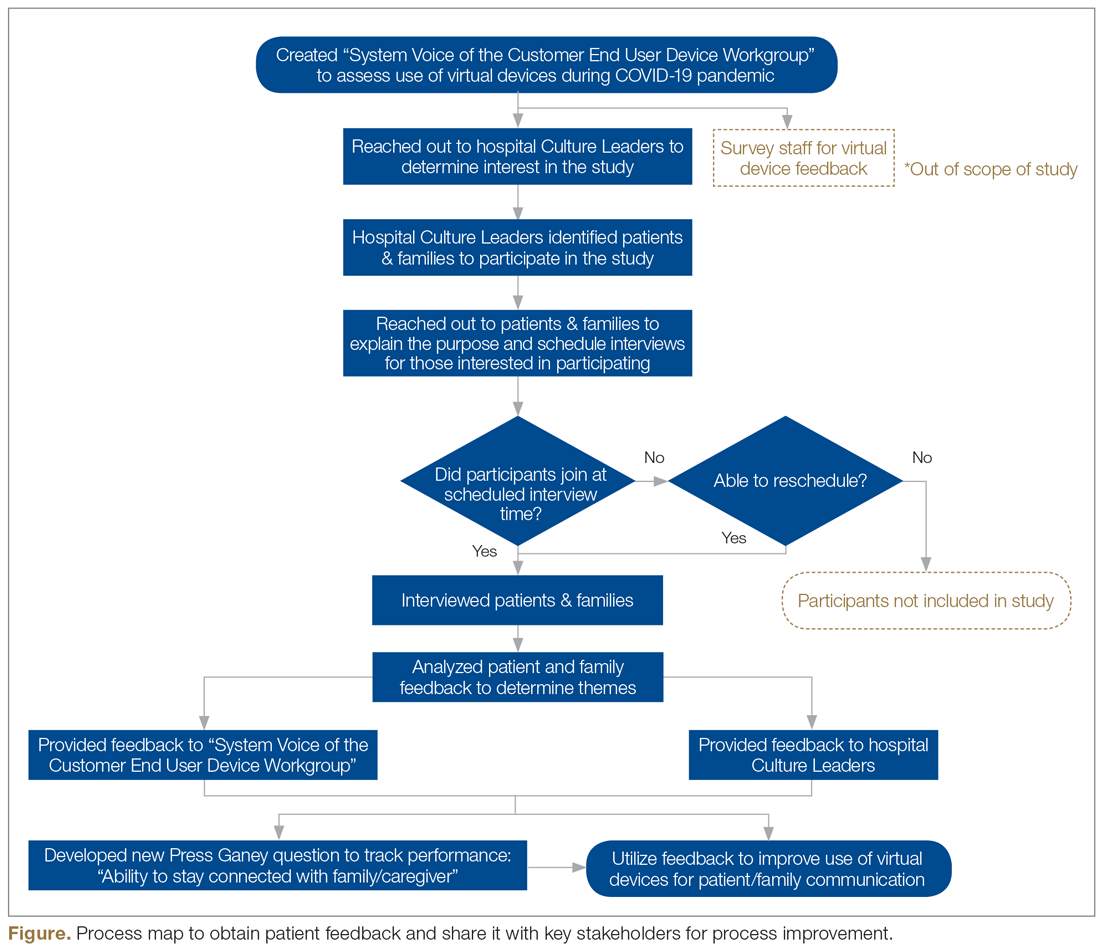
User login
Improving Hospital Metrics Through the Implementation of a Comorbidity Capture Tool and Other Quality Initiatives
From the University of Miami Miller School of Medicine (Drs. Sosa, Ferreira, Gershengorn, Soto, Parekh, and Suarez), and the Quality Department of the University of Miami Hospital and Clinics (Estin Kelly, Ameena Shrestha, Julianne Burgos, and Sandeep Devabhaktuni), Miami, FL.
Abstract
Background: Case mix index (CMI) and expected mortality are determined based on comorbidities. Improving documentation and coding can impact performance indicators. During and prior to 2018, our patient acuity was under-represented, with low expected mortality and CMI. Those metrics motivated our quality team to develop the quality initiatives reported here.
Objectives: We sought to assess the impact of quality initiatives on number of comorbidities, diagnoses, CMI, and expected mortality at the University of Miami Health System.
Design: We conducted an observational study of a series of quality initiatives: (1) education of clinical documentation specialists (CDS) to capture comorbidities (10/2019); (2) facilitating the process for physician query response (2/2020); (3) implementation of computer logic to capture electrolyte disturbances and renal dysfunction (8/2020); (4) development of a tool to capture Elixhauser comorbidities (11/2020); and (5) provider education and electronic health record reviews by the quality team.
Setting and participants: All admissions during 2019 and 2020 at University of Miami Health System. The health system includes 2 academic inpatient facilities, a 560-bed tertiary hospital, and a 40-bed cancer facility. Our hospital is 1 of the 11 PPS-Exempt Cancer Hospitals and is the South Florida’s only NCI-Designated Cancer Center.
Conclusion:
Keywords: PS/QI, coding, case mix index, comorbidities, mortality.
Adoption of comprehensive electronic health record (EHR) systems by US hospitals, defined as an EHR capable of meeting all core meaningful-use metrics including evaluation and tracking of quality metrics, has been steadily increasing.3,4 Many institutions have looked to EHR system transitions as an inflection point to expand clinical documentation improvement (CDI) efforts. Over the past several years, our institution, an academic medical center, has endeavored to fully transition to a comprehensive EHR system (Epic from Epic Systems Corporation). Part of the purpose of this transition was to help study and improve outcomes, reduce readmissions, improve quality of care, and meet performance indicators.
Prior to 2019, our hospital’s patient acuity was low, with a CMI consistently below 2, ranging from 1.81 to 1.99, and an expected mortality consistently below 1.9%, ranging from 1.65% to 1.85%. Our concern that these values underestimated the real severity of illness of our patient population prompted the development of a quality improvement plan. In this report, we describe the processes we undertook to improve documentation and coding of comorbid illness, and report on the impact of these initiatives on performance indicators. We hypothesized that our initiatives would have a significant impact on our ability to capture patient complexity, and thus impact our CMI and expected mortality.
Methods
In the fall of 2019, we embarked on a multifaceted quality improvement project aimed at improving comorbidity capture for patients hospitalized at our institution. The health system includes 2 academic inpatient facilities, a 560-bed tertiary hospital and a 40-bed cancer facility. Since September 2017, we have used Epic as our EHR. In August 2019, we started working with Vizient Clinical Data Base5 to allow benchmarking with peer institutions. We assessed the impact of this initiative with a pre/post study design.
Quality Initiatives
This quality improvement project consisted of a series of 5 targeted interventions coupled with continuous monitoring and education.
1. Comorbidity coding. In October 2019, we met with the clinical documentation specialists (CDS) and the coding team to educate them on the value of coding all comorbidities that have an impact on
2. Physician query. In October 2019, we modified the process for physician query response, allowing physicians to answer queries in the EHR through a reply tool incorporated into the query and accept answers in the body of the Epic message as an active part of the EHR.
3. EHR logic. In August 2020, we developed an EHR smart logic to automatically capture fluid and electrolyte disturbances and renal dysfunction, based on the most recent laboratory values. The logic automatically populated potentially appropriate diagnoses in the assessment and plan of provider notes, which require provider acknowledgment and which providers are able to modify

