User login
Effect of In-Office Samples on Dermatologists’ Prescribing Habits: A Retrospective Review
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
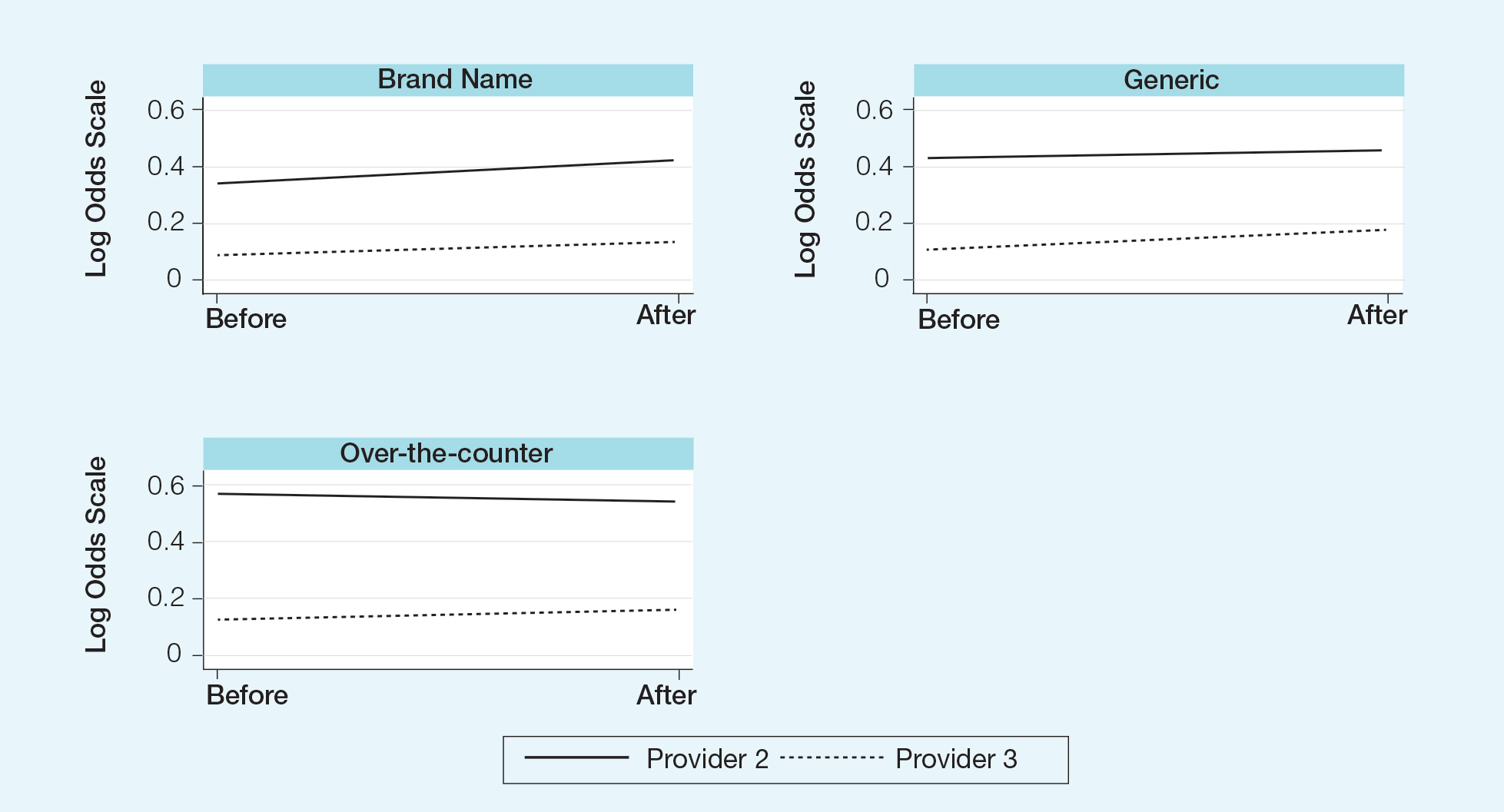
