User login
Lithium and kidney disease: Understand the risks
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
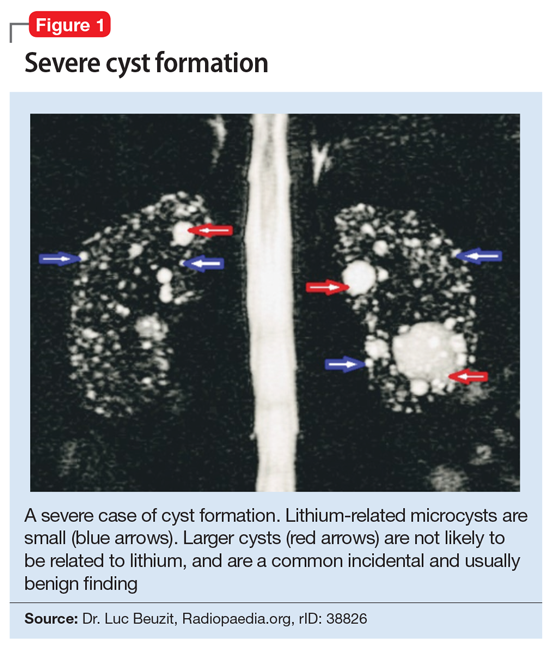
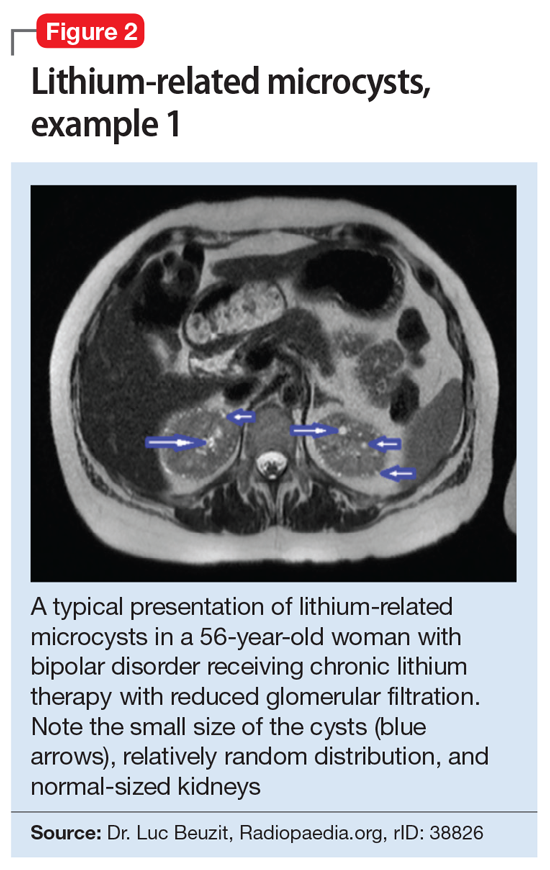
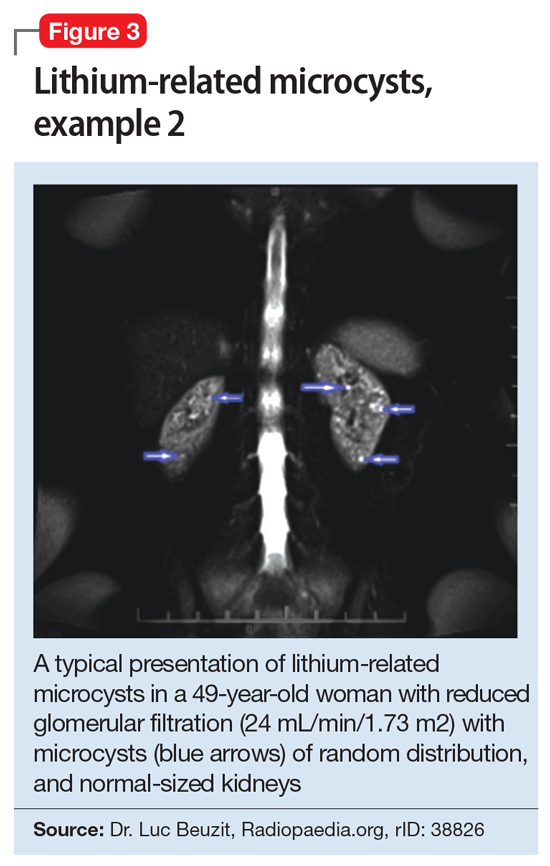
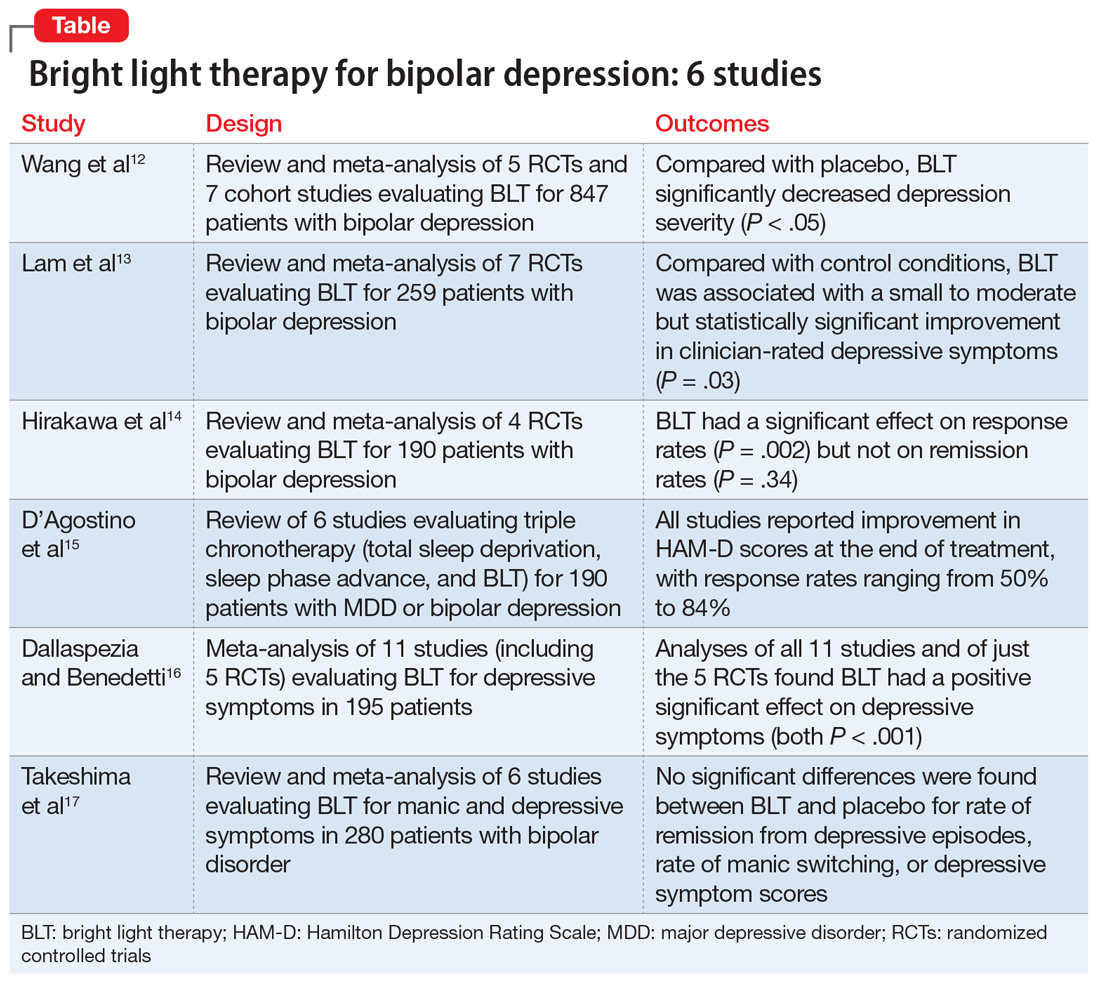
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
APA, AMA, others move to stop insurer from overturning mental health claims ruling
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
Prevalence of psychiatric disorders higher in adult cerebral palsy patients
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
FROM RESEARCH IN DEVELOPMENTAL DISABILITIES
Novel drug offers rapid relief from agitation in serious mental illness
An investigational, orally dissolving film formulation of dexmedetomidine (BXCL501, BioXcel Therapeutics) may offer rapid relief from acute agitation related to schizophrenia or bipolar disorder (BD), results of two phase 3, randomized, placebo-controlled trials show.
For both disorders, BXCL501 showed “superiority over placebo” by meeting the primary endpoint of reduction of agitation as measured by the excited component of the Positive and Negative Syndrome Scale (PANSS), study investigator Leslie Citrome, MD, MPH, department of psychiatry and behavioral sciences, New York Medical College, Valhalla, said in an interview.
The findings were presented at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Noninvasive option
Acute agitation in patients with schizophrenia or BD is often encountered in emergency departments (EDs) and inpatient units. When nondrug tactics fail to calm the patient, drug options include injectable antipsychotics or benzodiazepines. BXCL501 is a thin, orally dissolving film for sublingual or buccal use.
“Dexmedetomidine is a highly-selective alpha-2a receptor agonist and we haven’t really had one of those before in psychiatry for this purpose. And we haven’t had much in the way of orally dissolving thin films that are absorbed in the oral mucosa so this represents an opportunity to provide a potential intervention that does not require an injection and yet could possibly be of use in people who are agitated,” Dr. Citrome said.
The study, known as SERENITY I, included 380 adults (mean age 45.6 years, 63% male) with schizophrenia, schizoaffective disorder, or schizophreniform disorder, and acute agitation in the ED (total score ≥ 14 on the PANSS-Excited Component (PEC) scale at baseline and a score ≥ 4 on at least one of the five PEC items).
Patients were randomly allocated to a single oral dose of BXCL501: 120 mcg, 180 mcg, or placebo. A total of 372 patients (97.9%) completed the study.
Mean PEC total score was 17.6 at baseline. The mean change from baseline in the PEC total score at 2 hours (primary endpoint) was -8.5 and -10.3 with BXCL501 120 mcg and 180 mcg, respectively, versus -4.8 for placebo (P < .0001 vs. placebo).
PEC response rates (≥ 40% reduction from baseline) were 80.6% and 89.6% with BXCL501 120 mcg and 180 mcg versus 47.6% with placebo (P < .0001 vs. placebo).
Compared with placebo, and in the Agitation and Calmness Evaluation Scale (ACES) at 2 hours post dosing.
The incidence of adverse events (AE) was 39.5%, 37.3%, and 15.1% with BXCL501 120 mg, 180 mg, and placebo groups.
All AEs were mild or moderate. The most common AEs with BXCL501 were somnolence, dizziness, dry mouth, hypotension, orthostatic hypotension, hypoesthesia, and paresthesia. No drug-related severe or serious AEs occurred.
Nipping it in the bud
SERENITY II had a similar design. This study included 380 adults (mean age 48, 55% female) with bipolar I or II disorder and acute agitation in the ED (total score ≥14 on the PEC scale at baseline and a score ≥4 on at least one PEC item). A total of 362 (95.3%) of patients completed the study.
Mean PEC total score was 18 at baseline. The mean change from baseline in the PEC total score at 2 hours (primary endpoint) was -9.0 and -10.4 with BXCL501 120 mcg and 180 mcg, respectively, versus -4.9 for placebo (P < .0001 vs. placebo).