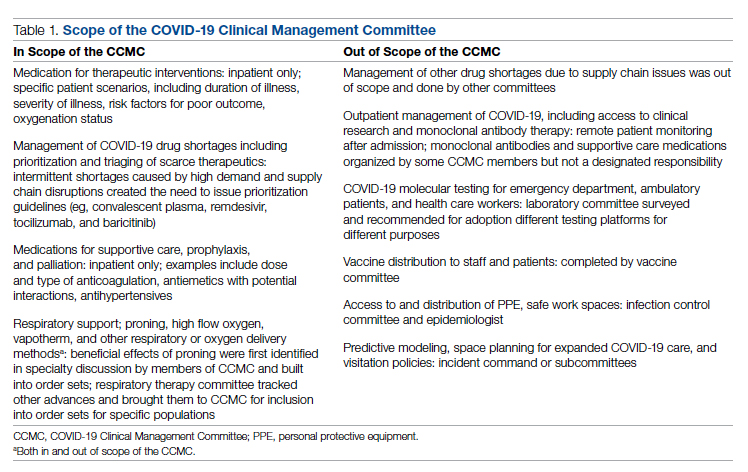
4. Comorbidity capture tool. In November 2020, we developed a standardized tool to allow providers to easily capture Elixhauser comorbidities (eFigure 2). The Elixhauser index is a method for measuring comorbidities based on International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Disease, Tenth Revision diagnosis codes found in administrative data1-6 and is used by US News & World Report and Vizient to assess comorbidity burden. Our tool automatically captures diagnoses recorded in previous documentation and allows providers to easily provide the management plan for each; this information is automatically pulled into the provider note.

The development of this tool used an existing functionality within the Epic EHR called SmartForms, SmartData Elements, and SmartLinks. The only cost of tool development was the time invested—124 hours inclusive of 4 hours of staff education. Specifically, a panel of experts (including physicians of different specialties, an analyst, and representatives from the quality office) met weekly for 30 minutes per week over 5 weeks to agree on specific clinical criteria and guide the EHR build analyst. Individual panel members confirmed and validated design requirements (in 15 hours over 5 weeks). Our senior clinical analyst II dedicated 80 hours to actual build time, 15 hours to design time, and 25 hours to tailor the function to our institution’s workflow. This tool was introduced in November 2020; completion was optional at the time of hospital admission but mandatory at discharge to ensure compliance.
5. Quality team
Assessment of Quality Initiatives’ Impact
Data on the number of comorbidities and performance indicators were obtained retrospectively. The data included all hospital admissions from 2019 and 2020 divided into 2 periods: pre-intervention from January 1, 2019 through September 30, 2019, and intervention from October 1, 2019 through December 31, 2020. The primary outcome of this observational study was the rate of comorbidity capture during the intervention period. Comorbidity capture was assessed using the Vizient Clinical Data Base (CDB) health care performance tool.5 Vizient CDB uses the Agency for Healthcare Research and Quality Elixhauser index, which includes 29 of the initial 31 comorbidities described by Elixhauser,6 as it combines hypertension with and without complications into one. We secondarily aimed to examine the impact of the quality improvement initiatives on several institutional-level performance indicators, including total number of diagnoses, comorbidities or complications (CC), major comorbidities or complications (MCC), CMI, and expected mortality.
Case mix index is the average Medicare Severity-DRG (MS-DRG) weighted across all hospital discharges (appropriate to their discharge date). The expected mortality represents the average expected number of deaths based on diagnosed conditions, age, and gender within the same time frame, and it is based on coded diagnosis; we obtained the mortality index by dividing the observed mortality by the expected mortality. The Vizient CDB Mortality Risk Adjustment Model was used to assign an expected mortality (0%-100%) to each case based on factors such as demographics, admission type, diagnoses, and procedures.
Standard statistics were used to measure the outcomes. We used Excel to compare pre-intervention and intervention period characteristics and outcomes, using t-testing for continuous variables and Chi-square testing for categorial outcomes. P values <0.05 were considered statistically significant.
The study was reviewed by the institutional review board (IRB) of our institution (IRB ID: 20210070). The IRB determined that the proposed activity was not research involving human subjects, as defined by the Department of Health and Human Services and US Food and Drug Administration regulations, and that IRB review and approval by the organization were not required.
Results
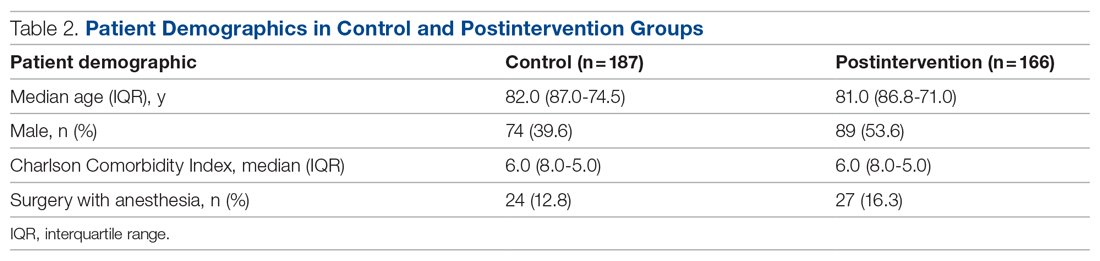
The health system had a total of 33 066 admissions during the study period—13 689 pre-intervention (January 1, 2019 through September 30, 2019) and 19,377 during the intervention period (October 1, 2019 to December 31, 2020). Demographics were similar among the pre-intervention and intervention periods: mean age was 60 years and 61 years, 52% and 51% of patients were male, 72% and 71% were White, and 20% and 19% were Black, respectively (Table 1).

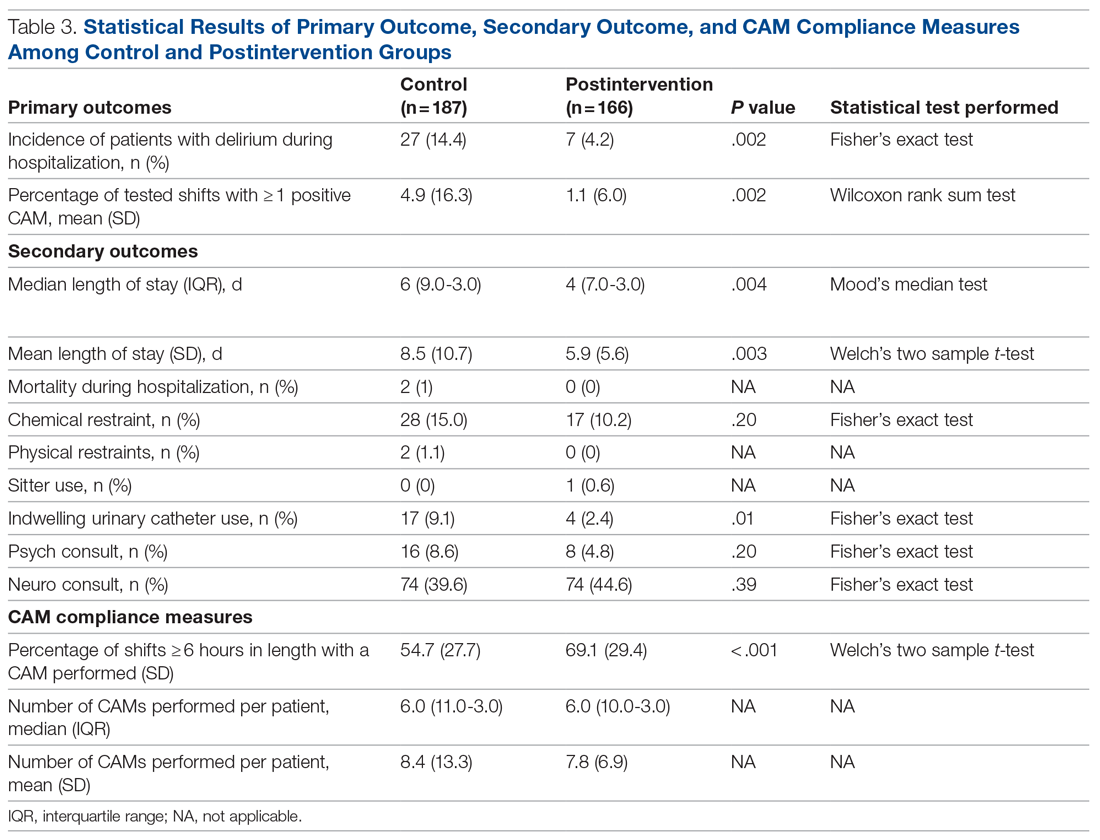
The multifaceted intervention resulted in a significant improvement in the primary outcome: mean comorbidity capture increased from 2.5 (SD, 1.7) before the intervention to 3.1 (SD, 2.0) during the intervention (P < .00001). Secondary outcomes also improved. The mean number of secondary diagnoses for admissions increased from 11.3 (SD, 7.3) prior to the intervention to 18.5 (SD, 10.4) (P < .00001) during the intervention period. The mean CMI increased from 2.1 (SD, 1.9) to 2.4 (SD, 2.2) post intervention (P < .00001), an increase during the intervention period of 14%. The expected mortality increased from 1.8% (SD, 6.1%) to 3.1% (SD, 9.2%) after the intervention (P < .00001) (Table 2).

