User login
Preventing ASCVD Events: Using Coronary Artery Calcification Scores to Personalize Risk and Guide Statin Therapy
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
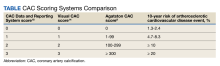
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
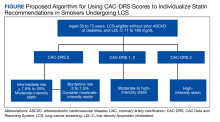
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
Poor Oral Health Tied to Worse Brain Health
In a large observational study of middle-aged adults without stroke or dementia, poor oral health was strongly associated with multiple neuroimaging markers of white matter injury.
“Because the neuroimaging markers evaluated in this study precede and are established risk factors of stroke and dementia, our results suggest that oral health, an easily modifiable process, may be a promising target for very early interventions focused on improving brain health,” wrote the authors, led by Cyprien A. Rivier, MD, MS, with the Department of Neurology, Yale University School of Medicine, New Haven, Connecticut.
The study was published online on December 20, 2023, in Neurology.
Research data came from 40,175 adults (mean age, 55 years; 53% women) with no history of stroke or dementia who enrolled in the UK Biobank from 2006 to 2010 and had brain MRI between 2014 and 2016.
Altogether, 5470 (14%) participants had poor oral health, defined as the presence of dentures or loose teeth. Those with poor (vs optimal) oral health were older, more likely to be male, and had higher prevalence of hypertension, hypercholesterolemia, diabetes, overweight/obesity, and current or past smoking history.
In a multivariable model, poor oral health was associated with a 9% increase in white matter hyperintensity (WMH) volume (P < .001), a well-established marker of clinically silent cerebrovascular disease.
Poor oral health was also associated with a 10% change in aggregate fractional anisotropy (FA) score (P < .001) and a 5% change in aggregate mean diffusivity (MD) score (P < .001), two diffusion tensor imaging metrics that accurately represent white matter disintegrity.
Genetic analyses using Mendelian randomization confirmed these associations. Individuals who were genetically prone to poor oral health had a 30% increase in WMH volume (P < .001), 43% change in aggregate FA score (P < .001), and 10% change in aggregate MD score (P < .01), the researchers reported.
These findings, they noted, add to prior epidemiologic evidence for an association between poor oral health and a higher risk for clinical outcomes related to brain health, including cognitive decline.
‘Huge Dividends’
The authors of an accompanying editorial praised the authors for looking at the consequences of poor oral health in a “new and powerful way by using as their outcome MRI-defined white matter injury, which is associated with, but antedates by many years, cognitive decline and stroke.”
“The fact that these imaging changes are seen in asymptomatic persons offers the hope that if the association is causal, interventions to improve oral health could pay huge dividends in subsequent brain health,” wrote Steven J. Kittner, MD, MPH, and Breana L. Taylor, MD, with the Department of Neurology, University of Maryland School of Medicine in Baltimore.
“The mechanisms mediating the relationship between the oral health genetic risk score and white matter injury are likely to be complex, but the authors have taken an important step forward in addressing a hypothesis of immense public health importance,” they added.
Data from the World Health Organization suggested that oral diseases, which are largely preventable, affect nearly 3.5 billion people globally, with three out of four people affected in middle-income countries.
Funding for the study was provided in part by grants from the National Institutes of Health, the American Heart Association, and the Neurocritical Care Society Research Fellowship. The authors and editorialists disclosed no relevant conflicts of interest.
Megan Brooks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a large observational study of middle-aged adults without stroke or dementia, poor oral health was strongly associated with multiple neuroimaging markers of white matter injury.
“Because the neuroimaging markers evaluated in this study precede and are established risk factors of stroke and dementia, our results suggest that oral health, an easily modifiable process, may be a promising target for very early interventions focused on improving brain health,” wrote the authors, led by Cyprien A. Rivier, MD, MS, with the Department of Neurology, Yale University School of Medicine, New Haven, Connecticut.
The study was published online on December 20, 2023, in Neurology.
Research data came from 40,175 adults (mean age, 55 years; 53% women) with no history of stroke or dementia who enrolled in the UK Biobank from 2006 to 2010 and had brain MRI between 2014 and 2016.
Altogether, 5470 (14%) participants had poor oral health, defined as the presence of dentures or loose teeth. Those with poor (vs optimal) oral health were older, more likely to be male, and had higher prevalence of hypertension, hypercholesterolemia, diabetes, overweight/obesity, and current or past smoking history.
In a multivariable model, poor oral health was associated with a 9% increase in white matter hyperintensity (WMH) volume (P < .001), a well-established marker of clinically silent cerebrovascular disease.
Poor oral health was also associated with a 10% change in aggregate fractional anisotropy (FA) score (P < .001) and a 5% change in aggregate mean diffusivity (MD) score (P < .001), two diffusion tensor imaging metrics that accurately represent white matter disintegrity.
Genetic analyses using Mendelian randomization confirmed these associations. Individuals who were genetically prone to poor oral health had a 30% increase in WMH volume (P < .001), 43% change in aggregate FA score (P < .001), and 10% change in aggregate MD score (P < .01), the researchers reported.
These findings, they noted, add to prior epidemiologic evidence for an association between poor oral health and a higher risk for clinical outcomes related to brain health, including cognitive decline.
‘Huge Dividends’
The authors of an accompanying editorial praised the authors for looking at the consequences of poor oral health in a “new and powerful way by using as their outcome MRI-defined white matter injury, which is associated with, but antedates by many years, cognitive decline and stroke.”
“The fact that these imaging changes are seen in asymptomatic persons offers the hope that if the association is causal, interventions to improve oral health could pay huge dividends in subsequent brain health,” wrote Steven J. Kittner, MD, MPH, and Breana L. Taylor, MD, with the Department of Neurology, University of Maryland School of Medicine in Baltimore.
“The mechanisms mediating the relationship between the oral health genetic risk score and white matter injury are likely to be complex, but the authors have taken an important step forward in addressing a hypothesis of immense public health importance,” they added.
Data from the World Health Organization suggested that oral diseases, which are largely preventable, affect nearly 3.5 billion people globally, with three out of four people affected in middle-income countries.
Funding for the study was provided in part by grants from the National Institutes of Health, the American Heart Association, and the Neurocritical Care Society Research Fellowship. The authors and editorialists disclosed no relevant conflicts of interest.
Megan Brooks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a large observational study of middle-aged adults without stroke or dementia, poor oral health was strongly associated with multiple neuroimaging markers of white matter injury.
“Because the neuroimaging markers evaluated in this study precede and are established risk factors of stroke and dementia, our results suggest that oral health, an easily modifiable process, may be a promising target for very early interventions focused on improving brain health,” wrote the authors, led by Cyprien A. Rivier, MD, MS, with the Department of Neurology, Yale University School of Medicine, New Haven, Connecticut.
The study was published online on December 20, 2023, in Neurology.
Research data came from 40,175 adults (mean age, 55 years; 53% women) with no history of stroke or dementia who enrolled in the UK Biobank from 2006 to 2010 and had brain MRI between 2014 and 2016.
Altogether, 5470 (14%) participants had poor oral health, defined as the presence of dentures or loose teeth. Those with poor (vs optimal) oral health were older, more likely to be male, and had higher prevalence of hypertension, hypercholesterolemia, diabetes, overweight/obesity, and current or past smoking history.
In a multivariable model, poor oral health was associated with a 9% increase in white matter hyperintensity (WMH) volume (P < .001), a well-established marker of clinically silent cerebrovascular disease.
Poor oral health was also associated with a 10% change in aggregate fractional anisotropy (FA) score (P < .001) and a 5% change in aggregate mean diffusivity (MD) score (P < .001), two diffusion tensor imaging metrics that accurately represent white matter disintegrity.
Genetic analyses using Mendelian randomization confirmed these associations. Individuals who were genetically prone to poor oral health had a 30% increase in WMH volume (P < .001), 43% change in aggregate FA score (P < .001), and 10% change in aggregate MD score (P < .01), the researchers reported.
These findings, they noted, add to prior epidemiologic evidence for an association between poor oral health and a higher risk for clinical outcomes related to brain health, including cognitive decline.
‘Huge Dividends’
The authors of an accompanying editorial praised the authors for looking at the consequences of poor oral health in a “new and powerful way by using as their outcome MRI-defined white matter injury, which is associated with, but antedates by many years, cognitive decline and stroke.”
“The fact that these imaging changes are seen in asymptomatic persons offers the hope that if the association is causal, interventions to improve oral health could pay huge dividends in subsequent brain health,” wrote Steven J. Kittner, MD, MPH, and Breana L. Taylor, MD, with the Department of Neurology, University of Maryland School of Medicine in Baltimore.
“The mechanisms mediating the relationship between the oral health genetic risk score and white matter injury are likely to be complex, but the authors have taken an important step forward in addressing a hypothesis of immense public health importance,” they added.
Data from the World Health Organization suggested that oral diseases, which are largely preventable, affect nearly 3.5 billion people globally, with three out of four people affected in middle-income countries.
Funding for the study was provided in part by grants from the National Institutes of Health, the American Heart Association, and the Neurocritical Care Society Research Fellowship. The authors and editorialists disclosed no relevant conflicts of interest.
Megan Brooks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Counterintuitive Approach to Lowering Cholesterol in Children
With the flip of the calendar a few short weeks ago, gyms and fitness centers began ramping up their advertising campaigns in hopes of attracting the horde of resolution makers searching for a place where they can inject some exercise into their sedentary lives. A recent survey by C.S. Mott’s Children’s Hospital found that even young people are setting health-related goals with more than half of the parents of 11- to 18-year-olds reporting their children were setting personal goals for themselves. More than 40% of the young people listed more exercise as a target.
However, our personal and professional experiences have taught us that achieving goals, particularly when it comes to exercise, is far more difficult than setting the target. Finding an exercise buddy can be an important motivator on the days when just lacing up one’s sneakers is a stumbling block. Investing in a gym membership and sweating with a peer group can help. However, it is an investment that rarely pays a dividend. Exercise isn’t fun for everyone. For adults, showing up at a gym may be just one more reminder of how they have already lost their competitive edge over their leaner and fitter peers. If they aren’t lucky enough to find a sport or activity that they enjoy, the loneliness of the long-distance runner has little appeal.
A recent study on children in the United Kingdom suggests that at least when it comes to teens and young adults we as physicians may actually have been making things worse for our obese patients by urging them to accept unrealistic activity goals. While it is already known that sedentary time is responsible for 70% of the total increase in cholesterol as children advance to young adulthood an unqualified recommendation for more exercise may not be the best advice.
In an interview with the study author, Andre O. Agbaje MD, MPH, said that in his large study population “light physical activity outperforms moderate to vigorous physical activity by five to eight times in lowering lipids”. While we may be surprised by this counterintuitive finding, Dr. Agbaje points out that an increase in sedentariness from 6 to 9 hours per day translates into a loss of 3 hours of light physical activity. In other words if you’re not sedentary you must be standing at attention or engaged in some light activity.
In my experience, and I suspect yours, it is difficult to get adults to do something, particularly if that something involves exerting energy, even a small amount of energy. The general admonishment of “be more active” is often met with a blank stare and the sometimes unspoken question “Like what?”
You could fall into a bottomless trap with them by suggesting a long list of activities, many of which are probably ones you do or would enjoy but don’t happen to fit with any of their interests or capabilities. Your chances of hitting on a perfect activity that the patient will attempt, let alone adopt, is very slim. Those of you with more patience than I have may choose to persist with this strategy. You could argue that even if the patient only dabbles briefly in one of your recommended activities, this is a minor victory worth celebrating. Who knows? The brief jolt of energy they received from this activity may prompt them to seek and find something else that works.
My interpretation of Dr. Agbaje’s findings is this: If we are going to suggest more activity, aim low. Don’t even mention the heavily weighted words “sport” or “exercise,” which are likely to dredge up bad memories. For adults, “Go shopping” or “Visit a friend” may be sufficient to at least get the person off the couch and on their feet and moving, even if very briefly.
The second message from this study applies more to children and adolescents and is one of those unusual instances in which a negative intervention may be more effective than a positive approach. Acknowledging that we are likely to have difficulty finding even a light activity that the child enjoys, why not pivot to the other side of the equation? Make a list of the child’s primary sedentary “activities.” Then suggest the parents put the child on a couch potato diet by immediately cutting in half the time he or she spends being sedentary. By definition, this will automatically increase his or her light physical activity by 50%. According to Dr. Agbaje’s data, this should be more effective in lowering lipids than in the unlikely event of finding a moderate activity the child accepts.
You can argue that the child will hound his or her parents unmercifully asking to be entertained. This may be true and this persistent complaining will be more likely to come from the older the child and the longer that the child has been allowed to be sedentary. Although the child may appear to have lost the ability to self amuse, I contend this isn’t a permanent loss and, This is another example of how saying “No!” in the right circumstances is often the most effective remedy for an unhealthy situation. I would never claim saying “No” is easy and helping parents to learn how to say “No” is one of our most difficult challenges. But, nothing else seems to be working.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
With the flip of the calendar a few short weeks ago, gyms and fitness centers began ramping up their advertising campaigns in hopes of attracting the horde of resolution makers searching for a place where they can inject some exercise into their sedentary lives. A recent survey by C.S. Mott’s Children’s Hospital found that even young people are setting health-related goals with more than half of the parents of 11- to 18-year-olds reporting their children were setting personal goals for themselves. More than 40% of the young people listed more exercise as a target.
However, our personal and professional experiences have taught us that achieving goals, particularly when it comes to exercise, is far more difficult than setting the target. Finding an exercise buddy can be an important motivator on the days when just lacing up one’s sneakers is a stumbling block. Investing in a gym membership and sweating with a peer group can help. However, it is an investment that rarely pays a dividend. Exercise isn’t fun for everyone. For adults, showing up at a gym may be just one more reminder of how they have already lost their competitive edge over their leaner and fitter peers. If they aren’t lucky enough to find a sport or activity that they enjoy, the loneliness of the long-distance runner has little appeal.
A recent study on children in the United Kingdom suggests that at least when it comes to teens and young adults we as physicians may actually have been making things worse for our obese patients by urging them to accept unrealistic activity goals. While it is already known that sedentary time is responsible for 70% of the total increase in cholesterol as children advance to young adulthood an unqualified recommendation for more exercise may not be the best advice.
In an interview with the study author, Andre O. Agbaje MD, MPH, said that in his large study population “light physical activity outperforms moderate to vigorous physical activity by five to eight times in lowering lipids”. While we may be surprised by this counterintuitive finding, Dr. Agbaje points out that an increase in sedentariness from 6 to 9 hours per day translates into a loss of 3 hours of light physical activity. In other words if you’re not sedentary you must be standing at attention or engaged in some light activity.
In my experience, and I suspect yours, it is difficult to get adults to do something, particularly if that something involves exerting energy, even a small amount of energy. The general admonishment of “be more active” is often met with a blank stare and the sometimes unspoken question “Like what?”
You could fall into a bottomless trap with them by suggesting a long list of activities, many of which are probably ones you do or would enjoy but don’t happen to fit with any of their interests or capabilities. Your chances of hitting on a perfect activity that the patient will attempt, let alone adopt, is very slim. Those of you with more patience than I have may choose to persist with this strategy. You could argue that even if the patient only dabbles briefly in one of your recommended activities, this is a minor victory worth celebrating. Who knows? The brief jolt of energy they received from this activity may prompt them to seek and find something else that works.
My interpretation of Dr. Agbaje’s findings is this: If we are going to suggest more activity, aim low. Don’t even mention the heavily weighted words “sport” or “exercise,” which are likely to dredge up bad memories. For adults, “Go shopping” or “Visit a friend” may be sufficient to at least get the person off the couch and on their feet and moving, even if very briefly.
The second message from this study applies more to children and adolescents and is one of those unusual instances in which a negative intervention may be more effective than a positive approach. Acknowledging that we are likely to have difficulty finding even a light activity that the child enjoys, why not pivot to the other side of the equation? Make a list of the child’s primary sedentary “activities.” Then suggest the parents put the child on a couch potato diet by immediately cutting in half the time he or she spends being sedentary. By definition, this will automatically increase his or her light physical activity by 50%. According to Dr. Agbaje’s data, this should be more effective in lowering lipids than in the unlikely event of finding a moderate activity the child accepts.
