User login
Influence of Patient Demographics and Facility Type on Overall Survival in Sezary Syndrome
Background
This study investigates the effects of patient characteristics on overall survival in Sezary Syndrome (SS), addressing a gap in the current literature. SS is a rare and aggressive form of cutaneous T-cell lymphoma (CTCL). SS is presumed to be related to service exposure, and veterans have a 6-8 times higher incidence of CTCL than the general population. A study investigating the socio-demographic factors at diagnosis on overall survival in SS has yet to be done.
Methods
This is a retrospective study of patients diagnosed with SS (ICD- 9701/3) between 2004 and 2020 in the National Cancer Database (NCDB), highlighting patient demographics on overall survival in SS (N = 809). Exclusion criteria included missing data. Descriptive statistics were collected for all patients with SS. Overall survival was determined via KaplanMeier test. Multivariate analysis via Cox regression was performed to determine factors leading to decreased survival in SS. All statistical tests were evaluated for a significance of P < 0.05.
Results
Of 809 patients with SS, the majority were White (77.3%), male (57.8%), and had an average age at diagnosis of 66.9 years (SD=13.0). Age at diagnosis was associated with decreased overall survival (HR 0.028; 95% CI, 1.016 – 1.042, P< 0.05). Patients with SS treated at nonacademic facilities had a HR of 0.41 (95% CI, 1.171 – 1.932, P< 0.05) compared to academic facilities. Those with private insurance had improved survival with a HR of -0.83 [95% CI, (-0.241) - (-0.781), P< 0.05] compared to those who were non-insured. The average survival time for patients with SS was found to be 73.1 months. The average survival time for patients treated at academic facilities was 8.8 months longer than those treated at nonacademic facilities (75.0 vs 66.2 months, P< 0.05). Patients with private insurance had higher overall survival compared to government-insured and non-insured patients (100.4 versus 56.9 and 54.2 months, respectively). Age, facility type, and primary payor are significant factors that affect survival in SS. Further studies should address the influence of these factors on treatments received by SS patients to decrease disparity related to care.
Background
This study investigates the effects of patient characteristics on overall survival in Sezary Syndrome (SS), addressing a gap in the current literature. SS is a rare and aggressive form of cutaneous T-cell lymphoma (CTCL). SS is presumed to be related to service exposure, and veterans have a 6-8 times higher incidence of CTCL than the general population. A study investigating the socio-demographic factors at diagnosis on overall survival in SS has yet to be done.
Methods
This is a retrospective study of patients diagnosed with SS (ICD- 9701/3) between 2004 and 2020 in the National Cancer Database (NCDB), highlighting patient demographics on overall survival in SS (N = 809). Exclusion criteria included missing data. Descriptive statistics were collected for all patients with SS. Overall survival was determined via KaplanMeier test. Multivariate analysis via Cox regression was performed to determine factors leading to decreased survival in SS. All statistical tests were evaluated for a significance of P < 0.05.
Results
Of 809 patients with SS, the majority were White (77.3%), male (57.8%), and had an average age at diagnosis of 66.9 years (SD=13.0). Age at diagnosis was associated with decreased overall survival (HR 0.028; 95% CI, 1.016 – 1.042, P< 0.05). Patients with SS treated at nonacademic facilities had a HR of 0.41 (95% CI, 1.171 – 1.932, P< 0.05) compared to academic facilities. Those with private insurance had improved survival with a HR of -0.83 [95% CI, (-0.241) - (-0.781), P< 0.05] compared to those who were non-insured. The average survival time for patients with SS was found to be 73.1 months. The average survival time for patients treated at academic facilities was 8.8 months longer than those treated at nonacademic facilities (75.0 vs 66.2 months, P< 0.05). Patients with private insurance had higher overall survival compared to government-insured and non-insured patients (100.4 versus 56.9 and 54.2 months, respectively). Age, facility type, and primary payor are significant factors that affect survival in SS. Further studies should address the influence of these factors on treatments received by SS patients to decrease disparity related to care.
Background
This study investigates the effects of patient characteristics on overall survival in Sezary Syndrome (SS), addressing a gap in the current literature. SS is a rare and aggressive form of cutaneous T-cell lymphoma (CTCL). SS is presumed to be related to service exposure, and veterans have a 6-8 times higher incidence of CTCL than the general population. A study investigating the socio-demographic factors at diagnosis on overall survival in SS has yet to be done.
Methods
This is a retrospective study of patients diagnosed with SS (ICD- 9701/3) between 2004 and 2020 in the National Cancer Database (NCDB), highlighting patient demographics on overall survival in SS (N = 809). Exclusion criteria included missing data. Descriptive statistics were collected for all patients with SS. Overall survival was determined via KaplanMeier test. Multivariate analysis via Cox regression was performed to determine factors leading to decreased survival in SS. All statistical tests were evaluated for a significance of P < 0.05.
Results
Of 809 patients with SS, the majority were White (77.3%), male (57.8%), and had an average age at diagnosis of 66.9 years (SD=13.0). Age at diagnosis was associated with decreased overall survival (HR 0.028; 95% CI, 1.016 – 1.042, P< 0.05). Patients with SS treated at nonacademic facilities had a HR of 0.41 (95% CI, 1.171 – 1.932, P< 0.05) compared to academic facilities. Those with private insurance had improved survival with a HR of -0.83 [95% CI, (-0.241) - (-0.781), P< 0.05] compared to those who were non-insured. The average survival time for patients with SS was found to be 73.1 months. The average survival time for patients treated at academic facilities was 8.8 months longer than those treated at nonacademic facilities (75.0 vs 66.2 months, P< 0.05). Patients with private insurance had higher overall survival compared to government-insured and non-insured patients (100.4 versus 56.9 and 54.2 months, respectively). Age, facility type, and primary payor are significant factors that affect survival in SS. Further studies should address the influence of these factors on treatments received by SS patients to decrease disparity related to care.
Surgical Management of SDH-Deficient Gastrointestinal Stromal Tumors (GIST): A National Cancer Database Review
Background
To evaluate factors predicting surgical resection of SDH-deficient GIST and outcomes of adjuvant therapies. SDH-deficient GIST are very rare, comprising 5-7.5% of all GIST and most frequently occurring in the stomach or small bowel. Veterans who were exposed to burn pit toxins have an increased risk of developing these tumors. While most patients undergo surgery, there is limited information available regarding prognosis and survivability
Methods
The National Cancer Database was used to identify patients diagnosed with SDH-deficient GIST from 2004 to 2019 using histology code 8936 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA Chi-Square, and Logistic Regression tests were performed, and data were analyzed using SPSS version 29. Statistical significance was set at α = 0.05.
Results
721 patients with SDH-deficient GIST were queried, with 606 (84.05%) receiving surgical resection. Surgical patients experienced longer overall survival than non-surgical patients (116.3 months vs. 48.05 months, p< 0.001), with 248 (40.9%) patients undergoing a lobectomy and 29 (4.79%) patients undergoing a wedge/segmental resection. Patients who received wedge/segmental resection survived for 109.5 months while those who received a lobectomy survived for 108.6 months. Both surgeries showed a greater survival than other types of resections (p< 0.001). Of the initial sample, 42 (6.93%) patients received adjuvant chemotherapy, 3 (0.50%) patients received adjuvant radiation, and 3 (0.50%) patients received both. None of these adjuvants impacted overall survival. Stage I-II disease and well to moderately differentiated disease predicted an increased likelihood of receiving surgery (p< 0.001), while liver metastases predicted a decreased likelihood of receiving surgery (p< 0.001). Income status, race, insurance, facility type, and age were not significant predicting factors of receiving surgery.
Conclusions
Surgical resection of SDH-deficient GIST is associated with improved overall survival. Adjuvant therapies do not significantly improve survival over surgery alone. Patients with lower stage and grade of disease are more likely to receive surgery, while other social, economic, and demographic factors do not significantly affect the likelihood of receiving surgery. Surgical resection of SDH-deficient GIST is significantly associated with improved overall survival without the need for adjuvant therapies.
Background
To evaluate factors predicting surgical resection of SDH-deficient GIST and outcomes of adjuvant therapies. SDH-deficient GIST are very rare, comprising 5-7.5% of all GIST and most frequently occurring in the stomach or small bowel. Veterans who were exposed to burn pit toxins have an increased risk of developing these tumors. While most patients undergo surgery, there is limited information available regarding prognosis and survivability
Methods
The National Cancer Database was used to identify patients diagnosed with SDH-deficient GIST from 2004 to 2019 using histology code 8936 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA Chi-Square, and Logistic Regression tests were performed, and data were analyzed using SPSS version 29. Statistical significance was set at α = 0.05.
Results
721 patients with SDH-deficient GIST were queried, with 606 (84.05%) receiving surgical resection. Surgical patients experienced longer overall survival than non-surgical patients (116.3 months vs. 48.05 months, p< 0.001), with 248 (40.9%) patients undergoing a lobectomy and 29 (4.79%) patients undergoing a wedge/segmental resection. Patients who received wedge/segmental resection survived for 109.5 months while those who received a lobectomy survived for 108.6 months. Both surgeries showed a greater survival than other types of resections (p< 0.001). Of the initial sample, 42 (6.93%) patients received adjuvant chemotherapy, 3 (0.50%) patients received adjuvant radiation, and 3 (0.50%) patients received both. None of these adjuvants impacted overall survival. Stage I-II disease and well to moderately differentiated disease predicted an increased likelihood of receiving surgery (p< 0.001), while liver metastases predicted a decreased likelihood of receiving surgery (p< 0.001). Income status, race, insurance, facility type, and age were not significant predicting factors of receiving surgery.
Conclusions
Surgical resection of SDH-deficient GIST is associated with improved overall survival. Adjuvant therapies do not significantly improve survival over surgery alone. Patients with lower stage and grade of disease are more likely to receive surgery, while other social, economic, and demographic factors do not significantly affect the likelihood of receiving surgery. Surgical resection of SDH-deficient GIST is significantly associated with improved overall survival without the need for adjuvant therapies.
Background
To evaluate factors predicting surgical resection of SDH-deficient GIST and outcomes of adjuvant therapies. SDH-deficient GIST are very rare, comprising 5-7.5% of all GIST and most frequently occurring in the stomach or small bowel. Veterans who were exposed to burn pit toxins have an increased risk of developing these tumors. While most patients undergo surgery, there is limited information available regarding prognosis and survivability
Methods
The National Cancer Database was used to identify patients diagnosed with SDH-deficient GIST from 2004 to 2019 using histology code 8936 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA Chi-Square, and Logistic Regression tests were performed, and data were analyzed using SPSS version 29. Statistical significance was set at α = 0.05.
Results
721 patients with SDH-deficient GIST were queried, with 606 (84.05%) receiving surgical resection. Surgical patients experienced longer overall survival than non-surgical patients (116.3 months vs. 48.05 months, p< 0.001), with 248 (40.9%) patients undergoing a lobectomy and 29 (4.79%) patients undergoing a wedge/segmental resection. Patients who received wedge/segmental resection survived for 109.5 months while those who received a lobectomy survived for 108.6 months. Both surgeries showed a greater survival than other types of resections (p< 0.001). Of the initial sample, 42 (6.93%) patients received adjuvant chemotherapy, 3 (0.50%) patients received adjuvant radiation, and 3 (0.50%) patients received both. None of these adjuvants impacted overall survival. Stage I-II disease and well to moderately differentiated disease predicted an increased likelihood of receiving surgery (p< 0.001), while liver metastases predicted a decreased likelihood of receiving surgery (p< 0.001). Income status, race, insurance, facility type, and age were not significant predicting factors of receiving surgery.
Conclusions
Surgical resection of SDH-deficient GIST is associated with improved overall survival. Adjuvant therapies do not significantly improve survival over surgery alone. Patients with lower stage and grade of disease are more likely to receive surgery, while other social, economic, and demographic factors do not significantly affect the likelihood of receiving surgery. Surgical resection of SDH-deficient GIST is significantly associated with improved overall survival without the need for adjuvant therapies.
Factors Affecting Academic Center Access in Merkel Cell Carcinoma: An NCDB Analysis
Background
To identify socio-demographic factors affecting academic center access in Merkel cell carcinoma (MCC) patients. MCC is a leading cause of skin cancer death, disproportionately affecting males over 65. Treatment at academic cancer centers has been shown to improve survival in MCC, but there is limited literature on factors affecting accessibility. The National Cancer Database (NCDB) was analyzed to determine variables impacting treatment at academic versus non-academic facility types in MCC.
Methods
A retrospective cohort analysis using the NCDB from 2004 to 2021 included 21,866 patients with histologically confirmed MCC. Socio-demographic factors collected include sex, age, race, Hispanic status, population density, education level, income level, and distance to the treatment facility. Multivariate analysis of factors on academic center facility type was performed via binary logistic regression. Kaplan-Meier curves were used to estimate overall survival. A significance level of 0.05 was used.
