User login
What works best for genitourinary syndrome of menopause: vaginal estrogen, vaginal laser, or combined laser and estrogen therapy?
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
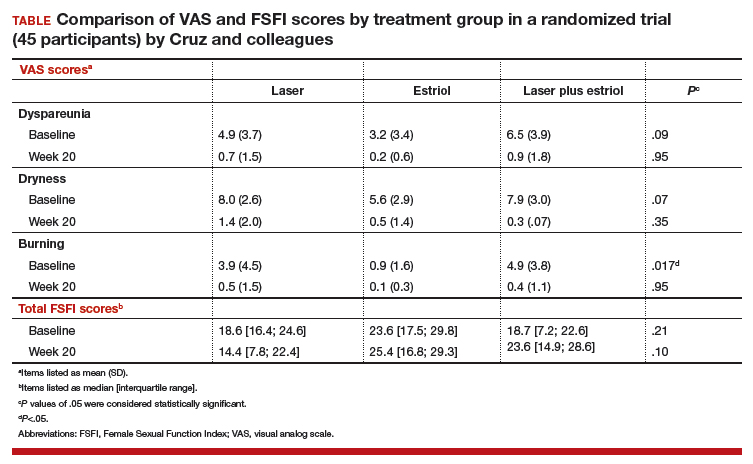
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
A multimodal treatment for vestibulodynia: TENS plus diazepam
and with placebo in a randomized, double-blind, placebo-controlled trial.
In the TENS/diazepam and TENS/placebo groups, participants reported significant improvements from baseline in pain and sexual functioning by questionnaire and visual analog scale. They also improved in measurements of pelvic floor muscle tone and vestibular nerve fiber current perception threshold.
The study had two groups of 21 women each, all aged 18 years or older and diagnosed (by physical exam) with moderate or severe pelvic floor hypertonic dysfunction. The diazepam was a tablet inserted vaginally daily before bed.
The TENS therapy was also self-administered (after six or seven supervised trial sessions), a recommended three times per week. The device is a 20-mm diameter plastic vaginal probe with gold metallic transversal rings as electrodes, inserted 20 mm, with 30 minutes of electrical stimulation increased slowly until sensation “reached a level described as the maximum tolerable without experiencing pain.” Vulvar pain was assessed on a on a 10-cm visual analog scale and dyspareunia on the Marinoff dyspareunia scale.
At the primary endpoint, the mean change from baseline to 60 days, the diazepam combination improved from 7.5 on the visual scale to 4.7, while the placebo combination improved from 7.2 to 4.3 (P not significant between the groups). Marinoff dyspareunia scores, however, improved from 2.5 to 1.6 and from 2.0 to 1.3, respectively (P less than .01).
Though “very few statistically significant differences in outcomes between the two groups were observed ... our results indicate that diazepam is able to positively change the functions of the pelvic floor muscle often highlighted” in women with vestibulodynia, reported Filippo Murina, MD, of the University of Milan and his coauthors. This conclusion followed from the Marinoff scores and from vaginal surface electromyography. In the latter measure, the diazepam group showed a significantly greater ability to relax the pelvic floor muscle after contraction (3.8 vs. 2.4 microvolts; P = .01), compared with the placebo group.
“We also observed that TENS itself is essential in reducing vulvar pain and the action of diazepam is useful but not decisive. ... It is possible that vaginal diazepam alone is insufficient to resolve the symptoms related to pelvic floor muscle dysfunction, while vaginal diazepam and TENS together provide a synergistic benefit in vestibulodynia patients,” wrote Dr. Murina and his coauthors.
This study was supported by the Associazione Italiana Vulvodinia. The authors declared no conflicts of interest.
SOURCE: Murina F et al. Eur J Obstet Gynecol Reprod Biol. 2018 Jun;228:148-53.
and with placebo in a randomized, double-blind, placebo-controlled trial.
In the TENS/diazepam and TENS/placebo groups, participants reported significant improvements from baseline in pain and sexual functioning by questionnaire and visual analog scale. They also improved in measurements of pelvic floor muscle tone and vestibular nerve fiber current perception threshold.
The study had two groups of 21 women each, all aged 18 years or older and diagnosed (by physical exam) with moderate or severe pelvic floor hypertonic dysfunction. The diazepam was a tablet inserted vaginally daily before bed.
The TENS therapy was also self-administered (after six or seven supervised trial sessions), a recommended three times per week. The device is a 20-mm diameter plastic vaginal probe with gold metallic transversal rings as electrodes, inserted 20 mm, with 30 minutes of electrical stimulation increased slowly until sensation “reached a level described as the maximum tolerable without experiencing pain.” Vulvar pain was assessed on a on a 10-cm visual analog scale and dyspareunia on the Marinoff dyspareunia scale.
At the primary endpoint, the mean change from baseline to 60 days, the diazepam combination improved from 7.5 on the visual scale to 4.7, while the placebo combination improved from 7.2 to 4.3 (P not significant between the groups). Marinoff dyspareunia scores, however, improved from 2.5 to 1.6 and from 2.0 to 1.3, respectively (P less than .01).
Though “very few statistically significant differences in outcomes between the two groups were observed ... our results indicate that diazepam is able to positively change the functions of the pelvic floor muscle often highlighted” in women with vestibulodynia, reported Filippo Murina, MD, of the University of Milan and his coauthors. This conclusion followed from the Marinoff scores and from vaginal surface electromyography. In the latter measure, the diazepam group showed a significantly greater ability to relax the pelvic floor muscle after contraction (3.8 vs. 2.4 microvolts; P = .01), compared with the placebo group.
“We also observed that TENS itself is essential in reducing vulvar pain and the action of diazepam is useful but not decisive. ... It is possible that vaginal diazepam alone is insufficient to resolve the symptoms related to pelvic floor muscle dysfunction, while vaginal diazepam and TENS together provide a synergistic benefit in vestibulodynia patients,” wrote Dr. Murina and his coauthors.
This study was supported by the Associazione Italiana Vulvodinia. The authors declared no conflicts of interest.
SOURCE: Murina F et al. Eur J Obstet Gynecol Reprod Biol. 2018 Jun;228:148-53.
and with placebo in a randomized, double-blind, placebo-controlled trial.
In the TENS/diazepam and TENS/placebo groups, participants reported significant improvements from baseline in pain and sexual functioning by questionnaire and visual analog scale. They also improved in measurements of pelvic floor muscle tone and vestibular nerve fiber current perception threshold.
The study had two groups of 21 women each, all aged 18 years or older and diagnosed (by physical exam) with moderate or severe pelvic floor hypertonic dysfunction. The diazepam was a tablet inserted vaginally daily before bed.
The TENS therapy was also self-administered (after six or seven supervised trial sessions), a recommended three times per week. The device is a 20-mm diameter plastic vaginal probe with gold metallic transversal rings as electrodes, inserted 20 mm, with 30 minutes of electrical stimulation increased slowly until sensation “reached a level described as the maximum tolerable without experiencing pain.” Vulvar pain was assessed on a on a 10-cm visual analog scale and dyspareunia on the Marinoff dyspareunia scale.
At the primary endpoint, the mean change from baseline to 60 days, the diazepam combination improved from 7.5 on the visual scale to 4.7, while the placebo combination improved from 7.2 to 4.3 (P not significant between the groups). Marinoff dyspareunia scores, however, improved from 2.5 to 1.6 and from 2.0 to 1.3, respectively (P less than .01).
Though “very few statistically significant differences in outcomes between the two groups were observed ... our results indicate that diazepam is able to positively change the functions of the pelvic floor muscle often highlighted” in women with vestibulodynia, reported Filippo Murina, MD, of the University of Milan and his coauthors. This conclusion followed from the Marinoff scores and from vaginal surface electromyography. In the latter measure, the diazepam group showed a significantly greater ability to relax the pelvic floor muscle after contraction (3.8 vs. 2.4 microvolts; P = .01), compared with the placebo group.
“We also observed that TENS itself is essential in reducing vulvar pain and the action of diazepam is useful but not decisive. ... It is possible that vaginal diazepam alone is insufficient to resolve the symptoms related to pelvic floor muscle dysfunction, while vaginal diazepam and TENS together provide a synergistic benefit in vestibulodynia patients,” wrote Dr. Murina and his coauthors.
This study was supported by the Associazione Italiana Vulvodinia. The authors declared no conflicts of interest.
SOURCE: Murina F et al. Eur J Obstet Gynecol Reprod Biol. 2018 Jun;228:148-53.
FROM THE EUROPEAN JOURNAL OF OBSTETRICS & GYNECOLOGY AND REPRODUCTIVE BIOLOGY
2018 Update on menopause
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
Optimal surgical management of stage 3 and 4 pelvic organ prolapse
Effective surgical management of advanced pelvic organ prolapse (POP) depends on prolapse location and stage, presence of urinary incontinence, need for hysterectomy, the patient’s desire to maintain sexual function, type of surgery, and the surgeon’s skill and experience, among other factors. For these reasons, POP repair is not a one-size-fits all procedure.
In this article, experts in minimally invasive prolapse repair offer their perspectives on 3 surgical approaches: use of native tissue (Drs. White, Aguilar, and Rogers), abdominal sacrocolpopexy (Drs. Huber and Culligan), and transvaginal mesh (Drs. Lucente and Ton). They evaluate the evidence on these procedures and provide recommendations based on their experience of best practices for achieving surgical success and minimizing adverse events.
Using native tissue for vaginal anatomy repair
Amanda White, MD; Vivian Aguilar, MD; and Rebecca G. Rogers, MD
Dr. Rogers reports that she receives royalties from UpToDate. Drs. White and Aguilar report no financial relationships relevant to this article.
Surgical therapy is the mainstay of treatment for POP, and 20% of US women will undergo prolapse and/or stress incontinence surgery by age 80.1 Prolapse surgery either restores the vaginal anatomy (reconstructive surgery) or obliterates the vaginal canal (obliterative surgery). Vaginal reconstruction can be performed using the patient's native tissue or mesh. Because of concerns associated with mesh use, native tissue repairs continue to be commonly performed.
Unfortunately, not all prolapse surgeries result in prolapse cure, and recurrent prolapse that necessitates repeat operation is not rare, regardless of whether or not mesh is used.2,3 Native tissue repairs are most commonly performed through the vaginal route, the first minimally invasive approach to prolapse surgery. Restoration of the vaginal apex has been identified as critically important in these surgeries. Apical native tissue repairs include reconstructive procedures, such as sacrospinous ligament suspension (SSLS) or uterosacral ligament suspension (USLS), and obliterative procedures, such as colpocleisis.
