User login
Vedolizumab-Induced Acne Fulminans: An Uncommon and Severe Adverse Effect
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
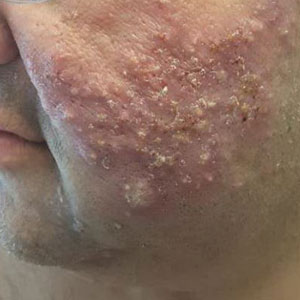
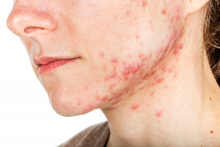
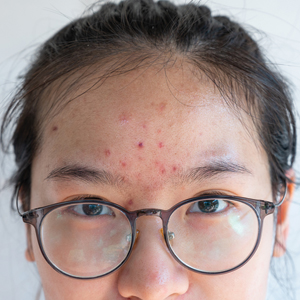
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.
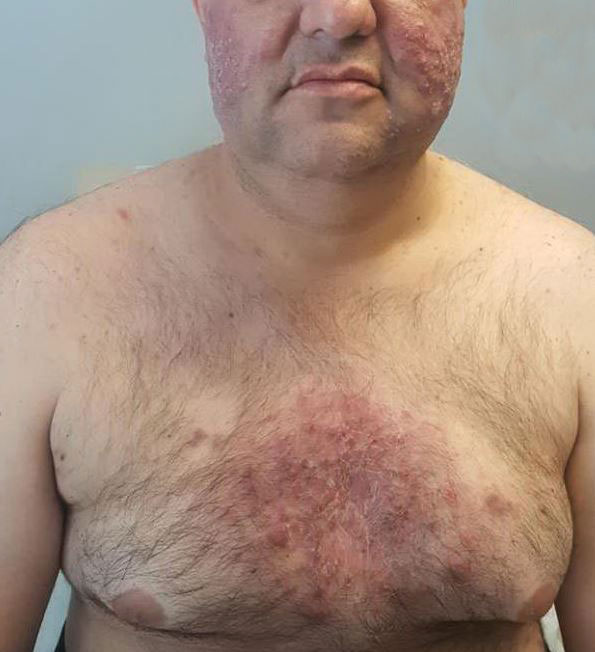
Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.
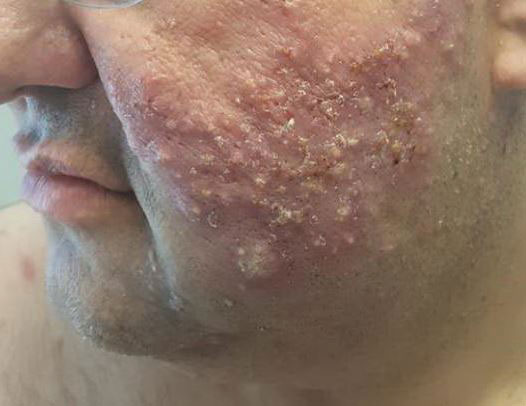
The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
Practice Points
- Vedolizumab, a monoclonal antibody for the treatment of refractory inflammatory bowel disease, was found to cause acne fulminans without systemic symptoms.
- Vedolizumab previously was believed to be a gut-limited immune modulator.
- Off-target cutaneous effects may indicate wider expression of the target integrin of vedolizumab and should be recognized as the drug becomes more widely used.
Consider the mnemonic ‘CLEAR’ when counseling acne patients
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Expert calls for thoughtful approach to curbing costs in dermatology
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
AT PDA 2022
Isotretinoin prescribers need better education on emergency contraception
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
FROM PEDIATRIC DERMATOLOGY
Hormonal therapy a safe, long term option for older women with recalcitrant acne
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
AT PDA 2022
What are your treatment options when isotretinoin fails?
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
AT SPD 2022
Study eyes characteristics of pediatric patients with hidradenitis suppurativa
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.
“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.
“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.
“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
AT SPD 2022
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
What’s Diet Got to Do With It? Basic and Clinical Science Behind Diet and Acne
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
Practice Points
- Patients are frequently interested in the role that diet plays in acne, and dermatologists should be aware of the current evidence to answer these questions effectively.
- One of the primary pathways in acne pathogenesis, mTORC1 (mammalian target of rapamycin complex 1), is partially regulated by nutrient availability, insulin, and insulinlike growth factor 1.
- Dietary recommendations for acne based on available evidence may include a low glycemic index diet and avoidance of certain dairy products.
- Insulin resistance may underlie the pathogenesis of acne in a subset of patients, and assessing insulin resistance in acne patients should be considered.
Adapting to Changes in Acne Management: Take One Step at a Time
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.