Results
The majority of MCC patients were male (63.9%) and treated at a non-academic center (56.7%) with an average age of 74.4 (SD=10.8). Overall survival was significantly better at academic versus non-academic centers (97.1 months vs 80.1 months, P< 0.05). Those who were treated at a non-academic center were older (OR=1.004; CI, 1.001-1.007). Native Americans were 2.06 times as likely to be treated at a non-academic center relative to White patients (95% CI, 1.00-4.24). Patients with median household incomes in the top first or second quartile were 0.62 and 0.86 times as likely to receive treatment at a non-academic center compared to those in the bottom quartile (95% CI, 0.55-0.69 and CI, 0.77- 0.95, respectively). Those who were treated at an academic center lived in an urban area but traveled further for care (P< 0.05; 64.9 miles vs 20.7 miles, respectively).
Conclusions
Treatment at academic centers significantly improves survival in MCC patients. The results show that Native American, low-income, and older patients are undertreated at academic facilities, highlighting inequalities in access to care. Addressing these disparities is crucial for improving overall outcomes in MCC.
Background
To identify socio-demographic factors affecting academic center access in Merkel cell carcinoma (MCC) patients. MCC is a leading cause of skin cancer death, disproportionately affecting males over 65. Treatment at academic cancer centers has been shown to improve survival in MCC, but there is limited literature on factors affecting accessibility. The National Cancer Database (NCDB) was analyzed to determine variables impacting treatment at academic versus non-academic facility types in MCC.
Methods
A retrospective cohort analysis using the NCDB from 2004 to 2021 included 21,866 patients with histologically confirmed MCC. Socio-demographic factors collected include sex, age, race, Hispanic status, population density, education level, income level, and distance to the treatment facility. Multivariate analysis of factors on academic center facility type was performed via binary logistic regression. Kaplan-Meier curves were used to estimate overall survival. A significance level of 0.05 was used.
Results
The majority of MCC patients were male (63.9%) and treated at a non-academic center (56.7%) with an average age of 74.4 (SD=10.8). Overall survival was significantly better at academic versus non-academic centers (97.1 months vs 80.1 months, P< 0.05). Those who were treated at a non-academic center were older (OR=1.004; CI, 1.001-1.007). Native Americans were 2.06 times as likely to be treated at a non-academic center relative to White patients (95% CI, 1.00-4.24). Patients with median household incomes in the top first or second quartile were 0.62 and 0.86 times as likely to receive treatment at a non-academic center compared to those in the bottom quartile (95% CI, 0.55-0.69 and CI, 0.77- 0.95, respectively). Those who were treated at an academic center lived in an urban area but traveled further for care (P< 0.05; 64.9 miles vs 20.7 miles, respectively).
Conclusions
Treatment at academic centers significantly improves survival in MCC patients. The results show that Native American, low-income, and older patients are undertreated at academic facilities, highlighting inequalities in access to care. Addressing these disparities is crucial for improving overall outcomes in MCC.
Background
To identify socio-demographic factors affecting academic center access in Merkel cell carcinoma (MCC) patients. MCC is a leading cause of skin cancer death, disproportionately affecting males over 65. Treatment at academic cancer centers has been shown to improve survival in MCC, but there is limited literature on factors affecting accessibility. The National Cancer Database (NCDB) was analyzed to determine variables impacting treatment at academic versus non-academic facility types in MCC.
Methods
A retrospective cohort analysis using the NCDB from 2004 to 2021 included 21,866 patients with histologically confirmed MCC. Socio-demographic factors collected include sex, age, race, Hispanic status, population density, education level, income level, and distance to the treatment facility. Multivariate analysis of factors on academic center facility type was performed via binary logistic regression. Kaplan-Meier curves were used to estimate overall survival. A significance level of 0.05 was used.
Results
The majority of MCC patients were male (63.9%) and treated at a non-academic center (56.7%) with an average age of 74.4 (SD=10.8). Overall survival was significantly better at academic versus non-academic centers (97.1 months vs 80.1 months, P< 0.05). Those who were treated at a non-academic center were older (OR=1.004; CI, 1.001-1.007). Native Americans were 2.06 times as likely to be treated at a non-academic center relative to White patients (95% CI, 1.00-4.24). Patients with median household incomes in the top first or second quartile were 0.62 and 0.86 times as likely to receive treatment at a non-academic center compared to those in the bottom quartile (95% CI, 0.55-0.69 and CI, 0.77- 0.95, respectively). Those who were treated at an academic center lived in an urban area but traveled further for care (P< 0.05; 64.9 miles vs 20.7 miles, respectively).
Conclusions
Treatment at academic centers significantly improves survival in MCC patients. The results show that Native American, low-income, and older patients are undertreated at academic facilities, highlighting inequalities in access to care. Addressing these disparities is crucial for improving overall outcomes in MCC.
Impact of Facility Type on Survival Outcomes in Pancreatic Neuroendocrine Carcinoma: An Analysis of the National Cancer Database
Background
This study aims to evaluate the impact of treatment facility type on the long-term survival rates of patients with pancreatic neuroendocrine tumors, as well as the demographic and treatment differences between these groups. Pancreatic neuroendocrine tumors are a rare form of pancreatic cancer with a highly variable survival rate. While existing cancer research indicates that patients treated at academic facilities generally experience improved survival outcomes compared to low income patients, there is little research on this topic in the context of pancreatic neuroendocrine tumors.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with pancreatic neuroendocrine carcinoma from 2004 to 2019 using the histology code 8246 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA, and Chi-Square tests were performed. Data was analyzed using SPSS version 27 and statistical significance was set at α = 0.05.
Results
In this analysis of 13,987 patients, 6,957 (49.7%) were treated at academic facilities, while 7,012 (50.3%) were treated at non-academic facilities. Patients treated at academic facilities experienced a significantly increased mean survival rate of 100.5 months following diagnosis compared to the 75.6 month mean survival rate of patients treated at non-academic facilities (p< 0.05). Additionally, patients treated at academic facilities were more likely to be black, have private insurance, undergo surgery, and live in a metropolitan area with a population larger than 1 million (p< 0.05). Conversely, patients treated at non-academic facilities experienced a worse 30-day and 90-day mortality, had a higher average Charlson-Deyo Comorbidity Index, and lived closer to their treatment facility (p< 0.05). Patients’ income did not differ significantly.
Conclusions
This study showed that patients with pancreatic neuroendocrine carcinomas treated at academic facilities experienced a significantly improved overall survival rate compared to low income patients. This disparity may be attributed to differences in rates of surgical intervention or insurance status, among other factors. These observations are based on correlational data, and they underscore the necessity for further investigation to establish causality.
Background
This study aims to evaluate the impact of treatment facility type on the long-term survival rates of patients with pancreatic neuroendocrine tumors, as well as the demographic and treatment differences between these groups. Pancreatic neuroendocrine tumors are a rare form of pancreatic cancer with a highly variable survival rate. While existing cancer research indicates that patients treated at academic facilities generally experience improved survival outcomes compared to low income patients, there is little research on this topic in the context of pancreatic neuroendocrine tumors.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with pancreatic neuroendocrine carcinoma from 2004 to 2019 using the histology code 8246 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA, and Chi-Square tests were performed. Data was analyzed using SPSS version 27 and statistical significance was set at α = 0.05.
Results
In this analysis of 13,987 patients, 6,957 (49.7%) were treated at academic facilities, while 7,012 (50.3%) were treated at non-academic facilities. Patients treated at academic facilities experienced a significantly increased mean survival rate of 100.5 months following diagnosis compared to the 75.6 month mean survival rate of patients treated at non-academic facilities (p< 0.05). Additionally, patients treated at academic facilities were more likely to be black, have private insurance, undergo surgery, and live in a metropolitan area with a population larger than 1 million (p< 0.05). Conversely, patients treated at non-academic facilities experienced a worse 30-day and 90-day mortality, had a higher average Charlson-Deyo Comorbidity Index, and lived closer to their treatment facility (p< 0.05). Patients’ income did not differ significantly.
Conclusions
This study showed that patients with pancreatic neuroendocrine carcinomas treated at academic facilities experienced a significantly improved overall survival rate compared to low income patients. This disparity may be attributed to differences in rates of surgical intervention or insurance status, among other factors. These observations are based on correlational data, and they underscore the necessity for further investigation to establish causality.
Background
This study aims to evaluate the impact of treatment facility type on the long-term survival rates of patients with pancreatic neuroendocrine tumors, as well as the demographic and treatment differences between these groups. Pancreatic neuroendocrine tumors are a rare form of pancreatic cancer with a highly variable survival rate. While existing cancer research indicates that patients treated at academic facilities generally experience improved survival outcomes compared to low income patients, there is little research on this topic in the context of pancreatic neuroendocrine tumors.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with pancreatic neuroendocrine carcinoma from 2004 to 2019 using the histology code 8246 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA, and Chi-Square tests were performed. Data was analyzed using SPSS version 27 and statistical significance was set at α = 0.05.
Results
In this analysis of 13,987 patients, 6,957 (49.7%) were treated at academic facilities, while 7,012 (50.3%) were treated at non-academic facilities. Patients treated at academic facilities experienced a significantly increased mean survival rate of 100.5 months following diagnosis compared to the 75.6 month mean survival rate of patients treated at non-academic facilities (p< 0.05). Additionally, patients treated at academic facilities were more likely to be black, have private insurance, undergo surgery, and live in a metropolitan area with a population larger than 1 million (p< 0.05). Conversely, patients treated at non-academic facilities experienced a worse 30-day and 90-day mortality, had a higher average Charlson-Deyo Comorbidity Index, and lived closer to their treatment facility (p< 0.05). Patients’ income did not differ significantly.
Conclusions
This study showed that patients with pancreatic neuroendocrine carcinomas treated at academic facilities experienced a significantly improved overall survival rate compared to low income patients. This disparity may be attributed to differences in rates of surgical intervention or insurance status, among other factors. These observations are based on correlational data, and they underscore the necessity for further investigation to establish causality.
An NCDB Analysis of Factors Associated With the Receipt of Surgery in Myxoid/Round Cell Liposarcoma
Background
Myxoid/round cell liposarcoma (MRCLS) is a rare soft tissue sarcoma originating from adipocytes and most commonly occurs in patients aged 20 to 40. Though slow-growing, MRCLS has a high propensity to metastasize. Complete surgical resection is central in the treatment of MRCLS. However, no significant study has analyzed the factors that predict the utilization of surgical therapy in MRCLS patients. This study also aims to characterize the effect of different treatment modalities on overall survival of these patients.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with MRCLS from 2004 to 2019 using the histology code 8852 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA Chi-Square, and Multilevel Logistic Regression were performed, and data were analyzed using SPSS version 29. Statistical significance was set at α = 0.05.
Results
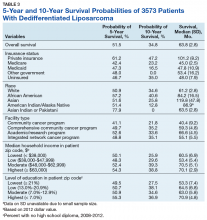
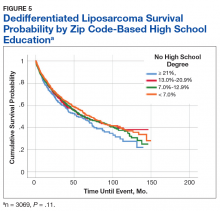
5365 patients with MRCLS were queried. 4811 (89.8%) patients received surgery. Surgical patients experienced greater overall survival compared to nonsurgical patients (159.17 vs 93.72 months, p < 0.001). Wedge/segmental resection (1551 patients, 32.2%) and lobectomy resection (2724 patients, 56.6%) were associated with improved survival over other surgery types (OS =161.0 months, p < 0.001). Private insurance status and care at an academic facility were associated with an increased likelihood of receiving surgery (p< 0.001). Metastasis was associated with a decreased likelihood of receiving surgery (p< 0.001). On nominal regression, grades I-II, stages 1-3, and histologically well to moderately differentiated disease were associated with a greater likelihood of receiving surgery. Adjuvant therapy did not appear to impact survival.
Conclusions
This study reaffirms that tumor resection is associated with increased overall survival in MRCLS patients. Specifically, wedge/segmental and lobectomy surgery types are associated with improved outcomes. It appears that care at an academic facility, private insurance status, lower stage and grade of disease, and well-differentiated histology are correlated to an increased likelihood of receiving surgical treatment. Metastasis is associated with a decreased chance of receiving surgery. This research serves as the start to a better understanding of the factors involved in the receipt of tumor resection, as it is the mainstay of MRCLS treatment.