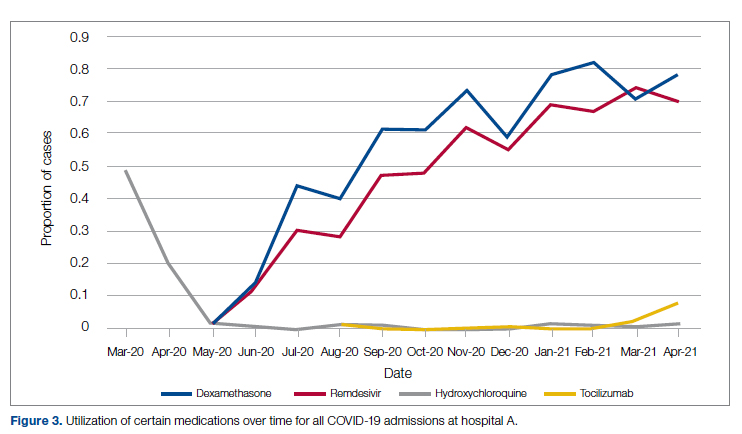
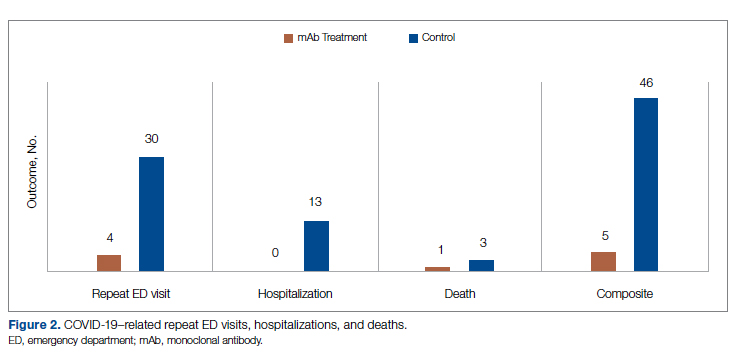
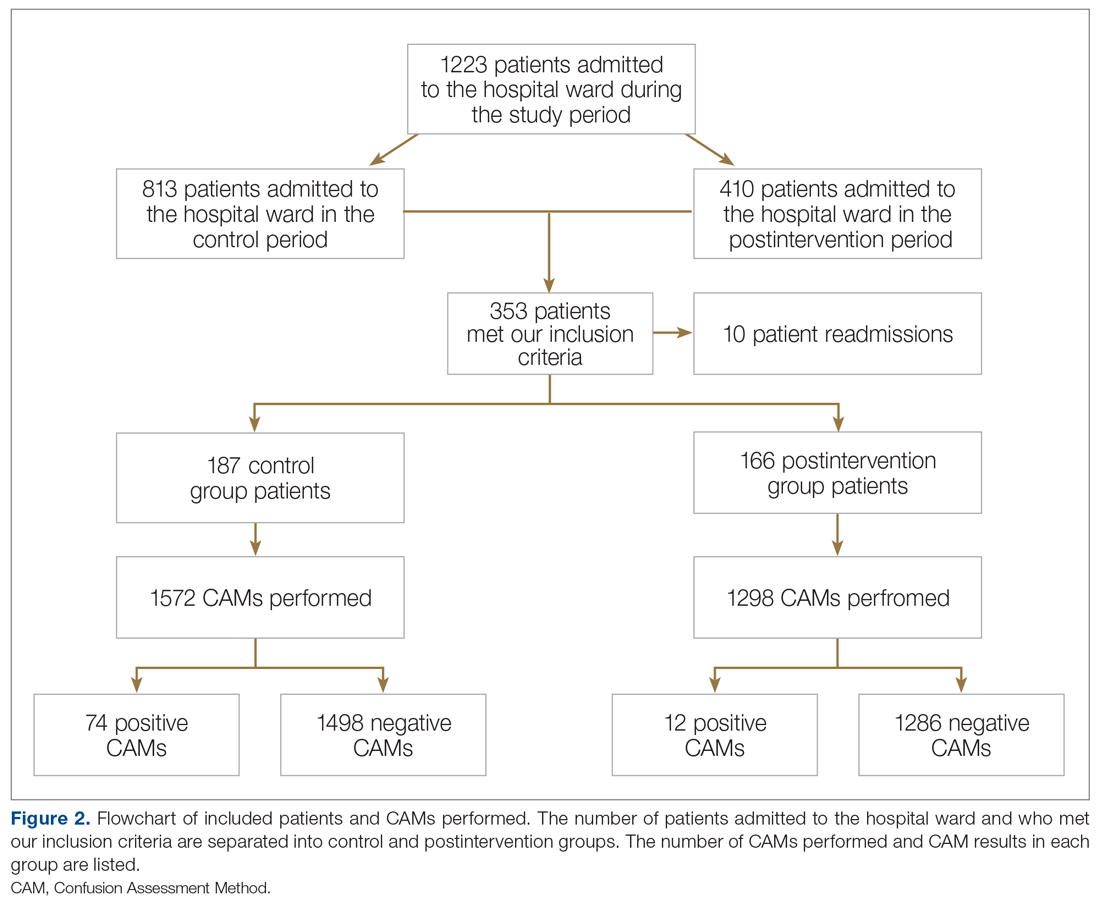
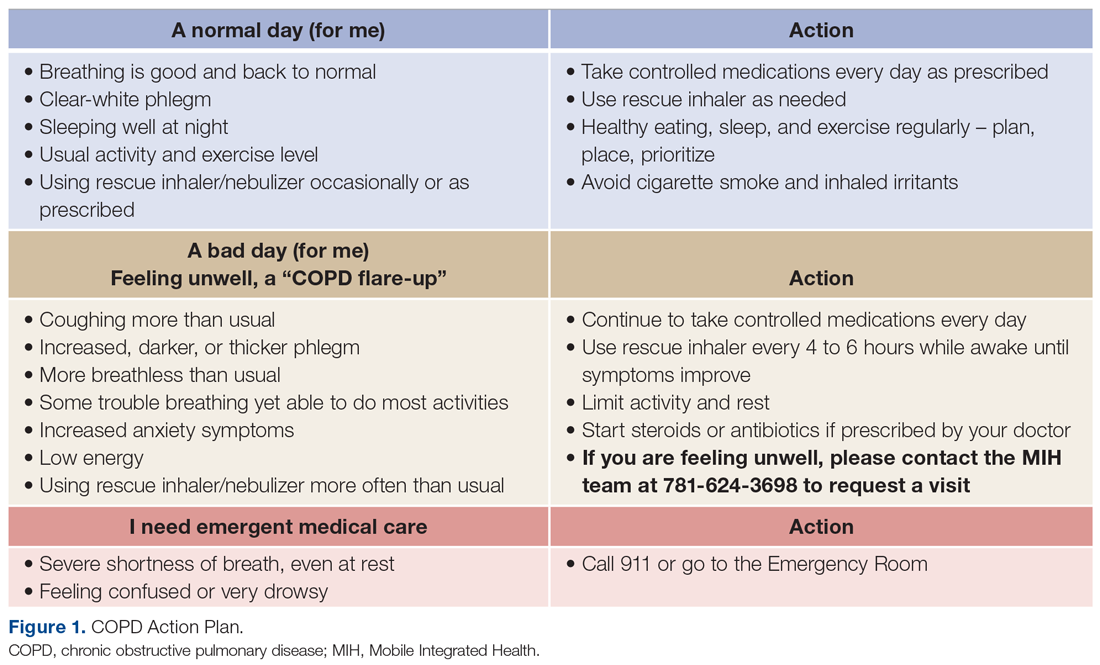
There was an overall observed improvement in percentage of discharges with documented CC and MCC for both surgical and medical specialties. Both CC and MCC increased for surgical specialties, from 54.4% to 68.5%, and for medical specialties, from 68.9% to 76.4%. (Figure 1). The diagnoses that were captured more consistently included deficiency anemia, obesity, diabetes with complications, fluid and electrolyte disorders and renal failure, hypertension, weight loss, depression, and hypothyroidism (Figure 2).