In this discussion, we present 2 case vignettes that highlight surgical decision making for repair of stage 3 or 4 pelvic organ prolapse utilizing these techniques.
- Native tissue repair offers a minimally invasive approach to prolapse repair.
- Sacrospinous and uterosacral ligament suspensions have equivalent success rates.
- Prophylactic midurethral slings reduce postoperative incontinence at the time of transvaginal native tissue repair.
- Hysterectomy at the time of colpocleisis should not be performed routinely.
CASE 1 Active woman with prolapse
A 65-year-old woman (G2P2) presents with stage 3 prolapse, with the anterior compartment at +3 and the cervix at the hymen with straining. She is sexually active and desires to retain coital function. A trial of pessary has failed.
What surgical options can be considered for this patient?
Reconstruction procedures for prolapse
This patient presents with a typical configuration of prolapse; the anterior and apical compartments are the most likely to prolapse.4 Importantly, conservative management of her prolapse has failed. While it is not required that women have a trial with pessary prior to undergoing surgery, all women should be offered conservative management of prolapse, according to the American Urogynecologic Society (AUGS) and the American College of Obstetricians and Gynecologists (ACOG).4,5
Apical suspension
Since this patient desires to retain coital function, her gynecologist recommends a reconstructive procedure. The combination of apical and anterior vaginal wall prolapse will require an apical suspension procedure (FIGURES 1 and 2). If suspension of the apex does not correct the anterior wall prolapse, the patient also may require anterior compartment reconstruction.
The 2 most commonly performed native tissue apical suspension procedures, SSLS and USLS, have equivalent outcomes at 2 years, according to a multicenter randomized trial.6 Therefore, the choice of procedure is at the surgeon's discretion. USLS is most commonly performed at the time of hysterectomy via an intraperitoneal approach, while SSLS is often selected for posthysterectomy vault prolapse, given its extraperitoneal location.
Suture type. Whether to use permanent suture at the time of SSLS or USLS is controversial. Some data suggest that permanent suture provides greater long-term success compared with delayed absorbable suture.7 However, permanent suture has been reported to be associated with higher rates of suture complications--up to 44% in USLS and 36% in SSLS--compared with a 3.5% complication rate in a USLS cohort treated with absorbable suture.8-10
Hysterectomy versus hysteropexy. Considerable debate exists regarding whether a patient requires hysterectomy at the time of prolapse repair. In a randomized trial at 12 months' follow-up, uterine preservation by sacrospinous hysteropexy was noninferior to vaginal hysterectomy with suspension of the uterosacral ligaments for surgical failure of the apical compartment.11 A recent meta-analysis found that apical failure rates after sacrospinous hysteropexy versus vaginal hysterectomy were not different.12 Repeat surgery rates for prolapse also were not different between groups. The most significant disadvantage of uterine-preservation prolapse surgery, when compared with hysterectomy, is the lack of prevention and diagnosis of uterine malignancy.12 From 2002 to 2012, rates of hysteropexy significantly increased in the United States, although rates remain low.13
Sling procedure pros and cons. This case patient did not report urinary incontinence, but she may develop incontinence with reduction of the anterior wall prolapse. A large randomized controlled trial that included 337 women compared sling with no sling procedures among women with prolapse undergoing transvaginal prolapse repair.14 Management with a prophylactic sling resulted in less incontinence (27.3% and 43.0%, respectively, at 12 months postoperatively) but higher rates of urinary tract infection (31.0% vs 18.3%), major bleeding complications (3.1% vs 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs 0%) (P≤.05 for all).14
CASE 1 Recommendations for this patient
For this case, we would offer the patient a transvaginal hysterectomy and USLS. At the time of repair, we would assess whether she needed an anterior repair as well. We would offer a prophylactic sling procedure and also would discuss the risks and benefits of concomitant versus interval incontinence procedures.
CASE 2 Elderly woman with severe prolapse
An 85-year-old woman (G3P3) presents with procidentia, or complete eversion of the vagina, with the cervix 10 cm outside of the hymen. She has difficulty voiding, and the prolapse is uncomfortable when walking. A trial of pessary has failed. The patient denies vaginal bleeding. She is not sexually active and does not desire to retain coital function.
What treatment options would be appropriate for this patient?
Obliterative surgery
This elderly patient presents with advanced pelvic organ prolapse, and conservative management has failed. She is not sexually active and does not desire coital function in the future, so an obliterative procedure is indicated. Colpocleisis is a minimally invasive procedure that has cure rates ranging from 91% to 100%.15 It is likely that this patient's voiding dysfunction will improve after surgery and that she will be highly satisfied with the surgery.16
The question of hysterectomy with colpocleisis
The role of hysterectomy at the time of colpocleisis is controversial. LeFort colpocleisis preserves the uterus, with the anterior and posterior vaginal walls sutured together (FIGURE 3). Hysterectomy at the time of vaginal closure increases the operative time and blood loss.15 On the other hand, closure without hysterectomy prohibits future endometrial or cervical cancer screening.
In a recent review using the American College of Surgeons National Surgical Quality Improvement Program database, investigators compared women who underwent colopocleisis alone with those who underwent colpocleisis with hysterectomy.17 They found that the incidence of major complications was greater among women who underwent concomitant hysterectomy, and they concluded that hysterectomy should not be performed routinely at the time of colpocleisis.17
Among 322 urogynecologists who responded to a web-based survey, only 18% routinely performed hysterectomy at the time of colpocleisis.18 Further, in a decision analysis model, the utility for colpocleisis without hysterectomy was higher in women older than age 40, suggesting that hysterectomy should be performed only in special circumstances.19
Evaluating the endometrium. If the uterus remains in situ, should endometrial evaluation be performed? If so, should ultrasonography or endometrial biopsy be used? Authors of a decision analysis model found that among women at low risk for cancer and without abnormal uterine bleeding, endometrial biopsy was not favored until the probability of cancer reached 64%.20 Specifically, no evaluation or evaluation by transvaginal ultrasonography is adequate in the majority of cases.20 When screened by transvaginal ultrasonography, the high, 99% negative predictive value for endometrial disease, using a cutoff value of 5 mm for endometrial stripe width, will allow most patients to avoid unnecessary tissue sampling.
Stress incontinence. It is likely that this patient's voiding dysfunction will resolve with reduction of the prolapse, and she may develop stress incontinence symptoms. In up to 68% of women, occult stress incontinence will be revealed with reduction of stage 3 or stage 4 prolapse.21 If the patient demonstrates stress incontinence, a midurethral sling is likely to treat her incontinence effectively, with little added risk from the procedure.22 Even among women who have an elevated postvoid residual urine volume, the incidence of sling revision is low.15
CASE 2 Procedure recommendation for this patient
For this case, we would perform a LeFort colpocleisis and discuss whether or not the patient would prefer a midurethral sling if stress incontinence was demonstrated on examination. We would not perform endometrial evaluation in this patient, as she has not been bleeding and her risk for endometrial cancer is low.
Weighing the benefits of native tissue repair
Native tissue repair when performed transvaginally is a minimally invasive approach to prolapse repair. In a multicenter randomized trial, anatomic success was reported to be 64.5% at 2 years.6 Long-term follow up of patients undergoing mesh sacrocolpopexy shows a similar anatomic failure rate, with up to one-third of patients meeting the definition of composite failure.3 Unlike mesh-augmented repairs, however, adverse events, including bowel obstruction, mesh exposure, and thromboembolism, are more likely to occur in the mesh sacrocolpopexy group.23
Obliterative procedures have the highest success rates of all prolapse repairs and carry with them low morbidity. However, women must forego the ability for coitus in the future. For all native tissue vaginal repairs, the surgeon and patient must weigh the risks and benefits of concomitant anti-incontinence procedures.
Read about using abdominal sacrocolpopexy for apical prolapse repair.
Abdominal sacrocolpopexy: A tried-and-true approach for apical prolapse repair
Sarah Huber, MD, and Patrick Culligan, MD
Dr. Culligan reports that he is a shareholder in Oragami Surgical LLC and a consultant and speaker for Coloplast and Intuitive Surgical Inc. Dr. Huber reports no financial relationships relevant to this article.
CASE Woman with advanced prolapse desires surgical repair
A 55-year-old woman (G2P2) presents to her gynecologist's office reporting a vaginal bulge and pressure that has been worsening for the past year. She describes a nontender ball of tissue the size of an orange protruding past the introitus that worsens with ambulating and lifting heavy objects. She reports some urinary urgency and increased frequency and at times feels as though her bladder does not empty completely with voiding. She denies any urinary incontinence. The patient has regular bowel movements but does report some difficulty with stool evacuation. She has a history of 2 vaginal deliveries and is sexually active. She is postmenopausal, with the last menses about 4 years ago. She is active and exercises regularly.
The patient's Pap smears, mammograms, and colonoscopy are up to date and test results have been normal. She has no significant medical or surgical history and no significant family history of cancer. On examination, her body mass index is normal, as is the cardiopulmonary exam. Her pelvic organ prolapse quantification system (POP-Q) score is Aa +3, Ba +3, C +4, GH 3, PB 3, TVL 10, Ap +2, Bp +2, and D +2. The patient is interested in surgical management.
What urodynamic tests would be appropriate for this patient, and what treatment options would you recommend?
- Robot-assisted laparoscopic sacrocolpopexy is a safe, effective, and durable treatment for advanced-stage pelvic organ prolapse.
- This procedure can completely correct stage 3 or 4 prolapse when the dissection of the anterior vaginal wall extends to the bladder neck and the dissection of the posterior vaginal wall extends to the perineal body.
- One can avoid the need for concomitant vaginal prolapse repair by gathering up stretched out vaginal epithelium while suturing to the mesh arms.
- Sacral attachment sutures should be placed in the anterior longitudinal ligament distal to the sacral promontory to avoid the L5-S1 disc.
- Unless contraindicated, lightweight macroporous polypropylene mesh is the current implant of choice.
Additional tests needed
Patients with advanced-stage pelvic organ prolapse are at an increased risk for stress urinary incontinence that may be masked by urethral "kinking" due to anatomic distortion of the periurethral support mechanism. Based on recommendations from the American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), we routinely perform a postvoid residual urine volume measurement, urinalysis, urine culture, and a prolapse reduction stress test.24 If the urinalysis is positive for blood, then a preoperative cystoscopy would be indicated.
If stress incontinence is confirmed by reduction stress testing, the patient should be offered an anti-incontinence procedure, such as a mesh midurethral sling.
