User login
Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate (FULL)
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
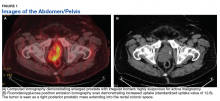
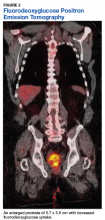
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Scientific Roundtable Focuses Efforts on Leiomyosarcoma Research
Research clinicians from the US and abroad participated in a scientific research roundtable this past September to establish the most important issues facing leiomyosarcoma (LMS) research and clinical trials. The workshop, expected to be an annual event, is a joint effort of the National Leiomyosarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN).
The roundtable’s mission is to bring together sarcoma experts for a meeting dedicated to LMS, where participants discuss the present state of LMS and the continued challenges of diagnosis and treatment. Its goal is to develop working plans to close the gaps in LMS patient care and improve LMS patient-care protocols. They also advise the NLMSF on worthy and important research projects that deserve the foundation’s future funding efforts.
Plans for roundtable meetings in 2020 and 2021 are already underway. Between the annual meetings, workgroups continue to take steps toward addressing the issues identified by the roundtable. For more information on the roundtable and NLMSF, visit: https://nlmsf.org.
Research clinicians from the US and abroad participated in a scientific research roundtable this past September to establish the most important issues facing leiomyosarcoma (LMS) research and clinical trials. The workshop, expected to be an annual event, is a joint effort of the National Leiomyosarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN).
The roundtable’s mission is to bring together sarcoma experts for a meeting dedicated to LMS, where participants discuss the present state of LMS and the continued challenges of diagnosis and treatment. Its goal is to develop working plans to close the gaps in LMS patient care and improve LMS patient-care protocols. They also advise the NLMSF on worthy and important research projects that deserve the foundation’s future funding efforts.
Plans for roundtable meetings in 2020 and 2021 are already underway. Between the annual meetings, workgroups continue to take steps toward addressing the issues identified by the roundtable. For more information on the roundtable and NLMSF, visit: https://nlmsf.org.
Research clinicians from the US and abroad participated in a scientific research roundtable this past September to establish the most important issues facing leiomyosarcoma (LMS) research and clinical trials. The workshop, expected to be an annual event, is a joint effort of the National Leiomyosarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN).
The roundtable’s mission is to bring together sarcoma experts for a meeting dedicated to LMS, where participants discuss the present state of LMS and the continued challenges of diagnosis and treatment. Its goal is to develop working plans to close the gaps in LMS patient care and improve LMS patient-care protocols. They also advise the NLMSF on worthy and important research projects that deserve the foundation’s future funding efforts.
Plans for roundtable meetings in 2020 and 2021 are already underway. Between the annual meetings, workgroups continue to take steps toward addressing the issues identified by the roundtable. For more information on the roundtable and NLMSF, visit: https://nlmsf.org.
Predicting treatment response in leiomyosarcoma, liposarcoma
Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma, based on an analysis of whole-exome sequencing of tumor samples from patients in a completed phase 3 randomized trial comparing trabectedin and dacarbazine.
In that trial, trabectedin benefit was mostly seen in patients with leiomyosarcoma, as well as in patients with myxoid/round cell sarcomas, and less so in those with dedifferentiated and pleomorphic liposarcomas.
Gurpreet Kapoor, PhD, of LabConnect, Seattle, and colleagues examined aberrations in oncogenic pathways (DNA damage response, PI3K, MDM2-p53) and in immune modulation and then correlated the genomic aberrations with prospective data on clinical outcomes in the trial.
For the study, presented at the annual meeting of the American Society of Clinical Oncology in Chicago, archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas.
Peripheral blood samples from a subset of 346 patients were also analyzed as matched normal to filter noise from nonpathogenic variants in the whole-exome sequencing.
Consistent with sarcoma data from The Cancer Genome Atlas, frequent homozygous gene deletions with relatively low mutational load were noted in these leiomyosarcoma and liposarcoma samples. TP53 and RB1 alterations were more frequent in leiomyosarcomas than in liposarcomas and were not associated with clinical outcomes. Analyses of 103 DNA damage-response genes found somatic alterations exceeded 20% across subtypes and correlated with improved progression-free survival in only uterine leiomyosarcomas (hazard ratio, 0.63; P = .03).
Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
A trend towards better overall survival was noted in dedifferentiated liposarcoma patients with MDM2 amplification as compared with normal MDM2 copy number.
Certain subtype-specific genomic aberrations in immune modulation pathways were associated with worse clinical outcomes in patients with uterine leiomyosarcoma or dedifferentiated liposarcoma. Alterations in immune suppressors were associated with improved clinical outcomes in nonuterine leiomyosarcomas and alterations in lipid metabolism were associated with improved clinical outcomes in dedifferentiated liposarcomas.
The invited discussant for the study, Mark Andrew Dickson, MD, of Memorial Sloan Kettering Cancer Center, New York, noted that “the real take-home here is that the TMBs (tumor mutation burdens) are relatively low across all of the L-type sarcomas.
“The pattern and prevalence of genomic aberrations that we’re seeing in this cohort of patients prospectively analyzed on a clinical trial are consistent with prior reports. ... including CDK4 and MDM2 in dedifferentiated liposarcoma, PI3-kinase in some myxoid/round cells, p53 in leiomyosarcoma and liposarcoma, and so on.”
Generally, tumor mutation burden is low in L-type sarcomas, and there are some intriguing associations with benefit to therapies, such as PI3-kinase pathway and potential resistance to trabectedin and high tumor mutation burden and potential sensitivity to trabectedin, that need to be explored and validated in another larger cohort, he said.
“I also am increasingly coming to terms with the fact that the tumors like leiomyosarcoma, which have low tumor mutation burden, and which so far have proven fairly immune to immunotherapy, based on all of the negative PD-1 data that we’ve seen, and that also have recurrent, relatively unactionable mutations, like p53 and Rb, remain very difficult to treat,” Dr. Dickson concluded.
SOURCE: Kapoor G et al. ASCO 2018, Abstract 11513.
Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma, based on an analysis of whole-exome sequencing of tumor samples from patients in a completed phase 3 randomized trial comparing trabectedin and dacarbazine.
In that trial, trabectedin benefit was mostly seen in patients with leiomyosarcoma, as well as in patients with myxoid/round cell sarcomas, and less so in those with dedifferentiated and pleomorphic liposarcomas.
Gurpreet Kapoor, PhD, of LabConnect, Seattle, and colleagues examined aberrations in oncogenic pathways (DNA damage response, PI3K, MDM2-p53) and in immune modulation and then correlated the genomic aberrations with prospective data on clinical outcomes in the trial.
For the study, presented at the annual meeting of the American Society of Clinical Oncology in Chicago, archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas.
Peripheral blood samples from a subset of 346 patients were also analyzed as matched normal to filter noise from nonpathogenic variants in the whole-exome sequencing.
Consistent with sarcoma data from The Cancer Genome Atlas, frequent homozygous gene deletions with relatively low mutational load were noted in these leiomyosarcoma and liposarcoma samples. TP53 and RB1 alterations were more frequent in leiomyosarcomas than in liposarcomas and were not associated with clinical outcomes. Analyses of 103 DNA damage-response genes found somatic alterations exceeded 20% across subtypes and correlated with improved progression-free survival in only uterine leiomyosarcomas (hazard ratio, 0.63; P = .03).
Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
A trend towards better overall survival was noted in dedifferentiated liposarcoma patients with MDM2 amplification as compared with normal MDM2 copy number.
Certain subtype-specific genomic aberrations in immune modulation pathways were associated with worse clinical outcomes in patients with uterine leiomyosarcoma or dedifferentiated liposarcoma. Alterations in immune suppressors were associated with improved clinical outcomes in nonuterine leiomyosarcomas and alterations in lipid metabolism were associated with improved clinical outcomes in dedifferentiated liposarcomas.
The invited discussant for the study, Mark Andrew Dickson, MD, of Memorial Sloan Kettering Cancer Center, New York, noted that “the real take-home here is that the TMBs (tumor mutation burdens) are relatively low across all of the L-type sarcomas.
“The pattern and prevalence of genomic aberrations that we’re seeing in this cohort of patients prospectively analyzed on a clinical trial are consistent with prior reports. ... including CDK4 and MDM2 in dedifferentiated liposarcoma, PI3-kinase in some myxoid/round cells, p53 in leiomyosarcoma and liposarcoma, and so on.”
Generally, tumor mutation burden is low in L-type sarcomas, and there are some intriguing associations with benefit to therapies, such as PI3-kinase pathway and potential resistance to trabectedin and high tumor mutation burden and potential sensitivity to trabectedin, that need to be explored and validated in another larger cohort, he said.
“I also am increasingly coming to terms with the fact that the tumors like leiomyosarcoma, which have low tumor mutation burden, and which so far have proven fairly immune to immunotherapy, based on all of the negative PD-1 data that we’ve seen, and that also have recurrent, relatively unactionable mutations, like p53 and Rb, remain very difficult to treat,” Dr. Dickson concluded.
SOURCE: Kapoor G et al. ASCO 2018, Abstract 11513.
Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma, based on an analysis of whole-exome sequencing of tumor samples from patients in a completed phase 3 randomized trial comparing trabectedin and dacarbazine.
In that trial, trabectedin benefit was mostly seen in patients with leiomyosarcoma, as well as in patients with myxoid/round cell sarcomas, and less so in those with dedifferentiated and pleomorphic liposarcomas.
Gurpreet Kapoor, PhD, of LabConnect, Seattle, and colleagues examined aberrations in oncogenic pathways (DNA damage response, PI3K, MDM2-p53) and in immune modulation and then correlated the genomic aberrations with prospective data on clinical outcomes in the trial.
For the study, presented at the annual meeting of the American Society of Clinical Oncology in Chicago, archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas.
Peripheral blood samples from a subset of 346 patients were also analyzed as matched normal to filter noise from nonpathogenic variants in the whole-exome sequencing.
Consistent with sarcoma data from The Cancer Genome Atlas, frequent homozygous gene deletions with relatively low mutational load were noted in these leiomyosarcoma and liposarcoma samples. TP53 and RB1 alterations were more frequent in leiomyosarcomas than in liposarcomas and were not associated with clinical outcomes. Analyses of 103 DNA damage-response genes found somatic alterations exceeded 20% across subtypes and correlated with improved progression-free survival in only uterine leiomyosarcomas (hazard ratio, 0.63; P = .03).
Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
A trend towards better overall survival was noted in dedifferentiated liposarcoma patients with MDM2 amplification as compared with normal MDM2 copy number.
Certain subtype-specific genomic aberrations in immune modulation pathways were associated with worse clinical outcomes in patients with uterine leiomyosarcoma or dedifferentiated liposarcoma. Alterations in immune suppressors were associated with improved clinical outcomes in nonuterine leiomyosarcomas and alterations in lipid metabolism were associated with improved clinical outcomes in dedifferentiated liposarcomas.
The invited discussant for the study, Mark Andrew Dickson, MD, of Memorial Sloan Kettering Cancer Center, New York, noted that “the real take-home here is that the TMBs (tumor mutation burdens) are relatively low across all of the L-type sarcomas.
“The pattern and prevalence of genomic aberrations that we’re seeing in this cohort of patients prospectively analyzed on a clinical trial are consistent with prior reports. ... including CDK4 and MDM2 in dedifferentiated liposarcoma, PI3-kinase in some myxoid/round cells, p53 in leiomyosarcoma and liposarcoma, and so on.”
Generally, tumor mutation burden is low in L-type sarcomas, and there are some intriguing associations with benefit to therapies, such as PI3-kinase pathway and potential resistance to trabectedin and high tumor mutation burden and potential sensitivity to trabectedin, that need to be explored and validated in another larger cohort, he said.