Background
Myxoid/round cell liposarcoma (MRCLS) is a rare soft tissue sarcoma originating from adipocytes and most commonly occurs in patients aged 20 to 40. Though slow-growing, MRCLS has a high propensity to metastasize. Complete surgical resection is central in the treatment of MRCLS. However, no significant study has analyzed the factors that predict the utilization of surgical therapy in MRCLS patients. This study also aims to characterize the effect of different treatment modalities on overall survival of these patients.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with MRCLS from 2004 to 2019 using the histology code 8852 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA Chi-Square, and Multilevel Logistic Regression were performed, and data were analyzed using SPSS version 29. Statistical significance was set at α = 0.05.
Results
5365 patients with MRCLS were queried. 4811 (89.8%) patients received surgery. Surgical patients experienced greater overall survival compared to nonsurgical patients (159.17 vs 93.72 months, p < 0.001). Wedge/segmental resection (1551 patients, 32.2%) and lobectomy resection (2724 patients, 56.6%) were associated with improved survival over other surgery types (OS =161.0 months, p < 0.001). Private insurance status and care at an academic facility were associated with an increased likelihood of receiving surgery (p< 0.001). Metastasis was associated with a decreased likelihood of receiving surgery (p< 0.001). On nominal regression, grades I-II, stages 1-3, and histologically well to moderately differentiated disease were associated with a greater likelihood of receiving surgery. Adjuvant therapy did not appear to impact survival.
Conclusions
This study reaffirms that tumor resection is associated with increased overall survival in MRCLS patients. Specifically, wedge/segmental and lobectomy surgery types are associated with improved outcomes. It appears that care at an academic facility, private insurance status, lower stage and grade of disease, and well-differentiated histology are correlated to an increased likelihood of receiving surgical treatment. Metastasis is associated with a decreased chance of receiving surgery. This research serves as the start to a better understanding of the factors involved in the receipt of tumor resection, as it is the mainstay of MRCLS treatment.
Background
Myxoid/round cell liposarcoma (MRCLS) is a rare soft tissue sarcoma originating from adipocytes and most commonly occurs in patients aged 20 to 40. Though slow-growing, MRCLS has a high propensity to metastasize. Complete surgical resection is central in the treatment of MRCLS. However, no significant study has analyzed the factors that predict the utilization of surgical therapy in MRCLS patients. This study also aims to characterize the effect of different treatment modalities on overall survival of these patients.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with MRCLS from 2004 to 2019 using the histology code 8852 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, ANOVA Chi-Square, and Multilevel Logistic Regression were performed, and data were analyzed using SPSS version 29. Statistical significance was set at α = 0.05.
Results
5365 patients with MRCLS were queried. 4811 (89.8%) patients received surgery. Surgical patients experienced greater overall survival compared to nonsurgical patients (159.17 vs 93.72 months, p < 0.001). Wedge/segmental resection (1551 patients, 32.2%) and lobectomy resection (2724 patients, 56.6%) were associated with improved survival over other surgery types (OS =161.0 months, p < 0.001). Private insurance status and care at an academic facility were associated with an increased likelihood of receiving surgery (p< 0.001). Metastasis was associated with a decreased likelihood of receiving surgery (p< 0.001). On nominal regression, grades I-II, stages 1-3, and histologically well to moderately differentiated disease were associated with a greater likelihood of receiving surgery. Adjuvant therapy did not appear to impact survival.
Conclusions
This study reaffirms that tumor resection is associated with increased overall survival in MRCLS patients. Specifically, wedge/segmental and lobectomy surgery types are associated with improved outcomes. It appears that care at an academic facility, private insurance status, lower stage and grade of disease, and well-differentiated histology are correlated to an increased likelihood of receiving surgical treatment. Metastasis is associated with a decreased chance of receiving surgery. This research serves as the start to a better understanding of the factors involved in the receipt of tumor resection, as it is the mainstay of MRCLS treatment.
Clear Cell Sarcoma Incidence and Survival: A SEER Database Analysis
Background
Clear cell sarcoma (CCS) is a rare soft tissue cancer that predominantly affects young to middle-aged adults. Current literature lacks recent accurate estimates of patient outcomes due to the disease’s low incidence and the small sample sizes in studies, particularly at a national registry level. This study aims to examine the incidence and survival of patients with CCS.
Methods
Patients from the Surveillance, Epidemiology, and End Results (SEER) database diagnosed with CCS between 2000-2020 were selected. Additional variables were collected including age, sex, race, stage, presence of metastases, tumor size, treatment status for surgery, radiation, and chemotherapy, time to treatment, median household income, and population size. Descriptive statistics, population-based incidence, log-rank tests with Kaplan-Meier curves, and Cox regression analyses were performed.
Results
A total of 287 patients were included. The population-adjusted incidence ranged from 0.012/100000 in 2004 to 0.027/100000 in 2010. The total percent change over the study period was 16.751% and the annual percent change, which did not change significantly over the study period, was 0.561%. The survival rate was 78.4% at one year, 62.0% at three years, and 57.1% at five years. Log-rank results showed Black patients survived shorter than White and Hispanic patients. Further, greater staging and tumor size >4.0cm were associated with shorter survival (p’s< 0.001). After controlling for covariates, Cox regression results showed Black patients were associated with shorter survival compared to White patients (p=0.038, hazard ratio=2.590). No other covariates were significantly associated with survival.
Conclusions
The findings showed CCS incidence is unchanged in recent years and prognosis is poor. Additionally, Black patients were associated with shorter survival duration compared to White patients. Contrary to prior findings on CCS, staging and tumor size were only significantly associated during univariate analyses, but not on Cox regression. The study was limited by a small sample size and variables found in the SEER database. Nonetheless, future research will benefit from assessing how race is an independent risk factor for CCS survival and how the prognosis of CCS patients can be improved.
Background
Clear cell sarcoma (CCS) is a rare soft tissue cancer that predominantly affects young to middle-aged adults. Current literature lacks recent accurate estimates of patient outcomes due to the disease’s low incidence and the small sample sizes in studies, particularly at a national registry level. This study aims to examine the incidence and survival of patients with CCS.
Methods
Patients from the Surveillance, Epidemiology, and End Results (SEER) database diagnosed with CCS between 2000-2020 were selected. Additional variables were collected including age, sex, race, stage, presence of metastases, tumor size, treatment status for surgery, radiation, and chemotherapy, time to treatment, median household income, and population size. Descriptive statistics, population-based incidence, log-rank tests with Kaplan-Meier curves, and Cox regression analyses were performed.
Results
A total of 287 patients were included. The population-adjusted incidence ranged from 0.012/100000 in 2004 to 0.027/100000 in 2010. The total percent change over the study period was 16.751% and the annual percent change, which did not change significantly over the study period, was 0.561%. The survival rate was 78.4% at one year, 62.0% at three years, and 57.1% at five years. Log-rank results showed Black patients survived shorter than White and Hispanic patients. Further, greater staging and tumor size >4.0cm were associated with shorter survival (p’s< 0.001). After controlling for covariates, Cox regression results showed Black patients were associated with shorter survival compared to White patients (p=0.038, hazard ratio=2.590). No other covariates were significantly associated with survival.
Conclusions
The findings showed CCS incidence is unchanged in recent years and prognosis is poor. Additionally, Black patients were associated with shorter survival duration compared to White patients. Contrary to prior findings on CCS, staging and tumor size were only significantly associated during univariate analyses, but not on Cox regression. The study was limited by a small sample size and variables found in the SEER database. Nonetheless, future research will benefit from assessing how race is an independent risk factor for CCS survival and how the prognosis of CCS patients can be improved.
Background
Clear cell sarcoma (CCS) is a rare soft tissue cancer that predominantly affects young to middle-aged adults. Current literature lacks recent accurate estimates of patient outcomes due to the disease’s low incidence and the small sample sizes in studies, particularly at a national registry level. This study aims to examine the incidence and survival of patients with CCS.
Methods
Patients from the Surveillance, Epidemiology, and End Results (SEER) database diagnosed with CCS between 2000-2020 were selected. Additional variables were collected including age, sex, race, stage, presence of metastases, tumor size, treatment status for surgery, radiation, and chemotherapy, time to treatment, median household income, and population size. Descriptive statistics, population-based incidence, log-rank tests with Kaplan-Meier curves, and Cox regression analyses were performed.
Results
A total of 287 patients were included. The population-adjusted incidence ranged from 0.012/100000 in 2004 to 0.027/100000 in 2010. The total percent change over the study period was 16.751% and the annual percent change, which did not change significantly over the study period, was 0.561%. The survival rate was 78.4% at one year, 62.0% at three years, and 57.1% at five years. Log-rank results showed Black patients survived shorter than White and Hispanic patients. Further, greater staging and tumor size >4.0cm were associated with shorter survival (p’s< 0.001). After controlling for covariates, Cox regression results showed Black patients were associated with shorter survival compared to White patients (p=0.038, hazard ratio=2.590). No other covariates were significantly associated with survival.
Conclusions
The findings showed CCS incidence is unchanged in recent years and prognosis is poor. Additionally, Black patients were associated with shorter survival duration compared to White patients. Contrary to prior findings on CCS, staging and tumor size were only significantly associated during univariate analyses, but not on Cox regression. The study was limited by a small sample size and variables found in the SEER database. Nonetheless, future research will benefit from assessing how race is an independent risk factor for CCS survival and how the prognosis of CCS patients can be improved.
Survival and Incidence of Gastric Neuroendocrine Tumors: A SEER Database Analysis
Background
Gastric neuroendocrine tumors (GNETs) are slow-growing tumors derived from enterochromaffinlike cells whose prognosis depends on the type. Prior GNET studies have shown an increasing incidence but survival analyses have been more limited. This study aims to investigate if the increasing incidence trend continues and better describe factors associated with survival for GNET patients.
Methods
Patients diagnosed with GNET between 2000-2020 were selected from the Surveillance, Epidemiology, and End Results (SEER) database. Additional variables collected were age, sex, race, stage, presence of metastases, tumor size, treatment status for surgery, radiation, and chemotherapy, median household income, and population size. Descriptive statistics, population-based incidence, log-rank tests with Kaplan-Meier curves, and Cox regression analyses were performed.
Results
A total of 6512 patients were included. The one-, three-, and five-year survival rates were 90.4%, 85.0%, and 83.8%, respectively. The population-adjusted incidence ranged from 0.272/100000 in 2000 to 0.680/100000 in 2018. The total percent change in incidence over the study range was 104.1% with an annual percent change of 4.27%, which met significance <2.0cm and >5.0cm were associated with shorter survival (p’s< 0.05). Additionally, females, Hispanic patients, and recipients of surgery were associated with longer survival (p’s< 0.05).
Conclusions
The findings show GNET incidence has continued to increase over the past two decades. Additionally, clinical factors including stage, extent of metastasis, tumor size and socioeconomic factors like age, gender, and race were associated with changes in GNET survival. In the context of increasing incidence of GNET these findings describe factors associated with lower- and higher-risk tumors. Further assessment of these risk factors can benefit future research to better understand why GNET incidence is increasing, aid in risk stratification of GNET patients, and improve the prognosis of GNET.
Background
Gastric neuroendocrine tumors (GNETs) are slow-growing tumors derived from enterochromaffinlike cells whose prognosis depends on the type. Prior GNET studies have shown an increasing incidence but survival analyses have been more limited. This study aims to investigate if the increasing incidence trend continues and better describe factors associated with survival for GNET patients.
Methods
Patients diagnosed with GNET between 2000-2020 were selected from the Surveillance, Epidemiology, and End Results (SEER) database. Additional variables collected were age, sex, race, stage, presence of metastases, tumor size, treatment status for surgery, radiation, and chemotherapy, median household income, and population size. Descriptive statistics, population-based incidence, log-rank tests with Kaplan-Meier curves, and Cox regression analyses were performed.
Results
A total of 6512 patients were included. The one-, three-, and five-year survival rates were 90.4%, 85.0%, and 83.8%, respectively. The population-adjusted incidence ranged from 0.272/100000 in 2000 to 0.680/100000 in 2018. The total percent change in incidence over the study range was 104.1% with an annual percent change of 4.27%, which met significance <2.0cm and >5.0cm were associated with shorter survival (p’s< 0.05). Additionally, females, Hispanic patients, and recipients of surgery were associated with longer survival (p’s< 0.05).
Conclusions
The findings show GNET incidence has continued to increase over the past two decades. Additionally, clinical factors including stage, extent of metastasis, tumor size and socioeconomic factors like age, gender, and race were associated with changes in GNET survival. In the context of increasing incidence of GNET these findings describe factors associated with lower- and higher-risk tumors. Further assessment of these risk factors can benefit future research to better understand why GNET incidence is increasing, aid in risk stratification of GNET patients, and improve the prognosis of GNET.
Background
Gastric neuroendocrine tumors (GNETs) are slow-growing tumors derived from enterochromaffinlike cells whose prognosis depends on the type. Prior GNET studies have shown an increasing incidence but survival analyses have been more limited. This study aims to investigate if the increasing incidence trend continues and better describe factors associated with survival for GNET patients.
