User login
Reproductive safety of treatments for women with bipolar disorder
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Blood pressure meds tied to increased schizophrenia risk
ACE inhibitors may be associated with an increased risk for schizophrenia and may affect psychiatric symptoms, new research suggests.
Investigators found individuals who carry a genetic variant associated with lower levels of the ACE gene and protein have increased liability to schizophrenia, suggesting that drugs that lower ACE levels or activity may do the same.
“Our findings warrant further investigation into the role of ACE in schizophrenia and closer monitoring by clinicians of individuals, especially those with schizophrenia, who may be on medication that lower ACE activity, such as ACE inhibitors,” Sonia Shah, PhD, Institute for Biomedical Sciences, University of Queensland, Brisbane, Australia, said in an interview.
The study was published online March 10, 2021, in JAMA Psychiatry.
Antihypertensives and mental illness
Hypertension is common in patients with psychiatric disorders and observational studies have reported associations between antihypertensive medication and these disorders, although the findings have been mixed.
Dr. Shah and colleagues estimated the potential of different antihypertensive drug classes on schizophrenia, bipolar disorder, and major depressive disorder.
In a two-sample Mendelian randomization study, they evaluated ties between a single-nucleotide variant and drug-target gene expression derived from expression quantitative trait loci data in blood (sample 1) and the SNV disease association from published case-control, genomewide association studies (sample 2).
The analyses included 40,675 patients with schizophrenia and 64,643 controls; 20,352 with bipolar disorder and 31,358 controls; and 135,458 with major depressive disorder and 344,901 controls.
The major finding was that a one standard deviation–lower expression of the ACE gene in blood was associated with lower systolic blood pressure of 4.0 mm Hg (95% confidence interval, 2.7-5.3), but also an increased risk of schizophrenia (odds ratio, 1.75; 95% CI, 1.28-2.38).
Could ACE inhibitors worsen symptoms or trigger episodes?
In their article, the researchers noted that, in most patients, onset of schizophrenia occurs in late adolescence or early adult life, ruling out ACE inhibitor treatment as a potential causal factor for most cases.
“However, if lower ACE levels play a causal role for schizophrenia risk, it would be reasonable to hypothesize that further lowering of ACE activity in existing patients could worsen symptoms or trigger a new episode,” they wrote.
Dr. Shah emphasized that evidence from genetic analyses alone is “not sufficient to justify changes in prescription guidelines.”
“Patients should not stop taking these medications if they are effective at controlling their blood pressure and they don’t suffer any adverse effects. But it would be reasonable to encourage greater pharmacovigilance,” she said in an interview.
“One way in which we are hoping to follow up these findings,” said Dr. Shah, “is to access electronic health record data for millions of individuals to investigate if there is evidence of increased rates of psychotic episodes in individuals who use ACE inhibitors, compared to other classes of blood pressure–lowering medication.”
Caution warranted
Reached for comment, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Staten Island University Hospital in New York, noted that this is an “extremely complicated” study and urged caution in interpreting the results.
“Since most people develop schizophrenia earlier in life, before they usually develop problems with blood pressure, it’s not so much that these drugs might cause schizophrenia,” Dr. Sullivan said.
“But because of their effects on this particular gene, there’s a possibility that they might worsen symptoms or in somebody with borderline risk might cause them to develop symptoms later in life. This may apply to a relatively small number of people who develop symptoms of schizophrenia in their 40s and beyond,” he added.
That’s where “pharmacovigilance” comes into play, Dr. Sullivan said. “In other words, that they otherwise wouldn’t experience?”
Support for the study was provided by the National Health and Medical Research Council (Australia) and U.S. National Institute for Mental Health. Dr. Shah and Dr. Sullivan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ACE inhibitors may be associated with an increased risk for schizophrenia and may affect psychiatric symptoms, new research suggests.
Investigators found individuals who carry a genetic variant associated with lower levels of the ACE gene and protein have increased liability to schizophrenia, suggesting that drugs that lower ACE levels or activity may do the same.
“Our findings warrant further investigation into the role of ACE in schizophrenia and closer monitoring by clinicians of individuals, especially those with schizophrenia, who may be on medication that lower ACE activity, such as ACE inhibitors,” Sonia Shah, PhD, Institute for Biomedical Sciences, University of Queensland, Brisbane, Australia, said in an interview.
The study was published online March 10, 2021, in JAMA Psychiatry.
Antihypertensives and mental illness
Hypertension is common in patients with psychiatric disorders and observational studies have reported associations between antihypertensive medication and these disorders, although the findings have been mixed.
Dr. Shah and colleagues estimated the potential of different antihypertensive drug classes on schizophrenia, bipolar disorder, and major depressive disorder.
In a two-sample Mendelian randomization study, they evaluated ties between a single-nucleotide variant and drug-target gene expression derived from expression quantitative trait loci data in blood (sample 1) and the SNV disease association from published case-control, genomewide association studies (sample 2).
The analyses included 40,675 patients with schizophrenia and 64,643 controls; 20,352 with bipolar disorder and 31,358 controls; and 135,458 with major depressive disorder and 344,901 controls.
The major finding was that a one standard deviation–lower expression of the ACE gene in blood was associated with lower systolic blood pressure of 4.0 mm Hg (95% confidence interval, 2.7-5.3), but also an increased risk of schizophrenia (odds ratio, 1.75; 95% CI, 1.28-2.38).
Could ACE inhibitors worsen symptoms or trigger episodes?
In their article, the researchers noted that, in most patients, onset of schizophrenia occurs in late adolescence or early adult life, ruling out ACE inhibitor treatment as a potential causal factor for most cases.
“However, if lower ACE levels play a causal role for schizophrenia risk, it would be reasonable to hypothesize that further lowering of ACE activity in existing patients could worsen symptoms or trigger a new episode,” they wrote.
Dr. Shah emphasized that evidence from genetic analyses alone is “not sufficient to justify changes in prescription guidelines.”
“Patients should not stop taking these medications if they are effective at controlling their blood pressure and they don’t suffer any adverse effects. But it would be reasonable to encourage greater pharmacovigilance,” she said in an interview.
“One way in which we are hoping to follow up these findings,” said Dr. Shah, “is to access electronic health record data for millions of individuals to investigate if there is evidence of increased rates of psychotic episodes in individuals who use ACE inhibitors, compared to other classes of blood pressure–lowering medication.”
Caution warranted
Reached for comment, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Staten Island University Hospital in New York, noted that this is an “extremely complicated” study and urged caution in interpreting the results.
“Since most people develop schizophrenia earlier in life, before they usually develop problems with blood pressure, it’s not so much that these drugs might cause schizophrenia,” Dr. Sullivan said.
“But because of their effects on this particular gene, there’s a possibility that they might worsen symptoms or in somebody with borderline risk might cause them to develop symptoms later in life. This may apply to a relatively small number of people who develop symptoms of schizophrenia in their 40s and beyond,” he added.
That’s where “pharmacovigilance” comes into play, Dr. Sullivan said. “In other words, that they otherwise wouldn’t experience?”
Support for the study was provided by the National Health and Medical Research Council (Australia) and U.S. National Institute for Mental Health. Dr. Shah and Dr. Sullivan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ACE inhibitors may be associated with an increased risk for schizophrenia and may affect psychiatric symptoms, new research suggests.
Investigators found individuals who carry a genetic variant associated with lower levels of the ACE gene and protein have increased liability to schizophrenia, suggesting that drugs that lower ACE levels or activity may do the same.
“Our findings warrant further investigation into the role of ACE in schizophrenia and closer monitoring by clinicians of individuals, especially those with schizophrenia, who may be on medication that lower ACE activity, such as ACE inhibitors,” Sonia Shah, PhD, Institute for Biomedical Sciences, University of Queensland, Brisbane, Australia, said in an interview.
The study was published online March 10, 2021, in JAMA Psychiatry.
Antihypertensives and mental illness
Hypertension is common in patients with psychiatric disorders and observational studies have reported associations between antihypertensive medication and these disorders, although the findings have been mixed.
Dr. Shah and colleagues estimated the potential of different antihypertensive drug classes on schizophrenia, bipolar disorder, and major depressive disorder.
In a two-sample Mendelian randomization study, they evaluated ties between a single-nucleotide variant and drug-target gene expression derived from expression quantitative trait loci data in blood (sample 1) and the SNV disease association from published case-control, genomewide association studies (sample 2).
The analyses included 40,675 patients with schizophrenia and 64,643 controls; 20,352 with bipolar disorder and 31,358 controls; and 135,458 with major depressive disorder and 344,901 controls.
The major finding was that a one standard deviation–lower expression of the ACE gene in blood was associated with lower systolic blood pressure of 4.0 mm Hg (95% confidence interval, 2.7-5.3), but also an increased risk of schizophrenia (odds ratio, 1.75; 95% CI, 1.28-2.38).
Could ACE inhibitors worsen symptoms or trigger episodes?
In their article, the researchers noted that, in most patients, onset of schizophrenia occurs in late adolescence or early adult life, ruling out ACE inhibitor treatment as a potential causal factor for most cases.
“However, if lower ACE levels play a causal role for schizophrenia risk, it would be reasonable to hypothesize that further lowering of ACE activity in existing patients could worsen symptoms or trigger a new episode,” they wrote.
Dr. Shah emphasized that evidence from genetic analyses alone is “not sufficient to justify changes in prescription guidelines.”
“Patients should not stop taking these medications if they are effective at controlling their blood pressure and they don’t suffer any adverse effects. But it would be reasonable to encourage greater pharmacovigilance,” she said in an interview.
“One way in which we are hoping to follow up these findings,” said Dr. Shah, “is to access electronic health record data for millions of individuals to investigate if there is evidence of increased rates of psychotic episodes in individuals who use ACE inhibitors, compared to other classes of blood pressure–lowering medication.”
Caution warranted
Reached for comment, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Staten Island University Hospital in New York, noted that this is an “extremely complicated” study and urged caution in interpreting the results.
