User login
VIDEO: Rivaroxaban plus aspirin halves ischemic strokes
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
REPORTING FROM ISC 2018
Key clinical point: Rivaroxaban plus aspirin cuts strokes in patients with stable atherosclerotic vascular disease.
Major finding: Rivaroxaban plus aspirin cut the rate of ischemic strokes by 49%, compared with aspirin only.
Study details: Secondary analysis from the COMPASS trial, a multicenter, randomized trial with 27,395 patients.
Disclosures: COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
Source: Sharma M et al. ISC 2018, Abstract LB7.
Asymptomatic carotid in-stent restenosis? Think medical management
CHICAGO – with a carotid in-stent restenosis in excess of 70%, Jayer Chung, MD, asserted at a symposium on vascular surgery sponsored by Northwestern University.
That, in his view, makes medical management the clear preferred strategy.
A growing body of evidence, mainly from nested cohorts within randomized controlled trials, indicates that the late ipsilateral stroke rate associated with post–carotid endarterectomy restenosis (CEA) is much higher than that for carotid in-stent restenosis (C-ISR). In a recent meta-analysis of nine randomized trials, the difference in risk was more than 10-fold, with a 9.2% stroke rate at a mean of 37 months of follow-up in patients with post-CEA restenosis, compared with a 0.8% rate with 50 months of follow-up in patients with C-ISR (Eur J Vasc Endovasc Surg. 2017 Jun;53[6]:766-75).
“These pathologies behave very, very differently,” Dr. Chung observed. “The C-ISR lesions tend to be less embologenic.”
C-ISR is an uncommon event. Extrapolating from the landmark CREST (Carotid Revascularization Endarterectomy versus Stenting Trial) and other randomized trials, about 6% of patients who undergo percutaneous carotid stenting will be develop C-ISR within 2 years. But since the proportion of all carotid revascularizations that are done by carotid stent angioplasty has steadily increased over the past 15 years as the frequency of CEA has dropped, C-ISR is a problem that vascular specialists will continue to encounter on a regular basis.
Symptomatic C-ISR warrants reintervention; broad agreement exists on that. But there is a paucity of data to guide treatment decisions regarding asymptomatic yet angiographically severe C-ISR. Indeed, Dr. Chung was lead author of the only retrospective study of the natural history of untreated C-ISR, as opposed to carefully selected cohorts from randomized trials involving highly experienced operators. This study was a retrospective review of 59 patients with 75 C-ISRs of at least 50% seen at a single Veterans Affairs medical center over a 13-year period. Three-quarters of the ISRs were asymptomatic.
Forty of the 79 C-ISRs underwent percutaneous intervention at the physician’s discretion. Those patients did not differ from the observation-only group in age, comorbid conditions, type of original stent, or clopidogrel use. Reintervention was safe: There was one stent thrombosis resulting in stroke and death within 30 days in the reintervention group and no 30-day strokes in the observation-only group. During a mean 2.6 years of follow-up, the composite rate of death, stroke, or MI was low and not statistically significantly different between the two groups. Indeed, during up to 13 years of follow-up only one patient with untreated C-ISR experienced an ipsilateral stroke, as did two patients in the percutaneous intervention group (J Vasc Surg. 2016 Nov;64[5]:1286-94).
Dr. Chung does the math
According to data from the National Inpatient Sample, vascular surgeons do an average of 15 carotid angioplasty and stenting procedures per year. If 6% of those stents develop in-stent restenosis, and with a number needed to treat with revascularization of 25 to prevent 1 stroke, Dr. Chung estimated that hypothetically it would take the typical vascular surgeon 27 years to prevent one stroke due to C-ISR.
“That’s a very long time to prevent one stroke, in my opinion,” he said.
How his study has affected his own practice
Dr. Chung now intervenes only for symptomatic C-ISRs, and only after an affected patient is on optimal medical therapy, including a statin and dual-antiplatelet therapy.
“I try to do an open procedure if possible, especially if the restenosis is above C-2. The ones I tend to do percutaneously are the post-irradiation stenoses or those with excessive scarring, and I use a cerebral protection device,” the surgeon explained.