Methods
Patients diagnosed with GNET between 2000-2020 were selected from the Surveillance, Epidemiology, and End Results (SEER) database. Additional variables collected were age, sex, race, stage, presence of metastases, tumor size, treatment status for surgery, radiation, and chemotherapy, median household income, and population size. Descriptive statistics, population-based incidence, log-rank tests with Kaplan-Meier curves, and Cox regression analyses were performed.
Results
A total of 6512 patients were included. The one-, three-, and five-year survival rates were 90.4%, 85.0%, and 83.8%, respectively. The population-adjusted incidence ranged from 0.272/100000 in 2000 to 0.680/100000 in 2018. The total percent change in incidence over the study range was 104.1% with an annual percent change of 4.27%, which met significance <2.0cm and >5.0cm were associated with shorter survival (p’s< 0.05). Additionally, females, Hispanic patients, and recipients of surgery were associated with longer survival (p’s< 0.05).
Conclusions
The findings show GNET incidence has continued to increase over the past two decades. Additionally, clinical factors including stage, extent of metastasis, tumor size and socioeconomic factors like age, gender, and race were associated with changes in GNET survival. In the context of increasing incidence of GNET these findings describe factors associated with lower- and higher-risk tumors. Further assessment of these risk factors can benefit future research to better understand why GNET incidence is increasing, aid in risk stratification of GNET patients, and improve the prognosis of GNET.
Multimodal Treatment Approaches for Basaloid Squamous Cell Carcinoma of the Larynx
Background
Basaloid squamous cell carcinoma (BSCC) is an aggressive laryngeal cancer with high recurrence and metastasis rates. Its rarity complicates diagnosis and optimal treatment selection, underscoring the significance of comprehensive data collection through national cancer registries. Historically, surgical intervention has been the primary approach to management.The RTOG 91-11 randomized trial catalyzed a paradigm shift, prioritizing laryngealpreserving treatments. The study provided evidence for radiotherapy in early-stage disease (stages 1-2) and combined chemoradiotherapy in advanced disease (stages 3-4). Consequently, organ preservation protocols gained traction, maintaining laryngeal anatomy while achieving comparable oncologic outcomes to total laryngectomy. This shift emphasizes exploring multimodal, laryngeal-sparing regimens to optimize quality of life without compromising disease control. However, further research utilizing large databases is needed to elucidate survival outcomes associated with these approaches.
Methods
We used the National Cancer Database to identify patients diagnosed with BSCC of the larynx (ICD-O-3 histology code 8083) between 2004-2019 (Nf1487). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p< 0.05.
Results
For early-stage patients, the estimated survival was 93.179 months. Surgery demonstrated the most favorable outcome with a median survival of 100.957 months, significantly higher than non-surgical patients (85.895 months, p=0.028). Survival did not differ between patients who received only chemotherapy (p=0.281), radiation (p=0.326), or chemoradiation (p=0.919) and those received other treatment modalities. In late-stage patients, the estimated survival was 61.993 months. Surgery yielded the most favorable outcome with a median survival of 70.484 months, significantly higher than non-surgical patients (54.153 months, p< 0.001). Patients who received only chemotherapy (p< 0.001), radiation (p< 0.001) and chemoradiation (p=0.24) had a worse survival outcome compared to those who received other treatment modalities.
Conclusions
The study results indicate that surgical resection could potentially improve survival outcomes for patients diagnosed with advanced-stage laryngeal BSCC. Conversely, for those with earlystage BSCC, larynx-preserving treatment modalities such as radiation, chemotherapy or concurrent chemoradiation appear to achieve comparable survival rates to primary surgical management. These results highlight the importance of careful consideration of treatment modalities based on disease staging at initial presentation.
Background
Basaloid squamous cell carcinoma (BSCC) is an aggressive laryngeal cancer with high recurrence and metastasis rates. Its rarity complicates diagnosis and optimal treatment selection, underscoring the significance of comprehensive data collection through national cancer registries. Historically, surgical intervention has been the primary approach to management.The RTOG 91-11 randomized trial catalyzed a paradigm shift, prioritizing laryngealpreserving treatments. The study provided evidence for radiotherapy in early-stage disease (stages 1-2) and combined chemoradiotherapy in advanced disease (stages 3-4). Consequently, organ preservation protocols gained traction, maintaining laryngeal anatomy while achieving comparable oncologic outcomes to total laryngectomy. This shift emphasizes exploring multimodal, laryngeal-sparing regimens to optimize quality of life without compromising disease control. However, further research utilizing large databases is needed to elucidate survival outcomes associated with these approaches.
Methods
We used the National Cancer Database to identify patients diagnosed with BSCC of the larynx (ICD-O-3 histology code 8083) between 2004-2019 (Nf1487). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p< 0.05.
Results
For early-stage patients, the estimated survival was 93.179 months. Surgery demonstrated the most favorable outcome with a median survival of 100.957 months, significantly higher than non-surgical patients (85.895 months, p=0.028). Survival did not differ between patients who received only chemotherapy (p=0.281), radiation (p=0.326), or chemoradiation (p=0.919) and those received other treatment modalities. In late-stage patients, the estimated survival was 61.993 months. Surgery yielded the most favorable outcome with a median survival of 70.484 months, significantly higher than non-surgical patients (54.153 months, p< 0.001). Patients who received only chemotherapy (p< 0.001), radiation (p< 0.001) and chemoradiation (p=0.24) had a worse survival outcome compared to those who received other treatment modalities.
Conclusions
The study results indicate that surgical resection could potentially improve survival outcomes for patients diagnosed with advanced-stage laryngeal BSCC. Conversely, for those with earlystage BSCC, larynx-preserving treatment modalities such as radiation, chemotherapy or concurrent chemoradiation appear to achieve comparable survival rates to primary surgical management. These results highlight the importance of careful consideration of treatment modalities based on disease staging at initial presentation.
Background
Basaloid squamous cell carcinoma (BSCC) is an aggressive laryngeal cancer with high recurrence and metastasis rates. Its rarity complicates diagnosis and optimal treatment selection, underscoring the significance of comprehensive data collection through national cancer registries. Historically, surgical intervention has been the primary approach to management.The RTOG 91-11 randomized trial catalyzed a paradigm shift, prioritizing laryngealpreserving treatments. The study provided evidence for radiotherapy in early-stage disease (stages 1-2) and combined chemoradiotherapy in advanced disease (stages 3-4). Consequently, organ preservation protocols gained traction, maintaining laryngeal anatomy while achieving comparable oncologic outcomes to total laryngectomy. This shift emphasizes exploring multimodal, laryngeal-sparing regimens to optimize quality of life without compromising disease control. However, further research utilizing large databases is needed to elucidate survival outcomes associated with these approaches.
Methods
We used the National Cancer Database to identify patients diagnosed with BSCC of the larynx (ICD-O-3 histology code 8083) between 2004-2019 (Nf1487). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p< 0.05.
Results
For early-stage patients, the estimated survival was 93.179 months. Surgery demonstrated the most favorable outcome with a median survival of 100.957 months, significantly higher than non-surgical patients (85.895 months, p=0.028). Survival did not differ between patients who received only chemotherapy (p=0.281), radiation (p=0.326), or chemoradiation (p=0.919) and those received other treatment modalities. In late-stage patients, the estimated survival was 61.993 months. Surgery yielded the most favorable outcome with a median survival of 70.484 months, significantly higher than non-surgical patients (54.153 months, p< 0.001). Patients who received only chemotherapy (p< 0.001), radiation (p< 0.001) and chemoradiation (p=0.24) had a worse survival outcome compared to those who received other treatment modalities.
Conclusions
The study results indicate that surgical resection could potentially improve survival outcomes for patients diagnosed with advanced-stage laryngeal BSCC. Conversely, for those with earlystage BSCC, larynx-preserving treatment modalities such as radiation, chemotherapy or concurrent chemoradiation appear to achieve comparable survival rates to primary surgical management. These results highlight the importance of careful consideration of treatment modalities based on disease staging at initial presentation.
Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate (FULL)
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
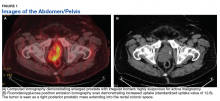
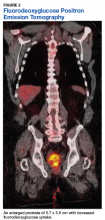
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Treatment Facility: An Important Prognostic Factor for Dedifferentiated Liposarcoma Survival (FULL)
Approximately 17% to 25% of all softtissue sarcomas (STS) are liposarcomas, making liposarcoma the most common type of STS.1 The 2013 World Health Organization (WHO) classification separates liposarcoma into 4 histologic subtypes: atypical lipomatous tumor/well-differentiated (ALT/ WDLPS), dedifferentiated (DDLPS), myxoid, and pleomorphic.2 Each subtype has unique histology, morphology, and natural history. WDLPS and DDLPS are the most common histologic subtypes, comprising approximately 50% of all sarcomas that arise in the retroperitoneum.3 DDLPS represents 18% of all liposarcomas, making it the second most common subtype of liposarcoma.4
In 1979, DDLPS was first characterized.5 Most (90%) cases of DDLPS present de novo, whereas the other 10% transform from preexisting low-grade WDLPS.2 DDLPSs are formed by an amplification of 12q14-15 involving the MDM2 gene.4 These malignancies most commonly present in the retroperitoneum as a large painless mass, consisting of both fatty and nonfatty components.2 Primary site has been previously reported as a major prognostic factor for DDLPSs, with retroperitoneal DDLPSs demonstrating the worst prognosis.6 DDLPSs have a high risk of local recurrence, with some reports estimating recurrence rates approaching 40%.2 Overall mortality at 5 years for DDLPS is estimated to be between 30% and 40%.4
Previous literature has determined that median income, race, health insurance, and facility type are related to survival outcomes for patients with DDLPS.7-9 When comparing the most common types of cancers, residents of poorer US counties consistently had a higher risk of mortality than residents in affluent US counties, and all racial minorities showed worse survival outcomes when compared with white patients.7 Differences in survival outcomes have been reported in patients attending different treatment facilities for other cancers including pancreatic cancers, glioblastomas, and oral cancers, with multiple studies concluding that academic and research programs are associated with the longest survival outcomes.10-12 For many cancers, insurance status has been shown to be a significant prognostic factor, with private insurance typically resulting in the best prognosis.8,9
The goal of this retrospective study was to assess the prognostic effects of socioeconomic variables on the overall survival (OS) probabilities in a large cohort of DDLPS patients in order to inform clinicians about a potentially at-risk population.
Method
The National Cancer Database (NCDB) was created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is the largest cancer database in the US and includes data on almost 70% of US patients with cancer. CoC-accredited cancer programs add data on patients with cancer to the NCDB. The authors accessed the NCDB data through the use of the NCDB Participant Use File program.
Patients’ data from 2004 through 2015 were abstracted. Only patients with the International Classification of Diseases for Oncology histology code 8858, corresponding to DDLPS, were analyzed. Patients with other comorbid malignant tumors were excluded to accurately capture the true survival rates for DDLPS. Variables analyzed included age, sex, race, insurance status, treatment facility type, median household income by zip code, and percentage of adults in the patient’s zip code with no high school (HS) education.
Median survival, 5- and 10-year OS probabilities, and Kaplan-Meier survival curves were calculated for multiple variables, specifically race, insurance status, treatment facility type, median family income, and percentage of adults without a HS degree. Both 5- and 10-year OS probabilities were determined by race with the patients separated into white, African American, Asian, American Indian/Alaska Native (AI/AN), and Asian Indian or Pakistani groups. Our study categorized Chinese, Japanese, Filipino, Hmong, Korean, Vietnamese, Thai, Guamanian, Asian not otherwise specified, and other Asian ethnicity patients together into one collective Asian group. Insurance status was classified into Medicare, Medicaid, other government insurance, and private insurance groups. Other government insurance consisted of US Department of Veterans Affairs, Indian Health Service, Public Health Service, and other government health care programs. Further analysis could not be performed into the distribution of the other government insurance variable.
Facility types were divided into 4 groups: community, comprehensive community, academic/ research, and integrated network cancer treatment facilities. Median income quartiles and the percentage of adults with no high school degree were estimated by comparison of the patient’s zip code with US Census Bureau data. Median household income was separated into 4 groups, including lowest level of household income (< $38,000), low level of household income ($38,000 to $47,999), moderate level of household income ($48,000 to $62,999), and highest level of household income (≥ $63,000). The percentages of adults with no high school degree were divided into 4 groups: lowest level of HS education (≥ 21% ), low level of HS education (13.0% to 20.9%), moderate level of HS education (7.0% to 12.9%), and highest level of HS education (≤ 7%). The 5- and 10-year survival probabilities were calculated using the number of months between the date of diagnosis and the date of death or last known contact.