This patient's overactive bladder symptoms warrant investigation via complex urodynamic testing to allow for comprehensive counseling about her postoperative expectations.
Counseling the patient on the sacrocolpopexy option
Abdominal sacrocolpopexy initially was described in 1962 by Lane as a technique to affix the vaginal apex to the sacral promontory using a graft. Although the procedure has been modified over the years, the principles of using an implanted strengthening material to permanently attach the apex to the anterior longitudinal ligament at the sacrum has proven to be a highly effective and safe treatment, establishing it as the gold standard for apical prolapse repair.25,26
Compared with other methods of apical prolapse repair, sacrocolpopexy via any approach is superior to vaginal surgery in terms of subjective and objective outcomes. In a recent systematic review comparing apical prolapse repairs, patients who underwent a vaginal approach were more likely to report awareness of their prolapse after surgery, undergo repeat surgery, have objective recurrent prolapse, and were at increased risk for postoperative stress urinary incontinence and dyspareunia.26 Prospective studies within our practice have shown 1-year composite subjective and objective cure rates of 94% to 95%.27,28
Selecting a route for sacrocolpopexy
Although sacrocolpopexy can be approached via laparotomy or conventional laparoscopy, we routinely use a robot-assisted approach, as it has been shown to be especially beneficial for complex situations, such as in patients with prior pelvic surgery, a foreshortened vagina, or obesity.29,30
Potential complications
Sacrocolpopexy complications are rare, especially when a minimally invasive approach is used.31 Reported complications of minimally invasive sacrocolpopexy include gastrointestinal or genitourinary injury, bowel obstruction or ileus, incisional hernia, vascular injury, discitis or osteomyelitis, conversion to open procedure, and mesh exposure.
Vaginal mesh exposure is rare following sacrocolpopexy, but it can occur at any time following surgery.31 Some risk factors include mesh material selection (specifically polytetrafluoroethylene [PTFE] mesh), concurrent total hysterectomy, vaginal atrophy, and smoking.32,33 As a result, recent recommendations have advised the use of polypropylene mesh with uterine preservation or supracervical hysterectomy at the time of sacrocolpopexy.34 In fact, supracervical hysterectomy alone appears to cut down or eliminate the risk of mesh exposure in laparoscopic sacrocolpopexy.35
In our practice, avoiding split-thickness vaginal dissection, employing supracervical hysterectomy techniques, and using ultralightweight mesh has resulted in mesh exposure rates approaching zero.28
For atrophic vaginal tissue, one can consider prescribing preoperative vaginal estrogen for 4 to 6 weeks, but this is not essential and should not routinely delay pelvic reconstructive surgery.
What type of implant material is best?
While various materials have been used as the fixation media in sacrocolpopexy, loosely knitted synthetic type I macroporous polypropylene mesh is the best choice due to its efficacy, availability, and low adverse effect profile. We recommend a lightweight mesh with a maximum weight of 25 g/m2. Two such products currently available are the UPsylon Y-Mesh (Boston Scientific, Marlborough, Massachusetts) and Restorelle Y mesh (Coloplast, Minneapolis, Minnesota). Lightweight mesh has been proven to maintain integrity, guaranteeing a successful outcome, while reducing the "mesh load" on the attached tissue.27,28
Comparative studies with fascia lata or cross-linked porcine dermal grafts demonstrated inferior outcomes versus synthetic mesh, and currently the only biologic material on the market indicated for prolapse repair augmentation, ACell Pelvic Floor Matrix (ACell, Columbia, Maryland), has not been extensively tested in sacrocolpopexy.36-38
Vaginal anatomy restored by sacrocolpopexy
Abdominal sacrocolpopexy, specifically via a minimally invasive approach, is an effective and long-lasting treatment that should be offered to women with advanced-stage prolapse.
Using the surgical techniques described below, including attachment of the mesh along the lengths of the anterior and posterior vaginal walls and gathering up excess tissue with mesh attachment, can provide women with adequate support for the entire vagina with restoration of normal vaginal anatomy and caliber.
Step-by-step tips for surgical efficiency
Robotic port placement
- Place the trocars in a "W" layout for the da Vinci Si Surgical System (FIGURE 4, VIDEO 1) or in a linear layout for the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). Both Si and Xi port placement includes a 3- to 5-mm assistant port in the right upper quadrant of the abdomen.
Supracervical hysterectomy, if indicated
- Maneuver the uterus with the robotic tenaculum, which obviates the need for a uterine manipulator during the hysterectomy (VIDEO 2).
- Create the bladder flap just above the upper edge of the bladder to facilitate the upcoming anterior wall dissection. This helps to prevent the development of a split-thickness dissection plane.
- 1.5 to 2 cm of cervix should be left in place, and conization should be avoided.
Anterior vaginal wall dissection
- The key to a good full-thickness dissection is sustained tissue traction and countertraction. The bedside assistant pulls the anterior peritoneal cut edge anteriorly for "gross" traction, and further "fine" traction can be created by pulling the areolar tissue with robotic forceps. The cervix is grasped with the tenaculum, which applies a constant midline cephalad countertraction (VIDEO 3).
- Sharp dissection with cold scissors allows for creation of the dissection plane, while cautery is judiciously applied only for hemostasis. If bleeding is encountered, this usually indicates that a split thickness of the vaginal wall has been created, and the surgeon should correct to the proper dissection plane.
- Dissection is made easier by taking down the bladder pillars before advancing down toward the bladder neck.
- The anterior dissection is always carried down to level of the trigone, confirmed by visualization of the Foley bulb (FIGURE 5).
Posterior vaginal wall dissection
- Begin dissection just above the rectal reflection, leaving peritoneum on the posterior cervix (VIDEO 4).
- Extend the incision bilaterally to the uterosacral ligaments only after the correct dissection plane is confirmed by visualization of the areolar tissue.
- Apply cervical traction using the tenaculum in a cephalad midline direction, and place traction on the cut edge of the posterior peritoneum using the bipolar forceps. The tenaculum wrist must be turned away from the working instruments to avoid internal clashing.
- Completely transect the right uterosacral ligament to better facilitate the creation of a contiguous peritoneal opening for burying the mesh. The remainder of the opening will be created later.
- While it is important to avoid split-thickness dissection, the vaginal plane must be "clean" (that is, without fat or adventitia) to allow for robust suturing.
- Dissection at least halfway down the posterior vaginal wall is recommended but proceeding down to the perineal body provides the most optimal support (FIGURE 6).
Sacral dissection
- Use a noncrushing instrument to laterally sweep the bowel to the left side, effectively "plastering" the peritoneum over the sacral promontory (FIGURE 7; VIDEO 5).
- Extend the superficial peritoneal incision down the right paracolic gutter halfway between the ureter and colon until it communicates with the incised posterior peritoneal edge created during the posterior dissection.
- Identify the middle sacral artery to avoid vascular injury, but there is no need to prophylactically coagulate it.
Vaginal mesh attachment
- Cut a lightweight Y-mesh to a length of 6 to 8 cm anteriorly and 8 to 11 cm posteriorly and place it into the surgical field (FIGURE 8; VIDEO 6). The length is determined based on the preoperative office examination and examination under anesthesia prior to starting the procedure.
- Attach the mesh securely and evenly to the anterior and posterior vaginal walls using multiple interrupted monofilament sutures. We aim to place sutures that provide mesh stability without excess vaginal wall incorporation to avoid "through-and-through" suturing.
- The posterior wall suturing is performed first, starting at the perineal body and continuing cephalad (VIDEO 7). We find it easiest to tie the knots between the mesh and the vagina in this space.
- Suture the crotch of the Y-mesh to the cervix so that no gap exists between tissue and mesh.
- For advanced-stage prolapse with significant anterior prolapse, the stretched out vaginal epithelium can be systematically gathered up to reconfigure the tissue to conform to the desired mesh dimensions (VIDEO 8). This tissue remodeling is evident even at the 2- to 4-week postoperative visit.
Peritoneal closure: Step 1
- Reapproximate the cut edges of peritoneum surrounding the vagina and cervix using a continuous purse-string suture of 0 Monocryl (poliglecaprone 25) on an SH needle (Ethicon, Somerville, New Jersey) with a fisherman's knot tied at the end (VIDEO 9). The needle passes are placed close together and close to the incised edge of the cut peritoneum.
- We typically start our peritoneal suture at the 5 o'clock position of the posterior peritoneum, extending in a clockwise direction and ultimately jumping anteriorly around the sacral arm of the mesh.
- Place the mesh within the paracolic peritoneal canal, and secure the needle for later use.
Sacral mesh attachment
- The mesh is tensioned so that a vaginal examination confirms adequate support of all the walls without excess tension or tissue banding. Some laxity of the anterior vaginal wall consistent with a mild cystocele is appropriate.
- Place 2 permanent PTFE sutures along the slope of the sacral promontory into the anterior longitudinal ligament (VIDEO 10). This avoids injury to the disc space that sits at the edge of the promontory. We do not advise the use of bone anchors as they increase the risk for discitis and osteomyelitis.
- Secure the mesh to the anterior longitudinal ligament without any tension. This is facilitated by creating mesh slack via cephalad pressure from a vaginal probe.
Peritoneal closure: Step 2
- Close the remaining paracolic peritoneal incision, completely burying the mesh within the created canal (FIGURE 9).
- At the end of the procedure, perform a repeat vaginal exam, rectal exam, and cystoscopy.
Technique with prior total hysterectomy
- In patients with a prior total hysterectomy, place a 13 x 3.5 cm Breisky vaginal retractor and/or coated nonconductive stent (Marina Medical, Sunrise, Florida) into the vagina to delineate the anterior and posterior walls at the vaginal apex during dissection.
- Some surgeons may opt to retrograde fill the bladder to better identify its location.
- We routinely leave a segment of peritoneum attached to the dome of the vaginal apex for added tissue integrity to prevent erosion.
Read about using transvaginal mesh for POP repair.
Transvaginal mesh: An effective, durable option for POP repair
Vincent R. Lucente, MD, MBA, and Jessica B. Ton, MD
Dr. Lucente reports that he has received grant or research support from Advanced Tactile Imaging, Boston Scientific, Coloplast, and Valencia; is a consultant to Coloplast; is a speaker for Allergan, Boston Scientific, Coloplast, and Shionogi; and serves as an expert witness for American Medical Systems and C.R. Bard. Dr. Ton reports no financial relationships relevant to this article.