He emphasized, however, that the final word on the appropriate management of C-ISRs isn’t in yet. A standardized definition of C-ISR is needed, as are multicenter prospective registries of medically managed patients as well as those undergoing various forms of reintervention. And a pathologic study is warranted to confirm the hypothesis that the histopathology of post-CEA and post-stent restenosis – and hence the natural history – is markedly different.
Dr. Chung reported having no financial conflicts regarding his presentation.
CHICAGO – with a carotid in-stent restenosis in excess of 70%, Jayer Chung, MD, asserted at a symposium on vascular surgery sponsored by Northwestern University.
That, in his view, makes medical management the clear preferred strategy.
A growing body of evidence, mainly from nested cohorts within randomized controlled trials, indicates that the late ipsilateral stroke rate associated with post–carotid endarterectomy restenosis (CEA) is much higher than that for carotid in-stent restenosis (C-ISR). In a recent meta-analysis of nine randomized trials, the difference in risk was more than 10-fold, with a 9.2% stroke rate at a mean of 37 months of follow-up in patients with post-CEA restenosis, compared with a 0.8% rate with 50 months of follow-up in patients with C-ISR (Eur J Vasc Endovasc Surg. 2017 Jun;53[6]:766-75).
“These pathologies behave very, very differently,” Dr. Chung observed. “The C-ISR lesions tend to be less embologenic.”
C-ISR is an uncommon event. Extrapolating from the landmark CREST (Carotid Revascularization Endarterectomy versus Stenting Trial) and other randomized trials, about 6% of patients who undergo percutaneous carotid stenting will be develop C-ISR within 2 years. But since the proportion of all carotid revascularizations that are done by carotid stent angioplasty has steadily increased over the past 15 years as the frequency of CEA has dropped, C-ISR is a problem that vascular specialists will continue to encounter on a regular basis.
Symptomatic C-ISR warrants reintervention; broad agreement exists on that. But there is a paucity of data to guide treatment decisions regarding asymptomatic yet angiographically severe C-ISR. Indeed, Dr. Chung was lead author of the only retrospective study of the natural history of untreated C-ISR, as opposed to carefully selected cohorts from randomized trials involving highly experienced operators. This study was a retrospective review of 59 patients with 75 C-ISRs of at least 50% seen at a single Veterans Affairs medical center over a 13-year period. Three-quarters of the ISRs were asymptomatic.
Forty of the 79 C-ISRs underwent percutaneous intervention at the physician’s discretion. Those patients did not differ from the observation-only group in age, comorbid conditions, type of original stent, or clopidogrel use. Reintervention was safe: There was one stent thrombosis resulting in stroke and death within 30 days in the reintervention group and no 30-day strokes in the observation-only group. During a mean 2.6 years of follow-up, the composite rate of death, stroke, or MI was low and not statistically significantly different between the two groups. Indeed, during up to 13 years of follow-up only one patient with untreated C-ISR experienced an ipsilateral stroke, as did two patients in the percutaneous intervention group (J Vasc Surg. 2016 Nov;64[5]:1286-94).
Dr. Chung does the math
According to data from the National Inpatient Sample, vascular surgeons do an average of 15 carotid angioplasty and stenting procedures per year. If 6% of those stents develop in-stent restenosis, and with a number needed to treat with revascularization of 25 to prevent 1 stroke, Dr. Chung estimated that hypothetically it would take the typical vascular surgeon 27 years to prevent one stroke due to C-ISR.
“That’s a very long time to prevent one stroke, in my opinion,” he said.
How his study has affected his own practice
Dr. Chung now intervenes only for symptomatic C-ISRs, and only after an affected patient is on optimal medical therapy, including a statin and dual-antiplatelet therapy.
“I try to do an open procedure if possible, especially if the restenosis is above C-2. The ones I tend to do percutaneously are the post-irradiation stenoses or those with excessive scarring, and I use a cerebral protection device,” the surgeon explained.
He emphasized, however, that the final word on the appropriate management of C-ISRs isn’t in yet. A standardized definition of C-ISR is needed, as are multicenter prospective registries of medically managed patients as well as those undergoing various forms of reintervention. And a pathologic study is warranted to confirm the hypothesis that the histopathology of post-CEA and post-stent restenosis – and hence the natural history – is markedly different.
Dr. Chung reported having no financial conflicts regarding his presentation.
CHICAGO – with a carotid in-stent restenosis in excess of 70%, Jayer Chung, MD, asserted at a symposium on vascular surgery sponsored by Northwestern University.