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
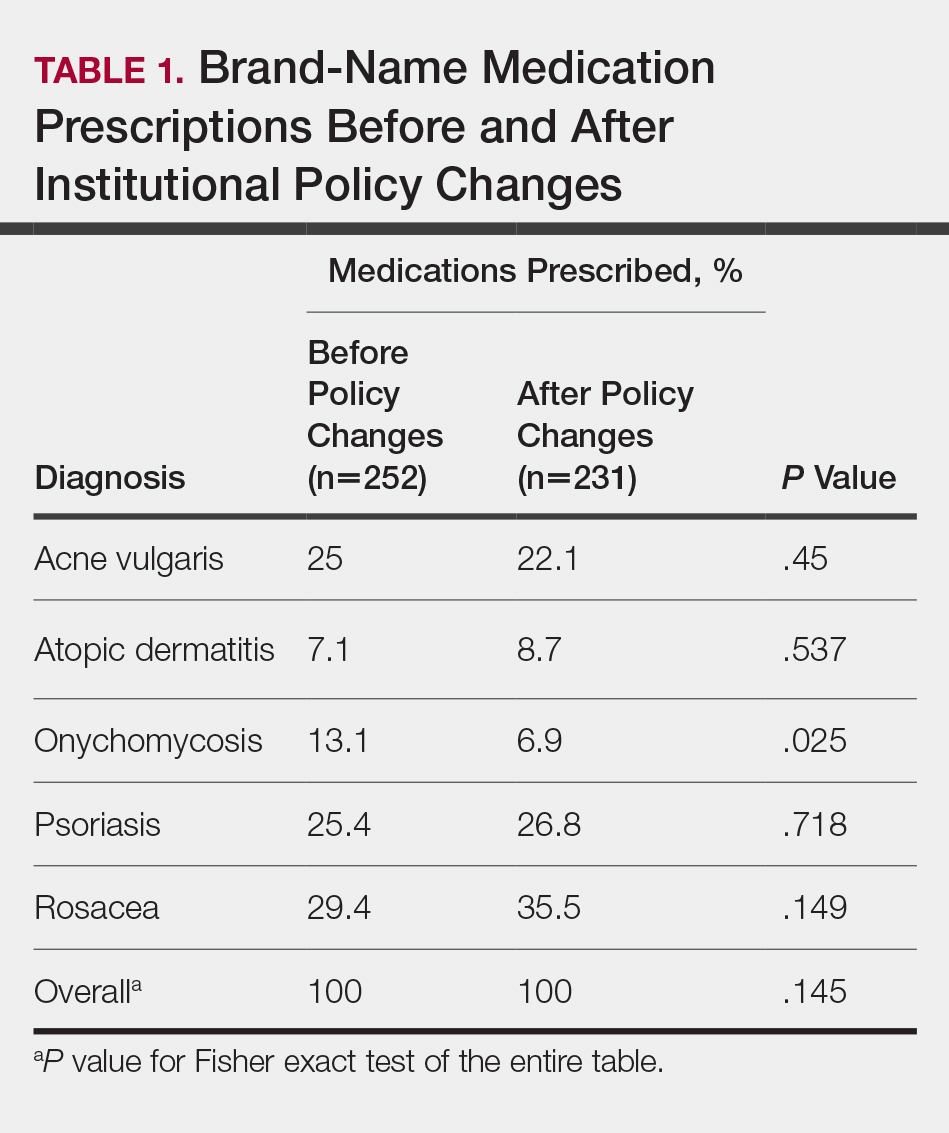
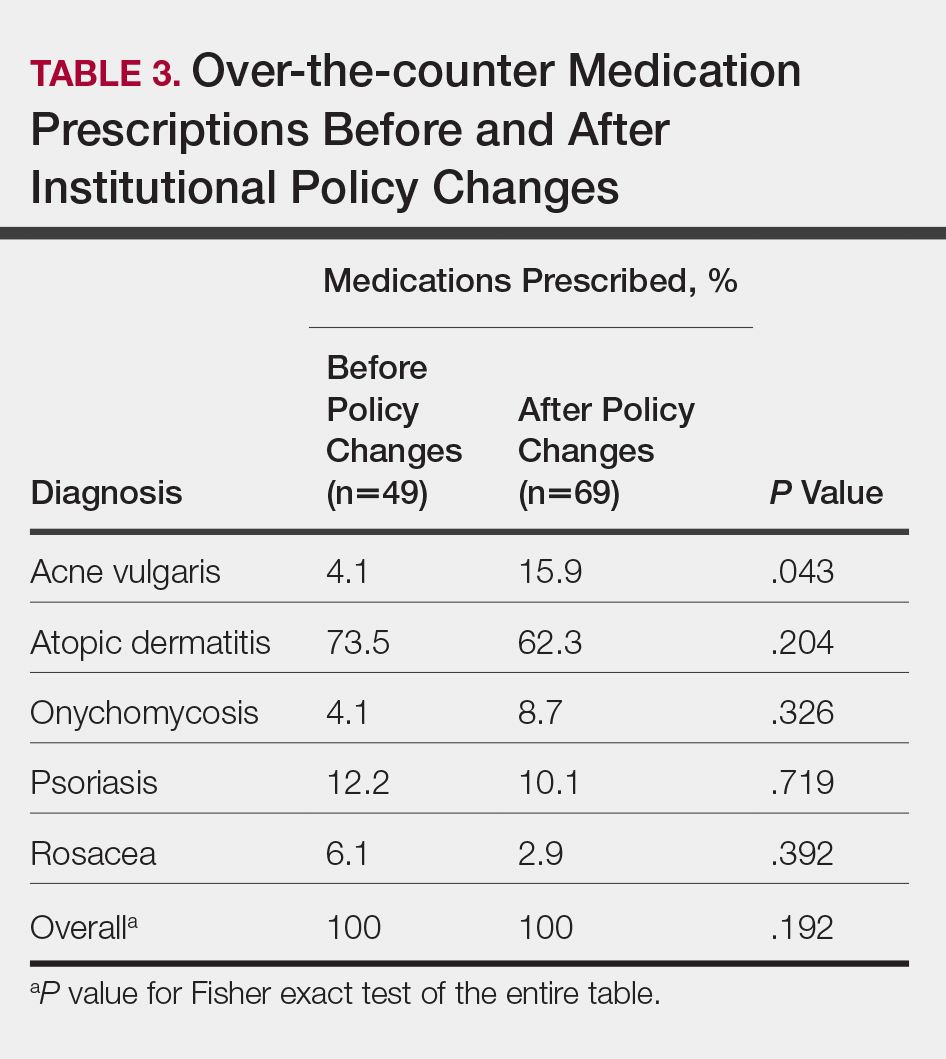
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).
Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).
Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
Over the years, there has been growing concern about the relationship between physicians and pharmaceutical companies. Many studies have demonstrated that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.1-3 As a result, many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians. Although these policies can vary greatly, they generally limit access of pharmaceutical representatives to providers and restrict pharmaceutical samples.4,5 This policy shift has even been reported in private practice.6
At the heart of the matter is the question: What really influences physicians to write a prescription for a particular medication? Is it cost, efficacy, or representatives pushing a product? Prior studies illustrate that generic medications are equivalent to their brand-name counterparts. In fact, current regulations require no more than 5% to 7% difference in bioequivalence.7-9 Although most generic medications are bioequivalent, it may not be universal.10
Garrison and Levin11 distributed a survey to US-based prescribers in family practice, psychiatry, and internal medicine and found that prescribers deemed patient response and success as the highest priority when determining which drugs to prescribe. In contrast, drug representatives and free samples only slightly contributed.11 Considering the minimum duration for efficacy of a medication such as an antidepressant vs a topical steroid, this pattern may differ with samples in dermatologic settings. Interestingly, another survey concluded that samples were associated with “sticky” prescribing habits, noting that physicians would prescribe a brand-name medication after using a sample, despite increased cost to the patient.12 Further, it has been suggested that recipients of free samples may experience increased costs in the long run, which contrasts a stated goal of affordability to patients.12,13
Physician interaction with pharmaceutical companies begins as early as medical school,14 with physicians reporting interactions as often as 4 times each month.14-18 Interactions can include meetings with pharmaceutical representatives, sponsored meals, gifts, continuing medical education sponsorship, funding for travel, pharmaceutical representative speakers, research funding, and drug samples.3
A 2014 study reported that prescribing habits are influenced by the free drug samples provided by nongeneric pharmaceutical companies.19 Nationally, the number of brand-name and branded generic medications constitute 79% of prescriptions, yet together they only comprise 17% of medications prescribed at an academic medical clinic that does not provide samples. The number of medications with samples being prescribed by dermatologists increased by 15% over 9 years, which may correlate with the wider availability of medication samples, more specifically an increase in branded generic samples.19 This potential interaction is the reason why institutions question the current influence of pharmaceutical companies. Samples may appear convenient, allowing a patient to test the medication prior to committing; however, with brand-name samples being provided to the physician, he/she may become more inclined to prescribe the branded medication.12,15,19-22 Because brand-name medications are more expensive than generic medications, this practice can increase the cost of health care.13 One study found that over 1 year, the overuse of nongeneric medications led to a loss of potential savings throughout 49 states, equating to $229 million just through Medicaid; interestingly, it was noted that in some states, a maximum reimbursement is set by Medicaid, regardless of whether the generic or branded medication is dispensed. The authors also noted variability in the potential savings by state, which may be a function of the state-by-state maximum reimbursements for certain medications.23 Another study on oral combination medications estimated Medicare spending on branded drugs relative to the cost if generic combinations had been purchased instead. This study examined branded medications for which the active components were available as over-the-counter (OTC), generic, or same-class generic, and the authors estimated that $925 million could have been saved in 2016 by purchasing a generic substitute.24 The overuse of nongeneric medications when generic alternatives are available becomes an issue that not only financially impacts patients but all taxpayers. However, this pattern may differ if limited only to dermatologic medications, which was not the focus of the prior studies.
To limit conflicts of interest in interactions with the pharmaceutical, medical device, and biotechnology industries, the University of South Florida (USF) Morsani College of Medicine (COM)(Tampa, Florida) implemented its own set of regulations that eliminated in-office pharmaceutical samples, in addition to other restrictions. This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after their medical school implemented these new policies.
We hypothesized that the number of brand-name drugs prescribed by physicians in the Department of Dermatology & Cutaneous Surgery would change following USF Morsani COM pharmaceutical policy changes. We sought to determine how physician prescribing practices within the Department of Dermatology & Cutaneous Surgery changed following USF Morsani COM pharmaceutical policy changes.
Methods
Data Collection
A retrospective review of medical records was conducted to investigate the effect of the USF Morsani COM pharmaceutical policy changes on physician prescribing practices within the Department of Dermatology & Cutaneous Surgery. Medical records of patients seen for common dermatology diagnoses before (January 1, 2010, to May 30, 2010) and after (August 1, 2011, to December 31, 2011) the pharmaceutical policy changes were reviewed, and all medications prescribed were recorded. Data were collected from medical records within the USF Health electronic medical record system and included visits with each of the department’s 3 attending dermatologists. The diagnoses included in the study—acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, and rosacea—were chosen because in-office samples were available. Prescribing data from the first 100 consecutive medical records were collected from each time period, and a medical record was included only if it contained at least 1 of the following diagnoses: acne vulgaris, atopic dermatitis, onychomycosis, psoriasis, or rosacea. The assessment and plan of each progress note were reviewed, and the exact medication name and associated diagnosis were recorded for each prescription. Subsequently, each medication was reviewed and placed in 1 of 3 categories: brand name, generic, and OTC. The total number of prescriptions for each diagnosis (per visit/note); the specific number of brand, generic, and OTC medications prescribed (per visit/note); and the percentage of brand, generic, and OTC medications prescribed (per visit/note and per diagnosis in total) were calculated. To ensure only intended medications were included, each medication recorded in the medical record note was cross-referenced with the prescribed medication in the electronic medical record. The primary objective of this study was to capture the prescribing physician’s intent as proxied by the pattern of prescription. Thus, changes made in prescriptions after the initial plan—whether insurance related or otherwise—were not relevant to this investigation.
The data were collected to compare the percentage of brand vs generic or OTC prescriptions per diagnosis to see if there was a difference in the prescribing habits before and after the pharmaceutical policy changes. Of note, several other pieces of data were collected from each medical record, including age, race, class of insurance (ie, Medicare, Medicaid, private health maintenance organization, private preferred provider organization), subtype diagnoses, and whether the prescription was new or a refill. The information gathered from the written record on the assessment and plan was verified using prescriptions ordered in the Allscripts electronic record, and any difference was noted. No identifying information that could be used to easily identify study participants was recorded.
Differences in prescribing habits across diagnoses before and after the policy changes were ascertained using a Fisher exact test and were further assessed using a mixed effects ordinal logistic regression model that accounted for within-provider clustering and baseline patient characteristics. An ordinal model was chosen to recognize differences in average cost among brand-name, generic, and OTC medications.