“Since most people develop schizophrenia earlier in life, before they usually develop problems with blood pressure, it’s not so much that these drugs might cause schizophrenia,” Dr. Sullivan said.
“But because of their effects on this particular gene, there’s a possibility that they might worsen symptoms or in somebody with borderline risk might cause them to develop symptoms later in life. This may apply to a relatively small number of people who develop symptoms of schizophrenia in their 40s and beyond,” he added.
That’s where “pharmacovigilance” comes into play, Dr. Sullivan said. “In other words, that they otherwise wouldn’t experience?”
Support for the study was provided by the National Health and Medical Research Council (Australia) and U.S. National Institute for Mental Health. Dr. Shah and Dr. Sullivan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Inflammatory immune findings likely in acute schizophrenia, MDD, bipolar
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
FROM NPA 2021
Cannabis tied to self-harm, death in youth with mood disorders
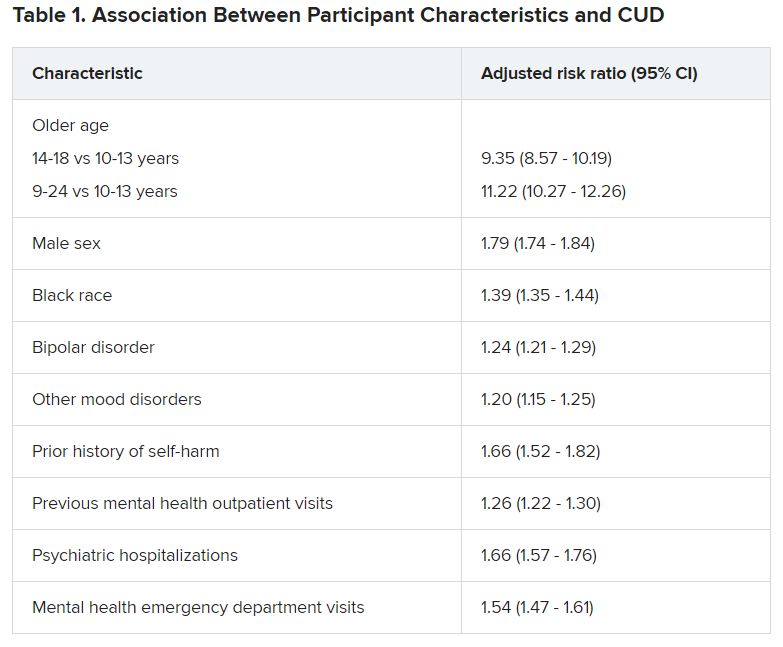
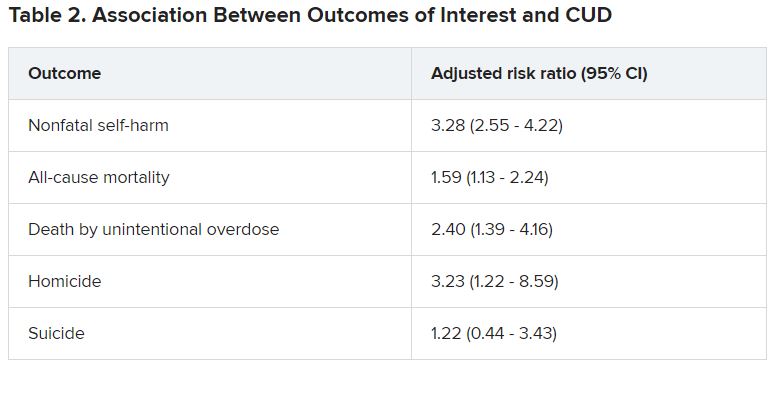
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Give women's mental health a seat at the health care table
Why it’s time for women’s mental health to be recognized as the subspecialty it already is
It wasn’t until I (Dr. Leistikow) finished my psychiatry residency that I realized the training I had received in women’s mental health was unusual. It was simply a required experience for PGY-3 residents at Johns Hopkins University, Baltimore.
All of us, regardless of interest, spent 1 afternoon a week over 6 months caring for patients in a specialty psychiatric clinic for women (run by Dr. Payne and Dr. Osborne). We discussed cases and received didactics on such topics as risk factors for postpartum depression; the risks of untreated mental illness in pregnancy, compared with the risks of various psychiatric medications; how to choose and dose medications for bipolar disorder as blood levels change across pregnancy; which resources to consult to determine the amounts and risks of various medications passed on in breast milk; and how to diagnose and treat premenstrual dysphoric disorder, to name a few lecture subjects.
By the time we were done, all residents had received more than 20 hours of teaching about how to treat mental illness in women across the reproductive life cycle. This was 20 hours more than is currently required by the American College of Graduate Medical Education, the accrediting body for all residencies, including psychiatry.1 It is time for that to change.
Women’s need for psychiatric treatment that addresses reproductive transitions is not new; it is as old as time. Not only do women who previously needed psychiatric treatment continue to need treatment when they get pregnant or are breastfeeding, but it is now well recognized that times of reproductive transition or flux – whether premenstrual, post partum, or perimenopausal – confer increased risk for both new-onset and exacerbations of prior mental illnesses.
What has changed is psychiatry’s ability to finally meet that need. Previously, despite the fact that women make up the majority of patients presenting for treatment, that nearly all women will menstruate and go through menopause, and that more than 80% of American women will have at least one pregnancy during their lifetime,psychiatrists practice as if these reproductive transitions were unfortunate blips getting in the doctor’s way.2 We mostly threw up our hands when our patients became pregnant, reflexively stopped all medications, and expected women to suffer for the sake of their babies.
with a large and growing research base, with both agreed-upon best practices and evolving standards of care informed by and responsive to the scientific literature. We now know that untreated maternal psychiatric illness carries its own risks for infants both before and after delivery; that many maternal pharmacologic treatments are lower risk for infants than previously thought; that protecting and treating women’s mental health in pregnancy has benefits for women, their babies, and the families that depend on them; and that there is now a growing evidence base informing both new and older treatments and enabling women and their doctors to make complex decisions balancing risk and benefit across the life cycle.
Many psychiatrists-in-training are hungry for this knowledge. At last count, in the United States alone, there were 16 women’s mental health fellowships available, up from just 3 in 2008.3 The problem is that none of them are accredited or funded by the ACGME, because reproductive psychiatry (here used interchangeably with the term women’s mental health) has not been officially recognized as a subspecialty. This means that current funding frequently rests on philanthropy, which often cannot be sustained, and clinical billing, which gives fellows in some programs such heavy clinical responsibilities that little time is left for scholarly work. Lack of subspecialty status also blocks numerous important downstream effects that would flow from this recognition.
Reproductive psychiatry clearly already meets criteria laid out by the American Board of Medical Specialties for defining a subspecialty field. As argued elsewhere, it has a distinct patient population with definable care needs and a standalone body of scientific medical knowledge as well as a national (and international) community of experts that has already done much to improve women’s access to care they desperately need.4 It also meets the ACGME’s criteria for a new subspecialty except for approval by the American Board of Psychiatry and Neurology.5 Finally, it also meets the requirements of the ABPN except for having 25 fellowship programs with 50 fellowship positions and 50 trainees per year completing fellowships, a challenging Catch-22 without the necessary funding that would accrue from accreditation.6
Despite growing awareness and demand, there remains a shortage of psychiatrists trained to treat women during times of reproductive transition and to pass their recommendations and knowledge on to their primary care and ob.gyn. colleagues. What official recognition would bring, in addition to funding for fellowships post residency, is a guaranteed seat at the table in psychiatry residencies, in terms of a required number of hours devoted to these topics for trainees, ensuring that all graduating psychiatrists have at least some exposure to the knowledge and practices so material to their patients.
It isn’t enough to wait for residencies to see the writing on the wall and voluntarily carve out a slice of pie devoted to women’s mental health from the limited time and resources available to train residents. A 2017 survey of psychiatry residency program training directors found that 23%, or almost a quarter of programs that responded, offered no reproductive psychiatry training at all, that 49% required 5 hours or less across all 4 years of training, and that 75% of programs had no required clinical exposure to reproductive psychiatry patients.7 Despite the fact that 87% of training directors surveyed agreed either that reproductive psychiatry was “an important area of education” or a subject general residents should be competent in, ACGME-recognized specialties take precedence.
A system so patchy and insufficient won’t do. It’s not good enough for the trainees who frequently have to look outside of their own institutions for the training they know they need. It’s not good enough for the pregnant or postpartum patient looking for evidence-based advice, who is currently left on her own to determine, prior to booking an appointment, whether a specific psychiatrist has received any training relevant to treating her. Adding reproductive psychiatry to the topics a graduating psychiatrist must have some proficiency in also signals to recent graduates and experienced attendings, as well as the relevant examining boards and producers of continuing medical education content, that women’s mental health is no longer a fringe topic but rather foundational to all practicing psychiatrists.
The oil needed to prime this pump is official recognition of the subspecialty that reproductive psychiatry already is. The women’s mental health community is ready. The research base is well established and growing exponentially. The number of women’s mental health fellowships is healthy and would increase significantly with ACGME funding. Psychiatry residency training programs can turn to recent graduates of these fellowships as well as their own faculty with reproductive psychiatry experience to teach trainees. In addition, the National Curriculum in Reproductive Psychiatry, over the last 4 years, has created a repository of free online modules dedicated to facilitating this type of training, with case discussions across numerous topics for use by both educators and trainees. The American Psychiatric Association recently formed the Committee on Women’s Mental Health in 2020 and will be publishing a textbook based on work done by the NCRP within the coming year.
Imagine the changed world that would open to all psychiatrists if reproductive psychiatry were given the credentials it deserves. When writing prescriptions, we would view pregnancy as the potential outcome it is in any woman of reproductive age, given that 50% of pregnancies are unplanned, and let women know ahead of time how to think about possible fetal effects rather than waiting for their panicked phone messages or hearing that they have stopped their medications abruptly. We would work to identify our patient’s individual risk factors for postpartum depression predelivery to reduce that risk and prevent or limit illness. We would plan ahead for close follow-up post partum during the window of greatest risk, rather than expecting women to drop out of care while taking care of their infants or languish on scheduling waiting lists. We would feel confident in giving evidence-based advice to our patients around times of reproductive transition across the life cycle, but especially in pregnancy and lactation, empowering women to make healthy decisions for themselves and their families, no longer abandoning them just when they need us most.