That, in his view, makes medical management the clear preferred strategy.
A growing body of evidence, mainly from nested cohorts within randomized controlled trials, indicates that the late ipsilateral stroke rate associated with post–carotid endarterectomy restenosis (CEA) is much higher than that for carotid in-stent restenosis (C-ISR). In a recent meta-analysis of nine randomized trials, the difference in risk was more than 10-fold, with a 9.2% stroke rate at a mean of 37 months of follow-up in patients with post-CEA restenosis, compared with a 0.8% rate with 50 months of follow-up in patients with C-ISR (Eur J Vasc Endovasc Surg. 2017 Jun;53[6]:766-75).
“These pathologies behave very, very differently,” Dr. Chung observed. “The C-ISR lesions tend to be less embologenic.”
C-ISR is an uncommon event. Extrapolating from the landmark CREST (Carotid Revascularization Endarterectomy versus Stenting Trial) and other randomized trials, about 6% of patients who undergo percutaneous carotid stenting will be develop C-ISR within 2 years. But since the proportion of all carotid revascularizations that are done by carotid stent angioplasty has steadily increased over the past 15 years as the frequency of CEA has dropped, C-ISR is a problem that vascular specialists will continue to encounter on a regular basis.
Symptomatic C-ISR warrants reintervention; broad agreement exists on that. But there is a paucity of data to guide treatment decisions regarding asymptomatic yet angiographically severe C-ISR. Indeed, Dr. Chung was lead author of the only retrospective study of the natural history of untreated C-ISR, as opposed to carefully selected cohorts from randomized trials involving highly experienced operators. This study was a retrospective review of 59 patients with 75 C-ISRs of at least 50% seen at a single Veterans Affairs medical center over a 13-year period. Three-quarters of the ISRs were asymptomatic.
Forty of the 79 C-ISRs underwent percutaneous intervention at the physician’s discretion. Those patients did not differ from the observation-only group in age, comorbid conditions, type of original stent, or clopidogrel use. Reintervention was safe: There was one stent thrombosis resulting in stroke and death within 30 days in the reintervention group and no 30-day strokes in the observation-only group. During a mean 2.6 years of follow-up, the composite rate of death, stroke, or MI was low and not statistically significantly different between the two groups. Indeed, during up to 13 years of follow-up only one patient with untreated C-ISR experienced an ipsilateral stroke, as did two patients in the percutaneous intervention group (J Vasc Surg. 2016 Nov;64[5]:1286-94).
Dr. Chung does the math
According to data from the National Inpatient Sample, vascular surgeons do an average of 15 carotid angioplasty and stenting procedures per year. If 6% of those stents develop in-stent restenosis, and with a number needed to treat with revascularization of 25 to prevent 1 stroke, Dr. Chung estimated that hypothetically it would take the typical vascular surgeon 27 years to prevent one stroke due to C-ISR.
“That’s a very long time to prevent one stroke, in my opinion,” he said.
How his study has affected his own practice
Dr. Chung now intervenes only for symptomatic C-ISRs, and only after an affected patient is on optimal medical therapy, including a statin and dual-antiplatelet therapy.
“I try to do an open procedure if possible, especially if the restenosis is above C-2. The ones I tend to do percutaneously are the post-irradiation stenoses or those with excessive scarring, and I use a cerebral protection device,” the surgeon explained.
He emphasized, however, that the final word on the appropriate management of C-ISRs isn’t in yet. A standardized definition of C-ISR is needed, as are multicenter prospective registries of medically managed patients as well as those undergoing various forms of reintervention. And a pathologic study is warranted to confirm the hypothesis that the histopathology of post-CEA and post-stent restenosis – and hence the natural history – is markedly different.
Dr. Chung reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Carotid stenting isn’t safer than endarterectomy with contralateral carotid occlusion
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Watchman device PREVAILs for stroke prevention
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: All-cause mortality was reduced by 27% in patients randomized to left atrial appendage closure with the Watchman device, compared with those assigned to warfarin.
Study details: This patient-level meta-analysis included 5-year follow-up data on 1,114 patients with atrial fibrillation at increased stroke risk who were randomized 2:1 to the Watchman device or warfarin.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to study sponsor Boston Scientific.
