User login
Metastatic angiosarcoma arising in a patient with long-standing treatment-refractory hemangioma
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
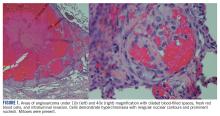
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
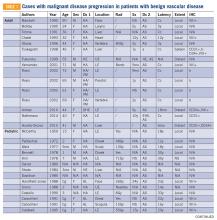
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
Adult soft tissue sarcoma: Professional resources from the National Cancer Institute
Adult Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Adult Soft Tissue Sarcoma
Incidence and Mortality
Estimated new cases and deaths from soft tissue sarcoma in the United States in 2018:[1]
- New cases: 13,040.
- Deaths: 5,150.
Soft tissue sarcomas are malignant tumors that arise in any of the mesodermal tissues of the extremities (50%), trunk and retroperitoneum (40%), or head and neck (10%). The reported international incidence rates range from 1.8 to 5 per 100,000 individuals per year.[2]
Risk Factors and Genetic Factors
The risk of sporadic soft tissue sarcomas is increased by previous radiation therapy and, in the case of lymphangiosarcoma, by chronic lymphedema. The chemicals Thorotrast (thorium dioxide), vinyl chloride, and arsenic are also established carcinogens for hepatic angiosarcomas.[3-5]
Soft tissue sarcomas occur with greater frequency in patients with the following inherited syndromes:[3-5]
- Nevoid basal cell carcinoma syndrome (Gorlin syndrome: PTC gene mutation).
- Gardner syndrome (APC mutation).
- Li-Fraumeni syndrome (p53 mutation).
- Tuberous sclerosis (Bourneville disease: TSC1 or TSC2 mutation).
- von Recklinghausen disease (neurofibromatosis type 1: NF1 mutation).
- Werner syndrome (adult progeria: WRN mutation).
Diagnosis
Soft tissue sarcomas may be heterogeneous, so adequate tissue should be obtained via either core-needle or incisional biopsy for microscopic examination to determine histologic type and tumor grade. Careful planning of the initial biopsy is important to avoid compromising subsequent curative resection. Since the selection of treatment is determined by the grade of the tumor, it is essential to have a careful review of the biopsy tissue by a pathologist who is experienced in diagnosing sarcomas. Complete staging and treatment planning by a multidisciplinary team of cancer specialists is required to determine the optimal treatment for patients with this disease.
There is evidence that at least some favorable clinical outcomes may be associated with referral to a specialized sarcoma treatment center. In a population-based consecutive series of 375 soft tissue sarcoma patients in Sweden, local recurrence rates of resected tumors were higher in patients who were not referred to the specialized center: in 35 of 78 (45%) patients not referred; in 24 of 102 (24%) patients referred after initial surgery or incisional biopsy; and in 36 of 195 (18%) patients referred before any surgical procedure (P = .0001 for the difference between those never referred vs. those referred before any surgical procedure).[6][Level of evidence: 3iDii] However, there were no statistically significant differences in death from sarcoma between the groups of patients.
Prognostic Factors
The prognosis for patients with adult soft tissue sarcomas depends on several factors, including:[3-5,7,8]
- Patient’s age.
- Size, sarcoma subtype, histologic grade, mitotic activity, and stage of the tumor.
Factors associated with a poorer prognosis include the following:[9]
- Age older than 60 years.
- Tumors larger than 5 cm in greatest dimension.
- High-grade histology with high mitotic activity.
- Positive margins after resection.[10]
Although low-grade tumors are usually curable by surgery alone, higher-grade sarcomas (as determined by the mitotic index and by the presence of hemorrhage and necrosis) are associated with higher local-treatment failure rates and increased metastatic potential.
Surveillance for Relapse
A retrospective review included 174 consecutive patients with a soft tissue sarcoma of the limb who underwent follow-up by oncologists at a single center from 2003 to 2009.[11] The rate and site of recurrence and mode of detection were analyzed. Eighty-two patients (47%) experienced relapse. Isolated local recurrences occurred in 26 patients and local relapse with synchronous pulmonary metastases occurred in 5 patients. Local recurrences were detected clinically in 30 of the 31 patients; magnetic resonance imaging identified only one local recurrence. Twenty-eight patients developed isolated lung metastases; in 9 patients, the lung metastases were amenable to resections, 7 of whom were free of disease after treatment. Lung metastases were detected by chest x-ray in 19 patients, by computed tomography scanning in 3 patients, and clinically in 11 patients. Twenty-three patients developed nonpulmonary metastases. More than 80% of the relapses occurred in the first 2 years of follow-up; however, later recurrences were also observed.[11][Level of evidence: 3iiDi] This study supports imaging surveillance for detection of lung metastases, whereas local recurrences at the primary site were usually detected by clinical examination. The impact of picking up metastases from overall survival or quality-of-life data is unknown.
Related Summaries
Other PDQ summaries containing information about soft tissue sarcoma include:
References
- American Cancer Society: Cancer Facts and Figures 2018. Atlanta, Ga: American Cancer Society, 2018. Available online. Last accessed January 5, 2018.
- Wibmer C, Leithner A, Zielonke N, et al.: Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol 21 (5): 1106-11, 2010. [PUBMED Abstract]
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Gustafson P, Dreinhöfer KE, Rydholm A: Soft tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand 65 (1): 47-50, 1994. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Kasper B, Ouali M, van Glabbeke M, et al.: Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer 49 (2): 449-56, 2013. [PUBMED Abstract]
- Vraa S, Keller J, Nielsen OS, et al.: Prognostic factors in soft tissue sarcomas: the Aarhus experience. Eur J Cancer 34 (12): 1876-82, 1998. [PUBMED Abstract]
- Trovik LH, Ovrebo K, Almquist M, et al.: Adjuvant radiotherapy in retroperitoneal sarcomas. A Scandinavian Sarcoma Group study of 97 patients. Acta Oncol 53 (9): 1165-72, 2014. [PUBMED Abstract]
- Rothermundt C, Whelan JS, Dileo P, et al.: What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 110 (10): 2420-6, 2014. [PUBMED Abstract]
Cellular Classification of Adult Soft Tissue Sarcoma
Soft tissue sarcomas are classified histologically according to the soft tissue cell of origin. Additional studies, including electron microscopy, specialized immunohistochemistry, flow cytometry, cytogenetics, and tissue culture studies may allow identification of particular subtypes within the major histologic categories. For example, S100 antigen suggests neural sheath origin, cytokeratin suggests epithelioid or synovial cell origin, and factor VIII-related antigen suggests endothelial origin. Likewise, some subtypes of sarcomas have characteristic genetic markers, but these markers are not generally used in the routine clinical setting (e.g., translocation t(X;18)(p11;q11) in synovial sarcomas and translocation t(12;16)(q13;p11) in myxoid and round-cell sarcomas).[1-3]
The histologic grade reflects the metastatic potential of these tumors more accurately than the classic cellular classification listed below. Pathologists assign a grade based on the number of mitoses per high-powered field, the presence of necrosis, cellular and nuclear morphology, and the degree of cellularity; discordance among expert pathologists regarding tumor grade, and even histologic subtype, can be substantial.[4]
The World Health Organization lists the following cell types in its classification of soft tissue sarcomas:[5,6]
- Adipocytic tumors.
- Dedifferentiated liposarcoma.*
- Myxoid/round cell liposarcoma.
- Pleomorphic liposarcoma.
- Fibroblastic/myofibroblastic tumors.
- Fibrosarcoma.**
- Myxofibrosarcoma, low grade.
- Low-grade fibromyxoid sarcoma.
- Sclerosing epithelioid fibrosarcoma.
- So-called fibrohistiocytic tumors.
- Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (including pleomorphic, giant cell, myxoid/high-grade myxofibrosarcoma, and inflammatory forms).
- Smooth muscle tumors.
- Leiomyosarcoma.
- Skeletal muscle tumors.
- Rhabdomyosarcoma (embryonal, alveolar, and pleomorphic forms).
- Vascular tumors.
- Epithelioid hemangioendothelioma.
- Angiosarcoma, deep.***
- Tumors of peripheral nerves.
- Malignant peripheral nerve sheath tumor.
- Chondro-osseous tumors.
- Extraskeletal chondrosarcoma (mesenchymal and other variants).
- Extraskeletal osteosarcoma.
- Tumors of uncertain differentiation.
- Synovial sarcoma.
- Epithelioid sarcoma.
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Extraskeletal myxoid chondrosarcoma.
- Primitive neuroectodermal tumor/extraskeletal Ewing tumor.
- Desmoplastic small round cell tumor.
- Extrarenal rhabdoid tumor.
- Undifferentiated sarcoma; sarcoma, not otherwise specified.
[Note: *It is recognized that dedifferentiated liposarcoma primarily arises in the context of deep atypical lipomatous tumor/well-differentiated liposarcoma, a sarcoma of intermediate malignancy because of the lack of metastatic capacity. **The category of fibrosarcoma can be inclusive of fibrosarcomatous differentiation in dermatofibrosarcoma protuberans. ***Cutaneous angiosarcoma may be difficult to stage using the American Joint Committee on Cancer system. (Refer to the PDQ summary on Gastrointestinal Stromal Tumors for more information.)]
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Alvegård TA, Berg NO: Histopathology peer review of high-grade soft tissue sarcoma: the Scandinavian Sarcoma Group experience. J Clin Oncol 7 (12): 1845-51, 1989. [PUBMED Abstract]
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
Stage Information for Adult Soft Tissue Sarcoma
Note: The American Joint Committee on Cancer (AJCC) has published the 8th edition of the AJCC Cancer Staging Manual, which includes revisions to the staging for this disease. Implementation of the 8th edition began in January 2018. The PDQ Adult Treatment Editorial Board, which maintains this summary, is reviewing the revised staging and will make appropriate changes as needed.
Staging has an important role in determining the most effective treatment for soft tissue sarcoma. Clinical staging involves magnetic resonance imaging (MRI) or computed tomography (CT) of the primary tumor area and a chest CT to look for metastasis to the lung (the most common site of distant spread). An abdominal CT scan is done in the case of retroperitoneal sarcomas because the liver may be the site of initial clinical metastasis for these tumors.
The stage is determined by the size of the tumor, the histologic grade, and whether there is spread to lymph nodes or distant sites. Intracompartmental or extracompartmental extension of extremity sarcomas is also important for surgical decision making. For complete staging, a thorough review of all biopsy specimens (including those from the primary tumor, lymph nodes, or other suspicious lesions) is essential. CT scan of the chest is recommended for sarcomas larger than 5 cm (T2) or with moderate to poor differentiation (grades 2–4). Nodal involvement is rare, occurring in fewer than 3% of patients with sarcoma.[1]
Lymph node involvement in soft tissue sarcomas of adulthood is rare but is somewhat more frequent in some subtypes (e.g., rhabdomyosarcoma, vascular sarcomas, clear cell sarcomas, and epithelioid sarcomas) when they are high grade.[2] Because treatment decisions are predicated on pathology staging, patients should be staged before, and again after, any neoadjuvant therapy. The assessment of tumor grade can be affected in either direction, but more frequently decreased because of differential cellular loss related to the neoadjuvant chemotherapy or radiation.[3] Grade, which is based on cellular differentiation, mitotic rate, and extent of necrosis, should be recorded for all soft tissue sarcomas. A three-grade system (G1–G3) is preferred. (See below.)
The AJCC has designated staging by the four criteria of tumor size, nodal status, metastasis, and grade (TNMG).[3] The characteristic molecular markers of some sarcomas are not formally incorporated in the staging system pending further evaluation of their impact on prognosis. Recurrent sarcomas are restaged using the same system as for primary tumors with the specification that the tumor is recurrent.
Definitions of TNM and Grade
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| bSuperficial tumor is located exclusively above the superficial fascia without invasion of the fascia; deep tumor is located either exclusively beneath the superficial fascia, superficial to the fascia with invasion of or through the fascia, or both superficial yet beneath the fascia. | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| T1 | Tumor ≤5 cm in greatest dimension. (Size should be regarded as a continuous variable, and the measurement should be provided.) |
| T1a | Superficial tumor.b |
| T1b | Deep tumor.b |
| T2 | Tumor >5 cm in greatest dimension.b |
| T2a | Superficial tumor.b |
| T2b | Deep tumor. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| bPresence of positive nodes (N1) in M0 tumors is considered Stage III. | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1b | Regional lymph node metastasis. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| M0 | No distant metastasis. |
| M1 | Distant metastasis. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | ||||
| Stage IA | T1a | N0 | M0 | G1, GX |
| T1b | N0 | M0 | G1, GX | |
| Stage IB | T2a | N0 | M0 | G1, GX |
| T2b | N0 | M0 | G1, GX | |
| Stage IIA | T1a | N0 | M0 | G2, G3 |
| T1b | N0 | M0 | G2, G3 | |
| Stage IIB | T2a | N0 | M0 | G2 |
| T2b | N0 | M0 | G2 | |
| Stage III | T2a, T2b | N0 | M0 | G3 |
| Any T | N1 | M0 | Any G | |
| Stage IV | Any T | Any N | M1 | Any G |
Neurovascular and bone invasion are indicators of poor prognosis, but they are not incorporated into the formal staging system.
References
- Fong Y, Coit DG, Woodruff JM, et al.: Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 217 (1): 72-7, 1993. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
Treatment Option Overview
Multimodality Approach
In most cases, a combined modality approach of preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT) is used, rather than the radical surgical procedures, such as amputation, that were used in the past. It may even be possible to use surgery without PORT in selected cases. For example, a case series was reported from a specialized sarcoma treatment referral center in which 74 selected patients with primary extremity and trunk tumors 5 cm or smaller were found to have no histologic involvement of the surgical margins. The patients were observed without radiation therapy, and the estimated local recurrence rate after 10 years was 11%.[1][Level of evidence: 3iiiDiv] The role of chemotherapy is not as well defined as is the role for radiation therapy. Because of the evolving nature of the treatment options for this disease, patients should be considered when available. Information about ongoing clinical trials is available from the NCI website.
Role of Surgery
Surgical resection is the mainstay of therapy for soft tissue sarcomas. When feasible, wide-margin function–sparing surgical excision is the cornerstone of effective treatment for extremity tumors. This may be facilitated by soft tissue reconstructive surgery, which generally permits wider margins than those obtained when the surgical plan involves direct closure of the excision site.[2] Cutting into the tumor mass or shelling out the gross tumor along the plane of the pseudocapsule of compressed tumor cells and reactive tissue that often surrounds soft tissue sarcomas are associated with an elevated risk of local recurrence. Even high-grade, soft tissue sarcomas of the extremities can usually be effectively treated while preserving the limb with combined-modality treatment consisting of preRT or PORT to reduce local recurrence. (Refer to the Role of Radiation Therapy section of this summary for more information.)
Only one small, single-institution, randomized trial has directly compared amputation to limb-sparing surgery for soft tissue sarcomas of the extremities.[3] In a 2:1 randomization ratio, 27 patients with high-grade extremity sarcomas were assigned to a wide excision plus PORT (45 Gy–50 Gy to the wide local excision area, and a total of 60 Gy–70 Gy to the tumor bed over 6–7 weeks), and 16 were assigned to amputation at or above the joint proximal to the tumor. Both groups received adjuvant chemotherapy (i.e., doxorubicin, cyclophosphamide, and high-dose methotrexate). At 63 months, with a median follow-up of 56 months, there were four local recurrences in the 27 patients who underwent limb-sparing surgery and no recurrences in the 16 patients who underwent amputation P2 = .12. Overall survival (OS) rates were not statistically significantly different (actuarial 5-year survival rate, 83% vs. 88%, P2 = .99).[3][Level of evidence: 1iiA]
Local control of high-grade soft tissue sarcomas of the trunk and the head and neck can be achieved with surgery in combination with radiation therapy.[4] It may be possible to use surgery without PORT in selected cases. For example, a case series was reported from a specialized sarcoma treatment referral center in which 74 selected patients with primary extremity and trunk tumors 5 cm or smaller were found to have no histologic involvement of the surgical margins.[1] They were observed without radiation therapy, and the estimated local recurrence rate after 10 years was 11%.[1][Level of evidence: 3iiiDiv] The role of chemotherapy is not as well defined as is the role of radiation therapy. Because of the evolving nature of the treatment options for this disease, patients should be offered the option of clinical trials when available.
Effective treatment of retroperitoneal sarcomas requires removal of all gross disease while sparing adjacent viscera not invaded by tumor. The prognosis for patients with high-grade retroperitoneal sarcomas is less favorable than for patients with tumors at other sites, partly because of the difficulty in completely resecting these tumors and the dose-limiting toxicity of high-dose radiation therapy on visceral organs.[5-8]
In the setting of distant metastasis, surgery may be associated with long-term, disease-free survival in patients with pulmonary metastasis and optimal underlying disease biology (i.e., patients with a limited number of metastases and slow nodule growth) who have undergone or are undergoing complete resection of the primary tumor.[9-11] It is not clear to what degree the favorable outcomes are attributable to the efficacy of surgery or the careful selection of patients based on factors that are associated with less-virulent disease.
Role of Radiation Therapy
Radiation plays an important role in limb-sparing therapy. Pre- and postoperative external-beam radiation therapies (EBRT), as well as brachytherapy, have been shown to decrease the risk of local recurrence. They have not been shown to increase OS but are used to avoid amputation for all but the most locally advanced tumors or for limbs seriously compromised by vascular disease, where acceptable functional preservation is not possible. In the case of EBRT, irradiation of the entire limb circumference is avoided to preserve vascular and nerve structures that are critical to function and preservation of the limb.
PORT
PORT has been tested in a single-institution, randomized trial of 141 patients with extremity sarcomas who were treated with limb-sparing surgery. Patients with high-grade tumors (n = 91) also received adjuvant chemotherapy (i.e., five 28-day cycles of doxorubicin and cyclophosphamide). All patients were randomly assigned to receive radiation (45 Gy to a wide field, plus a tumor-bed boost of 18 Gy over 6–7 weeks), concurrent with chemotherapy in the case of high-grade tumors versus no radiation.[12] At up to 12 years of follow-up, there was one local recurrence in the 70 patients randomly assigned to receive radiation versus 17 recurrences in the 71 control patients (P = .0001), with similar reduction in risk of local recurrence for both high- and low-grade tumors. However, there was no difference in OS between the radiation and control groups.[12][Level of evidence: 1iiDiii] Global quality of life was similar in the two groups, but the radiation therapy group had substantially worse functional deficits resulting from reduced strength and joint motion as well as increased edema.
To limit acute toxicity with preRT, smaller fields and lower doses are generally given than is the case with PORT. PreRT has been directly compared with PORT for extremity soft tissue sarcomas in a multicenter randomized trial.[13-15] Designed to include 266 patients, the trial was stopped early after 190 patients had been accrued because of an increase in wound complications in the preRT group. The scheduled radiation in the preRT group was a wide field of 50 Gy in 2-Gy fractions (first phase of the trial) with an additional 16 Gy to 20 Gy to the tumor bed and a 2-cm margin (second phase of the trial) only if tumor cells were found at the surgical margins.