References
1. ACGME Program Requirements for Graduate Medical Education in Psychiatry. Accreditation Counsel for Graduate Medical Education. 2020 Jul 1.
2. Livingston G. “They’re waiting longer, but U.S. women today more likely to have children than a decade ago.” Pew Research Center’s Social & Demographic Trends Project. pewsocialtrends.org. 2018 Jan 18.
3. Nagle-Yang S et al. Acad Psychiatry. 2018 Apr;42(2):202-6.
4. Payne JL. Int Rev Psychiatry. 2019 May;31(3):207-9.
5. Accreditation Council for Graduate Medical Education Policies and Procedures. 2020 Sep 26.
6. American Board of Psychiatry and Neurology. Requirements for Subspecialty Recognition, Attachment A. 2008.
7. Osborne LM et al. Acad Psychiatry. 2018 Apr;42(2):197-201.
Dr. Leistikow is a reproductive psychiatrist and clinical assistant professor in the department of psychiatry at the University of Maryland, Baltimore, where she sees patients and helps train residents and fellows. She is on the education committee of the National Curriculum in Reproductive Psychiatry (NCRPtraining.org) and has written about women’s mental health for textbooks, scientific journals and on her private practice blog at www.womenspsychiatrybaltimore.com. Dr. Leistikow has no conflicts of interest.
Dr. Payne is associate professor of psychiatry and behavioral sciences and director of the Women’s Mood Disorders Center at Johns Hopkins University, Baltimore. In addition to providing outstanding clinical care for women with mood disorders, she conducts research into the genetic, biological, and environmental factors involved in postpartum depression. She and her colleagues have recently identified two epigenetic biomarkers of postpartum depression and are working hard to replicate this work with National Institutes of Health funding. Most recently, she was appointed to the American Psychiatric Association’s committee on women’s mental health and is serving as president-elect for both the Marcé of North America and the International Marcé Perinatal Mental Health Societies. She disclosed the following relevant financial relationships: serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Sage Therapeutics and Janssen Pharmaceuticals.
Dr. Osborne is associate professor of psychiatry and behavioral sciences and of gynecology and obstetrics at Johns Hopkins University, where she directs a postdoctoral fellowship program in reproductive psychiatry. She is an expert on the diagnosis and treatment of mood and anxiety disorders during pregnancy, the post partum, the premenstrual period, and perimenopause. Her work is supported by the Brain and Behavior Foundation, the Doris Duke Foundation, the American Board of Psychiatry and Neurology, and the National Institute of Mental Health. She has no conflicts of interest.
Why it’s time for women’s mental health to be recognized as the subspecialty it already is
Why it’s time for women’s mental health to be recognized as the subspecialty it already is
It wasn’t until I (Dr. Leistikow) finished my psychiatry residency that I realized the training I had received in women’s mental health was unusual. It was simply a required experience for PGY-3 residents at Johns Hopkins University, Baltimore.
All of us, regardless of interest, spent 1 afternoon a week over 6 months caring for patients in a specialty psychiatric clinic for women (run by Dr. Payne and Dr. Osborne). We discussed cases and received didactics on such topics as risk factors for postpartum depression; the risks of untreated mental illness in pregnancy, compared with the risks of various psychiatric medications; how to choose and dose medications for bipolar disorder as blood levels change across pregnancy; which resources to consult to determine the amounts and risks of various medications passed on in breast milk; and how to diagnose and treat premenstrual dysphoric disorder, to name a few lecture subjects.
By the time we were done, all residents had received more than 20 hours of teaching about how to treat mental illness in women across the reproductive life cycle. This was 20 hours more than is currently required by the American College of Graduate Medical Education, the accrediting body for all residencies, including psychiatry.1 It is time for that to change.
Women’s need for psychiatric treatment that addresses reproductive transitions is not new; it is as old as time. Not only do women who previously needed psychiatric treatment continue to need treatment when they get pregnant or are breastfeeding, but it is now well recognized that times of reproductive transition or flux – whether premenstrual, post partum, or perimenopausal – confer increased risk for both new-onset and exacerbations of prior mental illnesses.
What has changed is psychiatry’s ability to finally meet that need. Previously, despite the fact that women make up the majority of patients presenting for treatment, that nearly all women will menstruate and go through menopause, and that more than 80% of American women will have at least one pregnancy during their lifetime,psychiatrists practice as if these reproductive transitions were unfortunate blips getting in the doctor’s way.2 We mostly threw up our hands when our patients became pregnant, reflexively stopped all medications, and expected women to suffer for the sake of their babies.
with a large and growing research base, with both agreed-upon best practices and evolving standards of care informed by and responsive to the scientific literature. We now know that untreated maternal psychiatric illness carries its own risks for infants both before and after delivery; that many maternal pharmacologic treatments are lower risk for infants than previously thought; that protecting and treating women’s mental health in pregnancy has benefits for women, their babies, and the families that depend on them; and that there is now a growing evidence base informing both new and older treatments and enabling women and their doctors to make complex decisions balancing risk and benefit across the life cycle.
Many psychiatrists-in-training are hungry for this knowledge. At last count, in the United States alone, there were 16 women’s mental health fellowships available, up from just 3 in 2008.3 The problem is that none of them are accredited or funded by the ACGME, because reproductive psychiatry (here used interchangeably with the term women’s mental health) has not been officially recognized as a subspecialty. This means that current funding frequently rests on philanthropy, which often cannot be sustained, and clinical billing, which gives fellows in some programs such heavy clinical responsibilities that little time is left for scholarly work. Lack of subspecialty status also blocks numerous important downstream effects that would flow from this recognition.
Reproductive psychiatry clearly already meets criteria laid out by the American Board of Medical Specialties for defining a subspecialty field. As argued elsewhere, it has a distinct patient population with definable care needs and a standalone body of scientific medical knowledge as well as a national (and international) community of experts that has already done much to improve women’s access to care they desperately need.4 It also meets the ACGME’s criteria for a new subspecialty except for approval by the American Board of Psychiatry and Neurology.5 Finally, it also meets the requirements of the ABPN except for having 25 fellowship programs with 50 fellowship positions and 50 trainees per year completing fellowships, a challenging Catch-22 without the necessary funding that would accrue from accreditation.6
Despite growing awareness and demand, there remains a shortage of psychiatrists trained to treat women during times of reproductive transition and to pass their recommendations and knowledge on to their primary care and ob.gyn. colleagues. What official recognition would bring, in addition to funding for fellowships post residency, is a guaranteed seat at the table in psychiatry residencies, in terms of a required number of hours devoted to these topics for trainees, ensuring that all graduating psychiatrists have at least some exposure to the knowledge and practices so material to their patients.
It isn’t enough to wait for residencies to see the writing on the wall and voluntarily carve out a slice of pie devoted to women’s mental health from the limited time and resources available to train residents. A 2017 survey of psychiatry residency program training directors found that 23%, or almost a quarter of programs that responded, offered no reproductive psychiatry training at all, that 49% required 5 hours or less across all 4 years of training, and that 75% of programs had no required clinical exposure to reproductive psychiatry patients.7 Despite the fact that 87% of training directors surveyed agreed either that reproductive psychiatry was “an important area of education” or a subject general residents should be competent in, ACGME-recognized specialties take precedence.
A system so patchy and insufficient won’t do. It’s not good enough for the trainees who frequently have to look outside of their own institutions for the training they know they need. It’s not good enough for the pregnant or postpartum patient looking for evidence-based advice, who is currently left on her own to determine, prior to booking an appointment, whether a specific psychiatrist has received any training relevant to treating her. Adding reproductive psychiatry to the topics a graduating psychiatrist must have some proficiency in also signals to recent graduates and experienced attendings, as well as the relevant examining boards and producers of continuing medical education content, that women’s mental health is no longer a fringe topic but rather foundational to all practicing psychiatrists.
The oil needed to prime this pump is official recognition of the subspecialty that reproductive psychiatry already is. The women’s mental health community is ready. The research base is well established and growing exponentially. The number of women’s mental health fellowships is healthy and would increase significantly with ACGME funding. Psychiatry residency training programs can turn to recent graduates of these fellowships as well as their own faculty with reproductive psychiatry experience to teach trainees. In addition, the National Curriculum in Reproductive Psychiatry, over the last 4 years, has created a repository of free online modules dedicated to facilitating this type of training, with case discussions across numerous topics for use by both educators and trainees. The American Psychiatric Association recently formed the Committee on Women’s Mental Health in 2020 and will be publishing a textbook based on work done by the NCRP within the coming year.
Imagine the changed world that would open to all psychiatrists if reproductive psychiatry were given the credentials it deserves. When writing prescriptions, we would view pregnancy as the potential outcome it is in any woman of reproductive age, given that 50% of pregnancies are unplanned, and let women know ahead of time how to think about possible fetal effects rather than waiting for their panicked phone messages or hearing that they have stopped their medications abruptly. We would work to identify our patient’s individual risk factors for postpartum depression predelivery to reduce that risk and prevent or limit illness. We would plan ahead for close follow-up post partum during the window of greatest risk, rather than expecting women to drop out of care while taking care of their infants or languish on scheduling waiting lists. We would feel confident in giving evidence-based advice to our patients around times of reproductive transition across the life cycle, but especially in pregnancy and lactation, empowering women to make healthy decisions for themselves and their families, no longer abandoning them just when they need us most.
References
1. ACGME Program Requirements for Graduate Medical Education in Psychiatry. Accreditation Counsel for Graduate Medical Education. 2020 Jul 1.
2. Livingston G. “They’re waiting longer, but U.S. women today more likely to have children than a decade ago.” Pew Research Center’s Social & Demographic Trends Project. pewsocialtrends.org. 2018 Jan 18.
