User login
Expert calls for thoughtful approach to curbing costs in dermatology
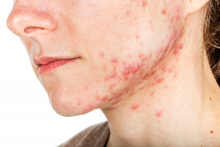
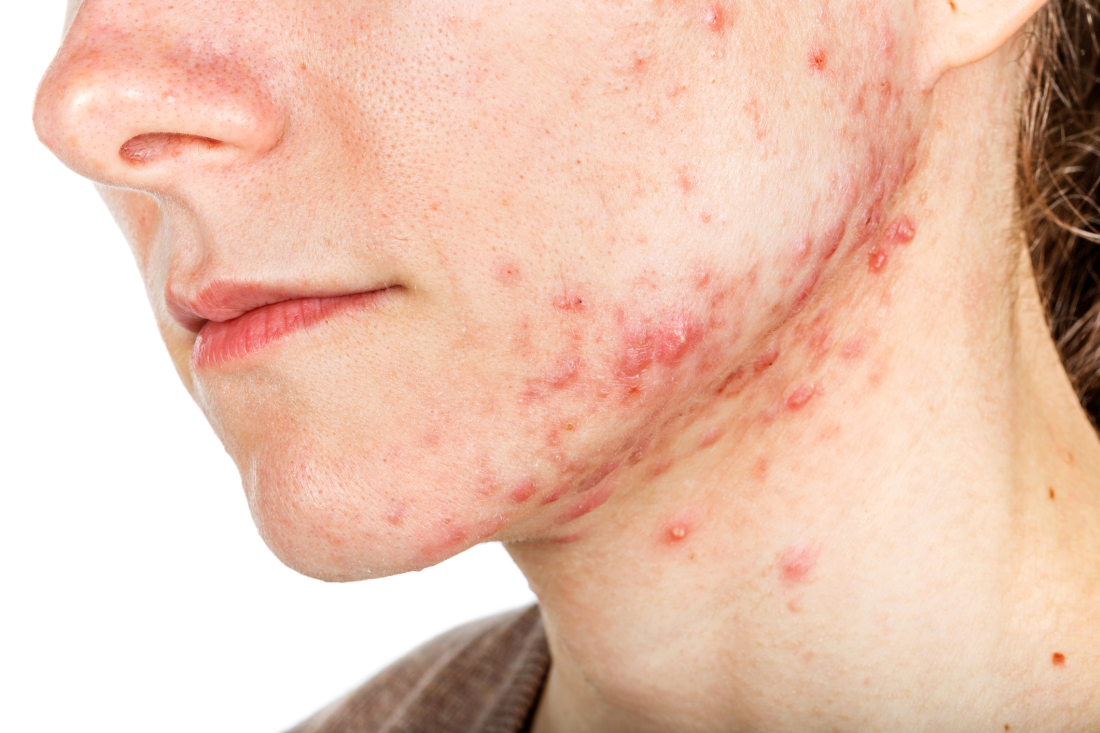
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
AT PDA 2022
Pandemic has helped clinicians to gain better insight on pernio, expert says
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
AT PDA 2022
Expert shares tips on hair disorders and photoprotection for patients of color
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
AT PDA 2022
Hormonal therapy a safe, long term option for older women with recalcitrant acne
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
AT PDA 2022
Consider the ‘long game’ in tumor management following Mohs surgery
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
AT PDA 2022
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
Consider essential oil allergy in patient with dermatitis
PORTLAND, ORE. – When patients present to Brandon L. Adler, MD, with dermatitis on the eyelid, face, or neck, he routinely asks them if they apply essential oils on their skin, or if they have an essential oil diffuser or nebulizer in their home.
“The answer is frequently ‘yes,’ ” Dr. Adler, clinical assistant professor of dermatology at the University of Southern California, Los Angeles, said at the annual meeting of the Pacific Dermatologic Association. “Essential oils are widely used throughout the wellness industry. They are contained in personal care products, beauty products, natural cleaning products, and they’re being diffused by our patients into the air. More than 75 essential oils are reported to cause allergic contact dermatitis.”
“Linalool is most classically associated with lavender, while limonene is associated with citrus, but they’re found in many different plants,” said Dr. Adler, who directs USC’s contact dermatitis clinic. “On their own, linalool and limonene are not particularly allergenic; they’re not a big deal in the patch test clinic. The problem comes when we add air to the mix, because they oxidize to hydroperoxides of linalool and limonene. These are quite potent allergens.”