You can argue that the child will hound his or her parents unmercifully asking to be entertained. This may be true and this persistent complaining will be more likely to come from the older the child and the longer that the child has been allowed to be sedentary. Although the child may appear to have lost the ability to self amuse, I contend this isn’t a permanent loss and, This is another example of how saying “No!” in the right circumstances is often the most effective remedy for an unhealthy situation. I would never claim saying “No” is easy and helping parents to learn how to say “No” is one of our most difficult challenges. But, nothing else seems to be working.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
With the flip of the calendar a few short weeks ago, gyms and fitness centers began ramping up their advertising campaigns in hopes of attracting the horde of resolution makers searching for a place where they can inject some exercise into their sedentary lives. A recent survey by C.S. Mott’s Children’s Hospital found that even young people are setting health-related goals with more than half of the parents of 11- to 18-year-olds reporting their children were setting personal goals for themselves. More than 40% of the young people listed more exercise as a target.
However, our personal and professional experiences have taught us that achieving goals, particularly when it comes to exercise, is far more difficult than setting the target. Finding an exercise buddy can be an important motivator on the days when just lacing up one’s sneakers is a stumbling block. Investing in a gym membership and sweating with a peer group can help. However, it is an investment that rarely pays a dividend. Exercise isn’t fun for everyone. For adults, showing up at a gym may be just one more reminder of how they have already lost their competitive edge over their leaner and fitter peers. If they aren’t lucky enough to find a sport or activity that they enjoy, the loneliness of the long-distance runner has little appeal.
A recent study on children in the United Kingdom suggests that at least when it comes to teens and young adults we as physicians may actually have been making things worse for our obese patients by urging them to accept unrealistic activity goals. While it is already known that sedentary time is responsible for 70% of the total increase in cholesterol as children advance to young adulthood an unqualified recommendation for more exercise may not be the best advice.
In an interview with the study author, Andre O. Agbaje MD, MPH, said that in his large study population “light physical activity outperforms moderate to vigorous physical activity by five to eight times in lowering lipids”. While we may be surprised by this counterintuitive finding, Dr. Agbaje points out that an increase in sedentariness from 6 to 9 hours per day translates into a loss of 3 hours of light physical activity. In other words if you’re not sedentary you must be standing at attention or engaged in some light activity.
In my experience, and I suspect yours, it is difficult to get adults to do something, particularly if that something involves exerting energy, even a small amount of energy. The general admonishment of “be more active” is often met with a blank stare and the sometimes unspoken question “Like what?”
You could fall into a bottomless trap with them by suggesting a long list of activities, many of which are probably ones you do or would enjoy but don’t happen to fit with any of their interests or capabilities. Your chances of hitting on a perfect activity that the patient will attempt, let alone adopt, is very slim. Those of you with more patience than I have may choose to persist with this strategy. You could argue that even if the patient only dabbles briefly in one of your recommended activities, this is a minor victory worth celebrating. Who knows? The brief jolt of energy they received from this activity may prompt them to seek and find something else that works.
My interpretation of Dr. Agbaje’s findings is this: If we are going to suggest more activity, aim low. Don’t even mention the heavily weighted words “sport” or “exercise,” which are likely to dredge up bad memories. For adults, “Go shopping” or “Visit a friend” may be sufficient to at least get the person off the couch and on their feet and moving, even if very briefly.
The second message from this study applies more to children and adolescents and is one of those unusual instances in which a negative intervention may be more effective than a positive approach. Acknowledging that we are likely to have difficulty finding even a light activity that the child enjoys, why not pivot to the other side of the equation? Make a list of the child’s primary sedentary “activities.” Then suggest the parents put the child on a couch potato diet by immediately cutting in half the time he or she spends being sedentary. By definition, this will automatically increase his or her light physical activity by 50%. According to Dr. Agbaje’s data, this should be more effective in lowering lipids than in the unlikely event of finding a moderate activity the child accepts.
You can argue that the child will hound his or her parents unmercifully asking to be entertained. This may be true and this persistent complaining will be more likely to come from the older the child and the longer that the child has been allowed to be sedentary. Although the child may appear to have lost the ability to self amuse, I contend this isn’t a permanent loss and, This is another example of how saying “No!” in the right circumstances is often the most effective remedy for an unhealthy situation. I would never claim saying “No” is easy and helping parents to learn how to say “No” is one of our most difficult challenges. But, nothing else seems to be working.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Sudden Cardiac Deaths Down Among NCAA Athletes
TOPLINE:
A new study shows sudden cardiac deaths among collegiate athletes decreased over a recent 20-year period, but risks are still elevated among males, Black players, and basketball players, suggesting more intensive screening among these groups is needed.
METHODOLOGY:
- The study examined incidence and surrounding circumstances of sudden cardiac death (SCD) among student athletes who competed in at least one varsity sport at National Collegiate Athletic Association (NCAA) Division I, II, or III institutions in the 20 years from July 1, 2002, to June 30, 2022.
- Researchers determined causes of death and gathered demographic characteristics using multiple methods, including review of autopsy and other official documents, Internet searches, and contacts to next of kin, coaches, athletic trainers, coroners, medical examiners, scholarship foundations, and physicians involved in the case.
- SCD was defined as sudden unexpected death attributable to a cardiac cause, or a sudden death in a structurally normal heart with no other explanation for death and a history consistent with cardiac-related death that occurred within an hour of symptom onset, or an unwitnessed death occurring within 24 hours of the person being alive.
- Researchers calculated incidence rates over a typical 4-year collegiate career and reported these as athlete-years.
TAKEAWAY:
- The incidence of SCD, which accounted for 13% of the 1102 total deaths during the study period, decreased over time, with a 5-year incidence rate ratio (IRR) of 0.71 (95% CI, 0.61-0.82), while noncardiovascular deaths remained stable.
- IRR for males versus females was 3.79 (95% CI, 2.45-5.88) and for Black versus White athletes was 2.79 (95% CI, 1.98-3.94).
- Basketball and football players were at increased risk of SCD; for example, the incidence rate among Division I Black male basketball athletes was 1:1924 per 4-year athlete-years.
- The most common postmortem finding was autopsy-negative sudden unexplained death, at 19%, followed by idiopathic left ventricular hypertrophy/possible cardiomyopathy (17%) and hypertrophic cardiomyopathy (13%), with no cases of death attributable to COVID-19 myocarditis.
IN PRACTICE:
Although the reason for the decrease in SCD is unknown, “our data suggest that strategies to reduce SCD among competing athletes may be having a positive effect,” wrote the authors. More intensive screening strategies among groups with high SCD incidence may be warranted, they added.
SOURCE:
The study was conducted by Bradley J. Petek, MD, Sports Cardiology Program, Knight Cardiovascular Institute, Oregon Health & Science University, Portland. It was published online November 13 in Circulation and presented at the American Heart Association scientific sessions (abstract 479).
LIMITATIONS:
Some cases of SCD may have been missed as there is no mandatory reporting system in the United States. Approaches to cardiac autopsy and reporting varied significantly. The cause of death was unknown in 16 cases, and postmortem genetic testing was available for only 3% of athletes. As the study didn’t have data on resuscitated sudden cardiac arrest or preparticipation cardiovascular screening practices and findings, definitive conclusions couldn’t be drawn regarding causal factors underlying the decreased incidence of SCD.
DISCLOSURES:
There was no outside funding source. Dr. Petek has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article appeared on Medscape.com.
TOPLINE:
A new study shows sudden cardiac deaths among collegiate athletes decreased over a recent 20-year period, but risks are still elevated among males, Black players, and basketball players, suggesting more intensive screening among these groups is needed.
METHODOLOGY:
- The study examined incidence and surrounding circumstances of sudden cardiac death (SCD) among student athletes who competed in at least one varsity sport at National Collegiate Athletic Association (NCAA) Division I, II, or III institutions in the 20 years from July 1, 2002, to June 30, 2022.
- Researchers determined causes of death and gathered demographic characteristics using multiple methods, including review of autopsy and other official documents, Internet searches, and contacts to next of kin, coaches, athletic trainers, coroners, medical examiners, scholarship foundations, and physicians involved in the case.
- SCD was defined as sudden unexpected death attributable to a cardiac cause, or a sudden death in a structurally normal heart with no other explanation for death and a history consistent with cardiac-related death that occurred within an hour of symptom onset, or an unwitnessed death occurring within 24 hours of the person being alive.
- Researchers calculated incidence rates over a typical 4-year collegiate career and reported these as athlete-years.
TAKEAWAY:
- The incidence of SCD, which accounted for 13% of the 1102 total deaths during the study period, decreased over time, with a 5-year incidence rate ratio (IRR) of 0.71 (95% CI, 0.61-0.82), while noncardiovascular deaths remained stable.
- IRR for males versus females was 3.79 (95% CI, 2.45-5.88) and for Black versus White athletes was 2.79 (95% CI, 1.98-3.94).
- Basketball and football players were at increased risk of SCD; for example, the incidence rate among Division I Black male basketball athletes was 1:1924 per 4-year athlete-years.
- The most common postmortem finding was autopsy-negative sudden unexplained death, at 19%, followed by idiopathic left ventricular hypertrophy/possible cardiomyopathy (17%) and hypertrophic cardiomyopathy (13%), with no cases of death attributable to COVID-19 myocarditis.
IN PRACTICE:
Although the reason for the decrease in SCD is unknown, “our data suggest that strategies to reduce SCD among competing athletes may be having a positive effect,” wrote the authors. More intensive screening strategies among groups with high SCD incidence may be warranted, they added.
SOURCE:
The study was conducted by Bradley J. Petek, MD, Sports Cardiology Program, Knight Cardiovascular Institute, Oregon Health & Science University, Portland. It was published online November 13 in Circulation and presented at the American Heart Association scientific sessions (abstract 479).
LIMITATIONS:
Some cases of SCD may have been missed as there is no mandatory reporting system in the United States. Approaches to cardiac autopsy and reporting varied significantly. The cause of death was unknown in 16 cases, and postmortem genetic testing was available for only 3% of athletes. As the study didn’t have data on resuscitated sudden cardiac arrest or preparticipation cardiovascular screening practices and findings, definitive conclusions couldn’t be drawn regarding causal factors underlying the decreased incidence of SCD.
DISCLOSURES:
There was no outside funding source. Dr. Petek has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article appeared on Medscape.com.
TOPLINE:
A new study shows sudden cardiac deaths among collegiate athletes decreased over a recent 20-year period, but risks are still elevated among males, Black players, and basketball players, suggesting more intensive screening among these groups is needed.
METHODOLOGY:
- The study examined incidence and surrounding circumstances of sudden cardiac death (SCD) among student athletes who competed in at least one varsity sport at National Collegiate Athletic Association (NCAA) Division I, II, or III institutions in the 20 years from July 1, 2002, to June 30, 2022.
- Researchers determined causes of death and gathered demographic characteristics using multiple methods, including review of autopsy and other official documents, Internet searches, and contacts to next of kin, coaches, athletic trainers, coroners, medical examiners, scholarship foundations, and physicians involved in the case.
- SCD was defined as sudden unexpected death attributable to a cardiac cause, or a sudden death in a structurally normal heart with no other explanation for death and a history consistent with cardiac-related death that occurred within an hour of symptom onset, or an unwitnessed death occurring within 24 hours of the person being alive.
- Researchers calculated incidence rates over a typical 4-year collegiate career and reported these as athlete-years.
TAKEAWAY:
- The incidence of SCD, which accounted for 13% of the 1102 total deaths during the study period, decreased over time, with a 5-year incidence rate ratio (IRR) of 0.71 (95% CI, 0.61-0.82), while noncardiovascular deaths remained stable.
- IRR for males versus females was 3.79 (95% CI, 2.45-5.88) and for Black versus White athletes was 2.79 (95% CI, 1.98-3.94).
- Basketball and football players were at increased risk of SCD; for example, the incidence rate among Division I Black male basketball athletes was 1:1924 per 4-year athlete-years.
- The most common postmortem finding was autopsy-negative sudden unexplained death, at 19%, followed by idiopathic left ventricular hypertrophy/possible cardiomyopathy (17%) and hypertrophic cardiomyopathy (13%), with no cases of death attributable to COVID-19 myocarditis.
IN PRACTICE:
Although the reason for the decrease in SCD is unknown, “our data suggest that strategies to reduce SCD among competing athletes may be having a positive effect,” wrote the authors. More intensive screening strategies among groups with high SCD incidence may be warranted, they added.
SOURCE:
The study was conducted by Bradley J. Petek, MD, Sports Cardiology Program, Knight Cardiovascular Institute, Oregon Health & Science University, Portland. It was published online November 13 in Circulation and presented at the American Heart Association scientific sessions (abstract 479).
LIMITATIONS:
Some cases of SCD may have been missed as there is no mandatory reporting system in the United States. Approaches to cardiac autopsy and reporting varied significantly. The cause of death was unknown in 16 cases, and postmortem genetic testing was available for only 3% of athletes. As the study didn’t have data on resuscitated sudden cardiac arrest or preparticipation cardiovascular screening practices and findings, definitive conclusions couldn’t be drawn regarding causal factors underlying the decreased incidence of SCD.
DISCLOSURES:
There was no outside funding source. Dr. Petek has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article appeared on Medscape.com.
Anticoagulants Safe With Enzyme-Inducing Meds for Epilepsy
ORLANDO — Combining an enzyme-inducing antiseizure medication with a direct-acting oral anticoagulant (DOAC) does not significantly increase the risk of thromboembolic events in patients with epilepsy, preliminary results of a new study show.
These new data are important, “particularly when we’re talking about a more global perspective, given the vital role of enzyme-inducing antiseizure medications in epilepsy care across many middle- and low-income countries where they may be the only readily available treatment options,” said study investigator Emily K. Acton, PhD candidate in epidemiology and a medical student, University of Pennsylvania Perelman School of Medicine, Philadelphia, and University of Illinois College of Medicine, Chicago.
The findings also suggest that use of enzyme-inducing antiseizure medication with DOACs may be associated with a reduction in major bleeding events, although Ms. Acton stressed this requires more research.
The findings were presented at the American Epilepsy Society annual meeting.
Important Implications
Enzyme-inducing antiseizure medications may induce key drug metabolizing enzymes that result in wide-ranging interactions, Ms. Acton told this news organization. “But, in many cases, the clinical significance of these pharmacokinetic interactions is not completely understood.”
This has important implications for managing anticoagulation, said Ms. Acton. “The ease of DOAC use, and growing evidence of the drugs’ safety and efficacy compared to vitamin K antagonists, has led to widespread shifts in clinical practice towards DOACs.”
Due to the relative novelty of DOACs, their interaction profiles have been less than complete, she explained. Evidence that enzyme-inducing antiseizure medications may reduce absorption and accelerate metabolism of DOACs, potentially lowering DOAC levels and elevating thromboembolism risk, comes mainly from in vitro and animal studies.
“Research in humans is lacking and complicated in interpretation by inconsistent findings and methodological limitations,” she said.
The investigators wanted to address the “clinical uncertainty” surrounding the real-world relevance of enzyme-inducing antiseizure medications and DOAC interactions but conducting a randomized trial “would be neither feasible nor ethical,” said Ms. Acton.
Using healthcare claims data from October 2010 to September 2021, the researchers conducted an active comparator, new-user cohort study among a nationally representative sample of adults with epilepsy who had been co-prescribed these drugs.
They compared thromboembolic and major bleeding event rates between exposure to DOACs with enzyme-inducing antiseizure medications vs exposure to DOACs with non-enzyme inducing antiseizure medications.
Enzyme-inducing antiseizure medications included in the study were carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate. Non-enzyme-inducing antiseizure medications included gabapentin, lacosamide, lamotrigine, levetiracetam, and pregabalin.
The researchers used data-adaptive high-dimensional propensity score matching to control for “hundreds and hundreds” of observed confounders, and proxies for unobserved confounders, said Ms. Acton. They identified outcomes based on validated diagnostic coding algorithms for thromboembolic and major bleeding events and estimated adjusted hazard ratios (aHRs) using Cox proportional hazard models with robust variance estimators to account for clustering within matched pairs.
Reduced Risk of Major Bleeding
Outcomes were analyzed in three separate cohorts. These included patients on DOACs for any indication (indication-agnostic); those on DOACs for atrial fibrillation (AF); and those taking DOACs for deep vein thrombus/pulmonary embolism (DVT/PE).
In the indication-agnostic analysis, the investigators examined thromboembolic events among 5989 episodes in patients taking both DOACs and enzyme-inducing antiseizure medications, compared witha reference group of 14,671 episodes in patients taking DOACs and non-enzyme-inducing antiseizure medications.
The reference group was generally older and had a greater prevalence of a number of major comorbidities compared with the exposed group, noted Ms. Acton.