As baseline health in the elderly population continues to improve, the number of women in the United States with symptomatic POP will increase by approximately 50% by 2050.39 Unfortunately, after native tissue repair (NTR) the rate of prolapse recurrence is extremely high: approximately 40% regardless of approach, as demonstrated in the OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial by Barber and colleagues.6 The authors of that clinical trial recently revealed that at the 5-year follow-up, these failure rates progressed to 70% for sacrospinous ligament fixation and 61% for uterosacral ligament suspension (data presented at the Society of Gynecologic Surgeons Annual Scientific Meeting 2018, Orlando, Florida). This establishes that NTR is not durable enough to meet the increasing physical demands of this age group and that mesh augmentation must be considered.
For patients at increased risk of prolapse recurrence, using transvaginal mesh (TVM) is the most minimally invasive approach and is an excellent option for mesh augmentation. Avoiding adverse events during placement of TVM depends largely on optimal surgical technique.40 (VIDEO: “Demonstration of an anterior vaginal wall dissection into the true vesicovaginal space”)
- Active advanced age requires a durable reconstructive pelvic surgery for pelvic organ prolapse, and native tissue repair does not meet that demand.
- Mesh augmentation reduces the risk of prolapse recurrence, and vaginal placement of mesh is the most minimally invasive approach.
- Rates of exposure with transvaginal mesh would be minimized with use of a full-thickness vaginal wall dissection.
- Optimal surgical technique could be highly reproducible with better surgical training.
The evidence on TVM versus NTR
Several studies have examined whether TVM has a measurable benefit over NTR.
A 2016 Cochrane review by Maher and colleagues included 37 randomized trials (4,023 women) that compared TVM and biologic grafts with NTR.41 Three primary outcomes were defined: awareness of prolapse, recurrence, and repeat surgery. Compared with women treated with NTR, those treated with synthetic nonabsorbable TVM exhibited a greater reduction in awareness of prolapse (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54-0.81), decreased recurrence in the anterior compartment (RR, 0.33; 95% CI, 0.26-0.40), and decreased reoperation for prolapse (RR, 0.53; 95% CI, 0.31-0.88). The overall calculated exposure rate was 12%, with a range of 3.2% to 20.8%.41 As we will discuss, this wide range most likely is attributed to a suboptimal, split-thickness dissection. There were no differences in other key secondary outcomes, including dyspareunia, operating time, and estimated blood loss.41
Longitudinal studies are emerging as almost 2 decades have passed since TVM was introduced. In a study of 5-year follow-up after TVM placement, Meyer and colleagues reported that patients had continued significant improvements in both subjective and objective outcomes.42 The mesh exposure rate was 6%, attributed to severe vaginal atrophy.42 A 10-year observational study by Weintraub and colleagues demonstrated a recurrence rate of only 2.6% in the anterior compartment, 7.6% in the posterior (nonaugmented) compartment, and no exposures or extrusions after anterior TVM placement.43
In contrast to the Cochrane review, in the 2017 multicenter PROSPECT (Prolapse surgery: Pragmatic evaluation and randomized controlled trials) trial, Glazener and colleagues found no difference in desired outcomes with TVM compared with NTR.44 There was an overall 6% to 7% exposure rate over 2 years.44 To reflect "real-world" practice, however, this study was intentionally designed without rigorous standardization of surgical technique. The authors reported that "appropriately experienced surgeons" performed the procedure, but it is unclear how experience was determined given that 20% of the cases were performed by "registrars," the equivalent of US residents or fellows.45
The PROSPECT study protocol described the TVM procedure as "a standard repair with a nonabsorbable mesh inlay to support the stitches," implying that there was no apical attachment of the mesh to the sacrospinous ligament.45 This is a suboptimal use of TVM because it does not address a detachment-type defect common in advanced prolapse. The PROSPECT study reinforces the need for better surgical training and standardization of the TVM procedure.44
How TVM compares with sacrocolpopexy
When comparing the use of TVM with sacrocolpopexy, our experience has been that TVM yields similar outcomes to sacrocolpopexy with additional benefits. We completed a 1-year retrospective cohort study comparing robot-assisted laparoscopic sacrocolpopexy (RALS) with TVM in a total of 86 patients, with both approaches performed by the same surgeon. Both treatment groups showed statistically significant improvements in nearly all functional and quality-of-life measures, including urinary symptoms, sexual function, and POP-Q scores.40 In particular, points Aa and Ba on the POP-Q score were significantly improved with TVM as compared to RALS. This suggests that TVM can achieve both lateral and apical support, where sacrocolpopexy addresses only the apex.40 This has clinical significance when considering DeLancey and colleagues' dynamic magnetic resonance imaging study, which demonstrated advanced prolapse results from both lateral and apical detachment.46 In addition, TVM placement also was considerably faster than RALS by approximately 96 minutes and could be performed using regional anesthesia. Only 1 mesh exposure in each study arm was reported.40
Finally, as with other vaginal procedures, patients who undergo TVM placement require minimal to no pain medication postoperatively and report faster return to daily activities. Almost none of our patients require narcotics, which is a significant benefit in the face of the ongoing national opioid crisis.
Gutman and colleagues compared laparoscopic mesh hysteropexy with TVM; they demonstrated comparable cure rates and, again, significantly longer operative times for the laparoscopic approach (174 vs 64 minutes; P<.0001).47 This multicenter study reported mesh exposure rates of 2.7% for laparoscopy and 6.6% for TVM,47 again likely due to a split-thickness dissection.
Safety of TVM depends on the surgeon factor
Because of the reported complications associated with TVM, in 2011 the US Food and Drug Administration (FDA) issued an update on the safety and efficacy of TVM augmentation and mandated postmarket studies.48 While we do not dispute that the mesh exposure rates were accurate at the time the FDA document was issued, we recognize that exposure has been erroneously attributed to inherent properties of the mesh.
Mesh exposure rates reported in the literature vary widely, ranging from 0% to 30%, even when surgeons used identical mesh products.49 This clearly establishes that the main contributing variable is surgical technique. It is critically important to recognize the "surgeon factor" as a confounder in trials that compare surgical procedures.50 Studies on TVM have shown that low-volume surgeons had significantly higher reoperation rates, while high-volume surgeons achieved a 41% reduction in reoperations.51,52 When TVM is performed by expert surgeons, the reported mesh exposure rates for TVM are noticeably lower.40,42,43,53,54
Decreasing mesh exposure rates would reduce the most common adverse event associated with TVM, thus improving its safety. The critical step to successful TVM placement is the initial dissection. Gynecologists traditionally have performed a split-thickness, colporrhaphy-style dissection to place the mesh within the layers of the vaginal wall.55 Placement within these planes, however, is too superficial and increases the risk of exposure. By contrast, by consistently performing a full-thickness vaginal wall dissection (FIGURE 10) and placing the mesh in the true vesicovaginal space,56 we have achieved a TVM exposure rate as low as 0% to 3%.40,54 If we can standardize the dissection component across our subspecialty, the rate of mesh exposure undoubtedly will decrease.
The PROSPECT investigators readily admitted what the study was not: a trial conducted "exclusively by the most experienced surgeons in the highest volume centres¬with a highly protocolised technique."44 In reality, that is the kind of rigorous study on TVM that our subspecialty demands. We must hold ourselves accountable and ensure that only the most qualified surgeons are placing TVM.
Keep the mesh option available
We support the position of the American Urogynecologic Society in opposing an outright ban of TVM because such a restriction would deny our patients access to an effective, durable, and minimally invasive approach for prolapse repair.57
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201-1206.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-506.
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016-2024.
- American College of Obstetricians and Gynecologists, American Urogynecologic Society. Practice Bulletin No. 185 Summary: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):1170-1172.
- American Urogynecologic Society Best Practice Statement: Evaluation and counseling of patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2017;23(5):281-287.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023-1034.
- Chung CP, Miskimins R, Kuehl TJ, Yandell PM, Shull BL. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23(2):223-227.
- Yazdany T, Yip S, Bhatia NN, Nguyen JN. Suture complications in a teaching institution among patients undergoing uterosacral ligament suspension with permanent braided suture. Int Urogynecol J. 2010;21(7):813-818.
- Toglia MR, Fagan MJ. Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol. 2008;198(5):600.e1-e4.
- Wong MJ, Rezvan A, Bhatia NN, Yazdany T. Uterosacral ligament vaginal vault suspension using delayed absorbable monofilament suture. Int Urogynecol J. 2011;22(11):1389-1394.
- Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- Kapoor S, Sivanesan K, Robertson JA, Veerasingham M, Kapoor V. Sacrospinous hysteropexy: review and meta-analysis of outcomes. Int Urogynecol J. 2017;28(9):1285-1294.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365-371.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
- Buchsbaum GM, Lee TG. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175-183.
- Zebede S, Smith AL, Plowright LN, Hegde A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol. 2013;121(2 pt 1):279-284.
- Bochenska K, Leader-Cramer A, Mueller M, Dave B, Alverdy A, Kenton K. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671-1675.
- Jones K, Wang G, Romano R, St Marie P, Harmanli O. Colpocleisis: a survey of current practice patterns. Female Pelvic Med Reconstr Surg. 2017;23(4):276-280.
- Jones KA, Zhuo Y, Solak S, Harmanli O. Hysterectomy at the time of colpocleisis: a decision analysis. Int Urogynecol J. 2016;27(5):805-810.
- Kandadai P, Flynn M, Zweizig S, Patterson D. Cost-utility of routine endometrial evaluation before le fort colpocleisis. Female Pelvic Med Reconstr Surg. 2014;20(3):168-173.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97(1):31-34.
- Oliphant SS, Shepherd JP, Lowder JL. Midurethral sling for treatment of occult stress urinary incontinence at the time of colpocleisis: a decision analysis. Female Pelvic Med Reconstr Surg. 2012;18(4):216-220.
- Siddiqui NY, Grimes CL, Casiano ER, et al; Society of Gynecologic Surgeons Systematic Review Group. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):44-55.
- Winters JC, Dmochowski RR, Goldman HB, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2464-2472.
- Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24(11):1815-1833.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376.
- Salamon CG, Lewis C, Priestley J, Gurshumov E, Culligan PJ. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371-1375.
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J. 2014;25(6):731-735.
- Eddib A, Danakas A, Hughes S, et al. Influence of morbid obesity on surgical outcomes in robotic-assisted gynecologic surgery. J Gynecol Surg. 2014;30(2):81-86.
- Gallo T, Kashani S, Patel DA, Elsahwi K, Silasi D-A, Azodi M. Robotic-assisted laparoscopic hysterectomy: outcomes in obese and morbidly obese patients. JSLS. 2012;16(3):421-427.