According to the most recent North American Contact Dermatitis Group data, 8.9% of patients undergoing patch testing tested positive to linalool hydroperoxides and 2.6% were positive to limonene hydroperoxides.
Dr. Adler discussed the case of a female massage therapist who presented with refractory hand dermatitis and was on methotrexate and dupilumab at the time of consultation but was still symptomatic. She patch-tested positive to limonene and linalool hydroperoxides as well as multiple essential oils that she had been using with her clients, ranging from sacred frankincense oil to basil oil, and she was advised to massage using only coconut or vegetable oils.
Essential oil allergy may also be related to cannabis allergy. According to Dr. Adler, allergic contact dermatitis to cannabis has been rarely reported, but in an analysis of 103 commercial topical cannabinoid preparations that he published with Vincent DeLeo, MD, also with USC, 84% contained a NACDG allergen, frequently essential oils.
More recently, Dr. Adler and colleagues reported the case of a 40-year-old woman who was referred for patch testing for nummular dermatitis that wasn’t responding to treatment. The patient was found to be using topical cannabis and also grew cannabis at home. “She asked to be patch-tested to her homegrown cannabis and had a strong positive patch test to the cannabis, linalool and limonene hydroperoxides, and other essential oils,” Dr. Adler recalled. “We sent her cannabis sample for analysis at a commercial lab and found that it contained limonene and other allergenic terpene chemicals.
“We’re just starting to unravel what this means in terms of our patients and how to manage them, but many are using topical cannabis and topical CBD. I suspect this is a lot less rare than we realize.”
Another recent case from Europe reported allergic contact dermatitis to Cannabis sativa (hemp) seed oil following topical application, with positive patch testing.
Dr. Adler disclosed that he has received research grants from the American Contact Dermatitis Society. He is also an investigator for AbbVie and a consultant for the Skin Research Institute.
PORTLAND, ORE. – When patients present to Brandon L. Adler, MD, with dermatitis on the eyelid, face, or neck, he routinely asks them if they apply essential oils on their skin, or if they have an essential oil diffuser or nebulizer in their home.
“The answer is frequently ‘yes,’ ” Dr. Adler, clinical assistant professor of dermatology at the University of Southern California, Los Angeles, said at the annual meeting of the Pacific Dermatologic Association. “Essential oils are widely used throughout the wellness industry. They are contained in personal care products, beauty products, natural cleaning products, and they’re being diffused by our patients into the air. More than 75 essential oils are reported to cause allergic contact dermatitis.”
“Linalool is most classically associated with lavender, while limonene is associated with citrus, but they’re found in many different plants,” said Dr. Adler, who directs USC’s contact dermatitis clinic. “On their own, linalool and limonene are not particularly allergenic; they’re not a big deal in the patch test clinic. The problem comes when we add air to the mix, because they oxidize to hydroperoxides of linalool and limonene. These are quite potent allergens.”
According to the most recent North American Contact Dermatitis Group data, 8.9% of patients undergoing patch testing tested positive to linalool hydroperoxides and 2.6% were positive to limonene hydroperoxides.
Dr. Adler discussed the case of a female massage therapist who presented with refractory hand dermatitis and was on methotrexate and dupilumab at the time of consultation but was still symptomatic. She patch-tested positive to limonene and linalool hydroperoxides as well as multiple essential oils that she had been using with her clients, ranging from sacred frankincense oil to basil oil, and she was advised to massage using only coconut or vegetable oils.
Essential oil allergy may also be related to cannabis allergy. According to Dr. Adler, allergic contact dermatitis to cannabis has been rarely reported, but in an analysis of 103 commercial topical cannabinoid preparations that he published with Vincent DeLeo, MD, also with USC, 84% contained a NACDG allergen, frequently essential oils.
More recently, Dr. Adler and colleagues reported the case of a 40-year-old woman who was referred for patch testing for nummular dermatitis that wasn’t responding to treatment. The patient was found to be using topical cannabis and also grew cannabis at home. “She asked to be patch-tested to her homegrown cannabis and had a strong positive patch test to the cannabis, linalool and limonene hydroperoxides, and other essential oils,” Dr. Adler recalled. “We sent her cannabis sample for analysis at a commercial lab and found that it contained limonene and other allergenic terpene chemicals.
“We’re just starting to unravel what this means in terms of our patients and how to manage them, but many are using topical cannabis and topical CBD. I suspect this is a lot less rare than we realize.”