Patients in the PORT group were scheduled to receive radiation during both phases of the trial. The wound-complication rates were 35% versus 17% in the preRT and PORT groups, respectively (P = .01). In addition, limb function at 6 weeks after surgery was worse in the preRT group (P = .01).[13] At 5 years, the two groups had similar local control rates (93% vs. 92%) and OS (73% vs. 67%, P = .48).[14] Of the 129 patients evaluated for limb function at 21 to 27 months after surgery (n = 73 for preRT and n = 56 for PORT), limb function was similar in both groups, but there was a statistical trend for less fibrosis in the preRT group (P = .07).[15]
Brachytherapy
Brachytherapy has also been investigated as an adjuvant therapy for soft tissue sarcomas. Although it has possible advantages of convenience and less radiation to normal surrounding tissue relative to EBRT, the two treatment strategies have not been directly compared in terms of efficacy or morbidity. However, adjuvant brachytherapy has been compared with surgery without radiation. The time interval between preRT and surgical excision in extremity soft tissue sarcoma had minimal influence on the development of wound complications. Four- or 5-week intervals showed equivalent complication rates between patients who did or did not develop wound complications, suggesting an optimal interval to reduce potential complications.[16]
In a single-institution trial, 164 patients with sarcomas of the extremity or superficial trunk were randomly assigned during surgery, if all gross tumor could be excised, to receive an iridium Ir 192 implant (delivering 42 Gy–45 Gy over 4–6 days; 78 patients) or to a control arm of no radiation (86 patients).[17,18] Some of the patients with high-grade tumors received adjuvant doxorubicin-based chemotherapy if they were thought to be at a high risk for metastasis (34 patients in each study arm). With a median follow-up of 76 months, the 5-year actuarial local recurrence rates were 18% and 31% in the brachytherapy and control arms, respectively (P = .04). This difference was limited to patients with high-grade tumors. There was no discernible difference in sarcoma-specific survival rates between the brachytherapy and control arms (84% and 81%, respectively; P = .65), and there was no difference in the high tumor-grade group.[17][Level of evidence: 1iiDiii] The rates of clinically important wound complications (e.g., need for operative revision or repeated seroma drainage, wound separation, large hematomas, or purulent infection) were 24% and 14% in the radiation and control arms, respectively (P = .13); wound reoperation rates were 10% and 0%, respectively (P = .006).[18]
Intensity-modulated radiation therapy
Intensity-modulated radiation therapy (IMRT) has been used to deliver preRT or PORT to patients with extremity soft tissue sarcomas in an effort to spare the femur, joints, and selected other normal tissues from the full prescription dose and to maintain local control while potentially reducing radiation therapy-related morbidity. Initial single-institution reports suggest that high rates of local control with some reduction in morbidity are possible with this technique.[19,20] Retrospective comparison of IMRT compared with 3-dimensional, conformal radiation therapy demonstrates that local recurrence for primary soft tissue sarcomas of the extremity was worse in the non-IMRT group.[21][Level of evidence: 3iiiDiv]
Surgery and radiation therapy
In some tumors of the extremities or trunk, surgery alone can be performed without the use of radiation. Evidence for this approach is limited to single-institution, relatively small, case series [1,22,23] or analysis of outcomes in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) tumor registry.[24] However, these comparisons suffer from low statistical power and differential evaluability rates that could have introduced bias.[1] Patient selection factors may vary among surgeons. In general, this approach is considered in patients with low-grade tumors of the extremity or superficial trunk that are 5 cm or smaller in diameter (T1) and have microscopically negative surgical margins; long-term local tumor control is about 90% in such patients.[25]
A patterns-of-care study using SEER data was queried to identify patients undergoing surgery for truncal and extremity soft tissue sarcomas from 2004 to 2009.[26] Of 5,075 patients, 50% received radiation therapy. Radiation was considered to be underused in a significant portion of patients undergoing treatment for soft tissue sarcoma in the United States. Although routine radiation therapy is not recommended for stage I patients, 25% of them still underwent radiation. Even though routine radiation therapy is recommended for patients with stage II and III tumors, only 60% of them underwent radiation. On multivariate analysis, predictors of radiation therapy included age younger than 50 years (odds ratio [OR], 1.57; 95% confidence interval [CI], 1.28–1.91), malignant fibrous histiocytoma histology (OR, 1.47; 95% CI, 1.3–1.92), T2 classification (OR, 1.88; 95% CI, 1.60–2.20), and G3 (OR, 6.27; 95% CI, 5.10–7.72). Patients with stage III soft tissue sarcoma who received radiation therapy showed improved disease-specific survival at 5 years compared with those who did not (68% vs. 46%, P < .001).[26][Level of evidence: 3iDii]
On occasion, surgical excision cannot be performed in the initial management of soft tissue sarcomas because the morbidity would be unacceptable or nearby critical organs make complete resection impossible. In such circumstances, radiation has been used as the primary therapy.[27] However, this must be considered a treatment of last resort. Experience is limited to retrospective case series from single centers.[27][Level of evidence: 3iiiDiv]
Role of Adjuvant or Neoadjuvant Chemotherapy for Clinically Localized Tumors
The role of adjuvant chemotherapy is not completely clear. The investigation of its use falls into two categories or generations—pre- and post-ifosfamide regimens. In discussions with a patient, any potential benefits should be considered in the context of the short- and long-term toxicities of the chemotherapy.
First-generation trials (preifosfamide)
Several prospective, randomized trials were unable to determine conclusively whether doxorubicin-based adjuvant chemotherapy benefits adults with resectable soft tissue sarcomas. The majority of these studies accrued small numbers of patients and did not demonstrate a metastasis-free survival or an OS benefit for adjuvant chemotherapy.[4] A small study of adjuvant chemotherapy showed a positive effect on both disease-free survival (DFS) and OS in patients treated with postoperative chemotherapy.[28] There was wide interstudy variability among the reported trials, including differences in therapeutic regimens, drug doses, sample size, tumor site, and histologic grade.
A quantitative meta-analysis of updated data from 1,568 individual patients in 14 trials of doxorubicin-based adjuvant therapy showed an absolute benefit from adjuvant therapy of 6% for a local relapse-free interval (95% CI, 1%–10%), 10% for a distant relapse-free interval (95% CI, 5%–15%), and 10% for recurrence-free survival (95% CI, 5%–15%). A statistically significant OS benefit at 10 years was not detected: absolute difference 4% (95% CI, -1%–+9%).[29,30][Level of evidence: 1iiDii] However, only a small proportion of patients in this meta-analysis were treated with ifosfamide, an agent with demonstrated activity against soft tissue sarcoma. In addition, a subset analysis suggested that patients with sarcomas of the extremities may have benefited from adjuvant chemotherapy (hazard ratio [HR] for death, 0.8, P = .029), but there was no clear evidence that patients with extremity sarcomas had outcomes that were statistically significantly different from the outcomes of patients with tumors at other sites (P = .58).[30]
Second-generation trials (postifosfamide)
Subsequent chemotherapy trials were performed using anthracycline and ifosfamide combinations in patients who primarily had extremity or truncal soft tissue sarcomas. The data are conflicting, and the issue is still not settled. In a small feasibility study, 59 patients with high-risk, soft tissue sarcomas, 58 of whom had an extremity or the trunk as the primary site, underwent primary resection plus PORT and were randomly assigned to observation versus a dose-dense regimen of six 14-day courses of ifosfamide, dacarbazine (DTIC), and doxorubicin (IFADIC regimen) with granulocyte colony-stimulating factor (G-CSF) bone marrow support and mesna uroprotection.[31] There were no statistically significant differences in OS or relapse-free survival (RFS), but the study was severely underpowered.
In a second trial performed by the Italian National Council for Research, high-risk patients were treated with local therapy (i.e., wide resection plus preRT or PORT, or amputation as clinically necessary) and were then randomly assigned to observation versus five 21-day cycles of 4-epidoxorubicin (epirubicin) plus ifosfamide (with mesna and G-CSF).[28,32] Based on power calculations, the planned study size was 190 patients, but the trial was stopped after 104 patients had been entered because an interim analysis revealed a statistically significant (P = .001) difference in DFS favoring the chemotherapy arm. By the time of the initial peer-reviewed report of the study, the DFS still favored the chemotherapy group (median DFS of 48 months vs. 16 months), but the P value had risen to .04.[28]
Although there was no difference in metastasis-free survival at the time of the report, there was an improvement in median OS (75 months vs. 46 months, P = .03). However, at the follow-up report (at a median of 89.6 months in a range of 56–119 months), OS differences were no longer statistically significant (58.5% vs. 43.1% [P = .07]). The DFS difference had also lost statistical significance (47.2% vs. 16.0% [P = .09]).[32] In summary, the trial was underpowered because it was stopped early, and the early promising results that led to stopping the trial diminished as the trial matured.
In a third, underpowered, single-center trial, 88 patients with high-risk, soft tissue sarcomas (64 of whom had extremity or truncal primary tumors) underwent surgery (with or without radiation) and were then randomly assigned to receive four 21-day cycles of chemotherapy (epirubicin [n = 26] or epirubicin plus ifosfamide [n = 19]) versus no adjuvant chemotherapy (n = 43).[33] The trial was closed prematurely because of a slow accrual rate. After a median follow-up of 94 months, the 5-year DFS in the chemotherapy and control arms was 69% versus 44%, respectively (P = .01); the 5-year OS rates were 72% versus 47% (P = .06). All of the benefit associated with chemotherapy appeared restricted to the 19 patients who received epirubicin plus ifosfamide.
In yet another underpowered trial, 137 patients with high-risk, soft tissue sarcomas (93% with extremity or truncal primary tumors) who met the eligibility criteria were randomly assigned to undergo surgical resection (with or without radiation) or to receive three preoperative 21-day cycles of doxorubicin plus ifosfamide.[34] This multicenter European Organization for Research and Treatment of Cancer trial (EORTC-62874) was closed because of slow accrual and results that were not promising enough to continue. With a median follow-up of 7.3 years, the 5-year DFS in the surgery alone and chemotherapy plus surgery arms was 52% and 56%, respectively (P = .35); and OS was 64% and 65%, respectively (P = .22).
These last four trials have been combined with the 14 first-generation trials in a trial-level meta-analysis.[35] Of the 18 randomized trials of patients with resectable soft tissue sarcomas, five trials used a combination of doxorubicin (50–90 mg/m2 per cycle) plus ifosfamide (1,500–5,000 mg/m2 per cycle). The remaining 13 trials used doxorubicin (50–70 mg/m2 per cycle) alone or with other drugs. The absolute risk reduction in local recurrence rates associated with any chemotherapy added to local therapy was 4 percentage points (95% CI, 0%–7%), and it was 5 percentage points (95% CI, 1%–12%) when ifosfamide was combined with doxorubicin. The absolute reduction in overall mortality was 6 percentage points with any chemotherapy (95% CI, 2%–11%; [i.e., a reduction from 46%–40%]), 11 percentage points for doxorubicin plus ifosfamide (95% CI, 3%–19%; [i.e., a reduction from 41%–30%]), and 5 percentage points for doxorubicin without ifosfamide.[35][Level of evidence: 1iiA]
An additional multicenter randomized trial (EORTC-62931 [NCT00002641]), the largest trial reported to date using adjuvant doxorubicin (75 mg/m2) plus ifosfamide (5,000 mg/m2), was subsequently published in abstract form and was not included in the above meta-analysis.[36] The results differed from those reported in the meta-analysis.[35] After local therapy, 351 patients were randomly assigned to five 21-day cycles of adjuvant therapy versus observation. The trial was stopped for futility because the 5-year RFS was 52% in both arms. OS was 64% in the chemotherapy arm versus 69% in the observation arm. In a subsequent abstract, the EORTC investigators reported a combined analysis of this trial and their previous trial (EORTC-62771) [37] of adjuvant cyclophosphamide plus doxorubicin plus DTIC (CYVADIC), representing the two largest trials of adjuvant therapy for adult soft tissue sarcoma in the literature.[38] The combined analysis showed no improvement in either RFS or OS associated with adjuvant chemotherapy.[38][Level of evidence: 1iiA]
In summary, the impact of adjuvant chemotherapy on survival is not clear but is likely to be small in absolute magnitude. Therefore, in discussions with a patient, any potential benefits should be considered in the context of the short- and long-term toxicities of the chemotherapy.
Role of regional hyperthermia
The use of regional hyperthermia to enhance the local effects of systemic chemotherapy in the neoadjuvant and adjuvant setting is under investigation. In a multicenter phase III trial, 341 patients with high-risk (tumor ≥5 cm, grade 2–3, and deep to fascia), soft tissue sarcomas (149 extremity tumors and 192 nonextremity tumors) were randomly allocated to receive four 21-day cycles of chemotherapy (etoposide 125 mg/m2 on days 1 and 4; ifosfamide 1,500 mg/m2 on days 1–4; doxorubicin 50 mg/m2 on day 1) with or without regional hyperthermia both before and after local therapy.[39] Approximately 11% of the patients were being treated for recurrent tumors. The regional hyperthermia was designed to produce tumor temperatures of 42°C for 60 minutes and was given on days 1 and 4 of each chemotherapy cycle. After the first four cycles of chemotherapy, definitive surgical excision of the tumor was performed, if possible, followed by radiation therapy, if indicated (i.e., a 52.7 Gy median dose delivered), and then the last four cycles of chemotherapy plus or minus hyperthermia. Three of the nine treatment centers with particular expertise in hyperthermia treated 91% of the patients in the trial.
The median duration of follow-up was 34 months. Local progression occurred in 56 patients in the hyperthermia group and 76 patients in the control group. The relative HR for local progression or death was 0.58 (95% CI, 0.41–0.84), with an absolute difference at 2 years of 15% (76% vs. 61%; 95% CI of the difference 6–26). The decreased risk of local progression or death was seen in both extremity and nonextremity tumors. However, hyperthermia had no effect on distant failure rates nor was there a statistically significant effect on OS (HR, .88, 95% CI, 0.64–1.21; P = .43).[39][Level of evidence: 1iiDiii] There was a higher rate of grade 3 to 4 leucopenia in the hyperthermia group: 77.6% versus 63.5% (P = .005). Since a large proportion of the patients were treated at centers with special expertise, there is no certainty that the finding can be generalized to apply to other settings.
Role of isolated limb perfusion
Isolated limb perfusion is under investigation as a means to deliver high doses of chemotherapy and permit limb salvage in unresectable primary or recurrent extremity soft tissue sarcomas that would otherwise require amputation, in the opinion of the surgeon.[40,41] Common drugs used in the procedure are TNF-alpha, melphalan, and interferon-gamma. Experience is limited to case series with response rates and reported avoidance of amputation as the outcome.[40,41][Level of evidence: 3iiiDiv] The technique requires specialized expertise to avoid severe local and systemic toxicity including systemic effects of TNF-alpha. The technique has not been directly compared with standard approaches using combined systemic and local therapy.
Role of chemotherapy for advanced disease
Doxorubicin is a mainstay of systemic therapy in the management of locally advanced and metastatic soft tissue sarcoma. Pegylated liposomal encapsulated doxorubicin is a formulation of doxorubicin designed to prolong the half-life of circulating doxorubicin and slow the release of active drugs.[42] The changed pharmacokinetics result in less myelosuppression and possibly less cardiotoxic effects, but there is a substantial incidence of hypersensitivity-like reactions and hand-foot syndrome. Its clinical activity relative to unencapsulated doxorubicin is not clear.[42][Level of evidence: 3iiiDiv] Other drugs that are thought to have clinical activity as single agents are ifosfamide, epirubicin, gemcitabine, and paclitaxel.[43-46][Level of Evidence: 3iiiDiv] Their clinical activity relative to single-agent doxorubicin is not clear, and they are not known to have superior activity.
There is controversy about the clinical benefit of adding other drugs to doxorubicin as a single agent. A systematic evidence review and meta-analysis conducted by the Cochrane Collaboration summarized the eight randomized trials reported from 1976 to 1995.[47] No additional randomized trials had been reported or were known to be in progress between 1995 and the 2002 literature search. Single-agent doxorubicin had been compared with a variety of doxorubicin-containing combinations that included vincristine, vindesine, cyclophosphamide, streptozotocin, mitomycin-C, cisplatin, and/or ifosfamide. Combination regimens consistently caused more nausea and hematologic toxicity. However, the better response rates associated with combination therapy were marginal and depended on the statistical model used (fixed effects model ORresponse = 1.29; 95% CI, 1.03–1.60, P = .03; random effects model ORresp = 1.26; 95% CI, 0.96–1.67, P = .10) There was no statistically significant difference in the 1- (ORmortality = 0.87; 95% CI, 0.73–1.05, P = .14) or 2-year mortality rates (ORmortality = 0.84; 95% CI, 0.67–1.06, P = .13).
These results were very similar even when the analyses were restricted to the four trials that used DTIC and/or ifosfamide as part of the combination regimen with doxorubicin agents that were postulated to have greater activity than the others tested. A subsequent meta-analysis of all three published randomized trials of chemotherapy regimens that contained ifosfamide versus those that did not came to similar conclusions: tumor response rates were better when the regimen included ifosfamide (RRresponse = 1.52; 95% CI, 1.11–2.08), but mortality at 1 year was not (RRmortality = 0.98; 95% CI, 0.85–1.13).[48][Level of evidence: 1iiDiv]. Therefore, response rate was a poor surrogate for OS. Quality-of-life outcomes were not reported in any of the above-mentioned randomized trials, but toxicity was worse when agents were added to doxorubicin.
References
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Lohman RF, Nabawi AS, Reece GP, et al.: Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 94 (8): 2256-64, 2002. [PUBMED Abstract]
- Rosenberg SA, Tepper J, Glatstein E, et al.: The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305-15, 1982. [PUBMED Abstract]
- O'Byrne K, Steward WP: The role of adjuvant chemotherapy in the treatment of adult soft tissue sarcomas. Crit Rev Oncol Hematol 27 (3): 221-7, 1998. [PUBMED Abstract]
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Lewis JJ, Leung D, Woodruff JM, et al.: Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 228 (3): 355-65, 1998. [PUBMED Abstract]
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Griffin AM, Dickie CI, Catton CN, et al.: The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann Surg Oncol 22 (9): 2824-30, 2015. [PUBMED Abstract]
- Pisters PW, Harrison LB, Leung DH, et al.: Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 14 (3): 859-68, 1996. [PUBMED Abstract]
- Alektiar KM, Zelefsky MJ, Brennan MF: Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys 47 (5): 1273-9, 2000. [PUBMED Abstract]
- Alektiar KM, Brennan MF, Healey JH, et al.: Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 26 (20): 3440-4, 2008. [PUBMED Abstract]
- Alektiar KM, Brennan MF, Singer S: Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer 117 (14): 3229-34, 2011. [PUBMED Abstract]
- Folkert MR, Singer S, Brennan MF, et al.: Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol 32 (29): 3236-41, 2014. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Bagaria SP, Ashman JB, Daugherty LC, et al.: Compliance with National Comprehensive Cancer Network guidelines in the use of radiation therapy for extremity and superficial trunk soft tissue sarcoma in the United States. J Surg Oncol 109 (7): 633-8, 2014. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
- Frustaci S, Gherlinzoni F, De Paoli A, et al.: Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 19 (5): 1238-47, 2001. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Sarcoma Meta-analysis Collaboration (SMAC): Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev (4): CD001419, 2000. [PUBMED Abstract]
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
- Frustaci S, De Paoli A, Bidoli E, et al.: Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology 65 (Suppl 2): 80-4, 2003. [PUBMED Abstract]
- Petrioli R, Coratti A, Correale P, et al.: Adjuvant epirubicin with or without Ifosfamide for adult soft-tissue sarcoma. Am J Clin Oncol 25 (5): 468-73, 2002. [PUBMED Abstract]
- Gortzak E, Azzarelli A, Buesa J, et al.: A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. Eur J Cancer 37 (9): 1096-103, 2001. [PUBMED Abstract]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al.: A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113 (3): 573-81, 2008. [PUBMED Abstract]
- Woll PJ, van Glabbeke M, Hohenberger P, et al.: Adjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): Interim analysis of a randomised phase III trial. [Abstract] J Clin Oncol 25 (Suppl 18): A-10008, 2007.
- Bramwell V, Rouesse J, Steward W, et al.: Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 12 (6): 1137-49, 1994. [PUBMED Abstract]
- Le Cesne A, Van Glabbeke M, Woll PJ, et al.: The end of adjuvant chemotherapy (adCT) era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma (STS): pooled analysis of the two STBSG-EORTC phase III clinical trials. [Abstract] J Clin Oncol 26 (Suppl 15): A-10525, 2008.
- Issels RD, Lindner LH, Verweij J, et al.: Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 11 (6): 561-70, 2010. [PUBMED Abstract]
- Eggermont AM, de Wilt JH, ten Hagen TL: Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 4 (7): 429-37, 2003. [PUBMED Abstract]
- Bonvalot S, Laplanche A, Lejeune F, et al.: Limb salvage with isolated perfusion for soft tissue sarcoma: could less TNF-alpha be better? Ann Oncol 16 (7): 1061-8, 2005. [PUBMED Abstract]
- Grenader T, Goldberg A, Hadas-Halperin I, et al.: Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20 (1): 15-20, 2009. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
- Bramwell VH, Anderson D, Charette ML, et al.: Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev (3): CD003293, 2003. [PUBMED Abstract]
- Verma S, Younus J, Stys-Norman D, et al.: Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 34 (4): 339-47, 2008. [PUBMED Abstract]
Stage I Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery and radiation therapy.