“I also am increasingly coming to terms with the fact that the tumors like leiomyosarcoma, which have low tumor mutation burden, and which so far have proven fairly immune to immunotherapy, based on all of the negative PD-1 data that we’ve seen, and that also have recurrent, relatively unactionable mutations, like p53 and Rb, remain very difficult to treat,” Dr. Dickson concluded.
SOURCE: Kapoor G et al. ASCO 2018, Abstract 11513.
REPORTING FROM ASCO 2018
Key clinical point: Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma.
Major finding: Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
Study details: Archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas in the completed phase 3 randomized trial comparing trabectedin and dacarbazine.
Disclosures: Dr. Kapoor is employed by LabConnect, Seattle. Research funding was supplied by Janssen Research & Development.
Source: Kapoor G et al. ASCO 2018, Abstract 11513.
Trabectedin bests supportive care in advanced soft-tissue sarcomas
CHICAGO – Trabectedin (Yondelis) was superior to best supportive care at prolonging progression-free survival in patients with heavily pretreated advanced leiomyosarcomas and liposarcomas, investigators in the randomized phase 3 T-SAR trial reported.
Among 103 patients with soft-tissue sarcomas that had progressed after two to four lines of standard chemotherapy, median progression-free survival (PFS) for patients randomized to trabectedin was 3.12 months, compared with 1.51 for patients randomized to best supportive care.
This difference translated into a hazard ratio (HR) favoring trabectedin of 0.39 (P less than .0001), Axel Le Cesne, MD, of Gustave Roussy Cancer Institute in Villejuif, France, reported on behalf of colleagues in the French Sarcoma Group.
All of the benefit was apparently among patients with what he termed “L-sarcomas” – leiomyosarcoma and liposarcoma – compared with other sarcoma histologies.
“The tumor control rate after six courses of trabectedin is similar to previous studies. As already reported, trabectedin is well-tolerated,” he said at the annual meeting of the American Society of Clinical Oncology.
Trabectedin was shown to be superior to best supportive care at delaying disease progression among patients with advanced translocation-related sarcomas in a randomized phase 2 trial in Japan, but had not been studied in this setting against other sarcoma histologies, Dr. Le Cesne said.
The investigators enrolled 103 patients and randomly assigned them to receive either best supportive care or trabectedin in a 1.5 mg/m2 infusion over 24 hours every 3 weeks. Patients in the best supportive care arm could be crossed over to the trabectedin arm at the time of progression.
Sarcoma histologies included liposarcoma, leiomyosarcoma, undifferentiated sarcomas, myxofibrosarcoma, synovial sarcoma, and others. The L-sarcomas accounted for 60.2% of the patient population.
Fifty-two patients were randomized to trabectedin and 51 to best supportive care, but 2 patients assigned to best supportive care dropped out soon after randomization, leaving 52 and 49 patients, respectively, for the as-treated analysis. All 103 patients were assessable for efficacy.
After a median follow-up of 26 months, median PFS for all patients, as noted before, was 3.12 months in the trabectedin arm and 1.51 months in the best supportive care arm.
The overall response rate in the trabectedin arm was 13.7%, composed of seven partial responses. There were no responses in the best supportive care arm. In all, 66.7% of patients in the trabectedin arm and 61.2% of patients in the best supportive care arm had stable disease, and 19.6% and 38.8%, respectively, had disease progression.
An analysis of PFS by sarcoma histology showed that all of the benefit appeared to be in patients with L-sarcomas, with a median PFS for trabectedin-treated patients of 5.13 months compared with 1.41 months for controls (HR 0.29, P less than .0001).
In contrast, there was no significant difference between the groups among patients with non–L sarcomas, with respective median PFS of 1.81 and 1.51 months (HR 0.60, P = .16). There were no treatment responses among patients in either treatment arm in this subgroup.
Not surprisingly, there were more grade 3 or 4 adverse events among patients in the trabectedin arm. Neutropenia was seen in 23 patients given trabectedin and 1 given best supportive care; leukopenia in 18 patients vs. 0, thrombocytopenia in 13 vs. 0, and elevated liver transaminases in 17 vs. 1, respectively.
In all, 45 of the 49 patients who were treated in the best supportive care arm were crossed over to trabectedin.
Median overall survival was 13.6 months in the trabectedin arm and 10.8 months in the best supportive care arm. This difference was not statistically significant.
Dr Le Cesne noted that the tumor control rate of 30% with trabectedin was similar to that seen in an earlier French trial (Lancet Oncol. 2015 Mar 1;16[3]:312-19).
Pharmamar supplied trabectedin for the study. Dr. Le Cesne disclosed honoraria from the company and from Amgen, Bayer, Lilly, Novartis, and Pfizer.
SOURCE: Le Cesne A et al. ASCO 2018. Abstract 11508.
CHICAGO – Trabectedin (Yondelis) was superior to best supportive care at prolonging progression-free survival in patients with heavily pretreated advanced leiomyosarcomas and liposarcomas, investigators in the randomized phase 3 T-SAR trial reported.
Among 103 patients with soft-tissue sarcomas that had progressed after two to four lines of standard chemotherapy, median progression-free survival (PFS) for patients randomized to trabectedin was 3.12 months, compared with 1.51 for patients randomized to best supportive care.
This difference translated into a hazard ratio (HR) favoring trabectedin of 0.39 (P less than .0001), Axel Le Cesne, MD, of Gustave Roussy Cancer Institute in Villejuif, France, reported on behalf of colleagues in the French Sarcoma Group.
All of the benefit was apparently among patients with what he termed “L-sarcomas” – leiomyosarcoma and liposarcoma – compared with other sarcoma histologies.
“The tumor control rate after six courses of trabectedin is similar to previous studies. As already reported, trabectedin is well-tolerated,” he said at the annual meeting of the American Society of Clinical Oncology.
Trabectedin was shown to be superior to best supportive care at delaying disease progression among patients with advanced translocation-related sarcomas in a randomized phase 2 trial in Japan, but had not been studied in this setting against other sarcoma histologies, Dr. Le Cesne said.
The investigators enrolled 103 patients and randomly assigned them to receive either best supportive care or trabectedin in a 1.5 mg/m2 infusion over 24 hours every 3 weeks. Patients in the best supportive care arm could be crossed over to the trabectedin arm at the time of progression.
Sarcoma histologies included liposarcoma, leiomyosarcoma, undifferentiated sarcomas, myxofibrosarcoma, synovial sarcoma, and others. The L-sarcomas accounted for 60.2% of the patient population.
Fifty-two patients were randomized to trabectedin and 51 to best supportive care, but 2 patients assigned to best supportive care dropped out soon after randomization, leaving 52 and 49 patients, respectively, for the as-treated analysis. All 103 patients were assessable for efficacy.
After a median follow-up of 26 months, median PFS for all patients, as noted before, was 3.12 months in the trabectedin arm and 1.51 months in the best supportive care arm.
The overall response rate in the trabectedin arm was 13.7%, composed of seven partial responses. There were no responses in the best supportive care arm. In all, 66.7% of patients in the trabectedin arm and 61.2% of patients in the best supportive care arm had stable disease, and 19.6% and 38.8%, respectively, had disease progression.
An analysis of PFS by sarcoma histology showed that all of the benefit appeared to be in patients with L-sarcomas, with a median PFS for trabectedin-treated patients of 5.13 months compared with 1.41 months for controls (HR 0.29, P less than .0001).
In contrast, there was no significant difference between the groups among patients with non–L sarcomas, with respective median PFS of 1.81 and 1.51 months (HR 0.60, P = .16). There were no treatment responses among patients in either treatment arm in this subgroup.
Not surprisingly, there were more grade 3 or 4 adverse events among patients in the trabectedin arm. Neutropenia was seen in 23 patients given trabectedin and 1 given best supportive care; leukopenia in 18 patients vs. 0, thrombocytopenia in 13 vs. 0, and elevated liver transaminases in 17 vs. 1, respectively.
In all, 45 of the 49 patients who were treated in the best supportive care arm were crossed over to trabectedin.
Median overall survival was 13.6 months in the trabectedin arm and 10.8 months in the best supportive care arm. This difference was not statistically significant.
Dr Le Cesne noted that the tumor control rate of 30% with trabectedin was similar to that seen in an earlier French trial (Lancet Oncol. 2015 Mar 1;16[3]:312-19).
Pharmamar supplied trabectedin for the study. Dr. Le Cesne disclosed honoraria from the company and from Amgen, Bayer, Lilly, Novartis, and Pfizer.
SOURCE: Le Cesne A et al. ASCO 2018. Abstract 11508.
CHICAGO – Trabectedin (Yondelis) was superior to best supportive care at prolonging progression-free survival in patients with heavily pretreated advanced leiomyosarcomas and liposarcomas, investigators in the randomized phase 3 T-SAR trial reported.
Among 103 patients with soft-tissue sarcomas that had progressed after two to four lines of standard chemotherapy, median progression-free survival (PFS) for patients randomized to trabectedin was 3.12 months, compared with 1.51 for patients randomized to best supportive care.
This difference translated into a hazard ratio (HR) favoring trabectedin of 0.39 (P less than .0001), Axel Le Cesne, MD, of Gustave Roussy Cancer Institute in Villejuif, France, reported on behalf of colleagues in the French Sarcoma Group.
All of the benefit was apparently among patients with what he termed “L-sarcomas” – leiomyosarcoma and liposarcoma – compared with other sarcoma histologies.
“The tumor control rate after six courses of trabectedin is similar to previous studies. As already reported, trabectedin is well-tolerated,” he said at the annual meeting of the American Society of Clinical Oncology.
Trabectedin was shown to be superior to best supportive care at delaying disease progression among patients with advanced translocation-related sarcomas in a randomized phase 2 trial in Japan, but had not been studied in this setting against other sarcoma histologies, Dr. Le Cesne said.
The investigators enrolled 103 patients and randomly assigned them to receive either best supportive care or trabectedin in a 1.5 mg/m2 infusion over 24 hours every 3 weeks. Patients in the best supportive care arm could be crossed over to the trabectedin arm at the time of progression.
Sarcoma histologies included liposarcoma, leiomyosarcoma, undifferentiated sarcomas, myxofibrosarcoma, synovial sarcoma, and others. The L-sarcomas accounted for 60.2% of the patient population.
Fifty-two patients were randomized to trabectedin and 51 to best supportive care, but 2 patients assigned to best supportive care dropped out soon after randomization, leaving 52 and 49 patients, respectively, for the as-treated analysis. All 103 patients were assessable for efficacy.
After a median follow-up of 26 months, median PFS for all patients, as noted before, was 3.12 months in the trabectedin arm and 1.51 months in the best supportive care arm.
The overall response rate in the trabectedin arm was 13.7%, composed of seven partial responses. There were no responses in the best supportive care arm. In all, 66.7% of patients in the trabectedin arm and 61.2% of patients in the best supportive care arm had stable disease, and 19.6% and 38.8%, respectively, had disease progression.
An analysis of PFS by sarcoma histology showed that all of the benefit appeared to be in patients with L-sarcomas, with a median PFS for trabectedin-treated patients of 5.13 months compared with 1.41 months for controls (HR 0.29, P less than .0001).
In contrast, there was no significant difference between the groups among patients with non–L sarcomas, with respective median PFS of 1.81 and 1.51 months (HR 0.60, P = .16). There were no treatment responses among patients in either treatment arm in this subgroup.
Not surprisingly, there were more grade 3 or 4 adverse events among patients in the trabectedin arm. Neutropenia was seen in 23 patients given trabectedin and 1 given best supportive care; leukopenia in 18 patients vs. 0, thrombocytopenia in 13 vs. 0, and elevated liver transaminases in 17 vs. 1, respectively.