3. Nagle-Yang S et al. Acad Psychiatry. 2018 Apr;42(2):202-6.
4. Payne JL. Int Rev Psychiatry. 2019 May;31(3):207-9.
5. Accreditation Council for Graduate Medical Education Policies and Procedures. 2020 Sep 26.
6. American Board of Psychiatry and Neurology. Requirements for Subspecialty Recognition, Attachment A. 2008.
7. Osborne LM et al. Acad Psychiatry. 2018 Apr;42(2):197-201.
Dr. Leistikow is a reproductive psychiatrist and clinical assistant professor in the department of psychiatry at the University of Maryland, Baltimore, where she sees patients and helps train residents and fellows. She is on the education committee of the National Curriculum in Reproductive Psychiatry (NCRPtraining.org) and has written about women’s mental health for textbooks, scientific journals and on her private practice blog at www.womenspsychiatrybaltimore.com. Dr. Leistikow has no conflicts of interest.
Dr. Payne is associate professor of psychiatry and behavioral sciences and director of the Women’s Mood Disorders Center at Johns Hopkins University, Baltimore. In addition to providing outstanding clinical care for women with mood disorders, she conducts research into the genetic, biological, and environmental factors involved in postpartum depression. She and her colleagues have recently identified two epigenetic biomarkers of postpartum depression and are working hard to replicate this work with National Institutes of Health funding. Most recently, she was appointed to the American Psychiatric Association’s committee on women’s mental health and is serving as president-elect for both the Marcé of North America and the International Marcé Perinatal Mental Health Societies. She disclosed the following relevant financial relationships: serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Sage Therapeutics and Janssen Pharmaceuticals.
Dr. Osborne is associate professor of psychiatry and behavioral sciences and of gynecology and obstetrics at Johns Hopkins University, where she directs a postdoctoral fellowship program in reproductive psychiatry. She is an expert on the diagnosis and treatment of mood and anxiety disorders during pregnancy, the post partum, the premenstrual period, and perimenopause. Her work is supported by the Brain and Behavior Foundation, the Doris Duke Foundation, the American Board of Psychiatry and Neurology, and the National Institute of Mental Health. She has no conflicts of interest.
It wasn’t until I (Dr. Leistikow) finished my psychiatry residency that I realized the training I had received in women’s mental health was unusual. It was simply a required experience for PGY-3 residents at Johns Hopkins University, Baltimore.
All of us, regardless of interest, spent 1 afternoon a week over 6 months caring for patients in a specialty psychiatric clinic for women (run by Dr. Payne and Dr. Osborne). We discussed cases and received didactics on such topics as risk factors for postpartum depression; the risks of untreated mental illness in pregnancy, compared with the risks of various psychiatric medications; how to choose and dose medications for bipolar disorder as blood levels change across pregnancy; which resources to consult to determine the amounts and risks of various medications passed on in breast milk; and how to diagnose and treat premenstrual dysphoric disorder, to name a few lecture subjects.
By the time we were done, all residents had received more than 20 hours of teaching about how to treat mental illness in women across the reproductive life cycle. This was 20 hours more than is currently required by the American College of Graduate Medical Education, the accrediting body for all residencies, including psychiatry.1 It is time for that to change.
Women’s need for psychiatric treatment that addresses reproductive transitions is not new; it is as old as time. Not only do women who previously needed psychiatric treatment continue to need treatment when they get pregnant or are breastfeeding, but it is now well recognized that times of reproductive transition or flux – whether premenstrual, post partum, or perimenopausal – confer increased risk for both new-onset and exacerbations of prior mental illnesses.
What has changed is psychiatry’s ability to finally meet that need. Previously, despite the fact that women make up the majority of patients presenting for treatment, that nearly all women will menstruate and go through menopause, and that more than 80% of American women will have at least one pregnancy during their lifetime,psychiatrists practice as if these reproductive transitions were unfortunate blips getting in the doctor’s way.2 We mostly threw up our hands when our patients became pregnant, reflexively stopped all medications, and expected women to suffer for the sake of their babies.
with a large and growing research base, with both agreed-upon best practices and evolving standards of care informed by and responsive to the scientific literature. We now know that untreated maternal psychiatric illness carries its own risks for infants both before and after delivery; that many maternal pharmacologic treatments are lower risk for infants than previously thought; that protecting and treating women’s mental health in pregnancy has benefits for women, their babies, and the families that depend on them; and that there is now a growing evidence base informing both new and older treatments and enabling women and their doctors to make complex decisions balancing risk and benefit across the life cycle.
Many psychiatrists-in-training are hungry for this knowledge. At last count, in the United States alone, there were 16 women’s mental health fellowships available, up from just 3 in 2008.3 The problem is that none of them are accredited or funded by the ACGME, because reproductive psychiatry (here used interchangeably with the term women’s mental health) has not been officially recognized as a subspecialty. This means that current funding frequently rests on philanthropy, which often cannot be sustained, and clinical billing, which gives fellows in some programs such heavy clinical responsibilities that little time is left for scholarly work. Lack of subspecialty status also blocks numerous important downstream effects that would flow from this recognition.
Reproductive psychiatry clearly already meets criteria laid out by the American Board of Medical Specialties for defining a subspecialty field. As argued elsewhere, it has a distinct patient population with definable care needs and a standalone body of scientific medical knowledge as well as a national (and international) community of experts that has already done much to improve women’s access to care they desperately need.4 It also meets the ACGME’s criteria for a new subspecialty except for approval by the American Board of Psychiatry and Neurology.5 Finally, it also meets the requirements of the ABPN except for having 25 fellowship programs with 50 fellowship positions and 50 trainees per year completing fellowships, a challenging Catch-22 without the necessary funding that would accrue from accreditation.6
Despite growing awareness and demand, there remains a shortage of psychiatrists trained to treat women during times of reproductive transition and to pass their recommendations and knowledge on to their primary care and ob.gyn. colleagues. What official recognition would bring, in addition to funding for fellowships post residency, is a guaranteed seat at the table in psychiatry residencies, in terms of a required number of hours devoted to these topics for trainees, ensuring that all graduating psychiatrists have at least some exposure to the knowledge and practices so material to their patients.
It isn’t enough to wait for residencies to see the writing on the wall and voluntarily carve out a slice of pie devoted to women’s mental health from the limited time and resources available to train residents. A 2017 survey of psychiatry residency program training directors found that 23%, or almost a quarter of programs that responded, offered no reproductive psychiatry training at all, that 49% required 5 hours or less across all 4 years of training, and that 75% of programs had no required clinical exposure to reproductive psychiatry patients.7 Despite the fact that 87% of training directors surveyed agreed either that reproductive psychiatry was “an important area of education” or a subject general residents should be competent in, ACGME-recognized specialties take precedence.
A system so patchy and insufficient won’t do. It’s not good enough for the trainees who frequently have to look outside of their own institutions for the training they know they need. It’s not good enough for the pregnant or postpartum patient looking for evidence-based advice, who is currently left on her own to determine, prior to booking an appointment, whether a specific psychiatrist has received any training relevant to treating her. Adding reproductive psychiatry to the topics a graduating psychiatrist must have some proficiency in also signals to recent graduates and experienced attendings, as well as the relevant examining boards and producers of continuing medical education content, that women’s mental health is no longer a fringe topic but rather foundational to all practicing psychiatrists.
The oil needed to prime this pump is official recognition of the subspecialty that reproductive psychiatry already is. The women’s mental health community is ready. The research base is well established and growing exponentially. The number of women’s mental health fellowships is healthy and would increase significantly with ACGME funding. Psychiatry residency training programs can turn to recent graduates of these fellowships as well as their own faculty with reproductive psychiatry experience to teach trainees. In addition, the National Curriculum in Reproductive Psychiatry, over the last 4 years, has created a repository of free online modules dedicated to facilitating this type of training, with case discussions across numerous topics for use by both educators and trainees. The American Psychiatric Association recently formed the Committee on Women’s Mental Health in 2020 and will be publishing a textbook based on work done by the NCRP within the coming year.
Imagine the changed world that would open to all psychiatrists if reproductive psychiatry were given the credentials it deserves. When writing prescriptions, we would view pregnancy as the potential outcome it is in any woman of reproductive age, given that 50% of pregnancies are unplanned, and let women know ahead of time how to think about possible fetal effects rather than waiting for their panicked phone messages or hearing that they have stopped their medications abruptly. We would work to identify our patient’s individual risk factors for postpartum depression predelivery to reduce that risk and prevent or limit illness. We would plan ahead for close follow-up post partum during the window of greatest risk, rather than expecting women to drop out of care while taking care of their infants or languish on scheduling waiting lists. We would feel confident in giving evidence-based advice to our patients around times of reproductive transition across the life cycle, but especially in pregnancy and lactation, empowering women to make healthy decisions for themselves and their families, no longer abandoning them just when they need us most.
References
1. ACGME Program Requirements for Graduate Medical Education in Psychiatry. Accreditation Counsel for Graduate Medical Education. 2020 Jul 1.
2. Livingston G. “They’re waiting longer, but U.S. women today more likely to have children than a decade ago.” Pew Research Center’s Social & Demographic Trends Project. pewsocialtrends.org. 2018 Jan 18.
3. Nagle-Yang S et al. Acad Psychiatry. 2018 Apr;42(2):202-6.
4. Payne JL. Int Rev Psychiatry. 2019 May;31(3):207-9.
5. Accreditation Council for Graduate Medical Education Policies and Procedures. 2020 Sep 26.
6. American Board of Psychiatry and Neurology. Requirements for Subspecialty Recognition, Attachment A. 2008.
7. Osborne LM et al. Acad Psychiatry. 2018 Apr;42(2):197-201.
Dr. Leistikow is a reproductive psychiatrist and clinical assistant professor in the department of psychiatry at the University of Maryland, Baltimore, where she sees patients and helps train residents and fellows. She is on the education committee of the National Curriculum in Reproductive Psychiatry (NCRPtraining.org) and has written about women’s mental health for textbooks, scientific journals and on her private practice blog at www.womenspsychiatrybaltimore.com. Dr. Leistikow has no conflicts of interest.