Bipolar patients also saw significant improvement on the secondary outcomes of CGI-I and ACES, with an adverse event profile similar to that seen in patients with schizophrenia.
BXCL501 demonstrated “rapid, robust and clinically meaningful efficacy” in both patient populations and represents a “novel, noninvasive and well tolerated treatment of agitation,” the investigators concluded in their APA abstracts.
“Patients who are agitated are in psychic pain and they want relief from this psychic pain. We’re also worried that they might get worse and that agitation escalates to aggression potentially requiring restraints. We want to avoid that,” Dr. Citrome said.
“By nipping it in the bud with a pharmacological intervention, we can ease their psychic pain and we can manage a potentially dangerous situation. Offering an oral medicine that would work quickly would be ideal in my mind and patients might potentially be more accepting of that than an injection,” Dr. Citrome said.
Based on the SERENITY I and II data, BioXcel Therapeutics has submitted a new drug application to the Food and Drug Administration.
Negotiation first, medication second
Reached for comment, Samoon Ahmad, MD, professor, department of psychiatry, New York (N.Y.) University, cautioned that, “when we talk about treating an agitated patient, medication is only part of the picture.
“There is a negotiating process with the patient. Number one, you offer them an environment that is conducive to making them feel calm, safe, and secure and that they are being listened to. Providing all of those things sometimes can be very helpful,” said Dr. Ahmad, who serves as unit chief of inpatient psychiatry at Bellevue Hospital Center in New York City.
“If someone starts throwing chairs at you or assaulting you, that is not really the time to negotiate a medicine; you basically have to restrain the patient, and many times give them intramuscular medicine,” Dr. Ahmad said.
He also noted that patients in the SERENITY trials had moderate to severe acute agitation.
“These are people you can potentially negotiate with. But again, when a patient crosses a certain line, you have to immediately do something and that could be intramuscular injection or something oral, which they may spit right in your face, which has happened numerous times,” Dr. Ahmad said.
“I don’t think intramuscular options will ever go away but an oral agent could be a useful tool as well,” said Dr. Ahmad, founder of the Integrative Center for Wellness in New York City.
He cautioned that clinicians are not going to be using this medicine in their offices. “If a patient walks in and is floridly psychotic, you will need to call 911. We’re really talking about its use either in the ED or acute inpatient setting,” Dr. Ahmad said.
A version of this article first appeared on Medscape.com.
An investigational, orally dissolving film formulation of dexmedetomidine (BXCL501, BioXcel Therapeutics) may offer rapid relief from acute agitation related to schizophrenia or bipolar disorder (BD), results of two phase 3, randomized, placebo-controlled trials show.
For both disorders, BXCL501 showed “superiority over placebo” by meeting the primary endpoint of reduction of agitation as measured by the excited component of the Positive and Negative Syndrome Scale (PANSS), study investigator Leslie Citrome, MD, MPH, department of psychiatry and behavioral sciences, New York Medical College, Valhalla, said in an interview.
The findings were presented at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Noninvasive option
Acute agitation in patients with schizophrenia or BD is often encountered in emergency departments (EDs) and inpatient units. When nondrug tactics fail to calm the patient, drug options include injectable antipsychotics or benzodiazepines. BXCL501 is a thin, orally dissolving film for sublingual or buccal use.
“Dexmedetomidine is a highly-selective alpha-2a receptor agonist and we haven’t really had one of those before in psychiatry for this purpose. And we haven’t had much in the way of orally dissolving thin films that are absorbed in the oral mucosa so this represents an opportunity to provide a potential intervention that does not require an injection and yet could possibly be of use in people who are agitated,” Dr. Citrome said.
The study, known as SERENITY I, included 380 adults (mean age 45.6 years, 63% male) with schizophrenia, schizoaffective disorder, or schizophreniform disorder, and acute agitation in the ED (total score ≥ 14 on the PANSS-Excited Component (PEC) scale at baseline and a score ≥ 4 on at least one of the five PEC items).
Patients were randomly allocated to a single oral dose of BXCL501: 120 mcg, 180 mcg, or placebo. A total of 372 patients (97.9%) completed the study.
Mean PEC total score was 17.6 at baseline. The mean change from baseline in the PEC total score at 2 hours (primary endpoint) was -8.5 and -10.3 with BXCL501 120 mcg and 180 mcg, respectively, versus -4.8 for placebo (P < .0001 vs. placebo).
PEC response rates (≥ 40% reduction from baseline) were 80.6% and 89.6% with BXCL501 120 mcg and 180 mcg versus 47.6% with placebo (P < .0001 vs. placebo).
Compared with placebo, and in the Agitation and Calmness Evaluation Scale (ACES) at 2 hours post dosing.
The incidence of adverse events (AE) was 39.5%, 37.3%, and 15.1% with BXCL501 120 mg, 180 mg, and placebo groups.
All AEs were mild or moderate. The most common AEs with BXCL501 were somnolence, dizziness, dry mouth, hypotension, orthostatic hypotension, hypoesthesia, and paresthesia. No drug-related severe or serious AEs occurred.
Nipping it in the bud
SERENITY II had a similar design. This study included 380 adults (mean age 48, 55% female) with bipolar I or II disorder and acute agitation in the ED (total score ≥14 on the PEC scale at baseline and a score ≥4 on at least one PEC item). A total of 362 (95.3%) of patients completed the study.
Mean PEC total score was 18 at baseline. The mean change from baseline in the PEC total score at 2 hours (primary endpoint) was -9.0 and -10.4 with BXCL501 120 mcg and 180 mcg, respectively, versus -4.9 for placebo (P < .0001 vs. placebo).
Bipolar patients also saw significant improvement on the secondary outcomes of CGI-I and ACES, with an adverse event profile similar to that seen in patients with schizophrenia.
BXCL501 demonstrated “rapid, robust and clinically meaningful efficacy” in both patient populations and represents a “novel, noninvasive and well tolerated treatment of agitation,” the investigators concluded in their APA abstracts.
“Patients who are agitated are in psychic pain and they want relief from this psychic pain. We’re also worried that they might get worse and that agitation escalates to aggression potentially requiring restraints. We want to avoid that,” Dr. Citrome said.
“By nipping it in the bud with a pharmacological intervention, we can ease their psychic pain and we can manage a potentially dangerous situation. Offering an oral medicine that would work quickly would be ideal in my mind and patients might potentially be more accepting of that than an injection,” Dr. Citrome said.
Based on the SERENITY I and II data, BioXcel Therapeutics has submitted a new drug application to the Food and Drug Administration.
Negotiation first, medication second
Reached for comment, Samoon Ahmad, MD, professor, department of psychiatry, New York (N.Y.) University, cautioned that, “when we talk about treating an agitated patient, medication is only part of the picture.
“There is a negotiating process with the patient. Number one, you offer them an environment that is conducive to making them feel calm, safe, and secure and that they are being listened to. Providing all of those things sometimes can be very helpful,” said Dr. Ahmad, who serves as unit chief of inpatient psychiatry at Bellevue Hospital Center in New York City.
“If someone starts throwing chairs at you or assaulting you, that is not really the time to negotiate a medicine; you basically have to restrain the patient, and many times give them intramuscular medicine,” Dr. Ahmad said.
He also noted that patients in the SERENITY trials had moderate to severe acute agitation.
“These are people you can potentially negotiate with. But again, when a patient crosses a certain line, you have to immediately do something and that could be intramuscular injection or something oral, which they may spit right in your face, which has happened numerous times,” Dr. Ahmad said.
“I don’t think intramuscular options will ever go away but an oral agent could be a useful tool as well,” said Dr. Ahmad, founder of the Integrative Center for Wellness in New York City.
He cautioned that clinicians are not going to be using this medicine in their offices. “If a patient walks in and is floridly psychotic, you will need to call 911. We’re really talking about its use either in the ED or acute inpatient setting,” Dr. Ahmad said.
A version of this article first appeared on Medscape.com.
An investigational, orally dissolving film formulation of dexmedetomidine (BXCL501, BioXcel Therapeutics) may offer rapid relief from acute agitation related to schizophrenia or bipolar disorder (BD), results of two phase 3, randomized, placebo-controlled trials show.
For both disorders, BXCL501 showed “superiority over placebo” by meeting the primary endpoint of reduction of agitation as measured by the excited component of the Positive and Negative Syndrome Scale (PANSS), study investigator Leslie Citrome, MD, MPH, department of psychiatry and behavioral sciences, New York Medical College, Valhalla, said in an interview.
The findings were presented at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Noninvasive option
Acute agitation in patients with schizophrenia or BD is often encountered in emergency departments (EDs) and inpatient units. When nondrug tactics fail to calm the patient, drug options include injectable antipsychotics or benzodiazepines. BXCL501 is a thin, orally dissolving film for sublingual or buccal use.
“Dexmedetomidine is a highly-selective alpha-2a receptor agonist and we haven’t really had one of those before in psychiatry for this purpose. And we haven’t had much in the way of orally dissolving thin films that are absorbed in the oral mucosa so this represents an opportunity to provide a potential intervention that does not require an injection and yet could possibly be of use in people who are agitated,” Dr. Citrome said.
The study, known as SERENITY I, included 380 adults (mean age 45.6 years, 63% male) with schizophrenia, schizoaffective disorder, or schizophreniform disorder, and acute agitation in the ED (total score ≥ 14 on the PANSS-Excited Component (PEC) scale at baseline and a score ≥ 4 on at least one of the five PEC items).
Patients were randomly allocated to a single oral dose of BXCL501: 120 mcg, 180 mcg, or placebo. A total of 372 patients (97.9%) completed the study.
Mean PEC total score was 17.6 at baseline. The mean change from baseline in the PEC total score at 2 hours (primary endpoint) was -8.5 and -10.3 with BXCL501 120 mcg and 180 mcg, respectively, versus -4.8 for placebo (P < .0001 vs. placebo).
PEC response rates (≥ 40% reduction from baseline) were 80.6% and 89.6% with BXCL501 120 mcg and 180 mcg versus 47.6% with placebo (P < .0001 vs. placebo).
Compared with placebo, and in the Agitation and Calmness Evaluation Scale (ACES) at 2 hours post dosing.
The incidence of adverse events (AE) was 39.5%, 37.3%, and 15.1% with BXCL501 120 mg, 180 mg, and placebo groups.
All AEs were mild or moderate. The most common AEs with BXCL501 were somnolence, dizziness, dry mouth, hypotension, orthostatic hypotension, hypoesthesia, and paresthesia. No drug-related severe or serious AEs occurred.
Nipping it in the bud
SERENITY II had a similar design. This study included 380 adults (mean age 48, 55% female) with bipolar I or II disorder and acute agitation in the ED (total score ≥14 on the PEC scale at baseline and a score ≥4 on at least one PEC item). A total of 362 (95.3%) of patients completed the study.
Mean PEC total score was 18 at baseline. The mean change from baseline in the PEC total score at 2 hours (primary endpoint) was -9.0 and -10.4 with BXCL501 120 mcg and 180 mcg, respectively, versus -4.9 for placebo (P < .0001 vs. placebo).
Bipolar patients also saw significant improvement on the secondary outcomes of CGI-I and ACES, with an adverse event profile similar to that seen in patients with schizophrenia.