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303-318.
- Cundiff GW, Varner E, Visco AG, et al; Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1-e5.
- Wu JM, Wells EC, Hundley AF, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
- Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60-65.
- Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205-212.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol. 2013;121(1):143-151.
- Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137-143.
- Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
- ACOG Committee on Practice Bulletins-Gynecology, American Urogynecologic Society. ACOG Practice Bulletin No. 185: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):e234-e250.
- Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015;21(2):87-92.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database System Rev. 2016:CD012079.
- Meyer I, McGwin G, Swain T, Alvarez MD, Ellington DR, Richter HE. Synthetic graft augmentation in vaginal prolapse surgery: long-term objective and subjective outcomes. J Minim Invasive Gynecol. 2016;23(4):614-621.
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long‐term functional outcomes following mesh‐augmented posterior vaginal prolapse repair. Int J Gynecol Obstet. 2016;135(1):107-111.
- Glazener CM, Breeman S, Elders A, et al; PROSPECT Study Group. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381-392.
- Clinical and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomized controlled trials within Comprehensive Cohort Study. PROSPECT study protocol. The National Institute for Health Research. https://www.journalslibrary.nihr.ac.uk/programmes/hta/076018. Accessed January 17, 2018.
- Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural failure sites in anterior vaginal wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853-862.
- Gutman RE, Rardin CR, Sokol ER, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1-e11.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf. Published July 2011. Accessed January 9, 2017.
- Murphy M, Holzberg A, van Raalte H, et al; Pelvic Surgeons Network. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication: "Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse." Int Urogynecol J. 2012;23(1):5-9.
- Roman H, Marpeau L, Hulsey TC. Surgeons' experience and interaction effect in randomized controlled trials regarding new surgical procedures. Am J Obstet Gynecol. 2008;199(2):108.e1-e6.
- Eilber KS, Alperin M, Khan A, et al. The role of the surgeon on outcomes of vaginal prolapse surgery with mesh. Female Pelvic Med Reconstr Surg. 2017;23 (5):293-296.
- Kelly EC, Winick-Ng J, Welk B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet Gynecol. 2016;128(1):65-72.
- Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364(19):1826-1836.
- van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008:199(6):694.e1-e6.
- Iyer S, Botros SM. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J. 2017;28(4):527-535.
- Ting M, Gonzalez A, Ephraim S, Murphy M, Lucente V. The importance of a full thickness vaginal wall dissection. Comment on "Transvaginal mesh: a historical review and update of the current state of affairs in the United States." Int Urogynecol J. 2017;28(10):1609-1610.
- American Urogynecologic Society. Position statement on restriction of surgical options for pelvic floor disorders. https://www.augs.org/assets/1/6/Position_Statement_Surgical_Options_for_PFDs.pdf. Published March 26, 2013. Accessed January 9, 2017.
Effective surgical management of advanced pelvic organ prolapse (POP) depends on prolapse location and stage, presence of urinary incontinence, need for hysterectomy, the patient’s desire to maintain sexual function, type of surgery, and the surgeon’s skill and experience, among other factors. For these reasons, POP repair is not a one-size-fits all procedure.
In this article, experts in minimally invasive prolapse repair offer their perspectives on 3 surgical approaches: use of native tissue (Drs. White, Aguilar, and Rogers), abdominal sacrocolpopexy (Drs. Huber and Culligan), and transvaginal mesh (Drs. Lucente and Ton). They evaluate the evidence on these procedures and provide recommendations based on their experience of best practices for achieving surgical success and minimizing adverse events.
Using native tissue for vaginal anatomy repair
Amanda White, MD; Vivian Aguilar, MD; and Rebecca G. Rogers, MD
Dr. Rogers reports that she receives royalties from UpToDate. Drs. White and Aguilar report no financial relationships relevant to this article.
Surgical therapy is the mainstay of treatment for POP, and 20% of US women will undergo prolapse and/or stress incontinence surgery by age 80.1 Prolapse surgery either restores the vaginal anatomy (reconstructive surgery) or obliterates the vaginal canal (obliterative surgery). Vaginal reconstruction can be performed using the patient's native tissue or mesh. Because of concerns associated with mesh use, native tissue repairs continue to be commonly performed.
Unfortunately, not all prolapse surgeries result in prolapse cure, and recurrent prolapse that necessitates repeat operation is not rare, regardless of whether or not mesh is used.2,3 Native tissue repairs are most commonly performed through the vaginal route, the first minimally invasive approach to prolapse surgery. Restoration of the vaginal apex has been identified as critically important in these surgeries. Apical native tissue repairs include reconstructive procedures, such as sacrospinous ligament suspension (SSLS) or uterosacral ligament suspension (USLS), and obliterative procedures, such as colpocleisis.
In this discussion, we present 2 case vignettes that highlight surgical decision making for repair of stage 3 or 4 pelvic organ prolapse utilizing these techniques.
- Native tissue repair offers a minimally invasive approach to prolapse repair.
- Sacrospinous and uterosacral ligament suspensions have equivalent success rates.
- Prophylactic midurethral slings reduce postoperative incontinence at the time of transvaginal native tissue repair.
- Hysterectomy at the time of colpocleisis should not be performed routinely.
CASE 1 Active woman with prolapse
A 65-year-old woman (G2P2) presents with stage 3 prolapse, with the anterior compartment at +3 and the cervix at the hymen with straining. She is sexually active and desires to retain coital function. A trial of pessary has failed.
What surgical options can be considered for this patient?
Reconstruction procedures for prolapse
This patient presents with a typical configuration of prolapse; the anterior and apical compartments are the most likely to prolapse.4 Importantly, conservative management of her prolapse has failed. While it is not required that women have a trial with pessary prior to undergoing surgery, all women should be offered conservative management of prolapse, according to the American Urogynecologic Society (AUGS) and the American College of Obstetricians and Gynecologists (ACOG).4,5
Apical suspension
Since this patient desires to retain coital function, her gynecologist recommends a reconstructive procedure. The combination of apical and anterior vaginal wall prolapse will require an apical suspension procedure (FIGURES 1 and 2). If suspension of the apex does not correct the anterior wall prolapse, the patient also may require anterior compartment reconstruction.
The 2 most commonly performed native tissue apical suspension procedures, SSLS and USLS, have equivalent outcomes at 2 years, according to a multicenter randomized trial.6 Therefore, the choice of procedure is at the surgeon's discretion. USLS is most commonly performed at the time of hysterectomy via an intraperitoneal approach, while SSLS is often selected for posthysterectomy vault prolapse, given its extraperitoneal location.
Suture type. Whether to use permanent suture at the time of SSLS or USLS is controversial. Some data suggest that permanent suture provides greater long-term success compared with delayed absorbable suture.7 However, permanent suture has been reported to be associated with higher rates of suture complications--up to 44% in USLS and 36% in SSLS--compared with a 3.5% complication rate in a USLS cohort treated with absorbable suture.8-10
Hysterectomy versus hysteropexy. Considerable debate exists regarding whether a patient requires hysterectomy at the time of prolapse repair. In a randomized trial at 12 months' follow-up, uterine preservation by sacrospinous hysteropexy was noninferior to vaginal hysterectomy with suspension of the uterosacral ligaments for surgical failure of the apical compartment.11 A recent meta-analysis found that apical failure rates after sacrospinous hysteropexy versus vaginal hysterectomy were not different.12 Repeat surgery rates for prolapse also were not different between groups. The most significant disadvantage of uterine-preservation prolapse surgery, when compared with hysterectomy, is the lack of prevention and diagnosis of uterine malignancy.12 From 2002 to 2012, rates of hysteropexy significantly increased in the United States, although rates remain low.13
Sling procedure pros and cons. This case patient did not report urinary incontinence, but she may develop incontinence with reduction of the anterior wall prolapse. A large randomized controlled trial that included 337 women compared sling with no sling procedures among women with prolapse undergoing transvaginal prolapse repair.14 Management with a prophylactic sling resulted in less incontinence (27.3% and 43.0%, respectively, at 12 months postoperatively) but higher rates of urinary tract infection (31.0% vs 18.3%), major bleeding complications (3.1% vs 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs 0%) (P≤.05 for all).14
CASE 1 Recommendations for this patient
For this case, we would offer the patient a transvaginal hysterectomy and USLS. At the time of repair, we would assess whether she needed an anterior repair as well. We would offer a prophylactic sling procedure and also would discuss the risks and benefits of concomitant versus interval incontinence procedures.
CASE 2 Elderly woman with severe prolapse
An 85-year-old woman (G3P3) presents with procidentia, or complete eversion of the vagina, with the cervix 10 cm outside of the hymen. She has difficulty voiding, and the prolapse is uncomfortable when walking. A trial of pessary has failed. The patient denies vaginal bleeding. She is not sexually active and does not desire to retain coital function.
What treatment options would be appropriate for this patient?
Obliterative surgery
This elderly patient presents with advanced pelvic organ prolapse, and conservative management has failed. She is not sexually active and does not desire coital function in the future, so an obliterative procedure is indicated. Colpocleisis is a minimally invasive procedure that has cure rates ranging from 91% to 100%.15 It is likely that this patient's voiding dysfunction will improve after surgery and that she will be highly satisfied with the surgery.16
The question of hysterectomy with colpocleisis
The role of hysterectomy at the time of colpocleisis is controversial. LeFort colpocleisis preserves the uterus, with the anterior and posterior vaginal walls sutured together (FIGURE 3). Hysterectomy at the time of vaginal closure increases the operative time and blood loss.15 On the other hand, closure without hysterectomy prohibits future endometrial or cervical cancer screening.
In a recent review using the American College of Surgeons National Surgical Quality Improvement Program database, investigators compared women who underwent colopocleisis alone with those who underwent colpocleisis with hysterectomy.17 They found that the incidence of major complications was greater among women who underwent concomitant hysterectomy, and they concluded that hysterectomy should not be performed routinely at the time of colpocleisis.17
Among 322 urogynecologists who responded to a web-based survey, only 18% routinely performed hysterectomy at the time of colpocleisis.18 Further, in a decision analysis model, the utility for colpocleisis without hysterectomy was higher in women older than age 40, suggesting that hysterectomy should be performed only in special circumstances.19
Evaluating the endometrium. If the uterus remains in situ, should endometrial evaluation be performed? If so, should ultrasonography or endometrial biopsy be used? Authors of a decision analysis model found that among women at low risk for cancer and without abnormal uterine bleeding, endometrial biopsy was not favored until the probability of cancer reached 64%.20 Specifically, no evaluation or evaluation by transvaginal ultrasonography is adequate in the majority of cases.20 When screened by transvaginal ultrasonography, the high, 99% negative predictive value for endometrial disease, using a cutoff value of 5 mm for endometrial stripe width, will allow most patients to avoid unnecessary tissue sampling.