Results
In total, 200 medical records were collected. For the period analyzed before the policy change, 252 brand-name medications were prescribed compared to 231 prescribed for the period analyzed after the policy changes. There was insufficient evidence of an overall difference in brand-name medications prescribed before and after the policy changes (P=.145; Fisher exact test)(Table 1). There also was insufficient evidence of an overall difference in generic prescriptions, which totaled 153 before and 134 after the policy changes (P=.872; Fisher exact test)(Table 2). Over-the-counter prescriptions totaled 49 before and 69 after the policy changes. There was insufficient evidence of an overall difference before and after the policy changes for OTC medications (P=.192; Fisher exact test)(Table 3).
Comment
Although some medical institutions are diligently working to limit the potential influence pharmaceutical companies have on physician prescribing habits,4,5,25 the effect on physician prescribing habits is only now being established.15 Prior studies12,19,21 have found evidence that medication samples may lead to overuse of brand-name medications, but these findings do not hold true for the USF dermatologists included in this study, perhaps due to the difference in pharmaceutical company interactions or physicians maintaining prior prescription habits that were unrelated to the policy. Although this study focused on policy changes for in-office samples, prior studies either included other forms of interaction21 or did not include samples.22
Pharmaceutical samples allow patients to try a medication before committing to a long-term course of treatment with a particular medication, which has utility for physicians and patients. Although brand-name prescriptions may cost more, a trial period may assist the patient in deciding whether the medication is worth purchasing. Furthermore, physicians may feel more comfortable prescribing a medication once the individual patient has demonstrated a benefit from the sample, which may be particularly true in a specialty such as dermatology in which many branded topical medications contain a different vehicle than generic formulations, resulting in notable variations in active medication delivery and efficacy. Given the higher cost of branded topical medications, proving efficacy in patients through samples can provide a useful tool to the physician to determine the need for a branded formulation.
The benefits described are subjective but should not be disregarded. Although Hurley et al19 found that the number of brand-name medications prescribed increases as more samples are given out, our study demonstrated that after eliminating medication samples, there was no significant difference in the percentage of brand-name medications prescribed compared to generic and OTC medications.
Physician education concerning the price of each brand-name medication prescribed in office may be one method of reducing the amount of such prescriptions. Physicians generally are uninformed of the cost of the medications being prescribed26 and may not recognize the financial burden one medication may have compared to its alternative. However, educating physicians will empower them to make the conscious decision to prefer or not prefer a brand-name medication. With some generic medications shown to have a difference in bioequivalence compared to their brand-name counterparts, a physician may find more success prescribing the brand-name medications, regardless of pharmaceutical company influence, which is an alternative solution to policy changes that eliminate samples entirely. Although this study found insufficient evidence that removing samples decreases brand-name medication prescriptions, it is imperative that solutions are established to reduce the country’s increasing burden of medical costs.
Possible shortfalls of this study include the short period of time between which prepolicy data and postpolicy data were collected. It is possible that providers did not have enough time to adjust their prescribing habits or that providers would not have changed a prescribing pattern or preference simply because of a policy change. Future studies could allow a time period greater than 2 years to compare prepolicy and postpolicy prescribing habits, or a future study might make comparisons of prescriber patterns at different institutions that have different policies. Another possible shortfall is that providers and patients were limited to those at the Department of Dermatology & Cutaneous Surgery at the USF Morsani COM. Although this study has found insufficient evidence of a difference in prescribing habits, it may be beneficial to conduct a larger study that encompasses multiple academic institutions with similar policy changes. Most importantly, this study only investigated the influence of in-office pharmaceutical samples on prescribing patterns. This study did not look at the many other ways in which providers may be influenced by pharmaceutical companies, which likely is a significant confounding variable in this study. Continued additional studies that specifically examine other methods through which providers may be influenced would be helpful in further examining the many ways in which physician prescription habits are influenced.
Conclusion
Changes in pharmaceutical policy in 2011 at USF Morsani COM specifically banned in-office samples. The totality of evidence in this study shows modest observational evidence of a change in the postpolicy odds relative to prepolicy odds, but the data also are compatible with no change between prescribing habits before and after the policy changes. Further study is needed to fully understand this relationship.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
- Sondergaard J, Vach K, Kragstrup J, et al. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009;26:204-209.
- Jelinek GA, Neate SL. The influence of the pharmaceutical industry in medicine. J Law Med. 2009;17:216-223.
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380.
- Coleman DL. Establishing policies for the relationship between industry and clinicians: lessons learned from two academic health centers. Acad Med. 2008;83:882-887.
- Coleman DL, Kazdin AE, Miller LA, et al. Guidelines for interactions between clinical faculty and the pharmaceutical industry: one medical school’s approach. Acad Med. 2006;81:154-160.
- Evans D, Hartung DM, Beasley D, et al. Breaking up is hard to do: lessons learned from a pharma-free practice transformation. J Am Board Fam Med. 2013;26:332-338.
- Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583-1597.
- Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526.
- McCormack J, Chmelicek JT. Generic versus brand name: the other drug war. Can Fam Physician. 2014;60:911.
- Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578-1592.
- Garrison GD, Levin GM. Factors affecting prescribing of the newer antidepressants. Ann Pharmacother. 2000;34:10-14.
- Rafique S, Sarwar W, Rashid A, et al. Influence of free drug samples on prescribing by physicians: a cross sectional survey. J Pak Med Assoc. 2017;67:465-467.
- Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394-402.
- Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553-559.
- Brotzman GL, Mark DH. The effect on resident attitudes of regulatory policies regarding pharmaceutical representative activities. J Gen Intern Med. 1993;8:130-134.
- Keim SM, Sanders AB, Witzke DB, et al. Beliefs and practices of emergency medicine faculty and residents regarding professional interactions with the biomedical industry. Ann Emerg Med. 1993;22:1576-1581.
- Thomson AN, Craig BJ, Barham PM. Attitudes of general practitioners in New Zealand to pharmaceutical representatives. Br J Gen Pract. 1994;44:220-223.
- Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296-1298.
- Hurley MP, Stafford RS, Lane AT. Characterizing the relationship between free drug samples and prescription patterns for acne vulgaris and rosacea. JAMA Dermatol. 2014;150:487-493.
- Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ. 1993;149:1401-1407.
- Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9:e110130.
- Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352.
- Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051-1064.
- Sacks CA, Lee CC, Kesselheim AS, et al. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320:650-656.
- Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295:429-433.
- Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4:e283.
Practice Points
- There has been growing concern that pharmaceutical interactions and incentives can influence physicians’ prescribing habits.
- Many academic centers have adopted policies that attempt to limit the pharmaceutical industry’s influence on faculty and in-training physicians.
- This study aimed to investigate if there was a change in the prescribing habits of academic dermatologists after the medical school implemented new policies that banned in-office samples.
Social media may negatively influence acne treatment
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
FROM PEDIATRIC DERMATOLOGY
Infographic: Applications for the Ketogenic Diet in Dermatology
This infographic is available in the PDF above.
This infographic is available in the PDF above.
This infographic is available in the PDF above.
Celebrating 50 years of Dermatology News
The first issue of Skin & Allergy News, now Dermatology News, was published in January 1970. One front-page story highlighted the "continued improvement and more widespread use of steroids" as the most important development of the 1960s in dermatology. Another covered the launch of a national program for dermatology "to design a pattern for its future instead of simply drifting and letting its fate be determined by others."
Throughout 2020, look for articles and features marking the publication's golden anniversary. And read the first ever issue in the PDF above.
The first issue of Skin & Allergy News, now Dermatology News, was published in January 1970. One front-page story highlighted the "continued improvement and more widespread use of steroids" as the most important development of the 1960s in dermatology. Another covered the launch of a national program for dermatology "to design a pattern for its future instead of simply drifting and letting its fate be determined by others."
Throughout 2020, look for articles and features marking the publication's golden anniversary. And read the first ever issue in the PDF above.
The first issue of Skin & Allergy News, now Dermatology News, was published in January 1970. One front-page story highlighted the "continued improvement and more widespread use of steroids" as the most important development of the 1960s in dermatology. Another covered the launch of a national program for dermatology "to design a pattern for its future instead of simply drifting and letting its fate be determined by others."
Throughout 2020, look for articles and features marking the publication's golden anniversary. And read the first ever issue in the PDF above.
Frequent lab testing is common, but low-yield, for isotretinoin patients
Abnormalities in lipids, liver enzymes, and blood counts were rare, and
In a review of 1,863 patients receiving isotretinoin, there were no cases of grade 4 abnormalities of lipids, liver enzymes, or complete blood count (CBC). Further, fewer than 1% of patients had grade 2-3 laboratory abnormalities, and no patients had cholesterol or CBC abnormalities of grade 3 or higher.
The retrospective cohort study used an electronic database to identify patients who were prescribed isotretinoin for acne from 2007 to 2017, with inclusion criteria structured to “increase the likelihood of capturing a complete course of isotretinoin therapy,” wrote John Barbieri, MD, and coauthors. The database allowed the investigators to group lab values into baseline testing, and testing by month of therapy for individual deidentified patient records.
Dr. Barbieri, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, and coinvestigators found that over half of all patients had baseline triglyceride, total cholesterol, AST, ALT, and platelet and white blood cell count levels.
Though the number of patients who had any of these levels checked in a given month of treatment declined over time, as did the total number of patients still on isotretinoin therapy, monthly AST and ALT monitoring occurred in 37.6%-58.5% of patients. Monthly triglyceride monitoring was conducted in between 39.6% and 61.4% of participants, and CBCs were obtained in 26.8%-37.4% of participants.
In terms of the abnormalities that were seen, grade 1 triglyceride elevations of 150-300 mg/dL were present in about 13% of patients at baseline, rising to 39% of participants who were still receiving isotretinoin at month 6. However, grade 2 elevations of up to 500 mg/dL were seen in 1.4% of patients at baseline and 2.4%-5.6% of patients during subsequent months.
Grade 1 liver enzyme abnormalities of less than three times the upper limit of normal values were seen at baseline in under 4% of patients, and in no more than 6.7% of patients through the course of treatment.
Leukopenia of between 3 x 103/mcL and the lower limit of normal occurred in 4.1% of baseline tests and in 6.6%-10.1% of tests in subsequent months. Grade 1 thrombocytopenia (values between 75 x 103/mcL and the lower limit of normal) occurred in 1.9% of baseline tests and no more than 2.9% of tests in the following months.
The results, wrote Dr. Barbieri and coauthors, affirm that most patients fare well on isotretinoin, and frequent laboratory testing is likely to be low-yield. Even using relatively low Medicare reimbursement rates for these tests yielded an estimated $134 in per-patient charges for the studied population. If baseline lipid and liver functions were followed only by repeat testing when peak isotretinoin dose was reached, charges would drop to about $87 per patient. Using the iPLEDGE database figures, this would save $17.4 million in monitoring costs annually, they wrote.
They also calculated that the monitoring regimen they observed puts the cost of detecting one single grade 3 hepatic enzyme elevation at $6,000; one grade 3 triglyceride elevation would cost $7,750.
Of the patients, 49% were female, the median age was 18.2 years, and the median duration of isotretinoin therapy was under 5 months (148 days). Nearly 90% of patients were white and non-Hispanic; 2.5% were black.
The data used for the analysis did not give the investigators access to clinician notes, but they did observe that, even when abnormal test values were seen, isotretinoin prescribing continued. This, they added, pointed toward reassuring clinical scenarios, even in cases of abnormal lab values.
“These findings are consistent with prior studies and suggest that extensive laboratory monitoring observed in this population may be of low value,” concluded Dr. Barbieri and colleagues. “In addition, changes to lipid levels observed in this study typically occurred during the first 2-3 months of therapy before stabilizing, which is consistent with findings in prior studies.”