During the 9-month pre-intervention period (January 1 through September 30, 2019), there were 2795 queries, with an agreed volume of 1823; the agreement rate was 65% and the average provider turnaround time was 12.53 days. In the 15-month postintervention period, there were 10 216 queries, with an agreed volume of 6802 at 66%. We created a policy to encourage responses no later than 10 days after the query, and our average turnaround time decreased by more than 50% to 5.86 days. The average number of monthly queries increased by 55%, from an average of 311 monthly queries in the pre-intervention period to an average of 681 per month in the postintervention period. The more common queries that had an impact on CMI included sepsis, antineoplastic chemotherapy–induced pancytopenia, acute posthemorrhagic anemia, malnutrition, hyponatremia, and metabolic encephalopathy.
Discussion
The need for accurate documentation by physicians has been recognized for many years.7
With the growing complexity of the documentation and coding process, it is difficult for clinicians to keep up with the terminology required by the Centers for Medicare and Medicaid Services (CMS). Several different methods to improve documentation have been proposed. Prior interventions to standardize documentation templates in the trauma service have shown improvement in CMI.8 An educational program on coding for internal medicine that included a lecture series and creation of a laminated pocket card listing common CMS diagnoses, CC, and MCC has been implemented, with an improvement in the capture rate of CC and MCC from 42% to 48% and an impact on expected mortality.9 This program resulted in a 30% decrease in the median quarterly mortality index and an increase in CMI from 1.27 to 1.36.
Our results show that there was an increase in comorbidities documentation of admitted patients after all interventions were implemented, more accurately reflecting the complexity of our patient population in a tertiary care academic medical center. Our CMI increased by 14% during the intervention period. The estimated CMI dollar impact increased by 75% from the pre-intervention period (adjusted for PPS-exempt hospital). The hospital-expected mortality increased from 1.77 to 3.07 (peak at 4.74 during third quarter of 2020) during the implementation period, which is a key driver of quality rankings for national outcomes reporting services such as US News & World Report.
There was increased physician satisfaction as a result of the change of functionality of the query response system, and no additional monetary provider incentive for complete documentation was allocated, apart from education and 1:1 support that improved physician engagement. Our next steps include the implementation of an advanced program to concurrently and automatically capture and nudge providers to respond and complete their documentation in real time.
Limitations
The limitations of our study include those inherent to a retrospective review and are associative and observational in nature. Although we used expected mortality and CMI as a surrogate for patient acuity for comparison, there was no way to control for actual changes in patient acuity that contributed to the increase in CMI, although we believe that the population we served and the services provided and their structure did not change significantly during the intervention period. Additionally, the observed increase in CMI during the implementation period may be a result of described variabilities in CMI and would be better studied over a longer period. Also, during the year of our interventions, 2020, we were affected by the COVID-19 pandemic. Patients with COVID-19 are known to carry a lower-than-expected mortality, and that could have had a negative impact on our results. In fact, we did observe a decrease in our expected mortality during the last quarter of 2020, which correlated with one of our regional peaks for COVID-19, and that could be a confounding factor. While the described intervention process is potentially applicable to multiple EHR systems, the exact form to capture the Elixhauser comorbidities was built into the Epic EHR, limiting external applicability of this tool to other EHR software.
Conclusion
A continuous comprehensive series of interventions substantially increased our patient acuity scores. The increased scores have implications for reimbursement and quality comparisons for hospitals and physicians. Our institution can now be stratified more accurately with our peers and other hospitals. Accurate medical record documentation has become increasingly important, but also increasingly complex. Leveraging the EHR through quality initiatives that facilitate the workflow for providers can have an impact on documentation, coding, and ultimately risk-adjusted outcomes data that influence institutional reputation.
Corresponding author: Marie Anne Sosa, MD; 1120 NW 14th St., Suite 809, Miami, FL, 33134; mxs2157@med.miami.edu
Disclosures: None reported.
doi:10.12788/jcom.0088
1. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
2. Sehgal AR. The role of reputation in U.S. News & World Report’s rankings of the top 50 American hospitals. Ann Intern Med. 2010;152(8):521-525. doi:10.7326/0003-4819-152-8-201004200-00009
3. Jha AK, DesRoches CM, Campbell EG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med. 2009;360(16):1628-1638. doi:10.1056/NEJMsa0900592.
4. Adler-Milstein J, DesRoches CM, Kralovec, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi:10.1377/hlthaff.2015.0992
5. Vizient Clinical Data Base/Resource ManagerTM. Irving, TX: Vizient, Inc.; 2019. Accessed March 10, 2022. https://www.vizientinc.com
6. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. doi:10.1097/MLR.0000000000000735
7. Payne T. Improving clinical documentation in an EMR world. Healthc Financ Manage. 2010;64(2):70-74.
8. Barnes SL, Waterman M, Macintyre D, Coughenour J, Kessel J. Impact of standardized trauma documentation to the hospital’s bottom line. Surgery. 2010;148(4):793-797. doi:10.1016/j.surg.2010.07.040
9. Spellberg B, Harrington D, Black S, Sue D, Stringer W, Witt M. Capturing the diagnosis: an internal medicine education program to improve documentation. Am J Med. 2013;126(8):739-743.e1. doi:10.1016/j.amjmed.2012.11.035
From the University of Miami Miller School of Medicine (Drs. Sosa, Ferreira, Gershengorn, Soto, Parekh, and Suarez), and the Quality Department of the University of Miami Hospital and Clinics (Estin Kelly, Ameena Shrestha, Julianne Burgos, and Sandeep Devabhaktuni), Miami, FL.
Abstract
Background: Case mix index (CMI) and expected mortality are determined based on comorbidities. Improving documentation and coding can impact performance indicators. During and prior to 2018, our patient acuity was under-represented, with low expected mortality and CMI. Those metrics motivated our quality team to develop the quality initiatives reported here.
Objectives: We sought to assess the impact of quality initiatives on number of comorbidities, diagnoses, CMI, and expected mortality at the University of Miami Health System.
Design: We conducted an observational study of a series of quality initiatives: (1) education of clinical documentation specialists (CDS) to capture comorbidities (10/2019); (2) facilitating the process for physician query response (2/2020); (3) implementation of computer logic to capture electrolyte disturbances and renal dysfunction (8/2020); (4) development of a tool to capture Elixhauser comorbidities (11/2020); and (5) provider education and electronic health record reviews by the quality team.
Setting and participants: All admissions during 2019 and 2020 at University of Miami Health System. The health system includes 2 academic inpatient facilities, a 560-bed tertiary hospital, and a 40-bed cancer facility. Our hospital is 1 of the 11 PPS-Exempt Cancer Hospitals and is the South Florida’s only NCI-Designated Cancer Center.
Conclusion:
Keywords: PS/QI, coding, case mix index, comorbidities, mortality.
Adoption of comprehensive electronic health record (EHR) systems by US hospitals, defined as an EHR capable of meeting all core meaningful-use metrics including evaluation and tracking of quality metrics, has been steadily increasing.3,4 Many institutions have looked to EHR system transitions as an inflection point to expand clinical documentation improvement (CDI) efforts. Over the past several years, our institution, an academic medical center, has endeavored to fully transition to a comprehensive EHR system (Epic from Epic Systems Corporation). Part of the purpose of this transition was to help study and improve outcomes, reduce readmissions, improve quality of care, and meet performance indicators.
Prior to 2019, our hospital’s patient acuity was low, with a CMI consistently below 2, ranging from 1.81 to 1.99, and an expected mortality consistently below 1.9%, ranging from 1.65% to 1.85%. Our concern that these values underestimated the real severity of illness of our patient population prompted the development of a quality improvement plan. In this report, we describe the processes we undertook to improve documentation and coding of comorbid illness, and report on the impact of these initiatives on performance indicators. We hypothesized that our initiatives would have a significant impact on our ability to capture patient complexity, and thus impact our CMI and expected mortality.
Methods
In the fall of 2019, we embarked on a multifaceted quality improvement project aimed at improving comorbidity capture for patients hospitalized at our institution. The health system includes 2 academic inpatient facilities, a 560-bed tertiary hospital and a 40-bed cancer facility. Since September 2017, we have used Epic as our EHR. In August 2019, we started working with Vizient Clinical Data Base5 to allow benchmarking with peer institutions. We assessed the impact of this initiative with a pre/post study design.
Quality Initiatives
This quality improvement project consisted of a series of 5 targeted interventions coupled with continuous monitoring and education.
1. Comorbidity coding. In October 2019, we met with the clinical documentation specialists (CDS) and the coding team to educate them on the value of coding all comorbidities that have an impact on
2. Physician query. In October 2019, we modified the process for physician query response, allowing physicians to answer queries in the EHR through a reply tool incorporated into the query and accept answers in the body of the Epic message as an active part of the EHR.
3. EHR logic. In August 2020, we developed an EHR smart logic to automatically capture fluid and electrolyte disturbances and renal dysfunction, based on the most recent laboratory values. The logic automatically populated potentially appropriate diagnoses in the assessment and plan of provider notes, which require provider acknowledgment and which providers are able to modify