In all, 45 of the 49 patients who were treated in the best supportive care arm were crossed over to trabectedin.
Median overall survival was 13.6 months in the trabectedin arm and 10.8 months in the best supportive care arm. This difference was not statistically significant.
Dr Le Cesne noted that the tumor control rate of 30% with trabectedin was similar to that seen in an earlier French trial (Lancet Oncol. 2015 Mar 1;16[3]:312-19).
Pharmamar supplied trabectedin for the study. Dr. Le Cesne disclosed honoraria from the company and from Amgen, Bayer, Lilly, Novartis, and Pfizer.
SOURCE: Le Cesne A et al. ASCO 2018. Abstract 11508.
REPORTING FROM ASCO 2018
Key clinical point: Trabectedin was superior to best supportive care in delaying disease progression among patients with advanced soft tissue sarcomas.
Major finding: Median progression-free survival for patients with leiomyosarcoma or liposarcoma treated with trabectedin was 5.13 months vs. 1.41 months for patients treated with best supportive care.
Study details: Randomized open-label trial of 103 patients with histologically proven advanced soft-tissue sarcoma who progressed after at least 1 anthracycline-containing regimen.
Disclosures: Pharmamar supplied trabectedin for the study. Dr. Le Cesne disclosed receiving honoraria from the company and from Amgen, Bayer, Lilly, Novartis, and Pfizer.
Source: Le Cesne A et al. ASCO 2018. Abstract 11508.
Targetable genetic alterations found in 41% of soft tissue sarcomas
, reported Carlo Lucchesi, PhD, of Institut Bergonié in Bordeaux, France, and his associates.
In a cross-sectional study of next-generation sequencing results from 584 patients with soft tissue sarcomas in the American Association for Cancer Research’s GENIE Database, 57% of patients had complex genomics sarcomas (sarcomas with multiple, complex karyotypic abnormalities with no specific pattern), 25% had translocation-related sarcomas (sarcomas with specific reciprocal translocations resulting in oncogenic fusion transcripts), and 18% had simple amplicon sarcomas or sarcomas with inactivating mutations.
A total of 2,697 alterations (1,154 substitutions, 765 gene amplifications, 364 short indels and splicing variants, 346 gene homozygous deletions, and 68 gene rearrangements) were identified in 451 genes. A median of four alterations per case were detected, the researchers wrote in a study published online May 3 in JAMA Oncology.
The researchers identified the 20 genes that were most often altered. The top 5 were TP53, MDM2, CDK4, RB1, and ATRX.
Among these 584 samples, 85% had at least one alteration. The proportions of affected patients in each sarcoma group varied significantly among groups, with the other sarcomas group being the most altered (90.8%) and translocation-related sarcomas being the least mutated (77.8%).
At least one relevant gene alteration that could potentially be used to guide targeted therapy was found in 239 cases (41%) with a statistically significant higher number in other sarcomas (89 cases) and complex genomics sarcomas (131 cases) than in translocation-related sarcomas (19 cases).
This finding of an “unexpectedly high frequency” of clinically relevant genetic alterations supports the premise of the soon-to-be-launched MULTISARC trial, which posits that next-generation sequencing results can be used to guide and improve the treatment outcomes of patients with advanced soft tissue sarcomas. For MULTISARC, such patients will be randomized either to an experimental group that will undergo exome and RNA sequencing – and their results will be discussed in a molecular tumor board to tailor the treatment – or to a control group that will not undergo molecular profiling and will receive conventional therapy. The program will include 16 targeted therapies.
The researchers reported having no relevant financial conflicts of interest.
SOURCE: Lucchesi C et al. JAMA Oncol. doi: 10.1001/jamaoncol.2018.0723.
, reported Carlo Lucchesi, PhD, of Institut Bergonié in Bordeaux, France, and his associates.
In a cross-sectional study of next-generation sequencing results from 584 patients with soft tissue sarcomas in the American Association for Cancer Research’s GENIE Database, 57% of patients had complex genomics sarcomas (sarcomas with multiple, complex karyotypic abnormalities with no specific pattern), 25% had translocation-related sarcomas (sarcomas with specific reciprocal translocations resulting in oncogenic fusion transcripts), and 18% had simple amplicon sarcomas or sarcomas with inactivating mutations.
A total of 2,697 alterations (1,154 substitutions, 765 gene amplifications, 364 short indels and splicing variants, 346 gene homozygous deletions, and 68 gene rearrangements) were identified in 451 genes. A median of four alterations per case were detected, the researchers wrote in a study published online May 3 in JAMA Oncology.
The researchers identified the 20 genes that were most often altered. The top 5 were TP53, MDM2, CDK4, RB1, and ATRX.
Among these 584 samples, 85% had at least one alteration. The proportions of affected patients in each sarcoma group varied significantly among groups, with the other sarcomas group being the most altered (90.8%) and translocation-related sarcomas being the least mutated (77.8%).
At least one relevant gene alteration that could potentially be used to guide targeted therapy was found in 239 cases (41%) with a statistically significant higher number in other sarcomas (89 cases) and complex genomics sarcomas (131 cases) than in translocation-related sarcomas (19 cases).
This finding of an “unexpectedly high frequency” of clinically relevant genetic alterations supports the premise of the soon-to-be-launched MULTISARC trial, which posits that next-generation sequencing results can be used to guide and improve the treatment outcomes of patients with advanced soft tissue sarcomas. For MULTISARC, such patients will be randomized either to an experimental group that will undergo exome and RNA sequencing – and their results will be discussed in a molecular tumor board to tailor the treatment – or to a control group that will not undergo molecular profiling and will receive conventional therapy. The program will include 16 targeted therapies.
The researchers reported having no relevant financial conflicts of interest.
SOURCE: Lucchesi C et al. JAMA Oncol. doi: 10.1001/jamaoncol.2018.0723.
, reported Carlo Lucchesi, PhD, of Institut Bergonié in Bordeaux, France, and his associates.
In a cross-sectional study of next-generation sequencing results from 584 patients with soft tissue sarcomas in the American Association for Cancer Research’s GENIE Database, 57% of patients had complex genomics sarcomas (sarcomas with multiple, complex karyotypic abnormalities with no specific pattern), 25% had translocation-related sarcomas (sarcomas with specific reciprocal translocations resulting in oncogenic fusion transcripts), and 18% had simple amplicon sarcomas or sarcomas with inactivating mutations.
A total of 2,697 alterations (1,154 substitutions, 765 gene amplifications, 364 short indels and splicing variants, 346 gene homozygous deletions, and 68 gene rearrangements) were identified in 451 genes. A median of four alterations per case were detected, the researchers wrote in a study published online May 3 in JAMA Oncology.
The researchers identified the 20 genes that were most often altered. The top 5 were TP53, MDM2, CDK4, RB1, and ATRX.
Among these 584 samples, 85% had at least one alteration. The proportions of affected patients in each sarcoma group varied significantly among groups, with the other sarcomas group being the most altered (90.8%) and translocation-related sarcomas being the least mutated (77.8%).
At least one relevant gene alteration that could potentially be used to guide targeted therapy was found in 239 cases (41%) with a statistically significant higher number in other sarcomas (89 cases) and complex genomics sarcomas (131 cases) than in translocation-related sarcomas (19 cases).
This finding of an “unexpectedly high frequency” of clinically relevant genetic alterations supports the premise of the soon-to-be-launched MULTISARC trial, which posits that next-generation sequencing results can be used to guide and improve the treatment outcomes of patients with advanced soft tissue sarcomas. For MULTISARC, such patients will be randomized either to an experimental group that will undergo exome and RNA sequencing – and their results will be discussed in a molecular tumor board to tailor the treatment – or to a control group that will not undergo molecular profiling and will receive conventional therapy. The program will include 16 targeted therapies.
The researchers reported having no relevant financial conflicts of interest.
SOURCE: Lucchesi C et al. JAMA Oncol. doi: 10.1001/jamaoncol.2018.0723.
FROM JAMA ONCOLOGY
Key clinical point: Next-generation sequencing results might prove useful for guiding targeted therapy that could improve the treatment outcomes of patients with advanced soft tissue sarcomas.
Major finding: At least one targetable genetic alteration was found in 41% of 584 soft tissue sarcomas, and the probability of an alteration was higher in sarcomas with complex genomics than in translocation-related sarcomas.
Study details: A cross-sectional study of next-generation sequencing results from 584 patients with soft tissue sarcomas in the AACR GENIE Database.
Disclosures: The researchers reported having no relevant financial conflicts of interest.
Source: Lucchesi C et al. JAMA Oncol. doi: 10.1001/jamaoncol.2018.0723.
Uterine sarcoma: Professional resources from the National Cancer Institute
Uterine Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Uterine Sarcoma
Uterine sarcomas comprise less than 1% of gynecologic malignancies and 2% to 5% of all uterine malignancies.[1] The following tumors arise primarily from three distinct tissues:
- Carcinosarcomas arising in the endometrium, in other organs of mullerian origin, and accounting for 40% to 50% of all uterine sarcomas.
- Leiomyosarcomas arising from myometrial muscle, with a peak incidence occurring at age 50, and accounting for 30% of all uterine sarcomas.
- Sarcomas arising in the endometrial stroma, with a peak incidence occurring before menopause for the low-grade tumors and after menopause for the high-grade tumors, and accounting for 15% of all uterine sarcomas.
The three distinct entities are often grouped under uterine sarcomas; however, each type of tumor is currently being studied in separate clinical trials.
Carcinosarcomas (the preferred designation by the World Health Organization [WHO]) are also referred to as mixed mesodermal sarcomas or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial-cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements, such as malignant mesenchymal tissue considered possibly native to the uterus, or heterologous elements, such as striated muscle, cartilage, or bone, which are foreign to the uterus. Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Other rare forms of uterine sarcomas also fall under the WHO classification of mesenchymal and mixed tumors of the uterus. These include:[2,3]
- Mixed endometrial stromal and smooth muscle tumors.
- Adenosarcomas, in which the epithelial elements appear benign within a malignant mesenchymal background.
- Embryonal botryoides or rhabdomyosarcomas, which are found almost exclusively in infants.
- PEComa—a perivascular epithelial-cell tumor that may behave in a malignant fashion, which is the latest to be added.
(Refer to the PDQ summary on Childhood Rhabdomyosarcoma for more information.)
Risk Factors
The only documented etiologic factor in 10% to 25% of these malignancies is prior pelvic radiation therapy, which is often administered for benign uterine bleeding that began 5 to 25 years earlier. An increased incidence of uterine sarcoma has been associated with tamoxifen in the treatment of breast cancer. Subsequently, increases have also been noted when tamoxifen was given to prevent breast cancer in women at increased risk—a possible result of the estrogenic effect of tamoxifen on the uterus. Because of this increase, patients on tamoxifen should have follow-up pelvic examinations and should undergo endometrial biopsy if there is any abnormal uterine bleeding.[4-6]
Prognosis
The prognosis for women with uterine sarcoma is primarily dependent on the extent of disease at the time of diagnosis.[7] For women with carcinosarcomas, significant predictors of metastatic disease at initial surgery include:[7]
- Isthmic or cervical location.
- Lymphatic vascular space invasion.
- Serous and clear cell histology.
- Grade 2 or 3 carcinoma.
The above factors in addition to the following ones correlate with a progression-free interval:[7]
- Adnexal spread.
- Lymph node metastases.
- Tumor size.
- Peritoneal cytologic findings.
- Depth of myometrial invasion.