For the indication-agnostic analysis, the aHR was 1.11 (95% CI 0.89-1.39). Results were similar for the AF indication (aHR 1.10; 95% CI 0.82-1.46) and for the DVT/PE indication (aHR 1.11; 95% CI 0.81-1.51).
“This research provides large-scale, real-world evidence enzyme-inducing antiseizure medication use alongside DOACs does not significantly elevate risk of thromboembolic events among a nationally representative epilepsy population,” said Ms. Acton.
However, “it’s always important to consider risk factors for thromboembolic and bleeding events at the level of the individual patient,” she added.
With respect to major bleeding events, there was a slightly reduced risk in the exposed group, specifically in the analysis of subjects with atrial fibrillation, where the aHR was 0.63 (95% CI 0.44-0.89).
“A potential explanation may be pharmacokinetic interaction with enzyme-inducing antiseizure medications occurring to a degree that lowers DOAC levels without necessarily negating therapeutic effects,” said Ms. Acton.
However, she cautioned that more research is needed.
As for the differential potency among the various enzyme-inducing antiseizure medications studied, Ms. Acton said results from a secondary analysis in the atrial fibrillation assessment that removed the potentially less potent enzyme inducers, oxcarbazepine and topiramate, didn’t significantly change the study results.
‘Really Great News’
Commenting on the findings for this news organization, epilepsy expert Daniel M. Goldenholz, MD, PhD, assistant professor of Neurology, Harvard Beth Israel Deaconess Medical Center, Boston, Massachusetts, said the finding of no meaningful difference between DOAC plus enzyme-inducing medications vs DOACs plus non-enzyme-inducing medications is encouraging.
“This study asks a very important question at the population level and appropriately tries to control for present and hidden factors using a propensity matching approach,” he said.
The fact that the data support no difference in terms of thromboembolic events “is really great news” for patients taking an enzyme-inducing antiseizure medication who need to use a DOAC, he said.
While some patients or clinicians might consider transitioning off an enzyme-inducing antiseizure medication, this can lead to new side effects and potentially higher drug costs. “Knowing that a transition may be unnecessary is exciting,” said Dr. Goldenholz.
However, he’s concerned the 1.5-year observation period may not be long enough to see a true effect of these drug combinations.
He also noted that due to the “theoretical higher risk,” patients combining DOACs with enzyme-inducing drugs typically need extra monitoring, which may be less practical outside the US. This suggests “the result may not necessarily generalize outside high-income countries,” he said.
Dr. Goldenholz emphasized that the data are preliminary. “As always, I look forward to a full peer-reviewed study before forming final conclusions.”
The study was supported by the US Department of Health and Human Services’ National Institute of Neurological Disorders and Stroke.
Ms. Acton and Dr. Goldenholz report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO — Combining an enzyme-inducing antiseizure medication with a direct-acting oral anticoagulant (DOAC) does not significantly increase the risk of thromboembolic events in patients with epilepsy, preliminary results of a new study show.
These new data are important, “particularly when we’re talking about a more global perspective, given the vital role of enzyme-inducing antiseizure medications in epilepsy care across many middle- and low-income countries where they may be the only readily available treatment options,” said study investigator Emily K. Acton, PhD candidate in epidemiology and a medical student, University of Pennsylvania Perelman School of Medicine, Philadelphia, and University of Illinois College of Medicine, Chicago.
The findings also suggest that use of enzyme-inducing antiseizure medication with DOACs may be associated with a reduction in major bleeding events, although Ms. Acton stressed this requires more research.
The findings were presented at the American Epilepsy Society annual meeting.
Important Implications
Enzyme-inducing antiseizure medications may induce key drug metabolizing enzymes that result in wide-ranging interactions, Ms. Acton told this news organization. “But, in many cases, the clinical significance of these pharmacokinetic interactions is not completely understood.”
This has important implications for managing anticoagulation, said Ms. Acton. “The ease of DOAC use, and growing evidence of the drugs’ safety and efficacy compared to vitamin K antagonists, has led to widespread shifts in clinical practice towards DOACs.”
Due to the relative novelty of DOACs, their interaction profiles have been less than complete, she explained. Evidence that enzyme-inducing antiseizure medications may reduce absorption and accelerate metabolism of DOACs, potentially lowering DOAC levels and elevating thromboembolism risk, comes mainly from in vitro and animal studies.
“Research in humans is lacking and complicated in interpretation by inconsistent findings and methodological limitations,” she said.
The investigators wanted to address the “clinical uncertainty” surrounding the real-world relevance of enzyme-inducing antiseizure medications and DOAC interactions but conducting a randomized trial “would be neither feasible nor ethical,” said Ms. Acton.
Using healthcare claims data from October 2010 to September 2021, the researchers conducted an active comparator, new-user cohort study among a nationally representative sample of adults with epilepsy who had been co-prescribed these drugs.
They compared thromboembolic and major bleeding event rates between exposure to DOACs with enzyme-inducing antiseizure medications vs exposure to DOACs with non-enzyme inducing antiseizure medications.
Enzyme-inducing antiseizure medications included in the study were carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate. Non-enzyme-inducing antiseizure medications included gabapentin, lacosamide, lamotrigine, levetiracetam, and pregabalin.
The researchers used data-adaptive high-dimensional propensity score matching to control for “hundreds and hundreds” of observed confounders, and proxies for unobserved confounders, said Ms. Acton. They identified outcomes based on validated diagnostic coding algorithms for thromboembolic and major bleeding events and estimated adjusted hazard ratios (aHRs) using Cox proportional hazard models with robust variance estimators to account for clustering within matched pairs.
Reduced Risk of Major Bleeding
Outcomes were analyzed in three separate cohorts. These included patients on DOACs for any indication (indication-agnostic); those on DOACs for atrial fibrillation (AF); and those taking DOACs for deep vein thrombus/pulmonary embolism (DVT/PE).
In the indication-agnostic analysis, the investigators examined thromboembolic events among 5989 episodes in patients taking both DOACs and enzyme-inducing antiseizure medications, compared witha reference group of 14,671 episodes in patients taking DOACs and non-enzyme-inducing antiseizure medications.
The reference group was generally older and had a greater prevalence of a number of major comorbidities compared with the exposed group, noted Ms. Acton.
For the indication-agnostic analysis, the aHR was 1.11 (95% CI 0.89-1.39). Results were similar for the AF indication (aHR 1.10; 95% CI 0.82-1.46) and for the DVT/PE indication (aHR 1.11; 95% CI 0.81-1.51).
“This research provides large-scale, real-world evidence enzyme-inducing antiseizure medication use alongside DOACs does not significantly elevate risk of thromboembolic events among a nationally representative epilepsy population,” said Ms. Acton.
However, “it’s always important to consider risk factors for thromboembolic and bleeding events at the level of the individual patient,” she added.
With respect to major bleeding events, there was a slightly reduced risk in the exposed group, specifically in the analysis of subjects with atrial fibrillation, where the aHR was 0.63 (95% CI 0.44-0.89).
“A potential explanation may be pharmacokinetic interaction with enzyme-inducing antiseizure medications occurring to a degree that lowers DOAC levels without necessarily negating therapeutic effects,” said Ms. Acton.
However, she cautioned that more research is needed.
As for the differential potency among the various enzyme-inducing antiseizure medications studied, Ms. Acton said results from a secondary analysis in the atrial fibrillation assessment that removed the potentially less potent enzyme inducers, oxcarbazepine and topiramate, didn’t significantly change the study results.
‘Really Great News’
Commenting on the findings for this news organization, epilepsy expert Daniel M. Goldenholz, MD, PhD, assistant professor of Neurology, Harvard Beth Israel Deaconess Medical Center, Boston, Massachusetts, said the finding of no meaningful difference between DOAC plus enzyme-inducing medications vs DOACs plus non-enzyme-inducing medications is encouraging.
“This study asks a very important question at the population level and appropriately tries to control for present and hidden factors using a propensity matching approach,” he said.
The fact that the data support no difference in terms of thromboembolic events “is really great news” for patients taking an enzyme-inducing antiseizure medication who need to use a DOAC, he said.
While some patients or clinicians might consider transitioning off an enzyme-inducing antiseizure medication, this can lead to new side effects and potentially higher drug costs. “Knowing that a transition may be unnecessary is exciting,” said Dr. Goldenholz.
However, he’s concerned the 1.5-year observation period may not be long enough to see a true effect of these drug combinations.
He also noted that due to the “theoretical higher risk,” patients combining DOACs with enzyme-inducing drugs typically need extra monitoring, which may be less practical outside the US. This suggests “the result may not necessarily generalize outside high-income countries,” he said.
Dr. Goldenholz emphasized that the data are preliminary. “As always, I look forward to a full peer-reviewed study before forming final conclusions.”
The study was supported by the US Department of Health and Human Services’ National Institute of Neurological Disorders and Stroke.
Ms. Acton and Dr. Goldenholz report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO — Combining an enzyme-inducing antiseizure medication with a direct-acting oral anticoagulant (DOAC) does not significantly increase the risk of thromboembolic events in patients with epilepsy, preliminary results of a new study show.
These new data are important, “particularly when we’re talking about a more global perspective, given the vital role of enzyme-inducing antiseizure medications in epilepsy care across many middle- and low-income countries where they may be the only readily available treatment options,” said study investigator Emily K. Acton, PhD candidate in epidemiology and a medical student, University of Pennsylvania Perelman School of Medicine, Philadelphia, and University of Illinois College of Medicine, Chicago.
The findings also suggest that use of enzyme-inducing antiseizure medication with DOACs may be associated with a reduction in major bleeding events, although Ms. Acton stressed this requires more research.
The findings were presented at the American Epilepsy Society annual meeting.
Important Implications
Enzyme-inducing antiseizure medications may induce key drug metabolizing enzymes that result in wide-ranging interactions, Ms. Acton told this news organization. “But, in many cases, the clinical significance of these pharmacokinetic interactions is not completely understood.”
This has important implications for managing anticoagulation, said Ms. Acton. “The ease of DOAC use, and growing evidence of the drugs’ safety and efficacy compared to vitamin K antagonists, has led to widespread shifts in clinical practice towards DOACs.”
Due to the relative novelty of DOACs, their interaction profiles have been less than complete, she explained. Evidence that enzyme-inducing antiseizure medications may reduce absorption and accelerate metabolism of DOACs, potentially lowering DOAC levels and elevating thromboembolism risk, comes mainly from in vitro and animal studies.
“Research in humans is lacking and complicated in interpretation by inconsistent findings and methodological limitations,” she said.
The investigators wanted to address the “clinical uncertainty” surrounding the real-world relevance of enzyme-inducing antiseizure medications and DOAC interactions but conducting a randomized trial “would be neither feasible nor ethical,” said Ms. Acton.
Using healthcare claims data from October 2010 to September 2021, the researchers conducted an active comparator, new-user cohort study among a nationally representative sample of adults with epilepsy who had been co-prescribed these drugs.
They compared thromboembolic and major bleeding event rates between exposure to DOACs with enzyme-inducing antiseizure medications vs exposure to DOACs with non-enzyme inducing antiseizure medications.
Enzyme-inducing antiseizure medications included in the study were carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate. Non-enzyme-inducing antiseizure medications included gabapentin, lacosamide, lamotrigine, levetiracetam, and pregabalin.
The researchers used data-adaptive high-dimensional propensity score matching to control for “hundreds and hundreds” of observed confounders, and proxies for unobserved confounders, said Ms. Acton. They identified outcomes based on validated diagnostic coding algorithms for thromboembolic and major bleeding events and estimated adjusted hazard ratios (aHRs) using Cox proportional hazard models with robust variance estimators to account for clustering within matched pairs.
Reduced Risk of Major Bleeding
Outcomes were analyzed in three separate cohorts. These included patients on DOACs for any indication (indication-agnostic); those on DOACs for atrial fibrillation (AF); and those taking DOACs for deep vein thrombus/pulmonary embolism (DVT/PE).
In the indication-agnostic analysis, the investigators examined thromboembolic events among 5989 episodes in patients taking both DOACs and enzyme-inducing antiseizure medications, compared witha reference group of 14,671 episodes in patients taking DOACs and non-enzyme-inducing antiseizure medications.
The reference group was generally older and had a greater prevalence of a number of major comorbidities compared with the exposed group, noted Ms. Acton.
For the indication-agnostic analysis, the aHR was 1.11 (95% CI 0.89-1.39). Results were similar for the AF indication (aHR 1.10; 95% CI 0.82-1.46) and for the DVT/PE indication (aHR 1.11; 95% CI 0.81-1.51).
“This research provides large-scale, real-world evidence enzyme-inducing antiseizure medication use alongside DOACs does not significantly elevate risk of thromboembolic events among a nationally representative epilepsy population,” said Ms. Acton.
However, “it’s always important to consider risk factors for thromboembolic and bleeding events at the level of the individual patient,” she added.
With respect to major bleeding events, there was a slightly reduced risk in the exposed group, specifically in the analysis of subjects with atrial fibrillation, where the aHR was 0.63 (95% CI 0.44-0.89).
“A potential explanation may be pharmacokinetic interaction with enzyme-inducing antiseizure medications occurring to a degree that lowers DOAC levels without necessarily negating therapeutic effects,” said Ms. Acton.
However, she cautioned that more research is needed.
As for the differential potency among the various enzyme-inducing antiseizure medications studied, Ms. Acton said results from a secondary analysis in the atrial fibrillation assessment that removed the potentially less potent enzyme inducers, oxcarbazepine and topiramate, didn’t significantly change the study results.
‘Really Great News’
Commenting on the findings for this news organization, epilepsy expert Daniel M. Goldenholz, MD, PhD, assistant professor of Neurology, Harvard Beth Israel Deaconess Medical Center, Boston, Massachusetts, said the finding of no meaningful difference between DOAC plus enzyme-inducing medications vs DOACs plus non-enzyme-inducing medications is encouraging.
“This study asks a very important question at the population level and appropriately tries to control for present and hidden factors using a propensity matching approach,” he said.
The fact that the data support no difference in terms of thromboembolic events “is really great news” for patients taking an enzyme-inducing antiseizure medication who need to use a DOAC, he said.
While some patients or clinicians might consider transitioning off an enzyme-inducing antiseizure medication, this can lead to new side effects and potentially higher drug costs. “Knowing that a transition may be unnecessary is exciting,” said Dr. Goldenholz.
However, he’s concerned the 1.5-year observation period may not be long enough to see a true effect of these drug combinations.
He also noted that due to the “theoretical higher risk,” patients combining DOACs with enzyme-inducing drugs typically need extra monitoring, which may be less practical outside the US. This suggests “the result may not necessarily generalize outside high-income countries,” he said.
Dr. Goldenholz emphasized that the data are preliminary. “As always, I look forward to a full peer-reviewed study before forming final conclusions.”
The study was supported by the US Department of Health and Human Services’ National Institute of Neurological Disorders and Stroke.
Ms. Acton and Dr. Goldenholz report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AES 2023
Circadian Blood Pressure Shifts Earlier in Children With Moderate to Severe OSA
TOPLINE:
The time arrived at peak blood pressure (BP) velocity (TAPV) was significantly earlier in children with moderate to severe (MS) obstructive sleep apnea (OSA) than in controls.
METHODOLOGY:
- The researchers compared 24-hour circadian BP in children with OSA and controls to examine the impact of OSA on circadian BP.
- The study population included 219 children aged 5-14 years: 52 with mild OSA, 50 with MS OSA, and 117 controls.
- Participants underwent 24-hour BP monitoring and actigraphy; models included the times of BP peaks and TAPV.
TAKEAWAY:
- Children with MS OSA had a TAPV for diastolic BP in the morning, an average of 51 minutes earlier than controls (P < .001).
- Evening TAPV was significantly earlier in the children with MS OSA than in controls for both systolic BP (SBP) and diastolic BP (DBP) (95 min, P < .001 and 28 min, P = .028, respectively).
- Midday SBP and DBP velocity nadirs were significantly earlier in the children with MS OSA than in controls (57 min, P < .001 and 38 min, P < .01, respectively).
- Overall, children with MS OSA reached most BP values significantly earlier than controls, and both SBP and DBP were significantly elevated in the MS OSA group compared with the control group.
IN PRACTICE:
“The findings provide an essential puzzle piece in our understanding of the cardiovascular effects of OSA in children,” wrote the authors of an accompanying editorial.