BXCL501 demonstrated “rapid, robust and clinically meaningful efficacy” in both patient populations and represents a “novel, noninvasive and well tolerated treatment of agitation,” the investigators concluded in their APA abstracts.
“Patients who are agitated are in psychic pain and they want relief from this psychic pain. We’re also worried that they might get worse and that agitation escalates to aggression potentially requiring restraints. We want to avoid that,” Dr. Citrome said.
“By nipping it in the bud with a pharmacological intervention, we can ease their psychic pain and we can manage a potentially dangerous situation. Offering an oral medicine that would work quickly would be ideal in my mind and patients might potentially be more accepting of that than an injection,” Dr. Citrome said.
Based on the SERENITY I and II data, BioXcel Therapeutics has submitted a new drug application to the Food and Drug Administration.
Negotiation first, medication second
Reached for comment, Samoon Ahmad, MD, professor, department of psychiatry, New York (N.Y.) University, cautioned that, “when we talk about treating an agitated patient, medication is only part of the picture.
“There is a negotiating process with the patient. Number one, you offer them an environment that is conducive to making them feel calm, safe, and secure and that they are being listened to. Providing all of those things sometimes can be very helpful,” said Dr. Ahmad, who serves as unit chief of inpatient psychiatry at Bellevue Hospital Center in New York City.
“If someone starts throwing chairs at you or assaulting you, that is not really the time to negotiate a medicine; you basically have to restrain the patient, and many times give them intramuscular medicine,” Dr. Ahmad said.
He also noted that patients in the SERENITY trials had moderate to severe acute agitation.
“These are people you can potentially negotiate with. But again, when a patient crosses a certain line, you have to immediately do something and that could be intramuscular injection or something oral, which they may spit right in your face, which has happened numerous times,” Dr. Ahmad said.
“I don’t think intramuscular options will ever go away but an oral agent could be a useful tool as well,” said Dr. Ahmad, founder of the Integrative Center for Wellness in New York City.
He cautioned that clinicians are not going to be using this medicine in their offices. “If a patient walks in and is floridly psychotic, you will need to call 911. We’re really talking about its use either in the ED or acute inpatient setting,” Dr. Ahmad said.
A version of this article first appeared on Medscape.com.
Bright light therapy for bipolar depression: A review of 6 studies
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Tic disorders proliferate in bipolar patients with OCD
Bipolar disorder patients with comorbid obsessive-compulsive disorder were significantly more likely to suffer from tic disorders, as well as hoarding, excoriation, and body dysmorphic disorder, than were those without comorbid OCD, data from 70 patients suggest.
Between 10% and 20% of patients with bipolar disorder (BD) also meet criteria for obsessive-compulsive disorder (OCD), and these patients are more likely to experience treatment resistance and poor prognosis than are BD patients without OCD. In addition, preliminary indications suggest a specific association between OCD and bipolar depression (BP-D) in particular, wrote Leonid Braverman, MD, of Ma’ale HaCarmel Mental Health Center, Tirat Carmel, Israel, and colleagues.
In addition, “there is compelling evidence indicating that OCD-spectrum and tic disorders share with OCD clinical characteristics, familial inheritance, neurobiological underpinnings and some aspects of pharmacotherapy,” and investigations into the clinical characteristics of OCD spectrum behaviors in BP-D patients with and without OCD are ongoing, they said.
In a study published in the Journal of Obsessive-Compulsive and Related Disorders (2021 Mar 21. doi: 10.1016/j.jocrd.2021.100643), the researchers reviewed data from 87 adults who met the DSM-5 criteria for BP-D. Of these, 27 also met criteria for OCD, 17 for subthreshold OCD, and 43 had neither OCD nor subthreshold OCD. The researchers compared the 27 OCD patients and the 43 non-OCD patients; the OCD patients had significantly higher rates overall of body dysmorphic disorder, hoarding disorder, excoriation disorder, and tic disorder, compared with non-OCD patients (P range from < .05-0.01 for all). No differences between the groups appeared for trichotillomania.
Also, the researchers found significant between-group differences in the number of patients with at least one OCD spectrum disorder and tic disorders (13 of 19 patients in the OCD group vs. 3 of 37 patients in the non-OCD group) and in the co-occurrence of two OCD-spectrum and tic disorders (3 of 19 patients in the OCD group vs. 1 patient in the non-OCD group).
The most common comorbid psychiatric disorders in both groups were substance use and combined anxiety disorders, followed by eating disorders, but no between-group differences were found in the frequencies of any of these conditions.
“From the clinical perspective, in BP-D patients,” the researchers noted.
The study findings were limited by several factors, including the small sample size, cross-sectional design, and exclusion of subsyndromic disorders, the researchers noted. However, the results support findings from previous studies, and the study emphasizes the clinical complexity and poor prognosis for these patients. Therefore, additional research is needed in patients with BP-D verse the manic/hypomanic phases of bipolar illness to determine similar patterns, they said. Medication trials are needed to address functional impairments in these patients, given the differences in treatment of BDD, hoarding, excoriation, and tic disorders, compared with “pure” OCD, they concluded.
The study received no outside funding. The researchers reported no financial conflicts.
Bipolar disorder patients with comorbid obsessive-compulsive disorder were significantly more likely to suffer from tic disorders, as well as hoarding, excoriation, and body dysmorphic disorder, than were those without comorbid OCD, data from 70 patients suggest.
Between 10% and 20% of patients with bipolar disorder (BD) also meet criteria for obsessive-compulsive disorder (OCD), and these patients are more likely to experience treatment resistance and poor prognosis than are BD patients without OCD. In addition, preliminary indications suggest a specific association between OCD and bipolar depression (BP-D) in particular, wrote Leonid Braverman, MD, of Ma’ale HaCarmel Mental Health Center, Tirat Carmel, Israel, and colleagues.
In addition, “there is compelling evidence indicating that OCD-spectrum and tic disorders share with OCD clinical characteristics, familial inheritance, neurobiological underpinnings and some aspects of pharmacotherapy,” and investigations into the clinical characteristics of OCD spectrum behaviors in BP-D patients with and without OCD are ongoing, they said.
In a study published in the Journal of Obsessive-Compulsive and Related Disorders (2021 Mar 21. doi: 10.1016/j.jocrd.2021.100643), the researchers reviewed data from 87 adults who met the DSM-5 criteria for BP-D. Of these, 27 also met criteria for OCD, 17 for subthreshold OCD, and 43 had neither OCD nor subthreshold OCD. The researchers compared the 27 OCD patients and the 43 non-OCD patients; the OCD patients had significantly higher rates overall of body dysmorphic disorder, hoarding disorder, excoriation disorder, and tic disorder, compared with non-OCD patients (P range from < .05-0.01 for all). No differences between the groups appeared for trichotillomania.
Also, the researchers found significant between-group differences in the number of patients with at least one OCD spectrum disorder and tic disorders (13 of 19 patients in the OCD group vs. 3 of 37 patients in the non-OCD group) and in the co-occurrence of two OCD-spectrum and tic disorders (3 of 19 patients in the OCD group vs. 1 patient in the non-OCD group).
The most common comorbid psychiatric disorders in both groups were substance use and combined anxiety disorders, followed by eating disorders, but no between-group differences were found in the frequencies of any of these conditions.
“From the clinical perspective, in BP-D patients,” the researchers noted.
The study findings were limited by several factors, including the small sample size, cross-sectional design, and exclusion of subsyndromic disorders, the researchers noted. However, the results support findings from previous studies, and the study emphasizes the clinical complexity and poor prognosis for these patients. Therefore, additional research is needed in patients with BP-D verse the manic/hypomanic phases of bipolar illness to determine similar patterns, they said. Medication trials are needed to address functional impairments in these patients, given the differences in treatment of BDD, hoarding, excoriation, and tic disorders, compared with “pure” OCD, they concluded.
The study received no outside funding. The researchers reported no financial conflicts.
Bipolar disorder patients with comorbid obsessive-compulsive disorder were significantly more likely to suffer from tic disorders, as well as hoarding, excoriation, and body dysmorphic disorder, than were those without comorbid OCD, data from 70 patients suggest.
Between 10% and 20% of patients with bipolar disorder (BD) also meet criteria for obsessive-compulsive disorder (OCD), and these patients are more likely to experience treatment resistance and poor prognosis than are BD patients without OCD. In addition, preliminary indications suggest a specific association between OCD and bipolar depression (BP-D) in particular, wrote Leonid Braverman, MD, of Ma’ale HaCarmel Mental Health Center, Tirat Carmel, Israel, and colleagues.
In addition, “there is compelling evidence indicating that OCD-spectrum and tic disorders share with OCD clinical characteristics, familial inheritance, neurobiological underpinnings and some aspects of pharmacotherapy,” and investigations into the clinical characteristics of OCD spectrum behaviors in BP-D patients with and without OCD are ongoing, they said.
In a study published in the Journal of Obsessive-Compulsive and Related Disorders (2021 Mar 21. doi: 10.1016/j.jocrd.2021.100643), the researchers reviewed data from 87 adults who met the DSM-5 criteria for BP-D. Of these, 27 also met criteria for OCD, 17 for subthreshold OCD, and 43 had neither OCD nor subthreshold OCD. The researchers compared the 27 OCD patients and the 43 non-OCD patients; the OCD patients had significantly higher rates overall of body dysmorphic disorder, hoarding disorder, excoriation disorder, and tic disorder, compared with non-OCD patients (P range from < .05-0.01 for all). No differences between the groups appeared for trichotillomania.
Also, the researchers found significant between-group differences in the number of patients with at least one OCD spectrum disorder and tic disorders (13 of 19 patients in the OCD group vs. 3 of 37 patients in the non-OCD group) and in the co-occurrence of two OCD-spectrum and tic disorders (3 of 19 patients in the OCD group vs. 1 patient in the non-OCD group).
The most common comorbid psychiatric disorders in both groups were substance use and combined anxiety disorders, followed by eating disorders, but no between-group differences were found in the frequencies of any of these conditions.
“From the clinical perspective, in BP-D patients,” the researchers noted.
The study findings were limited by several factors, including the small sample size, cross-sectional design, and exclusion of subsyndromic disorders, the researchers noted. However, the results support findings from previous studies, and the study emphasizes the clinical complexity and poor prognosis for these patients. Therefore, additional research is needed in patients with BP-D verse the manic/hypomanic phases of bipolar illness to determine similar patterns, they said. Medication trials are needed to address functional impairments in these patients, given the differences in treatment of BDD, hoarding, excoriation, and tic disorders, compared with “pure” OCD, they concluded.
The study received no outside funding. The researchers reported no financial conflicts.
FROM THE JOURNAL OF OBSESSIVE-COMPULSIVE AND RELATED DISORDERS
Helping psychiatric patients heal holistically
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
Contradictions abound in ‘The End of Mental Illness’
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Antidepressants, TMS, and the risk of affective switch in bipolar depression
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).
In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.