Factors that bear no relationship to the presence or absence of metastases at surgical exploration are:
- The presence or absence of stromal heterologous elements.
- The types of such elements.
- The grade of the stromal components.
- The mitotic activity of the stromal components.
In one study, women with a well-differentiated sarcomatous component or carcinosarcomas had significantly longer progression-free intervals than those with moderately to poorly differentiated sarcomas for the homologous and heterologous types. The recurrence rate was 44% for homologous tumors and 63% for heterologous tumors. The type of heterologous sarcoma had no effect on the progression-free interval.
For women with leiomyosarcomas, some investigators consider tumor size to be the most important prognostic factor; women with tumors greater than 5.0 cm in maximum diameter have a poor prognosis.[8] However, in a Gynecologic Oncology Group study, the mitotic index was the only factor significantly related to progression-free interval.[7] Leiomyosarcomas matched for other known prognostic factors may be more aggressive than their carcinosarcoma counterparts.[9] The 5-year survival rate for women with stage I disease, which is confined to the corpus, is approximately 50% versus 0% to 20% for the remaining stages.
Surgery alone can be curative if the malignancy is contained within the uterus. The value of pelvic radiation therapy is not established. Current studies consist primarily of phase II chemotherapy trials for patients with advanced disease. Adjuvant chemotherapy following complete resection for patients with stage I or II disease was not established to be effective in a randomized trial.[10] Yet, other nonrandomized trials have reported improved survival following adjuvant chemotherapy with or without radiation therapy.[11-13]
Related Summaries
Other PDQ summaries containing information related to uterine sarcoma include the following:
References
- Forney JP, Buschbaum HJ: Classifying, staging, and treating uterine sarcomas. Contemp Ob Gyn 18(3):47, 50, 55-56, 61-62, 64, 69, 1981.
- Gershenson D, McGuire W, Gore Martin, et al.: Gynecologic Cancer: Controversies in Management. 3rd ed. New York, NY: Churchill Livingstone, 2004.
- Tavassoéli F, Devilee P, et al.: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France: International Agency for Research on Cancer, 2004.
- Bergman L, Beelen ML, Gallee MP, et al.: Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 356 (9233): 881-7, 2000. [PUBMED Abstract]
- Cohen I: Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 94 (2): 256-66, 2004. [PUBMED Abstract]
- Wickerham DL, Fisher B, Wolmark N, et al.: Association of tamoxifen and uterine sarcoma. J Clin Oncol 20 (11): 2758-60, 2002. [PUBMED Abstract]
- Major FJ, Blessing JA, Silverberg SG, et al.: Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 71 (4 Suppl): 1702-9, 1993. [PUBMED Abstract]
- Evans HL, Chawla SP, Simpson C, et al.: Smooth muscle neoplasms of the uterus other than ordinary leiomyoma. A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer 62 (10): 2239-47, 1988. [PUBMED Abstract]
- Oláh KS, Dunn JA, Gee H: Leiomyosarcomas have a poorer prognosis than mixed mesodermal tumours when adjusting for known prognostic factors: the result of a retrospective study of 423 cases of uterine sarcoma. Br J Obstet Gynaecol 99 (7): 590-4, 1992. [PUBMED Abstract]
- Omura GA, Blessing JA, Major F, et al.: A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 3 (9): 1240-5, 1985. [PUBMED Abstract]
- Piver MS, Lele SB, Marchetti DL, et al.: Effect of adjuvant chemotherapy on time to recurrence and survival of stage I uterine sarcomas. J Surg Oncol 38 (4): 233-9, 1988. [PUBMED Abstract]
- van Nagell JR Jr, Hanson MB, Donaldson ES, et al.: Adjuvant vincristine, dactinomycin, and cyclophosphamide therapy in stage I uterine sarcomas. A pilot study. Cancer 57 (8): 1451-4, 1986. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Cellular Classification of Uterine Sarcoma
The most common histologic types of uterine sarcomas include:
- Carcinosarcomas (mixed mesodermal sarcomas [40%–50%]).
- Leiomyosarcomas (30%).
- Endometrial stromal sarcomas (15%).
The uterine neoplasm classification of the International Society of Gynecologic Pathologists and the World Health Organization uses the term carcinosarcomas for all primary uterine neoplasms containing malignant elements of both epithelial and stromal light microscopic appearances, regardless of whether malignant heterologous elements are present.[1]
References
- Silverberg SG, Major FJ, Blessing JA, et al.: Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol 9 (1): 1-19, 1990. [PUBMED Abstract]
Stage Information for Uterine Sarcoma
Definitions: FIGO
The Féderation Internationale de Gynécologie et d’Obstétrique (FIGO) and the American Joint Committee on Cancer (AJCC) have designated staging to define carcinoma of the corpus uteri, which applies to uterine sarcoma; the FIGO system is most commonly used.[1,2]
Uterine sarcomas include leiomyosarcomas, endometrial stromal sarcomas, and adenosarcomas.
| Stage | Description |
|---|---|
| aAdapted from FIGO Committee on Gynecologic Oncology.[1] | |
| bEither G1, G2, or G3 (G = grade). | |
| cEndocervical glandular involvement only should be considered as stage I and no longer as stage II. | |
| dPositive cytology has to be reported separately without changing the stage. | |
| Ib | Tumor confined to the corpus uteri. |
| IAb | No or less than half myometrial invasion. |
| IBb | Invasion equal to or more than half of the myometrium. |
| IIb | Tumor invades cervical stroma but does not extend beyond the uterus.c |
| IIIb | Local and/or regional spread of the tumor. |
| IIIAb | Tumor invades the serosa of the corpus uteri and/or adnexae.d |
| IIIBb | Vaginal and/or parametrial involvement.d |
| IIICb | Metastases to pelvic and/or para-aortic lymph nodes.d |
| IIIC1b | Positive pelvic nodes. |
| IIIC2b | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes. |
| IVb | Tumor invades bladder and/or bowel mucosa, and/or distant metastases. |
| IVAb | Tumor invasion of bladder and/or bowel mucosa. |
| IVBb | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes. |
References
- Pecorelli S: Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105 (2): 103-4, 2009. [PUBMED Abstract]
- Corpus uteri. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 403-18.
Treatment Option Overview
Surgery is often the principal means of diagnosis and is the primary treatment for all patients with uterine sarcoma. If the diagnosis is known, the extent of surgery is planned according to the stage of the tumor. Hysterectomy is usually performed when a uterine malignancy is suspected, except for rare instances when preservation of the uterus in a young patient is deemed safe for the type of cancer (e.g., a totally confined low-grade leiomyosarcoma in a woman who desires to retain childbearing potential). Medically suitable patients with the preoperative diagnosis of uterine sarcoma are considered candidates for abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy. Cytologic washings are obtained from the pelvis and abdomen. Thorough examination of the diaphragm, omentum, and upper abdomen is performed.
There is no firm evidence from a prospective study that adjuvant chemotherapy or radiation therapy is of benefit for patients with uterine sarcoma.[1] In one Gynecologic Oncology Group (GOG) study, the use of adjuvant doxorubicin did not alter the survival rate of patients with resected stage I or stage II uterine sarcomas; however, interpretation of these results is difficult because this study included some patients who received radiation and three types of uterine sarcomas that have variable responses to doxorubicin.[1][Level of evidence: 1iiA] However, because the risk of disease recurrence is high even with localized presentations, many physicians have considered the use of adjuvant chemotherapy or radiation therapy.[2] A report of a study (GOG-0150 [NCT00002546]) that addressed radiation therapy versus adjuvant chemotherapy is awaited.[3]
References
- Omura GA, Blessing JA, Major F, et al.: A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 3 (9): 1240-5, 1985. [PUBMED Abstract]
- Kohorn EI, Schwartz PE, Chambers JT, et al.: Adjuvant therapy in mixed mullerian tumors of the uterus. Gynecol Oncol 23 (2): 212-21, 1986. [PUBMED Abstract]
- Wolfson AH, Brady MF, Mannel RS, et al.: A Gynecologic Oncology Group randomized trial of whole abdominal irradiation (WAI) vs cisplatin-ifosfamide+mesna (CIM) in optimally debulked stage I-IV carcinosarcoma (CS) of the uterus. [Abstract] J Clin Oncol 24 (Suppl 18): A-5001, 256s, 2006.
Stage I Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy).
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
- Surgery plus adjuvant radiation therapy as seen in the EORTC-55874 trial, for example.
In a nonrandomized, Gynecologic Oncology Group study in patients with stage I and II carcinosarcomas, those who had pelvic radiation therapy had a significant reduction of recurrences within the radiation treatment field but no alteration in survival.[1] A large nonrandomized study demonstrated improved survival and a lower local failure rate in patients with mixed mullerian tumors following postoperative external and intracavitary radiation therapy.[2] One nonrandomized study that predominantly included patients with carcinosarcomas appeared to show benefit for adjuvant therapy with cisplatin and doxorubicin.[3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986. [PUBMED Abstract]
- Larson B, Silfverswärd C, Nilsson B, et al.: Mixed müllerian tumours of the uterus--prognostic factors: a clinical and histopathologic study of 147 cases. Radiother Oncol 17 (2): 123-32, 1990. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Stage II Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy).
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
- Surgery plus adjuvant radiation therapy (EORTC-55874).
In a nonrandomized, Gynecologic Oncology Group study in patients with stage I and II carcinosarcomas, those who had pelvic radiation therapy had a significant reduction of recurrences within the radiation treatment field but no alteration in survival.[1] One nonrandomized study that predominantly included patients with carcinosarcomas appeared to show benefit for adjuvant therapy with cisplatin and doxorubicin.[2]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Stage III Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and periaortic selective lymphadenectomy, and resection of all gross tumor).
Treatment options under clinical evaluation:
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
Carcinosarcomas (the preferred designation by the World Health Organization) are also referred to as mixed mesodermal or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements (such as malignant mesenchymal tissue considered possibly native to the uterus) or heterologous elements (such as striated muscle, cartilage, or bone, which are foreign to the uterus). Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example.[1,2] These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas [3] and a 17.2% partial response rate in patients with leiomyosarcomas.[2]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[4][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[5] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[5][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
A role for chemotherapy as adjuvant to surgery has not yet been established.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
Stage IV Uterine Sarcoma
There is currently no standard therapy for patients with stage IV disease. These patients should be entered into an ongoing clinical trial.