SOURCE:
The lead author of the study was Md Tareq Ferdous Khan, MD, of the University of Cincinnati, Cincinnati, Ohio; the authors of the accompanying editorial were Kate Ching-Ching Chan, MD, and Albert Martin Li, MD, of the Chinese University of Hong Kong, China. The study was published online in the journal Sleep on December 13, 2023, along with the accompanying editorial.
LIMITATIONS:
More research is needed to investigate the potential mechanisms of action, optimize methodology, and investigate circadian biology via actigraphy and biomarkers, the authors of an accompanying editorial wrote.
DISCLOSURES:
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The time arrived at peak blood pressure (BP) velocity (TAPV) was significantly earlier in children with moderate to severe (MS) obstructive sleep apnea (OSA) than in controls.
METHODOLOGY:
- The researchers compared 24-hour circadian BP in children with OSA and controls to examine the impact of OSA on circadian BP.
- The study population included 219 children aged 5-14 years: 52 with mild OSA, 50 with MS OSA, and 117 controls.
- Participants underwent 24-hour BP monitoring and actigraphy; models included the times of BP peaks and TAPV.
TAKEAWAY:
- Children with MS OSA had a TAPV for diastolic BP in the morning, an average of 51 minutes earlier than controls (P < .001).
- Evening TAPV was significantly earlier in the children with MS OSA than in controls for both systolic BP (SBP) and diastolic BP (DBP) (95 min, P < .001 and 28 min, P = .028, respectively).
- Midday SBP and DBP velocity nadirs were significantly earlier in the children with MS OSA than in controls (57 min, P < .001 and 38 min, P < .01, respectively).
- Overall, children with MS OSA reached most BP values significantly earlier than controls, and both SBP and DBP were significantly elevated in the MS OSA group compared with the control group.
IN PRACTICE:
“The findings provide an essential puzzle piece in our understanding of the cardiovascular effects of OSA in children,” wrote the authors of an accompanying editorial.
SOURCE:
The lead author of the study was Md Tareq Ferdous Khan, MD, of the University of Cincinnati, Cincinnati, Ohio; the authors of the accompanying editorial were Kate Ching-Ching Chan, MD, and Albert Martin Li, MD, of the Chinese University of Hong Kong, China. The study was published online in the journal Sleep on December 13, 2023, along with the accompanying editorial.
LIMITATIONS:
More research is needed to investigate the potential mechanisms of action, optimize methodology, and investigate circadian biology via actigraphy and biomarkers, the authors of an accompanying editorial wrote.
DISCLOSURES:
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The time arrived at peak blood pressure (BP) velocity (TAPV) was significantly earlier in children with moderate to severe (MS) obstructive sleep apnea (OSA) than in controls.
METHODOLOGY:
- The researchers compared 24-hour circadian BP in children with OSA and controls to examine the impact of OSA on circadian BP.
- The study population included 219 children aged 5-14 years: 52 with mild OSA, 50 with MS OSA, and 117 controls.
- Participants underwent 24-hour BP monitoring and actigraphy; models included the times of BP peaks and TAPV.
TAKEAWAY:
- Children with MS OSA had a TAPV for diastolic BP in the morning, an average of 51 minutes earlier than controls (P < .001).
- Evening TAPV was significantly earlier in the children with MS OSA than in controls for both systolic BP (SBP) and diastolic BP (DBP) (95 min, P < .001 and 28 min, P = .028, respectively).
- Midday SBP and DBP velocity nadirs were significantly earlier in the children with MS OSA than in controls (57 min, P < .001 and 38 min, P < .01, respectively).
- Overall, children with MS OSA reached most BP values significantly earlier than controls, and both SBP and DBP were significantly elevated in the MS OSA group compared with the control group.
IN PRACTICE:
“The findings provide an essential puzzle piece in our understanding of the cardiovascular effects of OSA in children,” wrote the authors of an accompanying editorial.
SOURCE:
The lead author of the study was Md Tareq Ferdous Khan, MD, of the University of Cincinnati, Cincinnati, Ohio; the authors of the accompanying editorial were Kate Ching-Ching Chan, MD, and Albert Martin Li, MD, of the Chinese University of Hong Kong, China. The study was published online in the journal Sleep on December 13, 2023, along with the accompanying editorial.
LIMITATIONS:
More research is needed to investigate the potential mechanisms of action, optimize methodology, and investigate circadian biology via actigraphy and biomarkers, the authors of an accompanying editorial wrote.
DISCLOSURES:
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
What Causes One of Stroke’s Most Common Complications?
The mechanisms underlying poststroke depression (PSD), a common and debilitating complication of stroke, are unclear. Is it neurobiological, psychosocial, or both?
Two studies offer new insight into this question. In the first, in most dimensions of depressive symptoms. But surprisingly, anhedonia was less severe in patients with PSD compared with non-stroke controls, and those with PSD also showed greater emotional dysregulation.
“Our findings support previous recommendations that clinicians should adapt the provision of psychological support to the specific needs and difficulties of stroke survivors,” said lead author Joshua Blake, DClinPsy, lecturer in clinical psychology, University of East Anglia, Norwich, United Kingdom.
The study was published online in Neuropsychology Review
A second study used a machine learning algorithm to analyze blood samples from adults who had suffered a stroke, determining whether plasma protein data could predict mood and identifying potential proteins associated with mood in these patients.
“We can now look at a stroke survivor’s blood and predict their mood,” senior author Marion Buckwalter, MD, PhD, professor of neurology and neurosurgery at Stanford Medicine, California, said in a news release. “This means there is a genuine association between what’s happening in the blood and what’s happening with a person’s mood. It also means that, down the road, we may be able to develop new treatments for PSD.”
The study was published in November 2023 in Brain, Behavior, and Immunity.
‘Surprising’ Findings
“There has long been uncertainty over whether PSD might differ in its causes, phenomenology, and treatability, due to the presence of brain injury, related biological changes, and the psychosocial context unique to this population,” Dr. Blake said. “We felt that understanding symptomatologic similarities and differences would constructively contribute to this debate.”
The researchers reviewed 12 papers that sampled both stroke and non-stroke participants. “We compared profiles of depression symptoms, correlation strengths of individual depression symptoms with general depression, and latent item severity,” Dr. Blake reported.
They extracted 38 symptoms from five standardized depression tools and then organized the symptoms into nine dimensions.
They found mostly nonsignificant differences between patients with PSD and non-stroke controls in most dimensions, including negative affect, negative cognitions, somatic features, anxiety/worry, and suicidal ideation. Those with PSD more frequently had cognitive impairment, and “work inhibition” was more common in PSD.
But the most striking finding was greater severity/prevalence of emotional dysregulation in PSD vs non-stroke depression and also less anhedonia.
Dr. Blake acknowledged being “surprised.”
One possible explanation is that stroke recovery “appears to be a highly emotional journey, with extreme findings of both positive and negative emotions reported by survivors as they psychologically adjust,” which might be protective against anhedonia, he suggested.
Moreover, neurologically driven emotional dysregulation “may similarly reduce experiences of anhedonia.”
However, there was a “considerable risk of bias in many of the included studies, meaning it’s important that these findings are experimentally confirmed before stronger conclusions about phenomenological differences can be drawn,” he cautioned.
Common, Undertreated
Dr. Buckwalter said her team was motivated to conduct the research because PSD is among the top problems reported by chronic stroke patients, and for most, it is not adequately treated.
However, “despite the high prevalence of PSD, it is very poorly studied in the chronic time period.” In particular, PSD isn’t “well understood at a molecular level.”
She added that inflammation is a “promising candidate” as a mechanism, since neuroinflammation occurs in the stroke scar for decades, and chronic peripheral inflammation can produce neuroinflammation. Aberrant immune activation has also been implicated in major depression without stroke. But large studies with broad panels of plasma biomarkers are lacking in PSD.
To address this gap, the researchers used a proteomic approach. They recruited 85 chronic stroke patients (mean age, 65 years [interquartile range, 55-71], 41.2% female, 65.9% White, 17.6% Asian, and 0% Black) from the Stanford Stroke Recovery Program. Participants were between 5 months and 9 years after an ischemic stroke.
They analyzed a comprehensive panel of 1196 proteins in plasma samples, applying a machine learning algorithm to see whether the plasma protein levels “could be used to predict mood scores, using either the proteomics data alone or adding age and time since stroke.” The proteomics data were then incorporated into multivariable regression models, along with relevant clinical features, to ascertain the model’s predictive ability.
Mood was assessed using the Stroke Impact Scale mood questionnaire, with participants’ mood dichotomized into better mood (> 63) or worse mood (≤ 63).
‘Beautiful Mechanistic Model’
Machine learning verified a relationship between plasma proteomic data and mood, with the most accurate prediction occurring when the researchers added age and time since the stroke to the analysis.
Independent univariate analyses identified 202 proteins that were most highly correlated with mood in PSD. These were then organized into functional groups, including immune proteins, integrins, growth factors, synaptic function proteins, serotonin activity-related proteins, and cell death and stress-related functional groupings.
Although no single protein could predict depression, significant changes in levels of several proteins were found in PSD patients. A high proportion (45%) were proteins previously implicated in major depression, “likely providing a link to the underlying mechanisms of chronic PSD,” the authors stated.
Moreover, 80% of correlated immune proteins were higher in the plasma of people with worse mood, and several immune proteins known to have anti-inflammatory effects were reduced in those with worse mood.
And several pro-inflammatory cytokines were implicated. For example, interleukin 6, which has been extensively studied as a potential plasma marker of major depression in non-stroke cohorts, was significantly elevated in patients with worse mood after stroke (P = .0325), «implicating a broadly overactive immune system in PSD.»
“We demonstrated for the first time that we can use plasma protein measurements to predict mood in people with chronic stroke,” Dr. Buckwalter summarized. “This means there is a biological correlate of mood but [it] doesn’t tell us causality.”
To tease out causality, the researchers used their own data, as well as information from a literature review of previous studies, to assemble a model of how the immune response following a stroke could change both serotonin and brain plasticity.
“We used the most highly correlated proteins to construct a beautiful mechanistic model of how poststroke depression may work and how it may relate to mechanisms in major depression,” Dr. Buckwalter said.
The model “posits an increased inflammatory response that leads to decreased tryptophan, serotonin, and less synaptic function, all of which contribute to symptoms of depression.”
Currently, selective serotonin reuptake inhibitors represent the “best treatment” for people with PSD, but “unfortunately they don’t work for many patients,” Dr. Buckwalter noted. The findings “provide clues as to other molecular targets that are candidates novel therapies for poststroke depression.”
Dr. Blake commented that the proteomic study “complements the work by us and others interested in understanding PSD.”
Mood disorders “must be understood in terms of the dynamic relationships between structural neurological alterations, cellular and microbiological changes, psychological processes, and the person’s interactions with their social landscape,” Dr. Blake said.
New Treatments on the Horizon?
Gustavo C. Medeiros, MD, assistant professor, Department of Psychiatry, of the University of Maryland School of Medicine, Baltimore, said that knowing which individuals are more likely to develop PSD “allows treatment teams to implement earlier and more intensive interventions in those who are at higher risk.”
The findings [of the proteomic study] may also “help clarify the neurobiological correlates of PSD…[which] may help the development of new treatments that target these neurobiological changes,” said Dr. Medeiros, who wasn’t involved with either study.
However, he warned, “we should interpret their results with caution due to methodological reasons, including the relatively small sample size.”
Also commenting, Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, professor of neurology, UCSF Weill Institute for Neurosciences, California, said the proteomic study has some “clear limitations,” including the lack of Black or African American patients in the cohort, which limits generalizability, “since we know that Black and African American people are disproportionately affected by stroke and have very high rates of PSD and very severe presentation.”
The study by Dr. Blake et al. “was interesting because the phenotype of depressive symptoms after stroke differs from what’s seen in the general population, and the authors figured out a way to better understand the nuances of such differences,” said Dr. Ovbiagele, who wasn’t involved with either study.
He said he was also surprised by the finding regarding anhedonia and suggested that the findings be replicated in a study directly comparing patients with PSD and patients with depression from the general population.
The study by Bidoki et al. was funded by AHA/Paul Allen Foundation, the Leducq Stroke-IMPaCT Transatlantic Network of Excellence (MSB), the Wu Tsai Neurosciences Institute (MSB), the Alfred E. Mann Foundation (NA), and an Alzheimer’s Association Research Fellowship to one of the authors. No source of funding was listed for the study by Dr. Blake et al. The authors of both studies, Dr. Medeiros and Dr. Ovbiagele, declare no relevant financial relationships.
A version of this article appeared on Medscape.com.
The mechanisms underlying poststroke depression (PSD), a common and debilitating complication of stroke, are unclear. Is it neurobiological, psychosocial, or both?
Two studies offer new insight into this question. In the first, in most dimensions of depressive symptoms. But surprisingly, anhedonia was less severe in patients with PSD compared with non-stroke controls, and those with PSD also showed greater emotional dysregulation.
“Our findings support previous recommendations that clinicians should adapt the provision of psychological support to the specific needs and difficulties of stroke survivors,” said lead author Joshua Blake, DClinPsy, lecturer in clinical psychology, University of East Anglia, Norwich, United Kingdom.
The study was published online in Neuropsychology Review
A second study used a machine learning algorithm to analyze blood samples from adults who had suffered a stroke, determining whether plasma protein data could predict mood and identifying potential proteins associated with mood in these patients.
“We can now look at a stroke survivor’s blood and predict their mood,” senior author Marion Buckwalter, MD, PhD, professor of neurology and neurosurgery at Stanford Medicine, California, said in a news release. “This means there is a genuine association between what’s happening in the blood and what’s happening with a person’s mood. It also means that, down the road, we may be able to develop new treatments for PSD.”
The study was published in November 2023 in Brain, Behavior, and Immunity.
‘Surprising’ Findings
“There has long been uncertainty over whether PSD might differ in its causes, phenomenology, and treatability, due to the presence of brain injury, related biological changes, and the psychosocial context unique to this population,” Dr. Blake said. “We felt that understanding symptomatologic similarities and differences would constructively contribute to this debate.”
The researchers reviewed 12 papers that sampled both stroke and non-stroke participants. “We compared profiles of depression symptoms, correlation strengths of individual depression symptoms with general depression, and latent item severity,” Dr. Blake reported.
They extracted 38 symptoms from five standardized depression tools and then organized the symptoms into nine dimensions.
They found mostly nonsignificant differences between patients with PSD and non-stroke controls in most dimensions, including negative affect, negative cognitions, somatic features, anxiety/worry, and suicidal ideation. Those with PSD more frequently had cognitive impairment, and “work inhibition” was more common in PSD.
But the most striking finding was greater severity/prevalence of emotional dysregulation in PSD vs non-stroke depression and also less anhedonia.
Dr. Blake acknowledged being “surprised.”
One possible explanation is that stroke recovery “appears to be a highly emotional journey, with extreme findings of both positive and negative emotions reported by survivors as they psychologically adjust,” which might be protective against anhedonia, he suggested.
Moreover, neurologically driven emotional dysregulation “may similarly reduce experiences of anhedonia.”
However, there was a “considerable risk of bias in many of the included studies, meaning it’s important that these findings are experimentally confirmed before stronger conclusions about phenomenological differences can be drawn,” he cautioned.
Common, Undertreated
Dr. Buckwalter said her team was motivated to conduct the research because PSD is among the top problems reported by chronic stroke patients, and for most, it is not adequately treated.
However, “despite the high prevalence of PSD, it is very poorly studied in the chronic time period.” In particular, PSD isn’t “well understood at a molecular level.”
She added that inflammation is a “promising candidate” as a mechanism, since neuroinflammation occurs in the stroke scar for decades, and chronic peripheral inflammation can produce neuroinflammation. Aberrant immune activation has also been implicated in major depression without stroke. But large studies with broad panels of plasma biomarkers are lacking in PSD.
To address this gap, the researchers used a proteomic approach. They recruited 85 chronic stroke patients (mean age, 65 years [interquartile range, 55-71], 41.2% female, 65.9% White, 17.6% Asian, and 0% Black) from the Stanford Stroke Recovery Program. Participants were between 5 months and 9 years after an ischemic stroke.
They analyzed a comprehensive panel of 1196 proteins in plasma samples, applying a machine learning algorithm to see whether the plasma protein levels “could be used to predict mood scores, using either the proteomics data alone or adding age and time since stroke.” The proteomics data were then incorporated into multivariable regression models, along with relevant clinical features, to ascertain the model’s predictive ability.
Mood was assessed using the Stroke Impact Scale mood questionnaire, with participants’ mood dichotomized into better mood (> 63) or worse mood (≤ 63).