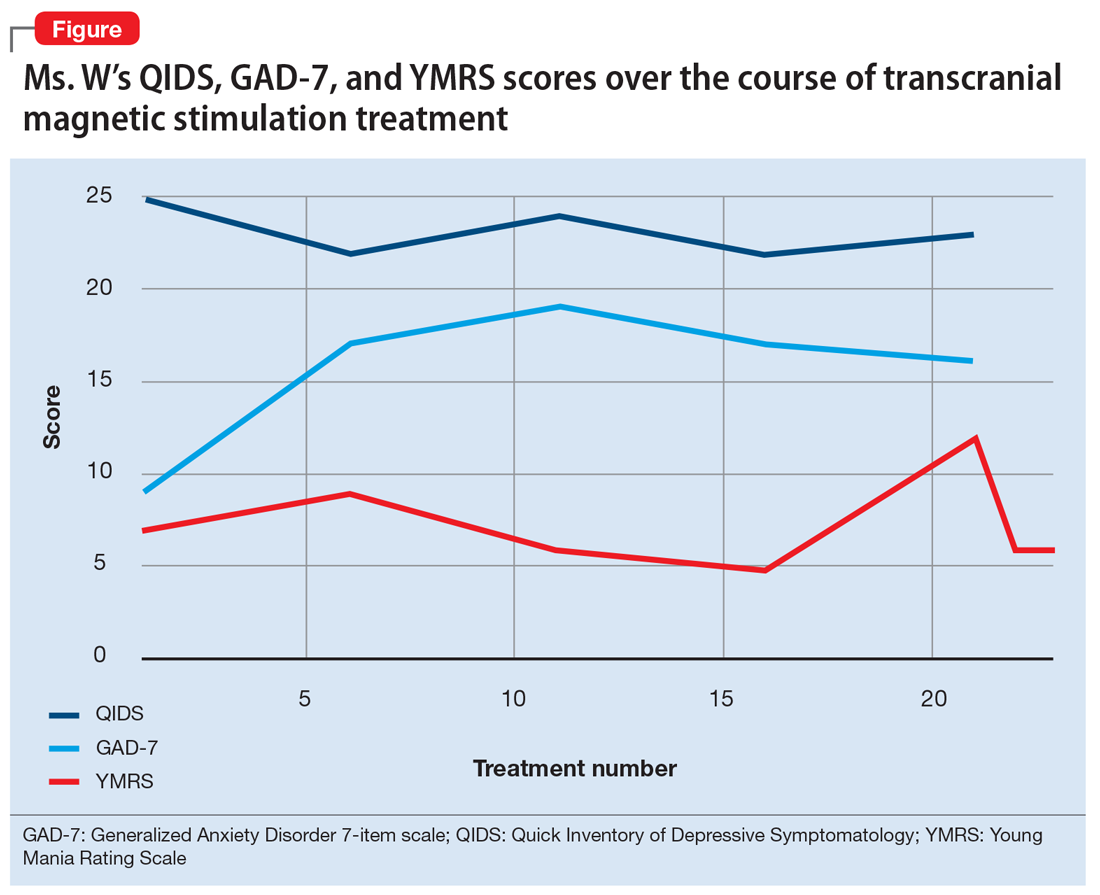
Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).
In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.

Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
Because treatment resistance is a pervasive problem in bipolar depression, the use of neuromodulation treatments such as transcranial magnetic stimulation (TMS) is increasing for patients with this disorder.1-7 Patients with bipolar disorder tend to spend the majority of the time with depressive symptoms, which underscores the importance of providing effective treatment for bipolar depression, especially given the chronicity of this disease.2,3,5 Only a few medications are FDA-approved for treating bipolar depression (Table).
In this article, we describe the case of a patient with treatment-resistant bipolar depression undergoing adjunctive TMS treatment who experienced an affective switch from depression to mania. We also discuss evidence regarding the likelihood of treatment-emergent mania for antidepressants vs TMS in bipolar depression.
CASE
Ms. W, a 60-year-old White female with a history of bipolar I disorder and attention-deficit/hyperactivity disorder (ADHD), presented for TMS evaluation during a depressive episode. Throughout her life, she had experienced numerous manic episodes, but as she got older she noted an increasing frequency of depressive episodes. Over the course of her illness, she had completed adequate trials at therapeutic doses of many medications, including second-generation antipsychotics (SGAs) (aripiprazole, lurasidone, olanzapine, quetiapine), mood stabilizers (lamotrigine, lithium), and antidepressants (bupropion, venlafaxine, fluoxetine, mirtazapine, trazodone). A course of electroconvulsive therapy was not effective. Ms. W had a long-standing diagnosis of ADHD and had been treated with stimulants for >10 years, although it was unclear whether formal neuropsychological testing had been conducted to confirm this diagnosis. She had >10 suicide attempts and multiple psychiatric hospitalizations.
At her initial evaluation for TMS, Ms. W said she had depressive symptoms predominating for the past 2 years, including low mood, hopelessness, poor sleep, poor appetite, anhedonia, and suicidal ideation without a plan. At the time, she was taking clonazepam, 0.5 mg twice a day; lurasidone, 40 mg/d at bedtime; fluoxetine, 60 mg/d; trazodone, 50 mg/d at bedtime; and methylphenidate, 40 mg/d, and was participating in psychotherapy consistently.
After Ms. W and her clinicians discussed alternatives, risks, benefits, and adverse effects, she consented to adjunctive TMS treatment and provided written informed consent. The treatment plan was outlined as 6 weeks of daily TMS therapy (NeuroStar; Neuronetics, Malvern, PA), 1 treatment per day, 5 days a week. Her clinical status was assessed weekly using the Quick Inventory of Depressive Symptomatology (QIDS) for depression, Generalized Anxiety Disorder 7-item scale (GAD-7) for anxiety, and Young Mania Rating Scale (YMRS) for mania. The Figure shows the trends in Ms. W’s QIDS, GAD-7, and YMRS scores over the course of TMS treatment.

Prior to initiating TMS, her baseline scores were QIDS: 25, GAD-7: 9, and YMRS: 7, indicating very severe depression, mild anxiety, and the absence of mania. Ms. W’s psychotropic regimen remained unchanged throughout the course of her TMS treatment. After her motor threshold was determined, her TMS treatment began at 80% of motor threshold and was titrated up to 95% at the first treatment. By the second treatment, it was titrated up to 110%. By the third treatment, it was titrated up to 120% of motor threshold, which is the percentage used for the remaining treatments.
Initially, Ms. W reported some improvement in her depression, but this improvement was short-lived, and she continued to have elevated QIDS scores throughout treatment. By treatment #21, her QIDS and GAD-7 scores remained elevated, and her YMRS score had increased to 12. Due to this increase in YMRS score, the YMRS was repeated on the next 2 treatment days (#22 and #23), and her score was 6 on both days. When Ms. W presented for treatment #25, she was disorganized, irritable, and endorsed racing thoughts and decreased sleep. She was involuntarily hospitalized for mania, and TMS was discontinued. Unfortunately, she did not complete any clinical scales on that day. Upon admission to the hospital, Ms. W reported that at approximately the time of treatment #21, she had a fluctuation in her mood that consisted of increased goal-directed activity, decreased need for sleep, racing thoughts, and increased frivolous spending. She was treated with lithium, 300 mg twice a day. Lurasidone was increased to 80 mg/d at bedtime, and she continued clonazepam, trazodone, and methylphenidate at the previous doses. Over 14 days, Ms. W’s mania gradually resolved, and she was discharged home.
Continue to: Mixed evidence on the risk of switching
Mixed evidence on the risk of switching
Currently, several TMS devices are FDA-cleared for treating unipolar major depressive disorder, obsessive-compulsive disorder, and certain types of migraine. In March 2020, the FDA granted Breakthrough Device Designation for one TMS device, the NeuroStar Advanced Therapy System, for the treatment of bipolar depression.8 This designation created an expedited pathway for prioritized FDA review of the NeuroStar Advanced Therapy clinical trial program.
Few published clinical studies have evaluated using TMS to treat patients with bipolar depression.9-15 As with any antidepressant treatment for bipolar depression, there is a risk of affective switch from depression to mania when using TMS. Most of the literature available regarding the treatment of bipolar depression focuses on the risk of antidepressant medications to induce an affective switch. This risk depends on the class of the antidepressant,16 and there is a paucity of studies examining the risk of switch with TMS.
Interpretation of available literature is limited due to inconsistencies in the definition of an affective switch, the variable length of treatment with antidepressants, the use of concurrent medications such as mood stabilizers, and confounders such as the natural course of switching in bipolar disorder.17 Overall, the evidence for treatment-emergent mania related to antidepressant use is mixed, and the reported rate of treatment-emergent mania varies. In a systematic review and meta-analysis of >20 randomized controlled trials that included 1,316 patients with bipolar disorder who received antidepressants, Fornaro et al18 found that the incidence of treatment-emergent mania was 11.8%. It is generally recommended that if antidepressants are used to treat patients with bipolar disorder, they should be given with a traditional mood stabilizer to prevent affective switches, although whether mood stabilizers can prevent such switches is unproven.19
In a literature review by Xia et al,20 the affective switch rate in patients with bipolar depression who were treated with TMS was 3.1%, which was not statistically different from the affective switch rate with sham treatment.However, most of the patients included in this analysis were receiving other medications concurrently, and the length of treatment was 2 weeks, which is shorter than the average length of TMS treatment in clinical practice. In a recent literature review by Rachid,21 TMS was found to possibly induce manic episodes when used as monotherapy or in combination with antidepressants in patients with bipolar depression. To reduce the risk of treatment-emergent mania, current recommendations advise the use of a mood stabilizer for a minimum of 2 weeks before initiating TMS.1
In our case, Ms. W was receiving antidepressants (fluoxetine and trazodone), lurasidone (an SGA that is FDA-approved for bipolar depression), and methylphenidate before starting TMS treatment. Fluoxetine, trazodone, and methylphenidate may possibly contribute to an increased risk of an affective switch.1,22 Further studies are needed to clarify whether mood stabilizers or SGAs can prevent the development of mania in patients with bipolar depression who undergo TMS treatment.20
Continue to: Because bipolar depression poses...
Because bipolar depression poses a major clinical challenge,23,24 it is imperative to consider alternate treatments. When evaluating alternative treatment strategies, one may consider TMS in conjunction with a traditional mood stabilizer because this regimen may have a lower risk of treatment-emergent mania compared with antidepressants.1,25
Acknowledgment
The authors thank Dr. Sy Saeed for his expertise and guidance on this article.
Bottom Line
For patients with bipolar depression, treatment with transcranial magnetic stimulation in conjunction with a mood stabilizer may have lower rates of treatment-emergent mania than treatment with antidepressants.
Related Resources
- Transcranial magnetic stimulation: clinical applications for psychiatric practice. Bermudes RA, Lanocha K, Janicak PG, eds. American Psychiatric Association Publishing; 2017.
- Gold AK, Ornelas AC, Cirillo P, et al. Clinical applications of transcranial magnetic stimulation in bipolar disorder. Brain Behav. 2019;9(10):e01419. doi: 10.1002/brb3.1419
Drug Brand Names
Aripiprazole • Abilify
Bupropion • Wellbutrin
Cariprazine • Vraylar
Clonazepam • Klonopin
Fluoxetine • Prozac
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Methylphenidate • Ritalin, Concerta
Mirtazapine • Remeron
Olanzapine • Zyprexa
Olanzapine-fluoxetine • Symbyax
Quetiapine • Seroquel
Trazodone • Desyrel
Venlafaxine • Effexor
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
1. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes RA, Lanocha K, Janicak PG, eds. Transcranial magnetic stimulation: clinical applications for psychiatric practice. American Psychiatric Association Publishing; 2017:127-156.
2. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
3. Gitlin M. Treatment-resistant bipolar disorder. Molecular Psychiatry. 2006;11(3):227-240.
4. Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41(1):18-30.
5. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68(3):241-251.
6. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235: 20-26.
7. Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593-2601.
8. Neuronetics. FDA grants NeuroStar® Advanced Therapy System Breakthrough Device Designation to treat bipolar depression. Accessed February 2, 2021. https://www.globenewswire.com/news-release/2020/03/06/1996447/0/en/FDA-Grants-NeuroStar-Advanced-Therapy-System-Breakthrough-Device-Designation-to-Treat-Bipolar-Depression.html
9. Cohen RB, Brunoni AR, Boggio PS, et al. Clinical predictors associated with duration of repetitive transcranial magnetic stimulation treatment for remission in bipolar depression: a naturalistic study. J Nerv Ment Dis. 2010;198(9):679-681.
10. Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567-e573.
11. Dell’osso B, D’Urso N, Castellano F, et al. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: a 1-year follow-up study. J ECT. 2011;27(2):141-144.
12. Dell’Osso B, Mundo E, D’Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11(1):76-81.
13. Harel EV, Zangen A, Roth Y, et al. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119-126.
14. Nahas Z, Kozel FA, Li X, et al. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord. 2003;5(1):40-47.
15. Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19(2):198-199.
16. Tundo A, Cavalieri P, Navari S, et al. Treating bipolar depression - antidepressants and alternatives: a critical review of the literature. Acta Neuropsychiatrica. 2011:23(3):94-105.
17. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161(9):1537-1547.
18. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant‐emergent mania in bipolar depression: a systematic review and meta‐analysis. Bipolar Disord. 2018;20(3):195-227.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
20. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
21. Rachid F. Repetitive transcranial magnetic stimulation and treatment-emergent mania and hypomania: a review of the literature. J Psychiatr Pract. 2017;23(2):150-159.
22. Victorin A, Rydén E, Thase M, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
23. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35.
24. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
25. Phillips AL, Burr RL, Dunner DL. Repetitive transcranial magnetic stimulation in the treatment of bipolar depression: Experience from a clinical setting. J Psychiatr Pract. 2020;26(1):37-45.
Steroid-induced psychiatric symptoms: What you need to know
Ms. N, age 30, presents to the emergency department for altered mental status, insomnia, and behavioral changes, which she has experienced for 1 week. On evaluation, she grabs a clinician’s hand and details her business ideas and life story with no prompting. Ms. N’s mental status examination is significant for hyperverbal speech with increased rate and volume; tangential thought process; and bright, expanded affect.
One week earlier, Ms. N was hospitalized for sudden-onset chest pain, weakness, and dizziness. She received 45 minutes of cardiopulmonary resuscitation prior to presentation and was found to have a ST-segment elevation myocardial infarction that required emergent left anterior descending coronary artery and right coronary artery percutaneous coronary intervention to place drug-eluting stents. Her recovery was complicated by acute cardiogenic shock, pulmonary edema, and hypoxic respiratory failure. Subsequently, she was intubated, admitted to the ICU, and received high-dose corticosteroids, including IV methylprednisolone, 40 mg every 12 hours, which was tapered prior to discharge. Her husband reports that since Ms. N came home, she has been more talkative and irritable, ruminating about past events, unable to sleep (<1 hour/night), and crying frequently. She has also been endorsing visual and auditory hallucinations, with increased praying and listening to religious music.
The frequent clinical use of steroids necessitates an understanding of these medications’ various adverse effects. The manifestations of steroid-induced psychiatric symptoms are broad and can involve affective, behavioral, and cognitive domains. While the current mechanism is unknown, this phenomenon may be related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity, as well as effects on brain regions such as the hippocampus and amygdala. The best interventions for steroid-induced psychiatric symptoms are awareness and early diagnosis. There are no FDA-approved treatments for steroid-induced psychiatric symptoms; initial measures should include tapering or discontinuing corticosteroids.
In this article, we review the literature on the incidence, characteristics, differential diagnoses, proposed mechanism, risk factors, and proposed treatments of steroid-induced psychiatric symptoms.
A wide range of presentations
Steroid use has increased over the past 2 decades, with 10% of medical and surgical inpatients and 1% to 3% of the general population taking long-term glucocorticoids.1 Even with topical application, steroid therapy is often systemically absorbed, and thus may lead to steroid-induced psychiatric symptoms. The incidence of steroid-induced psychiatric symptoms is difficult to assess because there can be a wide range of reactions that are dose- and time-related. Three reviews of a total of 122 cases reports found that an estimated 5% of patients treated with steroids experience severe psychiatric reactions.1-3
Steroid-induced psychopathology can include mood, behavioral, and/or cognitive impairments. Mania/hypomania is the most common overall psychiatric symptom; the most common mood manifestations are anxiety and depression.4,5 Other possible steroid-induced symptoms include psychosis, dementia, panic disorder, delirium, suicidal thinking and behavior, aggressive behavior, insomnia, agitation, depersonalization, and euphoria.5 The most common cognitive impairment is verbal or declarative memory deficit; others include distractibility and deficits in attention and psychomotor speed.5 These psychiatric symptoms can have a rapid onset, possibly within hours of starting steroids.1 However, studies have reported a median time to onset of 11.5 days; 39% of cases had onset during the first week and 62% within 2 weeks.3,6 After reducing or stopping the steroid, it may take days to weeks before symptoms start to subside.2
What to consider in the differential Dx
Psychiatric symptoms that are induced by steroids can mimic metabolic, neurologic, or toxic disorders. Other factors to consider include drug withdrawal/intoxication, infections, and paraneoplastic syndromes.4,5 Although there is no reported correlation between the location of neurologic lesions and the development of specific psychiatric symptoms, manic symptoms appear most commonly with lesions in the right frontal lobe. 4 Other factors to note include the presence of new-onset psychiatric illnesses such as bipolar, mood, or thought disorders,4 as well as psychosocial stressors that might be contributing to the patient’s presentation.5
Continue to: Proposed mechanisms
Proposed mechanisms
Although the exact mechanism by which steroids induce psychiatric symptoms is unknown, several mechanisms have been proposed. One hypothesis is that steroid-induced psychopathology is related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity.4,5,7 This may explain why many patients with major depressive disorder have elevated cortisol production and/or lack of suppression of cortisol secretion during a dexamethasone stimulation test, and why approximately one-half of patients with Cushing’s disease experience depressive symptoms.8 This is also likely why antipsychotics, which typically reduce cortisol, are efficacious treatments for some steroid-induced psychiatric symptoms.9
Cognitive impairments from steroid use may be related to these agents’ effects on certain brain regions. One such area is the hippocampus, an important mediator in the creation and maintenance of episodic and declarative memories.5,8,9 Acute glucocorticoid use is associated with decreased activity in the left hippocampus, reduced hippocampal glucose metabolism, and reduced cerebral blood flow in the posterior medial temporal lobe.10 Long-term glucocorticoid exposure is associated with smaller hippocampal volume and lower levels of temporal lobe N-acetylaspartate, a marker of neuronal viability.10 Because working memory depends on the prefrontal cortex and declarative memory relies on the hippocampus, deficits in these functions can be attributed to the effect of prolonged glucocorticoid exposure on glucocorticoid or mineralocorticoid receptors in the hippocampus, reduction of hippocampal volume, or elevated glutamate accumulation in that area.11 In addition, high cortisol levels inhibit brain-derived neurotrophic factor, which plays a crucial role in maintaining neural architecture in key brain regions such as the hippocampus and prefrontal cortex.11 There is also a correlation between the duration of prednisone treatment and atrophy of the right amygdala, which is an important regulator of mood and anxiety.11 Both the hippocampus and amygdala have dense collections of glucocorticoid receptors. This may explain why patients who receive high-dose corticosteroids can have reversible atrophy in the hypothalamus and amygdala, leading to deficits in emotional learning and the stress response.
Factors that increase risk
Several factors can increase the risk of steroid-induced psychopathology. The most significant is the dose; higher doses are more likely to produce psychiatric symptoms.1,5 Concurrent use of drugs that increase circulating levels of corticosteroids, such as inhibitors of the cytochrome P450 (CYP) enzyme (eg, clarithromycin), also increases the likelihood of developing psychiatric symptoms.1,5 Risk is also increased in patients with liver or renal dysfunction.1 Cerebral spinal fluid/serum albumin ratio, a marker of blood-brain barrier damage, and low serum complement levels were also reported to be independent risk factors,12 with the thought that increased permeability of the blood-brain barrier may allow hydrophobic steroid molecules to more easily penetrate the CNS, leading to increased neuropsychiatric effects. Hypoalbuminemia is another reported risk factor, perhaps because lower levels of serum albumin are related to higher levels of free and active glucocorticoids, which are normally inactive when bound to albumin.13 There also appears to be an increased prevalence of steroid-induced psychopathology in women, perhaps due to greater propensity in women to seek medical care or a higher prevalence of women with medical disorders that are treated with steroids.5 A previous history of psychiatric disorders may not increase risk.5
Several methods for reducing risk have been proposed, including using a divided-dosing regimens that may lower peak steroid plasma concentrations.13,14 However, the best prevention of steroid-induced psychiatric symptoms are awareness, early diagnosis, and intervention. Studies have suggested that N-methyl-
Treatment options
There are no FDA-approved medications for managing steroid-induced psychiatric symptoms.1,16 Treatment is based on evidence from case reports and a few small case series (Table2-5,17,18).
Continue to: When possible, initial treatment...
When possible, initial treatment should include discontinuing or tapering corticosteroids to <40 mg/d of prednisone-equivalent.1,4,10,18 Most studies have reported rapid reversal of deficits in declarative memory and of hippocampal volume loss once corticosteroids were tapered and discontinued.1,18 One study reported that >90% of patients recovered within 6 weeks, with patients with delirium recovering more quickly (mean: 5.4 days) than those with depression, mania, or psychosis (mean: 19.3 days).3 Another found that the vast majority (92%) of patients treated only with a steroid taper achieved clinical recovery, and 84% recovered with administration of antipsychotics without a steroid taper.3 In this study, all patients who received electroconvulsive therapy (ECT) recovered, as did those who received a steroid taper plus lithium or antipsychotics. Steroid tapering regimens are especially important for patients who have received long-term glucocorticoid treatment. Patients need to be closely monitored for signs of new or increased depression, delirium, or confusion during the taper. If these symptoms occur, the patient should be checked for adrenocortical insufficiency, which can be resolved by re-administering or increasing the dosage of the glucocorticoid.10
Mania. The treatment of mania/hypomania includes mood stabilizers (valproate, lithium, lamotrigine) and antipsychotics (quetiapine, olanzapine, haloperidol).2,4,5,10,14,18 Valproate has been reported to be an effective prophylactic of corticosteroid-induced mania,2 perhaps because it dampens neuronal hyperexcitability by attenuating NMDA receptors, blocking voltage-dependent sodium channels, and inhibiting the synthesis of cortical GABAergic steroids. Starting valproate while continuing corticosteroids (if necessary) may help lessen mania.2 Benzodiazepines also may be useful on a short-term basis.
Depression. Steroid-induced depression may be treated with sertraline or other first-line antidepressants.5,14 Consider ECT for patients with severe depression. Support for the use of antipsychotic medications stems from studies that reported steroids’ role in disrupting dopamine and 5HT2 activity. Lithium also has been used successfully to manage and prevent glucocorticoid-associated affective disorder.10,18 It can be used alone or in combination with selective serotonin reuptake inhibitors to alleviate depressive symptoms.10 Tricyclic antidepressants are generally avoided because their anticholinergic effects can exacerbate or worsen delirium.18 In general, ECT is an effective treatment for persistent and/or unresponsive steroid-induced depression,2,10 but may be difficult to use in patients with serious medical illnesses.