Dr. Payne is associate professor of psychiatry and behavioral sciences and director of the Women’s Mood Disorders Center at Johns Hopkins University, Baltimore. In addition to providing outstanding clinical care for women with mood disorders, she conducts research into the genetic, biological, and environmental factors involved in postpartum depression. She and her colleagues have recently identified two epigenetic biomarkers of postpartum depression and are working hard to replicate this work with National Institutes of Health funding. Most recently, she was appointed to the American Psychiatric Association’s committee on women’s mental health and is serving as president-elect for both the Marcé of North America and the International Marcé Perinatal Mental Health Societies. She disclosed the following relevant financial relationships: serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Sage Therapeutics and Janssen Pharmaceuticals.
Dr. Osborne is associate professor of psychiatry and behavioral sciences and of gynecology and obstetrics at Johns Hopkins University, where she directs a postdoctoral fellowship program in reproductive psychiatry. She is an expert on the diagnosis and treatment of mood and anxiety disorders during pregnancy, the post partum, the premenstrual period, and perimenopause. Her work is supported by the Brain and Behavior Foundation, the Doris Duke Foundation, the American Board of Psychiatry and Neurology, and the National Institute of Mental Health. She has no conflicts of interest.
‘Peer respites’ provide an alternative to psychiatric wards during pandemic
Mia McDermott is no stranger to isolation. Abandoned as an infant in China, she lived in an orphanage until a family in California adopted her as a toddler. She spent her adolescence in boarding schools and early adult years in and out of psychiatric hospitals, where she underwent treatment for bipolar disorder, anxiety, and anorexia.
The pandemic left Ms. McDermott feeling especially lonely. She restricted social interactions because her fatty liver disease put her at greater risk of complications should she contract COVID-19. The 26-year-old Santa Cruz, Calif., resident stopped regularly eating and taking her psychiatric medications, and contemplated suicide.
When Ms. McDermott’s thoughts grew increasingly dark in June, she checked into Second Story, a mental health program based in a home not far from her own, where she finds nonclinical support in a peaceful environment from people who have faced similar challenges.
Second Story is what is known as a “peer respite,” a welcoming place where people can stay when they’re experiencing or nearing a mental health crisis. Betting that a low-key wellness approach, coupled with empathy from people who have “been there,” can help people in distress recover, this unorthodox strategy has gained popularity in recent years as the nation grapples with a severe shortage of psychiatric beds that has been exacerbated by the pandemic.
Peer respites allow guests to avoid psychiatric hospitalization and ED visits. They now operate in at least 14 states. California has five, in the San Francisco Bay Area and Los Angeles County.
“When things are really tough and you need extra support but you don’t need hospitalization, where’s that middle ground?” asked Keris Myrick, founder of Hacienda of Hope, a peer respite in Long Beach, Calif.
People with serious mental illness are more likely to experience emotional distress in the pandemic than the general population, said Benjamin Druss, MD, a psychiatrist and professor at Emory University, Atlanta, elaborating that they tend to have smaller social networks and more medical problems.
That was the case with Ms. McDermott. “I don’t have a full-on relationship with my family. My friends are my family,” she said. She yearned to “give them a hug, see their smile, or stand close and take a selfie.”
The next best thing was Second Story, located in a pewter-gray split-level, five-bedroom house in Aptos, a quaint beach community near Ms. McDermott’s Santa Cruz home.
– people who have experienced mental health conditions and are trained and often certified by states to support others with similar issues – and activities like arts, meditation and support groups.
“You can’t tell who’s the guest and who’s the staff. We don’t wear uniforms or badges,” said Angelica Garcia-Guerrero, associate director of Hacienda of Hope’s parent organization.
Peer respites are free for guests but rarely covered by insurance. States and counties typically pick up the tab. Hacienda of Hope’s $900,000 annual operating costs are covered by Los Angeles County through the Mental Health Services Act, a policy that directs proceeds from a statewide tax on people who earn more than $1 million annually to behavioral health programs.
In September, California Gov. Gavin Newsom signed a bill that would establish a statewide certification process for mental health peer providers by July 2022.
For now, however, peer-respite staff members in California are not licensed or certified. Peer respites typically don’t offer clinical care or dispense psychiatric drugs, though guests can bring theirs. Peers share personal stories with guests but avoid labeling them with diagnoses. Guests must come – and can leave – voluntarily. Some respites have few restrictions on who can stay; others don’t allow guests who express suicidal thoughts or are homeless.
Peer respite is one of several types of programs that divert people facing behavioral health crises from the hospital, but the only one without clinical involvement, said Travis Atkinson, a consultant at TBD Solutions, a behavioral health care company. The first peer respites arose around 2000, said Laysha Ostrow, CEO of Live & Learn, which conducts behavioral health research.
The approach seems to be expanding. Live & Learn currently counts 33 peer respites in the United States, up from 19 6 years ago. All are overseen and staffed by people with histories of psychiatric disorders. About a dozen other programs employ a mix of peers and laypeople who don’t have psychiatric diagnoses, or aren’t peer led, Mr. Atkinson said.
Though she had stayed at Second Story several times over the past 5 years, Ms. McDermott hesitated to return during the pandemic. However, she felt reassured after learning that guests were required to wear a mask in common areas and get a COVID test before their stay. To ensure physical distancing, the respite reduced capacity from six to five guests at a time.
During her 2-week stay, Ms. McDermott played with the respite’s two cats and piano – activities she found therapeutic. But most helpful was talking to peers in a way she couldn’t with her mental health providers. In the past, Ms. McDermott said, she had been involuntarily admitted to a psychiatric hospital after she expressed suicidal thoughts. When she shared similar sentiments with Second Story peers, they offered to talk, or call the hospital if she wanted.
“They were willing to listen,” she said. “But they’re not forceful about helping.”
By the end of the visit, Ms. McDermott said that she felt understood and her loneliness and suicidal feelings had waned. She started eating and taking her medications more consistently.
The small number of studies on respites have found that guests had fewer hospitalizations and accounted for lower Medicaid spending for nearly a year after a respite stay than people with similar conditions who did not stay in a respite. Respite visitors spent less time in the hospital and emergency room the longer they stayed in the respite.
Financial struggles and opposition from neighbors have hindered the growth of respites, however. Live & Learn said that, although five peer respites have been created since 2018, at least two others closed because of budget cuts.
Neighbors have challenged nearby respite placements in a few instances. Santa Cruz–area media outlets reported in 2019 that Second Story neighbors had voiced safety concerns with the respite. Neighbor Tony Crane said in an interview that guests have used drugs and consumed alcohol in the neighborhood, and he worried that peers are not licensed or certified to support people in crisis. He felt it was too risky to let his children ride their bikes near the respite when they were younger.
In a written response, Monica Martinez, whose organization runs Second Story, said neighbors often target community mental health programs because of concerns that “come from misconceptions and stigma surrounding those seeking mental health support.”
Many respites are struggling with increased demand and decreased availability during the pandemic. Sherry Jenkins Tucker, executive director of Georgia Mental Health Consumer Network, said its four respites have had to reduce capacity to enable physical distancing, despite increased demand for services. Other respites have temporarily suspended stays because of the pandemic.
Ms. McDermott said her mental health had improved since staying at Second Story in June, but she still struggles with isolation amid the pandemic. “Holidays are hard for me,” said Ms. McDermott, who returned to Second Story in November. “I really wanted to be able to have Thanksgiving with people.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Mia McDermott is no stranger to isolation. Abandoned as an infant in China, she lived in an orphanage until a family in California adopted her as a toddler. She spent her adolescence in boarding schools and early adult years in and out of psychiatric hospitals, where she underwent treatment for bipolar disorder, anxiety, and anorexia.
The pandemic left Ms. McDermott feeling especially lonely. She restricted social interactions because her fatty liver disease put her at greater risk of complications should she contract COVID-19. The 26-year-old Santa Cruz, Calif., resident stopped regularly eating and taking her psychiatric medications, and contemplated suicide.
When Ms. McDermott’s thoughts grew increasingly dark in June, she checked into Second Story, a mental health program based in a home not far from her own, where she finds nonclinical support in a peaceful environment from people who have faced similar challenges.
Second Story is what is known as a “peer respite,” a welcoming place where people can stay when they’re experiencing or nearing a mental health crisis. Betting that a low-key wellness approach, coupled with empathy from people who have “been there,” can help people in distress recover, this unorthodox strategy has gained popularity in recent years as the nation grapples with a severe shortage of psychiatric beds that has been exacerbated by the pandemic.
Peer respites allow guests to avoid psychiatric hospitalization and ED visits. They now operate in at least 14 states. California has five, in the San Francisco Bay Area and Los Angeles County.
“When things are really tough and you need extra support but you don’t need hospitalization, where’s that middle ground?” asked Keris Myrick, founder of Hacienda of Hope, a peer respite in Long Beach, Calif.
People with serious mental illness are more likely to experience emotional distress in the pandemic than the general population, said Benjamin Druss, MD, a psychiatrist and professor at Emory University, Atlanta, elaborating that they tend to have smaller social networks and more medical problems.
That was the case with Ms. McDermott. “I don’t have a full-on relationship with my family. My friends are my family,” she said. She yearned to “give them a hug, see their smile, or stand close and take a selfie.”
The next best thing was Second Story, located in a pewter-gray split-level, five-bedroom house in Aptos, a quaint beach community near Ms. McDermott’s Santa Cruz home.
– people who have experienced mental health conditions and are trained and often certified by states to support others with similar issues – and activities like arts, meditation and support groups.
“You can’t tell who’s the guest and who’s the staff. We don’t wear uniforms or badges,” said Angelica Garcia-Guerrero, associate director of Hacienda of Hope’s parent organization.
Peer respites are free for guests but rarely covered by insurance. States and counties typically pick up the tab. Hacienda of Hope’s $900,000 annual operating costs are covered by Los Angeles County through the Mental Health Services Act, a policy that directs proceeds from a statewide tax on people who earn more than $1 million annually to behavioral health programs.