Continuous variables are presented as median and interquartile range (IQR) whereas categorical variables are presented as frequencies and proportion. IBM SPSS version 25.0 was used to produce Kaplan-Meier survival curves and descriptive statistics. This study used Kaplan- Meier survival tables and log-rank tests to analyze both the 5- and 10-year OS rates for the 5 variables listed above. This study also used a multivariable Cox regression model that accommodated the correlative nature of outcomes within facilities to study the association of the treatment facility type and other socioeconomic factors, while controlling for age, race (which was collapsed into 3 categories), sex, primary site, tumor stage, and treatment approaches. The proportional hazards assumption was individually checked for all pertinent variables. Any patient records that were missing data were excluded from the multivariable Cox regression model, which was analyzed with SAS version 9.4 (Cary, NC). P < 0.05 was used to indicate statistical significance for all analyses.
Results
Table 1 provides descriptive analysis for demographic characteristics of the 3573 patients including age, sex, and race. The median age at diagnosis was 64 years. There were 1073 more men (65%) than women (35%) in this analysis. Whites were the predominant racial category, comprising 87.7% of the patient population, followed by African Americans (6.5%) and Asians (2.5%).
Socioeconomic Variables
The largest proportion of the patient population (45.5%) had private insurance (Table 2). Medicare came in a close second covering almost 42.2% of the population, followed by Medicaid (5.0%), uninsured (2.8%), and other government insurance (1.5%). About half (53.7%) of the patients were treated at academic or research facilities, while the fewest number of patients (5.2%) underwent treatment at community cancer facilities. The largest percentage (36.6%) of patients lived in zip codes with the highest level of median household income, while 26.0% and 22.3% had moderate and low levels of income, respectively. About 14% of patients lived within an area of the lowest level of income. Similarly, almost 15% of patients lived in an area of lowest level of HS education. The greatest percentage of the patient population (34.5%) lived in a zip code with moderate level of HS education. Surgery was the most common treatment modality with 90.8% of the cohort undergoing surgery, while 35.4% and 16.5% were treated with radiation and chemotherapy, respectively (some patients received more than one type of treatment modality).
Survival Data
Survival data were available for 3112 patients. Kaplan-Meier survival curves were used to analyze OS according to insurance status, racial background, treatment facility type, median family income, and percentage of adults with no high school education. Overall 5- and 10- year OS probabilities were 51.5% and 34.8%, respectively, while the median OS (SD) was 63.57 (2.8) months (Table 3).
Private insurance showed significantly higher 5- and 10-year OS probabilities and median OS: 5-year OS was 61.2%, 10-year OS was 47.2%, and median survival (SD) was 101.2 (8.2) months compared with that of all other insurance groups (Medicare, Medicaid, other government insurance, and uninsured) (Figure 1). These other insurance types were fairly similar in their 5-year and median OS, but surprisingly, patients with no insurance had the second longest 10-year OS. The difference between the 5-year OS probabilities of private insurance compared with an average of the other insurances was 15.1%, which had almost doubled to 28.5% at 10 years, with a median OS difference of almost 5 years (56 months; data not shown).
Using the Kaplan-Meier survival curve, Asian Indians had the longest 5-year OS probability of 77.9% and African Americans had the longest 10-year OS probability of 40.6%. However, Asians as a group demonstrated the longest median (SD) OS outcome with 119.8 (47.8) months (Figure 2).
Overall, academic/research programs had the longest median OS and 5-year OS probability (SD) of 66.6 (4.5) months and 52.6%, respectively (Figure 3). Comprehensive community cancer programs and integrated network cancer programs had nearly identical 10-year OS rates (35.2% vs 35.1%, respectively). Community cancer programs had the worst 5- and 10-year OS probabilities (41.1% and 21.8%, respectively).
The top 2 income quartiles combined to demonstrate the longest median, 5-year, and 10-year OS probabilities and were very similar. Patients living in a zip code with the highest income level had the longest 5-year OS rates of 54.3%, while patients living in zip codes with a moderate income level had the longest 10-year OS at 39.3% and the longest median OS of about 71 months. Patients with the lowest level of median household income had the worst 5-year OS rates (48.3%) and a median (SD) OS of 53.4 (5.4) months (Figure 4).
A Kaplan-Meier curve for percentage of adults without a HS degree is displayed in Figure 5. Zip codes with the highest level of education had the longest 5-year OS rates and median (SD) OS of 55.3% and 70.9 (4.8) months, respectively. The longest 10-year OS outcomes at 38.1% were found in patients who lived in areas of low-education levels. The worst 5- and 10- year OS outcomes and median OS were found in the least educated zip codes.
Results from the Cox regression model of OS are displayed in Table 4. Race and ethnicity, zip code-level median household income, and zip code-level education were not associated with OS. Patients with no insurance had an increased risk of death (hazard ratio [HR], 1.84; 95% CI, 1.17-2.88; P < .01) when compared with patients with private insurance. Patients with other government insurance also had an increased risk of death (HR, 2.12; 95% CI, 1.27-3.54; P < .01) when compared with patients with private insurance while controlling for all other variables. Patients with Medicare had a decreased risk of death when compared with patients with other government insurance and no insurance (HR, 0.53; 95% CI, 0.31-0.92; P = .02 and HR, 0.62; 95% CI, 0.38-0.99; P = .05, respectively). Patients treated at academic centers had better OS when compared with patients treated at comprehensive treatment centers (HR, 0.77; 95% CI, 0.65-0.92;P < .01) and community treatment centers (HR, 0.62; 95% CI, 0.44-0.86; P < .01).
Discussion
This study is the largest study to date that specifically studies the type of treatment facilities and socioeconomic factors, including insurance status, race, income, and education, and how they affect survival of DDLPS. The overall 5- and 10-year OS probabilities for DDLPS in this study were 51.5% and 34.8%, respectively, with median OS of 63.6 months. These results were more encouraging than previous reports, which found a 5-year survival probability of 36.5% and a median OS of 45 months.13,14
The largest age grouping was aged 61 to 80 years (48.9% of the cohort), and the median age at diagnosis was 64 years. DDLPSs most typically present between the ages of 50 and 70 years.15 Our cohort was 65% male. Previous studies have indicated that DDLPSs affect the sexes equally; however, another study showed a similar male predominance (68.8%) at the MD Anderson Cancer Center in Houston, Texas.13,16
In our study, approximately 88% of patients were white, 6.5% were African American, and 2.5% were Asian, which differed from a previous study of 84 patients that had a 78.6% white, 4.8% Asian, and 1.2% African American patient population.14
Asian Indian or Pakistani patients had the best 5-year OS probability at 77.9%, followed by African American (57.2%), Asian (51.6%), AI/AN (51.4%), and white patients (50.9%). This trend had disappeared by 10 years and Asian, AI/AN, African American, and Asian Indian or Pakistani groups all demonstrated longer median OS than did white patients. In fact, Asian patients had the longest median OS at 119.8 months, which was almost double that of white patients with the lowest median OS of 61.2 months. This finding is contrary to previous studies, which reported that racial minorities typically had worse OS outcomes when compared with white patients in different types of cancer.7,17 Notably, these findings were not statistically significant in our current study in the log-rank or multivariable analyses.
Private insurance was the most common form of insurance followed in decreasing order by Medicare, Medicaid, uninsured, and other government insurance. About 42% of the cohort had Medicare, which is a federally funded US insurance program designated for patients aged ≥ 65 years and certain younger patients with disabilities.
Patients with private insurance demonstrated the longest OS, essentially twice the median OS of all other insured groups at 101 months. Medicare had the worst 5-year OS probability and median OS of all groups. A previous study of 77 patients with DDLPS reported that patients aged > 65 years had reduced OS.13 Medicare patients in this study were older, with a mean and median age at DDLPS diagnosis of 71 and 72 years, respectively, while private insurance had a mean and median age at diagnosis of 56 and 57 years, respectively. Medicare inherently covers older patients and this age difference could account for the decrease in overall survival.
Improved OS for privately insured patients was most notable compared with the uninsured or patients with other government insurance. Uninsured patients had an 83.7% increased risk of mortality when compared with patients with private insurance. When compared with patients with private insurance, patients with other government insurance had an 111.5% increased risk of mortality. Comparing patients with Medicare vs patients with no insurance or other government insurance, there was a decreased risk of mortality of 38.5% and 46.6%, respectively. This decreased OS in patients with other government insurance could be related to the choice of treatment facility, because only 31% of the patients with other government insurance went to academic or research centers when compared with the 58.4% and 50.8% of patients with private and Medicare insurance treated there (data not shown). Such centers often have access to more advanced technology and protocols that may not be available at other treatment facilities.
A little more than half of the patients in the cohort went to an academic or research center for treatment (53.7%); comprehensive community cancer programs were the second most common treatment facility at 28%. Patients treated at academic or research centers demonstrated the best outcomes with a 5-year OS of 52.6%, followed in decreasing order by comprehensive community cancer programs (49.7%), integrated network cancer programs (48.8%), and community cancer programs (41.1%). In our patient cocohort, patients treated at an academic/research center had slightly decreased 10-year OS rates compared with those patients treated at a comprehensive community cancer program, although the median OS for the academic/research centers were still the highest of all treatment facilities.
Treatment options varied significantly by facility, and the number of patients treated surgically followed a similar trend, with 92% undergoing surgery as the primary treatment at academic or research programs compared with 89% at comprehensive cancer programs and 82.7% at community cancer programs (data not shown). Another potential explaination for differing OS outcomes across facilities is the surgical margin outcome. Surgeries performed at community cancer programs or comprehensive cancer programs resulted with no residual tumor in 36% and 40% of cases, respectively, whereas cases performed at academic or research programs resulted with no residual tumor in 47% of cases (data not shown). Regardless, multivariate analysis demonstrated a marked decrease in the chance of mortality when comparing treatment received at academic facility centers with that received at comprehensive cancer centers (22.9%) and community cancer centers (38.3%) (data not shown).
A recent study demonstrated improved outcomes for patients with retroperitoneal or extremity STS treated at high-volume treatment centers.18 Patients treated at high-volume centers were found to have an 8% decreased risk of death compared with patients treated at low-volume centers. Notably, they found highvolume academic centers demonstrated the strongest improvement in survival, while highvolume community centers showed decreased survival.18 Similarly, we found that patients treated at academic/research institutions had improved 5-year OS and greater median OS than did patients treated at community cancer programs or comprehensive community cancer programs.
The top 2 income quartiles (≥ $48,000) combined to demonstrate the longest median, 5-year, and 10-year OS and were fairly similar between the quartiles. Patients living in zip codes with a median income of $38,000 to $47,999 had the worst 5-year OS and median OS. The log-rank analysis showed statistical evidence of differences in survival associated with income, but within the context of the multivariable analysis, there was no remaining evidence of a difference.
The longest 5-year OS outcomes were seen in patients living in zip codes with the highest level of education (55.3%). However, the difference in OS was not statistically significant using either the log-rank analysis or multivariate analysis.
Limitations
This study has certain inherent limitations in using a retrospective design and a large database such as the NCDB. Many different pathologists at CoC-accredited cancer programs perform the pathology that contributes to the data in the NCDB. There was no pathological review of these findings, which could potentially introduce error into the findings of this study. With the NCDB, potential selection bias is possible because patients in the database are added only from CoC-accredited cancer programs. This risk is minimized because NCDB contains data on most newly diagnosed cancer patients in the US. Further potential risks, which are unable to be controlled for, include potential interobserver error and data that may be incompletely, improperly, or inaccurately recorded from the patients’ charts. Without patient-specific information regarding income and education, it is challenging to utilize zip codes to estimate socioeconomic status and educational level. Even though a patient may live in a zip code identified with specific economic and educational characteristics, that patient may not share those characteristics. Furthermore, patients with Medicare tend to be older than patients with other forms of insurance, which limits the significance of comparisons across insurance groups. A future SEER (Surveillance, Epidemiology, and End Results) program study to confirm this study’s results and the effects of socioeconomic variables on DDLPS would be an excellent followup study.
Conclusion
This study used a large cohort of patients with DDLPS to study the effects of treatment facility, insurance status, and socioeconomic variables on survival outcomes. Although insurance status, median household income, and treatment facility were associated with differences in median OS and 5- and 10-year OS probabilities, evidence for a difference remained for only insurance status and facility type within the context of a multivariable analysis irrespective of age, race, sex, insurance status, education, and median income. Patients with private insurance and Medicaid had a decreased risk of mortality compared with other government insurance and no insurance. Patients receiving treatment at academic research programs had the highest median and 5-year OS of 66.6 months and 52.6%, respectively. Patients receiving treatment at academic centers had improved survival outcomes with a decrease in mortality of 23% and 38% compared to comprehensive or community cancer programs.
1. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol. 2012;40(12):1122-1131.