‘Beautiful Mechanistic Model’
Machine learning verified a relationship between plasma proteomic data and mood, with the most accurate prediction occurring when the researchers added age and time since the stroke to the analysis.
Independent univariate analyses identified 202 proteins that were most highly correlated with mood in PSD. These were then organized into functional groups, including immune proteins, integrins, growth factors, synaptic function proteins, serotonin activity-related proteins, and cell death and stress-related functional groupings.
Although no single protein could predict depression, significant changes in levels of several proteins were found in PSD patients. A high proportion (45%) were proteins previously implicated in major depression, “likely providing a link to the underlying mechanisms of chronic PSD,” the authors stated.
Moreover, 80% of correlated immune proteins were higher in the plasma of people with worse mood, and several immune proteins known to have anti-inflammatory effects were reduced in those with worse mood.
And several pro-inflammatory cytokines were implicated. For example, interleukin 6, which has been extensively studied as a potential plasma marker of major depression in non-stroke cohorts, was significantly elevated in patients with worse mood after stroke (P = .0325), «implicating a broadly overactive immune system in PSD.»
“We demonstrated for the first time that we can use plasma protein measurements to predict mood in people with chronic stroke,” Dr. Buckwalter summarized. “This means there is a biological correlate of mood but [it] doesn’t tell us causality.”
To tease out causality, the researchers used their own data, as well as information from a literature review of previous studies, to assemble a model of how the immune response following a stroke could change both serotonin and brain plasticity.
“We used the most highly correlated proteins to construct a beautiful mechanistic model of how poststroke depression may work and how it may relate to mechanisms in major depression,” Dr. Buckwalter said.
The model “posits an increased inflammatory response that leads to decreased tryptophan, serotonin, and less synaptic function, all of which contribute to symptoms of depression.”
Currently, selective serotonin reuptake inhibitors represent the “best treatment” for people with PSD, but “unfortunately they don’t work for many patients,” Dr. Buckwalter noted. The findings “provide clues as to other molecular targets that are candidates novel therapies for poststroke depression.”
Dr. Blake commented that the proteomic study “complements the work by us and others interested in understanding PSD.”
Mood disorders “must be understood in terms of the dynamic relationships between structural neurological alterations, cellular and microbiological changes, psychological processes, and the person’s interactions with their social landscape,” Dr. Blake said.
New Treatments on the Horizon?
Gustavo C. Medeiros, MD, assistant professor, Department of Psychiatry, of the University of Maryland School of Medicine, Baltimore, said that knowing which individuals are more likely to develop PSD “allows treatment teams to implement earlier and more intensive interventions in those who are at higher risk.”
The findings [of the proteomic study] may also “help clarify the neurobiological correlates of PSD…[which] may help the development of new treatments that target these neurobiological changes,” said Dr. Medeiros, who wasn’t involved with either study.
However, he warned, “we should interpret their results with caution due to methodological reasons, including the relatively small sample size.”
Also commenting, Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, professor of neurology, UCSF Weill Institute for Neurosciences, California, said the proteomic study has some “clear limitations,” including the lack of Black or African American patients in the cohort, which limits generalizability, “since we know that Black and African American people are disproportionately affected by stroke and have very high rates of PSD and very severe presentation.”
The study by Dr. Blake et al. “was interesting because the phenotype of depressive symptoms after stroke differs from what’s seen in the general population, and the authors figured out a way to better understand the nuances of such differences,” said Dr. Ovbiagele, who wasn’t involved with either study.
He said he was also surprised by the finding regarding anhedonia and suggested that the findings be replicated in a study directly comparing patients with PSD and patients with depression from the general population.
The study by Bidoki et al. was funded by AHA/Paul Allen Foundation, the Leducq Stroke-IMPaCT Transatlantic Network of Excellence (MSB), the Wu Tsai Neurosciences Institute (MSB), the Alfred E. Mann Foundation (NA), and an Alzheimer’s Association Research Fellowship to one of the authors. No source of funding was listed for the study by Dr. Blake et al. The authors of both studies, Dr. Medeiros and Dr. Ovbiagele, declare no relevant financial relationships.
A version of this article appeared on Medscape.com.
The mechanisms underlying poststroke depression (PSD), a common and debilitating complication of stroke, are unclear. Is it neurobiological, psychosocial, or both?
Two studies offer new insight into this question. In the first, in most dimensions of depressive symptoms. But surprisingly, anhedonia was less severe in patients with PSD compared with non-stroke controls, and those with PSD also showed greater emotional dysregulation.
“Our findings support previous recommendations that clinicians should adapt the provision of psychological support to the specific needs and difficulties of stroke survivors,” said lead author Joshua Blake, DClinPsy, lecturer in clinical psychology, University of East Anglia, Norwich, United Kingdom.
The study was published online in Neuropsychology Review
A second study used a machine learning algorithm to analyze blood samples from adults who had suffered a stroke, determining whether plasma protein data could predict mood and identifying potential proteins associated with mood in these patients.
“We can now look at a stroke survivor’s blood and predict their mood,” senior author Marion Buckwalter, MD, PhD, professor of neurology and neurosurgery at Stanford Medicine, California, said in a news release. “This means there is a genuine association between what’s happening in the blood and what’s happening with a person’s mood. It also means that, down the road, we may be able to develop new treatments for PSD.”
The study was published in November 2023 in Brain, Behavior, and Immunity.
‘Surprising’ Findings
“There has long been uncertainty over whether PSD might differ in its causes, phenomenology, and treatability, due to the presence of brain injury, related biological changes, and the psychosocial context unique to this population,” Dr. Blake said. “We felt that understanding symptomatologic similarities and differences would constructively contribute to this debate.”
The researchers reviewed 12 papers that sampled both stroke and non-stroke participants. “We compared profiles of depression symptoms, correlation strengths of individual depression symptoms with general depression, and latent item severity,” Dr. Blake reported.
They extracted 38 symptoms from five standardized depression tools and then organized the symptoms into nine dimensions.
They found mostly nonsignificant differences between patients with PSD and non-stroke controls in most dimensions, including negative affect, negative cognitions, somatic features, anxiety/worry, and suicidal ideation. Those with PSD more frequently had cognitive impairment, and “work inhibition” was more common in PSD.
But the most striking finding was greater severity/prevalence of emotional dysregulation in PSD vs non-stroke depression and also less anhedonia.
Dr. Blake acknowledged being “surprised.”
One possible explanation is that stroke recovery “appears to be a highly emotional journey, with extreme findings of both positive and negative emotions reported by survivors as they psychologically adjust,” which might be protective against anhedonia, he suggested.
Moreover, neurologically driven emotional dysregulation “may similarly reduce experiences of anhedonia.”
However, there was a “considerable risk of bias in many of the included studies, meaning it’s important that these findings are experimentally confirmed before stronger conclusions about phenomenological differences can be drawn,” he cautioned.
Common, Undertreated
Dr. Buckwalter said her team was motivated to conduct the research because PSD is among the top problems reported by chronic stroke patients, and for most, it is not adequately treated.
However, “despite the high prevalence of PSD, it is very poorly studied in the chronic time period.” In particular, PSD isn’t “well understood at a molecular level.”
She added that inflammation is a “promising candidate” as a mechanism, since neuroinflammation occurs in the stroke scar for decades, and chronic peripheral inflammation can produce neuroinflammation. Aberrant immune activation has also been implicated in major depression without stroke. But large studies with broad panels of plasma biomarkers are lacking in PSD.
To address this gap, the researchers used a proteomic approach. They recruited 85 chronic stroke patients (mean age, 65 years [interquartile range, 55-71], 41.2% female, 65.9% White, 17.6% Asian, and 0% Black) from the Stanford Stroke Recovery Program. Participants were between 5 months and 9 years after an ischemic stroke.
They analyzed a comprehensive panel of 1196 proteins in plasma samples, applying a machine learning algorithm to see whether the plasma protein levels “could be used to predict mood scores, using either the proteomics data alone or adding age and time since stroke.” The proteomics data were then incorporated into multivariable regression models, along with relevant clinical features, to ascertain the model’s predictive ability.
Mood was assessed using the Stroke Impact Scale mood questionnaire, with participants’ mood dichotomized into better mood (> 63) or worse mood (≤ 63).
‘Beautiful Mechanistic Model’
Machine learning verified a relationship between plasma proteomic data and mood, with the most accurate prediction occurring when the researchers added age and time since the stroke to the analysis.
Independent univariate analyses identified 202 proteins that were most highly correlated with mood in PSD. These were then organized into functional groups, including immune proteins, integrins, growth factors, synaptic function proteins, serotonin activity-related proteins, and cell death and stress-related functional groupings.
Although no single protein could predict depression, significant changes in levels of several proteins were found in PSD patients. A high proportion (45%) were proteins previously implicated in major depression, “likely providing a link to the underlying mechanisms of chronic PSD,” the authors stated.
Moreover, 80% of correlated immune proteins were higher in the plasma of people with worse mood, and several immune proteins known to have anti-inflammatory effects were reduced in those with worse mood.
And several pro-inflammatory cytokines were implicated. For example, interleukin 6, which has been extensively studied as a potential plasma marker of major depression in non-stroke cohorts, was significantly elevated in patients with worse mood after stroke (P = .0325), «implicating a broadly overactive immune system in PSD.»
“We demonstrated for the first time that we can use plasma protein measurements to predict mood in people with chronic stroke,” Dr. Buckwalter summarized. “This means there is a biological correlate of mood but [it] doesn’t tell us causality.”
To tease out causality, the researchers used their own data, as well as information from a literature review of previous studies, to assemble a model of how the immune response following a stroke could change both serotonin and brain plasticity.
“We used the most highly correlated proteins to construct a beautiful mechanistic model of how poststroke depression may work and how it may relate to mechanisms in major depression,” Dr. Buckwalter said.
The model “posits an increased inflammatory response that leads to decreased tryptophan, serotonin, and less synaptic function, all of which contribute to symptoms of depression.”
Currently, selective serotonin reuptake inhibitors represent the “best treatment” for people with PSD, but “unfortunately they don’t work for many patients,” Dr. Buckwalter noted. The findings “provide clues as to other molecular targets that are candidates novel therapies for poststroke depression.”
Dr. Blake commented that the proteomic study “complements the work by us and others interested in understanding PSD.”
Mood disorders “must be understood in terms of the dynamic relationships between structural neurological alterations, cellular and microbiological changes, psychological processes, and the person’s interactions with their social landscape,” Dr. Blake said.
New Treatments on the Horizon?
Gustavo C. Medeiros, MD, assistant professor, Department of Psychiatry, of the University of Maryland School of Medicine, Baltimore, said that knowing which individuals are more likely to develop PSD “allows treatment teams to implement earlier and more intensive interventions in those who are at higher risk.”
The findings [of the proteomic study] may also “help clarify the neurobiological correlates of PSD…[which] may help the development of new treatments that target these neurobiological changes,” said Dr. Medeiros, who wasn’t involved with either study.
However, he warned, “we should interpret their results with caution due to methodological reasons, including the relatively small sample size.”
Also commenting, Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, professor of neurology, UCSF Weill Institute for Neurosciences, California, said the proteomic study has some “clear limitations,” including the lack of Black or African American patients in the cohort, which limits generalizability, “since we know that Black and African American people are disproportionately affected by stroke and have very high rates of PSD and very severe presentation.”
The study by Dr. Blake et al. “was interesting because the phenotype of depressive symptoms after stroke differs from what’s seen in the general population, and the authors figured out a way to better understand the nuances of such differences,” said Dr. Ovbiagele, who wasn’t involved with either study.
He said he was also surprised by the finding regarding anhedonia and suggested that the findings be replicated in a study directly comparing patients with PSD and patients with depression from the general population.
The study by Bidoki et al. was funded by AHA/Paul Allen Foundation, the Leducq Stroke-IMPaCT Transatlantic Network of Excellence (MSB), the Wu Tsai Neurosciences Institute (MSB), the Alfred E. Mann Foundation (NA), and an Alzheimer’s Association Research Fellowship to one of the authors. No source of funding was listed for the study by Dr. Blake et al. The authors of both studies, Dr. Medeiros and Dr. Ovbiagele, declare no relevant financial relationships.
A version of this article appeared on Medscape.com.
Newborn Recipient of Partial Heart Transplant Doing Well
, researchers said.
The surgery was performed on the 18th day of life of a 5-pound newborn boy diagnosed prenatally with persistent truncus arteriosus and severe truncal valve dysfunction. The procedure involved transplantation of the part of the heart containing the aorta and pulmonary valves from an infant donor upon cardiac death.
The standard of care for neonatal heart valve implants are cadaver grafts. But these grafts are not viable and can’t grow or self-repair. Therefore, recipient neonates need to undergo repeated implant-exchange surgeries until an adult-sized heart valve can fit. Clinical outcomes generally are poor.
“We have learned that these partial heart transplant valves, when procured fresh and the [recipient] baby is placed on low-dose antirejection medicine, can grow with the child and function completely normally,” Joseph W. Turek, MD, PhD, MBA of Duke University Medical Center in Durham, North Carolina, told this news organization.
“This represents a new field in heart surgery that could dramatically change the way we care for children with poorly functioning heart valves by allowing valve implants that grow with them.”
A case report describing the novel intervention was published online on January 2, 2024, in JAMA.
‘Expected to Last a Lifetime’
The donor was a 2-day-old female weighing 8 pounds. Delivery had been complicated by hypoxic ischemic brain injury, but echocardiography showed structurally normal, functioning outflow heart valves. The heart was donated after cardiac death and procured using standard surgical techniques.
The recipient infant’s operation involved sternotomy, cardiopulmonary bypass, and cardioplegic arrest of the heart. The pulmonary artery ostia and coronary artery buttons were dissected, and the infant’s irreparable truncal valve was excised.
The donor aortic root was transplanted first, using donor tissue to close the ventricular septal defect. Then, the coronary artery buttons were reimplanted; the right ventricular outflow tract was enlarged; and the pulmonary root was transplanted. Postoperative immunosuppression followed.
On the follow-up at age 14 months, the transplanted valves showed no obstruction or insufficiency on echocardiography. Now, almost 21 months later, the recipient is doing well, Dr. Turek said. “His family has shared his many milestones with me, including eating his first birthday cake, videos of his first steps, and his newfound oral appetite (he was largely g-tube fed for a while).”
“The rationale for partial heart transplant is that pediatric heart transplants grow,” Dr. Turek and coauthors wrote. “Moreover, failure of heart transplant outflow valves is exceedingly rare. While heart transplant long-term outcomes are limited by inevitable ventricular dysfunction, partial heart transplants spare the native ventricles and are therefore expected to last a lifetime.”
‘Domino Hearts’
“While this particular baby had truncus arteriosus, this operation should prove to be beneficial for a host of congenital heart conditions with valves that are either too small or poorly functioning,” Dr. Turek said. “We have performed subsequent partial heart operations for babies with aortic stenosis, tetralogy of Fallot with pulmonary atresia, and biventricular outflow tract obstruction.”
The challenge is organ availability, he noted. “While this procedure does make use of hearts that would be otherwise unusable for full heart transplant, such as hearts with poor ventricular function or hearts removed from recipients of full heart transplants (aka domino hearts), the availability is still low compared to the need.”
With domino hearts, “you could potentially double the number of hearts that are used for the benefit of children with heart disease,” Dr. Turek said in a Duke communication released with the paper. In a domino heart procedure, a patient who has healthy valves but needs stronger heart muscle receives a full heart transplant, and the healthy valves are then donated to another patient in need, creating a domino effect.
Since this breakthrough procedure in 2022, partial heart transplants have been performed 13 times at four centers, including nine at Duke, three of which used the domino technique.
For now, Dr. Turek told this news organization, “we are hoping to receive funds for a clinical trial that will evaluate these partial heart transplant valves on a larger basis and determine an optimal antirejection dose necessary to maintain viability.”
Preclinical research leading to this case report was supported by the Brett Boyer Foundation. Dr. Turek reported no conflicts of interest.
A version of this article appeared on Medscape.com.
, researchers said.
The surgery was performed on the 18th day of life of a 5-pound newborn boy diagnosed prenatally with persistent truncus arteriosus and severe truncal valve dysfunction. The procedure involved transplantation of the part of the heart containing the aorta and pulmonary valves from an infant donor upon cardiac death.
The standard of care for neonatal heart valve implants are cadaver grafts. But these grafts are not viable and can’t grow or self-repair. Therefore, recipient neonates need to undergo repeated implant-exchange surgeries until an adult-sized heart valve can fit. Clinical outcomes generally are poor.