Carcinosarcomas (the preferred designation by the World Health Organization) are also referred to as mixed mesodermal or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements, such as malignant mesenchymal tissue considered possibly native to the uterus, or heterologous elements, such as striated muscle, cartilage, or bone, which is foreign to the uterus. Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example.[1] These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas,[2] a 33% response rate in patients with endometrial stromal cell sarcomas,[3], and a 17.2% partial response rate in patients with leiomyosarcomas.[4] Doxorubicin in combination with dacarbazine or cyclophosphamide is no more active than doxorubicin alone for advanced disease.[5,6] Cisplatin has activity as first-line therapy and minimal activity as second-line therapy for patients with carcinosarcomas, but cisplatin is inactive as first- or second-line therapy for patients with leiomyosarcomas.[1,7]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[8][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[9] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[9][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Blessing JA, Park R, et al.: Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol 87 (5 Pt 1): 747-50, 1996. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Omura GA, Major FJ, Blessing JA, et al.: A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 52 (4): 626-32, 1983. [PUBMED Abstract]
- Muss HB, Bundy B, DiSaia PJ, et al.: Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 55 (8): 1648-53, 1985. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Wilbanks GD: Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol 9 (1): 18-20, 1986. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
Recurrent Uterine Sarcoma
There is currently no standard therapy for patients with recurrent disease. These patients should be entered into an ongoing clinical trial.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example. These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas,[1] a 33% response rate in patients with endometrial stromal cell sarcomas,[2] and a 17.2% partial response rate in patients with leiomyosarcomas.[3] Doxorubicin in combination with dacarbazine or cyclophosphamide is no more active than doxorubicin alone for recurrent disease.[4,5] Cisplatin has activity as first-line therapy and minimal activity as second-line therapy for patients with carcinosarcomas, but cisplatin is inactive as first- or second-line therapy for patients with leiomyosarcomas.[6,7] A regimen of gemcitabine plus docetaxel had a 53% response rate in patients with unresectable leiomyosarcomas and is undergoing further study.[8]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[9][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[10] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[10][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
For patients with carcinosarcomas who have localized recurrence to the pelvis confirmed by computed tomographic scanning, radiation therapy may be effective palliation. Phase I and II clinical trials are appropriate for patients who recur with distant metastasis and are unresponsive to first-line phase II trials. High-dose progesterone hormone therapy may be of some benefit to patients with low-grade stromal sarcoma.[11]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Blessing JA, Park R, et al.: Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol 87 (5 Pt 1): 747-50, 1996. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Omura GA, Major FJ, Blessing JA, et al.: A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 52 (4): 626-32, 1983. [PUBMED Abstract]
- Muss HB, Bundy B, DiSaia PJ, et al.: Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 55 (8): 1648-53, 1985. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Wilbanks GD: Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol 9 (1): 18-20, 1986. [PUBMED Abstract]
- Hensley ML, Maki R, Venkatraman E, et al.: Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 20 (12): 2824-31, 2002. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
- Katz L, Merino MJ, Sakamoto H, et al.: Endometrial stromal sarcoma: a clinicopathologic study of 11 cases with determination of estrogen and progestin receptor levels in three tumors. Gynecol Oncol 26 (1): 87-97, 1987. [PUBMED Abstract]
Changes to This Summary (07/15/2015)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of uterine sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Uterine Sarcoma Treatment are:
- Leslie R. Boyd, MD (New York University Medical Center)
- Franco M. Muggia, MD (New York University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Uterine Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/uterine/hp/uterine-sarcoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389327]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Uterine Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Uterine Sarcoma
Uterine sarcomas comprise less than 1% of gynecologic malignancies and 2% to 5% of all uterine malignancies.[1] The following tumors arise primarily from three distinct tissues:
- Carcinosarcomas arising in the endometrium, in other organs of mullerian origin, and accounting for 40% to 50% of all uterine sarcomas.
- Leiomyosarcomas arising from myometrial muscle, with a peak incidence occurring at age 50, and accounting for 30% of all uterine sarcomas.
- Sarcomas arising in the endometrial stroma, with a peak incidence occurring before menopause for the low-grade tumors and after menopause for the high-grade tumors, and accounting for 15% of all uterine sarcomas.
The three distinct entities are often grouped under uterine sarcomas; however, each type of tumor is currently being studied in separate clinical trials.
Carcinosarcomas (the preferred designation by the World Health Organization [WHO]) are also referred to as mixed mesodermal sarcomas or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial-cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements, such as malignant mesenchymal tissue considered possibly native to the uterus, or heterologous elements, such as striated muscle, cartilage, or bone, which are foreign to the uterus. Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Other rare forms of uterine sarcomas also fall under the WHO classification of mesenchymal and mixed tumors of the uterus. These include:[2,3]
- Mixed endometrial stromal and smooth muscle tumors.
- Adenosarcomas, in which the epithelial elements appear benign within a malignant mesenchymal background.
- Embryonal botryoides or rhabdomyosarcomas, which are found almost exclusively in infants.
- PEComa—a perivascular epithelial-cell tumor that may behave in a malignant fashion, which is the latest to be added.
(Refer to the PDQ summary on Childhood Rhabdomyosarcoma for more information.)
Risk Factors
The only documented etiologic factor in 10% to 25% of these malignancies is prior pelvic radiation therapy, which is often administered for benign uterine bleeding that began 5 to 25 years earlier. An increased incidence of uterine sarcoma has been associated with tamoxifen in the treatment of breast cancer. Subsequently, increases have also been noted when tamoxifen was given to prevent breast cancer in women at increased risk—a possible result of the estrogenic effect of tamoxifen on the uterus. Because of this increase, patients on tamoxifen should have follow-up pelvic examinations and should undergo endometrial biopsy if there is any abnormal uterine bleeding.[4-6]
Prognosis
The prognosis for women with uterine sarcoma is primarily dependent on the extent of disease at the time of diagnosis.[7] For women with carcinosarcomas, significant predictors of metastatic disease at initial surgery include:[7]
- Isthmic or cervical location.
- Lymphatic vascular space invasion.
- Serous and clear cell histology.
- Grade 2 or 3 carcinoma.
The above factors in addition to the following ones correlate with a progression-free interval:[7]
- Adnexal spread.
- Lymph node metastases.
- Tumor size.
- Peritoneal cytologic findings.
- Depth of myometrial invasion.
Factors that bear no relationship to the presence or absence of metastases at surgical exploration are:
- The presence or absence of stromal heterologous elements.
- The types of such elements.
- The grade of the stromal components.
- The mitotic activity of the stromal components.
In one study, women with a well-differentiated sarcomatous component or carcinosarcomas had significantly longer progression-free intervals than those with moderately to poorly differentiated sarcomas for the homologous and heterologous types. The recurrence rate was 44% for homologous tumors and 63% for heterologous tumors. The type of heterologous sarcoma had no effect on the progression-free interval.
For women with leiomyosarcomas, some investigators consider tumor size to be the most important prognostic factor; women with tumors greater than 5.0 cm in maximum diameter have a poor prognosis.[8] However, in a Gynecologic Oncology Group study, the mitotic index was the only factor significantly related to progression-free interval.[7] Leiomyosarcomas matched for other known prognostic factors may be more aggressive than their carcinosarcoma counterparts.[9] The 5-year survival rate for women with stage I disease, which is confined to the corpus, is approximately 50% versus 0% to 20% for the remaining stages.
Surgery alone can be curative if the malignancy is contained within the uterus. The value of pelvic radiation therapy is not established. Current studies consist primarily of phase II chemotherapy trials for patients with advanced disease. Adjuvant chemotherapy following complete resection for patients with stage I or II disease was not established to be effective in a randomized trial.[10] Yet, other nonrandomized trials have reported improved survival following adjuvant chemotherapy with or without radiation therapy.[11-13]
Related Summaries
Other PDQ summaries containing information related to uterine sarcoma include the following:
References
- Forney JP, Buschbaum HJ: Classifying, staging, and treating uterine sarcomas. Contemp Ob Gyn 18(3):47, 50, 55-56, 61-62, 64, 69, 1981.
- Gershenson D, McGuire W, Gore Martin, et al.: Gynecologic Cancer: Controversies in Management. 3rd ed. New York, NY: Churchill Livingstone, 2004.
- Tavassoéli F, Devilee P, et al.: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France: International Agency for Research on Cancer, 2004.
- Bergman L, Beelen ML, Gallee MP, et al.: Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 356 (9233): 881-7, 2000. [PUBMED Abstract]
- Cohen I: Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 94 (2): 256-66, 2004. [PUBMED Abstract]
- Wickerham DL, Fisher B, Wolmark N, et al.: Association of tamoxifen and uterine sarcoma. J Clin Oncol 20 (11): 2758-60, 2002. [PUBMED Abstract]
- Major FJ, Blessing JA, Silverberg SG, et al.: Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 71 (4 Suppl): 1702-9, 1993. [PUBMED Abstract]
- Evans HL, Chawla SP, Simpson C, et al.: Smooth muscle neoplasms of the uterus other than ordinary leiomyoma. A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer 62 (10): 2239-47, 1988. [PUBMED Abstract]
- Oláh KS, Dunn JA, Gee H: Leiomyosarcomas have a poorer prognosis than mixed mesodermal tumours when adjusting for known prognostic factors: the result of a retrospective study of 423 cases of uterine sarcoma. Br J Obstet Gynaecol 99 (7): 590-4, 1992. [PUBMED Abstract]
- Omura GA, Blessing JA, Major F, et al.: A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 3 (9): 1240-5, 1985. [PUBMED Abstract]
- Piver MS, Lele SB, Marchetti DL, et al.: Effect of adjuvant chemotherapy on time to recurrence and survival of stage I uterine sarcomas. J Surg Oncol 38 (4): 233-9, 1988. [PUBMED Abstract]
- van Nagell JR Jr, Hanson MB, Donaldson ES, et al.: Adjuvant vincristine, dactinomycin, and cyclophosphamide therapy in stage I uterine sarcomas. A pilot study. Cancer 57 (8): 1451-4, 1986. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Cellular Classification of Uterine Sarcoma
The most common histologic types of uterine sarcomas include:
- Carcinosarcomas (mixed mesodermal sarcomas [40%–50%]).
- Leiomyosarcomas (30%).
- Endometrial stromal sarcomas (15%).
The uterine neoplasm classification of the International Society of Gynecologic Pathologists and the World Health Organization uses the term carcinosarcomas for all primary uterine neoplasms containing malignant elements of both epithelial and stromal light microscopic appearances, regardless of whether malignant heterologous elements are present.[1]
References
- Silverberg SG, Major FJ, Blessing JA, et al.: Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol 9 (1): 1-19, 1990. [PUBMED Abstract]
Stage Information for Uterine Sarcoma
Definitions: FIGO
The Féderation Internationale de Gynécologie et d’Obstétrique (FIGO) and the American Joint Committee on Cancer (AJCC) have designated staging to define carcinoma of the corpus uteri, which applies to uterine sarcoma; the FIGO system is most commonly used.[1,2]
Uterine sarcomas include leiomyosarcomas, endometrial stromal sarcomas, and adenosarcomas.
| Stage | Description |
|---|---|
| aAdapted from FIGO Committee on Gynecologic Oncology.[1] | |
| bEither G1, G2, or G3 (G = grade). | |
| cEndocervical glandular involvement only should be considered as stage I and no longer as stage II. | |
| dPositive cytology has to be reported separately without changing the stage. | |
| Ib | Tumor confined to the corpus uteri. |
| IAb | No or less than half myometrial invasion. |
| IBb | Invasion equal to or more than half of the myometrium. |
| IIb | Tumor invades cervical stroma but does not extend beyond the uterus.c |
| IIIb | Local and/or regional spread of the tumor. |
| IIIAb | Tumor invades the serosa of the corpus uteri and/or adnexae.d |
| IIIBb | Vaginal and/or parametrial involvement.d |
| IIICb | Metastases to pelvic and/or para-aortic lymph nodes.d |
| IIIC1b | Positive pelvic nodes. |
| IIIC2b | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes. |
| IVb | Tumor invades bladder and/or bowel mucosa, and/or distant metastases. |
| IVAb | Tumor invasion of bladder and/or bowel mucosa. |
| IVBb | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes. |
References
- Pecorelli S: Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105 (2): 103-4, 2009. [PUBMED Abstract]
- Corpus uteri. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 403-18.
Treatment Option Overview
Surgery is often the principal means of diagnosis and is the primary treatment for all patients with uterine sarcoma. If the diagnosis is known, the extent of surgery is planned according to the stage of the tumor. Hysterectomy is usually performed when a uterine malignancy is suspected, except for rare instances when preservation of the uterus in a young patient is deemed safe for the type of cancer (e.g., a totally confined low-grade leiomyosarcoma in a woman who desires to retain childbearing potential). Medically suitable patients with the preoperative diagnosis of uterine sarcoma are considered candidates for abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy. Cytologic washings are obtained from the pelvis and abdomen. Thorough examination of the diaphragm, omentum, and upper abdomen is performed.