Low-grade soft tissue sarcomas have little metastatic potential, but they have a propensity to recur locally. Accordingly, surgical excision with negative tissue margins of 1 cm to 2 cm or larger in all directions is the treatment of choice for patients with these early-stage sarcomas.[1-3] The Mohs surgical technique may be considered as an alternative to wide surgical excision for the very rare, small, well-differentiated primary sarcomas of the skin when cosmetic results are considered to be important, as margins can be assured with minimal normal tissue removal.[4]
Carefully executed high-dose radiation therapy using a shrinking-field technique may be beneficial for unresectable tumors or for resectable tumors in which a high likelihood of residual disease is thought to be present when margins are judged to be inadequate, and when wider resection would require either an amputation or the removal of a vital organ.[5] Because of the low metastatic potential of these tumors, chemotherapy is usually not given.[6,7]
Standard treatment options:
- Surgical excision of tumors 5 cm or smaller in diameter with negative tissue margins in all directions.[8-12]
- Surgical excision with preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT). Radiation decreases the risk of local recurrence but has not been shown to increase overall survival.[13-16]
- If the tumor is unresectable, high-dose preRT may be used.[17]
- For tumors of the retroperitoneum, trunk, and head and neck, the following are options:
- Surgical resection with the option of PORT if negative margins cannot be obtained. Wide margins are unusual in these sites, and radiation therapy is usually advocated for trunk and head and neck primary sites.[18]
- PreRT followed by maximal surgical resection. Radiation therapy may be used in sarcomas of the trunk and head and neck to maximize local control because of the inability to obtain wide surgical margins.[19]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Fish FS: Soft tissue sarcomas in dermatology. Dermatol Surg 22 (3): 268-73, 1996. [PUBMED Abstract]
- Temple WJ, Temple CL, Arthur K, et al.: Prospective cohort study of neoadjuvant treatment in conservative surgery of soft tissue sarcomas. Ann Surg Oncol 4 (7): 586-90, 1997 Oct-Nov. [PUBMED Abstract]
- Sarcoma Meta-analysis Collaboration (SMAC): Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev (4): CD001419, 2000. [PUBMED Abstract]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al.: A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113 (3): 573-81, 2008. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
- Brennan MF, Singer S, Maki RG: Sarcomas of the soft tissue and bone. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds.: Cancer: Principles and Practice of Oncology. Vols. 1 & 2. 8th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2008, pp 1741-1833.
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
Stage II and Node-Negative Stage III Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
High-grade localized soft tissue sarcomas have an increased potential for local recurrence and metastasis. For sarcomas of the extremities, local control comparable to that obtained with amputation may be achieved with limb-sparing surgery that involves wide local excision in combination with preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT).
Complete surgical resection is often difficult for sarcomas of the retroperitoneum because of their large size before detection and anatomical location.[1,2] As opposed to soft tissue sarcomas of the extremities, local recurrence is the most common cause of death in patients with retroperitoneal soft tissue sarcomas. Complete surgical resection (i.e., removal of the entire gross tumor) is the most important factor in preventing local recurrence and, in many instances, requires resection of adjacent viscera. For retroperitoneal sarcomas, retrospective comparison of surgery alone versus preRT review suggests that preRT is associated with improved local recurrence-free survival, but not disease-free survival.[3]
Standard treatment options:
- Surgical excision with preRT or PORT. Radiation decreases the risk of local recurrence but has not been shown to increase overall survival.[4-8]
- Surgical excision with negative tissue margins in all directions. This approach is generally restricted to low-grade tumors ( ≤5 cm in diameter) of the extremities or superficial trunk with microscopically negative surgical tumor margins.[9-13]
- If the tumor is unresectable, high-dose radiation therapy may be used, but poor local control is likely to result.[14]
- In some situations, radiation therapy and/or chemotherapy may be used before surgery in an attempt to convert a marginally resectable tumor to one that can be adequately resected with limb preservation; this treatment may be followed by PORT.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Heslin MJ, Lewis JJ, Nadler E, et al.: Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol 15 (8): 2832-9, 1997. [PUBMED Abstract]
- Jaques DP, Coit DG, Hajdu SI, et al.: Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 212 (1): 51-9, 1990. [PUBMED Abstract]
- Kelly KJ, Yoon SS, Kuk D, et al.: Comparison of Perioperative Radiation Therapy and Surgery Versus Surgery Alone in 204 Patients With Primary Retroperitoneal Sarcoma: A Retrospective 2-Institution Study. Ann Surg 262 (1): 156-62, 2015. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- Rosenberg SA, Tepper J, Glatstein E, et al.: The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305-15, 1982. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
Advanced Stage III (N1) Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
Regional lymph node involvement by soft tissue sarcomas of adulthood is very infrequent. However, sarcoma types that more commonly spread to lymph nodes include high-grade rhabdomyosarcoma, vascular sarcomas, and epithelioid sarcomas.[1]
Standard treatment options:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Watson DI, Coventry BJ, Langlois SL, et al.: Soft-tissue sarcoma of the extremity. Experience with limb-sparing surgery. Med J Aust 160 (7): 412-6, 1994. [PUBMED Abstract]
- Cormier JN, Huang X, Xing Y, et al.: Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol 22 (22): 4567-74, 2004. [PUBMED Abstract]
- O'Byrne K, Steward WP: The role of adjuvant chemotherapy in the treatment of adult soft tissue sarcomas. Crit Rev Oncol Hematol 27 (3): 221-7, 1998. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
Stage IV Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
In the setting of lung metastasis, resection of metastatic tumors may be associated with long-term disease-free survival in patients selected for optimal underlying disease biology (i.e., patients with a limited number of metastases and slow tumor growth).[1-3] It is not clear to what degree the favorable outcomes are attributable to the efficacy of surgery or to careful selection of patients based upon factors that are associated with less-virulent disease.[1-3] The value of resection of hepatic metastases is unclear.
As noted in the Treatment Option Overview section above, doxorubicin is the standard systemic therapy in the management of metastatic sarcomas.[4,5] Other drugs that may have clinical activity as single agents are ifosfamide, epirubicin, gemcitabine, and paclitaxel.[6-9] Their clinical activity relative to single-agent doxorubicin is not clear, and they are not known to have superior activity. There is controversy about whether adding drugs to doxorubicin offers clinical benefit beyond what is achieved by doxorubicin as a single agent. To avoid severe toxicity in older patients, sequential use of single agents may be the preferred strategy for palliation.
A randomized study assessed whether dose intensification of doxorubicin with ifosfamide improved the survival of patients with advanced soft-tissue sarcoma compared with doxorubicin alone.[10] Two hundred twenty-eight patients were randomly assigned to receive doxorubicin, and 227 patients were randomly assigned to receive doxorubicin and ifosfamide. Median follow-up was 56 months (interquartile range [IQR], 31–77) in the doxorubicin-only group and 59 months (IQR, 36–72) in the combination group.
There was no significant difference in overall survival (OS) between groups (median OS, 12.8 months; 95.5% confidence interval [CI], 10.5–14.3 in the doxorubicin-alone group vs. 14.3 months; range, 12.5–16.5 months in the doxorubicin and ifosfamide group; hazard ratio [HR], 0.83; 95.5% CI 0.67–1.03; stratified log-rank test P = .076). Median progression-free survival was significantly higher for the doxorubicin and ifosfamide group (7.4 months; 95% CI, 6.6-8.3) than for the doxorubicin-alone group (4.6 months; range, 2.9–5.6 months; HR, 0.74; 95% CI, 0.60–0.90; stratified log-rank test P = .003). More patients in the doxorubicin and ifosfamide group than in the doxorubicin-alone group had an overall response (60 [26%] of 227 patients vs. 31 [14%] of 228; P < .0006). The most common grade 3 and 4 toxic effects, which were all more common with doxorubicin and ifosfamide than with doxorubicin alone, were leucopenia (97 [43%] of 224 patients vs. 40 [18%] of 223 patients), neutropenia (93 [42%] vs. 83 [37%]), febrile neutropenia (103 (46%) vs. 30 [13%]), anemia (78 [35%] vs. 10 [5%]), and thrombocytopenia (75 [33%]) vs. 1 [<1%]).[10][Level of evidence: 1iiA] Treatment intensification with doxorubicin and ifosfamide for palliation of advanced soft tissue sarcoma is not indicated.
Standard treatment options
- Chemotherapy.
- Single-agent chemotherapy, with subsequent single agents for disease regrowth.[4-6,8,9,11] Doxorubicin is generally the first-line agent. Ifosfamide also has substantial single-agent activity.
- Doxorubicin-based combination chemotherapy. A variety of regimens have been used, but none has been proven to increase OS compared with doxorubicin alone.[4,5] There is some evidence that the addition of ifosfamide increases response rates (but not survival). Toxicity is increased with the addition of drugs to doxorubicin. No quality-of-life studies have been reported in comparisons of single-agent therapy versus combination therapy.
- Resection of pulmonary lesions may be performed if the primary tumor is under control.[1-3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Bramwell VH, Anderson D, Charette ML, et al.: Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev (3): CD003293, 2003. [PUBMED Abstract]
- Verma S, Younus J, Stys-Norman D, et al.: Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 34 (4): 339-47, 2008. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
- Judson I, Verweij J, Gelderblom H, et al.: Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 15 (4): 415-23, 2014. [PUBMED Abstract]
- Grenader T, Goldberg A, Hadas-Halperin I, et al.: Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20 (1): 15-20, 2009. [PUBMED Abstract]
Recurrent Adult Soft Tissue Sarcoma
Treatment of patients with recurrent soft tissue sarcoma depends on the type of initial presentation and treatment. Patients who develop a local recurrence often can be treated by local therapy: surgical excision plus radiation therapy after previous minimal therapy or amputation after previous aggressive treatment.[1-7] Resection of limited pulmonary metastases may be associated with favorable disease-free survival.[8-10][Level of evidence: 3iiiDiv] However, the contribution of selection factors, such as low tumor burden, slow tumor growth, and long disease-free interval, to these favorable outcomes is not known.
There is no standard chemotherapy for recurrent soft tissue sarcomas that have progressed after doxorubicin as a single agent or in combination with other agents that have clinical activity, such as ifosfamide, epirubicin, gemcitabine, and paclitaxel. Any of these agents not previously administered to the patient may be used sequentially at the time of recurrence or progression.[11-14][Level of Evidence: 3iiiDiv] None of these agents has been shown to increase overall survival in this setting, therefore, clinical trials are an appropriate option.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Midis GP, Pollock RE, Chen NP, et al.: Locally recurrent soft tissue sarcoma of the extremities. Surgery 123 (6): 666-71, 1998. [PUBMED Abstract]
- Essner R, Selch M, Eilber FR: Reirradiation for extremity soft tissue sarcomas. Local control and complications. Cancer 67 (11): 2813-7, 1991. [PUBMED Abstract]
- Singer S, Antman K, Corson JM, et al.: Long-term salvageability for patients with locally recurrent soft-tissue sarcomas. Arch Surg 127 (5): 548-53; discussion 553-4, 1992. [PUBMED Abstract]
- Lewis JJ, Leung D, Heslin M, et al.: Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 15 (2): 646-52, 1997. [PUBMED Abstract]
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
Changes to This Summary (02/01/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Adult Soft Tissue Sarcoma
Updated statistics with estimated new cases and deaths for 2018 (cited American Cancer Society as reference 1).
Stage Information for Adult Soft Tissue Sarcoma
An editorial change was made to this section.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult soft tissue sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Adult Soft Tissue Sarcoma Treatment are:
- Russell S. Berman, MD (New York University School of Medicine)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Adult Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/adult-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389481]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: February 1, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/2127.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:00:45.0
Adult Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Adult Soft Tissue Sarcoma
Incidence and Mortality
Estimated new cases and deaths from soft tissue sarcoma in the United States in 2018:[1]
- New cases: 13,040.
- Deaths: 5,150.
Soft tissue sarcomas are malignant tumors that arise in any of the mesodermal tissues of the extremities (50%), trunk and retroperitoneum (40%), or head and neck (10%). The reported international incidence rates range from 1.8 to 5 per 100,000 individuals per year.[2]
Risk Factors and Genetic Factors
The risk of sporadic soft tissue sarcomas is increased by previous radiation therapy and, in the case of lymphangiosarcoma, by chronic lymphedema. The chemicals Thorotrast (thorium dioxide), vinyl chloride, and arsenic are also established carcinogens for hepatic angiosarcomas.[3-5]
Soft tissue sarcomas occur with greater frequency in patients with the following inherited syndromes:[3-5]
- Nevoid basal cell carcinoma syndrome (Gorlin syndrome: PTC gene mutation).
- Gardner syndrome (APC mutation).
- Li-Fraumeni syndrome (p53 mutation).
- Tuberous sclerosis (Bourneville disease: TSC1 or TSC2 mutation).
- von Recklinghausen disease (neurofibromatosis type 1: NF1 mutation).
- Werner syndrome (adult progeria: WRN mutation).
Diagnosis
Soft tissue sarcomas may be heterogeneous, so adequate tissue should be obtained via either core-needle or incisional biopsy for microscopic examination to determine histologic type and tumor grade. Careful planning of the initial biopsy is important to avoid compromising subsequent curative resection. Since the selection of treatment is determined by the grade of the tumor, it is essential to have a careful review of the biopsy tissue by a pathologist who is experienced in diagnosing sarcomas. Complete staging and treatment planning by a multidisciplinary team of cancer specialists is required to determine the optimal treatment for patients with this disease.
There is evidence that at least some favorable clinical outcomes may be associated with referral to a specialized sarcoma treatment center. In a population-based consecutive series of 375 soft tissue sarcoma patients in Sweden, local recurrence rates of resected tumors were higher in patients who were not referred to the specialized center: in 35 of 78 (45%) patients not referred; in 24 of 102 (24%) patients referred after initial surgery or incisional biopsy; and in 36 of 195 (18%) patients referred before any surgical procedure (P = .0001 for the difference between those never referred vs. those referred before any surgical procedure).[6][Level of evidence: 3iDii] However, there were no statistically significant differences in death from sarcoma between the groups of patients.
Prognostic Factors
The prognosis for patients with adult soft tissue sarcomas depends on several factors, including:[3-5,7,8]
- Patient’s age.
- Size, sarcoma subtype, histologic grade, mitotic activity, and stage of the tumor.
Factors associated with a poorer prognosis include the following:[9]
- Age older than 60 years.
- Tumors larger than 5 cm in greatest dimension.
- High-grade histology with high mitotic activity.
- Positive margins after resection.[10]
Although low-grade tumors are usually curable by surgery alone, higher-grade sarcomas (as determined by the mitotic index and by the presence of hemorrhage and necrosis) are associated with higher local-treatment failure rates and increased metastatic potential.
Surveillance for Relapse
A retrospective review included 174 consecutive patients with a soft tissue sarcoma of the limb who underwent follow-up by oncologists at a single center from 2003 to 2009.[11] The rate and site of recurrence and mode of detection were analyzed. Eighty-two patients (47%) experienced relapse. Isolated local recurrences occurred in 26 patients and local relapse with synchronous pulmonary metastases occurred in 5 patients. Local recurrences were detected clinically in 30 of the 31 patients; magnetic resonance imaging identified only one local recurrence. Twenty-eight patients developed isolated lung metastases; in 9 patients, the lung metastases were amenable to resections, 7 of whom were free of disease after treatment. Lung metastases were detected by chest x-ray in 19 patients, by computed tomography scanning in 3 patients, and clinically in 11 patients. Twenty-three patients developed nonpulmonary metastases. More than 80% of the relapses occurred in the first 2 years of follow-up; however, later recurrences were also observed.[11][Level of evidence: 3iiDi] This study supports imaging surveillance for detection of lung metastases, whereas local recurrences at the primary site were usually detected by clinical examination. The impact of picking up metastases from overall survival or quality-of-life data is unknown.
Related Summaries
Other PDQ summaries containing information about soft tissue sarcoma include:
References
- American Cancer Society: Cancer Facts and Figures 2018. Atlanta, Ga: American Cancer Society, 2018. Available online. Last accessed January 5, 2018.
- Wibmer C, Leithner A, Zielonke N, et al.: Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol 21 (5): 1106-11, 2010. [PUBMED Abstract]
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Gustafson P, Dreinhöfer KE, Rydholm A: Soft tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand 65 (1): 47-50, 1994. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Kasper B, Ouali M, van Glabbeke M, et al.: Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer 49 (2): 449-56, 2013. [PUBMED Abstract]
- Vraa S, Keller J, Nielsen OS, et al.: Prognostic factors in soft tissue sarcomas: the Aarhus experience. Eur J Cancer 34 (12): 1876-82, 1998. [PUBMED Abstract]
- Trovik LH, Ovrebo K, Almquist M, et al.: Adjuvant radiotherapy in retroperitoneal sarcomas. A Scandinavian Sarcoma Group study of 97 patients. Acta Oncol 53 (9): 1165-72, 2014. [PUBMED Abstract]
- Rothermundt C, Whelan JS, Dileo P, et al.: What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 110 (10): 2420-6, 2014. [PUBMED Abstract]
Cellular Classification of Adult Soft Tissue Sarcoma
Soft tissue sarcomas are classified histologically according to the soft tissue cell of origin. Additional studies, including electron microscopy, specialized immunohistochemistry, flow cytometry, cytogenetics, and tissue culture studies may allow identification of particular subtypes within the major histologic categories. For example, S100 antigen suggests neural sheath origin, cytokeratin suggests epithelioid or synovial cell origin, and factor VIII-related antigen suggests endothelial origin. Likewise, some subtypes of sarcomas have characteristic genetic markers, but these markers are not generally used in the routine clinical setting (e.g., translocation t(X;18)(p11;q11) in synovial sarcomas and translocation t(12;16)(q13;p11) in myxoid and round-cell sarcomas).[1-3]
The histologic grade reflects the metastatic potential of these tumors more accurately than the classic cellular classification listed below. Pathologists assign a grade based on the number of mitoses per high-powered field, the presence of necrosis, cellular and nuclear morphology, and the degree of cellularity; discordance among expert pathologists regarding tumor grade, and even histologic subtype, can be substantial.[4]
The World Health Organization lists the following cell types in its classification of soft tissue sarcomas:[5,6]
- Adipocytic tumors.
- Dedifferentiated liposarcoma.*
- Myxoid/round cell liposarcoma.
- Pleomorphic liposarcoma.
- Fibroblastic/myofibroblastic tumors.
- Fibrosarcoma.**
- Myxofibrosarcoma, low grade.
- Low-grade fibromyxoid sarcoma.
- Sclerosing epithelioid fibrosarcoma.
- So-called fibrohistiocytic tumors.
- Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (including pleomorphic, giant cell, myxoid/high-grade myxofibrosarcoma, and inflammatory forms).
- Smooth muscle tumors.
- Leiomyosarcoma.
- Skeletal muscle tumors.
- Rhabdomyosarcoma (embryonal, alveolar, and pleomorphic forms).
- Vascular tumors.
- Epithelioid hemangioendothelioma.
- Angiosarcoma, deep.***
- Tumors of peripheral nerves.
- Malignant peripheral nerve sheath tumor.
- Chondro-osseous tumors.
- Extraskeletal chondrosarcoma (mesenchymal and other variants).
- Extraskeletal osteosarcoma.
- Tumors of uncertain differentiation.
- Synovial sarcoma.
- Epithelioid sarcoma.
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Extraskeletal myxoid chondrosarcoma.
- Primitive neuroectodermal tumor/extraskeletal Ewing tumor.
- Desmoplastic small round cell tumor.
- Extrarenal rhabdoid tumor.
- Undifferentiated sarcoma; sarcoma, not otherwise specified.
[Note: *It is recognized that dedifferentiated liposarcoma primarily arises in the context of deep atypical lipomatous tumor/well-differentiated liposarcoma, a sarcoma of intermediate malignancy because of the lack of metastatic capacity. **The category of fibrosarcoma can be inclusive of fibrosarcomatous differentiation in dermatofibrosarcoma protuberans. ***Cutaneous angiosarcoma may be difficult to stage using the American Joint Committee on Cancer system. (Refer to the PDQ summary on Gastrointestinal Stromal Tumors for more information.)]