“We have learned that these partial heart transplant valves, when procured fresh and the [recipient] baby is placed on low-dose antirejection medicine, can grow with the child and function completely normally,” Joseph W. Turek, MD, PhD, MBA of Duke University Medical Center in Durham, North Carolina, told this news organization.
“This represents a new field in heart surgery that could dramatically change the way we care for children with poorly functioning heart valves by allowing valve implants that grow with them.”
A case report describing the novel intervention was published online on January 2, 2024, in JAMA.
‘Expected to Last a Lifetime’
The donor was a 2-day-old female weighing 8 pounds. Delivery had been complicated by hypoxic ischemic brain injury, but echocardiography showed structurally normal, functioning outflow heart valves. The heart was donated after cardiac death and procured using standard surgical techniques.
The recipient infant’s operation involved sternotomy, cardiopulmonary bypass, and cardioplegic arrest of the heart. The pulmonary artery ostia and coronary artery buttons were dissected, and the infant’s irreparable truncal valve was excised.
The donor aortic root was transplanted first, using donor tissue to close the ventricular septal defect. Then, the coronary artery buttons were reimplanted; the right ventricular outflow tract was enlarged; and the pulmonary root was transplanted. Postoperative immunosuppression followed.
On the follow-up at age 14 months, the transplanted valves showed no obstruction or insufficiency on echocardiography. Now, almost 21 months later, the recipient is doing well, Dr. Turek said. “His family has shared his many milestones with me, including eating his first birthday cake, videos of his first steps, and his newfound oral appetite (he was largely g-tube fed for a while).”
“The rationale for partial heart transplant is that pediatric heart transplants grow,” Dr. Turek and coauthors wrote. “Moreover, failure of heart transplant outflow valves is exceedingly rare. While heart transplant long-term outcomes are limited by inevitable ventricular dysfunction, partial heart transplants spare the native ventricles and are therefore expected to last a lifetime.”
‘Domino Hearts’
“While this particular baby had truncus arteriosus, this operation should prove to be beneficial for a host of congenital heart conditions with valves that are either too small or poorly functioning,” Dr. Turek said. “We have performed subsequent partial heart operations for babies with aortic stenosis, tetralogy of Fallot with pulmonary atresia, and biventricular outflow tract obstruction.”
The challenge is organ availability, he noted. “While this procedure does make use of hearts that would be otherwise unusable for full heart transplant, such as hearts with poor ventricular function or hearts removed from recipients of full heart transplants (aka domino hearts), the availability is still low compared to the need.”
With domino hearts, “you could potentially double the number of hearts that are used for the benefit of children with heart disease,” Dr. Turek said in a Duke communication released with the paper. In a domino heart procedure, a patient who has healthy valves but needs stronger heart muscle receives a full heart transplant, and the healthy valves are then donated to another patient in need, creating a domino effect.
Since this breakthrough procedure in 2022, partial heart transplants have been performed 13 times at four centers, including nine at Duke, three of which used the domino technique.
For now, Dr. Turek told this news organization, “we are hoping to receive funds for a clinical trial that will evaluate these partial heart transplant valves on a larger basis and determine an optimal antirejection dose necessary to maintain viability.”
Preclinical research leading to this case report was supported by the Brett Boyer Foundation. Dr. Turek reported no conflicts of interest.
A version of this article appeared on Medscape.com.
, researchers said.
The surgery was performed on the 18th day of life of a 5-pound newborn boy diagnosed prenatally with persistent truncus arteriosus and severe truncal valve dysfunction. The procedure involved transplantation of the part of the heart containing the aorta and pulmonary valves from an infant donor upon cardiac death.
The standard of care for neonatal heart valve implants are cadaver grafts. But these grafts are not viable and can’t grow or self-repair. Therefore, recipient neonates need to undergo repeated implant-exchange surgeries until an adult-sized heart valve can fit. Clinical outcomes generally are poor.
“We have learned that these partial heart transplant valves, when procured fresh and the [recipient] baby is placed on low-dose antirejection medicine, can grow with the child and function completely normally,” Joseph W. Turek, MD, PhD, MBA of Duke University Medical Center in Durham, North Carolina, told this news organization.
“This represents a new field in heart surgery that could dramatically change the way we care for children with poorly functioning heart valves by allowing valve implants that grow with them.”
A case report describing the novel intervention was published online on January 2, 2024, in JAMA.
‘Expected to Last a Lifetime’
The donor was a 2-day-old female weighing 8 pounds. Delivery had been complicated by hypoxic ischemic brain injury, but echocardiography showed structurally normal, functioning outflow heart valves. The heart was donated after cardiac death and procured using standard surgical techniques.
The recipient infant’s operation involved sternotomy, cardiopulmonary bypass, and cardioplegic arrest of the heart. The pulmonary artery ostia and coronary artery buttons were dissected, and the infant’s irreparable truncal valve was excised.
The donor aortic root was transplanted first, using donor tissue to close the ventricular septal defect. Then, the coronary artery buttons were reimplanted; the right ventricular outflow tract was enlarged; and the pulmonary root was transplanted. Postoperative immunosuppression followed.
On the follow-up at age 14 months, the transplanted valves showed no obstruction or insufficiency on echocardiography. Now, almost 21 months later, the recipient is doing well, Dr. Turek said. “His family has shared his many milestones with me, including eating his first birthday cake, videos of his first steps, and his newfound oral appetite (he was largely g-tube fed for a while).”
“The rationale for partial heart transplant is that pediatric heart transplants grow,” Dr. Turek and coauthors wrote. “Moreover, failure of heart transplant outflow valves is exceedingly rare. While heart transplant long-term outcomes are limited by inevitable ventricular dysfunction, partial heart transplants spare the native ventricles and are therefore expected to last a lifetime.”
‘Domino Hearts’
“While this particular baby had truncus arteriosus, this operation should prove to be beneficial for a host of congenital heart conditions with valves that are either too small or poorly functioning,” Dr. Turek said. “We have performed subsequent partial heart operations for babies with aortic stenosis, tetralogy of Fallot with pulmonary atresia, and biventricular outflow tract obstruction.”
The challenge is organ availability, he noted. “While this procedure does make use of hearts that would be otherwise unusable for full heart transplant, such as hearts with poor ventricular function or hearts removed from recipients of full heart transplants (aka domino hearts), the availability is still low compared to the need.”
With domino hearts, “you could potentially double the number of hearts that are used for the benefit of children with heart disease,” Dr. Turek said in a Duke communication released with the paper. In a domino heart procedure, a patient who has healthy valves but needs stronger heart muscle receives a full heart transplant, and the healthy valves are then donated to another patient in need, creating a domino effect.
Since this breakthrough procedure in 2022, partial heart transplants have been performed 13 times at four centers, including nine at Duke, three of which used the domino technique.
For now, Dr. Turek told this news organization, “we are hoping to receive funds for a clinical trial that will evaluate these partial heart transplant valves on a larger basis and determine an optimal antirejection dose necessary to maintain viability.”
Preclinical research leading to this case report was supported by the Brett Boyer Foundation. Dr. Turek reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Evidence Grows for SGLT2 Inhibitors in Rheumatology
Over just a decade, sodium-glucose cotransporter-2 (SGLT2) inhibitors have revolutionized the second-line treatment of type 2 diabetes by improving the control of blood sugar, and they’re also being used to treat heart failure and chronic kidney disease. Now, there’s growing evidence that the medications have the potential to play a role in the treatment of a variety of rheumatologic diseases — gout, systemic lupus erythematosus (SLE), and lupus nephritis.
“I suspect that SGLT2 inhibitors may have a role in multiple rheumatic diseases,” said rheumatologist April Jorge, MD, of Harvard Medical School and Massachusetts General Hospital, Boston.
In gout, for example, “SGLT2 inhibitors hold great promise as a multipurpose treatment option,” said rheumatologist Chio Yokose, MD, MSc, also of Harvard Medical School and Massachusetts General Hospital. Both Dr. Jorge and Dr. Yokose spoke at recent medical conferences and in interviews about the potential value of the drugs in rheumatology.
There’s a big caveat. For the moment, SGLT2 inhibitors aren’t cleared for use in the treatment of rheumatologic conditions, and neither physician is ready to recommend prescribing them off-label outside of their FDA-approved indications.
But studies could pave the way toward more approved uses in rheumatology. And there’s good news for now: Many rheumatology patients may already be eligible to take the drugs because of other medical conditions. In gout, for example, “sizable proportions of patients have comorbidities for which they are already indicated,” Dr. Yokose said.
Research Hints at Gout-Busting Potential
The first SGLT2 inhibitor canagliflozin (Invokana), received FDA approval in 2013, followed by dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and bexagliflozin (Brenzavvy). The drugs “lower blood sugar by causing the kidneys to remove sugar from the body through urine,” reports the National Kidney Foundation, and they “help to protect the kidneys and heart in people with CKD [chronic kidney disease].”
As Dr. Yokose noted in a presentation at the 2023 Gout Hyperuricemia and Crystal Associated Disease Network research symposium, SGLT2 inhibitors “have really become blockbuster drugs, and they’ve now been integrated into multiple professional society guidelines and recommendations.”
These drugs should not be confused with the wildly popular medications known as glucagon-like peptide-1 (GLP1) agonists, which include medications such as semaglutide (Ozempic and Wegovy). These drugs are generally administered via injection — unlike the oral SGLT2 inhibitors — and they’re variously indicated for type 2 diabetes and obesity.
Dr. Yokose highlighted research findings about the drugs in gout. A 2020 study, for example, tracked 295,907 US adults with type 2 diabetes who received a new prescription for an SGLT2 inhibitor or GLP1 agonist during 2013-2017. Those in the SGLT2 inhibitor group had a 36% lower risk of newly diagnosed gout (hazard ratio [HR], 0.64; 95% CI, 0.57-0.72), the researchers reported.
A similar study, a 2021 report from Taiwan, also linked SGLT2 inhibitors to improvement in gout incidence vs. dipeptidyl peptidase 4 (DPP4) inhibitors, diabetes drugs that are not linked to lower serum urate levels. In an adjusted analysis, the risk of gout was 11% lower in the SGLT2 inhibitor group (adjusted HR, 0.86; 95% CI, 0.78-0.95).
What about recurrent gout? In a 2023 study, Dr. Yokose and colleagues tracked patients with type 2 diabetes who began SGLT2 inhibitors or DPP4 inhibitors. Over the period from 2013 to 2017, those who took SGLT2 inhibitors were less likely to have gout flares (rate ratio [RR], 0.66; 95% CI, 0.57-0.75) and gout-primary emergency department visits/hospitalizations (RR, 0.52; 95% CI, 0.32-0.84).
“This finding requires further replication in other populations and compared to other drugs,” Dr. Yokose cautioned.
Another 2023 study analyzed UK data and reached similar results regarding risk of recurrent gout.
Lower Urate Levels and Less Inflammation Could Be Key
How might SGLT2 inhibitors reduce the risk of gout? Multiple studies have linked the drugs to lower serum urate levels, Dr. Yokose said, but researchers often excluded patients with gout.
For a small new study presented at the 2023 annual meeting of the American College of Rheumatology but not yet published, Dr. Yokose and colleagues reported that patients with gout who began SGLT2 inhibitors had lower urate levels than those who began a sulfonylurea, another second-line agent for type 2 diabetes. During the study period, up to 3 months before and after initiation, 43.5% of patients in the SGLT2 inhibitor group reached a target serum urate of < 6 mg/dL vs. 4.2% of sulfonylurea initiators.
“The magnitude of this reduction, while not as large as what can be achieved with appropriately titrated urate-lowering therapy such as allopurinol or febuxostat, is also not negligible. It’s believed to be between 1.5-2.0 mg/dL among patients with gout,” Dr. Yokose said. “Also, SGLT2 inhibitors are purported to have some anti-inflammatory effects that may target the same pathways responsible for the profound inflammation associated with acute gout flares. However, both the exact mechanisms underlying the serum urate-lowering and anti-inflammatory effects of SGLT2 [inhibitors] require further research and clarification.”
Moving forward, she said, “I would love to see some prospective studies of SGLT2 inhibitor use among patients with gout, looking at serum urate and clinical gout endpoints, as well as biomarkers to understand better the beneficial effects of SGLT2 inhibitors as it pertains to patients with gout.”
In Lupus, Findings Are More Mixed
Studies of SGLT2 inhibitors have excluded patients with lupus, limiting insight into their benefits in that specific population, said Dr. Jorge of Massachusetts General Hospital and Harvard Medical School. However, “one small phase I/II trial showed an acceptable safety profile of dapagliflozin add-on therapy in adult patients with SLE,” she said.
Her team is working to expand understanding about the drugs in people with lupus. At the 2023 ACR annual meeting, she presented the findings of a study that tracked patients with SLE who took SGLT2 inhibitors (n = 426, including 154 with lupus nephritis) or DPP4 inhibitors (n = 865, including 270 with lupus nephritis). Patients who took SGLT2 inhibitors had lower risks of major adverse cardiac events (HR, 0.69; 95% CI, 0.48-0.99) and renal progression (HR, 0.71; 95% CI, 0.51-0.98).
“Our results are promising, but the majority of patient with lupus who had received SGLT2 inhibitors also had the comorbidity of type 2 diabetes as a separate indication for SGLT2 inhibitor use,” Dr. Jorge said. “We still need to study the impact of SGLT2 inhibitors in patients with SLE and lupus nephritis who do not have a separate indication for the medication.”
Dr. Jorge added that “we do not yet know the ideal time to initiate SGLT2 inhibitors in the treatment of lupus nephritis. Specifically, it is not yet known whether these medications should be used in patients with persistent proteinuria due to damage from lupus nephritis or whether there is also a role to start these medications in patients with active lupus nephritis who are undergoing induction immunosuppression regimens.”
However, another study released at the 2023 ACR annual meeting suggested that SGLT2 inhibitors may not have a beneficial effect in lupus nephritis: “We observed a reduction in decline in eGFR [estimated glomerular filtration rate] after starting SGLT2 inhibitors; however, this reduction was not statistically significant … early experience suggested marginal benefit of SGLT2 inhibitors in SLE,” researchers from Johns Hopkins University, and the University of Maryland, Baltimore, reported.
“My cohort is not showing miracles from SGLT2 inhibitors,” study lead author Michelle Petri, MD, MPH, of Johns Hopkins, said in an interview.
Still, new European Alliance of Associations for Rheumatology recommendations for SLE now advise to consider the use of the drugs in patients with lupus nephritis who have reduced eGFR. Meanwhile, “the American College of Rheumatology is currently developing new treatment guidelines for SLE and for lupus nephritis, and SGLT2 inhibitors will likely be a topic of consideration,” Dr. Jorge added.
As for mechanism, Dr. Jorge said it’s not clear how the drugs may affect lupus. “It’s proposed that they have benefits in hemodynamic effects as well as potentially anti-inflammatory effects. The hemodynamic effects, including reducing intraglomerular hyperfiltration and reducing blood pressure, likely have similar benefits in patients with chronic kidney disease due to diabetic nephropathy or due to lupus nephritis with damage/scarring and persistent proteinuria. Patients with SLE and other chronic, systemic rheumatic diseases such as ANCA [antineutrophilic cytoplasmic antibody]-associated vasculitis also develop kidney disease and cardiovascular events mediated by inflammatory processes.”
Side Effects and Cost: Where Do They Fit In?
According to Dr. Yokose, SGLT2 inhibitors “are generally quite well-tolerated, and very serious adverse effects are rare.” Side effects include disrupted urination, increased thirst, genital infections, flu-like symptoms, and swelling.
Urinary-related problems are understandable “because these drugs cause the kidneys to pass more glucose into the urine,” University of Hong Kong cardiac specialist Bernard Cheung, MBBCh, PhD, who has studied SGLT2 inhibitors, said in an interview.
In Dr. Yokose’s 2023 study of SGLT2 inhibitors in recurrent gout, patients who took the drugs were 2.15 times more likely than the comparison group to have genital infections (hazard ratio, 2.15; 95% CI, 1.39-3.30). This finding “was what we’d expect,” she said.
She added that genital infection rates were higher among patients with diabetes, women, and uncircumcised men. “Fortunately, most experienced just a single mild episode that can readily be treated with topical therapy. There does not appear to be an increased risk of urinary tract infections.”