There is no firm evidence from a prospective study that adjuvant chemotherapy or radiation therapy is of benefit for patients with uterine sarcoma.[1] In one Gynecologic Oncology Group (GOG) study, the use of adjuvant doxorubicin did not alter the survival rate of patients with resected stage I or stage II uterine sarcomas; however, interpretation of these results is difficult because this study included some patients who received radiation and three types of uterine sarcomas that have variable responses to doxorubicin.[1][Level of evidence: 1iiA] However, because the risk of disease recurrence is high even with localized presentations, many physicians have considered the use of adjuvant chemotherapy or radiation therapy.[2] A report of a study (GOG-0150 [NCT00002546]) that addressed radiation therapy versus adjuvant chemotherapy is awaited.[3]
References
- Omura GA, Blessing JA, Major F, et al.: A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 3 (9): 1240-5, 1985. [PUBMED Abstract]
- Kohorn EI, Schwartz PE, Chambers JT, et al.: Adjuvant therapy in mixed mullerian tumors of the uterus. Gynecol Oncol 23 (2): 212-21, 1986. [PUBMED Abstract]
- Wolfson AH, Brady MF, Mannel RS, et al.: A Gynecologic Oncology Group randomized trial of whole abdominal irradiation (WAI) vs cisplatin-ifosfamide+mesna (CIM) in optimally debulked stage I-IV carcinosarcoma (CS) of the uterus. [Abstract] J Clin Oncol 24 (Suppl 18): A-5001, 256s, 2006.
Stage I Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy).
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
- Surgery plus adjuvant radiation therapy as seen in the EORTC-55874 trial, for example.
In a nonrandomized, Gynecologic Oncology Group study in patients with stage I and II carcinosarcomas, those who had pelvic radiation therapy had a significant reduction of recurrences within the radiation treatment field but no alteration in survival.[1] A large nonrandomized study demonstrated improved survival and a lower local failure rate in patients with mixed mullerian tumors following postoperative external and intracavitary radiation therapy.[2] One nonrandomized study that predominantly included patients with carcinosarcomas appeared to show benefit for adjuvant therapy with cisplatin and doxorubicin.[3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986. [PUBMED Abstract]
- Larson B, Silfverswärd C, Nilsson B, et al.: Mixed müllerian tumours of the uterus--prognostic factors: a clinical and histopathologic study of 147 cases. Radiother Oncol 17 (2): 123-32, 1990. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Stage II Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy).
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
- Surgery plus adjuvant radiation therapy (EORTC-55874).
In a nonrandomized, Gynecologic Oncology Group study in patients with stage I and II carcinosarcomas, those who had pelvic radiation therapy had a significant reduction of recurrences within the radiation treatment field but no alteration in survival.[1] One nonrandomized study that predominantly included patients with carcinosarcomas appeared to show benefit for adjuvant therapy with cisplatin and doxorubicin.[2]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Stage III Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and periaortic selective lymphadenectomy, and resection of all gross tumor).
Treatment options under clinical evaluation:
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
Carcinosarcomas (the preferred designation by the World Health Organization) are also referred to as mixed mesodermal or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements (such as malignant mesenchymal tissue considered possibly native to the uterus) or heterologous elements (such as striated muscle, cartilage, or bone, which are foreign to the uterus). Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example.[1,2] These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas [3] and a 17.2% partial response rate in patients with leiomyosarcomas.[2]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[4][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[5] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[5][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
A role for chemotherapy as adjuvant to surgery has not yet been established.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
Stage IV Uterine Sarcoma
There is currently no standard therapy for patients with stage IV disease. These patients should be entered into an ongoing clinical trial.
Carcinosarcomas (the preferred designation by the World Health Organization) are also referred to as mixed mesodermal or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements, such as malignant mesenchymal tissue considered possibly native to the uterus, or heterologous elements, such as striated muscle, cartilage, or bone, which is foreign to the uterus. Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example.[1] These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas,[2] a 33% response rate in patients with endometrial stromal cell sarcomas,[3], and a 17.2% partial response rate in patients with leiomyosarcomas.[4] Doxorubicin in combination with dacarbazine or cyclophosphamide is no more active than doxorubicin alone for advanced disease.[5,6] Cisplatin has activity as first-line therapy and minimal activity as second-line therapy for patients with carcinosarcomas, but cisplatin is inactive as first- or second-line therapy for patients with leiomyosarcomas.[1,7]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[8][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[9] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[9][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Blessing JA, Park R, et al.: Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol 87 (5 Pt 1): 747-50, 1996. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Omura GA, Major FJ, Blessing JA, et al.: A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 52 (4): 626-32, 1983. [PUBMED Abstract]
- Muss HB, Bundy B, DiSaia PJ, et al.: Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 55 (8): 1648-53, 1985. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Wilbanks GD: Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol 9 (1): 18-20, 1986. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
Recurrent Uterine Sarcoma
There is currently no standard therapy for patients with recurrent disease. These patients should be entered into an ongoing clinical trial.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example. These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas,[1] a 33% response rate in patients with endometrial stromal cell sarcomas,[2] and a 17.2% partial response rate in patients with leiomyosarcomas.[3] Doxorubicin in combination with dacarbazine or cyclophosphamide is no more active than doxorubicin alone for recurrent disease.[4,5] Cisplatin has activity as first-line therapy and minimal activity as second-line therapy for patients with carcinosarcomas, but cisplatin is inactive as first- or second-line therapy for patients with leiomyosarcomas.[6,7] A regimen of gemcitabine plus docetaxel had a 53% response rate in patients with unresectable leiomyosarcomas and is undergoing further study.[8]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[9][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[10] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[10][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
For patients with carcinosarcomas who have localized recurrence to the pelvis confirmed by computed tomographic scanning, radiation therapy may be effective palliation. Phase I and II clinical trials are appropriate for patients who recur with distant metastasis and are unresponsive to first-line phase II trials. High-dose progesterone hormone therapy may be of some benefit to patients with low-grade stromal sarcoma.[11]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Blessing JA, Park R, et al.: Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol 87 (5 Pt 1): 747-50, 1996. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Omura GA, Major FJ, Blessing JA, et al.: A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 52 (4): 626-32, 1983. [PUBMED Abstract]
- Muss HB, Bundy B, DiSaia PJ, et al.: Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 55 (8): 1648-53, 1985. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Wilbanks GD: Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol 9 (1): 18-20, 1986. [PUBMED Abstract]
- Hensley ML, Maki R, Venkatraman E, et al.: Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 20 (12): 2824-31, 2002. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
- Katz L, Merino MJ, Sakamoto H, et al.: Endometrial stromal sarcoma: a clinicopathologic study of 11 cases with determination of estrogen and progestin receptor levels in three tumors. Gynecol Oncol 26 (1): 87-97, 1987. [PUBMED Abstract]
Changes to This Summary (07/15/2015)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of uterine sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Uterine Sarcoma Treatment are:
- Leslie R. Boyd, MD (New York University Medical Center)
- Franco M. Muggia, MD (New York University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Uterine Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/uterine/hp/uterine-sarcoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389327]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Uterine Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Uterine Sarcoma
Uterine sarcomas comprise less than 1% of gynecologic malignancies and 2% to 5% of all uterine malignancies.[1] The following tumors arise primarily from three distinct tissues:
- Carcinosarcomas arising in the endometrium, in other organs of mullerian origin, and accounting for 40% to 50% of all uterine sarcomas.
- Leiomyosarcomas arising from myometrial muscle, with a peak incidence occurring at age 50, and accounting for 30% of all uterine sarcomas.
- Sarcomas arising in the endometrial stroma, with a peak incidence occurring before menopause for the low-grade tumors and after menopause for the high-grade tumors, and accounting for 15% of all uterine sarcomas.
The three distinct entities are often grouped under uterine sarcomas; however, each type of tumor is currently being studied in separate clinical trials.
Carcinosarcomas (the preferred designation by the World Health Organization [WHO]) are also referred to as mixed mesodermal sarcomas or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial-cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements, such as malignant mesenchymal tissue considered possibly native to the uterus, or heterologous elements, such as striated muscle, cartilage, or bone, which are foreign to the uterus. Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Other rare forms of uterine sarcomas also fall under the WHO classification of mesenchymal and mixed tumors of the uterus. These include:[2,3]
- Mixed endometrial stromal and smooth muscle tumors.
- Adenosarcomas, in which the epithelial elements appear benign within a malignant mesenchymal background.
- Embryonal botryoides or rhabdomyosarcomas, which are found almost exclusively in infants.
- PEComa—a perivascular epithelial-cell tumor that may behave in a malignant fashion, which is the latest to be added.
(Refer to the PDQ summary on Childhood Rhabdomyosarcoma for more information.)
Risk Factors
The only documented etiologic factor in 10% to 25% of these malignancies is prior pelvic radiation therapy, which is often administered for benign uterine bleeding that began 5 to 25 years earlier. An increased incidence of uterine sarcoma has been associated with tamoxifen in the treatment of breast cancer. Subsequently, increases have also been noted when tamoxifen was given to prevent breast cancer in women at increased risk—a possible result of the estrogenic effect of tamoxifen on the uterus. Because of this increase, patients on tamoxifen should have follow-up pelvic examinations and should undergo endometrial biopsy if there is any abnormal uterine bleeding.[4-6]
Prognosis
The prognosis for women with uterine sarcoma is primarily dependent on the extent of disease at the time of diagnosis.[7] For women with carcinosarcomas, significant predictors of metastatic disease at initial surgery include:[7]
- Isthmic or cervical location.
- Lymphatic vascular space invasion.
- Serous and clear cell histology.
- Grade 2 or 3 carcinoma.
The above factors in addition to the following ones correlate with a progression-free interval:[7]
- Adnexal spread.
- Lymph node metastases.
- Tumor size.
- Peritoneal cytologic findings.
- Depth of myometrial invasion.
Factors that bear no relationship to the presence or absence of metastases at surgical exploration are:
- The presence or absence of stromal heterologous elements.
- The types of such elements.
- The grade of the stromal components.
- The mitotic activity of the stromal components.
In one study, women with a well-differentiated sarcomatous component or carcinosarcomas had significantly longer progression-free intervals than those with moderately to poorly differentiated sarcomas for the homologous and heterologous types. The recurrence rate was 44% for homologous tumors and 63% for heterologous tumors. The type of heterologous sarcoma had no effect on the progression-free interval.
For women with leiomyosarcomas, some investigators consider tumor size to be the most important prognostic factor; women with tumors greater than 5.0 cm in maximum diameter have a poor prognosis.[8] However, in a Gynecologic Oncology Group study, the mitotic index was the only factor significantly related to progression-free interval.[7] Leiomyosarcomas matched for other known prognostic factors may be more aggressive than their carcinosarcoma counterparts.[9] The 5-year survival rate for women with stage I disease, which is confined to the corpus, is approximately 50% versus 0% to 20% for the remaining stages.
Surgery alone can be curative if the malignancy is contained within the uterus. The value of pelvic radiation therapy is not established. Current studies consist primarily of phase II chemotherapy trials for patients with advanced disease. Adjuvant chemotherapy following complete resection for patients with stage I or II disease was not established to be effective in a randomized trial.[10] Yet, other nonrandomized trials have reported improved survival following adjuvant chemotherapy with or without radiation therapy.[11-13]
Related Summaries
Other PDQ summaries containing information related to uterine sarcoma include the following:
References
- Forney JP, Buschbaum HJ: Classifying, staging, and treating uterine sarcomas. Contemp Ob Gyn 18(3):47, 50, 55-56, 61-62, 64, 69, 1981.