CMS clinical trials raise cardiac mortality
Nearly 2 years ago I speculated in this column that health planners or health economists would attempt to manipulate the patterns of patient care to influence the cost and/or quality of clinical care. At that time I suggested that, in that event, the intervention should be managed as we have with drug or device trials to ensure the authenticity and accuracy and most of all assuring the safety of the patient. Furthermore, the design should be incorporated in the intervention, that equipoise be present in the arms of the trial and that a safety monitoring board be in place to alert investigators when and if patient safety is threatened. Patient consent should also be obtained.
Beginning in 2012, CMS, using claims data from 2008 to 2012, penalized hospitals if they did not achieve acceptable readmission rates. At the same time, the agency established the Hospital Admission Reduction Program to monitor 30-day mortality and standardize readmission data. The recent data indicate that the incentives did achieve some decrease in rehospitalization but this was associated with a 16.5% relative increase in 30-day mortality. It was of particular concern that in the previous decade there had been a progressive decrease in 30-day mortality (Circulation 2014;130:966-75). The increase in 30-day mortality observed in the 4-year observational period appears to have interrupted the progressive decrease in 30-day mortality, which would have decreased to 30% if not impacted by the plan.
My previous concerns with this type of social experimentation and manipulation of health care was carried out, and as far as I can tell, continues without any oversight and little insight into the possible risks of this process. A better designed study would have provided better understanding of these results and might have mitigated the adverse effects and mortality events. It is suggested that some hospitals actually gamed the system to their economic advantage. In addition, no oversight board was or is in place as we have with drug trials to allow monitors to become aware of adverse events before there any further loss of life occurs.
I would agree that a randomized trial in this environment would be difficult to achieve. Obtaining consent from thousands of patients would also be difficult. Nevertheless, .
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Nearly 2 years ago I speculated in this column that health planners or health economists would attempt to manipulate the patterns of patient care to influence the cost and/or quality of clinical care. At that time I suggested that, in that event, the intervention should be managed as we have with drug or device trials to ensure the authenticity and accuracy and most of all assuring the safety of the patient. Furthermore, the design should be incorporated in the intervention, that equipoise be present in the arms of the trial and that a safety monitoring board be in place to alert investigators when and if patient safety is threatened. Patient consent should also be obtained.
Beginning in 2012, CMS, using claims data from 2008 to 2012, penalized hospitals if they did not achieve acceptable readmission rates. At the same time, the agency established the Hospital Admission Reduction Program to monitor 30-day mortality and standardize readmission data. The recent data indicate that the incentives did achieve some decrease in rehospitalization but this was associated with a 16.5% relative increase in 30-day mortality. It was of particular concern that in the previous decade there had been a progressive decrease in 30-day mortality (Circulation 2014;130:966-75). The increase in 30-day mortality observed in the 4-year observational period appears to have interrupted the progressive decrease in 30-day mortality, which would have decreased to 30% if not impacted by the plan.
My previous concerns with this type of social experimentation and manipulation of health care was carried out, and as far as I can tell, continues without any oversight and little insight into the possible risks of this process. A better designed study would have provided better understanding of these results and might have mitigated the adverse effects and mortality events. It is suggested that some hospitals actually gamed the system to their economic advantage. In addition, no oversight board was or is in place as we have with drug trials to allow monitors to become aware of adverse events before there any further loss of life occurs.
I would agree that a randomized trial in this environment would be difficult to achieve. Obtaining consent from thousands of patients would also be difficult. Nevertheless, .
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Nearly 2 years ago I speculated in this column that health planners or health economists would attempt to manipulate the patterns of patient care to influence the cost and/or quality of clinical care. At that time I suggested that, in that event, the intervention should be managed as we have with drug or device trials to ensure the authenticity and accuracy and most of all assuring the safety of the patient. Furthermore, the design should be incorporated in the intervention, that equipoise be present in the arms of the trial and that a safety monitoring board be in place to alert investigators when and if patient safety is threatened. Patient consent should also be obtained.