In September, California Gov. Gavin Newsom signed a bill that would establish a statewide certification process for mental health peer providers by July 2022.
For now, however, peer-respite staff members in California are not licensed or certified. Peer respites typically don’t offer clinical care or dispense psychiatric drugs, though guests can bring theirs. Peers share personal stories with guests but avoid labeling them with diagnoses. Guests must come – and can leave – voluntarily. Some respites have few restrictions on who can stay; others don’t allow guests who express suicidal thoughts or are homeless.
Peer respite is one of several types of programs that divert people facing behavioral health crises from the hospital, but the only one without clinical involvement, said Travis Atkinson, a consultant at TBD Solutions, a behavioral health care company. The first peer respites arose around 2000, said Laysha Ostrow, CEO of Live & Learn, which conducts behavioral health research.
The approach seems to be expanding. Live & Learn currently counts 33 peer respites in the United States, up from 19 6 years ago. All are overseen and staffed by people with histories of psychiatric disorders. About a dozen other programs employ a mix of peers and laypeople who don’t have psychiatric diagnoses, or aren’t peer led, Mr. Atkinson said.
Though she had stayed at Second Story several times over the past 5 years, Ms. McDermott hesitated to return during the pandemic. However, she felt reassured after learning that guests were required to wear a mask in common areas and get a COVID test before their stay. To ensure physical distancing, the respite reduced capacity from six to five guests at a time.
During her 2-week stay, Ms. McDermott played with the respite’s two cats and piano – activities she found therapeutic. But most helpful was talking to peers in a way she couldn’t with her mental health providers. In the past, Ms. McDermott said, she had been involuntarily admitted to a psychiatric hospital after she expressed suicidal thoughts. When she shared similar sentiments with Second Story peers, they offered to talk, or call the hospital if she wanted.
“They were willing to listen,” she said. “But they’re not forceful about helping.”
By the end of the visit, Ms. McDermott said that she felt understood and her loneliness and suicidal feelings had waned. She started eating and taking her medications more consistently.
The small number of studies on respites have found that guests had fewer hospitalizations and accounted for lower Medicaid spending for nearly a year after a respite stay than people with similar conditions who did not stay in a respite. Respite visitors spent less time in the hospital and emergency room the longer they stayed in the respite.
Financial struggles and opposition from neighbors have hindered the growth of respites, however. Live & Learn said that, although five peer respites have been created since 2018, at least two others closed because of budget cuts.
Neighbors have challenged nearby respite placements in a few instances. Santa Cruz–area media outlets reported in 2019 that Second Story neighbors had voiced safety concerns with the respite. Neighbor Tony Crane said in an interview that guests have used drugs and consumed alcohol in the neighborhood, and he worried that peers are not licensed or certified to support people in crisis. He felt it was too risky to let his children ride their bikes near the respite when they were younger.
In a written response, Monica Martinez, whose organization runs Second Story, said neighbors often target community mental health programs because of concerns that “come from misconceptions and stigma surrounding those seeking mental health support.”
Many respites are struggling with increased demand and decreased availability during the pandemic. Sherry Jenkins Tucker, executive director of Georgia Mental Health Consumer Network, said its four respites have had to reduce capacity to enable physical distancing, despite increased demand for services. Other respites have temporarily suspended stays because of the pandemic.
Ms. McDermott said her mental health had improved since staying at Second Story in June, but she still struggles with isolation amid the pandemic. “Holidays are hard for me,” said Ms. McDermott, who returned to Second Story in November. “I really wanted to be able to have Thanksgiving with people.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Mia McDermott is no stranger to isolation. Abandoned as an infant in China, she lived in an orphanage until a family in California adopted her as a toddler. She spent her adolescence in boarding schools and early adult years in and out of psychiatric hospitals, where she underwent treatment for bipolar disorder, anxiety, and anorexia.
The pandemic left Ms. McDermott feeling especially lonely. She restricted social interactions because her fatty liver disease put her at greater risk of complications should she contract COVID-19. The 26-year-old Santa Cruz, Calif., resident stopped regularly eating and taking her psychiatric medications, and contemplated suicide.
When Ms. McDermott’s thoughts grew increasingly dark in June, she checked into Second Story, a mental health program based in a home not far from her own, where she finds nonclinical support in a peaceful environment from people who have faced similar challenges.
Second Story is what is known as a “peer respite,” a welcoming place where people can stay when they’re experiencing or nearing a mental health crisis. Betting that a low-key wellness approach, coupled with empathy from people who have “been there,” can help people in distress recover, this unorthodox strategy has gained popularity in recent years as the nation grapples with a severe shortage of psychiatric beds that has been exacerbated by the pandemic.
Peer respites allow guests to avoid psychiatric hospitalization and ED visits. They now operate in at least 14 states. California has five, in the San Francisco Bay Area and Los Angeles County.
“When things are really tough and you need extra support but you don’t need hospitalization, where’s that middle ground?” asked Keris Myrick, founder of Hacienda of Hope, a peer respite in Long Beach, Calif.
People with serious mental illness are more likely to experience emotional distress in the pandemic than the general population, said Benjamin Druss, MD, a psychiatrist and professor at Emory University, Atlanta, elaborating that they tend to have smaller social networks and more medical problems.
That was the case with Ms. McDermott. “I don’t have a full-on relationship with my family. My friends are my family,” she said. She yearned to “give them a hug, see their smile, or stand close and take a selfie.”
The next best thing was Second Story, located in a pewter-gray split-level, five-bedroom house in Aptos, a quaint beach community near Ms. McDermott’s Santa Cruz home.
– people who have experienced mental health conditions and are trained and often certified by states to support others with similar issues – and activities like arts, meditation and support groups.
“You can’t tell who’s the guest and who’s the staff. We don’t wear uniforms or badges,” said Angelica Garcia-Guerrero, associate director of Hacienda of Hope’s parent organization.
Peer respites are free for guests but rarely covered by insurance. States and counties typically pick up the tab. Hacienda of Hope’s $900,000 annual operating costs are covered by Los Angeles County through the Mental Health Services Act, a policy that directs proceeds from a statewide tax on people who earn more than $1 million annually to behavioral health programs.
In September, California Gov. Gavin Newsom signed a bill that would establish a statewide certification process for mental health peer providers by July 2022.
For now, however, peer-respite staff members in California are not licensed or certified. Peer respites typically don’t offer clinical care or dispense psychiatric drugs, though guests can bring theirs. Peers share personal stories with guests but avoid labeling them with diagnoses. Guests must come – and can leave – voluntarily. Some respites have few restrictions on who can stay; others don’t allow guests who express suicidal thoughts or are homeless.
Peer respite is one of several types of programs that divert people facing behavioral health crises from the hospital, but the only one without clinical involvement, said Travis Atkinson, a consultant at TBD Solutions, a behavioral health care company. The first peer respites arose around 2000, said Laysha Ostrow, CEO of Live & Learn, which conducts behavioral health research.
The approach seems to be expanding. Live & Learn currently counts 33 peer respites in the United States, up from 19 6 years ago. All are overseen and staffed by people with histories of psychiatric disorders. About a dozen other programs employ a mix of peers and laypeople who don’t have psychiatric diagnoses, or aren’t peer led, Mr. Atkinson said.
Though she had stayed at Second Story several times over the past 5 years, Ms. McDermott hesitated to return during the pandemic. However, she felt reassured after learning that guests were required to wear a mask in common areas and get a COVID test before their stay. To ensure physical distancing, the respite reduced capacity from six to five guests at a time.
During her 2-week stay, Ms. McDermott played with the respite’s two cats and piano – activities she found therapeutic. But most helpful was talking to peers in a way she couldn’t with her mental health providers. In the past, Ms. McDermott said, she had been involuntarily admitted to a psychiatric hospital after she expressed suicidal thoughts. When she shared similar sentiments with Second Story peers, they offered to talk, or call the hospital if she wanted.
“They were willing to listen,” she said. “But they’re not forceful about helping.”
By the end of the visit, Ms. McDermott said that she felt understood and her loneliness and suicidal feelings had waned. She started eating and taking her medications more consistently.
The small number of studies on respites have found that guests had fewer hospitalizations and accounted for lower Medicaid spending for nearly a year after a respite stay than people with similar conditions who did not stay in a respite. Respite visitors spent less time in the hospital and emergency room the longer they stayed in the respite.
Financial struggles and opposition from neighbors have hindered the growth of respites, however. Live & Learn said that, although five peer respites have been created since 2018, at least two others closed because of budget cuts.
Neighbors have challenged nearby respite placements in a few instances. Santa Cruz–area media outlets reported in 2019 that Second Story neighbors had voiced safety concerns with the respite. Neighbor Tony Crane said in an interview that guests have used drugs and consumed alcohol in the neighborhood, and he worried that peers are not licensed or certified to support people in crisis. He felt it was too risky to let his children ride their bikes near the respite when they were younger.
In a written response, Monica Martinez, whose organization runs Second Story, said neighbors often target community mental health programs because of concerns that “come from misconceptions and stigma surrounding those seeking mental health support.”
Many respites are struggling with increased demand and decreased availability during the pandemic. Sherry Jenkins Tucker, executive director of Georgia Mental Health Consumer Network, said its four respites have had to reduce capacity to enable physical distancing, despite increased demand for services. Other respites have temporarily suspended stays because of the pandemic.
Ms. McDermott said her mental health had improved since staying at Second Story in June, but she still struggles with isolation amid the pandemic. “Holidays are hard for me,” said Ms. McDermott, who returned to Second Story in November. “I really wanted to be able to have Thanksgiving with people.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Polydoctoring: The case against fragmented psychiatric care
How many providers does it take to depersonalize a patient? Nine? 1. A psychiatrist for transcranial magnetic stimulation (TMS). 2. A psychiatrist for ketamine. 3. A psychiatrist who specializes in substance use disorder medication. 4. A psychiatrist for the rest of the psychotropic medication. 5. An alternative medicine provider who prescribes supplements. 6. A therapist for depression who uses cognitive-behavioral therapy. 7. A therapist for posttraumatic stress disorder who uses eye movement desensitization and reprocessing. 8. An addiction counselor. 9. An equine therapist.