The investigators noted that, despite mounting evidence of isotretinoin’s safety, there was no trend toward decreased CBC testing over the decade-long period of the study, and there were only “modest” decreases in hepatic enzyme and lipid monitoring. They called for an awareness campaign on the part of professional societies, and consideration for “more specific guideline recommendations” that may ease the testing burden on the adolescent and young adult population receiving isotretinoin.
The study was funded in part by the National Institutes of Health, and Dr. Barbieri receives partial salary support from Pfizer through a grant to the University of Pennsylvania. He has received support for unrelated work from Eli Lilly and Novartis. The other authors reported no conflicts of interest.
SOURCE: Barbieri J et al. J Am Acad Dermatol. 2020 Jan;82(1):72-9.
Abnormalities in lipids, liver enzymes, and blood counts were rare, and
In a review of 1,863 patients receiving isotretinoin, there were no cases of grade 4 abnormalities of lipids, liver enzymes, or complete blood count (CBC). Further, fewer than 1% of patients had grade 2-3 laboratory abnormalities, and no patients had cholesterol or CBC abnormalities of grade 3 or higher.
The retrospective cohort study used an electronic database to identify patients who were prescribed isotretinoin for acne from 2007 to 2017, with inclusion criteria structured to “increase the likelihood of capturing a complete course of isotretinoin therapy,” wrote John Barbieri, MD, and coauthors. The database allowed the investigators to group lab values into baseline testing, and testing by month of therapy for individual deidentified patient records.
Dr. Barbieri, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, and coinvestigators found that over half of all patients had baseline triglyceride, total cholesterol, AST, ALT, and platelet and white blood cell count levels.
Though the number of patients who had any of these levels checked in a given month of treatment declined over time, as did the total number of patients still on isotretinoin therapy, monthly AST and ALT monitoring occurred in 37.6%-58.5% of patients. Monthly triglyceride monitoring was conducted in between 39.6% and 61.4% of participants, and CBCs were obtained in 26.8%-37.4% of participants.
In terms of the abnormalities that were seen, grade 1 triglyceride elevations of 150-300 mg/dL were present in about 13% of patients at baseline, rising to 39% of participants who were still receiving isotretinoin at month 6. However, grade 2 elevations of up to 500 mg/dL were seen in 1.4% of patients at baseline and 2.4%-5.6% of patients during subsequent months.
Grade 1 liver enzyme abnormalities of less than three times the upper limit of normal values were seen at baseline in under 4% of patients, and in no more than 6.7% of patients through the course of treatment.
Leukopenia of between 3 x 103/mcL and the lower limit of normal occurred in 4.1% of baseline tests and in 6.6%-10.1% of tests in subsequent months. Grade 1 thrombocytopenia (values between 75 x 103/mcL and the lower limit of normal) occurred in 1.9% of baseline tests and no more than 2.9% of tests in the following months.
The results, wrote Dr. Barbieri and coauthors, affirm that most patients fare well on isotretinoin, and frequent laboratory testing is likely to be low-yield. Even using relatively low Medicare reimbursement rates for these tests yielded an estimated $134 in per-patient charges for the studied population. If baseline lipid and liver functions were followed only by repeat testing when peak isotretinoin dose was reached, charges would drop to about $87 per patient. Using the iPLEDGE database figures, this would save $17.4 million in monitoring costs annually, they wrote.
They also calculated that the monitoring regimen they observed puts the cost of detecting one single grade 3 hepatic enzyme elevation at $6,000; one grade 3 triglyceride elevation would cost $7,750.
Of the patients, 49% were female, the median age was 18.2 years, and the median duration of isotretinoin therapy was under 5 months (148 days). Nearly 90% of patients were white and non-Hispanic; 2.5% were black.
The data used for the analysis did not give the investigators access to clinician notes, but they did observe that, even when abnormal test values were seen, isotretinoin prescribing continued. This, they added, pointed toward reassuring clinical scenarios, even in cases of abnormal lab values.
“These findings are consistent with prior studies and suggest that extensive laboratory monitoring observed in this population may be of low value,” concluded Dr. Barbieri and colleagues. “In addition, changes to lipid levels observed in this study typically occurred during the first 2-3 months of therapy before stabilizing, which is consistent with findings in prior studies.”
The investigators noted that, despite mounting evidence of isotretinoin’s safety, there was no trend toward decreased CBC testing over the decade-long period of the study, and there were only “modest” decreases in hepatic enzyme and lipid monitoring. They called for an awareness campaign on the part of professional societies, and consideration for “more specific guideline recommendations” that may ease the testing burden on the adolescent and young adult population receiving isotretinoin.
The study was funded in part by the National Institutes of Health, and Dr. Barbieri receives partial salary support from Pfizer through a grant to the University of Pennsylvania. He has received support for unrelated work from Eli Lilly and Novartis. The other authors reported no conflicts of interest.
SOURCE: Barbieri J et al. J Am Acad Dermatol. 2020 Jan;82(1):72-9.
Abnormalities in lipids, liver enzymes, and blood counts were rare, and
In a review of 1,863 patients receiving isotretinoin, there were no cases of grade 4 abnormalities of lipids, liver enzymes, or complete blood count (CBC). Further, fewer than 1% of patients had grade 2-3 laboratory abnormalities, and no patients had cholesterol or CBC abnormalities of grade 3 or higher.
The retrospective cohort study used an electronic database to identify patients who were prescribed isotretinoin for acne from 2007 to 2017, with inclusion criteria structured to “increase the likelihood of capturing a complete course of isotretinoin therapy,” wrote John Barbieri, MD, and coauthors. The database allowed the investigators to group lab values into baseline testing, and testing by month of therapy for individual deidentified patient records.
Dr. Barbieri, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, and coinvestigators found that over half of all patients had baseline triglyceride, total cholesterol, AST, ALT, and platelet and white blood cell count levels.