4. Comorbidity capture tool. In November 2020, we developed a standardized tool to allow providers to easily capture Elixhauser comorbidities (eFigure 2). The Elixhauser index is a method for measuring comorbidities based on International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Disease, Tenth Revision diagnosis codes found in administrative data1-6 and is used by US News & World Report and Vizient to assess comorbidity burden. Our tool automatically captures diagnoses recorded in previous documentation and allows providers to easily provide the management plan for each; this information is automatically pulled into the provider note.

The development of this tool used an existing functionality within the Epic EHR called SmartForms, SmartData Elements, and SmartLinks. The only cost of tool development was the time invested—124 hours inclusive of 4 hours of staff education. Specifically, a panel of experts (including physicians of different specialties, an analyst, and representatives from the quality office) met weekly for 30 minutes per week over 5 weeks to agree on specific clinical criteria and guide the EHR build analyst. Individual panel members confirmed and validated design requirements (in 15 hours over 5 weeks). Our senior clinical analyst II dedicated 80 hours to actual build time, 15 hours to design time, and 25 hours to tailor the function to our institution’s workflow. This tool was introduced in November 2020; completion was optional at the time of hospital admission but mandatory at discharge to ensure compliance.
5. Quality team
Assessment of Quality Initiatives’ Impact
Data on the number of comorbidities and performance indicators were obtained retrospectively. The data included all hospital admissions from 2019 and 2020 divided into 2 periods: pre-intervention from January 1, 2019 through September 30, 2019, and intervention from October 1, 2019 through December 31, 2020. The primary outcome of this observational study was the rate of comorbidity capture during the intervention period. Comorbidity capture was assessed using the Vizient Clinical Data Base (CDB) health care performance tool.5 Vizient CDB uses the Agency for Healthcare Research and Quality Elixhauser index, which includes 29 of the initial 31 comorbidities described by Elixhauser,6 as it combines hypertension with and without complications into one. We secondarily aimed to examine the impact of the quality improvement initiatives on several institutional-level performance indicators, including total number of diagnoses, comorbidities or complications (CC), major comorbidities or complications (MCC), CMI, and expected mortality.
Case mix index is the average Medicare Severity-DRG (MS-DRG) weighted across all hospital discharges (appropriate to their discharge date). The expected mortality represents the average expected number of deaths based on diagnosed conditions, age, and gender within the same time frame, and it is based on coded diagnosis; we obtained the mortality index by dividing the observed mortality by the expected mortality. The Vizient CDB Mortality Risk Adjustment Model was used to assign an expected mortality (0%-100%) to each case based on factors such as demographics, admission type, diagnoses, and procedures.
Standard statistics were used to measure the outcomes. We used Excel to compare pre-intervention and intervention period characteristics and outcomes, using t-testing for continuous variables and Chi-square testing for categorial outcomes. P values <0.05 were considered statistically significant.
The study was reviewed by the institutional review board (IRB) of our institution (IRB ID: 20210070). The IRB determined that the proposed activity was not research involving human subjects, as defined by the Department of Health and Human Services and US Food and Drug Administration regulations, and that IRB review and approval by the organization were not required.
Results
The health system had a total of 33 066 admissions during the study period—13 689 pre-intervention (January 1, 2019 through September 30, 2019) and 19,377 during the intervention period (October 1, 2019 to December 31, 2020). Demographics were similar among the pre-intervention and intervention periods: mean age was 60 years and 61 years, 52% and 51% of patients were male, 72% and 71% were White, and 20% and 19% were Black, respectively (Table 1).

The multifaceted intervention resulted in a significant improvement in the primary outcome: mean comorbidity capture increased from 2.5 (SD, 1.7) before the intervention to 3.1 (SD, 2.0) during the intervention (P < .00001). Secondary outcomes also improved. The mean number of secondary diagnoses for admissions increased from 11.3 (SD, 7.3) prior to the intervention to 18.5 (SD, 10.4) (P < .00001) during the intervention period. The mean CMI increased from 2.1 (SD, 1.9) to 2.4 (SD, 2.2) post intervention (P < .00001), an increase during the intervention period of 14%. The expected mortality increased from 1.8% (SD, 6.1%) to 3.1% (SD, 9.2%) after the intervention (P < .00001) (Table 2).

There was an overall observed improvement in percentage of discharges with documented CC and MCC for both surgical and medical specialties. Both CC and MCC increased for surgical specialties, from 54.4% to 68.5%, and for medical specialties, from 68.9% to 76.4%. (Figure 1). The diagnoses that were captured more consistently included deficiency anemia, obesity, diabetes with complications, fluid and electrolyte disorders and renal failure, hypertension, weight loss, depression, and hypothyroidism (Figure 2).