- Gershenson D, McGuire W, Gore Martin, et al.: Gynecologic Cancer: Controversies in Management. 3rd ed. New York, NY: Churchill Livingstone, 2004.
- Tavassoéli F, Devilee P, et al.: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France: International Agency for Research on Cancer, 2004.
- Bergman L, Beelen ML, Gallee MP, et al.: Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 356 (9233): 881-7, 2000. [PUBMED Abstract]
- Cohen I: Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 94 (2): 256-66, 2004. [PUBMED Abstract]
- Wickerham DL, Fisher B, Wolmark N, et al.: Association of tamoxifen and uterine sarcoma. J Clin Oncol 20 (11): 2758-60, 2002. [PUBMED Abstract]
- Major FJ, Blessing JA, Silverberg SG, et al.: Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 71 (4 Suppl): 1702-9, 1993. [PUBMED Abstract]
- Evans HL, Chawla SP, Simpson C, et al.: Smooth muscle neoplasms of the uterus other than ordinary leiomyoma. A study of 46 cases, with emphasis on diagnostic criteria and prognostic factors. Cancer 62 (10): 2239-47, 1988. [PUBMED Abstract]
- Oláh KS, Dunn JA, Gee H: Leiomyosarcomas have a poorer prognosis than mixed mesodermal tumours when adjusting for known prognostic factors: the result of a retrospective study of 423 cases of uterine sarcoma. Br J Obstet Gynaecol 99 (7): 590-4, 1992. [PUBMED Abstract]
- Omura GA, Blessing JA, Major F, et al.: A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 3 (9): 1240-5, 1985. [PUBMED Abstract]
- Piver MS, Lele SB, Marchetti DL, et al.: Effect of adjuvant chemotherapy on time to recurrence and survival of stage I uterine sarcomas. J Surg Oncol 38 (4): 233-9, 1988. [PUBMED Abstract]
- van Nagell JR Jr, Hanson MB, Donaldson ES, et al.: Adjuvant vincristine, dactinomycin, and cyclophosphamide therapy in stage I uterine sarcomas. A pilot study. Cancer 57 (8): 1451-4, 1986. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Cellular Classification of Uterine Sarcoma
The most common histologic types of uterine sarcomas include:
- Carcinosarcomas (mixed mesodermal sarcomas [40%–50%]).
- Leiomyosarcomas (30%).
- Endometrial stromal sarcomas (15%).
The uterine neoplasm classification of the International Society of Gynecologic Pathologists and the World Health Organization uses the term carcinosarcomas for all primary uterine neoplasms containing malignant elements of both epithelial and stromal light microscopic appearances, regardless of whether malignant heterologous elements are present.[1]
References
- Silverberg SG, Major FJ, Blessing JA, et al.: Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol 9 (1): 1-19, 1990. [PUBMED Abstract]
Stage Information for Uterine Sarcoma
Definitions: FIGO
The Féderation Internationale de Gynécologie et d’Obstétrique (FIGO) and the American Joint Committee on Cancer (AJCC) have designated staging to define carcinoma of the corpus uteri, which applies to uterine sarcoma; the FIGO system is most commonly used.[1,2]
Uterine sarcomas include leiomyosarcomas, endometrial stromal sarcomas, and adenosarcomas.
| Stage | Description |
|---|---|
| aAdapted from FIGO Committee on Gynecologic Oncology.[1] | |
| bEither G1, G2, or G3 (G = grade). | |
| cEndocervical glandular involvement only should be considered as stage I and no longer as stage II. | |
| dPositive cytology has to be reported separately without changing the stage. | |
| Ib | Tumor confined to the corpus uteri. |
| IAb | No or less than half myometrial invasion. |
| IBb | Invasion equal to or more than half of the myometrium. |
| IIb | Tumor invades cervical stroma but does not extend beyond the uterus.c |
| IIIb | Local and/or regional spread of the tumor. |
| IIIAb | Tumor invades the serosa of the corpus uteri and/or adnexae.d |
| IIIBb | Vaginal and/or parametrial involvement.d |
| IIICb | Metastases to pelvic and/or para-aortic lymph nodes.d |
| IIIC1b | Positive pelvic nodes. |
| IIIC2b | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes. |
| IVb | Tumor invades bladder and/or bowel mucosa, and/or distant metastases. |
| IVAb | Tumor invasion of bladder and/or bowel mucosa. |
| IVBb | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes. |
References
- Pecorelli S: Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105 (2): 103-4, 2009. [PUBMED Abstract]
- Corpus uteri. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 403-18.
Treatment Option Overview
Surgery is often the principal means of diagnosis and is the primary treatment for all patients with uterine sarcoma. If the diagnosis is known, the extent of surgery is planned according to the stage of the tumor. Hysterectomy is usually performed when a uterine malignancy is suspected, except for rare instances when preservation of the uterus in a young patient is deemed safe for the type of cancer (e.g., a totally confined low-grade leiomyosarcoma in a woman who desires to retain childbearing potential). Medically suitable patients with the preoperative diagnosis of uterine sarcoma are considered candidates for abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy. Cytologic washings are obtained from the pelvis and abdomen. Thorough examination of the diaphragm, omentum, and upper abdomen is performed.
There is no firm evidence from a prospective study that adjuvant chemotherapy or radiation therapy is of benefit for patients with uterine sarcoma.[1] In one Gynecologic Oncology Group (GOG) study, the use of adjuvant doxorubicin did not alter the survival rate of patients with resected stage I or stage II uterine sarcomas; however, interpretation of these results is difficult because this study included some patients who received radiation and three types of uterine sarcomas that have variable responses to doxorubicin.[1][Level of evidence: 1iiA] However, because the risk of disease recurrence is high even with localized presentations, many physicians have considered the use of adjuvant chemotherapy or radiation therapy.[2] A report of a study (GOG-0150 [NCT00002546]) that addressed radiation therapy versus adjuvant chemotherapy is awaited.[3]
References
- Omura GA, Blessing JA, Major F, et al.: A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. J Clin Oncol 3 (9): 1240-5, 1985. [PUBMED Abstract]
- Kohorn EI, Schwartz PE, Chambers JT, et al.: Adjuvant therapy in mixed mullerian tumors of the uterus. Gynecol Oncol 23 (2): 212-21, 1986. [PUBMED Abstract]
- Wolfson AH, Brady MF, Mannel RS, et al.: A Gynecologic Oncology Group randomized trial of whole abdominal irradiation (WAI) vs cisplatin-ifosfamide+mesna (CIM) in optimally debulked stage I-IV carcinosarcoma (CS) of the uterus. [Abstract] J Clin Oncol 24 (Suppl 18): A-5001, 256s, 2006.
Stage I Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy).
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
- Surgery plus adjuvant radiation therapy as seen in the EORTC-55874 trial, for example.
In a nonrandomized, Gynecologic Oncology Group study in patients with stage I and II carcinosarcomas, those who had pelvic radiation therapy had a significant reduction of recurrences within the radiation treatment field but no alteration in survival.[1] A large nonrandomized study demonstrated improved survival and a lower local failure rate in patients with mixed mullerian tumors following postoperative external and intracavitary radiation therapy.[2] One nonrandomized study that predominantly included patients with carcinosarcomas appeared to show benefit for adjuvant therapy with cisplatin and doxorubicin.[3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986. [PUBMED Abstract]
- Larson B, Silfverswärd C, Nilsson B, et al.: Mixed müllerian tumours of the uterus--prognostic factors: a clinical and histopathologic study of 147 cases. Radiother Oncol 17 (2): 123-32, 1990. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Stage II Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and periaortic selective lymphadenectomy).
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
- Surgery plus adjuvant radiation therapy (EORTC-55874).
In a nonrandomized, Gynecologic Oncology Group study in patients with stage I and II carcinosarcomas, those who had pelvic radiation therapy had a significant reduction of recurrences within the radiation treatment field but no alteration in survival.[1] One nonrandomized study that predominantly included patients with carcinosarcomas appeared to show benefit for adjuvant therapy with cisplatin and doxorubicin.[2]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986. [PUBMED Abstract]
- Peters WA 3rd, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989. [PUBMED Abstract]
Stage III Uterine Sarcoma
Standard treatment options:
- Surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and periaortic selective lymphadenectomy, and resection of all gross tumor).
Treatment options under clinical evaluation:
- Surgery plus pelvic radiation therapy.
- Surgery plus adjuvant chemotherapy.
Carcinosarcomas (the preferred designation by the World Health Organization) are also referred to as mixed mesodermal or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements (such as malignant mesenchymal tissue considered possibly native to the uterus) or heterologous elements (such as striated muscle, cartilage, or bone, which are foreign to the uterus). Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example.[1,2] These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas [3] and a 17.2% partial response rate in patients with leiomyosarcomas.[2]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[4][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[5] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[5][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
A role for chemotherapy as adjuvant to surgery has not yet been established.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
Stage IV Uterine Sarcoma
There is currently no standard therapy for patients with stage IV disease. These patients should be entered into an ongoing clinical trial.
Carcinosarcomas (the preferred designation by the World Health Organization) are also referred to as mixed mesodermal or mullerian tumors. Controversy exists about the following issues:
- Whether they are true sarcomas.
- Whether the sarcomatous elements are actually derived from a common epithelial cell precursor that also gives rise to the usually more abundant adenocarcinomatous elements.
The stromal components of the carcinosarcomas are further characterized by whether they contain homologous elements, such as malignant mesenchymal tissue considered possibly native to the uterus, or heterologous elements, such as striated muscle, cartilage, or bone, which is foreign to the uterus. Carcinosarcomas parallel endometrial cancer in its postmenopausal predominance and in other of its epidemiologic features; increasingly, the treatment of carcinosarcomas is becoming similar to combined modality approaches for endometrial adenocarcinomas.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example.[1] These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas,[2] a 33% response rate in patients with endometrial stromal cell sarcomas,[3], and a 17.2% partial response rate in patients with leiomyosarcomas.[4] Doxorubicin in combination with dacarbazine or cyclophosphamide is no more active than doxorubicin alone for advanced disease.[5,6] Cisplatin has activity as first-line therapy and minimal activity as second-line therapy for patients with carcinosarcomas, but cisplatin is inactive as first- or second-line therapy for patients with leiomyosarcomas.[1,7]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[8][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[9] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[9][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Blessing JA, Park R, et al.: Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol 87 (5 Pt 1): 747-50, 1996. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Omura GA, Major FJ, Blessing JA, et al.: A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 52 (4): 626-32, 1983. [PUBMED Abstract]
- Muss HB, Bundy B, DiSaia PJ, et al.: Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 55 (8): 1648-53, 1985. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Wilbanks GD: Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol 9 (1): 18-20, 1986. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
Recurrent Uterine Sarcoma
There is currently no standard therapy for patients with recurrent disease. These patients should be entered into an ongoing clinical trial.