Though the number of patients who had any of these levels checked in a given month of treatment declined over time, as did the total number of patients still on isotretinoin therapy, monthly AST and ALT monitoring occurred in 37.6%-58.5% of patients. Monthly triglyceride monitoring was conducted in between 39.6% and 61.4% of participants, and CBCs were obtained in 26.8%-37.4% of participants.
In terms of the abnormalities that were seen, grade 1 triglyceride elevations of 150-300 mg/dL were present in about 13% of patients at baseline, rising to 39% of participants who were still receiving isotretinoin at month 6. However, grade 2 elevations of up to 500 mg/dL were seen in 1.4% of patients at baseline and 2.4%-5.6% of patients during subsequent months.
Grade 1 liver enzyme abnormalities of less than three times the upper limit of normal values were seen at baseline in under 4% of patients, and in no more than 6.7% of patients through the course of treatment.
Leukopenia of between 3 x 103/mcL and the lower limit of normal occurred in 4.1% of baseline tests and in 6.6%-10.1% of tests in subsequent months. Grade 1 thrombocytopenia (values between 75 x 103/mcL and the lower limit of normal) occurred in 1.9% of baseline tests and no more than 2.9% of tests in the following months.
The results, wrote Dr. Barbieri and coauthors, affirm that most patients fare well on isotretinoin, and frequent laboratory testing is likely to be low-yield. Even using relatively low Medicare reimbursement rates for these tests yielded an estimated $134 in per-patient charges for the studied population. If baseline lipid and liver functions were followed only by repeat testing when peak isotretinoin dose was reached, charges would drop to about $87 per patient. Using the iPLEDGE database figures, this would save $17.4 million in monitoring costs annually, they wrote.
They also calculated that the monitoring regimen they observed puts the cost of detecting one single grade 3 hepatic enzyme elevation at $6,000; one grade 3 triglyceride elevation would cost $7,750.
Of the patients, 49% were female, the median age was 18.2 years, and the median duration of isotretinoin therapy was under 5 months (148 days). Nearly 90% of patients were white and non-Hispanic; 2.5% were black.
The data used for the analysis did not give the investigators access to clinician notes, but they did observe that, even when abnormal test values were seen, isotretinoin prescribing continued. This, they added, pointed toward reassuring clinical scenarios, even in cases of abnormal lab values.
“These findings are consistent with prior studies and suggest that extensive laboratory monitoring observed in this population may be of low value,” concluded Dr. Barbieri and colleagues. “In addition, changes to lipid levels observed in this study typically occurred during the first 2-3 months of therapy before stabilizing, which is consistent with findings in prior studies.”
The investigators noted that, despite mounting evidence of isotretinoin’s safety, there was no trend toward decreased CBC testing over the decade-long period of the study, and there were only “modest” decreases in hepatic enzyme and lipid monitoring. They called for an awareness campaign on the part of professional societies, and consideration for “more specific guideline recommendations” that may ease the testing burden on the adolescent and young adult population receiving isotretinoin.
The study was funded in part by the National Institutes of Health, and Dr. Barbieri receives partial salary support from Pfizer through a grant to the University of Pennsylvania. He has received support for unrelated work from Eli Lilly and Novartis. The other authors reported no conflicts of interest.
SOURCE: Barbieri J et al. J Am Acad Dermatol. 2020 Jan;82(1):72-9.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
The Ketogenic Diet and Dermatology: A Primer on Current Literature
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
Practice Points
- The ketogenic diet has been employed since antiquity for varying ailments and has a good safety and efficacy profile if administered by a knowledgeable provider.
- New literature is showing promising potential roles for the ketogenic diet as an adjunctive therapy, particularly in the realm of inflammatory disorders, metabolic diseases, and malignancy.
- The dermatologist should be aware of this diet because it is gaining popularity with physicians and patients alike. Dermatologists also should know how it can potentially benefit a number of patients with dermatologic diseases based on small clinical trials, population studies, and basic science research.
Annual Skin Check: Examining the Dermatology Headlines of 2019
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
Resident Pearls
- Chemical sunscreen made headlines in 2019 due to concerns over coral reef toxicity and systemic absorption in humans.
- With a total of 654 cases, New York City’s largest measles outbreak in nearly 30 years ended in September 2019.
- From dupilumab for adolescent atopic dermatitis to apremilast for Behçet disease, the US Food and Drug Administration approved several therapies for pediatric dermatology and rare dermatologic conditions in 2019.
- Two popular skin care products—the Neutrogena Light Therapy Acne Mask and Johnson’s Baby Powder—were involved in recalls in 2019.
Makeup is contaminated with pathogenic bacteria
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
iPLEDGE vexes dermatologists treating transgender patients
Physicians treating transgender patients – in particular, transgender men who were born female – are faced with a confusing process when prescribing isotretinoin for severe acne.
A research letter published in the Journal of the American Academy of Dermatology reports that established to prevent female patients from starting isotretinoin therapy while pregnant or from becoming pregnant while exposed to the teratogenic medication.
Nearly 90% of respondents favored changing the current gender-specific categories in iPLEDGE to gender-neutral ones, classifying patients only by whether or not they have the ability to become pregnant.
For their research, Courtney Ensslin, MD, of the department of dermatology at Johns Hopkins University, Baltimore, and colleagues, distributed an 18-point questionnaire to 385 members of the Association of Professors of Dermatology that included questions assessing clinicians’ knowledge about the reproductive potential of transgender men and women. The recipients were asked to distribute it to faculty members and residents. The survey also described three clinical scenarios in which the physician needed to decide how to register a patient in iPLEDGE. The clinicians largely opted to class transgender men as women with childbearing potential, even if the category conflicted with the patient’s self-identified and legally recognized male gender.
Of the 136 clinicians who responded, 60% were women, almost half were aged 25-34 years. About 12% of respondents said the complexities of prescribing isotretinoin to a transgender patient led them to choose alternative therapies. And the survey revealed some gaps on providers’ general literacy on transgender patients and their reproductive potential. For example, fewer than a third of respondents answered correctly as to whether testosterone treatment decreases the quality and development of an immature ovum.