During the 9-month pre-intervention period (January 1 through September 30, 2019), there were 2795 queries, with an agreed volume of 1823; the agreement rate was 65% and the average provider turnaround time was 12.53 days. In the 15-month postintervention period, there were 10 216 queries, with an agreed volume of 6802 at 66%. We created a policy to encourage responses no later than 10 days after the query, and our average turnaround time decreased by more than 50% to 5.86 days. The average number of monthly queries increased by 55%, from an average of 311 monthly queries in the pre-intervention period to an average of 681 per month in the postintervention period. The more common queries that had an impact on CMI included sepsis, antineoplastic chemotherapy–induced pancytopenia, acute posthemorrhagic anemia, malnutrition, hyponatremia, and metabolic encephalopathy.
Discussion
The need for accurate documentation by physicians has been recognized for many years.7
With the growing complexity of the documentation and coding process, it is difficult for clinicians to keep up with the terminology required by the Centers for Medicare and Medicaid Services (CMS). Several different methods to improve documentation have been proposed. Prior interventions to standardize documentation templates in the trauma service have shown improvement in CMI.8 An educational program on coding for internal medicine that included a lecture series and creation of a laminated pocket card listing common CMS diagnoses, CC, and MCC has been implemented, with an improvement in the capture rate of CC and MCC from 42% to 48% and an impact on expected mortality.9 This program resulted in a 30% decrease in the median quarterly mortality index and an increase in CMI from 1.27 to 1.36.
Our results show that there was an increase in comorbidities documentation of admitted patients after all interventions were implemented, more accurately reflecting the complexity of our patient population in a tertiary care academic medical center. Our CMI increased by 14% during the intervention period. The estimated CMI dollar impact increased by 75% from the pre-intervention period (adjusted for PPS-exempt hospital). The hospital-expected mortality increased from 1.77 to 3.07 (peak at 4.74 during third quarter of 2020) during the implementation period, which is a key driver of quality rankings for national outcomes reporting services such as US News & World Report.
There was increased physician satisfaction as a result of the change of functionality of the query response system, and no additional monetary provider incentive for complete documentation was allocated, apart from education and 1:1 support that improved physician engagement. Our next steps include the implementation of an advanced program to concurrently and automatically capture and nudge providers to respond and complete their documentation in real time.
Limitations
The limitations of our study include those inherent to a retrospective review and are associative and observational in nature. Although we used expected mortality and CMI as a surrogate for patient acuity for comparison, there was no way to control for actual changes in patient acuity that contributed to the increase in CMI, although we believe that the population we served and the services provided and their structure did not change significantly during the intervention period. Additionally, the observed increase in CMI during the implementation period may be a result of described variabilities in CMI and would be better studied over a longer period. Also, during the year of our interventions, 2020, we were affected by the COVID-19 pandemic. Patients with COVID-19 are known to carry a lower-than-expected mortality, and that could have had a negative impact on our results. In fact, we did observe a decrease in our expected mortality during the last quarter of 2020, which correlated with one of our regional peaks for COVID-19, and that could be a confounding factor. While the described intervention process is potentially applicable to multiple EHR systems, the exact form to capture the Elixhauser comorbidities was built into the Epic EHR, limiting external applicability of this tool to other EHR software.
Conclusion
A continuous comprehensive series of interventions substantially increased our patient acuity scores. The increased scores have implications for reimbursement and quality comparisons for hospitals and physicians. Our institution can now be stratified more accurately with our peers and other hospitals. Accurate medical record documentation has become increasingly important, but also increasingly complex. Leveraging the EHR through quality initiatives that facilitate the workflow for providers can have an impact on documentation, coding, and ultimately risk-adjusted outcomes data that influence institutional reputation.
Corresponding author: Marie Anne Sosa, MD; 1120 NW 14th St., Suite 809, Miami, FL, 33134; mxs2157@med.miami.edu
Disclosures: None reported.
doi:10.12788/jcom.0088
From the University of Miami Miller School of Medicine (Drs. Sosa, Ferreira, Gershengorn, Soto, Parekh, and Suarez), and the Quality Department of the University of Miami Hospital and Clinics (Estin Kelly, Ameena Shrestha, Julianne Burgos, and Sandeep Devabhaktuni), Miami, FL.
Abstract
Background: Case mix index (CMI) and expected mortality are determined based on comorbidities. Improving documentation and coding can impact performance indicators. During and prior to 2018, our patient acuity was under-represented, with low expected mortality and CMI. Those metrics motivated our quality team to develop the quality initiatives reported here.
Objectives: We sought to assess the impact of quality initiatives on number of comorbidities, diagnoses, CMI, and expected mortality at the University of Miami Health System.
Design: We conducted an observational study of a series of quality initiatives: (1) education of clinical documentation specialists (CDS) to capture comorbidities (10/2019); (2) facilitating the process for physician query response (2/2020); (3) implementation of computer logic to capture electrolyte disturbances and renal dysfunction (8/2020); (4) development of a tool to capture Elixhauser comorbidities (11/2020); and (5) provider education and electronic health record reviews by the quality team.
Setting and participants: All admissions during 2019 and 2020 at University of Miami Health System. The health system includes 2 academic inpatient facilities, a 560-bed tertiary hospital, and a 40-bed cancer facility. Our hospital is 1 of the 11 PPS-Exempt Cancer Hospitals and is the South Florida’s only NCI-Designated Cancer Center.
Conclusion:
Keywords: PS/QI, coding, case mix index, comorbidities, mortality.
Adoption of comprehensive electronic health record (EHR) systems by US hospitals, defined as an EHR capable of meeting all core meaningful-use metrics including evaluation and tracking of quality metrics, has been steadily increasing.3,4 Many institutions have looked to EHR system transitions as an inflection point to expand clinical documentation improvement (CDI) efforts. Over the past several years, our institution, an academic medical center, has endeavored to fully transition to a comprehensive EHR system (Epic from Epic Systems Corporation). Part of the purpose of this transition was to help study and improve outcomes, reduce readmissions, improve quality of care, and meet performance indicators.
Prior to 2019, our hospital’s patient acuity was low, with a CMI consistently below 2, ranging from 1.81 to 1.99, and an expected mortality consistently below 1.9%, ranging from 1.65% to 1.85%. Our concern that these values underestimated the real severity of illness of our patient population prompted the development of a quality improvement plan. In this report, we describe the processes we undertook to improve documentation and coding of comorbid illness, and report on the impact of these initiatives on performance indicators. We hypothesized that our initiatives would have a significant impact on our ability to capture patient complexity, and thus impact our CMI and expected mortality.
Methods
In the fall of 2019, we embarked on a multifaceted quality improvement project aimed at improving comorbidity capture for patients hospitalized at our institution. The health system includes 2 academic inpatient facilities, a 560-bed tertiary hospital and a 40-bed cancer facility. Since September 2017, we have used Epic as our EHR. In August 2019, we started working with Vizient Clinical Data Base5 to allow benchmarking with peer institutions. We assessed the impact of this initiative with a pre/post study design.
Quality Initiatives
This quality improvement project consisted of a series of 5 targeted interventions coupled with continuous monitoring and education.
1. Comorbidity coding. In October 2019, we met with the clinical documentation specialists (CDS) and the coding team to educate them on the value of coding all comorbidities that have an impact on
2. Physician query. In October 2019, we modified the process for physician query response, allowing physicians to answer queries in the EHR through a reply tool incorporated into the query and accept answers in the body of the Epic message as an active part of the EHR.
3. EHR logic. In August 2020, we developed an EHR smart logic to automatically capture fluid and electrolyte disturbances and renal dysfunction, based on the most recent laboratory values. The logic automatically populated potentially appropriate diagnoses in the assessment and plan of provider notes, which require provider acknowledgment and which providers are able to modify

4. Comorbidity capture tool. In November 2020, we developed a standardized tool to allow providers to easily capture Elixhauser comorbidities (eFigure 2). The Elixhauser index is a method for measuring comorbidities based on International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Disease, Tenth Revision diagnosis codes found in administrative data1-6 and is used by US News & World Report and Vizient to assess comorbidity burden. Our tool automatically captures diagnoses recorded in previous documentation and allows providers to easily provide the management plan for each; this information is automatically pulled into the provider note.