2. Mangham D. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Am. 2004;86(3):466.
3. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
4. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456(2):167-179.
5. Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
6. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271-281.
7. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93.
8. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231.
9. Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411.
10. Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103-110.
11. Chu Q, Medeiros K, Zhou M, et al. Effect of facility type on outcome following pancreatectomy for pancreatic adenocarcinoma: analysis of the National Cancer Data Base [Abstract FP26-02]. HPB (Oxford). 2016;18(suppl 1):E81-E82.
12. Rubin SJ, Cohen MB, Kirke DN, Qureshi MM, Truong MT, Jalisi S. Comparison of facility type outcomes for oral cavity cancer: analysis of the National Cancer Database. Laryngoscope. 2017;127(11):2551-2557.
13. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15(6):1585-1593.
14. Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836.
15. Brennan MF, Antonescu CR, Alektiar KM, Maki RG. Management of Soft Tissue Sarcoma. 2nd ed. New York, NY: Springer; 2016.
16. Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2014.
17. White A, Djenaba J, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123 (suppl 24):5014-5036.
18. Murphy JD, Padwal J, Guss ZD, Okamoto K, Sardar R. Impact of hospital volume on patterns of care and outcomes in soft tissue sarcoma [ASCO Abstract e23550]. J Clin Oncol. 2018;36(suppl 15):e23550
Approximately 17% to 25% of all softtissue sarcomas (STS) are liposarcomas, making liposarcoma the most common type of STS.1 The 2013 World Health Organization (WHO) classification separates liposarcoma into 4 histologic subtypes: atypical lipomatous tumor/well-differentiated (ALT/ WDLPS), dedifferentiated (DDLPS), myxoid, and pleomorphic.2 Each subtype has unique histology, morphology, and natural history. WDLPS and DDLPS are the most common histologic subtypes, comprising approximately 50% of all sarcomas that arise in the retroperitoneum.3 DDLPS represents 18% of all liposarcomas, making it the second most common subtype of liposarcoma.4
In 1979, DDLPS was first characterized.5 Most (90%) cases of DDLPS present de novo, whereas the other 10% transform from preexisting low-grade WDLPS.2 DDLPSs are formed by an amplification of 12q14-15 involving the MDM2 gene.4 These malignancies most commonly present in the retroperitoneum as a large painless mass, consisting of both fatty and nonfatty components.2 Primary site has been previously reported as a major prognostic factor for DDLPSs, with retroperitoneal DDLPSs demonstrating the worst prognosis.6 DDLPSs have a high risk of local recurrence, with some reports estimating recurrence rates approaching 40%.2 Overall mortality at 5 years for DDLPS is estimated to be between 30% and 40%.4
Previous literature has determined that median income, race, health insurance, and facility type are related to survival outcomes for patients with DDLPS.7-9 When comparing the most common types of cancers, residents of poorer US counties consistently had a higher risk of mortality than residents in affluent US counties, and all racial minorities showed worse survival outcomes when compared with white patients.7 Differences in survival outcomes have been reported in patients attending different treatment facilities for other cancers including pancreatic cancers, glioblastomas, and oral cancers, with multiple studies concluding that academic and research programs are associated with the longest survival outcomes.10-12 For many cancers, insurance status has been shown to be a significant prognostic factor, with private insurance typically resulting in the best prognosis.8,9
The goal of this retrospective study was to assess the prognostic effects of socioeconomic variables on the overall survival (OS) probabilities in a large cohort of DDLPS patients in order to inform clinicians about a potentially at-risk population.
Method
The National Cancer Database (NCDB) was created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is the largest cancer database in the US and includes data on almost 70% of US patients with cancer. CoC-accredited cancer programs add data on patients with cancer to the NCDB. The authors accessed the NCDB data through the use of the NCDB Participant Use File program.
Patients’ data from 2004 through 2015 were abstracted. Only patients with the International Classification of Diseases for Oncology histology code 8858, corresponding to DDLPS, were analyzed. Patients with other comorbid malignant tumors were excluded to accurately capture the true survival rates for DDLPS. Variables analyzed included age, sex, race, insurance status, treatment facility type, median household income by zip code, and percentage of adults in the patient’s zip code with no high school (HS) education.
Median survival, 5- and 10-year OS probabilities, and Kaplan-Meier survival curves were calculated for multiple variables, specifically race, insurance status, treatment facility type, median family income, and percentage of adults without a HS degree. Both 5- and 10-year OS probabilities were determined by race with the patients separated into white, African American, Asian, American Indian/Alaska Native (AI/AN), and Asian Indian or Pakistani groups. Our study categorized Chinese, Japanese, Filipino, Hmong, Korean, Vietnamese, Thai, Guamanian, Asian not otherwise specified, and other Asian ethnicity patients together into one collective Asian group. Insurance status was classified into Medicare, Medicaid, other government insurance, and private insurance groups. Other government insurance consisted of US Department of Veterans Affairs, Indian Health Service, Public Health Service, and other government health care programs. Further analysis could not be performed into the distribution of the other government insurance variable.
Facility types were divided into 4 groups: community, comprehensive community, academic/ research, and integrated network cancer treatment facilities. Median income quartiles and the percentage of adults with no high school degree were estimated by comparison of the patient’s zip code with US Census Bureau data. Median household income was separated into 4 groups, including lowest level of household income (< $38,000), low level of household income ($38,000 to $47,999), moderate level of household income ($48,000 to $62,999), and highest level of household income (≥ $63,000). The percentages of adults with no high school degree were divided into 4 groups: lowest level of HS education (≥ 21% ), low level of HS education (13.0% to 20.9%), moderate level of HS education (7.0% to 12.9%), and highest level of HS education (≤ 7%). The 5- and 10-year survival probabilities were calculated using the number of months between the date of diagnosis and the date of death or last known contact.
Continuous variables are presented as median and interquartile range (IQR) whereas categorical variables are presented as frequencies and proportion. IBM SPSS version 25.0 was used to produce Kaplan-Meier survival curves and descriptive statistics. This study used Kaplan- Meier survival tables and log-rank tests to analyze both the 5- and 10-year OS rates for the 5 variables listed above. This study also used a multivariable Cox regression model that accommodated the correlative nature of outcomes within facilities to study the association of the treatment facility type and other socioeconomic factors, while controlling for age, race (which was collapsed into 3 categories), sex, primary site, tumor stage, and treatment approaches. The proportional hazards assumption was individually checked for all pertinent variables. Any patient records that were missing data were excluded from the multivariable Cox regression model, which was analyzed with SAS version 9.4 (Cary, NC). P < 0.05 was used to indicate statistical significance for all analyses.
Results
Table 1 provides descriptive analysis for demographic characteristics of the 3573 patients including age, sex, and race. The median age at diagnosis was 64 years. There were 1073 more men (65%) than women (35%) in this analysis. Whites were the predominant racial category, comprising 87.7% of the patient population, followed by African Americans (6.5%) and Asians (2.5%).
Socioeconomic Variables
The largest proportion of the patient population (45.5%) had private insurance (Table 2). Medicare came in a close second covering almost 42.2% of the population, followed by Medicaid (5.0%), uninsured (2.8%), and other government insurance (1.5%). About half (53.7%) of the patients were treated at academic or research facilities, while the fewest number of patients (5.2%) underwent treatment at community cancer facilities. The largest percentage (36.6%) of patients lived in zip codes with the highest level of median household income, while 26.0% and 22.3% had moderate and low levels of income, respectively. About 14% of patients lived within an area of the lowest level of income. Similarly, almost 15% of patients lived in an area of lowest level of HS education. The greatest percentage of the patient population (34.5%) lived in a zip code with moderate level of HS education. Surgery was the most common treatment modality with 90.8% of the cohort undergoing surgery, while 35.4% and 16.5% were treated with radiation and chemotherapy, respectively (some patients received more than one type of treatment modality).
Survival Data
Survival data were available for 3112 patients. Kaplan-Meier survival curves were used to analyze OS according to insurance status, racial background, treatment facility type, median family income, and percentage of adults with no high school education. Overall 5- and 10- year OS probabilities were 51.5% and 34.8%, respectively, while the median OS (SD) was 63.57 (2.8) months (Table 3).
Private insurance showed significantly higher 5- and 10-year OS probabilities and median OS: 5-year OS was 61.2%, 10-year OS was 47.2%, and median survival (SD) was 101.2 (8.2) months compared with that of all other insurance groups (Medicare, Medicaid, other government insurance, and uninsured) (Figure 1). These other insurance types were fairly similar in their 5-year and median OS, but surprisingly, patients with no insurance had the second longest 10-year OS. The difference between the 5-year OS probabilities of private insurance compared with an average of the other insurances was 15.1%, which had almost doubled to 28.5% at 10 years, with a median OS difference of almost 5 years (56 months; data not shown).
Using the Kaplan-Meier survival curve, Asian Indians had the longest 5-year OS probability of 77.9% and African Americans had the longest 10-year OS probability of 40.6%. However, Asians as a group demonstrated the longest median (SD) OS outcome with 119.8 (47.8) months (Figure 2).
Overall, academic/research programs had the longest median OS and 5-year OS probability (SD) of 66.6 (4.5) months and 52.6%, respectively (Figure 3). Comprehensive community cancer programs and integrated network cancer programs had nearly identical 10-year OS rates (35.2% vs 35.1%, respectively). Community cancer programs had the worst 5- and 10-year OS probabilities (41.1% and 21.8%, respectively).
The top 2 income quartiles combined to demonstrate the longest median, 5-year, and 10-year OS probabilities and were very similar. Patients living in a zip code with the highest income level had the longest 5-year OS rates of 54.3%, while patients living in zip codes with a moderate income level had the longest 10-year OS at 39.3% and the longest median OS of about 71 months. Patients with the lowest level of median household income had the worst 5-year OS rates (48.3%) and a median (SD) OS of 53.4 (5.4) months (Figure 4).
A Kaplan-Meier curve for percentage of adults without a HS degree is displayed in Figure 5. Zip codes with the highest level of education had the longest 5-year OS rates and median (SD) OS of 55.3% and 70.9 (4.8) months, respectively. The longest 10-year OS outcomes at 38.1% were found in patients who lived in areas of low-education levels. The worst 5- and 10- year OS outcomes and median OS were found in the least educated zip codes.
Results from the Cox regression model of OS are displayed in Table 4. Race and ethnicity, zip code-level median household income, and zip code-level education were not associated with OS. Patients with no insurance had an increased risk of death (hazard ratio [HR], 1.84; 95% CI, 1.17-2.88; P < .01) when compared with patients with private insurance. Patients with other government insurance also had an increased risk of death (HR, 2.12; 95% CI, 1.27-3.54; P < .01) when compared with patients with private insurance while controlling for all other variables. Patients with Medicare had a decreased risk of death when compared with patients with other government insurance and no insurance (HR, 0.53; 95% CI, 0.31-0.92; P = .02 and HR, 0.62; 95% CI, 0.38-0.99; P = .05, respectively). Patients treated at academic centers had better OS when compared with patients treated at comprehensive treatment centers (HR, 0.77; 95% CI, 0.65-0.92;P < .01) and community treatment centers (HR, 0.62; 95% CI, 0.44-0.86; P < .01).
Discussion
This study is the largest study to date that specifically studies the type of treatment facilities and socioeconomic factors, including insurance status, race, income, and education, and how they affect survival of DDLPS. The overall 5- and 10-year OS probabilities for DDLPS in this study were 51.5% and 34.8%, respectively, with median OS of 63.6 months. These results were more encouraging than previous reports, which found a 5-year survival probability of 36.5% and a median OS of 45 months.13,14
The largest age grouping was aged 61 to 80 years (48.9% of the cohort), and the median age at diagnosis was 64 years. DDLPSs most typically present between the ages of 50 and 70 years.15 Our cohort was 65% male. Previous studies have indicated that DDLPSs affect the sexes equally; however, another study showed a similar male predominance (68.8%) at the MD Anderson Cancer Center in Houston, Texas.13,16
In our study, approximately 88% of patients were white, 6.5% were African American, and 2.5% were Asian, which differed from a previous study of 84 patients that had a 78.6% white, 4.8% Asian, and 1.2% African American patient population.14
Asian Indian or Pakistani patients had the best 5-year OS probability at 77.9%, followed by African American (57.2%), Asian (51.6%), AI/AN (51.4%), and white patients (50.9%). This trend had disappeared by 10 years and Asian, AI/AN, African American, and Asian Indian or Pakistani groups all demonstrated longer median OS than did white patients. In fact, Asian patients had the longest median OS at 119.8 months, which was almost double that of white patients with the lowest median OS of 61.2 months. This finding is contrary to previous studies, which reported that racial minorities typically had worse OS outcomes when compared with white patients in different types of cancer.7,17 Notably, these findings were not statistically significant in our current study in the log-rank or multivariable analyses.