Agitation. Medications that have been proposed for treating steroid-induced agitation include benzodiazepines, haloperidol, and second-generation antipsychotics.5,17
Other considerations. Clinicians, patients, and families should discuss in detail the risks of steroid-induced psychiatric symptoms so an early diagnosis and appropriate intervention can be implemented. Before starting steroids, it is important to review the patient’s current medication list to ensure that steroid treatment is indicated, and to check for potential drug–drug interactions. In addition, the medical condition that is being treated with steroids also needs to be carefully reviewed, because certain illnesses are associated with the development of psychiatric symptoms. 5,10
Continue to: Young children...
Young children (age <6) and older adults appear to be at greater risk for cognitive and memory disturbances from steroid use.10 In addition, patients have individual levels of susceptibility to steroid-induced psychiatric symptoms that can vary over time. The risk for adverse effects may be elevated based on response to previous courses of glucocorticoid treatment.10 While gender, age, dosage, and duration of treatment influence risk, it is not possible to predict which patients will experience psychiatric effects during a given course of glucocorticoid therapy. Therefore, all patients should be considered to have the potential of developing such effects, and should be monitored during glucocorticoid treatment and withdrawal.
Goals for future research
To help reduce the severity of and cost associated with steroid-induced psychiatric symptoms,5,14 future studies should focus on controlled trials of preventative strategies. In particular, recent advances in genetic mapping may help identify involvement of certain genes or polymorphisms.5 Because current guidelines for the prevention and treatment of steroid-induced psychiatric symptoms are not evidence-based, controlled clinical trials are needed to elucidate the optimal management of such symptoms. There is much interplay between many of the proposed mechanisms of steroid-induced psychiatric symptoms, and future studies can help uncover a deeper understanding of the intricacies of this phenomenon.
CASE CONTINUED
Mrs. N is admitted for altered mental status. Medical workup includes MRI of the brain, MRI of the neck, cardiac echocardiogram, and EEG. There is no evidence of acute structural pathology. She is started on olanzapine, 10 mg/d at bedtime for manic and psychotic symptoms, and is discharged after 5 days. After 1 month, the outpatient psychiatrist gradually decreases and discontinues olanzapine as Mrs. N steadily returns to baseline. One year after discharge, Mrs. N continues to report resolution of her manic and psychotic symptoms.
Bottom Line
Steroids can induce a wide range of psychiatric symptoms, including mania/ hypomania, anxiety, and depression. Initial treatment typically includes tapering or discontinuing the steroid when possible. Other proposed treatments include certain antipsychotics, antidepressants, and other psychotropics, but the supporting evidence is largely anecdotal or based on case studies. Additional research is needed to elucidate the mechanism and treatment recommendations.
Related Resources
- Janes M, Kuster S, Goldson TM, et al. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32(4):614-615.
- Mayo Clinic. Prednisone and other corticosteroids. https://www.mayoclinic.org/steroids/art-20045692
Drug Brand Names
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Methylprednisolone injection • Solu-Medrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproate • Depakote
1. Dubovsky AN, Arvikar S, Stern TA, et al. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
2. Roxanas MG, Hunt GE. Rapid reversal of corticosteroid-induced mania with sodium valproate: a case series of 20 patients. Psychosomatics. 2012;53(6):575-581.
3. Lewis DA, Smith RE. Steroid‐induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319-332.
4. Warren KN, Katakam J, Espiridion ED. Acute-onset mania in a patient with non-small cell lung cancer. Cureus. 2019;11(8):e5436.
5. Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
6. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen. Psychiatry. 1981;38(4):471-477.
7. Ularntinon S, Tzuang D, Dahl G, et al. Concurrent treatment of steroid-related mood and psychotic symptoms with risperidone. Pediatrics. 2010;125(5):e1241-e1245.
8. Pokladinkova J, Meyboom RH, Vlcek J, et al. Intranasally administered corticosteroids and neuropsychiatric disturbances: a review of the international pharmacovigilance programme of the World Health Organization. Ann Allergy Asthma Immunol. 2008;101(1):67-73.
9. Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410-417.
10. Wolkowitz OM, Reus UI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61(5):698-711.
11. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045-1051.
12. Appenzeller S, Cendes F, Costallat LT. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
13. Glynne-Jones R, Vernon CC, Bell G. Is steroid psychosis preventable by divided doses? Lancet. 1986;2(8520):1404.
14. Ismail MF, Lavelle C, Cassidy EM. Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719-2731.
15. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89-98.
16. Brown BS, Stuard G, Liggin JDM, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543-548.
17. Desai S, Khanani S, Shad MU, et al. Attenutation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. J Clin Psychopharmacol. 2009;29(3):284-287.
18. Gable M, Depry D. Sustained corticosteroid-induced mania and psychosis despite cessation: a case study and brief literature review. Int J Psychiatry Med. 2015;50(4):398-404.
Ms. N, age 30, presents to the emergency department for altered mental status, insomnia, and behavioral changes, which she has experienced for 1 week. On evaluation, she grabs a clinician’s hand and details her business ideas and life story with no prompting. Ms. N’s mental status examination is significant for hyperverbal speech with increased rate and volume; tangential thought process; and bright, expanded affect.
One week earlier, Ms. N was hospitalized for sudden-onset chest pain, weakness, and dizziness. She received 45 minutes of cardiopulmonary resuscitation prior to presentation and was found to have a ST-segment elevation myocardial infarction that required emergent left anterior descending coronary artery and right coronary artery percutaneous coronary intervention to place drug-eluting stents. Her recovery was complicated by acute cardiogenic shock, pulmonary edema, and hypoxic respiratory failure. Subsequently, she was intubated, admitted to the ICU, and received high-dose corticosteroids, including IV methylprednisolone, 40 mg every 12 hours, which was tapered prior to discharge. Her husband reports that since Ms. N came home, she has been more talkative and irritable, ruminating about past events, unable to sleep (<1 hour/night), and crying frequently. She has also been endorsing visual and auditory hallucinations, with increased praying and listening to religious music.
The frequent clinical use of steroids necessitates an understanding of these medications’ various adverse effects. The manifestations of steroid-induced psychiatric symptoms are broad and can involve affective, behavioral, and cognitive domains. While the current mechanism is unknown, this phenomenon may be related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity, as well as effects on brain regions such as the hippocampus and amygdala. The best interventions for steroid-induced psychiatric symptoms are awareness and early diagnosis. There are no FDA-approved treatments for steroid-induced psychiatric symptoms; initial measures should include tapering or discontinuing corticosteroids.
In this article, we review the literature on the incidence, characteristics, differential diagnoses, proposed mechanism, risk factors, and proposed treatments of steroid-induced psychiatric symptoms.
A wide range of presentations
Steroid use has increased over the past 2 decades, with 10% of medical and surgical inpatients and 1% to 3% of the general population taking long-term glucocorticoids.1 Even with topical application, steroid therapy is often systemically absorbed, and thus may lead to steroid-induced psychiatric symptoms. The incidence of steroid-induced psychiatric symptoms is difficult to assess because there can be a wide range of reactions that are dose- and time-related. Three reviews of a total of 122 cases reports found that an estimated 5% of patients treated with steroids experience severe psychiatric reactions.1-3
Steroid-induced psychopathology can include mood, behavioral, and/or cognitive impairments. Mania/hypomania is the most common overall psychiatric symptom; the most common mood manifestations are anxiety and depression.4,5 Other possible steroid-induced symptoms include psychosis, dementia, panic disorder, delirium, suicidal thinking and behavior, aggressive behavior, insomnia, agitation, depersonalization, and euphoria.5 The most common cognitive impairment is verbal or declarative memory deficit; others include distractibility and deficits in attention and psychomotor speed.5 These psychiatric symptoms can have a rapid onset, possibly within hours of starting steroids.1 However, studies have reported a median time to onset of 11.5 days; 39% of cases had onset during the first week and 62% within 2 weeks.3,6 After reducing or stopping the steroid, it may take days to weeks before symptoms start to subside.2
What to consider in the differential Dx
Psychiatric symptoms that are induced by steroids can mimic metabolic, neurologic, or toxic disorders. Other factors to consider include drug withdrawal/intoxication, infections, and paraneoplastic syndromes.4,5 Although there is no reported correlation between the location of neurologic lesions and the development of specific psychiatric symptoms, manic symptoms appear most commonly with lesions in the right frontal lobe. 4 Other factors to note include the presence of new-onset psychiatric illnesses such as bipolar, mood, or thought disorders,4 as well as psychosocial stressors that might be contributing to the patient’s presentation.5
Continue to: Proposed mechanisms
Proposed mechanisms
Although the exact mechanism by which steroids induce psychiatric symptoms is unknown, several mechanisms have been proposed. One hypothesis is that steroid-induced psychopathology is related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity.4,5,7 This may explain why many patients with major depressive disorder have elevated cortisol production and/or lack of suppression of cortisol secretion during a dexamethasone stimulation test, and why approximately one-half of patients with Cushing’s disease experience depressive symptoms.8 This is also likely why antipsychotics, which typically reduce cortisol, are efficacious treatments for some steroid-induced psychiatric symptoms.9
Cognitive impairments from steroid use may be related to these agents’ effects on certain brain regions. One such area is the hippocampus, an important mediator in the creation and maintenance of episodic and declarative memories.5,8,9 Acute glucocorticoid use is associated with decreased activity in the left hippocampus, reduced hippocampal glucose metabolism, and reduced cerebral blood flow in the posterior medial temporal lobe.10 Long-term glucocorticoid exposure is associated with smaller hippocampal volume and lower levels of temporal lobe N-acetylaspartate, a marker of neuronal viability.10 Because working memory depends on the prefrontal cortex and declarative memory relies on the hippocampus, deficits in these functions can be attributed to the effect of prolonged glucocorticoid exposure on glucocorticoid or mineralocorticoid receptors in the hippocampus, reduction of hippocampal volume, or elevated glutamate accumulation in that area.11 In addition, high cortisol levels inhibit brain-derived neurotrophic factor, which plays a crucial role in maintaining neural architecture in key brain regions such as the hippocampus and prefrontal cortex.11 There is also a correlation between the duration of prednisone treatment and atrophy of the right amygdala, which is an important regulator of mood and anxiety.11 Both the hippocampus and amygdala have dense collections of glucocorticoid receptors. This may explain why patients who receive high-dose corticosteroids can have reversible atrophy in the hypothalamus and amygdala, leading to deficits in emotional learning and the stress response.