This doesn’t include other providers and professionals who likely contribute to one’s mental well-being, including yoga instructors and personal trainers. In addition, any one of those psychiatrists may have one or more nurse practitioners who routinely step in to attend to appointments.
In our uncertain and lonely times, the value of human contact and interaction has become exponentially more precious. I long to see my patients in my private practice office. I am now much more aware of their grounding effect on my life, and I suspect I had a similar grounding effect on theirs. Few things provide me more comfort than sitting on my lounge chair with a curious gaze waiting for the patient to start the visit. I often wonder what makes a patient choose to go see a private practice physician. Yet a common reason offered is, “Wait! You do everything? Therapy and meds if I need them? You’ll see me every week?”
While I am realistic about the need and use of split-care, I have never been enamored with the concept. I think that few medical students choose psychiatry with the goal of referring all psychotherapeutic needs and intervention to “allied mental health providers” as my prior managed care organization liked to refer to psychologists, social workers, marriage and family therapists, and other counselors. I remember particularly as a chief resident being bombarded by complaints of therapists complaining about psychiatry residents. All of their patients’ symptoms allegedly required medication adjustment and residents were supposedly dismissing them. In return, residents would complain that the therapists did not address the psychological manifestations of the patient’s ailments. Herein lies my problem with split-care, it encourages psychotherapy to be about medication management, and medication management to be about psychotherapy.
However, this is not an article against split-treatment. Psychiatrists, for a variety of reasons, are not suited to perform psychotherapy in most management care models. The main reason being that psychiatrists’ time is too expensive to justify the expense, and psychiatrists are (for the most part), the only ones able to prescribe medications for which the wait-list is already long enough. This article is about the absurd levels at which we have fragmented care of certain patients. Split-treatment is relevant in that its negative side effects, we are almost all familiar with, exemplify the problem of the fragmentation of modern psychiatry. In many ways this fragmentation of care is similar to polypharmacy – the premise for each psychotropic intervention may be sound, but the end result is often incoherent.
My main concern with the fragmentation of modern psychiatry stems from my belief that the most important facet of our work is our relationship with our patients. It is the duty we owe them, the attention we give them, the unique nature of interactions. Who among the nine providers is responsible for writing a discharge summary? Who is responsible for calling an emergency contact in a critical situation? Who communicates with the new provider when someone is taken off an insurance panel? Who makes the patient feel cared for? I am often confronted by this situation when TMS or ketamine providers say, “I just give the procedure/medication that was ordered by the referring psychiatrist.” This response disturbs me in that I could not imagine myself being so hands off in the care of a patient. There is an implication of projected immunity and lack of responsibility that bothers me.
But my concerns are also practical. From my forensic experience, I am well aware that the larger the number of providers treating a patient, the larger the number of inconsistent diagnoses, the more likely medication reconciliations are not kept up to date or incorrect, and the more likely intervention recommendations are contrary to one another. A disengaged ketamine provider may not realize that the patient was more recently enrolled in a substance use disorder program, a potential contraindication for ketamine, if not well-abreast of the patient’s continued evolution. A substance use disorder psychiatric specialist may be at odds with a substance use disorder counselor who worries about the message of treating psychiatric symptoms with chemical substances if they don’t communicate.
As with polypharmacy, “polydoctoring” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to specialize, patients still desire and benefit from an intimate and close relationship with one provider who is warm, concerned, and hopeful. Those traits can theoretically be provided by anyone and there is not something inherently wrong with having more than one provider. However, psychiatry would be wise to recognize this concerning trend, especially at a time when we all feel lonely, disconnected, and depersonalized.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
How many providers does it take to depersonalize a patient? Nine? 1. A psychiatrist for transcranial magnetic stimulation (TMS). 2. A psychiatrist for ketamine. 3. A psychiatrist who specializes in substance use disorder medication. 4. A psychiatrist for the rest of the psychotropic medication. 5. An alternative medicine provider who prescribes supplements. 6. A therapist for depression who uses cognitive-behavioral therapy. 7. A therapist for posttraumatic stress disorder who uses eye movement desensitization and reprocessing. 8. An addiction counselor. 9. An equine therapist.
This doesn’t include other providers and professionals who likely contribute to one’s mental well-being, including yoga instructors and personal trainers. In addition, any one of those psychiatrists may have one or more nurse practitioners who routinely step in to attend to appointments.
In our uncertain and lonely times, the value of human contact and interaction has become exponentially more precious. I long to see my patients in my private practice office. I am now much more aware of their grounding effect on my life, and I suspect I had a similar grounding effect on theirs. Few things provide me more comfort than sitting on my lounge chair with a curious gaze waiting for the patient to start the visit. I often wonder what makes a patient choose to go see a private practice physician. Yet a common reason offered is, “Wait! You do everything? Therapy and meds if I need them? You’ll see me every week?”
While I am realistic about the need and use of split-care, I have never been enamored with the concept. I think that few medical students choose psychiatry with the goal of referring all psychotherapeutic needs and intervention to “allied mental health providers” as my prior managed care organization liked to refer to psychologists, social workers, marriage and family therapists, and other counselors. I remember particularly as a chief resident being bombarded by complaints of therapists complaining about psychiatry residents. All of their patients’ symptoms allegedly required medication adjustment and residents were supposedly dismissing them. In return, residents would complain that the therapists did not address the psychological manifestations of the patient’s ailments. Herein lies my problem with split-care, it encourages psychotherapy to be about medication management, and medication management to be about psychotherapy.
However, this is not an article against split-treatment. Psychiatrists, for a variety of reasons, are not suited to perform psychotherapy in most management care models. The main reason being that psychiatrists’ time is too expensive to justify the expense, and psychiatrists are (for the most part), the only ones able to prescribe medications for which the wait-list is already long enough. This article is about the absurd levels at which we have fragmented care of certain patients. Split-treatment is relevant in that its negative side effects, we are almost all familiar with, exemplify the problem of the fragmentation of modern psychiatry. In many ways this fragmentation of care is similar to polypharmacy – the premise for each psychotropic intervention may be sound, but the end result is often incoherent.
My main concern with the fragmentation of modern psychiatry stems from my belief that the most important facet of our work is our relationship with our patients. It is the duty we owe them, the attention we give them, the unique nature of interactions. Who among the nine providers is responsible for writing a discharge summary? Who is responsible for calling an emergency contact in a critical situation? Who communicates with the new provider when someone is taken off an insurance panel? Who makes the patient feel cared for? I am often confronted by this situation when TMS or ketamine providers say, “I just give the procedure/medication that was ordered by the referring psychiatrist.” This response disturbs me in that I could not imagine myself being so hands off in the care of a patient. There is an implication of projected immunity and lack of responsibility that bothers me.
But my concerns are also practical. From my forensic experience, I am well aware that the larger the number of providers treating a patient, the larger the number of inconsistent diagnoses, the more likely medication reconciliations are not kept up to date or incorrect, and the more likely intervention recommendations are contrary to one another. A disengaged ketamine provider may not realize that the patient was more recently enrolled in a substance use disorder program, a potential contraindication for ketamine, if not well-abreast of the patient’s continued evolution. A substance use disorder psychiatric specialist may be at odds with a substance use disorder counselor who worries about the message of treating psychiatric symptoms with chemical substances if they don’t communicate.
As with polypharmacy, “polydoctoring” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to specialize, patients still desire and benefit from an intimate and close relationship with one provider who is warm, concerned, and hopeful. Those traits can theoretically be provided by anyone and there is not something inherently wrong with having more than one provider. However, psychiatry would be wise to recognize this concerning trend, especially at a time when we all feel lonely, disconnected, and depersonalized.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
How many providers does it take to depersonalize a patient? Nine? 1. A psychiatrist for transcranial magnetic stimulation (TMS). 2. A psychiatrist for ketamine. 3. A psychiatrist who specializes in substance use disorder medication. 4. A psychiatrist for the rest of the psychotropic medication. 5. An alternative medicine provider who prescribes supplements. 6. A therapist for depression who uses cognitive-behavioral therapy. 7. A therapist for posttraumatic stress disorder who uses eye movement desensitization and reprocessing. 8. An addiction counselor. 9. An equine therapist.
This doesn’t include other providers and professionals who likely contribute to one’s mental well-being, including yoga instructors and personal trainers. In addition, any one of those psychiatrists may have one or more nurse practitioners who routinely step in to attend to appointments.
In our uncertain and lonely times, the value of human contact and interaction has become exponentially more precious. I long to see my patients in my private practice office. I am now much more aware of their grounding effect on my life, and I suspect I had a similar grounding effect on theirs. Few things provide me more comfort than sitting on my lounge chair with a curious gaze waiting for the patient to start the visit. I often wonder what makes a patient choose to go see a private practice physician. Yet a common reason offered is, “Wait! You do everything? Therapy and meds if I need them? You’ll see me every week?”
While I am realistic about the need and use of split-care, I have never been enamored with the concept. I think that few medical students choose psychiatry with the goal of referring all psychotherapeutic needs and intervention to “allied mental health providers” as my prior managed care organization liked to refer to psychologists, social workers, marriage and family therapists, and other counselors. I remember particularly as a chief resident being bombarded by complaints of therapists complaining about psychiatry residents. All of their patients’ symptoms allegedly required medication adjustment and residents were supposedly dismissing them. In return, residents would complain that the therapists did not address the psychological manifestations of the patient’s ailments. Herein lies my problem with split-care, it encourages psychotherapy to be about medication management, and medication management to be about psychotherapy.
However, this is not an article against split-treatment. Psychiatrists, for a variety of reasons, are not suited to perform psychotherapy in most management care models. The main reason being that psychiatrists’ time is too expensive to justify the expense, and psychiatrists are (for the most part), the only ones able to prescribe medications for which the wait-list is already long enough. This article is about the absurd levels at which we have fragmented care of certain patients. Split-treatment is relevant in that its negative side effects, we are almost all familiar with, exemplify the problem of the fragmentation of modern psychiatry. In many ways this fragmentation of care is similar to polypharmacy – the premise for each psychotropic intervention may be sound, but the end result is often incoherent.