Dr. Cheung added that “doctors should be aware of a rare adverse effect called euglycemic ketoacidosis, in which the patient has increased ketones in the blood causing it to be more acidic than normal, but the blood glucose remains within the normal range.”
As for cost, goodrx.com reports that several SGLT2 inhibitors run about $550-$683 per month, making them expensive but still cheaper than GLP-1 agonists, which can cost $1,000 or more per month. Unlike the most popular GLP-1 agonists such as Ozempic, none of the SGLT2 inhibitors are in short supply, according to the American Society of Health-System Pharmacists.
“If someone with gout already has a cardiovascular-kidney-metabolic indication for SGLT2 inhibitors and also stands to benefit in terms of lowering serum urate and risk of recurrent gout flares, there is potential for high benefit relative to cost,” Dr. Yokose said.
She added: “It is well-documented that current gout care is suboptimal, and many patients end up in the emergency room or hospitalized for gout, which in and of itself is quite costly both for the patient and the health care system. Therefore, streamlining or integrating gout and comorbidity care with SGLT2 inhibitors could potentially be quite beneficial for patients with gout.”
In regard to lupus, “many patients with lupus undergo multiple hospitalizations related to their disease, which is a source of high health care costs,” Dr. Jorge said. “Additionally, chronic kidney disease and cardiovascular disease are major causes of disability and premature mortality. Further studies will be needed to better understand whether benefits of SGLT2 inhibitors may outweigh the costs of treatment.”
As for prescribing the drugs in lupus now, Dr. Jorge said they can be an option in lupus nephritis. “There is not a clear consensus of the ideal timing to initiate SGLT2 inhibitors — e.g., degree of proteinuria or eGFR range,” she said. “However, it is less controversial that SGLT2 inhibitors should be considered in particular for patients with lupus nephritis with ongoing proteinuria despite adequate treatment with conventional therapies.”
As for gout, Dr. Yokose isn’t ready to prescribe the drugs to patients who don’t have comorbidities that can be treated by the medications. However, she noted that those patients are rare.
“If I see a patient with gout with one or more of these comorbidities, and I see that they are not already on an SGLT2 inhibitor, I definitely take the time to talk to the patient about this exciting class of drugs and will consult with their other physicians about getting them started on an SGLT2 inhibitor.”
Dr. Yokose, Dr. Petri, and Dr. Cheung have no relevant disclosures. Dr. Jorge disclosed serving as a site investigator for SLE clinical trials funded by Bristol-Myers Squibb and Cabaletta Bio; the trials are not related to SGLT2 inhibitors.
Over just a decade, sodium-glucose cotransporter-2 (SGLT2) inhibitors have revolutionized the second-line treatment of type 2 diabetes by improving the control of blood sugar, and they’re also being used to treat heart failure and chronic kidney disease. Now, there’s growing evidence that the medications have the potential to play a role in the treatment of a variety of rheumatologic diseases — gout, systemic lupus erythematosus (SLE), and lupus nephritis.
“I suspect that SGLT2 inhibitors may have a role in multiple rheumatic diseases,” said rheumatologist April Jorge, MD, of Harvard Medical School and Massachusetts General Hospital, Boston.
In gout, for example, “SGLT2 inhibitors hold great promise as a multipurpose treatment option,” said rheumatologist Chio Yokose, MD, MSc, also of Harvard Medical School and Massachusetts General Hospital. Both Dr. Jorge and Dr. Yokose spoke at recent medical conferences and in interviews about the potential value of the drugs in rheumatology.
There’s a big caveat. For the moment, SGLT2 inhibitors aren’t cleared for use in the treatment of rheumatologic conditions, and neither physician is ready to recommend prescribing them off-label outside of their FDA-approved indications.
But studies could pave the way toward more approved uses in rheumatology. And there’s good news for now: Many rheumatology patients may already be eligible to take the drugs because of other medical conditions. In gout, for example, “sizable proportions of patients have comorbidities for which they are already indicated,” Dr. Yokose said.
Research Hints at Gout-Busting Potential
The first SGLT2 inhibitor canagliflozin (Invokana), received FDA approval in 2013, followed by dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and bexagliflozin (Brenzavvy). The drugs “lower blood sugar by causing the kidneys to remove sugar from the body through urine,” reports the National Kidney Foundation, and they “help to protect the kidneys and heart in people with CKD [chronic kidney disease].”
As Dr. Yokose noted in a presentation at the 2023 Gout Hyperuricemia and Crystal Associated Disease Network research symposium, SGLT2 inhibitors “have really become blockbuster drugs, and they’ve now been integrated into multiple professional society guidelines and recommendations.”
These drugs should not be confused with the wildly popular medications known as glucagon-like peptide-1 (GLP1) agonists, which include medications such as semaglutide (Ozempic and Wegovy). These drugs are generally administered via injection — unlike the oral SGLT2 inhibitors — and they’re variously indicated for type 2 diabetes and obesity.
Dr. Yokose highlighted research findings about the drugs in gout. A 2020 study, for example, tracked 295,907 US adults with type 2 diabetes who received a new prescription for an SGLT2 inhibitor or GLP1 agonist during 2013-2017. Those in the SGLT2 inhibitor group had a 36% lower risk of newly diagnosed gout (hazard ratio [HR], 0.64; 95% CI, 0.57-0.72), the researchers reported.
A similar study, a 2021 report from Taiwan, also linked SGLT2 inhibitors to improvement in gout incidence vs. dipeptidyl peptidase 4 (DPP4) inhibitors, diabetes drugs that are not linked to lower serum urate levels. In an adjusted analysis, the risk of gout was 11% lower in the SGLT2 inhibitor group (adjusted HR, 0.86; 95% CI, 0.78-0.95).
What about recurrent gout? In a 2023 study, Dr. Yokose and colleagues tracked patients with type 2 diabetes who began SGLT2 inhibitors or DPP4 inhibitors. Over the period from 2013 to 2017, those who took SGLT2 inhibitors were less likely to have gout flares (rate ratio [RR], 0.66; 95% CI, 0.57-0.75) and gout-primary emergency department visits/hospitalizations (RR, 0.52; 95% CI, 0.32-0.84).
“This finding requires further replication in other populations and compared to other drugs,” Dr. Yokose cautioned.
Another 2023 study analyzed UK data and reached similar results regarding risk of recurrent gout.
Lower Urate Levels and Less Inflammation Could Be Key
How might SGLT2 inhibitors reduce the risk of gout? Multiple studies have linked the drugs to lower serum urate levels, Dr. Yokose said, but researchers often excluded patients with gout.
For a small new study presented at the 2023 annual meeting of the American College of Rheumatology but not yet published, Dr. Yokose and colleagues reported that patients with gout who began SGLT2 inhibitors had lower urate levels than those who began a sulfonylurea, another second-line agent for type 2 diabetes. During the study period, up to 3 months before and after initiation, 43.5% of patients in the SGLT2 inhibitor group reached a target serum urate of < 6 mg/dL vs. 4.2% of sulfonylurea initiators.
“The magnitude of this reduction, while not as large as what can be achieved with appropriately titrated urate-lowering therapy such as allopurinol or febuxostat, is also not negligible. It’s believed to be between 1.5-2.0 mg/dL among patients with gout,” Dr. Yokose said. “Also, SGLT2 inhibitors are purported to have some anti-inflammatory effects that may target the same pathways responsible for the profound inflammation associated with acute gout flares. However, both the exact mechanisms underlying the serum urate-lowering and anti-inflammatory effects of SGLT2 [inhibitors] require further research and clarification.”
Moving forward, she said, “I would love to see some prospective studies of SGLT2 inhibitor use among patients with gout, looking at serum urate and clinical gout endpoints, as well as biomarkers to understand better the beneficial effects of SGLT2 inhibitors as it pertains to patients with gout.”
In Lupus, Findings Are More Mixed
Studies of SGLT2 inhibitors have excluded patients with lupus, limiting insight into their benefits in that specific population, said Dr. Jorge of Massachusetts General Hospital and Harvard Medical School. However, “one small phase I/II trial showed an acceptable safety profile of dapagliflozin add-on therapy in adult patients with SLE,” she said.
Her team is working to expand understanding about the drugs in people with lupus. At the 2023 ACR annual meeting, she presented the findings of a study that tracked patients with SLE who took SGLT2 inhibitors (n = 426, including 154 with lupus nephritis) or DPP4 inhibitors (n = 865, including 270 with lupus nephritis). Patients who took SGLT2 inhibitors had lower risks of major adverse cardiac events (HR, 0.69; 95% CI, 0.48-0.99) and renal progression (HR, 0.71; 95% CI, 0.51-0.98).
“Our results are promising, but the majority of patient with lupus who had received SGLT2 inhibitors also had the comorbidity of type 2 diabetes as a separate indication for SGLT2 inhibitor use,” Dr. Jorge said. “We still need to study the impact of SGLT2 inhibitors in patients with SLE and lupus nephritis who do not have a separate indication for the medication.”
Dr. Jorge added that “we do not yet know the ideal time to initiate SGLT2 inhibitors in the treatment of lupus nephritis. Specifically, it is not yet known whether these medications should be used in patients with persistent proteinuria due to damage from lupus nephritis or whether there is also a role to start these medications in patients with active lupus nephritis who are undergoing induction immunosuppression regimens.”
However, another study released at the 2023 ACR annual meeting suggested that SGLT2 inhibitors may not have a beneficial effect in lupus nephritis: “We observed a reduction in decline in eGFR [estimated glomerular filtration rate] after starting SGLT2 inhibitors; however, this reduction was not statistically significant … early experience suggested marginal benefit of SGLT2 inhibitors in SLE,” researchers from Johns Hopkins University, and the University of Maryland, Baltimore, reported.
“My cohort is not showing miracles from SGLT2 inhibitors,” study lead author Michelle Petri, MD, MPH, of Johns Hopkins, said in an interview.
Still, new European Alliance of Associations for Rheumatology recommendations for SLE now advise to consider the use of the drugs in patients with lupus nephritis who have reduced eGFR. Meanwhile, “the American College of Rheumatology is currently developing new treatment guidelines for SLE and for lupus nephritis, and SGLT2 inhibitors will likely be a topic of consideration,” Dr. Jorge added.
As for mechanism, Dr. Jorge said it’s not clear how the drugs may affect lupus. “It’s proposed that they have benefits in hemodynamic effects as well as potentially anti-inflammatory effects. The hemodynamic effects, including reducing intraglomerular hyperfiltration and reducing blood pressure, likely have similar benefits in patients with chronic kidney disease due to diabetic nephropathy or due to lupus nephritis with damage/scarring and persistent proteinuria. Patients with SLE and other chronic, systemic rheumatic diseases such as ANCA [antineutrophilic cytoplasmic antibody]-associated vasculitis also develop kidney disease and cardiovascular events mediated by inflammatory processes.”
Side Effects and Cost: Where Do They Fit In?
According to Dr. Yokose, SGLT2 inhibitors “are generally quite well-tolerated, and very serious adverse effects are rare.” Side effects include disrupted urination, increased thirst, genital infections, flu-like symptoms, and swelling.
Urinary-related problems are understandable “because these drugs cause the kidneys to pass more glucose into the urine,” University of Hong Kong cardiac specialist Bernard Cheung, MBBCh, PhD, who has studied SGLT2 inhibitors, said in an interview.
In Dr. Yokose’s 2023 study of SGLT2 inhibitors in recurrent gout, patients who took the drugs were 2.15 times more likely than the comparison group to have genital infections (hazard ratio, 2.15; 95% CI, 1.39-3.30). This finding “was what we’d expect,” she said.
She added that genital infection rates were higher among patients with diabetes, women, and uncircumcised men. “Fortunately, most experienced just a single mild episode that can readily be treated with topical therapy. There does not appear to be an increased risk of urinary tract infections.”
Dr. Cheung added that “doctors should be aware of a rare adverse effect called euglycemic ketoacidosis, in which the patient has increased ketones in the blood causing it to be more acidic than normal, but the blood glucose remains within the normal range.”
As for cost, goodrx.com reports that several SGLT2 inhibitors run about $550-$683 per month, making them expensive but still cheaper than GLP-1 agonists, which can cost $1,000 or more per month. Unlike the most popular GLP-1 agonists such as Ozempic, none of the SGLT2 inhibitors are in short supply, according to the American Society of Health-System Pharmacists.
“If someone with gout already has a cardiovascular-kidney-metabolic indication for SGLT2 inhibitors and also stands to benefit in terms of lowering serum urate and risk of recurrent gout flares, there is potential for high benefit relative to cost,” Dr. Yokose said.
She added: “It is well-documented that current gout care is suboptimal, and many patients end up in the emergency room or hospitalized for gout, which in and of itself is quite costly both for the patient and the health care system. Therefore, streamlining or integrating gout and comorbidity care with SGLT2 inhibitors could potentially be quite beneficial for patients with gout.”
In regard to lupus, “many patients with lupus undergo multiple hospitalizations related to their disease, which is a source of high health care costs,” Dr. Jorge said. “Additionally, chronic kidney disease and cardiovascular disease are major causes of disability and premature mortality. Further studies will be needed to better understand whether benefits of SGLT2 inhibitors may outweigh the costs of treatment.”
As for prescribing the drugs in lupus now, Dr. Jorge said they can be an option in lupus nephritis. “There is not a clear consensus of the ideal timing to initiate SGLT2 inhibitors — e.g., degree of proteinuria or eGFR range,” she said. “However, it is less controversial that SGLT2 inhibitors should be considered in particular for patients with lupus nephritis with ongoing proteinuria despite adequate treatment with conventional therapies.”
As for gout, Dr. Yokose isn’t ready to prescribe the drugs to patients who don’t have comorbidities that can be treated by the medications. However, she noted that those patients are rare.
“If I see a patient with gout with one or more of these comorbidities, and I see that they are not already on an SGLT2 inhibitor, I definitely take the time to talk to the patient about this exciting class of drugs and will consult with their other physicians about getting them started on an SGLT2 inhibitor.”
Dr. Yokose, Dr. Petri, and Dr. Cheung have no relevant disclosures. Dr. Jorge disclosed serving as a site investigator for SLE clinical trials funded by Bristol-Myers Squibb and Cabaletta Bio; the trials are not related to SGLT2 inhibitors.
Over just a decade, sodium-glucose cotransporter-2 (SGLT2) inhibitors have revolutionized the second-line treatment of type 2 diabetes by improving the control of blood sugar, and they’re also being used to treat heart failure and chronic kidney disease. Now, there’s growing evidence that the medications have the potential to play a role in the treatment of a variety of rheumatologic diseases — gout, systemic lupus erythematosus (SLE), and lupus nephritis.
“I suspect that SGLT2 inhibitors may have a role in multiple rheumatic diseases,” said rheumatologist April Jorge, MD, of Harvard Medical School and Massachusetts General Hospital, Boston.
In gout, for example, “SGLT2 inhibitors hold great promise as a multipurpose treatment option,” said rheumatologist Chio Yokose, MD, MSc, also of Harvard Medical School and Massachusetts General Hospital. Both Dr. Jorge and Dr. Yokose spoke at recent medical conferences and in interviews about the potential value of the drugs in rheumatology.
There’s a big caveat. For the moment, SGLT2 inhibitors aren’t cleared for use in the treatment of rheumatologic conditions, and neither physician is ready to recommend prescribing them off-label outside of their FDA-approved indications.
But studies could pave the way toward more approved uses in rheumatology. And there’s good news for now: Many rheumatology patients may already be eligible to take the drugs because of other medical conditions. In gout, for example, “sizable proportions of patients have comorbidities for which they are already indicated,” Dr. Yokose said.
Research Hints at Gout-Busting Potential
The first SGLT2 inhibitor canagliflozin (Invokana), received FDA approval in 2013, followed by dapagliflozin (Farxiga), empagliflozin (Jardiance), ertugliflozin (Steglatro), and bexagliflozin (Brenzavvy). The drugs “lower blood sugar by causing the kidneys to remove sugar from the body through urine,” reports the National Kidney Foundation, and they “help to protect the kidneys and heart in people with CKD [chronic kidney disease].”
As Dr. Yokose noted in a presentation at the 2023 Gout Hyperuricemia and Crystal Associated Disease Network research symposium, SGLT2 inhibitors “have really become blockbuster drugs, and they’ve now been integrated into multiple professional society guidelines and recommendations.”
These drugs should not be confused with the wildly popular medications known as glucagon-like peptide-1 (GLP1) agonists, which include medications such as semaglutide (Ozempic and Wegovy). These drugs are generally administered via injection — unlike the oral SGLT2 inhibitors — and they’re variously indicated for type 2 diabetes and obesity.