The development of this tool used an existing functionality within the Epic EHR called SmartForms, SmartData Elements, and SmartLinks. The only cost of tool development was the time invested—124 hours inclusive of 4 hours of staff education. Specifically, a panel of experts (including physicians of different specialties, an analyst, and representatives from the quality office) met weekly for 30 minutes per week over 5 weeks to agree on specific clinical criteria and guide the EHR build analyst. Individual panel members confirmed and validated design requirements (in 15 hours over 5 weeks). Our senior clinical analyst II dedicated 80 hours to actual build time, 15 hours to design time, and 25 hours to tailor the function to our institution’s workflow. This tool was introduced in November 2020; completion was optional at the time of hospital admission but mandatory at discharge to ensure compliance.
5. Quality team
Assessment of Quality Initiatives’ Impact
Data on the number of comorbidities and performance indicators were obtained retrospectively. The data included all hospital admissions from 2019 and 2020 divided into 2 periods: pre-intervention from January 1, 2019 through September 30, 2019, and intervention from October 1, 2019 through December 31, 2020. The primary outcome of this observational study was the rate of comorbidity capture during the intervention period. Comorbidity capture was assessed using the Vizient Clinical Data Base (CDB) health care performance tool.5 Vizient CDB uses the Agency for Healthcare Research and Quality Elixhauser index, which includes 29 of the initial 31 comorbidities described by Elixhauser,6 as it combines hypertension with and without complications into one. We secondarily aimed to examine the impact of the quality improvement initiatives on several institutional-level performance indicators, including total number of diagnoses, comorbidities or complications (CC), major comorbidities or complications (MCC), CMI, and expected mortality.
Case mix index is the average Medicare Severity-DRG (MS-DRG) weighted across all hospital discharges (appropriate to their discharge date). The expected mortality represents the average expected number of deaths based on diagnosed conditions, age, and gender within the same time frame, and it is based on coded diagnosis; we obtained the mortality index by dividing the observed mortality by the expected mortality. The Vizient CDB Mortality Risk Adjustment Model was used to assign an expected mortality (0%-100%) to each case based on factors such as demographics, admission type, diagnoses, and procedures.
Standard statistics were used to measure the outcomes. We used Excel to compare pre-intervention and intervention period characteristics and outcomes, using t-testing for continuous variables and Chi-square testing for categorial outcomes. P values <0.05 were considered statistically significant.
The study was reviewed by the institutional review board (IRB) of our institution (IRB ID: 20210070). The IRB determined that the proposed activity was not research involving human subjects, as defined by the Department of Health and Human Services and US Food and Drug Administration regulations, and that IRB review and approval by the organization were not required.
Results
The health system had a total of 33 066 admissions during the study period—13 689 pre-intervention (January 1, 2019 through September 30, 2019) and 19,377 during the intervention period (October 1, 2019 to December 31, 2020). Demographics were similar among the pre-intervention and intervention periods: mean age was 60 years and 61 years, 52% and 51% of patients were male, 72% and 71% were White, and 20% and 19% were Black, respectively (Table 1).

The multifaceted intervention resulted in a significant improvement in the primary outcome: mean comorbidity capture increased from 2.5 (SD, 1.7) before the intervention to 3.1 (SD, 2.0) during the intervention (P < .00001). Secondary outcomes also improved. The mean number of secondary diagnoses for admissions increased from 11.3 (SD, 7.3) prior to the intervention to 18.5 (SD, 10.4) (P < .00001) during the intervention period. The mean CMI increased from 2.1 (SD, 1.9) to 2.4 (SD, 2.2) post intervention (P < .00001), an increase during the intervention period of 14%. The expected mortality increased from 1.8% (SD, 6.1%) to 3.1% (SD, 9.2%) after the intervention (P < .00001) (Table 2).

There was an overall observed improvement in percentage of discharges with documented CC and MCC for both surgical and medical specialties. Both CC and MCC increased for surgical specialties, from 54.4% to 68.5%, and for medical specialties, from 68.9% to 76.4%. (Figure 1). The diagnoses that were captured more consistently included deficiency anemia, obesity, diabetes with complications, fluid and electrolyte disorders and renal failure, hypertension, weight loss, depression, and hypothyroidism (Figure 2).