The researchers wrote that the survey results, while limited by a small sample of respondents that skewed toward younger women providers, suggest that “continued education on fertility in transgender patients is needed because prescribers must fully understand each patient’s reproductive potential to safely prescribe teratogenic medications.” Additionally, they pointed out, the results support ongoing efforts to reform iPLEDGE, as the current categories “do not offer an inclusive approach to care for transgender patients.”
Earlier this year the American Academy of Dermatology issued a position statement that described a number of ongoing initiatives aimed at improving treatment for patients who are members of gender and sexual minorities. These included the “revision of the AAD position statement on isotretinoin to support a gender-neutral categorization model for [iPLEDGE] … based on child-bearing potential rather than on gender identity,” the statement said.
Dr. Ensslin and colleagues reported conflicts of interest related to their research. The study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
SOURCE: Ensslin C et al. J Am Acad Dermatol. 2019 Dec;81(6):1426-9.
Physicians treating transgender patients – in particular, transgender men who were born female – are faced with a confusing process when prescribing isotretinoin for severe acne.
A research letter published in the Journal of the American Academy of Dermatology reports that established to prevent female patients from starting isotretinoin therapy while pregnant or from becoming pregnant while exposed to the teratogenic medication.
Nearly 90% of respondents favored changing the current gender-specific categories in iPLEDGE to gender-neutral ones, classifying patients only by whether or not they have the ability to become pregnant.
For their research, Courtney Ensslin, MD, of the department of dermatology at Johns Hopkins University, Baltimore, and colleagues, distributed an 18-point questionnaire to 385 members of the Association of Professors of Dermatology that included questions assessing clinicians’ knowledge about the reproductive potential of transgender men and women. The recipients were asked to distribute it to faculty members and residents. The survey also described three clinical scenarios in which the physician needed to decide how to register a patient in iPLEDGE. The clinicians largely opted to class transgender men as women with childbearing potential, even if the category conflicted with the patient’s self-identified and legally recognized male gender.
Of the 136 clinicians who responded, 60% were women, almost half were aged 25-34 years. About 12% of respondents said the complexities of prescribing isotretinoin to a transgender patient led them to choose alternative therapies. And the survey revealed some gaps on providers’ general literacy on transgender patients and their reproductive potential. For example, fewer than a third of respondents answered correctly as to whether testosterone treatment decreases the quality and development of an immature ovum.
The researchers wrote that the survey results, while limited by a small sample of respondents that skewed toward younger women providers, suggest that “continued education on fertility in transgender patients is needed because prescribers must fully understand each patient’s reproductive potential to safely prescribe teratogenic medications.” Additionally, they pointed out, the results support ongoing efforts to reform iPLEDGE, as the current categories “do not offer an inclusive approach to care for transgender patients.”
Earlier this year the American Academy of Dermatology issued a position statement that described a number of ongoing initiatives aimed at improving treatment for patients who are members of gender and sexual minorities. These included the “revision of the AAD position statement on isotretinoin to support a gender-neutral categorization model for [iPLEDGE] … based on child-bearing potential rather than on gender identity,” the statement said.
Dr. Ensslin and colleagues reported conflicts of interest related to their research. The study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
SOURCE: Ensslin C et al. J Am Acad Dermatol. 2019 Dec;81(6):1426-9.
Physicians treating transgender patients – in particular, transgender men who were born female – are faced with a confusing process when prescribing isotretinoin for severe acne.
A research letter published in the Journal of the American Academy of Dermatology reports that established to prevent female patients from starting isotretinoin therapy while pregnant or from becoming pregnant while exposed to the teratogenic medication.
Nearly 90% of respondents favored changing the current gender-specific categories in iPLEDGE to gender-neutral ones, classifying patients only by whether or not they have the ability to become pregnant.
For their research, Courtney Ensslin, MD, of the department of dermatology at Johns Hopkins University, Baltimore, and colleagues, distributed an 18-point questionnaire to 385 members of the Association of Professors of Dermatology that included questions assessing clinicians’ knowledge about the reproductive potential of transgender men and women. The recipients were asked to distribute it to faculty members and residents. The survey also described three clinical scenarios in which the physician needed to decide how to register a patient in iPLEDGE. The clinicians largely opted to class transgender men as women with childbearing potential, even if the category conflicted with the patient’s self-identified and legally recognized male gender.
Of the 136 clinicians who responded, 60% were women, almost half were aged 25-34 years. About 12% of respondents said the complexities of prescribing isotretinoin to a transgender patient led them to choose alternative therapies. And the survey revealed some gaps on providers’ general literacy on transgender patients and their reproductive potential. For example, fewer than a third of respondents answered correctly as to whether testosterone treatment decreases the quality and development of an immature ovum.
The researchers wrote that the survey results, while limited by a small sample of respondents that skewed toward younger women providers, suggest that “continued education on fertility in transgender patients is needed because prescribers must fully understand each patient’s reproductive potential to safely prescribe teratogenic medications.” Additionally, they pointed out, the results support ongoing efforts to reform iPLEDGE, as the current categories “do not offer an inclusive approach to care for transgender patients.”
Earlier this year the American Academy of Dermatology issued a position statement that described a number of ongoing initiatives aimed at improving treatment for patients who are members of gender and sexual minorities. These included the “revision of the AAD position statement on isotretinoin to support a gender-neutral categorization model for [iPLEDGE] … based on child-bearing potential rather than on gender identity,” the statement said.
Dr. Ensslin and colleagues reported conflicts of interest related to their research. The study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
SOURCE: Ensslin C et al. J Am Acad Dermatol. 2019 Dec;81(6):1426-9.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
5 Key Points on Dietary Counseling of Acne Patients