Beginning in 2012, CMS, using claims data from 2008 to 2012, penalized hospitals if they did not achieve acceptable readmission rates. At the same time, the agency established the Hospital Admission Reduction Program to monitor 30-day mortality and standardize readmission data. The recent data indicate that the incentives did achieve some decrease in rehospitalization but this was associated with a 16.5% relative increase in 30-day mortality. It was of particular concern that in the previous decade there had been a progressive decrease in 30-day mortality (Circulation 2014;130:966-75). The increase in 30-day mortality observed in the 4-year observational period appears to have interrupted the progressive decrease in 30-day mortality, which would have decreased to 30% if not impacted by the plan.
My previous concerns with this type of social experimentation and manipulation of health care was carried out, and as far as I can tell, continues without any oversight and little insight into the possible risks of this process. A better designed study would have provided better understanding of these results and might have mitigated the adverse effects and mortality events. It is suggested that some hospitals actually gamed the system to their economic advantage. In addition, no oversight board was or is in place as we have with drug trials to allow monitors to become aware of adverse events before there any further loss of life occurs.
I would agree that a randomized trial in this environment would be difficult to achieve. Obtaining consent from thousands of patients would also be difficult. Nevertheless, .
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Carotid-axillary bypass for revascularization of the left subclavian artery in zone-2 TEVAR
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
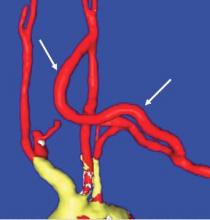
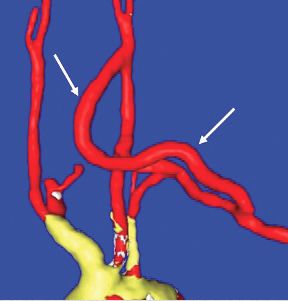
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Should carotid body tumors be embolized preoperatively?
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Benefit of dabigatran over warfarin persists in AF patient subgroups undergoing PCI
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After a mean follow-up of 14 months, the incidence of major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (HR, 0.52; 95% CI, 0.42-0.63, P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
Data source: Subgroup analysis of RE-DUAL PCI trial
Disclosures: The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Authors disclosed various financial ties to the company.
Bare metal stents: Rest in peace
DENVER – Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in the randomized, multicenter SENIOR trial.
“I think BMS [bare metal stents] should no longer be used as a strategy to reduce DAPT [dual antiplatelet therapy] duration in the elderly,” Olivier Varenne, MD, concluded in presenting the SENIOR findings at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“This trial adds to what I think is now a very large body of evidence showing that DES [drug-eluting stents] are the way to go, pretty much across the board, putting aside the economic factors that might come into play in certain regions of the world,” Deepak L. Bhatt, MD, commented at the meeting sponsored by the Cardiovascular Research Foundation.
“I think in general there’s really no good reason to use a BMS. I’d use a DES. I think DES is a winning strategy, whether it’s for a thrombotic lesion or an older patient,” added Dr. Bhatt, professor of medicine at Harvard Medical School in Boston and executive director of international cardiovascular programs at Brigham and Women’s Hospital.
Martin B. Leon, MD, concurred.
“I cannot think of any indication for using a BMS anymore, other than cost considerations. I think this study is yet another nail in the coffin of BMS. I think they should be relegated to the past because they really have very little role in environments where DES – which are becoming much more cost-efficient – can be used,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York. Yet in contemporary practice many cardiologists turn to BMS for percutaneous coronary intervention (PCI) in elderly patients with coronary artery disease (CAD), reasoning that the shorter DAPT duration recommended for BMS in the practice guidelines is attractive as a means of minimizing the risk of bleeding complications, which is typically high in the elderly.
The SENIOR trial demonstrates that using a modern DES in combination with the shortened 1- or 6-month DAPT duration typically reserved for BMS recipients results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with BMS. This is a finding of high clinical relevance because so many elderly patients undergo PCI; indeed, today one in four PCI patients in the United States is over age 75, explained Dr. Varenne of Cochin Hospital in Paris.
SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers in nine countries. Their mean age was 81.4 years. This was essentially an all-comers trial: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD. If they had stable CAD, they were slated for 1 month of DAPT. If they had ACS, they got 6 months of DAPT. All participants were then randomized to PCI with either the thin-strut everolimus-eluting bioabsorbable polymer Synergy DES or the thin-strut Omega or Rebel BMS.
The primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the DES group and 16.4% in the BMS group, for a 29% reduction in favor of the DES strategy and a favorably low number-needed-to-treat of 21. The difference in outcome was driven mainly by a higher ischemia-driven target lesion revascularization rate in the BMS group.