Patients who present with uterine sarcoma have been treated on a series of phase II studies by the Gynecologic Oncology Group, including the GOG-87B trial, for example. These chemotherapy studies have documented some antitumor activity for cisplatin, doxorubicin, and ifosfamide. These studies have also documented differences in response leading to separate trials for patients with carcinosarcomas and leiomyosarcomas. As an example, in patients previously untreated with chemotherapy, ifosfamide had a 32.2% response rate in patients with carcinosarcomas,[1] a 33% response rate in patients with endometrial stromal cell sarcomas,[2] and a 17.2% partial response rate in patients with leiomyosarcomas.[3] Doxorubicin in combination with dacarbazine or cyclophosphamide is no more active than doxorubicin alone for recurrent disease.[4,5] Cisplatin has activity as first-line therapy and minimal activity as second-line therapy for patients with carcinosarcomas, but cisplatin is inactive as first- or second-line therapy for patients with leiomyosarcomas.[6,7] A regimen of gemcitabine plus docetaxel had a 53% response rate in patients with unresectable leiomyosarcomas and is undergoing further study.[8]
A randomized comparison that was seen in the GOG-108 trial, for example, of ifosfamide with or without cisplatin for first-line therapy for patients with measurable advanced or recurrent carcinosarcomas demonstrated a higher response rate (54% vs. 34%) and longer progression-free survival (PFS) on the combination arm (6 months vs. 4 months), but there was no significant improvement in survival (9 months vs. 8 months).[9][Level of evidence: 1iiA] The follow-up GOG-0161 [NCT00003128] study utilized 3-day ifosfamide regimens (instead of the more toxic 5-day regimen in the preceding study) for the control and for a combination with paclitaxel (with filgrastim starting on day 4).[10] The combination was superior in response rates (45% vs. 29%), PFS (8.4 months vs. 5.8 months), and overall survival (13.5 months and 8.4 months). The hazard ratio for death favored the combination 0.69 (95% confidence interval, 0.49–0.97).[10][Level of evidence: 1iiA] In this study, 52% of 179 evaluable patients had recurrent disease, 18% had stage III disease, and 30% had stage IV disease. In addition, imbalances were present in the sites of disease and in the use of prior radiation therapy, and 30 patients were excluded for wrong pathology.
For patients with carcinosarcomas who have localized recurrence to the pelvis confirmed by computed tomographic scanning, radiation therapy may be effective palliation. Phase I and II clinical trials are appropriate for patients who recur with distant metastasis and are unresponsive to first-line phase II trials. High-dose progesterone hormone therapy may be of some benefit to patients with low-grade stromal sarcoma.[11]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Sutton GP, Blessing JA, Rosenshein N, et al.: Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). Am J Obstet Gynecol 161 (2): 309-12, 1989. [PUBMED Abstract]
- Sutton G, Blessing JA, Park R, et al.: Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol 87 (5 Pt 1): 747-50, 1996. [PUBMED Abstract]
- Sutton GP, Blessing JA, Barrett RJ, et al.: Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol 166 (2): 556-9, 1992. [PUBMED Abstract]
- Omura GA, Major FJ, Blessing JA, et al.: A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 52 (4): 626-32, 1983. [PUBMED Abstract]
- Muss HB, Bundy B, DiSaia PJ, et al.: Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 55 (8): 1648-53, 1985. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Beecham J, et al.: Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol 9 (11): 1962-6, 1991. [PUBMED Abstract]
- Thigpen JT, Blessing JA, Wilbanks GD: Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol 9 (1): 18-20, 1986. [PUBMED Abstract]
- Hensley ML, Maki R, Venkatraman E, et al.: Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 20 (12): 2824-31, 2002. [PUBMED Abstract]
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000. [PUBMED Abstract]
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007. [PUBMED Abstract]
- Katz L, Merino MJ, Sakamoto H, et al.: Endometrial stromal sarcoma: a clinicopathologic study of 11 cases with determination of estrogen and progestin receptor levels in three tumors. Gynecol Oncol 26 (1): 87-97, 1987. [PUBMED Abstract]
Changes to This Summary (07/15/2015)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of uterine sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Uterine Sarcoma Treatment are:
- Leslie R. Boyd, MD (New York University Medical Center)
- Franco M. Muggia, MD (New York University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Uterine Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/uterine/hp/uterine-sarcoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389327]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Onodera’s Prognostic Nutritional Index in soft tissue sarcoma patients as a predictor of wound complications
Background The ability to predict a wound complication after radiation therapy and surgery for soft tissue sarcomas remains difficult. Preoperative nutritional status, as determined by Onodera’s Prognostic Nutritional Index (OPNI), has been a predictor of complications in patients undergoing gastrointestinal surgery. However, the role OPNI has in predicting wound complications for soft tissue sarcoma remains unknown.
Objective To evaluate the role OPNI has in predicting wound complication in patients treated with radiation and surgery for soft tissue sarcomas.
Methods OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count). The albumin level and total lymphocyte counts closest to the index operation were chosen. Major and minor wound complications were identified. A receiver operating curve was calculated to identify a cut-off point value for OPNI and for age based on the best combination of sensitivity and specificity.
Results 44 patients were included in the study. Patients with an OPNI of <45.4 had a 7.5-times increased risk of a wound complication (P = .005; 95% confidence interval [CI], 1.8-31.0). An OPNI of <45.4 had a sensitivity of 62% and specificity of 82% of predicting a wound complication. Being older than 73 years was associated with a 6.8-times increased risk of wound complications (P = .01; 95% CI, 1.6-28.7).
Limitations Small sample size for patients with a rare condition
Conclusion An OPNI of <45.4 and being older than 73 years are strong predictors of which patients will have a wound complication after radiation therapy for soft tissue sarcomas. Preoperative nutritional status could be an important modifiable factor to help decrease wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2). In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications. TSJ
Correspondence
References
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Background The ability to predict a wound complication after radiation therapy and surgery for soft tissue sarcomas remains difficult. Preoperative nutritional status, as determined by Onodera’s Prognostic Nutritional Index (OPNI), has been a predictor of complications in patients undergoing gastrointestinal surgery. However, the role OPNI has in predicting wound complications for soft tissue sarcoma remains unknown.
Objective To evaluate the role OPNI has in predicting wound complication in patients treated with radiation and surgery for soft tissue sarcomas.
Methods OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count). The albumin level and total lymphocyte counts closest to the index operation were chosen. Major and minor wound complications were identified. A receiver operating curve was calculated to identify a cut-off point value for OPNI and for age based on the best combination of sensitivity and specificity.
Results 44 patients were included in the study. Patients with an OPNI of <45.4 had a 7.5-times increased risk of a wound complication (P = .005; 95% confidence interval [CI], 1.8-31.0). An OPNI of <45.4 had a sensitivity of 62% and specificity of 82% of predicting a wound complication. Being older than 73 years was associated with a 6.8-times increased risk of wound complications (P = .01; 95% CI, 1.6-28.7).
Limitations Small sample size for patients with a rare condition
Conclusion An OPNI of <45.4 and being older than 73 years are strong predictors of which patients will have a wound complication after radiation therapy for soft tissue sarcomas. Preoperative nutritional status could be an important modifiable factor to help decrease wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2). In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications. TSJ
Correspondence
References
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Background The ability to predict a wound complication after radiation therapy and surgery for soft tissue sarcomas remains difficult. Preoperative nutritional status, as determined by Onodera’s Prognostic Nutritional Index (OPNI), has been a predictor of complications in patients undergoing gastrointestinal surgery. However, the role OPNI has in predicting wound complications for soft tissue sarcoma remains unknown.
Objective To evaluate the role OPNI has in predicting wound complication in patients treated with radiation and surgery for soft tissue sarcomas.
Methods OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count). The albumin level and total lymphocyte counts closest to the index operation were chosen. Major and minor wound complications were identified. A receiver operating curve was calculated to identify a cut-off point value for OPNI and for age based on the best combination of sensitivity and specificity.
Results 44 patients were included in the study. Patients with an OPNI of <45.4 had a 7.5-times increased risk of a wound complication (P = .005; 95% confidence interval [CI], 1.8-31.0). An OPNI of <45.4 had a sensitivity of 62% and specificity of 82% of predicting a wound complication. Being older than 73 years was associated with a 6.8-times increased risk of wound complications (P = .01; 95% CI, 1.6-28.7).
Limitations Small sample size for patients with a rare condition
Conclusion An OPNI of <45.4 and being older than 73 years are strong predictors of which patients will have a wound complication after radiation therapy for soft tissue sarcomas. Preoperative nutritional status could be an important modifiable factor to help decrease wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2). In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications. TSJ
Correspondence
References
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Soft Tissue Sarcoma: Diagnosis and Treatment
Introduction
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
Epidemiology and Classification
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
Clinical Evaluation
› Case Presentation
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (
• What is the typical presentation for sarcomas?
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
Management
› Case Continued
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
Surgery
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
› Case Continued
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
Radiation THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
› Case Continued
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
Chemotherapy
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study.35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
› Case Continued
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dysp-nea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
› Case Continued
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51 A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
› Case Continued
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
Conclusion
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma. TSJ
CORRESPONDENCE
Ashley Pariser, MD, Resident, Department of Medicine, Northwestern University Feinberg School of Medicine Chicago, IL. Accepted for publication Jan/Feb 2017; Hosp Phys; Vol. 12, Part1
References
1. American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
2. National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
3. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
4. Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4: 252–66.
5. Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
6. Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
7. Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
8. Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
9. Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.

10. Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
11. Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
13. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
14. Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
15. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
16. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
17. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
18. Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
19. Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
20. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
21. Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
22. Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
23. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
24. Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
25. Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
26. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
27. Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
28. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
29. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
30. Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
31. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
32. Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
33. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
34. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
35. Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
36. Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
37. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
38. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.

39. Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
40. Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
41. Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
42. Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
43. Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
44. Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
45. Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
46. Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
47. Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
48. Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
49. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
50. Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
51. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
52. Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
53. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
54. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
55. Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
56. Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
57. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
58. Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
59. Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
Introduction
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
Epidemiology and Classification
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
Clinical Evaluation
› Case Presentation
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (
• What is the typical presentation for sarcomas?
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
Management
› Case Continued
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
Surgery
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
› Case Continued
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
Radiation THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
› Case Continued
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
Chemotherapy
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study.35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
› Case Continued
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dysp-nea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
› Case Continued
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51 A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
› Case Continued
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
Conclusion
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma. TSJ
CORRESPONDENCE
Ashley Pariser, MD, Resident, Department of Medicine, Northwestern University Feinberg School of Medicine Chicago, IL. Accepted for publication Jan/Feb 2017; Hosp Phys; Vol. 12, Part1
Introduction
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
Epidemiology and Classification
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
Clinical Evaluation
› Case Presentation
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (
• What is the typical presentation for sarcomas?
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
Management
› Case Continued
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
Surgery
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
› Case Continued
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
Radiation THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
› Case Continued
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
Chemotherapy
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study.35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
› Case Continued
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dysp-nea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
› Case Continued
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51 A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
› Case Continued
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
Conclusion
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma. TSJ
CORRESPONDENCE
Ashley Pariser, MD, Resident, Department of Medicine, Northwestern University Feinberg School of Medicine Chicago, IL. Accepted for publication Jan/Feb 2017; Hosp Phys; Vol. 12, Part1
References
1. American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
2. National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
3. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
4. Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4: 252–66.
5. Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
6. Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
7. Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
8. Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
9. Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.

10. Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
11. Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
13. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
14. Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
15. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
16. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
17. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
18. Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
19. Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
20. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
21. Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
22. Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
23. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
24. Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
25. Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
26. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
27. Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
28. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
29. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
30. Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
31. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
32. Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
33. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
34. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
35. Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
36. Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
37. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
38. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.

39. Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
40. Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
41. Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
42. Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
43. Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
44. Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
45. Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
46. Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
47. Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
48. Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
49. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
50. Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
51. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
52. Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
53. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
54. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
55. Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
56. Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
57. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
58. Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
59. Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
References
1. American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
2. National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
3. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
4. Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4: 252–66.
5. Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
6. Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
7. Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
8. Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
9. Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.

10. Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
11. Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
13. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
14. Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
15. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
16. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
17. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
18. Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
19. Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
20. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
21. Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
22. Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
23. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
24. Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
25. Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
26. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
27. Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
28. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
29. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
30. Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
31. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
32. Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
33. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
34. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
35. Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
36. Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
37. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
38. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.

39. Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
40. Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
41. Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
42. Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
43. Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
44. Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
45. Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
46. Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
47. Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
48. Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
49. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
50. Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
51. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
52. Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
53. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
54. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
55. Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
56. Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
57. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
58. Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
59. Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.