Private insurance was the most common form of insurance followed in decreasing order by Medicare, Medicaid, uninsured, and other government insurance. About 42% of the cohort had Medicare, which is a federally funded US insurance program designated for patients aged ≥ 65 years and certain younger patients with disabilities.
Patients with private insurance demonstrated the longest OS, essentially twice the median OS of all other insured groups at 101 months. Medicare had the worst 5-year OS probability and median OS of all groups. A previous study of 77 patients with DDLPS reported that patients aged > 65 years had reduced OS.13 Medicare patients in this study were older, with a mean and median age at DDLPS diagnosis of 71 and 72 years, respectively, while private insurance had a mean and median age at diagnosis of 56 and 57 years, respectively. Medicare inherently covers older patients and this age difference could account for the decrease in overall survival.
Improved OS for privately insured patients was most notable compared with the uninsured or patients with other government insurance. Uninsured patients had an 83.7% increased risk of mortality when compared with patients with private insurance. When compared with patients with private insurance, patients with other government insurance had an 111.5% increased risk of mortality. Comparing patients with Medicare vs patients with no insurance or other government insurance, there was a decreased risk of mortality of 38.5% and 46.6%, respectively. This decreased OS in patients with other government insurance could be related to the choice of treatment facility, because only 31% of the patients with other government insurance went to academic or research centers when compared with the 58.4% and 50.8% of patients with private and Medicare insurance treated there (data not shown). Such centers often have access to more advanced technology and protocols that may not be available at other treatment facilities.
A little more than half of the patients in the cohort went to an academic or research center for treatment (53.7%); comprehensive community cancer programs were the second most common treatment facility at 28%. Patients treated at academic or research centers demonstrated the best outcomes with a 5-year OS of 52.6%, followed in decreasing order by comprehensive community cancer programs (49.7%), integrated network cancer programs (48.8%), and community cancer programs (41.1%). In our patient cocohort, patients treated at an academic/research center had slightly decreased 10-year OS rates compared with those patients treated at a comprehensive community cancer program, although the median OS for the academic/research centers were still the highest of all treatment facilities.
Treatment options varied significantly by facility, and the number of patients treated surgically followed a similar trend, with 92% undergoing surgery as the primary treatment at academic or research programs compared with 89% at comprehensive cancer programs and 82.7% at community cancer programs (data not shown). Another potential explaination for differing OS outcomes across facilities is the surgical margin outcome. Surgeries performed at community cancer programs or comprehensive cancer programs resulted with no residual tumor in 36% and 40% of cases, respectively, whereas cases performed at academic or research programs resulted with no residual tumor in 47% of cases (data not shown). Regardless, multivariate analysis demonstrated a marked decrease in the chance of mortality when comparing treatment received at academic facility centers with that received at comprehensive cancer centers (22.9%) and community cancer centers (38.3%) (data not shown).
A recent study demonstrated improved outcomes for patients with retroperitoneal or extremity STS treated at high-volume treatment centers.18 Patients treated at high-volume centers were found to have an 8% decreased risk of death compared with patients treated at low-volume centers. Notably, they found highvolume academic centers demonstrated the strongest improvement in survival, while highvolume community centers showed decreased survival.18 Similarly, we found that patients treated at academic/research institutions had improved 5-year OS and greater median OS than did patients treated at community cancer programs or comprehensive community cancer programs.
The top 2 income quartiles (≥ $48,000) combined to demonstrate the longest median, 5-year, and 10-year OS and were fairly similar between the quartiles. Patients living in zip codes with a median income of $38,000 to $47,999 had the worst 5-year OS and median OS. The log-rank analysis showed statistical evidence of differences in survival associated with income, but within the context of the multivariable analysis, there was no remaining evidence of a difference.
The longest 5-year OS outcomes were seen in patients living in zip codes with the highest level of education (55.3%). However, the difference in OS was not statistically significant using either the log-rank analysis or multivariate analysis.
Limitations
This study has certain inherent limitations in using a retrospective design and a large database such as the NCDB. Many different pathologists at CoC-accredited cancer programs perform the pathology that contributes to the data in the NCDB. There was no pathological review of these findings, which could potentially introduce error into the findings of this study. With the NCDB, potential selection bias is possible because patients in the database are added only from CoC-accredited cancer programs. This risk is minimized because NCDB contains data on most newly diagnosed cancer patients in the US. Further potential risks, which are unable to be controlled for, include potential interobserver error and data that may be incompletely, improperly, or inaccurately recorded from the patients’ charts. Without patient-specific information regarding income and education, it is challenging to utilize zip codes to estimate socioeconomic status and educational level. Even though a patient may live in a zip code identified with specific economic and educational characteristics, that patient may not share those characteristics. Furthermore, patients with Medicare tend to be older than patients with other forms of insurance, which limits the significance of comparisons across insurance groups. A future SEER (Surveillance, Epidemiology, and End Results) program study to confirm this study’s results and the effects of socioeconomic variables on DDLPS would be an excellent followup study.
Conclusion
This study used a large cohort of patients with DDLPS to study the effects of treatment facility, insurance status, and socioeconomic variables on survival outcomes. Although insurance status, median household income, and treatment facility were associated with differences in median OS and 5- and 10-year OS probabilities, evidence for a difference remained for only insurance status and facility type within the context of a multivariable analysis irrespective of age, race, sex, insurance status, education, and median income. Patients with private insurance and Medicaid had a decreased risk of mortality compared with other government insurance and no insurance. Patients receiving treatment at academic research programs had the highest median and 5-year OS of 66.6 months and 52.6%, respectively. Patients receiving treatment at academic centers had improved survival outcomes with a decrease in mortality of 23% and 38% compared to comprehensive or community cancer programs.
Approximately 17% to 25% of all softtissue sarcomas (STS) are liposarcomas, making liposarcoma the most common type of STS.1 The 2013 World Health Organization (WHO) classification separates liposarcoma into 4 histologic subtypes: atypical lipomatous tumor/well-differentiated (ALT/ WDLPS), dedifferentiated (DDLPS), myxoid, and pleomorphic.2 Each subtype has unique histology, morphology, and natural history. WDLPS and DDLPS are the most common histologic subtypes, comprising approximately 50% of all sarcomas that arise in the retroperitoneum.3 DDLPS represents 18% of all liposarcomas, making it the second most common subtype of liposarcoma.4
In 1979, DDLPS was first characterized.5 Most (90%) cases of DDLPS present de novo, whereas the other 10% transform from preexisting low-grade WDLPS.2 DDLPSs are formed by an amplification of 12q14-15 involving the MDM2 gene.4 These malignancies most commonly present in the retroperitoneum as a large painless mass, consisting of both fatty and nonfatty components.2 Primary site has been previously reported as a major prognostic factor for DDLPSs, with retroperitoneal DDLPSs demonstrating the worst prognosis.6 DDLPSs have a high risk of local recurrence, with some reports estimating recurrence rates approaching 40%.2 Overall mortality at 5 years for DDLPS is estimated to be between 30% and 40%.4
Previous literature has determined that median income, race, health insurance, and facility type are related to survival outcomes for patients with DDLPS.7-9 When comparing the most common types of cancers, residents of poorer US counties consistently had a higher risk of mortality than residents in affluent US counties, and all racial minorities showed worse survival outcomes when compared with white patients.7 Differences in survival outcomes have been reported in patients attending different treatment facilities for other cancers including pancreatic cancers, glioblastomas, and oral cancers, with multiple studies concluding that academic and research programs are associated with the longest survival outcomes.10-12 For many cancers, insurance status has been shown to be a significant prognostic factor, with private insurance typically resulting in the best prognosis.8,9
The goal of this retrospective study was to assess the prognostic effects of socioeconomic variables on the overall survival (OS) probabilities in a large cohort of DDLPS patients in order to inform clinicians about a potentially at-risk population.
Method
The National Cancer Database (NCDB) was created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is the largest cancer database in the US and includes data on almost 70% of US patients with cancer. CoC-accredited cancer programs add data on patients with cancer to the NCDB. The authors accessed the NCDB data through the use of the NCDB Participant Use File program.
Patients’ data from 2004 through 2015 were abstracted. Only patients with the International Classification of Diseases for Oncology histology code 8858, corresponding to DDLPS, were analyzed. Patients with other comorbid malignant tumors were excluded to accurately capture the true survival rates for DDLPS. Variables analyzed included age, sex, race, insurance status, treatment facility type, median household income by zip code, and percentage of adults in the patient’s zip code with no high school (HS) education.
Median survival, 5- and 10-year OS probabilities, and Kaplan-Meier survival curves were calculated for multiple variables, specifically race, insurance status, treatment facility type, median family income, and percentage of adults without a HS degree. Both 5- and 10-year OS probabilities were determined by race with the patients separated into white, African American, Asian, American Indian/Alaska Native (AI/AN), and Asian Indian or Pakistani groups. Our study categorized Chinese, Japanese, Filipino, Hmong, Korean, Vietnamese, Thai, Guamanian, Asian not otherwise specified, and other Asian ethnicity patients together into one collective Asian group. Insurance status was classified into Medicare, Medicaid, other government insurance, and private insurance groups. Other government insurance consisted of US Department of Veterans Affairs, Indian Health Service, Public Health Service, and other government health care programs. Further analysis could not be performed into the distribution of the other government insurance variable.
Facility types were divided into 4 groups: community, comprehensive community, academic/ research, and integrated network cancer treatment facilities. Median income quartiles and the percentage of adults with no high school degree were estimated by comparison of the patient’s zip code with US Census Bureau data. Median household income was separated into 4 groups, including lowest level of household income (< $38,000), low level of household income ($38,000 to $47,999), moderate level of household income ($48,000 to $62,999), and highest level of household income (≥ $63,000). The percentages of adults with no high school degree were divided into 4 groups: lowest level of HS education (≥ 21% ), low level of HS education (13.0% to 20.9%), moderate level of HS education (7.0% to 12.9%), and highest level of HS education (≤ 7%). The 5- and 10-year survival probabilities were calculated using the number of months between the date of diagnosis and the date of death or last known contact.
Continuous variables are presented as median and interquartile range (IQR) whereas categorical variables are presented as frequencies and proportion. IBM SPSS version 25.0 was used to produce Kaplan-Meier survival curves and descriptive statistics. This study used Kaplan- Meier survival tables and log-rank tests to analyze both the 5- and 10-year OS rates for the 5 variables listed above. This study also used a multivariable Cox regression model that accommodated the correlative nature of outcomes within facilities to study the association of the treatment facility type and other socioeconomic factors, while controlling for age, race (which was collapsed into 3 categories), sex, primary site, tumor stage, and treatment approaches. The proportional hazards assumption was individually checked for all pertinent variables. Any patient records that were missing data were excluded from the multivariable Cox regression model, which was analyzed with SAS version 9.4 (Cary, NC). P < 0.05 was used to indicate statistical significance for all analyses.
Results
Table 1 provides descriptive analysis for demographic characteristics of the 3573 patients including age, sex, and race. The median age at diagnosis was 64 years. There were 1073 more men (65%) than women (35%) in this analysis. Whites were the predominant racial category, comprising 87.7% of the patient population, followed by African Americans (6.5%) and Asians (2.5%).
Socioeconomic Variables
The largest proportion of the patient population (45.5%) had private insurance (Table 2). Medicare came in a close second covering almost 42.2% of the population, followed by Medicaid (5.0%), uninsured (2.8%), and other government insurance (1.5%). About half (53.7%) of the patients were treated at academic or research facilities, while the fewest number of patients (5.2%) underwent treatment at community cancer facilities. The largest percentage (36.6%) of patients lived in zip codes with the highest level of median household income, while 26.0% and 22.3% had moderate and low levels of income, respectively. About 14% of patients lived within an area of the lowest level of income. Similarly, almost 15% of patients lived in an area of lowest level of HS education. The greatest percentage of the patient population (34.5%) lived in a zip code with moderate level of HS education. Surgery was the most common treatment modality with 90.8% of the cohort undergoing surgery, while 35.4% and 16.5% were treated with radiation and chemotherapy, respectively (some patients received more than one type of treatment modality).
Survival Data
Survival data were available for 3112 patients. Kaplan-Meier survival curves were used to analyze OS according to insurance status, racial background, treatment facility type, median family income, and percentage of adults with no high school education. Overall 5- and 10- year OS probabilities were 51.5% and 34.8%, respectively, while the median OS (SD) was 63.57 (2.8) months (Table 3).