Dr. Yokose highlighted research findings about the drugs in gout. A 2020 study, for example, tracked 295,907 US adults with type 2 diabetes who received a new prescription for an SGLT2 inhibitor or GLP1 agonist during 2013-2017. Those in the SGLT2 inhibitor group had a 36% lower risk of newly diagnosed gout (hazard ratio [HR], 0.64; 95% CI, 0.57-0.72), the researchers reported.
A similar study, a 2021 report from Taiwan, also linked SGLT2 inhibitors to improvement in gout incidence vs. dipeptidyl peptidase 4 (DPP4) inhibitors, diabetes drugs that are not linked to lower serum urate levels. In an adjusted analysis, the risk of gout was 11% lower in the SGLT2 inhibitor group (adjusted HR, 0.86; 95% CI, 0.78-0.95).
What about recurrent gout? In a 2023 study, Dr. Yokose and colleagues tracked patients with type 2 diabetes who began SGLT2 inhibitors or DPP4 inhibitors. Over the period from 2013 to 2017, those who took SGLT2 inhibitors were less likely to have gout flares (rate ratio [RR], 0.66; 95% CI, 0.57-0.75) and gout-primary emergency department visits/hospitalizations (RR, 0.52; 95% CI, 0.32-0.84).
“This finding requires further replication in other populations and compared to other drugs,” Dr. Yokose cautioned.
Another 2023 study analyzed UK data and reached similar results regarding risk of recurrent gout.
Lower Urate Levels and Less Inflammation Could Be Key
How might SGLT2 inhibitors reduce the risk of gout? Multiple studies have linked the drugs to lower serum urate levels, Dr. Yokose said, but researchers often excluded patients with gout.
For a small new study presented at the 2023 annual meeting of the American College of Rheumatology but not yet published, Dr. Yokose and colleagues reported that patients with gout who began SGLT2 inhibitors had lower urate levels than those who began a sulfonylurea, another second-line agent for type 2 diabetes. During the study period, up to 3 months before and after initiation, 43.5% of patients in the SGLT2 inhibitor group reached a target serum urate of < 6 mg/dL vs. 4.2% of sulfonylurea initiators.
“The magnitude of this reduction, while not as large as what can be achieved with appropriately titrated urate-lowering therapy such as allopurinol or febuxostat, is also not negligible. It’s believed to be between 1.5-2.0 mg/dL among patients with gout,” Dr. Yokose said. “Also, SGLT2 inhibitors are purported to have some anti-inflammatory effects that may target the same pathways responsible for the profound inflammation associated with acute gout flares. However, both the exact mechanisms underlying the serum urate-lowering and anti-inflammatory effects of SGLT2 [inhibitors] require further research and clarification.”
Moving forward, she said, “I would love to see some prospective studies of SGLT2 inhibitor use among patients with gout, looking at serum urate and clinical gout endpoints, as well as biomarkers to understand better the beneficial effects of SGLT2 inhibitors as it pertains to patients with gout.”
In Lupus, Findings Are More Mixed
Studies of SGLT2 inhibitors have excluded patients with lupus, limiting insight into their benefits in that specific population, said Dr. Jorge of Massachusetts General Hospital and Harvard Medical School. However, “one small phase I/II trial showed an acceptable safety profile of dapagliflozin add-on therapy in adult patients with SLE,” she said.
Her team is working to expand understanding about the drugs in people with lupus. At the 2023 ACR annual meeting, she presented the findings of a study that tracked patients with SLE who took SGLT2 inhibitors (n = 426, including 154 with lupus nephritis) or DPP4 inhibitors (n = 865, including 270 with lupus nephritis). Patients who took SGLT2 inhibitors had lower risks of major adverse cardiac events (HR, 0.69; 95% CI, 0.48-0.99) and renal progression (HR, 0.71; 95% CI, 0.51-0.98).
“Our results are promising, but the majority of patient with lupus who had received SGLT2 inhibitors also had the comorbidity of type 2 diabetes as a separate indication for SGLT2 inhibitor use,” Dr. Jorge said. “We still need to study the impact of SGLT2 inhibitors in patients with SLE and lupus nephritis who do not have a separate indication for the medication.”
Dr. Jorge added that “we do not yet know the ideal time to initiate SGLT2 inhibitors in the treatment of lupus nephritis. Specifically, it is not yet known whether these medications should be used in patients with persistent proteinuria due to damage from lupus nephritis or whether there is also a role to start these medications in patients with active lupus nephritis who are undergoing induction immunosuppression regimens.”
However, another study released at the 2023 ACR annual meeting suggested that SGLT2 inhibitors may not have a beneficial effect in lupus nephritis: “We observed a reduction in decline in eGFR [estimated glomerular filtration rate] after starting SGLT2 inhibitors; however, this reduction was not statistically significant … early experience suggested marginal benefit of SGLT2 inhibitors in SLE,” researchers from Johns Hopkins University, and the University of Maryland, Baltimore, reported.
“My cohort is not showing miracles from SGLT2 inhibitors,” study lead author Michelle Petri, MD, MPH, of Johns Hopkins, said in an interview.
Still, new European Alliance of Associations for Rheumatology recommendations for SLE now advise to consider the use of the drugs in patients with lupus nephritis who have reduced eGFR. Meanwhile, “the American College of Rheumatology is currently developing new treatment guidelines for SLE and for lupus nephritis, and SGLT2 inhibitors will likely be a topic of consideration,” Dr. Jorge added.
As for mechanism, Dr. Jorge said it’s not clear how the drugs may affect lupus. “It’s proposed that they have benefits in hemodynamic effects as well as potentially anti-inflammatory effects. The hemodynamic effects, including reducing intraglomerular hyperfiltration and reducing blood pressure, likely have similar benefits in patients with chronic kidney disease due to diabetic nephropathy or due to lupus nephritis with damage/scarring and persistent proteinuria. Patients with SLE and other chronic, systemic rheumatic diseases such as ANCA [antineutrophilic cytoplasmic antibody]-associated vasculitis also develop kidney disease and cardiovascular events mediated by inflammatory processes.”
Side Effects and Cost: Where Do They Fit In?
According to Dr. Yokose, SGLT2 inhibitors “are generally quite well-tolerated, and very serious adverse effects are rare.” Side effects include disrupted urination, increased thirst, genital infections, flu-like symptoms, and swelling.
Urinary-related problems are understandable “because these drugs cause the kidneys to pass more glucose into the urine,” University of Hong Kong cardiac specialist Bernard Cheung, MBBCh, PhD, who has studied SGLT2 inhibitors, said in an interview.
In Dr. Yokose’s 2023 study of SGLT2 inhibitors in recurrent gout, patients who took the drugs were 2.15 times more likely than the comparison group to have genital infections (hazard ratio, 2.15; 95% CI, 1.39-3.30). This finding “was what we’d expect,” she said.
She added that genital infection rates were higher among patients with diabetes, women, and uncircumcised men. “Fortunately, most experienced just a single mild episode that can readily be treated with topical therapy. There does not appear to be an increased risk of urinary tract infections.”
Dr. Cheung added that “doctors should be aware of a rare adverse effect called euglycemic ketoacidosis, in which the patient has increased ketones in the blood causing it to be more acidic than normal, but the blood glucose remains within the normal range.”
As for cost, goodrx.com reports that several SGLT2 inhibitors run about $550-$683 per month, making them expensive but still cheaper than GLP-1 agonists, which can cost $1,000 or more per month. Unlike the most popular GLP-1 agonists such as Ozempic, none of the SGLT2 inhibitors are in short supply, according to the American Society of Health-System Pharmacists.
“If someone with gout already has a cardiovascular-kidney-metabolic indication for SGLT2 inhibitors and also stands to benefit in terms of lowering serum urate and risk of recurrent gout flares, there is potential for high benefit relative to cost,” Dr. Yokose said.
She added: “It is well-documented that current gout care is suboptimal, and many patients end up in the emergency room or hospitalized for gout, which in and of itself is quite costly both for the patient and the health care system. Therefore, streamlining or integrating gout and comorbidity care with SGLT2 inhibitors could potentially be quite beneficial for patients with gout.”
In regard to lupus, “many patients with lupus undergo multiple hospitalizations related to their disease, which is a source of high health care costs,” Dr. Jorge said. “Additionally, chronic kidney disease and cardiovascular disease are major causes of disability and premature mortality. Further studies will be needed to better understand whether benefits of SGLT2 inhibitors may outweigh the costs of treatment.”
As for prescribing the drugs in lupus now, Dr. Jorge said they can be an option in lupus nephritis. “There is not a clear consensus of the ideal timing to initiate SGLT2 inhibitors — e.g., degree of proteinuria or eGFR range,” she said. “However, it is less controversial that SGLT2 inhibitors should be considered in particular for patients with lupus nephritis with ongoing proteinuria despite adequate treatment with conventional therapies.”
As for gout, Dr. Yokose isn’t ready to prescribe the drugs to patients who don’t have comorbidities that can be treated by the medications. However, she noted that those patients are rare.
“If I see a patient with gout with one or more of these comorbidities, and I see that they are not already on an SGLT2 inhibitor, I definitely take the time to talk to the patient about this exciting class of drugs and will consult with their other physicians about getting them started on an SGLT2 inhibitor.”
Dr. Yokose, Dr. Petri, and Dr. Cheung have no relevant disclosures. Dr. Jorge disclosed serving as a site investigator for SLE clinical trials funded by Bristol-Myers Squibb and Cabaletta Bio; the trials are not related to SGLT2 inhibitors.
New Stroke Prevention: Clopidogrel-Aspirin Within 72 Hours
TOPLINE:
Dual antiplatelet therapy (DAPT) with clopidogrel-aspirin given within 72 hours of a mild ischemic stroke or a high-risk transient ischemic attack (TIA) shows a greater risk reduction for new stroke than aspirin alone, although with a higher bleeding risk.
METHODOLOGY:
- The INSPIRES, a double-blind, placebo-controlled trial, involved patients with mild ischemic stroke or high-risk TIA of presumed atherosclerotic cause who had not undergone thrombolysis or thrombectomy.
- A total of 6100 patients were randomly assigned to receive clopidogrel plus aspirin or matching clopidogrel placebo plus aspirin within 72 hours after symptom onset.
- The occurrence of any new stroke (ischemic or hemorrhagic) within 90 days was the primary efficacy outcome.
- The primary safety outcome was moderate to severe bleeding, also assessed within 90 days.
TAKEAWAY:
- Within 24 hours of symptom onset, 12.8% of patients were assigned to each treatment group, and the remaining 87.2% were assigned within the time window of 24-72 hours.
- (7.3% vs 9.2%; marginal estimated hazard ratio [HR], 0.79; P =.008).
- The risk of a composite cardiovascular event and ischemic stroke were also 20%-25% lower with aspirin-clopidogrel combo vs aspirin alone.
- Moderate to severe bleeding was low in both groups (<1%), but the risk was double in patients who received DAPT vs aspirin alone (HR, 2.08; P =.03).
IN PRACTICE:
In an accompanying editorial, Anthony S. Kim, MD from the UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, commented, “The current trial provides evidence to support expanding the time window for dual antiplatelet therapy to 72 hours.” He also warned against administering DAPT to “patients with heightened bleeding risks, such as those with a history of cerebral or systemic hemorrhage.”
SOURCE:
Yilong Wang, MD, PhD, who held positions in the Department of Neurology, Beijing Tiantan Hospital, and several other institutions, was the corresponding author of this study. This study was published online December 28 in the New England Journal of Medicine.
LIMITATIONS:
- Patients with stroke of presumed cardioembolic origin, those with moderate or severe stroke, and those who had undergone thrombolysis or thrombectomy were excluded from this study.
- Of the enrolled participants, 98.5% belonged to the Han Chinese ethnic group.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the National Key R&D Program of China, and other sources. Some authors declared receiving grants or contracts or serving as consultants in various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Dual antiplatelet therapy (DAPT) with clopidogrel-aspirin given within 72 hours of a mild ischemic stroke or a high-risk transient ischemic attack (TIA) shows a greater risk reduction for new stroke than aspirin alone, although with a higher bleeding risk.
METHODOLOGY:
- The INSPIRES, a double-blind, placebo-controlled trial, involved patients with mild ischemic stroke or high-risk TIA of presumed atherosclerotic cause who had not undergone thrombolysis or thrombectomy.
- A total of 6100 patients were randomly assigned to receive clopidogrel plus aspirin or matching clopidogrel placebo plus aspirin within 72 hours after symptom onset.
- The occurrence of any new stroke (ischemic or hemorrhagic) within 90 days was the primary efficacy outcome.
- The primary safety outcome was moderate to severe bleeding, also assessed within 90 days.
TAKEAWAY:
- Within 24 hours of symptom onset, 12.8% of patients were assigned to each treatment group, and the remaining 87.2% were assigned within the time window of 24-72 hours.
- (7.3% vs 9.2%; marginal estimated hazard ratio [HR], 0.79; P =.008).
- The risk of a composite cardiovascular event and ischemic stroke were also 20%-25% lower with aspirin-clopidogrel combo vs aspirin alone.
- Moderate to severe bleeding was low in both groups (<1%), but the risk was double in patients who received DAPT vs aspirin alone (HR, 2.08; P =.03).
IN PRACTICE:
In an accompanying editorial, Anthony S. Kim, MD from the UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, commented, “The current trial provides evidence to support expanding the time window for dual antiplatelet therapy to 72 hours.” He also warned against administering DAPT to “patients with heightened bleeding risks, such as those with a history of cerebral or systemic hemorrhage.”
SOURCE:
Yilong Wang, MD, PhD, who held positions in the Department of Neurology, Beijing Tiantan Hospital, and several other institutions, was the corresponding author of this study. This study was published online December 28 in the New England Journal of Medicine.
LIMITATIONS:
- Patients with stroke of presumed cardioembolic origin, those with moderate or severe stroke, and those who had undergone thrombolysis or thrombectomy were excluded from this study.
- Of the enrolled participants, 98.5% belonged to the Han Chinese ethnic group.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the National Key R&D Program of China, and other sources. Some authors declared receiving grants or contracts or serving as consultants in various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Dual antiplatelet therapy (DAPT) with clopidogrel-aspirin given within 72 hours of a mild ischemic stroke or a high-risk transient ischemic attack (TIA) shows a greater risk reduction for new stroke than aspirin alone, although with a higher bleeding risk.
METHODOLOGY:
- The INSPIRES, a double-blind, placebo-controlled trial, involved patients with mild ischemic stroke or high-risk TIA of presumed atherosclerotic cause who had not undergone thrombolysis or thrombectomy.
- A total of 6100 patients were randomly assigned to receive clopidogrel plus aspirin or matching clopidogrel placebo plus aspirin within 72 hours after symptom onset.
- The occurrence of any new stroke (ischemic or hemorrhagic) within 90 days was the primary efficacy outcome.
- The primary safety outcome was moderate to severe bleeding, also assessed within 90 days.
TAKEAWAY:
- Within 24 hours of symptom onset, 12.8% of patients were assigned to each treatment group, and the remaining 87.2% were assigned within the time window of 24-72 hours.
- (7.3% vs 9.2%; marginal estimated hazard ratio [HR], 0.79; P =.008).
- The risk of a composite cardiovascular event and ischemic stroke were also 20%-25% lower with aspirin-clopidogrel combo vs aspirin alone.
- Moderate to severe bleeding was low in both groups (<1%), but the risk was double in patients who received DAPT vs aspirin alone (HR, 2.08; P =.03).
IN PRACTICE:
In an accompanying editorial, Anthony S. Kim, MD from the UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, commented, “The current trial provides evidence to support expanding the time window for dual antiplatelet therapy to 72 hours.” He also warned against administering DAPT to “patients with heightened bleeding risks, such as those with a history of cerebral or systemic hemorrhage.”
SOURCE:
Yilong Wang, MD, PhD, who held positions in the Department of Neurology, Beijing Tiantan Hospital, and several other institutions, was the corresponding author of this study. This study was published online December 28 in the New England Journal of Medicine.
LIMITATIONS:
- Patients with stroke of presumed cardioembolic origin, those with moderate or severe stroke, and those who had undergone thrombolysis or thrombectomy were excluded from this study.
- Of the enrolled participants, 98.5% belonged to the Han Chinese ethnic group.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the National Key R&D Program of China, and other sources. Some authors declared receiving grants or contracts or serving as consultants in various sources.
A version of this article appeared on Medscape.com.