Stress incontinence. It is likely that this patient's voiding dysfunction will resolve with reduction of the prolapse, and she may develop stress incontinence symptoms. In up to 68% of women, occult stress incontinence will be revealed with reduction of stage 3 or stage 4 prolapse.21 If the patient demonstrates stress incontinence, a midurethral sling is likely to treat her incontinence effectively, with little added risk from the procedure.22 Even among women who have an elevated postvoid residual urine volume, the incidence of sling revision is low.15
CASE 2 Procedure recommendation for this patient
For this case, we would perform a LeFort colpocleisis and discuss whether or not the patient would prefer a midurethral sling if stress incontinence was demonstrated on examination. We would not perform endometrial evaluation in this patient, as she has not been bleeding and her risk for endometrial cancer is low.
Weighing the benefits of native tissue repair
Native tissue repair when performed transvaginally is a minimally invasive approach to prolapse repair. In a multicenter randomized trial, anatomic success was reported to be 64.5% at 2 years.6 Long-term follow up of patients undergoing mesh sacrocolpopexy shows a similar anatomic failure rate, with up to one-third of patients meeting the definition of composite failure.3 Unlike mesh-augmented repairs, however, adverse events, including bowel obstruction, mesh exposure, and thromboembolism, are more likely to occur in the mesh sacrocolpopexy group.23
Obliterative procedures have the highest success rates of all prolapse repairs and carry with them low morbidity. However, women must forego the ability for coitus in the future. For all native tissue vaginal repairs, the surgeon and patient must weigh the risks and benefits of concomitant anti-incontinence procedures.
Read about using abdominal sacrocolpopexy for apical prolapse repair.
Abdominal sacrocolpopexy: A tried-and-true approach for apical prolapse repair
Sarah Huber, MD, and Patrick Culligan, MD
Dr. Culligan reports that he is a shareholder in Oragami Surgical LLC and a consultant and speaker for Coloplast and Intuitive Surgical Inc. Dr. Huber reports no financial relationships relevant to this article.
CASE Woman with advanced prolapse desires surgical repair
A 55-year-old woman (G2P2) presents to her gynecologist's office reporting a vaginal bulge and pressure that has been worsening for the past year. She describes a nontender ball of tissue the size of an orange protruding past the introitus that worsens with ambulating and lifting heavy objects. She reports some urinary urgency and increased frequency and at times feels as though her bladder does not empty completely with voiding. She denies any urinary incontinence. The patient has regular bowel movements but does report some difficulty with stool evacuation. She has a history of 2 vaginal deliveries and is sexually active. She is postmenopausal, with the last menses about 4 years ago. She is active and exercises regularly.
The patient's Pap smears, mammograms, and colonoscopy are up to date and test results have been normal. She has no significant medical or surgical history and no significant family history of cancer. On examination, her body mass index is normal, as is the cardiopulmonary exam. Her pelvic organ prolapse quantification system (POP-Q) score is Aa +3, Ba +3, C +4, GH 3, PB 3, TVL 10, Ap +2, Bp +2, and D +2. The patient is interested in surgical management.
What urodynamic tests would be appropriate for this patient, and what treatment options would you recommend?
- Robot-assisted laparoscopic sacrocolpopexy is a safe, effective, and durable treatment for advanced-stage pelvic organ prolapse.
- This procedure can completely correct stage 3 or 4 prolapse when the dissection of the anterior vaginal wall extends to the bladder neck and the dissection of the posterior vaginal wall extends to the perineal body.
- One can avoid the need for concomitant vaginal prolapse repair by gathering up stretched out vaginal epithelium while suturing to the mesh arms.
- Sacral attachment sutures should be placed in the anterior longitudinal ligament distal to the sacral promontory to avoid the L5-S1 disc.
- Unless contraindicated, lightweight macroporous polypropylene mesh is the current implant of choice.
Additional tests needed
Patients with advanced-stage pelvic organ prolapse are at an increased risk for stress urinary incontinence that may be masked by urethral "kinking" due to anatomic distortion of the periurethral support mechanism. Based on recommendations from the American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), we routinely perform a postvoid residual urine volume measurement, urinalysis, urine culture, and a prolapse reduction stress test.24 If the urinalysis is positive for blood, then a preoperative cystoscopy would be indicated.
If stress incontinence is confirmed by reduction stress testing, the patient should be offered an anti-incontinence procedure, such as a mesh midurethral sling.
This patient's overactive bladder symptoms warrant investigation via complex urodynamic testing to allow for comprehensive counseling about her postoperative expectations.
Counseling the patient on the sacrocolpopexy option
Abdominal sacrocolpopexy initially was described in 1962 by Lane as a technique to affix the vaginal apex to the sacral promontory using a graft. Although the procedure has been modified over the years, the principles of using an implanted strengthening material to permanently attach the apex to the anterior longitudinal ligament at the sacrum has proven to be a highly effective and safe treatment, establishing it as the gold standard for apical prolapse repair.25,26
Compared with other methods of apical prolapse repair, sacrocolpopexy via any approach is superior to vaginal surgery in terms of subjective and objective outcomes. In a recent systematic review comparing apical prolapse repairs, patients who underwent a vaginal approach were more likely to report awareness of their prolapse after surgery, undergo repeat surgery, have objective recurrent prolapse, and were at increased risk for postoperative stress urinary incontinence and dyspareunia.26 Prospective studies within our practice have shown 1-year composite subjective and objective cure rates of 94% to 95%.27,28
Selecting a route for sacrocolpopexy
Although sacrocolpopexy can be approached via laparotomy or conventional laparoscopy, we routinely use a robot-assisted approach, as it has been shown to be especially beneficial for complex situations, such as in patients with prior pelvic surgery, a foreshortened vagina, or obesity.29,30
Potential complications
Sacrocolpopexy complications are rare, especially when a minimally invasive approach is used.31 Reported complications of minimally invasive sacrocolpopexy include gastrointestinal or genitourinary injury, bowel obstruction or ileus, incisional hernia, vascular injury, discitis or osteomyelitis, conversion to open procedure, and mesh exposure.
Vaginal mesh exposure is rare following sacrocolpopexy, but it can occur at any time following surgery.31 Some risk factors include mesh material selection (specifically polytetrafluoroethylene [PTFE] mesh), concurrent total hysterectomy, vaginal atrophy, and smoking.32,33 As a result, recent recommendations have advised the use of polypropylene mesh with uterine preservation or supracervical hysterectomy at the time of sacrocolpopexy.34 In fact, supracervical hysterectomy alone appears to cut down or eliminate the risk of mesh exposure in laparoscopic sacrocolpopexy.35
In our practice, avoiding split-thickness vaginal dissection, employing supracervical hysterectomy techniques, and using ultralightweight mesh has resulted in mesh exposure rates approaching zero.28
For atrophic vaginal tissue, one can consider prescribing preoperative vaginal estrogen for 4 to 6 weeks, but this is not essential and should not routinely delay pelvic reconstructive surgery.
What type of implant material is best?
While various materials have been used as the fixation media in sacrocolpopexy, loosely knitted synthetic type I macroporous polypropylene mesh is the best choice due to its efficacy, availability, and low adverse effect profile. We recommend a lightweight mesh with a maximum weight of 25 g/m2. Two such products currently available are the UPsylon Y-Mesh (Boston Scientific, Marlborough, Massachusetts) and Restorelle Y mesh (Coloplast, Minneapolis, Minnesota). Lightweight mesh has been proven to maintain integrity, guaranteeing a successful outcome, while reducing the "mesh load" on the attached tissue.27,28
Comparative studies with fascia lata or cross-linked porcine dermal grafts demonstrated inferior outcomes versus synthetic mesh, and currently the only biologic material on the market indicated for prolapse repair augmentation, ACell Pelvic Floor Matrix (ACell, Columbia, Maryland), has not been extensively tested in sacrocolpopexy.36-38
Vaginal anatomy restored by sacrocolpopexy
Abdominal sacrocolpopexy, specifically via a minimally invasive approach, is an effective and long-lasting treatment that should be offered to women with advanced-stage prolapse.
Using the surgical techniques described below, including attachment of the mesh along the lengths of the anterior and posterior vaginal walls and gathering up excess tissue with mesh attachment, can provide women with adequate support for the entire vagina with restoration of normal vaginal anatomy and caliber.
Step-by-step tips for surgical efficiency
Robotic port placement
- Place the trocars in a "W" layout for the da Vinci Si Surgical System (FIGURE 4, VIDEO 1) or in a linear layout for the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). Both Si and Xi port placement includes a 3- to 5-mm assistant port in the right upper quadrant of the abdomen.
Supracervical hysterectomy, if indicated
- Maneuver the uterus with the robotic tenaculum, which obviates the need for a uterine manipulator during the hysterectomy (VIDEO 2).
- Create the bladder flap just above the upper edge of the bladder to facilitate the upcoming anterior wall dissection. This helps to prevent the development of a split-thickness dissection plane.
- 1.5 to 2 cm of cervix should be left in place, and conization should be avoided.
Anterior vaginal wall dissection
- The key to a good full-thickness dissection is sustained tissue traction and countertraction. The bedside assistant pulls the anterior peritoneal cut edge anteriorly for "gross" traction, and further "fine" traction can be created by pulling the areolar tissue with robotic forceps. The cervix is grasped with the tenaculum, which applies a constant midline cephalad countertraction (VIDEO 3).
- Sharp dissection with cold scissors allows for creation of the dissection plane, while cautery is judiciously applied only for hemostasis. If bleeding is encountered, this usually indicates that a split thickness of the vaginal wall has been created, and the surgeon should correct to the proper dissection plane.
- Dissection is made easier by taking down the bladder pillars before advancing down toward the bladder neck.
- The anterior dissection is always carried down to level of the trigone, confirmed by visualization of the Foley bulb (FIGURE 5).
Posterior vaginal wall dissection
- Begin dissection just above the rectal reflection, leaving peritoneum on the posterior cervix (VIDEO 4).
- Extend the incision bilaterally to the uterosacral ligaments only after the correct dissection plane is confirmed by visualization of the areolar tissue.