Private insurance showed significantly higher 5- and 10-year OS probabilities and median OS: 5-year OS was 61.2%, 10-year OS was 47.2%, and median survival (SD) was 101.2 (8.2) months compared with that of all other insurance groups (Medicare, Medicaid, other government insurance, and uninsured) (Figure 1). These other insurance types were fairly similar in their 5-year and median OS, but surprisingly, patients with no insurance had the second longest 10-year OS. The difference between the 5-year OS probabilities of private insurance compared with an average of the other insurances was 15.1%, which had almost doubled to 28.5% at 10 years, with a median OS difference of almost 5 years (56 months; data not shown).
Using the Kaplan-Meier survival curve, Asian Indians had the longest 5-year OS probability of 77.9% and African Americans had the longest 10-year OS probability of 40.6%. However, Asians as a group demonstrated the longest median (SD) OS outcome with 119.8 (47.8) months (Figure 2).
Overall, academic/research programs had the longest median OS and 5-year OS probability (SD) of 66.6 (4.5) months and 52.6%, respectively (Figure 3). Comprehensive community cancer programs and integrated network cancer programs had nearly identical 10-year OS rates (35.2% vs 35.1%, respectively). Community cancer programs had the worst 5- and 10-year OS probabilities (41.1% and 21.8%, respectively).
The top 2 income quartiles combined to demonstrate the longest median, 5-year, and 10-year OS probabilities and were very similar. Patients living in a zip code with the highest income level had the longest 5-year OS rates of 54.3%, while patients living in zip codes with a moderate income level had the longest 10-year OS at 39.3% and the longest median OS of about 71 months. Patients with the lowest level of median household income had the worst 5-year OS rates (48.3%) and a median (SD) OS of 53.4 (5.4) months (Figure 4).
A Kaplan-Meier curve for percentage of adults without a HS degree is displayed in Figure 5. Zip codes with the highest level of education had the longest 5-year OS rates and median (SD) OS of 55.3% and 70.9 (4.8) months, respectively. The longest 10-year OS outcomes at 38.1% were found in patients who lived in areas of low-education levels. The worst 5- and 10- year OS outcomes and median OS were found in the least educated zip codes.
Results from the Cox regression model of OS are displayed in Table 4. Race and ethnicity, zip code-level median household income, and zip code-level education were not associated with OS. Patients with no insurance had an increased risk of death (hazard ratio [HR], 1.84; 95% CI, 1.17-2.88; P < .01) when compared with patients with private insurance. Patients with other government insurance also had an increased risk of death (HR, 2.12; 95% CI, 1.27-3.54; P < .01) when compared with patients with private insurance while controlling for all other variables. Patients with Medicare had a decreased risk of death when compared with patients with other government insurance and no insurance (HR, 0.53; 95% CI, 0.31-0.92; P = .02 and HR, 0.62; 95% CI, 0.38-0.99; P = .05, respectively). Patients treated at academic centers had better OS when compared with patients treated at comprehensive treatment centers (HR, 0.77; 95% CI, 0.65-0.92;P < .01) and community treatment centers (HR, 0.62; 95% CI, 0.44-0.86; P < .01).
Discussion
This study is the largest study to date that specifically studies the type of treatment facilities and socioeconomic factors, including insurance status, race, income, and education, and how they affect survival of DDLPS. The overall 5- and 10-year OS probabilities for DDLPS in this study were 51.5% and 34.8%, respectively, with median OS of 63.6 months. These results were more encouraging than previous reports, which found a 5-year survival probability of 36.5% and a median OS of 45 months.13,14
The largest age grouping was aged 61 to 80 years (48.9% of the cohort), and the median age at diagnosis was 64 years. DDLPSs most typically present between the ages of 50 and 70 years.15 Our cohort was 65% male. Previous studies have indicated that DDLPSs affect the sexes equally; however, another study showed a similar male predominance (68.8%) at the MD Anderson Cancer Center in Houston, Texas.13,16
In our study, approximately 88% of patients were white, 6.5% were African American, and 2.5% were Asian, which differed from a previous study of 84 patients that had a 78.6% white, 4.8% Asian, and 1.2% African American patient population.14
Asian Indian or Pakistani patients had the best 5-year OS probability at 77.9%, followed by African American (57.2%), Asian (51.6%), AI/AN (51.4%), and white patients (50.9%). This trend had disappeared by 10 years and Asian, AI/AN, African American, and Asian Indian or Pakistani groups all demonstrated longer median OS than did white patients. In fact, Asian patients had the longest median OS at 119.8 months, which was almost double that of white patients with the lowest median OS of 61.2 months. This finding is contrary to previous studies, which reported that racial minorities typically had worse OS outcomes when compared with white patients in different types of cancer.7,17 Notably, these findings were not statistically significant in our current study in the log-rank or multivariable analyses.
Private insurance was the most common form of insurance followed in decreasing order by Medicare, Medicaid, uninsured, and other government insurance. About 42% of the cohort had Medicare, which is a federally funded US insurance program designated for patients aged ≥ 65 years and certain younger patients with disabilities.
Patients with private insurance demonstrated the longest OS, essentially twice the median OS of all other insured groups at 101 months. Medicare had the worst 5-year OS probability and median OS of all groups. A previous study of 77 patients with DDLPS reported that patients aged > 65 years had reduced OS.13 Medicare patients in this study were older, with a mean and median age at DDLPS diagnosis of 71 and 72 years, respectively, while private insurance had a mean and median age at diagnosis of 56 and 57 years, respectively. Medicare inherently covers older patients and this age difference could account for the decrease in overall survival.
Improved OS for privately insured patients was most notable compared with the uninsured or patients with other government insurance. Uninsured patients had an 83.7% increased risk of mortality when compared with patients with private insurance. When compared with patients with private insurance, patients with other government insurance had an 111.5% increased risk of mortality. Comparing patients with Medicare vs patients with no insurance or other government insurance, there was a decreased risk of mortality of 38.5% and 46.6%, respectively. This decreased OS in patients with other government insurance could be related to the choice of treatment facility, because only 31% of the patients with other government insurance went to academic or research centers when compared with the 58.4% and 50.8% of patients with private and Medicare insurance treated there (data not shown). Such centers often have access to more advanced technology and protocols that may not be available at other treatment facilities.
A little more than half of the patients in the cohort went to an academic or research center for treatment (53.7%); comprehensive community cancer programs were the second most common treatment facility at 28%. Patients treated at academic or research centers demonstrated the best outcomes with a 5-year OS of 52.6%, followed in decreasing order by comprehensive community cancer programs (49.7%), integrated network cancer programs (48.8%), and community cancer programs (41.1%). In our patient cocohort, patients treated at an academic/research center had slightly decreased 10-year OS rates compared with those patients treated at a comprehensive community cancer program, although the median OS for the academic/research centers were still the highest of all treatment facilities.
Treatment options varied significantly by facility, and the number of patients treated surgically followed a similar trend, with 92% undergoing surgery as the primary treatment at academic or research programs compared with 89% at comprehensive cancer programs and 82.7% at community cancer programs (data not shown). Another potential explaination for differing OS outcomes across facilities is the surgical margin outcome. Surgeries performed at community cancer programs or comprehensive cancer programs resulted with no residual tumor in 36% and 40% of cases, respectively, whereas cases performed at academic or research programs resulted with no residual tumor in 47% of cases (data not shown). Regardless, multivariate analysis demonstrated a marked decrease in the chance of mortality when comparing treatment received at academic facility centers with that received at comprehensive cancer centers (22.9%) and community cancer centers (38.3%) (data not shown).
A recent study demonstrated improved outcomes for patients with retroperitoneal or extremity STS treated at high-volume treatment centers.18 Patients treated at high-volume centers were found to have an 8% decreased risk of death compared with patients treated at low-volume centers. Notably, they found highvolume academic centers demonstrated the strongest improvement in survival, while highvolume community centers showed decreased survival.18 Similarly, we found that patients treated at academic/research institutions had improved 5-year OS and greater median OS than did patients treated at community cancer programs or comprehensive community cancer programs.
The top 2 income quartiles (≥ $48,000) combined to demonstrate the longest median, 5-year, and 10-year OS and were fairly similar between the quartiles. Patients living in zip codes with a median income of $38,000 to $47,999 had the worst 5-year OS and median OS. The log-rank analysis showed statistical evidence of differences in survival associated with income, but within the context of the multivariable analysis, there was no remaining evidence of a difference.
The longest 5-year OS outcomes were seen in patients living in zip codes with the highest level of education (55.3%). However, the difference in OS was not statistically significant using either the log-rank analysis or multivariate analysis.
Limitations
This study has certain inherent limitations in using a retrospective design and a large database such as the NCDB. Many different pathologists at CoC-accredited cancer programs perform the pathology that contributes to the data in the NCDB. There was no pathological review of these findings, which could potentially introduce error into the findings of this study. With the NCDB, potential selection bias is possible because patients in the database are added only from CoC-accredited cancer programs. This risk is minimized because NCDB contains data on most newly diagnosed cancer patients in the US. Further potential risks, which are unable to be controlled for, include potential interobserver error and data that may be incompletely, improperly, or inaccurately recorded from the patients’ charts. Without patient-specific information regarding income and education, it is challenging to utilize zip codes to estimate socioeconomic status and educational level. Even though a patient may live in a zip code identified with specific economic and educational characteristics, that patient may not share those characteristics. Furthermore, patients with Medicare tend to be older than patients with other forms of insurance, which limits the significance of comparisons across insurance groups. A future SEER (Surveillance, Epidemiology, and End Results) program study to confirm this study’s results and the effects of socioeconomic variables on DDLPS would be an excellent followup study.
Conclusion
This study used a large cohort of patients with DDLPS to study the effects of treatment facility, insurance status, and socioeconomic variables on survival outcomes. Although insurance status, median household income, and treatment facility were associated with differences in median OS and 5- and 10-year OS probabilities, evidence for a difference remained for only insurance status and facility type within the context of a multivariable analysis irrespective of age, race, sex, insurance status, education, and median income. Patients with private insurance and Medicaid had a decreased risk of mortality compared with other government insurance and no insurance. Patients receiving treatment at academic research programs had the highest median and 5-year OS of 66.6 months and 52.6%, respectively. Patients receiving treatment at academic centers had improved survival outcomes with a decrease in mortality of 23% and 38% compared to comprehensive or community cancer programs.
1. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol. 2012;40(12):1122-1131.
2. Mangham D. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Am. 2004;86(3):466.
3. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
4. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456(2):167-179.
5. Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
6. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271-281.
7. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93.
8. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231.
9. Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411.
10. Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103-110.
11. Chu Q, Medeiros K, Zhou M, et al. Effect of facility type on outcome following pancreatectomy for pancreatic adenocarcinoma: analysis of the National Cancer Data Base [Abstract FP26-02]. HPB (Oxford). 2016;18(suppl 1):E81-E82.
12. Rubin SJ, Cohen MB, Kirke DN, Qureshi MM, Truong MT, Jalisi S. Comparison of facility type outcomes for oral cavity cancer: analysis of the National Cancer Database. Laryngoscope. 2017;127(11):2551-2557.
13. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15(6):1585-1593.
14. Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836.
15. Brennan MF, Antonescu CR, Alektiar KM, Maki RG. Management of Soft Tissue Sarcoma. 2nd ed. New York, NY: Springer; 2016.
16. Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2014.
17. White A, Djenaba J, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123 (suppl 24):5014-5036.
18. Murphy JD, Padwal J, Guss ZD, Okamoto K, Sardar R. Impact of hospital volume on patterns of care and outcomes in soft tissue sarcoma [ASCO Abstract e23550]. J Clin Oncol. 2018;36(suppl 15):e23550
1. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol. 2012;40(12):1122-1131.
2. Mangham D. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Am. 2004;86(3):466.
3. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
4. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456(2):167-179.
5. Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
6. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271-281.
7. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93.
8. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231.
9. Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411.
10. Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103-110.
11. Chu Q, Medeiros K, Zhou M, et al. Effect of facility type on outcome following pancreatectomy for pancreatic adenocarcinoma: analysis of the National Cancer Data Base [Abstract FP26-02]. HPB (Oxford). 2016;18(suppl 1):E81-E82.
12. Rubin SJ, Cohen MB, Kirke DN, Qureshi MM, Truong MT, Jalisi S. Comparison of facility type outcomes for oral cavity cancer: analysis of the National Cancer Database. Laryngoscope. 2017;127(11):2551-2557.
13. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15(6):1585-1593.
14. Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836.
15. Brennan MF, Antonescu CR, Alektiar KM, Maki RG. Management of Soft Tissue Sarcoma. 2nd ed. New York, NY: Springer; 2016.
16. Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2014.
17. White A, Djenaba J, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123 (suppl 24):5014-5036.
18. Murphy JD, Padwal J, Guss ZD, Okamoto K, Sardar R. Impact of hospital volume on patterns of care and outcomes in soft tissue sarcoma [ASCO Abstract e23550]. J Clin Oncol. 2018;36(suppl 15):e23550