Factors that increase risk
Several factors can increase the risk of steroid-induced psychopathology. The most significant is the dose; higher doses are more likely to produce psychiatric symptoms.1,5 Concurrent use of drugs that increase circulating levels of corticosteroids, such as inhibitors of the cytochrome P450 (CYP) enzyme (eg, clarithromycin), also increases the likelihood of developing psychiatric symptoms.1,5 Risk is also increased in patients with liver or renal dysfunction.1 Cerebral spinal fluid/serum albumin ratio, a marker of blood-brain barrier damage, and low serum complement levels were also reported to be independent risk factors,12 with the thought that increased permeability of the blood-brain barrier may allow hydrophobic steroid molecules to more easily penetrate the CNS, leading to increased neuropsychiatric effects. Hypoalbuminemia is another reported risk factor, perhaps because lower levels of serum albumin are related to higher levels of free and active glucocorticoids, which are normally inactive when bound to albumin.13 There also appears to be an increased prevalence of steroid-induced psychopathology in women, perhaps due to greater propensity in women to seek medical care or a higher prevalence of women with medical disorders that are treated with steroids.5 A previous history of psychiatric disorders may not increase risk.5
Several methods for reducing risk have been proposed, including using a divided-dosing regimens that may lower peak steroid plasma concentrations.13,14 However, the best prevention of steroid-induced psychiatric symptoms are awareness, early diagnosis, and intervention. Studies have suggested that N-methyl-
Treatment options
There are no FDA-approved medications for managing steroid-induced psychiatric symptoms.1,16 Treatment is based on evidence from case reports and a few small case series (Table2-5,17,18).
Continue to: When possible, initial treatment...
When possible, initial treatment should include discontinuing or tapering corticosteroids to <40 mg/d of prednisone-equivalent.1,4,10,18 Most studies have reported rapid reversal of deficits in declarative memory and of hippocampal volume loss once corticosteroids were tapered and discontinued.1,18 One study reported that >90% of patients recovered within 6 weeks, with patients with delirium recovering more quickly (mean: 5.4 days) than those with depression, mania, or psychosis (mean: 19.3 days).3 Another found that the vast majority (92%) of patients treated only with a steroid taper achieved clinical recovery, and 84% recovered with administration of antipsychotics without a steroid taper.3 In this study, all patients who received electroconvulsive therapy (ECT) recovered, as did those who received a steroid taper plus lithium or antipsychotics. Steroid tapering regimens are especially important for patients who have received long-term glucocorticoid treatment. Patients need to be closely monitored for signs of new or increased depression, delirium, or confusion during the taper. If these symptoms occur, the patient should be checked for adrenocortical insufficiency, which can be resolved by re-administering or increasing the dosage of the glucocorticoid.10
Mania. The treatment of mania/hypomania includes mood stabilizers (valproate, lithium, lamotrigine) and antipsychotics (quetiapine, olanzapine, haloperidol).2,4,5,10,14,18 Valproate has been reported to be an effective prophylactic of corticosteroid-induced mania,2 perhaps because it dampens neuronal hyperexcitability by attenuating NMDA receptors, blocking voltage-dependent sodium channels, and inhibiting the synthesis of cortical GABAergic steroids. Starting valproate while continuing corticosteroids (if necessary) may help lessen mania.2 Benzodiazepines also may be useful on a short-term basis.
Depression. Steroid-induced depression may be treated with sertraline or other first-line antidepressants.5,14 Consider ECT for patients with severe depression. Support for the use of antipsychotic medications stems from studies that reported steroids’ role in disrupting dopamine and 5HT2 activity. Lithium also has been used successfully to manage and prevent glucocorticoid-associated affective disorder.10,18 It can be used alone or in combination with selective serotonin reuptake inhibitors to alleviate depressive symptoms.10 Tricyclic antidepressants are generally avoided because their anticholinergic effects can exacerbate or worsen delirium.18 In general, ECT is an effective treatment for persistent and/or unresponsive steroid-induced depression,2,10 but may be difficult to use in patients with serious medical illnesses.
Agitation. Medications that have been proposed for treating steroid-induced agitation include benzodiazepines, haloperidol, and second-generation antipsychotics.5,17
Other considerations. Clinicians, patients, and families should discuss in detail the risks of steroid-induced psychiatric symptoms so an early diagnosis and appropriate intervention can be implemented. Before starting steroids, it is important to review the patient’s current medication list to ensure that steroid treatment is indicated, and to check for potential drug–drug interactions. In addition, the medical condition that is being treated with steroids also needs to be carefully reviewed, because certain illnesses are associated with the development of psychiatric symptoms. 5,10
Continue to: Young children...
Young children (age <6) and older adults appear to be at greater risk for cognitive and memory disturbances from steroid use.10 In addition, patients have individual levels of susceptibility to steroid-induced psychiatric symptoms that can vary over time. The risk for adverse effects may be elevated based on response to previous courses of glucocorticoid treatment.10 While gender, age, dosage, and duration of treatment influence risk, it is not possible to predict which patients will experience psychiatric effects during a given course of glucocorticoid therapy. Therefore, all patients should be considered to have the potential of developing such effects, and should be monitored during glucocorticoid treatment and withdrawal.
Goals for future research
To help reduce the severity of and cost associated with steroid-induced psychiatric symptoms,5,14 future studies should focus on controlled trials of preventative strategies. In particular, recent advances in genetic mapping may help identify involvement of certain genes or polymorphisms.5 Because current guidelines for the prevention and treatment of steroid-induced psychiatric symptoms are not evidence-based, controlled clinical trials are needed to elucidate the optimal management of such symptoms. There is much interplay between many of the proposed mechanisms of steroid-induced psychiatric symptoms, and future studies can help uncover a deeper understanding of the intricacies of this phenomenon.
CASE CONTINUED
Mrs. N is admitted for altered mental status. Medical workup includes MRI of the brain, MRI of the neck, cardiac echocardiogram, and EEG. There is no evidence of acute structural pathology. She is started on olanzapine, 10 mg/d at bedtime for manic and psychotic symptoms, and is discharged after 5 days. After 1 month, the outpatient psychiatrist gradually decreases and discontinues olanzapine as Mrs. N steadily returns to baseline. One year after discharge, Mrs. N continues to report resolution of her manic and psychotic symptoms.
Bottom Line
Steroids can induce a wide range of psychiatric symptoms, including mania/ hypomania, anxiety, and depression. Initial treatment typically includes tapering or discontinuing the steroid when possible. Other proposed treatments include certain antipsychotics, antidepressants, and other psychotropics, but the supporting evidence is largely anecdotal or based on case studies. Additional research is needed to elucidate the mechanism and treatment recommendations.
Related Resources
- Janes M, Kuster S, Goldson TM, et al. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32(4):614-615.
- Mayo Clinic. Prednisone and other corticosteroids. https://www.mayoclinic.org/steroids/art-20045692
Drug Brand Names
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Methylprednisolone injection • Solu-Medrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproate • Depakote
Ms. N, age 30, presents to the emergency department for altered mental status, insomnia, and behavioral changes, which she has experienced for 1 week. On evaluation, she grabs a clinician’s hand and details her business ideas and life story with no prompting. Ms. N’s mental status examination is significant for hyperverbal speech with increased rate and volume; tangential thought process; and bright, expanded affect.
One week earlier, Ms. N was hospitalized for sudden-onset chest pain, weakness, and dizziness. She received 45 minutes of cardiopulmonary resuscitation prior to presentation and was found to have a ST-segment elevation myocardial infarction that required emergent left anterior descending coronary artery and right coronary artery percutaneous coronary intervention to place drug-eluting stents. Her recovery was complicated by acute cardiogenic shock, pulmonary edema, and hypoxic respiratory failure. Subsequently, she was intubated, admitted to the ICU, and received high-dose corticosteroids, including IV methylprednisolone, 40 mg every 12 hours, which was tapered prior to discharge. Her husband reports that since Ms. N came home, she has been more talkative and irritable, ruminating about past events, unable to sleep (<1 hour/night), and crying frequently. She has also been endorsing visual and auditory hallucinations, with increased praying and listening to religious music.
The frequent clinical use of steroids necessitates an understanding of these medications’ various adverse effects. The manifestations of steroid-induced psychiatric symptoms are broad and can involve affective, behavioral, and cognitive domains. While the current mechanism is unknown, this phenomenon may be related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity, as well as effects on brain regions such as the hippocampus and amygdala. The best interventions for steroid-induced psychiatric symptoms are awareness and early diagnosis. There are no FDA-approved treatments for steroid-induced psychiatric symptoms; initial measures should include tapering or discontinuing corticosteroids.
In this article, we review the literature on the incidence, characteristics, differential diagnoses, proposed mechanism, risk factors, and proposed treatments of steroid-induced psychiatric symptoms.
A wide range of presentations
Steroid use has increased over the past 2 decades, with 10% of medical and surgical inpatients and 1% to 3% of the general population taking long-term glucocorticoids.1 Even with topical application, steroid therapy is often systemically absorbed, and thus may lead to steroid-induced psychiatric symptoms. The incidence of steroid-induced psychiatric symptoms is difficult to assess because there can be a wide range of reactions that are dose- and time-related. Three reviews of a total of 122 cases reports found that an estimated 5% of patients treated with steroids experience severe psychiatric reactions.1-3
Steroid-induced psychopathology can include mood, behavioral, and/or cognitive impairments. Mania/hypomania is the most common overall psychiatric symptom; the most common mood manifestations are anxiety and depression.4,5 Other possible steroid-induced symptoms include psychosis, dementia, panic disorder, delirium, suicidal thinking and behavior, aggressive behavior, insomnia, agitation, depersonalization, and euphoria.5 The most common cognitive impairment is verbal or declarative memory deficit; others include distractibility and deficits in attention and psychomotor speed.5 These psychiatric symptoms can have a rapid onset, possibly within hours of starting steroids.1 However, studies have reported a median time to onset of 11.5 days; 39% of cases had onset during the first week and 62% within 2 weeks.3,6 After reducing or stopping the steroid, it may take days to weeks before symptoms start to subside.2
What to consider in the differential Dx
Psychiatric symptoms that are induced by steroids can mimic metabolic, neurologic, or toxic disorders. Other factors to consider include drug withdrawal/intoxication, infections, and paraneoplastic syndromes.4,5 Although there is no reported correlation between the location of neurologic lesions and the development of specific psychiatric symptoms, manic symptoms appear most commonly with lesions in the right frontal lobe. 4 Other factors to note include the presence of new-onset psychiatric illnesses such as bipolar, mood, or thought disorders,4 as well as psychosocial stressors that might be contributing to the patient’s presentation.5
Continue to: Proposed mechanisms
Proposed mechanisms
Although the exact mechanism by which steroids induce psychiatric symptoms is unknown, several mechanisms have been proposed. One hypothesis is that steroid-induced psychopathology is related to decreased levels of corticotropin, norepinephrine, and beta-endorphin immunoreactivity.4,5,7 This may explain why many patients with major depressive disorder have elevated cortisol production and/or lack of suppression of cortisol secretion during a dexamethasone stimulation test, and why approximately one-half of patients with Cushing’s disease experience depressive symptoms.8 This is also likely why antipsychotics, which typically reduce cortisol, are efficacious treatments for some steroid-induced psychiatric symptoms.9
Cognitive impairments from steroid use may be related to these agents’ effects on certain brain regions. One such area is the hippocampus, an important mediator in the creation and maintenance of episodic and declarative memories.5,8,9 Acute glucocorticoid use is associated with decreased activity in the left hippocampus, reduced hippocampal glucose metabolism, and reduced cerebral blood flow in the posterior medial temporal lobe.10 Long-term glucocorticoid exposure is associated with smaller hippocampal volume and lower levels of temporal lobe N-acetylaspartate, a marker of neuronal viability.10 Because working memory depends on the prefrontal cortex and declarative memory relies on the hippocampus, deficits in these functions can be attributed to the effect of prolonged glucocorticoid exposure on glucocorticoid or mineralocorticoid receptors in the hippocampus, reduction of hippocampal volume, or elevated glutamate accumulation in that area.11 In addition, high cortisol levels inhibit brain-derived neurotrophic factor, which plays a crucial role in maintaining neural architecture in key brain regions such as the hippocampus and prefrontal cortex.11 There is also a correlation between the duration of prednisone treatment and atrophy of the right amygdala, which is an important regulator of mood and anxiety.11 Both the hippocampus and amygdala have dense collections of glucocorticoid receptors. This may explain why patients who receive high-dose corticosteroids can have reversible atrophy in the hypothalamus and amygdala, leading to deficits in emotional learning and the stress response.