My main concern with the fragmentation of modern psychiatry stems from my belief that the most important facet of our work is our relationship with our patients. It is the duty we owe them, the attention we give them, the unique nature of interactions. Who among the nine providers is responsible for writing a discharge summary? Who is responsible for calling an emergency contact in a critical situation? Who communicates with the new provider when someone is taken off an insurance panel? Who makes the patient feel cared for? I am often confronted by this situation when TMS or ketamine providers say, “I just give the procedure/medication that was ordered by the referring psychiatrist.” This response disturbs me in that I could not imagine myself being so hands off in the care of a patient. There is an implication of projected immunity and lack of responsibility that bothers me.
But my concerns are also practical. From my forensic experience, I am well aware that the larger the number of providers treating a patient, the larger the number of inconsistent diagnoses, the more likely medication reconciliations are not kept up to date or incorrect, and the more likely intervention recommendations are contrary to one another. A disengaged ketamine provider may not realize that the patient was more recently enrolled in a substance use disorder program, a potential contraindication for ketamine, if not well-abreast of the patient’s continued evolution. A substance use disorder psychiatric specialist may be at odds with a substance use disorder counselor who worries about the message of treating psychiatric symptoms with chemical substances if they don’t communicate.
As with polypharmacy, “polydoctoring” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to specialize, patients still desire and benefit from an intimate and close relationship with one provider who is warm, concerned, and hopeful. Those traits can theoretically be provided by anyone and there is not something inherently wrong with having more than one provider. However, psychiatry would be wise to recognize this concerning trend, especially at a time when we all feel lonely, disconnected, and depersonalized.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Give psych patients the COVID vaccination now, experts say
With COVID-19 vaccinations now underway, mental health experts around the world continue to push for patients with serious mental illness (SMI) to be considered a high-priority group for the vaccine.
Research shows that patients with SMI are at increased risk of being infected with SARS-CoV-2 and have higher rates of hospitalization and poor outcomes, Nicola Warren, MBBS, University of Queensland, Brisbane, Australia, and coauthors write in a viewpoint published online Dec. 15 in JAMA Psychiatry
Factors behind the worse outcomes in individuals with SMI include concomitant medications, poorer premorbid general health, physical comorbidity, reduced access to medical care, and environmental and lifestyle factors such as lower socioeconomic status, overcrowding, smoking, and obesity.
“In light of these vulnerabilities, it is important that people with SMI are a priority group to receive a vaccination,” Dr. Warren and colleagues say.
Yet there are challenges at the individual and public health level in getting people with SMI vaccinated against COVID-19, they point out.
Challenges at the individual level include getting people with SMI to recognize the importance of the vaccine and combating negative beliefs about safety and misconceptions that the vaccine itself can make them sick with COVID-19.
Mental health professionals are “uniquely skilled” to deliver vaccine education, “being able to adapt for those with communication difficulties and balance factors influencing decision-making,” Dr. Warren and colleagues write.
, like getting to a vaccination clinic.
Research has shown that running vaccination clinics parallel to mental health services can boost vaccination rates by 25%, the authors note. Therefore, one solution may be to embed vaccination clinics within mental health services, Dr. Warren and colleagues suggest.
Join the chorus
Plans and policies to ensure rapid delivery of the COVID-19 vaccine are “vital,” they conclude. “Mental health clinicians have a key role in advocating for priority access to a COVID-19 vaccination for those with SMI, as well as facilitating its uptake,” they add.
Dr. Warren and her colleagues join a chorus of other mental health care providers who have sounded the alarm on the risks of COVID-19 for patients with SMI and the need to get them vaccinated early.
In a perspective article published last month in World Psychiatry, Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors called for individuals with SMI to have priority status for any COVID-19 vaccine, as reported by this news organization.
Dr. De Hert and colleagues noted that there is an ethical duty to prioritize vaccination for people with SMI given their increased risk of worse outcomes following COVID-19 infection and the structural barriers faced by people with SMI in accessing a vaccine.
Joining the chorus, Benjamin Druss, MD, MPH, from Emory University, Atlanta, Georgia, warned in a JAMA Psychiatry viewpoint in April that the COVID-19 pandemic represents a looming crisis for patients with SMI and the health care systems that serve them.
“Careful planning and execution at multiple levels will be essential for minimizing the adverse outcomes of this pandemic for this vulnerable population,” Dr. Druss wrote.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With COVID-19 vaccinations now underway, mental health experts around the world continue to push for patients with serious mental illness (SMI) to be considered a high-priority group for the vaccine.
Research shows that patients with SMI are at increased risk of being infected with SARS-CoV-2 and have higher rates of hospitalization and poor outcomes, Nicola Warren, MBBS, University of Queensland, Brisbane, Australia, and coauthors write in a viewpoint published online Dec. 15 in JAMA Psychiatry
Factors behind the worse outcomes in individuals with SMI include concomitant medications, poorer premorbid general health, physical comorbidity, reduced access to medical care, and environmental and lifestyle factors such as lower socioeconomic status, overcrowding, smoking, and obesity.
“In light of these vulnerabilities, it is important that people with SMI are a priority group to receive a vaccination,” Dr. Warren and colleagues say.
Yet there are challenges at the individual and public health level in getting people with SMI vaccinated against COVID-19, they point out.
Challenges at the individual level include getting people with SMI to recognize the importance of the vaccine and combating negative beliefs about safety and misconceptions that the vaccine itself can make them sick with COVID-19.
Mental health professionals are “uniquely skilled” to deliver vaccine education, “being able to adapt for those with communication difficulties and balance factors influencing decision-making,” Dr. Warren and colleagues write.
, like getting to a vaccination clinic.
Research has shown that running vaccination clinics parallel to mental health services can boost vaccination rates by 25%, the authors note. Therefore, one solution may be to embed vaccination clinics within mental health services, Dr. Warren and colleagues suggest.
Join the chorus
Plans and policies to ensure rapid delivery of the COVID-19 vaccine are “vital,” they conclude. “Mental health clinicians have a key role in advocating for priority access to a COVID-19 vaccination for those with SMI, as well as facilitating its uptake,” they add.
Dr. Warren and her colleagues join a chorus of other mental health care providers who have sounded the alarm on the risks of COVID-19 for patients with SMI and the need to get them vaccinated early.
In a perspective article published last month in World Psychiatry, Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors called for individuals with SMI to have priority status for any COVID-19 vaccine, as reported by this news organization.
Dr. De Hert and colleagues noted that there is an ethical duty to prioritize vaccination for people with SMI given their increased risk of worse outcomes following COVID-19 infection and the structural barriers faced by people with SMI in accessing a vaccine.
Joining the chorus, Benjamin Druss, MD, MPH, from Emory University, Atlanta, Georgia, warned in a JAMA Psychiatry viewpoint in April that the COVID-19 pandemic represents a looming crisis for patients with SMI and the health care systems that serve them.
“Careful planning and execution at multiple levels will be essential for minimizing the adverse outcomes of this pandemic for this vulnerable population,” Dr. Druss wrote.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With COVID-19 vaccinations now underway, mental health experts around the world continue to push for patients with serious mental illness (SMI) to be considered a high-priority group for the vaccine.
Research shows that patients with SMI are at increased risk of being infected with SARS-CoV-2 and have higher rates of hospitalization and poor outcomes, Nicola Warren, MBBS, University of Queensland, Brisbane, Australia, and coauthors write in a viewpoint published online Dec. 15 in JAMA Psychiatry
Factors behind the worse outcomes in individuals with SMI include concomitant medications, poorer premorbid general health, physical comorbidity, reduced access to medical care, and environmental and lifestyle factors such as lower socioeconomic status, overcrowding, smoking, and obesity.
“In light of these vulnerabilities, it is important that people with SMI are a priority group to receive a vaccination,” Dr. Warren and colleagues say.
Yet there are challenges at the individual and public health level in getting people with SMI vaccinated against COVID-19, they point out.
Challenges at the individual level include getting people with SMI to recognize the importance of the vaccine and combating negative beliefs about safety and misconceptions that the vaccine itself can make them sick with COVID-19.
Mental health professionals are “uniquely skilled” to deliver vaccine education, “being able to adapt for those with communication difficulties and balance factors influencing decision-making,” Dr. Warren and colleagues write.
, like getting to a vaccination clinic.
Research has shown that running vaccination clinics parallel to mental health services can boost vaccination rates by 25%, the authors note. Therefore, one solution may be to embed vaccination clinics within mental health services, Dr. Warren and colleagues suggest.
Join the chorus
Plans and policies to ensure rapid delivery of the COVID-19 vaccine are “vital,” they conclude. “Mental health clinicians have a key role in advocating for priority access to a COVID-19 vaccination for those with SMI, as well as facilitating its uptake,” they add.
Dr. Warren and her colleagues join a chorus of other mental health care providers who have sounded the alarm on the risks of COVID-19 for patients with SMI and the need to get them vaccinated early.
In a perspective article published last month in World Psychiatry, Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors called for individuals with SMI to have priority status for any COVID-19 vaccine, as reported by this news organization.
Dr. De Hert and colleagues noted that there is an ethical duty to prioritize vaccination for people with SMI given their increased risk of worse outcomes following COVID-19 infection and the structural barriers faced by people with SMI in accessing a vaccine.
Joining the chorus, Benjamin Druss, MD, MPH, from Emory University, Atlanta, Georgia, warned in a JAMA Psychiatry viewpoint in April that the COVID-19 pandemic represents a looming crisis for patients with SMI and the health care systems that serve them.
“Careful planning and execution at multiple levels will be essential for minimizing the adverse outcomes of this pandemic for this vulnerable population,” Dr. Druss wrote.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Global experts map the latest in bipolar management
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
Siblings of patients with bipolar disorder at increased risk
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
FROM ECNP 2020