The 1-year rate of bleeding complications was 5% in each group. Probable or definite stent thrombosis occurred in 0.5% of the DES group and 1.4% of the BMS group at 1 year. Ten of the 11 cases of stent thrombosis in the study occurred during the first 30 days after PCI while the patients were on DAPT; the other case occurred on day 31, the day after DAPT was stopped.
Discussant Eric D. Peterson, MD, applauded Dr. Varenne and his coinvestigators for conducting a study focused on elderly individuals, an understudied population largely excluded from the landmark clinical trials in interventional cardiology.
He found the study convincing: “My takeaway is DES is better than BMS. As it is in young people, it continues in old.”
But the study leaves two important questions unanswered: Is the Synergy stent the best DES in the elderly, and what is the best duration of DAPT therapy? Ideally, the SENIOR trial would have included a study arm with the standard 6- and 12-month DAPT durations recommended in the American guidelines for DES recipients with stable and unstable CAD, respectively, observed Dr. Peterson, professor of medicine at Duke University in Durham, N.C., and director of the Duke Clinical Research Institute.
Dr. Varenne replied that SENIOR wasn’t designed as a DAPT duration trial. DAPT duration wasn’t randomized. But other planned and ongoing studies are attempting to define the best DAPT durations in various patient subsets.
Dr. Bhatt said he believes the superiority of contemporary DES over modern BMS is a class effect.
The SENIOR trial was funded by Boston Scientific. Dr. Varenne reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
DENVER – Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in the randomized, multicenter SENIOR trial.
“I think BMS [bare metal stents] should no longer be used as a strategy to reduce DAPT [dual antiplatelet therapy] duration in the elderly,” Olivier Varenne, MD, concluded in presenting the SENIOR findings at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“This trial adds to what I think is now a very large body of evidence showing that DES [drug-eluting stents] are the way to go, pretty much across the board, putting aside the economic factors that might come into play in certain regions of the world,” Deepak L. Bhatt, MD, commented at the meeting sponsored by the Cardiovascular Research Foundation.
“I think in general there’s really no good reason to use a BMS. I’d use a DES. I think DES is a winning strategy, whether it’s for a thrombotic lesion or an older patient,” added Dr. Bhatt, professor of medicine at Harvard Medical School in Boston and executive director of international cardiovascular programs at Brigham and Women’s Hospital.
Martin B. Leon, MD, concurred.
“I cannot think of any indication for using a BMS anymore, other than cost considerations. I think this study is yet another nail in the coffin of BMS. I think they should be relegated to the past because they really have very little role in environments where DES – which are becoming much more cost-efficient – can be used,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York. Yet in contemporary practice many cardiologists turn to BMS for percutaneous coronary intervention (PCI) in elderly patients with coronary artery disease (CAD), reasoning that the shorter DAPT duration recommended for BMS in the practice guidelines is attractive as a means of minimizing the risk of bleeding complications, which is typically high in the elderly.
The SENIOR trial demonstrates that using a modern DES in combination with the shortened 1- or 6-month DAPT duration typically reserved for BMS recipients results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with BMS. This is a finding of high clinical relevance because so many elderly patients undergo PCI; indeed, today one in four PCI patients in the United States is over age 75, explained Dr. Varenne of Cochin Hospital in Paris.
SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers in nine countries. Their mean age was 81.4 years. This was essentially an all-comers trial: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD. If they had stable CAD, they were slated for 1 month of DAPT. If they had ACS, they got 6 months of DAPT. All participants were then randomized to PCI with either the thin-strut everolimus-eluting bioabsorbable polymer Synergy DES or the thin-strut Omega or Rebel BMS.
The primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the DES group and 16.4% in the BMS group, for a 29% reduction in favor of the DES strategy and a favorably low number-needed-to-treat of 21. The difference in outcome was driven mainly by a higher ischemia-driven target lesion revascularization rate in the BMS group.
The 1-year rate of bleeding complications was 5% in each group. Probable or definite stent thrombosis occurred in 0.5% of the DES group and 1.4% of the BMS group at 1 year. Ten of the 11 cases of stent thrombosis in the study occurred during the first 30 days after PCI while the patients were on DAPT; the other case occurred on day 31, the day after DAPT was stopped.