Another recent case from Europe reported allergic contact dermatitis to Cannabis sativa (hemp) seed oil following topical application, with positive patch testing.
Dr. Adler disclosed that he has received research grants from the American Contact Dermatitis Society. He is also an investigator for AbbVie and a consultant for the Skin Research Institute.
PORTLAND, ORE. – When patients present to Brandon L. Adler, MD, with dermatitis on the eyelid, face, or neck, he routinely asks them if they apply essential oils on their skin, or if they have an essential oil diffuser or nebulizer in their home.
“The answer is frequently ‘yes,’ ” Dr. Adler, clinical assistant professor of dermatology at the University of Southern California, Los Angeles, said at the annual meeting of the Pacific Dermatologic Association. “Essential oils are widely used throughout the wellness industry. They are contained in personal care products, beauty products, natural cleaning products, and they’re being diffused by our patients into the air. More than 75 essential oils are reported to cause allergic contact dermatitis.”
“Linalool is most classically associated with lavender, while limonene is associated with citrus, but they’re found in many different plants,” said Dr. Adler, who directs USC’s contact dermatitis clinic. “On their own, linalool and limonene are not particularly allergenic; they’re not a big deal in the patch test clinic. The problem comes when we add air to the mix, because they oxidize to hydroperoxides of linalool and limonene. These are quite potent allergens.”
According to the most recent North American Contact Dermatitis Group data, 8.9% of patients undergoing patch testing tested positive to linalool hydroperoxides and 2.6% were positive to limonene hydroperoxides.
Dr. Adler discussed the case of a female massage therapist who presented with refractory hand dermatitis and was on methotrexate and dupilumab at the time of consultation but was still symptomatic. She patch-tested positive to limonene and linalool hydroperoxides as well as multiple essential oils that she had been using with her clients, ranging from sacred frankincense oil to basil oil, and she was advised to massage using only coconut or vegetable oils.
Essential oil allergy may also be related to cannabis allergy. According to Dr. Adler, allergic contact dermatitis to cannabis has been rarely reported, but in an analysis of 103 commercial topical cannabinoid preparations that he published with Vincent DeLeo, MD, also with USC, 84% contained a NACDG allergen, frequently essential oils.
More recently, Dr. Adler and colleagues reported the case of a 40-year-old woman who was referred for patch testing for nummular dermatitis that wasn’t responding to treatment. The patient was found to be using topical cannabis and also grew cannabis at home. “She asked to be patch-tested to her homegrown cannabis and had a strong positive patch test to the cannabis, linalool and limonene hydroperoxides, and other essential oils,” Dr. Adler recalled. “We sent her cannabis sample for analysis at a commercial lab and found that it contained limonene and other allergenic terpene chemicals.
“We’re just starting to unravel what this means in terms of our patients and how to manage them, but many are using topical cannabis and topical CBD. I suspect this is a lot less rare than we realize.”
Another recent case from Europe reported allergic contact dermatitis to Cannabis sativa (hemp) seed oil following topical application, with positive patch testing.
Dr. Adler disclosed that he has received research grants from the American Contact Dermatitis Society. He is also an investigator for AbbVie and a consultant for the Skin Research Institute.
AT PDA 2022
Why it’s important for dermatologists to learn about JAK inhibitors
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
AT PDA 2022
Asking patients about their gender identity: ‘Normalize’ the discussion and other recommendations
PORTLAND, ORE. –
“From the patient perspective, there’s nothing more awkward than having an awkward provider asking awkward questions,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said at the annual meeting of the Pacific Dermatologic Association.
In 2014, Sean Cahill, PhD, and Harvey Makadon, MD, published an article recommending the inclusion of sexual orientation and gender identity questions in electronic medical records, a practice that Dr. Yeung characterized as “the most patient-centered way to collect sexual orientation and gender identity information. The most important thing is to ask routinely on an intake form where they fill it out themselves. All electronic medical records have the capacity to do so.”
On the other hand, when asking new patients about their sexual orientation and gender identity in person, it’s important to normalize the discussion and ask in an inclusive way, said Dr. Yeung, who was the lead author on published recommendations on dermatologic care for LGBTQ persons published in the Journal of the American Academy of Dermatology. “For example, I always say, ‘I’m Howa Yeung. I use him pronouns,’ ” he said. “ ‘How should I address you?’ Then they will tell you. Allow people to lead the way.”