Factors that increase risk
Several factors can increase the risk of steroid-induced psychopathology. The most significant is the dose; higher doses are more likely to produce psychiatric symptoms.1,5 Concurrent use of drugs that increase circulating levels of corticosteroids, such as inhibitors of the cytochrome P450 (CYP) enzyme (eg, clarithromycin), also increases the likelihood of developing psychiatric symptoms.1,5 Risk is also increased in patients with liver or renal dysfunction.1 Cerebral spinal fluid/serum albumin ratio, a marker of blood-brain barrier damage, and low serum complement levels were also reported to be independent risk factors,12 with the thought that increased permeability of the blood-brain barrier may allow hydrophobic steroid molecules to more easily penetrate the CNS, leading to increased neuropsychiatric effects. Hypoalbuminemia is another reported risk factor, perhaps because lower levels of serum albumin are related to higher levels of free and active glucocorticoids, which are normally inactive when bound to albumin.13 There also appears to be an increased prevalence of steroid-induced psychopathology in women, perhaps due to greater propensity in women to seek medical care or a higher prevalence of women with medical disorders that are treated with steroids.5 A previous history of psychiatric disorders may not increase risk.5
Several methods for reducing risk have been proposed, including using a divided-dosing regimens that may lower peak steroid plasma concentrations.13,14 However, the best prevention of steroid-induced psychiatric symptoms are awareness, early diagnosis, and intervention. Studies have suggested that N-methyl-
Treatment options
There are no FDA-approved medications for managing steroid-induced psychiatric symptoms.1,16 Treatment is based on evidence from case reports and a few small case series (Table2-5,17,18).
Continue to: When possible, initial treatment...
When possible, initial treatment should include discontinuing or tapering corticosteroids to <40 mg/d of prednisone-equivalent.1,4,10,18 Most studies have reported rapid reversal of deficits in declarative memory and of hippocampal volume loss once corticosteroids were tapered and discontinued.1,18 One study reported that >90% of patients recovered within 6 weeks, with patients with delirium recovering more quickly (mean: 5.4 days) than those with depression, mania, or psychosis (mean: 19.3 days).3 Another found that the vast majority (92%) of patients treated only with a steroid taper achieved clinical recovery, and 84% recovered with administration of antipsychotics without a steroid taper.3 In this study, all patients who received electroconvulsive therapy (ECT) recovered, as did those who received a steroid taper plus lithium or antipsychotics. Steroid tapering regimens are especially important for patients who have received long-term glucocorticoid treatment. Patients need to be closely monitored for signs of new or increased depression, delirium, or confusion during the taper. If these symptoms occur, the patient should be checked for adrenocortical insufficiency, which can be resolved by re-administering or increasing the dosage of the glucocorticoid.10
Mania. The treatment of mania/hypomania includes mood stabilizers (valproate, lithium, lamotrigine) and antipsychotics (quetiapine, olanzapine, haloperidol).2,4,5,10,14,18 Valproate has been reported to be an effective prophylactic of corticosteroid-induced mania,2 perhaps because it dampens neuronal hyperexcitability by attenuating NMDA receptors, blocking voltage-dependent sodium channels, and inhibiting the synthesis of cortical GABAergic steroids. Starting valproate while continuing corticosteroids (if necessary) may help lessen mania.2 Benzodiazepines also may be useful on a short-term basis.
Depression. Steroid-induced depression may be treated with sertraline or other first-line antidepressants.5,14 Consider ECT for patients with severe depression. Support for the use of antipsychotic medications stems from studies that reported steroids’ role in disrupting dopamine and 5HT2 activity. Lithium also has been used successfully to manage and prevent glucocorticoid-associated affective disorder.10,18 It can be used alone or in combination with selective serotonin reuptake inhibitors to alleviate depressive symptoms.10 Tricyclic antidepressants are generally avoided because their anticholinergic effects can exacerbate or worsen delirium.18 In general, ECT is an effective treatment for persistent and/or unresponsive steroid-induced depression,2,10 but may be difficult to use in patients with serious medical illnesses.
Agitation. Medications that have been proposed for treating steroid-induced agitation include benzodiazepines, haloperidol, and second-generation antipsychotics.5,17
Other considerations. Clinicians, patients, and families should discuss in detail the risks of steroid-induced psychiatric symptoms so an early diagnosis and appropriate intervention can be implemented. Before starting steroids, it is important to review the patient’s current medication list to ensure that steroid treatment is indicated, and to check for potential drug–drug interactions. In addition, the medical condition that is being treated with steroids also needs to be carefully reviewed, because certain illnesses are associated with the development of psychiatric symptoms. 5,10
Continue to: Young children...
Young children (age <6) and older adults appear to be at greater risk for cognitive and memory disturbances from steroid use.10 In addition, patients have individual levels of susceptibility to steroid-induced psychiatric symptoms that can vary over time. The risk for adverse effects may be elevated based on response to previous courses of glucocorticoid treatment.10 While gender, age, dosage, and duration of treatment influence risk, it is not possible to predict which patients will experience psychiatric effects during a given course of glucocorticoid therapy. Therefore, all patients should be considered to have the potential of developing such effects, and should be monitored during glucocorticoid treatment and withdrawal.
Goals for future research
To help reduce the severity of and cost associated with steroid-induced psychiatric symptoms,5,14 future studies should focus on controlled trials of preventative strategies. In particular, recent advances in genetic mapping may help identify involvement of certain genes or polymorphisms.5 Because current guidelines for the prevention and treatment of steroid-induced psychiatric symptoms are not evidence-based, controlled clinical trials are needed to elucidate the optimal management of such symptoms. There is much interplay between many of the proposed mechanisms of steroid-induced psychiatric symptoms, and future studies can help uncover a deeper understanding of the intricacies of this phenomenon.
CASE CONTINUED
Mrs. N is admitted for altered mental status. Medical workup includes MRI of the brain, MRI of the neck, cardiac echocardiogram, and EEG. There is no evidence of acute structural pathology. She is started on olanzapine, 10 mg/d at bedtime for manic and psychotic symptoms, and is discharged after 5 days. After 1 month, the outpatient psychiatrist gradually decreases and discontinues olanzapine as Mrs. N steadily returns to baseline. One year after discharge, Mrs. N continues to report resolution of her manic and psychotic symptoms.
Bottom Line
Steroids can induce a wide range of psychiatric symptoms, including mania/ hypomania, anxiety, and depression. Initial treatment typically includes tapering or discontinuing the steroid when possible. Other proposed treatments include certain antipsychotics, antidepressants, and other psychotropics, but the supporting evidence is largely anecdotal or based on case studies. Additional research is needed to elucidate the mechanism and treatment recommendations.
Related Resources
- Janes M, Kuster S, Goldson TM, et al. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32(4):614-615.
- Mayo Clinic. Prednisone and other corticosteroids. https://www.mayoclinic.org/steroids/art-20045692
Drug Brand Names
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Methylprednisolone injection • Solu-Medrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproate • Depakote
1. Dubovsky AN, Arvikar S, Stern TA, et al. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
2. Roxanas MG, Hunt GE. Rapid reversal of corticosteroid-induced mania with sodium valproate: a case series of 20 patients. Psychosomatics. 2012;53(6):575-581.
3. Lewis DA, Smith RE. Steroid‐induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319-332.
4. Warren KN, Katakam J, Espiridion ED. Acute-onset mania in a patient with non-small cell lung cancer. Cureus. 2019;11(8):e5436.
5. Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
6. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen. Psychiatry. 1981;38(4):471-477.
7. Ularntinon S, Tzuang D, Dahl G, et al. Concurrent treatment of steroid-related mood and psychotic symptoms with risperidone. Pediatrics. 2010;125(5):e1241-e1245.
8. Pokladinkova J, Meyboom RH, Vlcek J, et al. Intranasally administered corticosteroids and neuropsychiatric disturbances: a review of the international pharmacovigilance programme of the World Health Organization. Ann Allergy Asthma Immunol. 2008;101(1):67-73.
9. Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410-417.
10. Wolkowitz OM, Reus UI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61(5):698-711.
11. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045-1051.
12. Appenzeller S, Cendes F, Costallat LT. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
13. Glynne-Jones R, Vernon CC, Bell G. Is steroid psychosis preventable by divided doses? Lancet. 1986;2(8520):1404.
14. Ismail MF, Lavelle C, Cassidy EM. Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719-2731.
15. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89-98.
16. Brown BS, Stuard G, Liggin JDM, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543-548.
17. Desai S, Khanani S, Shad MU, et al. Attenutation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. J Clin Psychopharmacol. 2009;29(3):284-287.
18. Gable M, Depry D. Sustained corticosteroid-induced mania and psychosis despite cessation: a case study and brief literature review. Int J Psychiatry Med. 2015;50(4):398-404.
1. Dubovsky AN, Arvikar S, Stern TA, et al. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
2. Roxanas MG, Hunt GE. Rapid reversal of corticosteroid-induced mania with sodium valproate: a case series of 20 patients. Psychosomatics. 2012;53(6):575-581.
3. Lewis DA, Smith RE. Steroid‐induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5(4):319-332.
4. Warren KN, Katakam J, Espiridion ED. Acute-onset mania in a patient with non-small cell lung cancer. Cureus. 2019;11(8):e5436.
5. Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
6. Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen. Psychiatry. 1981;38(4):471-477.
7. Ularntinon S, Tzuang D, Dahl G, et al. Concurrent treatment of steroid-related mood and psychotic symptoms with risperidone. Pediatrics. 2010;125(5):e1241-e1245.
8. Pokladinkova J, Meyboom RH, Vlcek J, et al. Intranasally administered corticosteroids and neuropsychiatric disturbances: a review of the international pharmacovigilance programme of the World Health Organization. Ann Allergy Asthma Immunol. 2008;101(1):67-73.
9. Walker EF, Trotman HD, Pearce BD, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410-417.
10. Wolkowitz OM, Reus UI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61(5):698-711.
11. Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045-1051.
12. Appenzeller S, Cendes F, Costallat LT. Acute psychosis in systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
13. Glynne-Jones R, Vernon CC, Bell G. Is steroid psychosis preventable by divided doses? Lancet. 1986;2(8520):1404.
14. Ismail MF, Lavelle C, Cassidy EM. Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 2017;13(29):2719-2731.
15. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89-98.
16. Brown BS, Stuard G, Liggin JDM, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biol Psychiatry. 2005;57(5):543-548.
17. Desai S, Khanani S, Shad MU, et al. Attenutation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. J Clin Psychopharmacol. 2009;29(3):284-287.
18. Gable M, Depry D. Sustained corticosteroid-induced mania and psychosis despite cessation: a case study and brief literature review. Int J Psychiatry Med. 2015;50(4):398-404.