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Alvegård TA, Berg NO: Histopathology peer review of high-grade soft tissue sarcoma: the Scandinavian Sarcoma Group experience. J Clin Oncol 7 (12): 1845-51, 1989. [PUBMED Abstract]
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
Stage Information for Adult Soft Tissue Sarcoma
Note: The American Joint Committee on Cancer (AJCC) has published the 8th edition of the AJCC Cancer Staging Manual, which includes revisions to the staging for this disease. Implementation of the 8th edition began in January 2018. The PDQ Adult Treatment Editorial Board, which maintains this summary, is reviewing the revised staging and will make appropriate changes as needed.
Staging has an important role in determining the most effective treatment for soft tissue sarcoma. Clinical staging involves magnetic resonance imaging (MRI) or computed tomography (CT) of the primary tumor area and a chest CT to look for metastasis to the lung (the most common site of distant spread). An abdominal CT scan is done in the case of retroperitoneal sarcomas because the liver may be the site of initial clinical metastasis for these tumors.
The stage is determined by the size of the tumor, the histologic grade, and whether there is spread to lymph nodes or distant sites. Intracompartmental or extracompartmental extension of extremity sarcomas is also important for surgical decision making. For complete staging, a thorough review of all biopsy specimens (including those from the primary tumor, lymph nodes, or other suspicious lesions) is essential. CT scan of the chest is recommended for sarcomas larger than 5 cm (T2) or with moderate to poor differentiation (grades 2–4). Nodal involvement is rare, occurring in fewer than 3% of patients with sarcoma.[1]
Lymph node involvement in soft tissue sarcomas of adulthood is rare but is somewhat more frequent in some subtypes (e.g., rhabdomyosarcoma, vascular sarcomas, clear cell sarcomas, and epithelioid sarcomas) when they are high grade.[2] Because treatment decisions are predicated on pathology staging, patients should be staged before, and again after, any neoadjuvant therapy. The assessment of tumor grade can be affected in either direction, but more frequently decreased because of differential cellular loss related to the neoadjuvant chemotherapy or radiation.[3] Grade, which is based on cellular differentiation, mitotic rate, and extent of necrosis, should be recorded for all soft tissue sarcomas. A three-grade system (G1–G3) is preferred. (See below.)
The AJCC has designated staging by the four criteria of tumor size, nodal status, metastasis, and grade (TNMG).[3] The characteristic molecular markers of some sarcomas are not formally incorporated in the staging system pending further evaluation of their impact on prognosis. Recurrent sarcomas are restaged using the same system as for primary tumors with the specification that the tumor is recurrent.
Definitions of TNM and Grade
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| bSuperficial tumor is located exclusively above the superficial fascia without invasion of the fascia; deep tumor is located either exclusively beneath the superficial fascia, superficial to the fascia with invasion of or through the fascia, or both superficial yet beneath the fascia. | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| T1 | Tumor ≤5 cm in greatest dimension. (Size should be regarded as a continuous variable, and the measurement should be provided.) |
| T1a | Superficial tumor.b |
| T1b | Deep tumor.b |
| T2 | Tumor >5 cm in greatest dimension.b |
| T2a | Superficial tumor.b |
| T2b | Deep tumor. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| bPresence of positive nodes (N1) in M0 tumors is considered Stage III. | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1b | Regional lymph node metastasis. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| M0 | No distant metastasis. |
| M1 | Distant metastasis. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | ||||
| Stage IA | T1a | N0 | M0 | G1, GX |
| T1b | N0 | M0 | G1, GX | |
| Stage IB | T2a | N0 | M0 | G1, GX |
| T2b | N0 | M0 | G1, GX | |
| Stage IIA | T1a | N0 | M0 | G2, G3 |
| T1b | N0 | M0 | G2, G3 | |
| Stage IIB | T2a | N0 | M0 | G2 |
| T2b | N0 | M0 | G2 | |
| Stage III | T2a, T2b | N0 | M0 | G3 |
| Any T | N1 | M0 | Any G | |
| Stage IV | Any T | Any N | M1 | Any G |
Neurovascular and bone invasion are indicators of poor prognosis, but they are not incorporated into the formal staging system.
References
- Fong Y, Coit DG, Woodruff JM, et al.: Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 217 (1): 72-7, 1993. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
Treatment Option Overview
Multimodality Approach
In most cases, a combined modality approach of preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT) is used, rather than the radical surgical procedures, such as amputation, that were used in the past. It may even be possible to use surgery without PORT in selected cases. For example, a case series was reported from a specialized sarcoma treatment referral center in which 74 selected patients with primary extremity and trunk tumors 5 cm or smaller were found to have no histologic involvement of the surgical margins. The patients were observed without radiation therapy, and the estimated local recurrence rate after 10 years was 11%.[1][Level of evidence: 3iiiDiv] The role of chemotherapy is not as well defined as is the role for radiation therapy. Because of the evolving nature of the treatment options for this disease, patients should be considered when available. Information about ongoing clinical trials is available from the NCI website.
Role of Surgery
Surgical resection is the mainstay of therapy for soft tissue sarcomas. When feasible, wide-margin function–sparing surgical excision is the cornerstone of effective treatment for extremity tumors. This may be facilitated by soft tissue reconstructive surgery, which generally permits wider margins than those obtained when the surgical plan involves direct closure of the excision site.[2] Cutting into the tumor mass or shelling out the gross tumor along the plane of the pseudocapsule of compressed tumor cells and reactive tissue that often surrounds soft tissue sarcomas are associated with an elevated risk of local recurrence. Even high-grade, soft tissue sarcomas of the extremities can usually be effectively treated while preserving the limb with combined-modality treatment consisting of preRT or PORT to reduce local recurrence. (Refer to the Role of Radiation Therapy section of this summary for more information.)
Only one small, single-institution, randomized trial has directly compared amputation to limb-sparing surgery for soft tissue sarcomas of the extremities.[3] In a 2:1 randomization ratio, 27 patients with high-grade extremity sarcomas were assigned to a wide excision plus PORT (45 Gy–50 Gy to the wide local excision area, and a total of 60 Gy–70 Gy to the tumor bed over 6–7 weeks), and 16 were assigned to amputation at or above the joint proximal to the tumor. Both groups received adjuvant chemotherapy (i.e., doxorubicin, cyclophosphamide, and high-dose methotrexate). At 63 months, with a median follow-up of 56 months, there were four local recurrences in the 27 patients who underwent limb-sparing surgery and no recurrences in the 16 patients who underwent amputation P2 = .12. Overall survival (OS) rates were not statistically significantly different (actuarial 5-year survival rate, 83% vs. 88%, P2 = .99).[3][Level of evidence: 1iiA]
Local control of high-grade soft tissue sarcomas of the trunk and the head and neck can be achieved with surgery in combination with radiation therapy.[4] It may be possible to use surgery without PORT in selected cases. For example, a case series was reported from a specialized sarcoma treatment referral center in which 74 selected patients with primary extremity and trunk tumors 5 cm or smaller were found to have no histologic involvement of the surgical margins.[1] They were observed without radiation therapy, and the estimated local recurrence rate after 10 years was 11%.[1][Level of evidence: 3iiiDiv] The role of chemotherapy is not as well defined as is the role of radiation therapy. Because of the evolving nature of the treatment options for this disease, patients should be offered the option of clinical trials when available.
Effective treatment of retroperitoneal sarcomas requires removal of all gross disease while sparing adjacent viscera not invaded by tumor. The prognosis for patients with high-grade retroperitoneal sarcomas is less favorable than for patients with tumors at other sites, partly because of the difficulty in completely resecting these tumors and the dose-limiting toxicity of high-dose radiation therapy on visceral organs.[5-8]
In the setting of distant metastasis, surgery may be associated with long-term, disease-free survival in patients with pulmonary metastasis and optimal underlying disease biology (i.e., patients with a limited number of metastases and slow nodule growth) who have undergone or are undergoing complete resection of the primary tumor.[9-11] It is not clear to what degree the favorable outcomes are attributable to the efficacy of surgery or the careful selection of patients based on factors that are associated with less-virulent disease.
Role of Radiation Therapy
Radiation plays an important role in limb-sparing therapy. Pre- and postoperative external-beam radiation therapies (EBRT), as well as brachytherapy, have been shown to decrease the risk of local recurrence. They have not been shown to increase OS but are used to avoid amputation for all but the most locally advanced tumors or for limbs seriously compromised by vascular disease, where acceptable functional preservation is not possible. In the case of EBRT, irradiation of the entire limb circumference is avoided to preserve vascular and nerve structures that are critical to function and preservation of the limb.
PORT
PORT has been tested in a single-institution, randomized trial of 141 patients with extremity sarcomas who were treated with limb-sparing surgery. Patients with high-grade tumors (n = 91) also received adjuvant chemotherapy (i.e., five 28-day cycles of doxorubicin and cyclophosphamide). All patients were randomly assigned to receive radiation (45 Gy to a wide field, plus a tumor-bed boost of 18 Gy over 6–7 weeks), concurrent with chemotherapy in the case of high-grade tumors versus no radiation.[12] At up to 12 years of follow-up, there was one local recurrence in the 70 patients randomly assigned to receive radiation versus 17 recurrences in the 71 control patients (P = .0001), with similar reduction in risk of local recurrence for both high- and low-grade tumors. However, there was no difference in OS between the radiation and control groups.[12][Level of evidence: 1iiDiii] Global quality of life was similar in the two groups, but the radiation therapy group had substantially worse functional deficits resulting from reduced strength and joint motion as well as increased edema.
To limit acute toxicity with preRT, smaller fields and lower doses are generally given than is the case with PORT. PreRT has been directly compared with PORT for extremity soft tissue sarcomas in a multicenter randomized trial.[13-15] Designed to include 266 patients, the trial was stopped early after 190 patients had been accrued because of an increase in wound complications in the preRT group. The scheduled radiation in the preRT group was a wide field of 50 Gy in 2-Gy fractions (first phase of the trial) with an additional 16 Gy to 20 Gy to the tumor bed and a 2-cm margin (second phase of the trial) only if tumor cells were found at the surgical margins.
Patients in the PORT group were scheduled to receive radiation during both phases of the trial. The wound-complication rates were 35% versus 17% in the preRT and PORT groups, respectively (P = .01). In addition, limb function at 6 weeks after surgery was worse in the preRT group (P = .01).[13] At 5 years, the two groups had similar local control rates (93% vs. 92%) and OS (73% vs. 67%, P = .48).[14] Of the 129 patients evaluated for limb function at 21 to 27 months after surgery (n = 73 for preRT and n = 56 for PORT), limb function was similar in both groups, but there was a statistical trend for less fibrosis in the preRT group (P = .07).[15]
Brachytherapy
Brachytherapy has also been investigated as an adjuvant therapy for soft tissue sarcomas. Although it has possible advantages of convenience and less radiation to normal surrounding tissue relative to EBRT, the two treatment strategies have not been directly compared in terms of efficacy or morbidity. However, adjuvant brachytherapy has been compared with surgery without radiation. The time interval between preRT and surgical excision in extremity soft tissue sarcoma had minimal influence on the development of wound complications. Four- or 5-week intervals showed equivalent complication rates between patients who did or did not develop wound complications, suggesting an optimal interval to reduce potential complications.[16]
In a single-institution trial, 164 patients with sarcomas of the extremity or superficial trunk were randomly assigned during surgery, if all gross tumor could be excised, to receive an iridium Ir 192 implant (delivering 42 Gy–45 Gy over 4–6 days; 78 patients) or to a control arm of no radiation (86 patients).[17,18] Some of the patients with high-grade tumors received adjuvant doxorubicin-based chemotherapy if they were thought to be at a high risk for metastasis (34 patients in each study arm). With a median follow-up of 76 months, the 5-year actuarial local recurrence rates were 18% and 31% in the brachytherapy and control arms, respectively (P = .04). This difference was limited to patients with high-grade tumors. There was no discernible difference in sarcoma-specific survival rates between the brachytherapy and control arms (84% and 81%, respectively; P = .65), and there was no difference in the high tumor-grade group.[17][Level of evidence: 1iiDiii] The rates of clinically important wound complications (e.g., need for operative revision or repeated seroma drainage, wound separation, large hematomas, or purulent infection) were 24% and 14% in the radiation and control arms, respectively (P = .13); wound reoperation rates were 10% and 0%, respectively (P = .006).[18]
Intensity-modulated radiation therapy
Intensity-modulated radiation therapy (IMRT) has been used to deliver preRT or PORT to patients with extremity soft tissue sarcomas in an effort to spare the femur, joints, and selected other normal tissues from the full prescription dose and to maintain local control while potentially reducing radiation therapy-related morbidity. Initial single-institution reports suggest that high rates of local control with some reduction in morbidity are possible with this technique.[19,20] Retrospective comparison of IMRT compared with 3-dimensional, conformal radiation therapy demonstrates that local recurrence for primary soft tissue sarcomas of the extremity was worse in the non-IMRT group.[21][Level of evidence: 3iiiDiv]
Surgery and radiation therapy
In some tumors of the extremities or trunk, surgery alone can be performed without the use of radiation. Evidence for this approach is limited to single-institution, relatively small, case series [1,22,23] or analysis of outcomes in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) tumor registry.[24] However, these comparisons suffer from low statistical power and differential evaluability rates that could have introduced bias.[1] Patient selection factors may vary among surgeons. In general, this approach is considered in patients with low-grade tumors of the extremity or superficial trunk that are 5 cm or smaller in diameter (T1) and have microscopically negative surgical margins; long-term local tumor control is about 90% in such patients.[25]
A patterns-of-care study using SEER data was queried to identify patients undergoing surgery for truncal and extremity soft tissue sarcomas from 2004 to 2009.[26] Of 5,075 patients, 50% received radiation therapy. Radiation was considered to be underused in a significant portion of patients undergoing treatment for soft tissue sarcoma in the United States. Although routine radiation therapy is not recommended for stage I patients, 25% of them still underwent radiation. Even though routine radiation therapy is recommended for patients with stage II and III tumors, only 60% of them underwent radiation. On multivariate analysis, predictors of radiation therapy included age younger than 50 years (odds ratio [OR], 1.57; 95% confidence interval [CI], 1.28–1.91), malignant fibrous histiocytoma histology (OR, 1.47; 95% CI, 1.3–1.92), T2 classification (OR, 1.88; 95% CI, 1.60–2.20), and G3 (OR, 6.27; 95% CI, 5.10–7.72). Patients with stage III soft tissue sarcoma who received radiation therapy showed improved disease-specific survival at 5 years compared with those who did not (68% vs. 46%, P < .001).[26][Level of evidence: 3iDii]
On occasion, surgical excision cannot be performed in the initial management of soft tissue sarcomas because the morbidity would be unacceptable or nearby critical organs make complete resection impossible. In such circumstances, radiation has been used as the primary therapy.[27] However, this must be considered a treatment of last resort. Experience is limited to retrospective case series from single centers.[27][Level of evidence: 3iiiDiv]
Role of Adjuvant or Neoadjuvant Chemotherapy for Clinically Localized Tumors
The role of adjuvant chemotherapy is not completely clear. The investigation of its use falls into two categories or generations—pre- and post-ifosfamide regimens. In discussions with a patient, any potential benefits should be considered in the context of the short- and long-term toxicities of the chemotherapy.
First-generation trials (preifosfamide)
Several prospective, randomized trials were unable to determine conclusively whether doxorubicin-based adjuvant chemotherapy benefits adults with resectable soft tissue sarcomas. The majority of these studies accrued small numbers of patients and did not demonstrate a metastasis-free survival or an OS benefit for adjuvant chemotherapy.[4] A small study of adjuvant chemotherapy showed a positive effect on both disease-free survival (DFS) and OS in patients treated with postoperative chemotherapy.[28] There was wide interstudy variability among the reported trials, including differences in therapeutic regimens, drug doses, sample size, tumor site, and histologic grade.
A quantitative meta-analysis of updated data from 1,568 individual patients in 14 trials of doxorubicin-based adjuvant therapy showed an absolute benefit from adjuvant therapy of 6% for a local relapse-free interval (95% CI, 1%–10%), 10% for a distant relapse-free interval (95% CI, 5%–15%), and 10% for recurrence-free survival (95% CI, 5%–15%). A statistically significant OS benefit at 10 years was not detected: absolute difference 4% (95% CI, -1%–+9%).[29,30][Level of evidence: 1iiDii] However, only a small proportion of patients in this meta-analysis were treated with ifosfamide, an agent with demonstrated activity against soft tissue sarcoma. In addition, a subset analysis suggested that patients with sarcomas of the extremities may have benefited from adjuvant chemotherapy (hazard ratio [HR] for death, 0.8, P = .029), but there was no clear evidence that patients with extremity sarcomas had outcomes that were statistically significantly different from the outcomes of patients with tumors at other sites (P = .58).[30]
Second-generation trials (postifosfamide)
Subsequent chemotherapy trials were performed using anthracycline and ifosfamide combinations in patients who primarily had extremity or truncal soft tissue sarcomas. The data are conflicting, and the issue is still not settled. In a small feasibility study, 59 patients with high-risk, soft tissue sarcomas, 58 of whom had an extremity or the trunk as the primary site, underwent primary resection plus PORT and were randomly assigned to observation versus a dose-dense regimen of six 14-day courses of ifosfamide, dacarbazine (DTIC), and doxorubicin (IFADIC regimen) with granulocyte colony-stimulating factor (G-CSF) bone marrow support and mesna uroprotection.[31] There were no statistically significant differences in OS or relapse-free survival (RFS), but the study was severely underpowered.
In a second trial performed by the Italian National Council for Research, high-risk patients were treated with local therapy (i.e., wide resection plus preRT or PORT, or amputation as clinically necessary) and were then randomly assigned to observation versus five 21-day cycles of 4-epidoxorubicin (epirubicin) plus ifosfamide (with mesna and G-CSF).[28,32] Based on power calculations, the planned study size was 190 patients, but the trial was stopped after 104 patients had been entered because an interim analysis revealed a statistically significant (P = .001) difference in DFS favoring the chemotherapy arm. By the time of the initial peer-reviewed report of the study, the DFS still favored the chemotherapy group (median DFS of 48 months vs. 16 months), but the P value had risen to .04.[28]
Although there was no difference in metastasis-free survival at the time of the report, there was an improvement in median OS (75 months vs. 46 months, P = .03). However, at the follow-up report (at a median of 89.6 months in a range of 56–119 months), OS differences were no longer statistically significant (58.5% vs. 43.1% [P = .07]). The DFS difference had also lost statistical significance (47.2% vs. 16.0% [P = .09]).[32] In summary, the trial was underpowered because it was stopped early, and the early promising results that led to stopping the trial diminished as the trial matured.
In a third, underpowered, single-center trial, 88 patients with high-risk, soft tissue sarcomas (64 of whom had extremity or truncal primary tumors) underwent surgery (with or without radiation) and were then randomly assigned to receive four 21-day cycles of chemotherapy (epirubicin [n = 26] or epirubicin plus ifosfamide [n = 19]) versus no adjuvant chemotherapy (n = 43).[33] The trial was closed prematurely because of a slow accrual rate. After a median follow-up of 94 months, the 5-year DFS in the chemotherapy and control arms was 69% versus 44%, respectively (P = .01); the 5-year OS rates were 72% versus 47% (P = .06). All of the benefit associated with chemotherapy appeared restricted to the 19 patients who received epirubicin plus ifosfamide.
In yet another underpowered trial, 137 patients with high-risk, soft tissue sarcomas (93% with extremity or truncal primary tumors) who met the eligibility criteria were randomly assigned to undergo surgical resection (with or without radiation) or to receive three preoperative 21-day cycles of doxorubicin plus ifosfamide.[34] This multicenter European Organization for Research and Treatment of Cancer trial (EORTC-62874) was closed because of slow accrual and results that were not promising enough to continue. With a median follow-up of 7.3 years, the 5-year DFS in the surgery alone and chemotherapy plus surgery arms was 52% and 56%, respectively (P = .35); and OS was 64% and 65%, respectively (P = .22).