Other suggested tips in the JAAD article include to avoid using terms such as “sir” or “miss” until the patient’s gender identify is ascertained. Instead, use gender-neutral terms such as “they” or “the patient” when referring to new patients. Do not use the pronoun “it.” If a patient’s name does not match a name in the medical record, ask, “What is the name on your insurance/records?” and avoid assuming gender(s) of a patient’s partner or parents. Instead, consider asking, “Who did you bring with you today?” “Are you in a relationship?” “What are the names of your parents?”
Normalizing questions about the patient’s sexual history is also key. “I tell patients that I routinely ask about sexual history for patients with similar skin issues because it helps me provide the best care for them,” Dr. Yeung said. “I also discuss confidentiality and documentation.”
Dr. Yeung reported having no relevant disclosures.
PORTLAND, ORE. –
“From the patient perspective, there’s nothing more awkward than having an awkward provider asking awkward questions,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said at the annual meeting of the Pacific Dermatologic Association.
In 2014, Sean Cahill, PhD, and Harvey Makadon, MD, published an article recommending the inclusion of sexual orientation and gender identity questions in electronic medical records, a practice that Dr. Yeung characterized as “the most patient-centered way to collect sexual orientation and gender identity information. The most important thing is to ask routinely on an intake form where they fill it out themselves. All electronic medical records have the capacity to do so.”
On the other hand, when asking new patients about their sexual orientation and gender identity in person, it’s important to normalize the discussion and ask in an inclusive way, said Dr. Yeung, who was the lead author on published recommendations on dermatologic care for LGBTQ persons published in the Journal of the American Academy of Dermatology. “For example, I always say, ‘I’m Howa Yeung. I use him pronouns,’ ” he said. “ ‘How should I address you?’ Then they will tell you. Allow people to lead the way.”
Other suggested tips in the JAAD article include to avoid using terms such as “sir” or “miss” until the patient’s gender identify is ascertained. Instead, use gender-neutral terms such as “they” or “the patient” when referring to new patients. Do not use the pronoun “it.” If a patient’s name does not match a name in the medical record, ask, “What is the name on your insurance/records?” and avoid assuming gender(s) of a patient’s partner or parents. Instead, consider asking, “Who did you bring with you today?” “Are you in a relationship?” “What are the names of your parents?”
Normalizing questions about the patient’s sexual history is also key. “I tell patients that I routinely ask about sexual history for patients with similar skin issues because it helps me provide the best care for them,” Dr. Yeung said. “I also discuss confidentiality and documentation.”
Dr. Yeung reported having no relevant disclosures.
PORTLAND, ORE. –
“From the patient perspective, there’s nothing more awkward than having an awkward provider asking awkward questions,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said at the annual meeting of the Pacific Dermatologic Association.
In 2014, Sean Cahill, PhD, and Harvey Makadon, MD, published an article recommending the inclusion of sexual orientation and gender identity questions in electronic medical records, a practice that Dr. Yeung characterized as “the most patient-centered way to collect sexual orientation and gender identity information. The most important thing is to ask routinely on an intake form where they fill it out themselves. All electronic medical records have the capacity to do so.”
On the other hand, when asking new patients about their sexual orientation and gender identity in person, it’s important to normalize the discussion and ask in an inclusive way, said Dr. Yeung, who was the lead author on published recommendations on dermatologic care for LGBTQ persons published in the Journal of the American Academy of Dermatology. “For example, I always say, ‘I’m Howa Yeung. I use him pronouns,’ ” he said. “ ‘How should I address you?’ Then they will tell you. Allow people to lead the way.”
Other suggested tips in the JAAD article include to avoid using terms such as “sir” or “miss” until the patient’s gender identify is ascertained. Instead, use gender-neutral terms such as “they” or “the patient” when referring to new patients. Do not use the pronoun “it.” If a patient’s name does not match a name in the medical record, ask, “What is the name on your insurance/records?” and avoid assuming gender(s) of a patient’s partner or parents. Instead, consider asking, “Who did you bring with you today?” “Are you in a relationship?” “What are the names of your parents?”
Normalizing questions about the patient’s sexual history is also key. “I tell patients that I routinely ask about sexual history for patients with similar skin issues because it helps me provide the best care for them,” Dr. Yeung said. “I also discuss confidentiality and documentation.”
Dr. Yeung reported having no relevant disclosures.
AT PDA 2022
Does hidradenitis suppurativa worsen during pregnancy?
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
AT PDA 2022