Discussant Eric D. Peterson, MD, applauded Dr. Varenne and his coinvestigators for conducting a study focused on elderly individuals, an understudied population largely excluded from the landmark clinical trials in interventional cardiology.
He found the study convincing: “My takeaway is DES is better than BMS. As it is in young people, it continues in old.”
But the study leaves two important questions unanswered: Is the Synergy stent the best DES in the elderly, and what is the best duration of DAPT therapy? Ideally, the SENIOR trial would have included a study arm with the standard 6- and 12-month DAPT durations recommended in the American guidelines for DES recipients with stable and unstable CAD, respectively, observed Dr. Peterson, professor of medicine at Duke University in Durham, N.C., and director of the Duke Clinical Research Institute.
Dr. Varenne replied that SENIOR wasn’t designed as a DAPT duration trial. DAPT duration wasn’t randomized. But other planned and ongoing studies are attempting to define the best DAPT durations in various patient subsets.
Dr. Bhatt said he believes the superiority of contemporary DES over modern BMS is a class effect.
The SENIOR trial was funded by Boston Scientific. Dr. Varenne reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
DENVER – Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in the randomized, multicenter SENIOR trial.
“I think BMS [bare metal stents] should no longer be used as a strategy to reduce DAPT [dual antiplatelet therapy] duration in the elderly,” Olivier Varenne, MD, concluded in presenting the SENIOR findings at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“This trial adds to what I think is now a very large body of evidence showing that DES [drug-eluting stents] are the way to go, pretty much across the board, putting aside the economic factors that might come into play in certain regions of the world,” Deepak L. Bhatt, MD, commented at the meeting sponsored by the Cardiovascular Research Foundation.
“I think in general there’s really no good reason to use a BMS. I’d use a DES. I think DES is a winning strategy, whether it’s for a thrombotic lesion or an older patient,” added Dr. Bhatt, professor of medicine at Harvard Medical School in Boston and executive director of international cardiovascular programs at Brigham and Women’s Hospital.
Martin B. Leon, MD, concurred.
“I cannot think of any indication for using a BMS anymore, other than cost considerations. I think this study is yet another nail in the coffin of BMS. I think they should be relegated to the past because they really have very little role in environments where DES – which are becoming much more cost-efficient – can be used,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York. Yet in contemporary practice many cardiologists turn to BMS for percutaneous coronary intervention (PCI) in elderly patients with coronary artery disease (CAD), reasoning that the shorter DAPT duration recommended for BMS in the practice guidelines is attractive as a means of minimizing the risk of bleeding complications, which is typically high in the elderly.
The SENIOR trial demonstrates that using a modern DES in combination with the shortened 1- or 6-month DAPT duration typically reserved for BMS recipients results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with BMS. This is a finding of high clinical relevance because so many elderly patients undergo PCI; indeed, today one in four PCI patients in the United States is over age 75, explained Dr. Varenne of Cochin Hospital in Paris.
SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers in nine countries. Their mean age was 81.4 years. This was essentially an all-comers trial: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD. If they had stable CAD, they were slated for 1 month of DAPT. If they had ACS, they got 6 months of DAPT. All participants were then randomized to PCI with either the thin-strut everolimus-eluting bioabsorbable polymer Synergy DES or the thin-strut Omega or Rebel BMS.
The primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the DES group and 16.4% in the BMS group, for a 29% reduction in favor of the DES strategy and a favorably low number-needed-to-treat of 21. The difference in outcome was driven mainly by a higher ischemia-driven target lesion revascularization rate in the BMS group.
The 1-year rate of bleeding complications was 5% in each group. Probable or definite stent thrombosis occurred in 0.5% of the DES group and 1.4% of the BMS group at 1 year. Ten of the 11 cases of stent thrombosis in the study occurred during the first 30 days after PCI while the patients were on DAPT; the other case occurred on day 31, the day after DAPT was stopped.
Discussant Eric D. Peterson, MD, applauded Dr. Varenne and his coinvestigators for conducting a study focused on elderly individuals, an understudied population largely excluded from the landmark clinical trials in interventional cardiology.
He found the study convincing: “My takeaway is DES is better than BMS. As it is in young people, it continues in old.”