These last four trials have been combined with the 14 first-generation trials in a trial-level meta-analysis.[35] Of the 18 randomized trials of patients with resectable soft tissue sarcomas, five trials used a combination of doxorubicin (50–90 mg/m2 per cycle) plus ifosfamide (1,500–5,000 mg/m2 per cycle). The remaining 13 trials used doxorubicin (50–70 mg/m2 per cycle) alone or with other drugs. The absolute risk reduction in local recurrence rates associated with any chemotherapy added to local therapy was 4 percentage points (95% CI, 0%–7%), and it was 5 percentage points (95% CI, 1%–12%) when ifosfamide was combined with doxorubicin. The absolute reduction in overall mortality was 6 percentage points with any chemotherapy (95% CI, 2%–11%; [i.e., a reduction from 46%–40%]), 11 percentage points for doxorubicin plus ifosfamide (95% CI, 3%–19%; [i.e., a reduction from 41%–30%]), and 5 percentage points for doxorubicin without ifosfamide.[35][Level of evidence: 1iiA]
An additional multicenter randomized trial (EORTC-62931 [NCT00002641]), the largest trial reported to date using adjuvant doxorubicin (75 mg/m2) plus ifosfamide (5,000 mg/m2), was subsequently published in abstract form and was not included in the above meta-analysis.[36] The results differed from those reported in the meta-analysis.[35] After local therapy, 351 patients were randomly assigned to five 21-day cycles of adjuvant therapy versus observation. The trial was stopped for futility because the 5-year RFS was 52% in both arms. OS was 64% in the chemotherapy arm versus 69% in the observation arm. In a subsequent abstract, the EORTC investigators reported a combined analysis of this trial and their previous trial (EORTC-62771) [37] of adjuvant cyclophosphamide plus doxorubicin plus DTIC (CYVADIC), representing the two largest trials of adjuvant therapy for adult soft tissue sarcoma in the literature.[38] The combined analysis showed no improvement in either RFS or OS associated with adjuvant chemotherapy.[38][Level of evidence: 1iiA]
In summary, the impact of adjuvant chemotherapy on survival is not clear but is likely to be small in absolute magnitude. Therefore, in discussions with a patient, any potential benefits should be considered in the context of the short- and long-term toxicities of the chemotherapy.
Role of regional hyperthermia
The use of regional hyperthermia to enhance the local effects of systemic chemotherapy in the neoadjuvant and adjuvant setting is under investigation. In a multicenter phase III trial, 341 patients with high-risk (tumor ≥5 cm, grade 2–3, and deep to fascia), soft tissue sarcomas (149 extremity tumors and 192 nonextremity tumors) were randomly allocated to receive four 21-day cycles of chemotherapy (etoposide 125 mg/m2 on days 1 and 4; ifosfamide 1,500 mg/m2 on days 1–4; doxorubicin 50 mg/m2 on day 1) with or without regional hyperthermia both before and after local therapy.[39] Approximately 11% of the patients were being treated for recurrent tumors. The regional hyperthermia was designed to produce tumor temperatures of 42°C for 60 minutes and was given on days 1 and 4 of each chemotherapy cycle. After the first four cycles of chemotherapy, definitive surgical excision of the tumor was performed, if possible, followed by radiation therapy, if indicated (i.e., a 52.7 Gy median dose delivered), and then the last four cycles of chemotherapy plus or minus hyperthermia. Three of the nine treatment centers with particular expertise in hyperthermia treated 91% of the patients in the trial.
The median duration of follow-up was 34 months. Local progression occurred in 56 patients in the hyperthermia group and 76 patients in the control group. The relative HR for local progression or death was 0.58 (95% CI, 0.41–0.84), with an absolute difference at 2 years of 15% (76% vs. 61%; 95% CI of the difference 6–26). The decreased risk of local progression or death was seen in both extremity and nonextremity tumors. However, hyperthermia had no effect on distant failure rates nor was there a statistically significant effect on OS (HR, .88, 95% CI, 0.64–1.21; P = .43).[39][Level of evidence: 1iiDiii] There was a higher rate of grade 3 to 4 leucopenia in the hyperthermia group: 77.6% versus 63.5% (P = .005). Since a large proportion of the patients were treated at centers with special expertise, there is no certainty that the finding can be generalized to apply to other settings.
Role of isolated limb perfusion
Isolated limb perfusion is under investigation as a means to deliver high doses of chemotherapy and permit limb salvage in unresectable primary or recurrent extremity soft tissue sarcomas that would otherwise require amputation, in the opinion of the surgeon.[40,41] Common drugs used in the procedure are TNF-alpha, melphalan, and interferon-gamma. Experience is limited to case series with response rates and reported avoidance of amputation as the outcome.[40,41][Level of evidence: 3iiiDiv] The technique requires specialized expertise to avoid severe local and systemic toxicity including systemic effects of TNF-alpha. The technique has not been directly compared with standard approaches using combined systemic and local therapy.
Role of chemotherapy for advanced disease
Doxorubicin is a mainstay of systemic therapy in the management of locally advanced and metastatic soft tissue sarcoma. Pegylated liposomal encapsulated doxorubicin is a formulation of doxorubicin designed to prolong the half-life of circulating doxorubicin and slow the release of active drugs.[42] The changed pharmacokinetics result in less myelosuppression and possibly less cardiotoxic effects, but there is a substantial incidence of hypersensitivity-like reactions and hand-foot syndrome. Its clinical activity relative to unencapsulated doxorubicin is not clear.[42][Level of evidence: 3iiiDiv] Other drugs that are thought to have clinical activity as single agents are ifosfamide, epirubicin, gemcitabine, and paclitaxel.[43-46][Level of Evidence: 3iiiDiv] Their clinical activity relative to single-agent doxorubicin is not clear, and they are not known to have superior activity.
There is controversy about the clinical benefit of adding other drugs to doxorubicin as a single agent. A systematic evidence review and meta-analysis conducted by the Cochrane Collaboration summarized the eight randomized trials reported from 1976 to 1995.[47] No additional randomized trials had been reported or were known to be in progress between 1995 and the 2002 literature search. Single-agent doxorubicin had been compared with a variety of doxorubicin-containing combinations that included vincristine, vindesine, cyclophosphamide, streptozotocin, mitomycin-C, cisplatin, and/or ifosfamide. Combination regimens consistently caused more nausea and hematologic toxicity. However, the better response rates associated with combination therapy were marginal and depended on the statistical model used (fixed effects model ORresponse = 1.29; 95% CI, 1.03–1.60, P = .03; random effects model ORresp = 1.26; 95% CI, 0.96–1.67, P = .10) There was no statistically significant difference in the 1- (ORmortality = 0.87; 95% CI, 0.73–1.05, P = .14) or 2-year mortality rates (ORmortality = 0.84; 95% CI, 0.67–1.06, P = .13).
These results were very similar even when the analyses were restricted to the four trials that used DTIC and/or ifosfamide as part of the combination regimen with doxorubicin agents that were postulated to have greater activity than the others tested. A subsequent meta-analysis of all three published randomized trials of chemotherapy regimens that contained ifosfamide versus those that did not came to similar conclusions: tumor response rates were better when the regimen included ifosfamide (RRresponse = 1.52; 95% CI, 1.11–2.08), but mortality at 1 year was not (RRmortality = 0.98; 95% CI, 0.85–1.13).[48][Level of evidence: 1iiDiv]. Therefore, response rate was a poor surrogate for OS. Quality-of-life outcomes were not reported in any of the above-mentioned randomized trials, but toxicity was worse when agents were added to doxorubicin.
References
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Lohman RF, Nabawi AS, Reece GP, et al.: Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 94 (8): 2256-64, 2002. [PUBMED Abstract]
- Rosenberg SA, Tepper J, Glatstein E, et al.: The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305-15, 1982. [PUBMED Abstract]
- O'Byrne K, Steward WP: The role of adjuvant chemotherapy in the treatment of adult soft tissue sarcomas. Crit Rev Oncol Hematol 27 (3): 221-7, 1998. [PUBMED Abstract]
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Lewis JJ, Leung D, Woodruff JM, et al.: Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 228 (3): 355-65, 1998. [PUBMED Abstract]
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Griffin AM, Dickie CI, Catton CN, et al.: The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann Surg Oncol 22 (9): 2824-30, 2015. [PUBMED Abstract]
- Pisters PW, Harrison LB, Leung DH, et al.: Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 14 (3): 859-68, 1996. [PUBMED Abstract]
- Alektiar KM, Zelefsky MJ, Brennan MF: Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys 47 (5): 1273-9, 2000. [PUBMED Abstract]
- Alektiar KM, Brennan MF, Healey JH, et al.: Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 26 (20): 3440-4, 2008. [PUBMED Abstract]
- Alektiar KM, Brennan MF, Singer S: Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer 117 (14): 3229-34, 2011. [PUBMED Abstract]
- Folkert MR, Singer S, Brennan MF, et al.: Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol 32 (29): 3236-41, 2014. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Bagaria SP, Ashman JB, Daugherty LC, et al.: Compliance with National Comprehensive Cancer Network guidelines in the use of radiation therapy for extremity and superficial trunk soft tissue sarcoma in the United States. J Surg Oncol 109 (7): 633-8, 2014. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
- Frustaci S, Gherlinzoni F, De Paoli A, et al.: Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 19 (5): 1238-47, 2001. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Sarcoma Meta-analysis Collaboration (SMAC): Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev (4): CD001419, 2000. [PUBMED Abstract]
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
- Frustaci S, De Paoli A, Bidoli E, et al.: Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology 65 (Suppl 2): 80-4, 2003. [PUBMED Abstract]
- Petrioli R, Coratti A, Correale P, et al.: Adjuvant epirubicin with or without Ifosfamide for adult soft-tissue sarcoma. Am J Clin Oncol 25 (5): 468-73, 2002. [PUBMED Abstract]
- Gortzak E, Azzarelli A, Buesa J, et al.: A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. Eur J Cancer 37 (9): 1096-103, 2001. [PUBMED Abstract]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al.: A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113 (3): 573-81, 2008. [PUBMED Abstract]
- Woll PJ, van Glabbeke M, Hohenberger P, et al.: Adjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): Interim analysis of a randomised phase III trial. [Abstract] J Clin Oncol 25 (Suppl 18): A-10008, 2007.
- Bramwell V, Rouesse J, Steward W, et al.: Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 12 (6): 1137-49, 1994. [PUBMED Abstract]
- Le Cesne A, Van Glabbeke M, Woll PJ, et al.: The end of adjuvant chemotherapy (adCT) era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma (STS): pooled analysis of the two STBSG-EORTC phase III clinical trials. [Abstract] J Clin Oncol 26 (Suppl 15): A-10525, 2008.
- Issels RD, Lindner LH, Verweij J, et al.: Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 11 (6): 561-70, 2010. [PUBMED Abstract]
- Eggermont AM, de Wilt JH, ten Hagen TL: Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 4 (7): 429-37, 2003. [PUBMED Abstract]
- Bonvalot S, Laplanche A, Lejeune F, et al.: Limb salvage with isolated perfusion for soft tissue sarcoma: could less TNF-alpha be better? Ann Oncol 16 (7): 1061-8, 2005. [PUBMED Abstract]
- Grenader T, Goldberg A, Hadas-Halperin I, et al.: Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20 (1): 15-20, 2009. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
- Bramwell VH, Anderson D, Charette ML, et al.: Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev (3): CD003293, 2003. [PUBMED Abstract]
- Verma S, Younus J, Stys-Norman D, et al.: Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 34 (4): 339-47, 2008. [PUBMED Abstract]
Stage I Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery and radiation therapy.
Low-grade soft tissue sarcomas have little metastatic potential, but they have a propensity to recur locally. Accordingly, surgical excision with negative tissue margins of 1 cm to 2 cm or larger in all directions is the treatment of choice for patients with these early-stage sarcomas.[1-3] The Mohs surgical technique may be considered as an alternative to wide surgical excision for the very rare, small, well-differentiated primary sarcomas of the skin when cosmetic results are considered to be important, as margins can be assured with minimal normal tissue removal.[4]
Carefully executed high-dose radiation therapy using a shrinking-field technique may be beneficial for unresectable tumors or for resectable tumors in which a high likelihood of residual disease is thought to be present when margins are judged to be inadequate, and when wider resection would require either an amputation or the removal of a vital organ.[5] Because of the low metastatic potential of these tumors, chemotherapy is usually not given.[6,7]
Standard treatment options:
- Surgical excision of tumors 5 cm or smaller in diameter with negative tissue margins in all directions.[8-12]
- Surgical excision with preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT). Radiation decreases the risk of local recurrence but has not been shown to increase overall survival.[13-16]
- If the tumor is unresectable, high-dose preRT may be used.[17]
- For tumors of the retroperitoneum, trunk, and head and neck, the following are options:
- Surgical resection with the option of PORT if negative margins cannot be obtained. Wide margins are unusual in these sites, and radiation therapy is usually advocated for trunk and head and neck primary sites.[18]
- PreRT followed by maximal surgical resection. Radiation therapy may be used in sarcomas of the trunk and head and neck to maximize local control because of the inability to obtain wide surgical margins.[19]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Fish FS: Soft tissue sarcomas in dermatology. Dermatol Surg 22 (3): 268-73, 1996. [PUBMED Abstract]
- Temple WJ, Temple CL, Arthur K, et al.: Prospective cohort study of neoadjuvant treatment in conservative surgery of soft tissue sarcomas. Ann Surg Oncol 4 (7): 586-90, 1997 Oct-Nov. [PUBMED Abstract]
- Sarcoma Meta-analysis Collaboration (SMAC): Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev (4): CD001419, 2000. [PUBMED Abstract]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al.: A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113 (3): 573-81, 2008. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
- Brennan MF, Singer S, Maki RG: Sarcomas of the soft tissue and bone. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds.: Cancer: Principles and Practice of Oncology. Vols. 1 & 2. 8th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2008, pp 1741-1833.
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
Stage II and Node-Negative Stage III Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
High-grade localized soft tissue sarcomas have an increased potential for local recurrence and metastasis. For sarcomas of the extremities, local control comparable to that obtained with amputation may be achieved with limb-sparing surgery that involves wide local excision in combination with preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT).
Complete surgical resection is often difficult for sarcomas of the retroperitoneum because of their large size before detection and anatomical location.[1,2] As opposed to soft tissue sarcomas of the extremities, local recurrence is the most common cause of death in patients with retroperitoneal soft tissue sarcomas. Complete surgical resection (i.e., removal of the entire gross tumor) is the most important factor in preventing local recurrence and, in many instances, requires resection of adjacent viscera. For retroperitoneal sarcomas, retrospective comparison of surgery alone versus preRT review suggests that preRT is associated with improved local recurrence-free survival, but not disease-free survival.[3]
Standard treatment options:
- Surgical excision with preRT or PORT. Radiation decreases the risk of local recurrence but has not been shown to increase overall survival.[4-8]
- Surgical excision with negative tissue margins in all directions. This approach is generally restricted to low-grade tumors ( ≤5 cm in diameter) of the extremities or superficial trunk with microscopically negative surgical tumor margins.[9-13]
- If the tumor is unresectable, high-dose radiation therapy may be used, but poor local control is likely to result.[14]
- In some situations, radiation therapy and/or chemotherapy may be used before surgery in an attempt to convert a marginally resectable tumor to one that can be adequately resected with limb preservation; this treatment may be followed by PORT.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Heslin MJ, Lewis JJ, Nadler E, et al.: Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol 15 (8): 2832-9, 1997. [PUBMED Abstract]
- Jaques DP, Coit DG, Hajdu SI, et al.: Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 212 (1): 51-9, 1990. [PUBMED Abstract]
- Kelly KJ, Yoon SS, Kuk D, et al.: Comparison of Perioperative Radiation Therapy and Surgery Versus Surgery Alone in 204 Patients With Primary Retroperitoneal Sarcoma: A Retrospective 2-Institution Study. Ann Surg 262 (1): 156-62, 2015. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- Rosenberg SA, Tepper J, Glatstein E, et al.: The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305-15, 1982. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
Advanced Stage III (N1) Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
Regional lymph node involvement by soft tissue sarcomas of adulthood is very infrequent. However, sarcoma types that more commonly spread to lymph nodes include high-grade rhabdomyosarcoma, vascular sarcomas, and epithelioid sarcomas.[1]
Standard treatment options:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Watson DI, Coventry BJ, Langlois SL, et al.: Soft-tissue sarcoma of the extremity. Experience with limb-sparing surgery. Med J Aust 160 (7): 412-6, 1994. [PUBMED Abstract]
- Cormier JN, Huang X, Xing Y, et al.: Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol 22 (22): 4567-74, 2004. [PUBMED Abstract]
- O'Byrne K, Steward WP: The role of adjuvant chemotherapy in the treatment of adult soft tissue sarcomas. Crit Rev Oncol Hematol 27 (3): 221-7, 1998. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
Stage IV Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
In the setting of lung metastasis, resection of metastatic tumors may be associated with long-term disease-free survival in patients selected for optimal underlying disease biology (i.e., patients with a limited number of metastases and slow tumor growth).[1-3] It is not clear to what degree the favorable outcomes are attributable to the efficacy of surgery or to careful selection of patients based upon factors that are associated with less-virulent disease.[1-3] The value of resection of hepatic metastases is unclear.
As noted in the Treatment Option Overview section above, doxorubicin is the standard systemic therapy in the management of metastatic sarcomas.[4,5] Other drugs that may have clinical activity as single agents are ifosfamide, epirubicin, gemcitabine, and paclitaxel.[6-9] Their clinical activity relative to single-agent doxorubicin is not clear, and they are not known to have superior activity. There is controversy about whether adding drugs to doxorubicin offers clinical benefit beyond what is achieved by doxorubicin as a single agent. To avoid severe toxicity in older patients, sequential use of single agents may be the preferred strategy for palliation.
A randomized study assessed whether dose intensification of doxorubicin with ifosfamide improved the survival of patients with advanced soft-tissue sarcoma compared with doxorubicin alone.[10] Two hundred twenty-eight patients were randomly assigned to receive doxorubicin, and 227 patients were randomly assigned to receive doxorubicin and ifosfamide. Median follow-up was 56 months (interquartile range [IQR], 31–77) in the doxorubicin-only group and 59 months (IQR, 36–72) in the combination group.
There was no significant difference in overall survival (OS) between groups (median OS, 12.8 months; 95.5% confidence interval [CI], 10.5–14.3 in the doxorubicin-alone group vs. 14.3 months; range, 12.5–16.5 months in the doxorubicin and ifosfamide group; hazard ratio [HR], 0.83; 95.5% CI 0.67–1.03; stratified log-rank test P = .076). Median progression-free survival was significantly higher for the doxorubicin and ifosfamide group (7.4 months; 95% CI, 6.6-8.3) than for the doxorubicin-alone group (4.6 months; range, 2.9–5.6 months; HR, 0.74; 95% CI, 0.60–0.90; stratified log-rank test P = .003). More patients in the doxorubicin and ifosfamide group than in the doxorubicin-alone group had an overall response (60 [26%] of 227 patients vs. 31 [14%] of 228; P < .0006). The most common grade 3 and 4 toxic effects, which were all more common with doxorubicin and ifosfamide than with doxorubicin alone, were leucopenia (97 [43%] of 224 patients vs. 40 [18%] of 223 patients), neutropenia (93 [42%] vs. 83 [37%]), febrile neutropenia (103 (46%) vs. 30 [13%]), anemia (78 [35%] vs. 10 [5%]), and thrombocytopenia (75 [33%]) vs. 1 [<1%]).[10][Level of evidence: 1iiA] Treatment intensification with doxorubicin and ifosfamide for palliation of advanced soft tissue sarcoma is not indicated.
Standard treatment options
- Chemotherapy.
- Single-agent chemotherapy, with subsequent single agents for disease regrowth.[4-6,8,9,11] Doxorubicin is generally the first-line agent. Ifosfamide also has substantial single-agent activity.
- Doxorubicin-based combination chemotherapy. A variety of regimens have been used, but none has been proven to increase OS compared with doxorubicin alone.[4,5] There is some evidence that the addition of ifosfamide increases response rates (but not survival). Toxicity is increased with the addition of drugs to doxorubicin. No quality-of-life studies have been reported in comparisons of single-agent therapy versus combination therapy.