During the 9-month pre-intervention period (January 1 through September 30, 2019), there were 2795 queries, with an agreed volume of 1823; the agreement rate was 65% and the average provider turnaround time was 12.53 days. In the 15-month postintervention period, there were 10 216 queries, with an agreed volume of 6802 at 66%. We created a policy to encourage responses no later than 10 days after the query, and our average turnaround time decreased by more than 50% to 5.86 days. The average number of monthly queries increased by 55%, from an average of 311 monthly queries in the pre-intervention period to an average of 681 per month in the postintervention period. The more common queries that had an impact on CMI included sepsis, antineoplastic chemotherapy–induced pancytopenia, acute posthemorrhagic anemia, malnutrition, hyponatremia, and metabolic encephalopathy.
Discussion
The need for accurate documentation by physicians has been recognized for many years.7
With the growing complexity of the documentation and coding process, it is difficult for clinicians to keep up with the terminology required by the Centers for Medicare and Medicaid Services (CMS). Several different methods to improve documentation have been proposed. Prior interventions to standardize documentation templates in the trauma service have shown improvement in CMI.8 An educational program on coding for internal medicine that included a lecture series and creation of a laminated pocket card listing common CMS diagnoses, CC, and MCC has been implemented, with an improvement in the capture rate of CC and MCC from 42% to 48% and an impact on expected mortality.9 This program resulted in a 30% decrease in the median quarterly mortality index and an increase in CMI from 1.27 to 1.36.
Our results show that there was an increase in comorbidities documentation of admitted patients after all interventions were implemented, more accurately reflecting the complexity of our patient population in a tertiary care academic medical center. Our CMI increased by 14% during the intervention period. The estimated CMI dollar impact increased by 75% from the pre-intervention period (adjusted for PPS-exempt hospital). The hospital-expected mortality increased from 1.77 to 3.07 (peak at 4.74 during third quarter of 2020) during the implementation period, which is a key driver of quality rankings for national outcomes reporting services such as US News & World Report.
There was increased physician satisfaction as a result of the change of functionality of the query response system, and no additional monetary provider incentive for complete documentation was allocated, apart from education and 1:1 support that improved physician engagement. Our next steps include the implementation of an advanced program to concurrently and automatically capture and nudge providers to respond and complete their documentation in real time.
Limitations
The limitations of our study include those inherent to a retrospective review and are associative and observational in nature. Although we used expected mortality and CMI as a surrogate for patient acuity for comparison, there was no way to control for actual changes in patient acuity that contributed to the increase in CMI, although we believe that the population we served and the services provided and their structure did not change significantly during the intervention period. Additionally, the observed increase in CMI during the implementation period may be a result of described variabilities in CMI and would be better studied over a longer period. Also, during the year of our interventions, 2020, we were affected by the COVID-19 pandemic. Patients with COVID-19 are known to carry a lower-than-expected mortality, and that could have had a negative impact on our results. In fact, we did observe a decrease in our expected mortality during the last quarter of 2020, which correlated with one of our regional peaks for COVID-19, and that could be a confounding factor. While the described intervention process is potentially applicable to multiple EHR systems, the exact form to capture the Elixhauser comorbidities was built into the Epic EHR, limiting external applicability of this tool to other EHR software.
Conclusion
A continuous comprehensive series of interventions substantially increased our patient acuity scores. The increased scores have implications for reimbursement and quality comparisons for hospitals and physicians. Our institution can now be stratified more accurately with our peers and other hospitals. Accurate medical record documentation has become increasingly important, but also increasingly complex. Leveraging the EHR through quality initiatives that facilitate the workflow for providers can have an impact on documentation, coding, and ultimately risk-adjusted outcomes data that influence institutional reputation.
Corresponding author: Marie Anne Sosa, MD; 1120 NW 14th St., Suite 809, Miami, FL, 33134; mxs2157@med.miami.edu
Disclosures: None reported.
doi:10.12788/jcom.0088
1. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
2. Sehgal AR. The role of reputation in U.S. News & World Report’s rankings of the top 50 American hospitals. Ann Intern Med. 2010;152(8):521-525. doi:10.7326/0003-4819-152-8-201004200-00009
3. Jha AK, DesRoches CM, Campbell EG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med. 2009;360(16):1628-1638. doi:10.1056/NEJMsa0900592.
4. Adler-Milstein J, DesRoches CM, Kralovec, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi:10.1377/hlthaff.2015.0992
5. Vizient Clinical Data Base/Resource ManagerTM. Irving, TX: Vizient, Inc.; 2019. Accessed March 10, 2022. https://www.vizientinc.com
6. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. doi:10.1097/MLR.0000000000000735
7. Payne T. Improving clinical documentation in an EMR world. Healthc Financ Manage. 2010;64(2):70-74.
8. Barnes SL, Waterman M, Macintyre D, Coughenour J, Kessel J. Impact of standardized trauma documentation to the hospital’s bottom line. Surgery. 2010;148(4):793-797. doi:10.1016/j.surg.2010.07.040
9. Spellberg B, Harrington D, Black S, Sue D, Stringer W, Witt M. Capturing the diagnosis: an internal medicine education program to improve documentation. Am J Med. 2013;126(8):739-743.e1. doi:10.1016/j.amjmed.2012.11.035
1. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
2. Sehgal AR. The role of reputation in U.S. News & World Report’s rankings of the top 50 American hospitals. Ann Intern Med. 2010;152(8):521-525. doi:10.7326/0003-4819-152-8-201004200-00009
3. Jha AK, DesRoches CM, Campbell EG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med. 2009;360(16):1628-1638. doi:10.1056/NEJMsa0900592.
4. Adler-Milstein J, DesRoches CM, Kralovec, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi:10.1377/hlthaff.2015.0992
5. Vizient Clinical Data Base/Resource ManagerTM. Irving, TX: Vizient, Inc.; 2019. Accessed March 10, 2022. https://www.vizientinc.com
6. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. doi:10.1097/MLR.0000000000000735
7. Payne T. Improving clinical documentation in an EMR world. Healthc Financ Manage. 2010;64(2):70-74.
8. Barnes SL, Waterman M, Macintyre D, Coughenour J, Kessel J. Impact of standardized trauma documentation to the hospital’s bottom line. Surgery. 2010;148(4):793-797. doi:10.1016/j.surg.2010.07.040
9. Spellberg B, Harrington D, Black S, Sue D, Stringer W, Witt M. Capturing the diagnosis: an internal medicine education program to improve documentation. Am J Med. 2013;126(8):739-743.e1. doi:10.1016/j.amjmed.2012.11.035
A Practical and Cost-Effective Approach to the Diagnosis of Heparin-Induced Thrombocytopenia: A Single-Center Quality Improvement Study
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
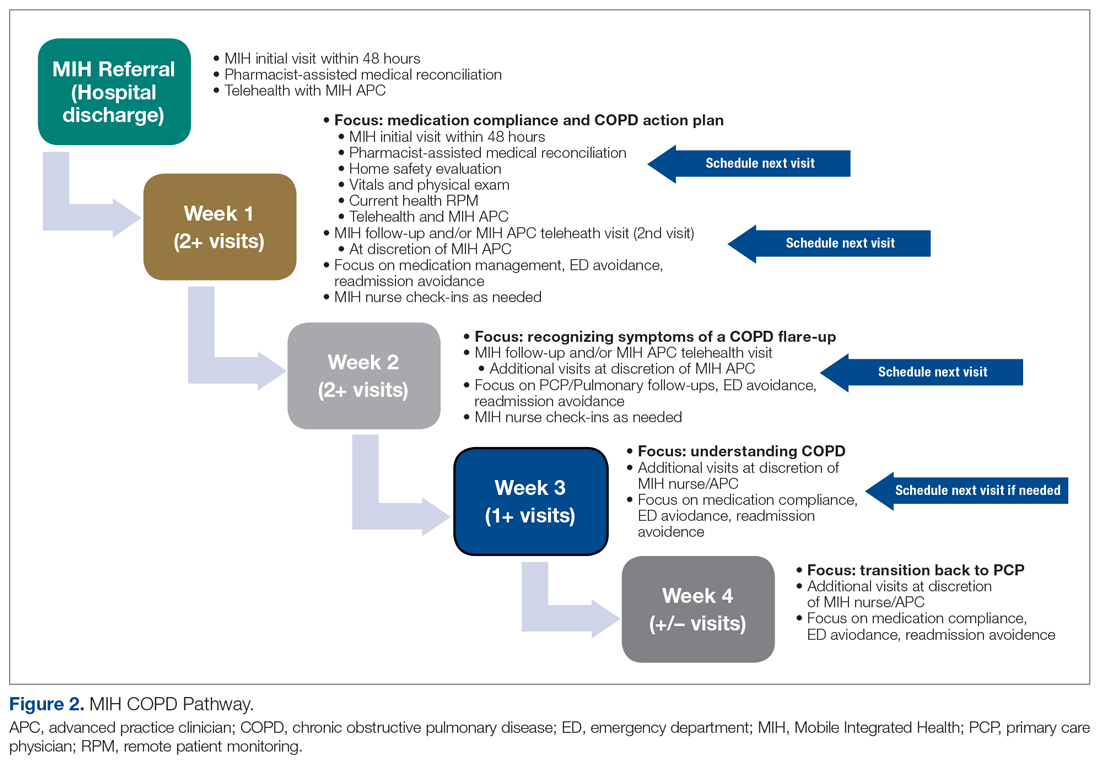
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; mccoyag@med.umich.edu
Disclosures: None reported.
doi: 10.12788/jcom.0087
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; mccoyag@med.umich.edu
Disclosures: None reported.
doi: 10.12788/jcom.0087
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; mccoyag@med.umich.edu
Disclosures: None reported.
doi: 10.12788/jcom.0087
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; meisenberg@AAHS.org.
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; meisenberg@AAHS.org.
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; meisenberg@AAHS.org.
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Successful COVID-19 Surge Management With Monoclonal Antibody Infusion in Emergency Department Patients
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
The Use of Nasogastric Tube Bridle Kits in COVID-19 Intensive Care Unit Patients
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).
The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; r.atkar@nhs.net.
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).
The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; r.atkar@nhs.net.
Financial disclosures: None.
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).
The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; r.atkar@nhs.net.
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
Positive Outcomes Following a Multidisciplinary Approach in the Diagnosis and Prevention of Hospital Delirium
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens
Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.
Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.
In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.
Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.
Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.
Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; jason.ching@cshs.org.
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens
Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.
Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.
In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.
Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.
Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.
Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; jason.ching@cshs.org.
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens
Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.
Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.
In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.
Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.
Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.
Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; jason.ching@cshs.org.
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
Mobile Integrated Health: Reducing Chronic Obstructive Pulmonary Disease Hospitalizations Through Novel Outpatient Care Initiatives
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; klannutti@southshorehealth.org.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; klannutti@southshorehealth.org.
Financial disclosures: None.
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; klannutti@southshorehealth.org.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
Virtual Visitation: Exploring the Impact on Patients and Families During COVID-19 and Beyond
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; nfilippa@northwell.edu.
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; nfilippa@northwell.edu.
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; nfilippa@northwell.edu.
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
Improving Physicians’ Bowel Documentation on Geriatric Wards
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002