- Apply cervical traction using the tenaculum in a cephalad midline direction, and place traction on the cut edge of the posterior peritoneum using the bipolar forceps. The tenaculum wrist must be turned away from the working instruments to avoid internal clashing.
- Completely transect the right uterosacral ligament to better facilitate the creation of a contiguous peritoneal opening for burying the mesh. The remainder of the opening will be created later.
- While it is important to avoid split-thickness dissection, the vaginal plane must be "clean" (that is, without fat or adventitia) to allow for robust suturing.
- Dissection at least halfway down the posterior vaginal wall is recommended but proceeding down to the perineal body provides the most optimal support (FIGURE 6).
Sacral dissection
- Use a noncrushing instrument to laterally sweep the bowel to the left side, effectively "plastering" the peritoneum over the sacral promontory (FIGURE 7; VIDEO 5).
- Extend the superficial peritoneal incision down the right paracolic gutter halfway between the ureter and colon until it communicates with the incised posterior peritoneal edge created during the posterior dissection.
- Identify the middle sacral artery to avoid vascular injury, but there is no need to prophylactically coagulate it.
Vaginal mesh attachment
- Cut a lightweight Y-mesh to a length of 6 to 8 cm anteriorly and 8 to 11 cm posteriorly and place it into the surgical field (FIGURE 8; VIDEO 6). The length is determined based on the preoperative office examination and examination under anesthesia prior to starting the procedure.
- Attach the mesh securely and evenly to the anterior and posterior vaginal walls using multiple interrupted monofilament sutures. We aim to place sutures that provide mesh stability without excess vaginal wall incorporation to avoid "through-and-through" suturing.
- The posterior wall suturing is performed first, starting at the perineal body and continuing cephalad (VIDEO 7). We find it easiest to tie the knots between the mesh and the vagina in this space.
- Suture the crotch of the Y-mesh to the cervix so that no gap exists between tissue and mesh.
- For advanced-stage prolapse with significant anterior prolapse, the stretched out vaginal epithelium can be systematically gathered up to reconfigure the tissue to conform to the desired mesh dimensions (VIDEO 8). This tissue remodeling is evident even at the 2- to 4-week postoperative visit.
Peritoneal closure: Step 1
- Reapproximate the cut edges of peritoneum surrounding the vagina and cervix using a continuous purse-string suture of 0 Monocryl (poliglecaprone 25) on an SH needle (Ethicon, Somerville, New Jersey) with a fisherman's knot tied at the end (VIDEO 9). The needle passes are placed close together and close to the incised edge of the cut peritoneum.
- We typically start our peritoneal suture at the 5 o'clock position of the posterior peritoneum, extending in a clockwise direction and ultimately jumping anteriorly around the sacral arm of the mesh.
- Place the mesh within the paracolic peritoneal canal, and secure the needle for later use.
Sacral mesh attachment
- The mesh is tensioned so that a vaginal examination confirms adequate support of all the walls without excess tension or tissue banding. Some laxity of the anterior vaginal wall consistent with a mild cystocele is appropriate.
- Place 2 permanent PTFE sutures along the slope of the sacral promontory into the anterior longitudinal ligament (VIDEO 10). This avoids injury to the disc space that sits at the edge of the promontory. We do not advise the use of bone anchors as they increase the risk for discitis and osteomyelitis.
- Secure the mesh to the anterior longitudinal ligament without any tension. This is facilitated by creating mesh slack via cephalad pressure from a vaginal probe.
Peritoneal closure: Step 2
- Close the remaining paracolic peritoneal incision, completely burying the mesh within the created canal (FIGURE 9).
- At the end of the procedure, perform a repeat vaginal exam, rectal exam, and cystoscopy.
Technique with prior total hysterectomy
- In patients with a prior total hysterectomy, place a 13 x 3.5 cm Breisky vaginal retractor and/or coated nonconductive stent (Marina Medical, Sunrise, Florida) into the vagina to delineate the anterior and posterior walls at the vaginal apex during dissection.
- Some surgeons may opt to retrograde fill the bladder to better identify its location.
- We routinely leave a segment of peritoneum attached to the dome of the vaginal apex for added tissue integrity to prevent erosion.
Read about using transvaginal mesh for POP repair.
Transvaginal mesh: An effective, durable option for POP repair
Vincent R. Lucente, MD, MBA, and Jessica B. Ton, MD
Dr. Lucente reports that he has received grant or research support from Advanced Tactile Imaging, Boston Scientific, Coloplast, and Valencia; is a consultant to Coloplast; is a speaker for Allergan, Boston Scientific, Coloplast, and Shionogi; and serves as an expert witness for American Medical Systems and C.R. Bard. Dr. Ton reports no financial relationships relevant to this article.
As baseline health in the elderly population continues to improve, the number of women in the United States with symptomatic POP will increase by approximately 50% by 2050.39 Unfortunately, after native tissue repair (NTR) the rate of prolapse recurrence is extremely high: approximately 40% regardless of approach, as demonstrated in the OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial by Barber and colleagues.6 The authors of that clinical trial recently revealed that at the 5-year follow-up, these failure rates progressed to 70% for sacrospinous ligament fixation and 61% for uterosacral ligament suspension (data presented at the Society of Gynecologic Surgeons Annual Scientific Meeting 2018, Orlando, Florida). This establishes that NTR is not durable enough to meet the increasing physical demands of this age group and that mesh augmentation must be considered.
For patients at increased risk of prolapse recurrence, using transvaginal mesh (TVM) is the most minimally invasive approach and is an excellent option for mesh augmentation. Avoiding adverse events during placement of TVM depends largely on optimal surgical technique.40 (VIDEO: “Demonstration of an anterior vaginal wall dissection into the true vesicovaginal space”)
- Active advanced age requires a durable reconstructive pelvic surgery for pelvic organ prolapse, and native tissue repair does not meet that demand.
- Mesh augmentation reduces the risk of prolapse recurrence, and vaginal placement of mesh is the most minimally invasive approach.
- Rates of exposure with transvaginal mesh would be minimized with use of a full-thickness vaginal wall dissection.
- Optimal surgical technique could be highly reproducible with better surgical training.
The evidence on TVM versus NTR
Several studies have examined whether TVM has a measurable benefit over NTR.
A 2016 Cochrane review by Maher and colleagues included 37 randomized trials (4,023 women) that compared TVM and biologic grafts with NTR.41 Three primary outcomes were defined: awareness of prolapse, recurrence, and repeat surgery. Compared with women treated with NTR, those treated with synthetic nonabsorbable TVM exhibited a greater reduction in awareness of prolapse (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54-0.81), decreased recurrence in the anterior compartment (RR, 0.33; 95% CI, 0.26-0.40), and decreased reoperation for prolapse (RR, 0.53; 95% CI, 0.31-0.88). The overall calculated exposure rate was 12%, with a range of 3.2% to 20.8%.41 As we will discuss, this wide range most likely is attributed to a suboptimal, split-thickness dissection. There were no differences in other key secondary outcomes, including dyspareunia, operating time, and estimated blood loss.41
Longitudinal studies are emerging as almost 2 decades have passed since TVM was introduced. In a study of 5-year follow-up after TVM placement, Meyer and colleagues reported that patients had continued significant improvements in both subjective and objective outcomes.42 The mesh exposure rate was 6%, attributed to severe vaginal atrophy.42 A 10-year observational study by Weintraub and colleagues demonstrated a recurrence rate of only 2.6% in the anterior compartment, 7.6% in the posterior (nonaugmented) compartment, and no exposures or extrusions after anterior TVM placement.43
In contrast to the Cochrane review, in the 2017 multicenter PROSPECT (Prolapse surgery: Pragmatic evaluation and randomized controlled trials) trial, Glazener and colleagues found no difference in desired outcomes with TVM compared with NTR.44 There was an overall 6% to 7% exposure rate over 2 years.44 To reflect "real-world" practice, however, this study was intentionally designed without rigorous standardization of surgical technique. The authors reported that "appropriately experienced surgeons" performed the procedure, but it is unclear how experience was determined given that 20% of the cases were performed by "registrars," the equivalent of US residents or fellows.45
The PROSPECT study protocol described the TVM procedure as "a standard repair with a nonabsorbable mesh inlay to support the stitches," implying that there was no apical attachment of the mesh to the sacrospinous ligament.45 This is a suboptimal use of TVM because it does not address a detachment-type defect common in advanced prolapse. The PROSPECT study reinforces the need for better surgical training and standardization of the TVM procedure.44
How TVM compares with sacrocolpopexy
When comparing the use of TVM with sacrocolpopexy, our experience has been that TVM yields similar outcomes to sacrocolpopexy with additional benefits. We completed a 1-year retrospective cohort study comparing robot-assisted laparoscopic sacrocolpopexy (RALS) with TVM in a total of 86 patients, with both approaches performed by the same surgeon. Both treatment groups showed statistically significant improvements in nearly all functional and quality-of-life measures, including urinary symptoms, sexual function, and POP-Q scores.40 In particular, points Aa and Ba on the POP-Q score were significantly improved with TVM as compared to RALS. This suggests that TVM can achieve both lateral and apical support, where sacrocolpopexy addresses only the apex.40 This has clinical significance when considering DeLancey and colleagues' dynamic magnetic resonance imaging study, which demonstrated advanced prolapse results from both lateral and apical detachment.46 In addition, TVM placement also was considerably faster than RALS by approximately 96 minutes and could be performed using regional anesthesia. Only 1 mesh exposure in each study arm was reported.40
Finally, as with other vaginal procedures, patients who undergo TVM placement require minimal to no pain medication postoperatively and report faster return to daily activities. Almost none of our patients require narcotics, which is a significant benefit in the face of the ongoing national opioid crisis.
Gutman and colleagues compared laparoscopic mesh hysteropexy with TVM; they demonstrated comparable cure rates and, again, significantly longer operative times for the laparoscopic approach (174 vs 64 minutes; P<.0001).47 This multicenter study reported mesh exposure rates of 2.7% for laparoscopy and 6.6% for TVM,47 again likely due to a split-thickness dissection.
Safety of TVM depends on the surgeon factor
Because of the reported complications associated with TVM, in 2011 the US Food and Drug Administration (FDA) issued an update on the safety and efficacy of TVM augmentation and mandated postmarket studies.48 While we do not dispute that the mesh exposure rates were accurate at the time the FDA document was issued, we recognize that exposure has been erroneously attributed to inherent properties of the mesh.