But the study leaves two important questions unanswered: Is the Synergy stent the best DES in the elderly, and what is the best duration of DAPT therapy? Ideally, the SENIOR trial would have included a study arm with the standard 6- and 12-month DAPT durations recommended in the American guidelines for DES recipients with stable and unstable CAD, respectively, observed Dr. Peterson, professor of medicine at Duke University in Durham, N.C., and director of the Duke Clinical Research Institute.
Dr. Varenne replied that SENIOR wasn’t designed as a DAPT duration trial. DAPT duration wasn’t randomized. But other planned and ongoing studies are attempting to define the best DAPT durations in various patient subsets.
Dr. Bhatt said he believes the superiority of contemporary DES over modern BMS is a class effect.
The SENIOR trial was funded by Boston Scientific. Dr. Varenne reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
AT TCT 2017
Key clinical point:
Major finding: The number of elderly patients with CAD who would need to be treated with a contemporary drug-eluting stent backed by a shortened DAPT regimen instead of a modern-era bare metal stent in order to avoid one additional major adverse cardiac and cerebrovascular event over the course of a year is 21.
Data source: This randomized, prospective, single-blind trial included 1,200 patients age 75 or older who underwent PCI at 44 centers in nine countries.
Disclosures: The SENIOR trial was funded by Boston Scientific. The presenter reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
VIDEO: Team approach boosts effective blood pressure control
ANAHEIM, CALIF. – Using a multidisciplinary team of clinicians “could be one of the most effective measures we have to improve blood pressure control,” Tracy Y. Wang, MD, said in a video interview during the American Heart Association scientific sessions.
Speaking a day after the release of revised guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults (J Am Coll Cardiol. 2017 Nov 13;doi: 10.1016/j.jacc.2017.11.006), Dr. Wang particularly highlighted the strong recommendation the guidelines made for a team-based approach for managing hypertension.
“I’m absolutely ecstatic that team management is embedded firmly in the new guidelines,” commented Dr. Wang, a cardiologist at Duke University in Durham, N.C. who has studied methods to optimize evidence-based treatment of cardiovascular diseases. “Endorsement of a team approach for blood pressure control was long overdue,” she said.
Dr. Wang discussed some approaches she believes would help better integrate team-based care into the routine management of patients with hypertension.
Dr. Wang has received honoraria from AstraZeneca, Eli Lilly, and Premier, and she has received research funding from several companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Using a multidisciplinary team of clinicians “could be one of the most effective measures we have to improve blood pressure control,” Tracy Y. Wang, MD, said in a video interview during the American Heart Association scientific sessions.
Speaking a day after the release of revised guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults (J Am Coll Cardiol. 2017 Nov 13;doi: 10.1016/j.jacc.2017.11.006), Dr. Wang particularly highlighted the strong recommendation the guidelines made for a team-based approach for managing hypertension.
“I’m absolutely ecstatic that team management is embedded firmly in the new guidelines,” commented Dr. Wang, a cardiologist at Duke University in Durham, N.C. who has studied methods to optimize evidence-based treatment of cardiovascular diseases. “Endorsement of a team approach for blood pressure control was long overdue,” she said.
Dr. Wang discussed some approaches she believes would help better integrate team-based care into the routine management of patients with hypertension.
Dr. Wang has received honoraria from AstraZeneca, Eli Lilly, and Premier, and she has received research funding from several companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Using a multidisciplinary team of clinicians “could be one of the most effective measures we have to improve blood pressure control,” Tracy Y. Wang, MD, said in a video interview during the American Heart Association scientific sessions.
Speaking a day after the release of revised guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults (J Am Coll Cardiol. 2017 Nov 13;doi: 10.1016/j.jacc.2017.11.006), Dr. Wang particularly highlighted the strong recommendation the guidelines made for a team-based approach for managing hypertension.
“I’m absolutely ecstatic that team management is embedded firmly in the new guidelines,” commented Dr. Wang, a cardiologist at Duke University in Durham, N.C. who has studied methods to optimize evidence-based treatment of cardiovascular diseases. “Endorsement of a team approach for blood pressure control was long overdue,” she said.
Dr. Wang discussed some approaches she believes would help better integrate team-based care into the routine management of patients with hypertension.
Dr. Wang has received honoraria from AstraZeneca, Eli Lilly, and Premier, and she has received research funding from several companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS