically use an application only once,6 and health care apps are not an exception. There are hundreds of thousands of mobile health apps on the market, but only 7% of these applications have more than 50,000 monthly active users. Further, 62% of digital health apps have less than 1,000 monthly active users.7
Using a new app daily or several times weekly requires new habit formation. Anyone who has tried to incorporate a new routine into their daily life knows how difficult habit formation can be. However, ObGyn patients may be particularly well suited to incorporate ObGyn-related apps into their care, given how many women already use mobile applications to track their menstrual cycles. A recent survey found that across all age groups, 47% of women use a mobile phone app to track their menstrual cycle,8 compared with 8% of US adults who regularly use an app to measure general health metrics.7 This removes one of the largest obstacles of market penetration since the habit of using an app for ObGyn purposes has already been established. As such, it presents an exciting opportunity to capitalize on the userbase already leveraging mobile apps to track their cycles and expand the patient engagement footprint into additional features that can broaden care to create a seamless, holistic patient application for ObGyn patient care.
One can envision a future in which a patient is “prescribed” an ObGyn app during their ObGyn appointment. Within the app, the patient can track their health data, engage with health providers, and gain access to educational materials. The clinician would be able to access data captured in the patient app at an aggregate level to analyze trends over time.
Current, patient-facing ObGyn mobile apps available for download on smartphones are for targeted aspects of ObGyn health. For example, there are separate apps for menstrual cycle tracking, contraception education, and medication adherence tracking. In the future, it would be ideal for clinicians and patients to have access to a single, holistic ObGyn mobile app that supports the end-to-end ObGyn patient journey, one in which clinicians could turn modules on or off given specific patient concerns. The technology for this type of holistic patient engagement platform exists, but unfortunately it is not as simple as downloading a mobile app. Standing up an end-to-end patient engagement platform requires enterprise-wide buy-in, tailoring user workflows, and building out integrations (eg, integrating provider dashboards into the existing electronic health record system). Full-scale solutions are powerful, but they can be expensive and time consuming to stand up. Until there is a more streamlined, outside-the-box ObGyn-tailored solution, there are patientfacing mobile apps available to support your patients for specific concerns.
Continue to: The top 3 recommended menstrual cycle tracking apps...
The top 3 recommended menstrual cycle tracking apps from Moglia and colleagues9 are listed in Dr. Chen’s article, “Top free menstrual cycle tracking apps for your patients.”10 The top 3 recommended contraception education mobile apps from Lunde and colleagues11 are listed in TABLE 1 and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, important special features).12 The top 3 recommended patient medication adherence mobile apps from the study by Santo and colleagues13 are listed in TABLE 2. The apps in the Stoyanov et al14 study were evaluated using the 23-item Mobile App Rating System scale. ●


- van Dijk MR, Koster MPH, et al. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod BioMed Online. 2017;35:453-460. doi:10.1016/j. rbmo.2017.06.014.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a metaanalysis. Diabet Med. 2011;28:455-463. doi:10.1111/j.1464- 5491.2010.03180.x.
- Laing BY, Mangione CM, Tseng C-H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients. Ann Intern Med. 2014;161(10 suppl):S5-S12. doi:10.7326/m13-3005.
- Dennison L, Morrison L, Conway G, et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15:E86. doi:10.2196/jmir.2583.
- Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi:10.1186/s12966- 016-0454-y.
- 25% of users abandon apps after one use. Upland Software website. Accessed May 6, 2021. https://uplandsoftware.com /localytics/resources/blog/25-of-users-abandon-apps-afterone-use/.
- mHealth economics 2017/2018 – connectivity in digital health. Published March 5, 2019. Accessed May 6, 2021. https:// research2guidance.com/product/connectivity-in-digitalhealth/.
- Epstein DA, Lee NB, Kang JH, et al. Examining menstrual tracking to inform the design of personal informatics tools. Proc SIGCHI Conf Hum Factor Comput Syst. 2017;2017:6876- 6888. doi:10.1145/3025453.3025635.
- Moglia M, Nguyen H, Chyjek K, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127:1153-1160. doi:10.1097/AOG.0000000000001444.
- Chen KT. Top free menstrual cycle tracking apps for your patients. OBG Manag. 2017;27:44-45.
- Lunde B, Perry R, Sridhar A, et al. An evaluation of contraception education and health promotion applications for patients. Womens Health Issues. 2017;27:29-35. doi:10.1016/j.whi.2016.09.012.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483. doi:10.1097 /AOG.0000000000000842.
- Santo K, Richtering SS, Chalmers J, et al. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4:E132. doi:10.2196/mhealth.6742.
- Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:E27. doi:10.2196 /mhealth.3422.
- van Dijk MR, Koster MPH, et al. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod BioMed Online. 2017;35:453-460. doi:10.1016/j. rbmo.2017.06.014.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a metaanalysis. Diabet Med. 2011;28:455-463. doi:10.1111/j.1464- 5491.2010.03180.x.
- Laing BY, Mangione CM, Tseng C-H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients. Ann Intern Med. 2014;161(10 suppl):S5-S12. doi:10.7326/m13-3005.
- Dennison L, Morrison L, Conway G, et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15:E86. doi:10.2196/jmir.2583.
- Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi:10.1186/s12966- 016-0454-y.
- 25% of users abandon apps after one use. Upland Software website. Accessed May 6, 2021. https://uplandsoftware.com /localytics/resources/blog/25-of-users-abandon-apps-afterone-use/.
- mHealth economics 2017/2018 – connectivity in digital health. Published March 5, 2019. Accessed May 6, 2021. https:// research2guidance.com/product/connectivity-in-digitalhealth/.
- Epstein DA, Lee NB, Kang JH, et al. Examining menstrual tracking to inform the design of personal informatics tools. Proc SIGCHI Conf Hum Factor Comput Syst. 2017;2017:6876- 6888. doi:10.1145/3025453.3025635.
- Moglia M, Nguyen H, Chyjek K, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127:1153-1160. doi:10.1097/AOG.0000000000001444.
- Chen KT. Top free menstrual cycle tracking apps for your patients. OBG Manag. 2017;27:44-45.
- Lunde B, Perry R, Sridhar A, et al. An evaluation of contraception education and health promotion applications for patients. Womens Health Issues. 2017;27:29-35. doi:10.1016/j.whi.2016.09.012.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483. doi:10.1097 /AOG.0000000000000842.
- Santo K, Richtering SS, Chalmers J, et al. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4:E132. doi:10.2196/mhealth.6742.
- Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:E27. doi:10.2196 /mhealth.3422.
Managing herpes simplex virus genital infection in pregnancy
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.
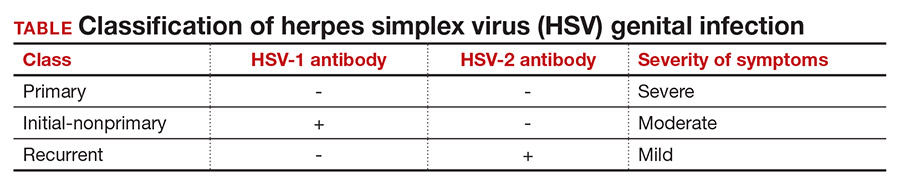
Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1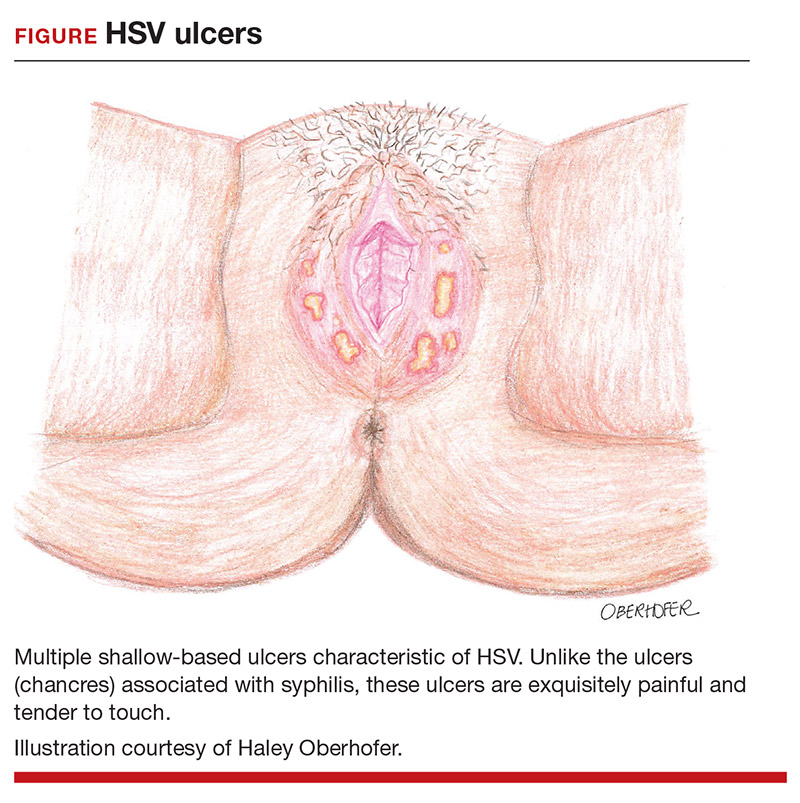
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.

