- Resection of pulmonary lesions may be performed if the primary tumor is under control.[1-3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Bramwell VH, Anderson D, Charette ML, et al.: Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev (3): CD003293, 2003. [PUBMED Abstract]
- Verma S, Younus J, Stys-Norman D, et al.: Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 34 (4): 339-47, 2008. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
- Judson I, Verweij J, Gelderblom H, et al.: Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 15 (4): 415-23, 2014. [PUBMED Abstract]
- Grenader T, Goldberg A, Hadas-Halperin I, et al.: Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20 (1): 15-20, 2009. [PUBMED Abstract]
Recurrent Adult Soft Tissue Sarcoma
Treatment of patients with recurrent soft tissue sarcoma depends on the type of initial presentation and treatment. Patients who develop a local recurrence often can be treated by local therapy: surgical excision plus radiation therapy after previous minimal therapy or amputation after previous aggressive treatment.[1-7] Resection of limited pulmonary metastases may be associated with favorable disease-free survival.[8-10][Level of evidence: 3iiiDiv] However, the contribution of selection factors, such as low tumor burden, slow tumor growth, and long disease-free interval, to these favorable outcomes is not known.
There is no standard chemotherapy for recurrent soft tissue sarcomas that have progressed after doxorubicin as a single agent or in combination with other agents that have clinical activity, such as ifosfamide, epirubicin, gemcitabine, and paclitaxel. Any of these agents not previously administered to the patient may be used sequentially at the time of recurrence or progression.[11-14][Level of Evidence: 3iiiDiv] None of these agents has been shown to increase overall survival in this setting, therefore, clinical trials are an appropriate option.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Midis GP, Pollock RE, Chen NP, et al.: Locally recurrent soft tissue sarcoma of the extremities. Surgery 123 (6): 666-71, 1998. [PUBMED Abstract]
- Essner R, Selch M, Eilber FR: Reirradiation for extremity soft tissue sarcomas. Local control and complications. Cancer 67 (11): 2813-7, 1991. [PUBMED Abstract]
- Singer S, Antman K, Corson JM, et al.: Long-term salvageability for patients with locally recurrent soft-tissue sarcomas. Arch Surg 127 (5): 548-53; discussion 553-4, 1992. [PUBMED Abstract]
- Lewis JJ, Leung D, Heslin M, et al.: Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 15 (2): 646-52, 1997. [PUBMED Abstract]
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
Changes to This Summary (02/01/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Adult Soft Tissue Sarcoma
Updated statistics with estimated new cases and deaths for 2018 (cited American Cancer Society as reference 1).
Stage Information for Adult Soft Tissue Sarcoma
An editorial change was made to this section.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult soft tissue sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Adult Soft Tissue Sarcoma Treatment are:
- Russell S. Berman, MD (New York University School of Medicine)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Adult Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/adult-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389481]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: February 1, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/2127.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:00:45.0
Adult Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Adult Soft Tissue Sarcoma
Incidence and Mortality
Estimated new cases and deaths from soft tissue sarcoma in the United States in 2018:[1]
- New cases: 13,040.
- Deaths: 5,150.
Soft tissue sarcomas are malignant tumors that arise in any of the mesodermal tissues of the extremities (50%), trunk and retroperitoneum (40%), or head and neck (10%). The reported international incidence rates range from 1.8 to 5 per 100,000 individuals per year.[2]
Risk Factors and Genetic Factors
The risk of sporadic soft tissue sarcomas is increased by previous radiation therapy and, in the case of lymphangiosarcoma, by chronic lymphedema. The chemicals Thorotrast (thorium dioxide), vinyl chloride, and arsenic are also established carcinogens for hepatic angiosarcomas.[3-5]
Soft tissue sarcomas occur with greater frequency in patients with the following inherited syndromes:[3-5]
- Nevoid basal cell carcinoma syndrome (Gorlin syndrome: PTC gene mutation).
- Gardner syndrome (APC mutation).
- Li-Fraumeni syndrome (p53 mutation).
- Tuberous sclerosis (Bourneville disease: TSC1 or TSC2 mutation).
- von Recklinghausen disease (neurofibromatosis type 1: NF1 mutation).
- Werner syndrome (adult progeria: WRN mutation).
Diagnosis
Soft tissue sarcomas may be heterogeneous, so adequate tissue should be obtained via either core-needle or incisional biopsy for microscopic examination to determine histologic type and tumor grade. Careful planning of the initial biopsy is important to avoid compromising subsequent curative resection. Since the selection of treatment is determined by the grade of the tumor, it is essential to have a careful review of the biopsy tissue by a pathologist who is experienced in diagnosing sarcomas. Complete staging and treatment planning by a multidisciplinary team of cancer specialists is required to determine the optimal treatment for patients with this disease.
There is evidence that at least some favorable clinical outcomes may be associated with referral to a specialized sarcoma treatment center. In a population-based consecutive series of 375 soft tissue sarcoma patients in Sweden, local recurrence rates of resected tumors were higher in patients who were not referred to the specialized center: in 35 of 78 (45%) patients not referred; in 24 of 102 (24%) patients referred after initial surgery or incisional biopsy; and in 36 of 195 (18%) patients referred before any surgical procedure (P = .0001 for the difference between those never referred vs. those referred before any surgical procedure).[6][Level of evidence: 3iDii] However, there were no statistically significant differences in death from sarcoma between the groups of patients.
Prognostic Factors
The prognosis for patients with adult soft tissue sarcomas depends on several factors, including:[3-5,7,8]
- Patient’s age.
- Size, sarcoma subtype, histologic grade, mitotic activity, and stage of the tumor.
Factors associated with a poorer prognosis include the following:[9]
- Age older than 60 years.
- Tumors larger than 5 cm in greatest dimension.
- High-grade histology with high mitotic activity.
- Positive margins after resection.[10]
Although low-grade tumors are usually curable by surgery alone, higher-grade sarcomas (as determined by the mitotic index and by the presence of hemorrhage and necrosis) are associated with higher local-treatment failure rates and increased metastatic potential.
Surveillance for Relapse
A retrospective review included 174 consecutive patients with a soft tissue sarcoma of the limb who underwent follow-up by oncologists at a single center from 2003 to 2009.[11] The rate and site of recurrence and mode of detection were analyzed. Eighty-two patients (47%) experienced relapse. Isolated local recurrences occurred in 26 patients and local relapse with synchronous pulmonary metastases occurred in 5 patients. Local recurrences were detected clinically in 30 of the 31 patients; magnetic resonance imaging identified only one local recurrence. Twenty-eight patients developed isolated lung metastases; in 9 patients, the lung metastases were amenable to resections, 7 of whom were free of disease after treatment. Lung metastases were detected by chest x-ray in 19 patients, by computed tomography scanning in 3 patients, and clinically in 11 patients. Twenty-three patients developed nonpulmonary metastases. More than 80% of the relapses occurred in the first 2 years of follow-up; however, later recurrences were also observed.[11][Level of evidence: 3iiDi] This study supports imaging surveillance for detection of lung metastases, whereas local recurrences at the primary site were usually detected by clinical examination. The impact of picking up metastases from overall survival or quality-of-life data is unknown.
Related Summaries
Other PDQ summaries containing information about soft tissue sarcoma include:
References
- American Cancer Society: Cancer Facts and Figures 2018. Atlanta, Ga: American Cancer Society, 2018. Available online. Last accessed January 5, 2018.
- Wibmer C, Leithner A, Zielonke N, et al.: Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol 21 (5): 1106-11, 2010. [PUBMED Abstract]
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Gustafson P, Dreinhöfer KE, Rydholm A: Soft tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand 65 (1): 47-50, 1994. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Kasper B, Ouali M, van Glabbeke M, et al.: Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer 49 (2): 449-56, 2013. [PUBMED Abstract]
- Vraa S, Keller J, Nielsen OS, et al.: Prognostic factors in soft tissue sarcomas: the Aarhus experience. Eur J Cancer 34 (12): 1876-82, 1998. [PUBMED Abstract]
- Trovik LH, Ovrebo K, Almquist M, et al.: Adjuvant radiotherapy in retroperitoneal sarcomas. A Scandinavian Sarcoma Group study of 97 patients. Acta Oncol 53 (9): 1165-72, 2014. [PUBMED Abstract]
- Rothermundt C, Whelan JS, Dileo P, et al.: What is the role of routine follow-up for localised limb soft tissue sarcomas? A retrospective analysis of 174 patients. Br J Cancer 110 (10): 2420-6, 2014. [PUBMED Abstract]
Cellular Classification of Adult Soft Tissue Sarcoma
Soft tissue sarcomas are classified histologically according to the soft tissue cell of origin. Additional studies, including electron microscopy, specialized immunohistochemistry, flow cytometry, cytogenetics, and tissue culture studies may allow identification of particular subtypes within the major histologic categories. For example, S100 antigen suggests neural sheath origin, cytokeratin suggests epithelioid or synovial cell origin, and factor VIII-related antigen suggests endothelial origin. Likewise, some subtypes of sarcomas have characteristic genetic markers, but these markers are not generally used in the routine clinical setting (e.g., translocation t(X;18)(p11;q11) in synovial sarcomas and translocation t(12;16)(q13;p11) in myxoid and round-cell sarcomas).[1-3]
The histologic grade reflects the metastatic potential of these tumors more accurately than the classic cellular classification listed below. Pathologists assign a grade based on the number of mitoses per high-powered field, the presence of necrosis, cellular and nuclear morphology, and the degree of cellularity; discordance among expert pathologists regarding tumor grade, and even histologic subtype, can be substantial.[4]
The World Health Organization lists the following cell types in its classification of soft tissue sarcomas:[5,6]
- Adipocytic tumors.
- Dedifferentiated liposarcoma.*
- Myxoid/round cell liposarcoma.
- Pleomorphic liposarcoma.
- Fibroblastic/myofibroblastic tumors.
- Fibrosarcoma.**
- Myxofibrosarcoma, low grade.
- Low-grade fibromyxoid sarcoma.
- Sclerosing epithelioid fibrosarcoma.
- So-called fibrohistiocytic tumors.
- Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (including pleomorphic, giant cell, myxoid/high-grade myxofibrosarcoma, and inflammatory forms).
- Smooth muscle tumors.
- Leiomyosarcoma.
- Skeletal muscle tumors.
- Rhabdomyosarcoma (embryonal, alveolar, and pleomorphic forms).
- Vascular tumors.
- Epithelioid hemangioendothelioma.
- Angiosarcoma, deep.***
- Tumors of peripheral nerves.
- Malignant peripheral nerve sheath tumor.
- Chondro-osseous tumors.
- Extraskeletal chondrosarcoma (mesenchymal and other variants).
- Extraskeletal osteosarcoma.
- Tumors of uncertain differentiation.
- Synovial sarcoma.
- Epithelioid sarcoma.
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Extraskeletal myxoid chondrosarcoma.
- Primitive neuroectodermal tumor/extraskeletal Ewing tumor.
- Desmoplastic small round cell tumor.
- Extrarenal rhabdoid tumor.
- Undifferentiated sarcoma; sarcoma, not otherwise specified.
[Note: *It is recognized that dedifferentiated liposarcoma primarily arises in the context of deep atypical lipomatous tumor/well-differentiated liposarcoma, a sarcoma of intermediate malignancy because of the lack of metastatic capacity. **The category of fibrosarcoma can be inclusive of fibrosarcomatous differentiation in dermatofibrosarcoma protuberans. ***Cutaneous angiosarcoma may be difficult to stage using the American Joint Committee on Cancer system. (Refer to the PDQ summary on Gastrointestinal Stromal Tumors for more information.)]
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Alvegård TA, Berg NO: Histopathology peer review of high-grade soft tissue sarcoma: the Scandinavian Sarcoma Group experience. J Clin Oncol 7 (12): 1845-51, 1989. [PUBMED Abstract]
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
Stage Information for Adult Soft Tissue Sarcoma
Note: The American Joint Committee on Cancer (AJCC) has published the 8th edition of the AJCC Cancer Staging Manual, which includes revisions to the staging for this disease. Implementation of the 8th edition began in January 2018. The PDQ Adult Treatment Editorial Board, which maintains this summary, is reviewing the revised staging and will make appropriate changes as needed.
Staging has an important role in determining the most effective treatment for soft tissue sarcoma. Clinical staging involves magnetic resonance imaging (MRI) or computed tomography (CT) of the primary tumor area and a chest CT to look for metastasis to the lung (the most common site of distant spread). An abdominal CT scan is done in the case of retroperitoneal sarcomas because the liver may be the site of initial clinical metastasis for these tumors.
The stage is determined by the size of the tumor, the histologic grade, and whether there is spread to lymph nodes or distant sites. Intracompartmental or extracompartmental extension of extremity sarcomas is also important for surgical decision making. For complete staging, a thorough review of all biopsy specimens (including those from the primary tumor, lymph nodes, or other suspicious lesions) is essential. CT scan of the chest is recommended for sarcomas larger than 5 cm (T2) or with moderate to poor differentiation (grades 2–4). Nodal involvement is rare, occurring in fewer than 3% of patients with sarcoma.[1]
Lymph node involvement in soft tissue sarcomas of adulthood is rare but is somewhat more frequent in some subtypes (e.g., rhabdomyosarcoma, vascular sarcomas, clear cell sarcomas, and epithelioid sarcomas) when they are high grade.[2] Because treatment decisions are predicated on pathology staging, patients should be staged before, and again after, any neoadjuvant therapy. The assessment of tumor grade can be affected in either direction, but more frequently decreased because of differential cellular loss related to the neoadjuvant chemotherapy or radiation.[3] Grade, which is based on cellular differentiation, mitotic rate, and extent of necrosis, should be recorded for all soft tissue sarcomas. A three-grade system (G1–G3) is preferred. (See below.)
The AJCC has designated staging by the four criteria of tumor size, nodal status, metastasis, and grade (TNMG).[3] The characteristic molecular markers of some sarcomas are not formally incorporated in the staging system pending further evaluation of their impact on prognosis. Recurrent sarcomas are restaged using the same system as for primary tumors with the specification that the tumor is recurrent.
Definitions of TNM and Grade
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| bSuperficial tumor is located exclusively above the superficial fascia without invasion of the fascia; deep tumor is located either exclusively beneath the superficial fascia, superficial to the fascia with invasion of or through the fascia, or both superficial yet beneath the fascia. | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| T1 | Tumor ≤5 cm in greatest dimension. (Size should be regarded as a continuous variable, and the measurement should be provided.) |
| T1a | Superficial tumor.b |
| T1b | Deep tumor.b |
| T2 | Tumor >5 cm in greatest dimension.b |
| T2a | Superficial tumor.b |
| T2b | Deep tumor. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| bPresence of positive nodes (N1) in M0 tumors is considered Stage III. | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1b | Regional lymph node metastasis. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | |
| M0 | No distant metastasis. |
| M1 | Distant metastasis. |
| aReprinted with permission from AJCC: Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-8. | ||||
| Stage IA | T1a | N0 | M0 | G1, GX |
| T1b | N0 | M0 | G1, GX | |
| Stage IB | T2a | N0 | M0 | G1, GX |
| T2b | N0 | M0 | G1, GX | |
| Stage IIA | T1a | N0 | M0 | G2, G3 |
| T1b | N0 | M0 | G2, G3 | |
| Stage IIB | T2a | N0 | M0 | G2 |
| T2b | N0 | M0 | G2 | |
| Stage III | T2a, T2b | N0 | M0 | G3 |
| Any T | N1 | M0 | Any G | |
| Stage IV | Any T | Any N | M1 | Any G |
Neurovascular and bone invasion are indicators of poor prognosis, but they are not incorporated into the formal staging system.
References
- Fong Y, Coit DG, Woodruff JM, et al.: Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 217 (1): 72-7, 1993. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
Treatment Option Overview
Multimodality Approach
In most cases, a combined modality approach of preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT) is used, rather than the radical surgical procedures, such as amputation, that were used in the past. It may even be possible to use surgery without PORT in selected cases. For example, a case series was reported from a specialized sarcoma treatment referral center in which 74 selected patients with primary extremity and trunk tumors 5 cm or smaller were found to have no histologic involvement of the surgical margins. The patients were observed without radiation therapy, and the estimated local recurrence rate after 10 years was 11%.[1][Level of evidence: 3iiiDiv] The role of chemotherapy is not as well defined as is the role for radiation therapy. Because of the evolving nature of the treatment options for this disease, patients should be considered when available. Information about ongoing clinical trials is available from the NCI website.
Role of Surgery
Surgical resection is the mainstay of therapy for soft tissue sarcomas. When feasible, wide-margin function–sparing surgical excision is the cornerstone of effective treatment for extremity tumors. This may be facilitated by soft tissue reconstructive surgery, which generally permits wider margins than those obtained when the surgical plan involves direct closure of the excision site.[2] Cutting into the tumor mass or shelling out the gross tumor along the plane of the pseudocapsule of compressed tumor cells and reactive tissue that often surrounds soft tissue sarcomas are associated with an elevated risk of local recurrence. Even high-grade, soft tissue sarcomas of the extremities can usually be effectively treated while preserving the limb with combined-modality treatment consisting of preRT or PORT to reduce local recurrence. (Refer to the Role of Radiation Therapy section of this summary for more information.)
Only one small, single-institution, randomized trial has directly compared amputation to limb-sparing surgery for soft tissue sarcomas of the extremities.[3] In a 2:1 randomization ratio, 27 patients with high-grade extremity sarcomas were assigned to a wide excision plus PORT (45 Gy–50 Gy to the wide local excision area, and a total of 60 Gy–70 Gy to the tumor bed over 6–7 weeks), and 16 were assigned to amputation at or above the joint proximal to the tumor. Both groups received adjuvant chemotherapy (i.e., doxorubicin, cyclophosphamide, and high-dose methotrexate). At 63 months, with a median follow-up of 56 months, there were four local recurrences in the 27 patients who underwent limb-sparing surgery and no recurrences in the 16 patients who underwent amputation P2 = .12. Overall survival (OS) rates were not statistically significantly different (actuarial 5-year survival rate, 83% vs. 88%, P2 = .99).[3][Level of evidence: 1iiA]
Local control of high-grade soft tissue sarcomas of the trunk and the head and neck can be achieved with surgery in combination with radiation therapy.[4] It may be possible to use surgery without PORT in selected cases. For example, a case series was reported from a specialized sarcoma treatment referral center in which 74 selected patients with primary extremity and trunk tumors 5 cm or smaller were found to have no histologic involvement of the surgical margins.[1] They were observed without radiation therapy, and the estimated local recurrence rate after 10 years was 11%.[1][Level of evidence: 3iiiDiv] The role of chemotherapy is not as well defined as is the role of radiation therapy. Because of the evolving nature of the treatment options for this disease, patients should be offered the option of clinical trials when available.
Effective treatment of retroperitoneal sarcomas requires removal of all gross disease while sparing adjacent viscera not invaded by tumor. The prognosis for patients with high-grade retroperitoneal sarcomas is less favorable than for patients with tumors at other sites, partly because of the difficulty in completely resecting these tumors and the dose-limiting toxicity of high-dose radiation therapy on visceral organs.[5-8]
In the setting of distant metastasis, surgery may be associated with long-term, disease-free survival in patients with pulmonary metastasis and optimal underlying disease biology (i.e., patients with a limited number of metastases and slow nodule growth) who have undergone or are undergoing complete resection of the primary tumor.[9-11] It is not clear to what degree the favorable outcomes are attributable to the efficacy of surgery or the careful selection of patients based on factors that are associated with less-virulent disease.
Role of Radiation Therapy
Radiation plays an important role in limb-sparing therapy. Pre- and postoperative external-beam radiation therapies (EBRT), as well as brachytherapy, have been shown to decrease the risk of local recurrence. They have not been shown to increase OS but are used to avoid amputation for all but the most locally advanced tumors or for limbs seriously compromised by vascular disease, where acceptable functional preservation is not possible. In the case of EBRT, irradiation of the entire limb circumference is avoided to preserve vascular and nerve structures that are critical to function and preservation of the limb.
PORT
PORT has been tested in a single-institution, randomized trial of 141 patients with extremity sarcomas who were treated with limb-sparing surgery. Patients with high-grade tumors (n = 91) also received adjuvant chemotherapy (i.e., five 28-day cycles of doxorubicin and cyclophosphamide). All patients were randomly assigned to receive radiation (45 Gy to a wide field, plus a tumor-bed boost of 18 Gy over 6–7 weeks), concurrent with chemotherapy in the case of high-grade tumors versus no radiation.[12] At up to 12 years of follow-up, there was one local recurrence in the 70 patients randomly assigned to receive radiation versus 17 recurrences in the 71 control patients (P = .0001), with similar reduction in risk of local recurrence for both high- and low-grade tumors. However, there was no difference in OS between the radiation and control groups.[12][Level of evidence: 1iiDiii] Global quality of life was similar in the two groups, but the radiation therapy group had substantially worse functional deficits resulting from reduced strength and joint motion as well as increased edema.
To limit acute toxicity with preRT, smaller fields and lower doses are generally given than is the case with PORT. PreRT has been directly compared with PORT for extremity soft tissue sarcomas in a multicenter randomized trial.[13-15] Designed to include 266 patients, the trial was stopped early after 190 patients had been accrued because of an increase in wound complications in the preRT group. The scheduled radiation in the preRT group was a wide field of 50 Gy in 2-Gy fractions (first phase of the trial) with an additional 16 Gy to 20 Gy to the tumor bed and a 2-cm margin (second phase of the trial) only if tumor cells were found at the surgical margins.
Patients in the PORT group were scheduled to receive radiation during both phases of the trial. The wound-complication rates were 35% versus 17% in the preRT and PORT groups, respectively (P = .01). In addition, limb function at 6 weeks after surgery was worse in the preRT group (P = .01).[13] At 5 years, the two groups had similar local control rates (93% vs. 92%) and OS (73% vs. 67%, P = .48).[14] Of the 129 patients evaluated for limb function at 21 to 27 months after surgery (n = 73 for preRT and n = 56 for PORT), limb function was similar in both groups, but there was a statistical trend for less fibrosis in the preRT group (P = .07).[15]
Brachytherapy
Brachytherapy has also been investigated as an adjuvant therapy for soft tissue sarcomas. Although it has possible advantages of convenience and less radiation to normal surrounding tissue relative to EBRT, the two treatment strategies have not been directly compared in terms of efficacy or morbidity. However, adjuvant brachytherapy has been compared with surgery without radiation. The time interval between preRT and surgical excision in extremity soft tissue sarcoma had minimal influence on the development of wound complications. Four- or 5-week intervals showed equivalent complication rates between patients who did or did not develop wound complications, suggesting an optimal interval to reduce potential complications.[16]
In a single-institution trial, 164 patients with sarcomas of the extremity or superficial trunk were randomly assigned during surgery, if all gross tumor could be excised, to receive an iridium Ir 192 implant (delivering 42 Gy–45 Gy over 4–6 days; 78 patients) or to a control arm of no radiation (86 patients).[17,18] Some of the patients with high-grade tumors received adjuvant doxorubicin-based chemotherapy if they were thought to be at a high risk for metastasis (34 patients in each study arm). With a median follow-up of 76 months, the 5-year actuarial local recurrence rates were 18% and 31% in the brachytherapy and control arms, respectively (P = .04). This difference was limited to patients with high-grade tumors. There was no discernible difference in sarcoma-specific survival rates between the brachytherapy and control arms (84% and 81%, respectively; P = .65), and there was no difference in the high tumor-grade group.[17][Level of evidence: 1iiDiii] The rates of clinically important wound complications (e.g., need for operative revision or repeated seroma drainage, wound separation, large hematomas, or purulent infection) were 24% and 14% in the radiation and control arms, respectively (P = .13); wound reoperation rates were 10% and 0%, respectively (P = .006).[18]
Intensity-modulated radiation therapy
Intensity-modulated radiation therapy (IMRT) has been used to deliver preRT or PORT to patients with extremity soft tissue sarcomas in an effort to spare the femur, joints, and selected other normal tissues from the full prescription dose and to maintain local control while potentially reducing radiation therapy-related morbidity. Initial single-institution reports suggest that high rates of local control with some reduction in morbidity are possible with this technique.[19,20] Retrospective comparison of IMRT compared with 3-dimensional, conformal radiation therapy demonstrates that local recurrence for primary soft tissue sarcomas of the extremity was worse in the non-IMRT group.[21][Level of evidence: 3iiiDiv]
Surgery and radiation therapy
In some tumors of the extremities or trunk, surgery alone can be performed without the use of radiation. Evidence for this approach is limited to single-institution, relatively small, case series [1,22,23] or analysis of outcomes in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) tumor registry.[24] However, these comparisons suffer from low statistical power and differential evaluability rates that could have introduced bias.[1] Patient selection factors may vary among surgeons. In general, this approach is considered in patients with low-grade tumors of the extremity or superficial trunk that are 5 cm or smaller in diameter (T1) and have microscopically negative surgical margins; long-term local tumor control is about 90% in such patients.[25]
A patterns-of-care study using SEER data was queried to identify patients undergoing surgery for truncal and extremity soft tissue sarcomas from 2004 to 2009.[26] Of 5,075 patients, 50% received radiation therapy. Radiation was considered to be underused in a significant portion of patients undergoing treatment for soft tissue sarcoma in the United States. Although routine radiation therapy is not recommended for stage I patients, 25% of them still underwent radiation. Even though routine radiation therapy is recommended for patients with stage II and III tumors, only 60% of them underwent radiation. On multivariate analysis, predictors of radiation therapy included age younger than 50 years (odds ratio [OR], 1.57; 95% confidence interval [CI], 1.28–1.91), malignant fibrous histiocytoma histology (OR, 1.47; 95% CI, 1.3–1.92), T2 classification (OR, 1.88; 95% CI, 1.60–2.20), and G3 (OR, 6.27; 95% CI, 5.10–7.72). Patients with stage III soft tissue sarcoma who received radiation therapy showed improved disease-specific survival at 5 years compared with those who did not (68% vs. 46%, P < .001).[26][Level of evidence: 3iDii]
On occasion, surgical excision cannot be performed in the initial management of soft tissue sarcomas because the morbidity would be unacceptable or nearby critical organs make complete resection impossible. In such circumstances, radiation has been used as the primary therapy.[27] However, this must be considered a treatment of last resort. Experience is limited to retrospective case series from single centers.[27][Level of evidence: 3iiiDiv]
Role of Adjuvant or Neoadjuvant Chemotherapy for Clinically Localized Tumors
The role of adjuvant chemotherapy is not completely clear. The investigation of its use falls into two categories or generations—pre- and post-ifosfamide regimens. In discussions with a patient, any potential benefits should be considered in the context of the short- and long-term toxicities of the chemotherapy.
First-generation trials (preifosfamide)
Several prospective, randomized trials were unable to determine conclusively whether doxorubicin-based adjuvant chemotherapy benefits adults with resectable soft tissue sarcomas. The majority of these studies accrued small numbers of patients and did not demonstrate a metastasis-free survival or an OS benefit for adjuvant chemotherapy.[4] A small study of adjuvant chemotherapy showed a positive effect on both disease-free survival (DFS) and OS in patients treated with postoperative chemotherapy.[28] There was wide interstudy variability among the reported trials, including differences in therapeutic regimens, drug doses, sample size, tumor site, and histologic grade.
A quantitative meta-analysis of updated data from 1,568 individual patients in 14 trials of doxorubicin-based adjuvant therapy showed an absolute benefit from adjuvant therapy of 6% for a local relapse-free interval (95% CI, 1%–10%), 10% for a distant relapse-free interval (95% CI, 5%–15%), and 10% for recurrence-free survival (95% CI, 5%–15%). A statistically significant OS benefit at 10 years was not detected: absolute difference 4% (95% CI, -1%–+9%).[29,30][Level of evidence: 1iiDii] However, only a small proportion of patients in this meta-analysis were treated with ifosfamide, an agent with demonstrated activity against soft tissue sarcoma. In addition, a subset analysis suggested that patients with sarcomas of the extremities may have benefited from adjuvant chemotherapy (hazard ratio [HR] for death, 0.8, P = .029), but there was no clear evidence that patients with extremity sarcomas had outcomes that were statistically significantly different from the outcomes of patients with tumors at other sites (P = .58).[30]
Second-generation trials (postifosfamide)
Subsequent chemotherapy trials were performed using anthracycline and ifosfamide combinations in patients who primarily had extremity or truncal soft tissue sarcomas. The data are conflicting, and the issue is still not settled. In a small feasibility study, 59 patients with high-risk, soft tissue sarcomas, 58 of whom had an extremity or the trunk as the primary site, underwent primary resection plus PORT and were randomly assigned to observation versus a dose-dense regimen of six 14-day courses of ifosfamide, dacarbazine (DTIC), and doxorubicin (IFADIC regimen) with granulocyte colony-stimulating factor (G-CSF) bone marrow support and mesna uroprotection.[31] There were no statistically significant differences in OS or relapse-free survival (RFS), but the study was severely underpowered.
In a second trial performed by the Italian National Council for Research, high-risk patients were treated with local therapy (i.e., wide resection plus preRT or PORT, or amputation as clinically necessary) and were then randomly assigned to observation versus five 21-day cycles of 4-epidoxorubicin (epirubicin) plus ifosfamide (with mesna and G-CSF).[28,32] Based on power calculations, the planned study size was 190 patients, but the trial was stopped after 104 patients had been entered because an interim analysis revealed a statistically significant (P = .001) difference in DFS favoring the chemotherapy arm. By the time of the initial peer-reviewed report of the study, the DFS still favored the chemotherapy group (median DFS of 48 months vs. 16 months), but the P value had risen to .04.[28]
Although there was no difference in metastasis-free survival at the time of the report, there was an improvement in median OS (75 months vs. 46 months, P = .03). However, at the follow-up report (at a median of 89.6 months in a range of 56–119 months), OS differences were no longer statistically significant (58.5% vs. 43.1% [P = .07]). The DFS difference had also lost statistical significance (47.2% vs. 16.0% [P = .09]).[32] In summary, the trial was underpowered because it was stopped early, and the early promising results that led to stopping the trial diminished as the trial matured.
In a third, underpowered, single-center trial, 88 patients with high-risk, soft tissue sarcomas (64 of whom had extremity or truncal primary tumors) underwent surgery (with or without radiation) and were then randomly assigned to receive four 21-day cycles of chemotherapy (epirubicin [n = 26] or epirubicin plus ifosfamide [n = 19]) versus no adjuvant chemotherapy (n = 43).[33] The trial was closed prematurely because of a slow accrual rate. After a median follow-up of 94 months, the 5-year DFS in the chemotherapy and control arms was 69% versus 44%, respectively (P = .01); the 5-year OS rates were 72% versus 47% (P = .06). All of the benefit associated with chemotherapy appeared restricted to the 19 patients who received epirubicin plus ifosfamide.
In yet another underpowered trial, 137 patients with high-risk, soft tissue sarcomas (93% with extremity or truncal primary tumors) who met the eligibility criteria were randomly assigned to undergo surgical resection (with or without radiation) or to receive three preoperative 21-day cycles of doxorubicin plus ifosfamide.[34] This multicenter European Organization for Research and Treatment of Cancer trial (EORTC-62874) was closed because of slow accrual and results that were not promising enough to continue. With a median follow-up of 7.3 years, the 5-year DFS in the surgery alone and chemotherapy plus surgery arms was 52% and 56%, respectively (P = .35); and OS was 64% and 65%, respectively (P = .22).
These last four trials have been combined with the 14 first-generation trials in a trial-level meta-analysis.[35] Of the 18 randomized trials of patients with resectable soft tissue sarcomas, five trials used a combination of doxorubicin (50–90 mg/m2 per cycle) plus ifosfamide (1,500–5,000 mg/m2 per cycle). The remaining 13 trials used doxorubicin (50–70 mg/m2 per cycle) alone or with other drugs. The absolute risk reduction in local recurrence rates associated with any chemotherapy added to local therapy was 4 percentage points (95% CI, 0%–7%), and it was 5 percentage points (95% CI, 1%–12%) when ifosfamide was combined with doxorubicin. The absolute reduction in overall mortality was 6 percentage points with any chemotherapy (95% CI, 2%–11%; [i.e., a reduction from 46%–40%]), 11 percentage points for doxorubicin plus ifosfamide (95% CI, 3%–19%; [i.e., a reduction from 41%–30%]), and 5 percentage points for doxorubicin without ifosfamide.[35][Level of evidence: 1iiA]
An additional multicenter randomized trial (EORTC-62931 [NCT00002641]), the largest trial reported to date using adjuvant doxorubicin (75 mg/m2) plus ifosfamide (5,000 mg/m2), was subsequently published in abstract form and was not included in the above meta-analysis.[36] The results differed from those reported in the meta-analysis.[35] After local therapy, 351 patients were randomly assigned to five 21-day cycles of adjuvant therapy versus observation. The trial was stopped for futility because the 5-year RFS was 52% in both arms. OS was 64% in the chemotherapy arm versus 69% in the observation arm. In a subsequent abstract, the EORTC investigators reported a combined analysis of this trial and their previous trial (EORTC-62771) [37] of adjuvant cyclophosphamide plus doxorubicin plus DTIC (CYVADIC), representing the two largest trials of adjuvant therapy for adult soft tissue sarcoma in the literature.[38] The combined analysis showed no improvement in either RFS or OS associated with adjuvant chemotherapy.[38][Level of evidence: 1iiA]
In summary, the impact of adjuvant chemotherapy on survival is not clear but is likely to be small in absolute magnitude. Therefore, in discussions with a patient, any potential benefits should be considered in the context of the short- and long-term toxicities of the chemotherapy.
Role of regional hyperthermia
The use of regional hyperthermia to enhance the local effects of systemic chemotherapy in the neoadjuvant and adjuvant setting is under investigation. In a multicenter phase III trial, 341 patients with high-risk (tumor ≥5 cm, grade 2–3, and deep to fascia), soft tissue sarcomas (149 extremity tumors and 192 nonextremity tumors) were randomly allocated to receive four 21-day cycles of chemotherapy (etoposide 125 mg/m2 on days 1 and 4; ifosfamide 1,500 mg/m2 on days 1–4; doxorubicin 50 mg/m2 on day 1) with or without regional hyperthermia both before and after local therapy.[39] Approximately 11% of the patients were being treated for recurrent tumors. The regional hyperthermia was designed to produce tumor temperatures of 42°C for 60 minutes and was given on days 1 and 4 of each chemotherapy cycle. After the first four cycles of chemotherapy, definitive surgical excision of the tumor was performed, if possible, followed by radiation therapy, if indicated (i.e., a 52.7 Gy median dose delivered), and then the last four cycles of chemotherapy plus or minus hyperthermia. Three of the nine treatment centers with particular expertise in hyperthermia treated 91% of the patients in the trial.
The median duration of follow-up was 34 months. Local progression occurred in 56 patients in the hyperthermia group and 76 patients in the control group. The relative HR for local progression or death was 0.58 (95% CI, 0.41–0.84), with an absolute difference at 2 years of 15% (76% vs. 61%; 95% CI of the difference 6–26). The decreased risk of local progression or death was seen in both extremity and nonextremity tumors. However, hyperthermia had no effect on distant failure rates nor was there a statistically significant effect on OS (HR, .88, 95% CI, 0.64–1.21; P = .43).[39][Level of evidence: 1iiDiii] There was a higher rate of grade 3 to 4 leucopenia in the hyperthermia group: 77.6% versus 63.5% (P = .005). Since a large proportion of the patients were treated at centers with special expertise, there is no certainty that the finding can be generalized to apply to other settings.
Role of isolated limb perfusion
Isolated limb perfusion is under investigation as a means to deliver high doses of chemotherapy and permit limb salvage in unresectable primary or recurrent extremity soft tissue sarcomas that would otherwise require amputation, in the opinion of the surgeon.[40,41] Common drugs used in the procedure are TNF-alpha, melphalan, and interferon-gamma. Experience is limited to case series with response rates and reported avoidance of amputation as the outcome.[40,41][Level of evidence: 3iiiDiv] The technique requires specialized expertise to avoid severe local and systemic toxicity including systemic effects of TNF-alpha. The technique has not been directly compared with standard approaches using combined systemic and local therapy.
Role of chemotherapy for advanced disease
Doxorubicin is a mainstay of systemic therapy in the management of locally advanced and metastatic soft tissue sarcoma. Pegylated liposomal encapsulated doxorubicin is a formulation of doxorubicin designed to prolong the half-life of circulating doxorubicin and slow the release of active drugs.[42] The changed pharmacokinetics result in less myelosuppression and possibly less cardiotoxic effects, but there is a substantial incidence of hypersensitivity-like reactions and hand-foot syndrome. Its clinical activity relative to unencapsulated doxorubicin is not clear.[42][Level of evidence: 3iiiDiv] Other drugs that are thought to have clinical activity as single agents are ifosfamide, epirubicin, gemcitabine, and paclitaxel.[43-46][Level of Evidence: 3iiiDiv] Their clinical activity relative to single-agent doxorubicin is not clear, and they are not known to have superior activity.
There is controversy about the clinical benefit of adding other drugs to doxorubicin as a single agent. A systematic evidence review and meta-analysis conducted by the Cochrane Collaboration summarized the eight randomized trials reported from 1976 to 1995.[47] No additional randomized trials had been reported or were known to be in progress between 1995 and the 2002 literature search. Single-agent doxorubicin had been compared with a variety of doxorubicin-containing combinations that included vincristine, vindesine, cyclophosphamide, streptozotocin, mitomycin-C, cisplatin, and/or ifosfamide. Combination regimens consistently caused more nausea and hematologic toxicity. However, the better response rates associated with combination therapy were marginal and depended on the statistical model used (fixed effects model ORresponse = 1.29; 95% CI, 1.03–1.60, P = .03; random effects model ORresp = 1.26; 95% CI, 0.96–1.67, P = .10) There was no statistically significant difference in the 1- (ORmortality = 0.87; 95% CI, 0.73–1.05, P = .14) or 2-year mortality rates (ORmortality = 0.84; 95% CI, 0.67–1.06, P = .13).
These results were very similar even when the analyses were restricted to the four trials that used DTIC and/or ifosfamide as part of the combination regimen with doxorubicin agents that were postulated to have greater activity than the others tested. A subsequent meta-analysis of all three published randomized trials of chemotherapy regimens that contained ifosfamide versus those that did not came to similar conclusions: tumor response rates were better when the regimen included ifosfamide (RRresponse = 1.52; 95% CI, 1.11–2.08), but mortality at 1 year was not (RRmortality = 0.98; 95% CI, 0.85–1.13).[48][Level of evidence: 1iiDiv]. Therefore, response rate was a poor surrogate for OS. Quality-of-life outcomes were not reported in any of the above-mentioned randomized trials, but toxicity was worse when agents were added to doxorubicin.
References
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Lohman RF, Nabawi AS, Reece GP, et al.: Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 94 (8): 2256-64, 2002. [PUBMED Abstract]
- Rosenberg SA, Tepper J, Glatstein E, et al.: The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305-15, 1982. [PUBMED Abstract]
- O'Byrne K, Steward WP: The role of adjuvant chemotherapy in the treatment of adult soft tissue sarcomas. Crit Rev Oncol Hematol 27 (3): 221-7, 1998. [PUBMED Abstract]
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Lewis JJ, Leung D, Woodruff JM, et al.: Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 228 (3): 355-65, 1998. [PUBMED Abstract]
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Griffin AM, Dickie CI, Catton CN, et al.: The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann Surg Oncol 22 (9): 2824-30, 2015. [PUBMED Abstract]
- Pisters PW, Harrison LB, Leung DH, et al.: Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 14 (3): 859-68, 1996. [PUBMED Abstract]
- Alektiar KM, Zelefsky MJ, Brennan MF: Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys 47 (5): 1273-9, 2000. [PUBMED Abstract]
- Alektiar KM, Brennan MF, Healey JH, et al.: Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 26 (20): 3440-4, 2008. [PUBMED Abstract]
- Alektiar KM, Brennan MF, Singer S: Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer 117 (14): 3229-34, 2011. [PUBMED Abstract]
- Folkert MR, Singer S, Brennan MF, et al.: Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol 32 (29): 3236-41, 2014. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Bagaria SP, Ashman JB, Daugherty LC, et al.: Compliance with National Comprehensive Cancer Network guidelines in the use of radiation therapy for extremity and superficial trunk soft tissue sarcoma in the United States. J Surg Oncol 109 (7): 633-8, 2014. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
- Frustaci S, Gherlinzoni F, De Paoli A, et al.: Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 19 (5): 1238-47, 2001. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Sarcoma Meta-analysis Collaboration (SMAC): Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev (4): CD001419, 2000. [PUBMED Abstract]
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
- Frustaci S, De Paoli A, Bidoli E, et al.: Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology 65 (Suppl 2): 80-4, 2003. [PUBMED Abstract]
- Petrioli R, Coratti A, Correale P, et al.: Adjuvant epirubicin with or without Ifosfamide for adult soft-tissue sarcoma. Am J Clin Oncol 25 (5): 468-73, 2002. [PUBMED Abstract]
- Gortzak E, Azzarelli A, Buesa J, et al.: A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. Eur J Cancer 37 (9): 1096-103, 2001. [PUBMED Abstract]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al.: A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113 (3): 573-81, 2008. [PUBMED Abstract]
- Woll PJ, van Glabbeke M, Hohenberger P, et al.: Adjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): Interim analysis of a randomised phase III trial. [Abstract] J Clin Oncol 25 (Suppl 18): A-10008, 2007.
- Bramwell V, Rouesse J, Steward W, et al.: Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 12 (6): 1137-49, 1994. [PUBMED Abstract]
- Le Cesne A, Van Glabbeke M, Woll PJ, et al.: The end of adjuvant chemotherapy (adCT) era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma (STS): pooled analysis of the two STBSG-EORTC phase III clinical trials. [Abstract] J Clin Oncol 26 (Suppl 15): A-10525, 2008.
- Issels RD, Lindner LH, Verweij J, et al.: Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 11 (6): 561-70, 2010. [PUBMED Abstract]
- Eggermont AM, de Wilt JH, ten Hagen TL: Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 4 (7): 429-37, 2003. [PUBMED Abstract]
- Bonvalot S, Laplanche A, Lejeune F, et al.: Limb salvage with isolated perfusion for soft tissue sarcoma: could less TNF-alpha be better? Ann Oncol 16 (7): 1061-8, 2005. [PUBMED Abstract]
- Grenader T, Goldberg A, Hadas-Halperin I, et al.: Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20 (1): 15-20, 2009. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
- Bramwell VH, Anderson D, Charette ML, et al.: Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev (3): CD003293, 2003. [PUBMED Abstract]
- Verma S, Younus J, Stys-Norman D, et al.: Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 34 (4): 339-47, 2008. [PUBMED Abstract]
Stage I Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery and radiation therapy.
Low-grade soft tissue sarcomas have little metastatic potential, but they have a propensity to recur locally. Accordingly, surgical excision with negative tissue margins of 1 cm to 2 cm or larger in all directions is the treatment of choice for patients with these early-stage sarcomas.[1-3] The Mohs surgical technique may be considered as an alternative to wide surgical excision for the very rare, small, well-differentiated primary sarcomas of the skin when cosmetic results are considered to be important, as margins can be assured with minimal normal tissue removal.[4]
Carefully executed high-dose radiation therapy using a shrinking-field technique may be beneficial for unresectable tumors or for resectable tumors in which a high likelihood of residual disease is thought to be present when margins are judged to be inadequate, and when wider resection would require either an amputation or the removal of a vital organ.[5] Because of the low metastatic potential of these tumors, chemotherapy is usually not given.[6,7]
Standard treatment options:
- Surgical excision of tumors 5 cm or smaller in diameter with negative tissue margins in all directions.[8-12]
- Surgical excision with preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT). Radiation decreases the risk of local recurrence but has not been shown to increase overall survival.[13-16]
- If the tumor is unresectable, high-dose preRT may be used.[17]
- For tumors of the retroperitoneum, trunk, and head and neck, the following are options:
- Surgical resection with the option of PORT if negative margins cannot be obtained. Wide margins are unusual in these sites, and radiation therapy is usually advocated for trunk and head and neck primary sites.[18]
- PreRT followed by maximal surgical resection. Radiation therapy may be used in sarcomas of the trunk and head and neck to maximize local control because of the inability to obtain wide surgical margins.[19]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Fish FS: Soft tissue sarcomas in dermatology. Dermatol Surg 22 (3): 268-73, 1996. [PUBMED Abstract]
- Temple WJ, Temple CL, Arthur K, et al.: Prospective cohort study of neoadjuvant treatment in conservative surgery of soft tissue sarcomas. Ann Surg Oncol 4 (7): 586-90, 1997 Oct-Nov. [PUBMED Abstract]
- Sarcoma Meta-analysis Collaboration (SMAC): Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev (4): CD001419, 2000. [PUBMED Abstract]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al.: A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113 (3): 573-81, 2008. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
- Brennan MF, Singer S, Maki RG: Sarcomas of the soft tissue and bone. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds.: Cancer: Principles and Practice of Oncology. Vols. 1 & 2. 8th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2008, pp 1741-1833.
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
Stage II and Node-Negative Stage III Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
High-grade localized soft tissue sarcomas have an increased potential for local recurrence and metastasis. For sarcomas of the extremities, local control comparable to that obtained with amputation may be achieved with limb-sparing surgery that involves wide local excision in combination with preoperative radiation therapy (preRT) or postoperative radiation therapy (PORT).
Complete surgical resection is often difficult for sarcomas of the retroperitoneum because of their large size before detection and anatomical location.[1,2] As opposed to soft tissue sarcomas of the extremities, local recurrence is the most common cause of death in patients with retroperitoneal soft tissue sarcomas. Complete surgical resection (i.e., removal of the entire gross tumor) is the most important factor in preventing local recurrence and, in many instances, requires resection of adjacent viscera. For retroperitoneal sarcomas, retrospective comparison of surgery alone versus preRT review suggests that preRT is associated with improved local recurrence-free survival, but not disease-free survival.[3]
Standard treatment options:
- Surgical excision with preRT or PORT. Radiation decreases the risk of local recurrence but has not been shown to increase overall survival.[4-8]
- Surgical excision with negative tissue margins in all directions. This approach is generally restricted to low-grade tumors ( ≤5 cm in diameter) of the extremities or superficial trunk with microscopically negative surgical tumor margins.[9-13]
- If the tumor is unresectable, high-dose radiation therapy may be used, but poor local control is likely to result.[14]
- In some situations, radiation therapy and/or chemotherapy may be used before surgery in an attempt to convert a marginally resectable tumor to one that can be adequately resected with limb preservation; this treatment may be followed by PORT.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Heslin MJ, Lewis JJ, Nadler E, et al.: Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol 15 (8): 2832-9, 1997. [PUBMED Abstract]
- Jaques DP, Coit DG, Hajdu SI, et al.: Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 212 (1): 51-9, 1990. [PUBMED Abstract]
- Kelly KJ, Yoon SS, Kuk D, et al.: Comparison of Perioperative Radiation Therapy and Surgery Versus Surgery Alone in 204 Patients With Primary Retroperitoneal Sarcoma: A Retrospective 2-Institution Study. Ann Surg 262 (1): 156-62, 2015. [PUBMED Abstract]
- Yang JC, Chang AE, Baker AR, et al.: Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16 (1): 197-203, 1998. [PUBMED Abstract]
- Rosenberg SA, Tepper J, Glatstein E, et al.: The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196 (3): 305-15, 1982. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- O'Sullivan B, Davis A, Turcotte R, et al.: Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. [Abstract] J Clin Oncol 22 (Suppl 14): A-9007, 819s, 2004.
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Al-Refaie WB, Habermann EB, Jensen EH, et al.: Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg 97 (5): 707-13, 2010. [PUBMED Abstract]
- Pisters PW, Pollock RE, Lewis VO, et al.: Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 246 (4): 675-81; discussion 681-2, 2007. [PUBMED Abstract]
- Fabrizio PL, Stafford SL, Pritchard DJ: Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys 48 (1): 227-32, 2000. [PUBMED Abstract]
- Rydholm A, Gustafson P, Rööser B, et al.: Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 9 (10): 1757-65, 1991. [PUBMED Abstract]
- Rydholm A: Surgery without radiotherapy in soft tissue sarcoma. Acta Orthop Scand Suppl 273: 117-9, 1997. [PUBMED Abstract]
- Kepka L, DeLaney TF, Suit HD, et al.: Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63 (3): 852-9, 2005. [PUBMED Abstract]
Advanced Stage III (N1) Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
Regional lymph node involvement by soft tissue sarcomas of adulthood is very infrequent. However, sarcoma types that more commonly spread to lymph nodes include high-grade rhabdomyosarcoma, vascular sarcomas, and epithelioid sarcomas.[1]
Standard treatment options:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Watson DI, Coventry BJ, Langlois SL, et al.: Soft-tissue sarcoma of the extremity. Experience with limb-sparing surgery. Med J Aust 160 (7): 412-6, 1994. [PUBMED Abstract]
- Cormier JN, Huang X, Xing Y, et al.: Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol 22 (22): 4567-74, 2004. [PUBMED Abstract]
- O'Byrne K, Steward WP: The role of adjuvant chemotherapy in the treatment of adult soft tissue sarcomas. Crit Rev Oncol Hematol 27 (3): 221-7, 1998. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
Stage IV Adult Soft Tissue Sarcoma
Refer to the Treatment Option Overview section of this summary for a more detailed discussion of the roles of surgery, radiation therapy, and chemotherapy.
In the setting of lung metastasis, resection of metastatic tumors may be associated with long-term disease-free survival in patients selected for optimal underlying disease biology (i.e., patients with a limited number of metastases and slow tumor growth).[1-3] It is not clear to what degree the favorable outcomes are attributable to the efficacy of surgery or to careful selection of patients based upon factors that are associated with less-virulent disease.[1-3] The value of resection of hepatic metastases is unclear.
As noted in the Treatment Option Overview section above, doxorubicin is the standard systemic therapy in the management of metastatic sarcomas.[4,5] Other drugs that may have clinical activity as single agents are ifosfamide, epirubicin, gemcitabine, and paclitaxel.[6-9] Their clinical activity relative to single-agent doxorubicin is not clear, and they are not known to have superior activity. There is controversy about whether adding drugs to doxorubicin offers clinical benefit beyond what is achieved by doxorubicin as a single agent. To avoid severe toxicity in older patients, sequential use of single agents may be the preferred strategy for palliation.
A randomized study assessed whether dose intensification of doxorubicin with ifosfamide improved the survival of patients with advanced soft-tissue sarcoma compared with doxorubicin alone.[10] Two hundred twenty-eight patients were randomly assigned to receive doxorubicin, and 227 patients were randomly assigned to receive doxorubicin and ifosfamide. Median follow-up was 56 months (interquartile range [IQR], 31–77) in the doxorubicin-only group and 59 months (IQR, 36–72) in the combination group.
There was no significant difference in overall survival (OS) between groups (median OS, 12.8 months; 95.5% confidence interval [CI], 10.5–14.3 in the doxorubicin-alone group vs. 14.3 months; range, 12.5–16.5 months in the doxorubicin and ifosfamide group; hazard ratio [HR], 0.83; 95.5% CI 0.67–1.03; stratified log-rank test P = .076). Median progression-free survival was significantly higher for the doxorubicin and ifosfamide group (7.4 months; 95% CI, 6.6-8.3) than for the doxorubicin-alone group (4.6 months; range, 2.9–5.6 months; HR, 0.74; 95% CI, 0.60–0.90; stratified log-rank test P = .003). More patients in the doxorubicin and ifosfamide group than in the doxorubicin-alone group had an overall response (60 [26%] of 227 patients vs. 31 [14%] of 228; P < .0006). The most common grade 3 and 4 toxic effects, which were all more common with doxorubicin and ifosfamide than with doxorubicin alone, were leucopenia (97 [43%] of 224 patients vs. 40 [18%] of 223 patients), neutropenia (93 [42%] vs. 83 [37%]), febrile neutropenia (103 (46%) vs. 30 [13%]), anemia (78 [35%] vs. 10 [5%]), and thrombocytopenia (75 [33%]) vs. 1 [<1%]).[10][Level of evidence: 1iiA] Treatment intensification with doxorubicin and ifosfamide for palliation of advanced soft tissue sarcoma is not indicated.
Standard treatment options
- Chemotherapy.
- Single-agent chemotherapy, with subsequent single agents for disease regrowth.[4-6,8,9,11] Doxorubicin is generally the first-line agent. Ifosfamide also has substantial single-agent activity.
- Doxorubicin-based combination chemotherapy. A variety of regimens have been used, but none has been proven to increase OS compared with doxorubicin alone.[4,5] There is some evidence that the addition of ifosfamide increases response rates (but not survival). Toxicity is increased with the addition of drugs to doxorubicin. No quality-of-life studies have been reported in comparisons of single-agent therapy versus combination therapy.
- Resection of pulmonary lesions may be performed if the primary tumor is under control.[1-3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Bramwell VH, Anderson D, Charette ML, et al.: Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev (3): CD003293, 2003. [PUBMED Abstract]
- Verma S, Younus J, Stys-Norman D, et al.: Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 34 (4): 339-47, 2008. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
- Judson I, Verweij J, Gelderblom H, et al.: Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 15 (4): 415-23, 2014. [PUBMED Abstract]
- Grenader T, Goldberg A, Hadas-Halperin I, et al.: Long-term response to pegylated liposomal doxorubicin in patients with metastatic soft tissue sarcomas. Anticancer Drugs 20 (1): 15-20, 2009. [PUBMED Abstract]
Recurrent Adult Soft Tissue Sarcoma
Treatment of patients with recurrent soft tissue sarcoma depends on the type of initial presentation and treatment. Patients who develop a local recurrence often can be treated by local therapy: surgical excision plus radiation therapy after previous minimal therapy or amputation after previous aggressive treatment.[1-7] Resection of limited pulmonary metastases may be associated with favorable disease-free survival.[8-10][Level of evidence: 3iiiDiv] However, the contribution of selection factors, such as low tumor burden, slow tumor growth, and long disease-free interval, to these favorable outcomes is not known.
There is no standard chemotherapy for recurrent soft tissue sarcomas that have progressed after doxorubicin as a single agent or in combination with other agents that have clinical activity, such as ifosfamide, epirubicin, gemcitabine, and paclitaxel. Any of these agents not previously administered to the patient may be used sequentially at the time of recurrence or progression.[11-14][Level of Evidence: 3iiiDiv] None of these agents has been shown to increase overall survival in this setting, therefore, clinical trials are an appropriate option.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Singer S, Nielsen T, Antonescu CR: Molecular biology of soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1522-32.
- Singer S, Maki RG, O'Sullivan B: Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1533-77.
- Malawer MM, Helman LJ, O'Sullivan B: Sarcomas of bone. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2011, pp 1578-1609.
- Midis GP, Pollock RE, Chen NP, et al.: Locally recurrent soft tissue sarcoma of the extremities. Surgery 123 (6): 666-71, 1998. [PUBMED Abstract]
- Essner R, Selch M, Eilber FR: Reirradiation for extremity soft tissue sarcomas. Local control and complications. Cancer 67 (11): 2813-7, 1991. [PUBMED Abstract]
- Singer S, Antman K, Corson JM, et al.: Long-term salvageability for patients with locally recurrent soft-tissue sarcomas. Arch Surg 127 (5): 548-53; discussion 553-4, 1992. [PUBMED Abstract]
- Lewis JJ, Leung D, Heslin M, et al.: Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 15 (2): 646-52, 1997. [PUBMED Abstract]
- van Geel AN, Pastorino U, Jauch KW, et al.: Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 77 (4): 675-82, 1996. [PUBMED Abstract]
- Casson AG, Putnam JB, Natarajan G, et al.: Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 69 (3): 662-8, 1992. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Lorigan P, Verweij J, Papai Z, et al.: Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 25 (21): 3144-50, 2007. [PUBMED Abstract]
- Nielsen OS, Dombernowsky P, Mouridsen H, et al.: High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 78 (12): 1634-9, 1998. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Okuno S, Ryan LM, Edmonson JH, et al.: Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97 (8): 1969-73, 2003. [PUBMED Abstract]
Changes to This Summary (02/01/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Adult Soft Tissue Sarcoma
Updated statistics with estimated new cases and deaths for 2018 (cited American Cancer Society as reference 1).
Stage Information for Adult Soft Tissue Sarcoma
An editorial change was made to this section.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult soft tissue sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Adult Soft Tissue Sarcoma Treatment are:
- Russell S. Berman, MD (New York University School of Medicine)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Adult Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/adult-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389481]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: February 1, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/2127.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:00:45.0
Onodera’s Prognostic Nutritional Index in soft tissue sarcoma patients as a predictor of wound complications
Background The ability to predict a wound complication after radiation therapy and surgery for soft tissue sarcomas remains difficult. Preoperative nutritional status, as determined by Onodera’s Prognostic Nutritional Index (OPNI), has been a predictor of complications in patients undergoing gastrointestinal surgery. However, the role OPNI has in predicting wound complications for soft tissue sarcoma remains unknown.
Objective To evaluate the role OPNI has in predicting wound complication in patients treated with radiation and surgery for soft tissue sarcomas.
Methods OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count). The albumin level and total lymphocyte counts closest to the index operation were chosen. Major and minor wound complications were identified. A receiver operating curve was calculated to identify a cut-off point value for OPNI and for age based on the best combination of sensitivity and specificity.
Results 44 patients were included in the study. Patients with an OPNI of <45.4 had a 7.5-times increased risk of a wound complication (P = .005; 95% confidence interval [CI], 1.8-31.0). An OPNI of <45.4 had a sensitivity of 62% and specificity of 82% of predicting a wound complication. Being older than 73 years was associated with a 6.8-times increased risk of wound complications (P = .01; 95% CI, 1.6-28.7).
Limitations Small sample size for patients with a rare condition
Conclusion An OPNI of <45.4 and being older than 73 years are strong predictors of which patients will have a wound complication after radiation therapy for soft tissue sarcomas. Preoperative nutritional status could be an important modifiable factor to help decrease wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2). In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications. TSJ
Correspondence
References
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Background The ability to predict a wound complication after radiation therapy and surgery for soft tissue sarcomas remains difficult. Preoperative nutritional status, as determined by Onodera’s Prognostic Nutritional Index (OPNI), has been a predictor of complications in patients undergoing gastrointestinal surgery. However, the role OPNI has in predicting wound complications for soft tissue sarcoma remains unknown.
Objective To evaluate the role OPNI has in predicting wound complication in patients treated with radiation and surgery for soft tissue sarcomas.
Methods OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count). The albumin level and total lymphocyte counts closest to the index operation were chosen. Major and minor wound complications were identified. A receiver operating curve was calculated to identify a cut-off point value for OPNI and for age based on the best combination of sensitivity and specificity.
Results 44 patients were included in the study. Patients with an OPNI of <45.4 had a 7.5-times increased risk of a wound complication (P = .005; 95% confidence interval [CI], 1.8-31.0). An OPNI of <45.4 had a sensitivity of 62% and specificity of 82% of predicting a wound complication. Being older than 73 years was associated with a 6.8-times increased risk of wound complications (P = .01; 95% CI, 1.6-28.7).
Limitations Small sample size for patients with a rare condition
Conclusion An OPNI of <45.4 and being older than 73 years are strong predictors of which patients will have a wound complication after radiation therapy for soft tissue sarcomas. Preoperative nutritional status could be an important modifiable factor to help decrease wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2). In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications. TSJ
Correspondence
References
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Background The ability to predict a wound complication after radiation therapy and surgery for soft tissue sarcomas remains difficult. Preoperative nutritional status, as determined by Onodera’s Prognostic Nutritional Index (OPNI), has been a predictor of complications in patients undergoing gastrointestinal surgery. However, the role OPNI has in predicting wound complications for soft tissue sarcoma remains unknown.
Objective To evaluate the role OPNI has in predicting wound complication in patients treated with radiation and surgery for soft tissue sarcomas.
Methods OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count). The albumin level and total lymphocyte counts closest to the index operation were chosen. Major and minor wound complications were identified. A receiver operating curve was calculated to identify a cut-off point value for OPNI and for age based on the best combination of sensitivity and specificity.
Results 44 patients were included in the study. Patients with an OPNI of <45.4 had a 7.5-times increased risk of a wound complication (P = .005; 95% confidence interval [CI], 1.8-31.0). An OPNI of <45.4 had a sensitivity of 62% and specificity of 82% of predicting a wound complication. Being older than 73 years was associated with a 6.8-times increased risk of wound complications (P = .01; 95% CI, 1.6-28.7).
Limitations Small sample size for patients with a rare condition
Conclusion An OPNI of <45.4 and being older than 73 years are strong predictors of which patients will have a wound complication after radiation therapy for soft tissue sarcomas. Preoperative nutritional status could be an important modifiable factor to help decrease wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2). In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications. TSJ
Correspondence
References
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.