Mesh exposure rates reported in the literature vary widely, ranging from 0% to 30%, even when surgeons used identical mesh products.49 This clearly establishes that the main contributing variable is surgical technique. It is critically important to recognize the "surgeon factor" as a confounder in trials that compare surgical procedures.50 Studies on TVM have shown that low-volume surgeons had significantly higher reoperation rates, while high-volume surgeons achieved a 41% reduction in reoperations.51,52 When TVM is performed by expert surgeons, the reported mesh exposure rates for TVM are noticeably lower.40,42,43,53,54
Decreasing mesh exposure rates would reduce the most common adverse event associated with TVM, thus improving its safety. The critical step to successful TVM placement is the initial dissection. Gynecologists traditionally have performed a split-thickness, colporrhaphy-style dissection to place the mesh within the layers of the vaginal wall.55 Placement within these planes, however, is too superficial and increases the risk of exposure. By contrast, by consistently performing a full-thickness vaginal wall dissection (FIGURE 10) and placing the mesh in the true vesicovaginal space,56 we have achieved a TVM exposure rate as low as 0% to 3%.40,54 If we can standardize the dissection component across our subspecialty, the rate of mesh exposure undoubtedly will decrease.
The PROSPECT investigators readily admitted what the study was not: a trial conducted "exclusively by the most experienced surgeons in the highest volume centres¬with a highly protocolised technique."44 In reality, that is the kind of rigorous study on TVM that our subspecialty demands. We must hold ourselves accountable and ensure that only the most qualified surgeons are placing TVM.
Keep the mesh option available
We support the position of the American Urogynecologic Society in opposing an outright ban of TVM because such a restriction would deny our patients access to an effective, durable, and minimally invasive approach for prolapse repair.57
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Effective surgical management of advanced pelvic organ prolapse (POP) depends on prolapse location and stage, presence of urinary incontinence, need for hysterectomy, the patient’s desire to maintain sexual function, type of surgery, and the surgeon’s skill and experience, among other factors. For these reasons, POP repair is not a one-size-fits all procedure.
In this article, experts in minimally invasive prolapse repair offer their perspectives on 3 surgical approaches: use of native tissue (Drs. White, Aguilar, and Rogers), abdominal sacrocolpopexy (Drs. Huber and Culligan), and transvaginal mesh (Drs. Lucente and Ton). They evaluate the evidence on these procedures and provide recommendations based on their experience of best practices for achieving surgical success and minimizing adverse events.
Using native tissue for vaginal anatomy repair
Amanda White, MD; Vivian Aguilar, MD; and Rebecca G. Rogers, MD
Dr. Rogers reports that she receives royalties from UpToDate. Drs. White and Aguilar report no financial relationships relevant to this article.
Surgical therapy is the mainstay of treatment for POP, and 20% of US women will undergo prolapse and/or stress incontinence surgery by age 80.1 Prolapse surgery either restores the vaginal anatomy (reconstructive surgery) or obliterates the vaginal canal (obliterative surgery). Vaginal reconstruction can be performed using the patient's native tissue or mesh. Because of concerns associated with mesh use, native tissue repairs continue to be commonly performed.
Unfortunately, not all prolapse surgeries result in prolapse cure, and recurrent prolapse that necessitates repeat operation is not rare, regardless of whether or not mesh is used.2,3 Native tissue repairs are most commonly performed through the vaginal route, the first minimally invasive approach to prolapse surgery. Restoration of the vaginal apex has been identified as critically important in these surgeries. Apical native tissue repairs include reconstructive procedures, such as sacrospinous ligament suspension (SSLS) or uterosacral ligament suspension (USLS), and obliterative procedures, such as colpocleisis.
In this discussion, we present 2 case vignettes that highlight surgical decision making for repair of stage 3 or 4 pelvic organ prolapse utilizing these techniques.
- Native tissue repair offers a minimally invasive approach to prolapse repair.
- Sacrospinous and uterosacral ligament suspensions have equivalent success rates.
- Prophylactic midurethral slings reduce postoperative incontinence at the time of transvaginal native tissue repair.
- Hysterectomy at the time of colpocleisis should not be performed routinely.
CASE 1 Active woman with prolapse
A 65-year-old woman (G2P2) presents with stage 3 prolapse, with the anterior compartment at +3 and the cervix at the hymen with straining. She is sexually active and desires to retain coital function. A trial of pessary has failed.
What surgical options can be considered for this patient?
Reconstruction procedures for prolapse
This patient presents with a typical configuration of prolapse; the anterior and apical compartments are the most likely to prolapse.4 Importantly, conservative management of her prolapse has failed. While it is not required that women have a trial with pessary prior to undergoing surgery, all women should be offered conservative management of prolapse, according to the American Urogynecologic Society (AUGS) and the American College of Obstetricians and Gynecologists (ACOG).4,5
Apical suspension
Since this patient desires to retain coital function, her gynecologist recommends a reconstructive procedure. The combination of apical and anterior vaginal wall prolapse will require an apical suspension procedure (FIGURES 1 and 2). If suspension of the apex does not correct the anterior wall prolapse, the patient also may require anterior compartment reconstruction.
The 2 most commonly performed native tissue apical suspension procedures, SSLS and USLS, have equivalent outcomes at 2 years, according to a multicenter randomized trial.6 Therefore, the choice of procedure is at the surgeon's discretion. USLS is most commonly performed at the time of hysterectomy via an intraperitoneal approach, while SSLS is often selected for posthysterectomy vault prolapse, given its extraperitoneal location.
Suture type. Whether to use permanent suture at the time of SSLS or USLS is controversial. Some data suggest that permanent suture provides greater long-term success compared with delayed absorbable suture.7 However, permanent suture has been reported to be associated with higher rates of suture complications--up to 44% in USLS and 36% in SSLS--compared with a 3.5% complication rate in a USLS cohort treated with absorbable suture.8-10
Hysterectomy versus hysteropexy. Considerable debate exists regarding whether a patient requires hysterectomy at the time of prolapse repair. In a randomized trial at 12 months' follow-up, uterine preservation by sacrospinous hysteropexy was noninferior to vaginal hysterectomy with suspension of the uterosacral ligaments for surgical failure of the apical compartment.11 A recent meta-analysis found that apical failure rates after sacrospinous hysteropexy versus vaginal hysterectomy were not different.12 Repeat surgery rates for prolapse also were not different between groups. The most significant disadvantage of uterine-preservation prolapse surgery, when compared with hysterectomy, is the lack of prevention and diagnosis of uterine malignancy.12 From 2002 to 2012, rates of hysteropexy significantly increased in the United States, although rates remain low.13
Sling procedure pros and cons. This case patient did not report urinary incontinence, but she may develop incontinence with reduction of the anterior wall prolapse. A large randomized controlled trial that included 337 women compared sling with no sling procedures among women with prolapse undergoing transvaginal prolapse repair.14 Management with a prophylactic sling resulted in less incontinence (27.3% and 43.0%, respectively, at 12 months postoperatively) but higher rates of urinary tract infection (31.0% vs 18.3%), major bleeding complications (3.1% vs 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs 0%) (P≤.05 for all).14
CASE 1 Recommendations for this patient
For this case, we would offer the patient a transvaginal hysterectomy and USLS. At the time of repair, we would assess whether she needed an anterior repair as well. We would offer a prophylactic sling procedure and also would discuss the risks and benefits of concomitant versus interval incontinence procedures.
CASE 2 Elderly woman with severe prolapse
An 85-year-old woman (G3P3) presents with procidentia, or complete eversion of the vagina, with the cervix 10 cm outside of the hymen. She has difficulty voiding, and the prolapse is uncomfortable when walking. A trial of pessary has failed. The patient denies vaginal bleeding. She is not sexually active and does not desire to retain coital function.
What treatment options would be appropriate for this patient?
Obliterative surgery
This elderly patient presents with advanced pelvic organ prolapse, and conservative management has failed. She is not sexually active and does not desire coital function in the future, so an obliterative procedure is indicated. Colpocleisis is a minimally invasive procedure that has cure rates ranging from 91% to 100%.15 It is likely that this patient's voiding dysfunction will improve after surgery and that she will be highly satisfied with the surgery.16
The question of hysterectomy with colpocleisis
The role of hysterectomy at the time of colpocleisis is controversial. LeFort colpocleisis preserves the uterus, with the anterior and posterior vaginal walls sutured together (FIGURE 3). Hysterectomy at the time of vaginal closure increases the operative time and blood loss.15 On the other hand, closure without hysterectomy prohibits future endometrial or cervical cancer screening.
In a recent review using the American College of Surgeons National Surgical Quality Improvement Program database, investigators compared women who underwent colopocleisis alone with those who underwent colpocleisis with hysterectomy.17 They found that the incidence of major complications was greater among women who underwent concomitant hysterectomy, and they concluded that hysterectomy should not be performed routinely at the time of colpocleisis.17
Among 322 urogynecologists who responded to a web-based survey, only 18% routinely performed hysterectomy at the time of colpocleisis.18 Further, in a decision analysis model, the utility for colpocleisis without hysterectomy was higher in women older than age 40, suggesting that hysterectomy should be performed only in special circumstances.19
Evaluating the endometrium. If the uterus remains in situ, should endometrial evaluation be performed? If so, should ultrasonography or endometrial biopsy be used? Authors of a decision analysis model found that among women at low risk for cancer and without abnormal uterine bleeding, endometrial biopsy was not favored until the probability of cancer reached 64%.20 Specifically, no evaluation or evaluation by transvaginal ultrasonography is adequate in the majority of cases.20 When screened by transvaginal ultrasonography, the high, 99% negative predictive value for endometrial disease, using a cutoff value of 5 mm for endometrial stripe width, will allow most patients to avoid unnecessary tissue sampling.
Stress incontinence. It is likely that this patient's voiding dysfunction will resolve with reduction of the prolapse, and she may develop stress incontinence symptoms. In up to 68% of women, occult stress incontinence will be revealed with reduction of stage 3 or stage 4 prolapse.21 If the patient demonstrates stress incontinence, a midurethral sling is likely to treat her incontinence effectively, with little added risk from the procedure.22 Even among women who have an elevated postvoid residual urine volume, the incidence of sling revision is low.15
CASE 2 Procedure recommendation for this patient
For this case, we would perform a LeFort colpocleisis and discuss whether or not the patient would prefer a midurethral sling if stress incontinence was demonstrated on examination. We would not perform endometrial evaluation in this patient, as she has not been bleeding and her risk for endometrial cancer is low.
Weighing the benefits of native tissue repair
Native tissue repair when performed transvaginally is a minimally invasive approach to prolapse repair. In a multicenter randomized trial, anatomic success was reported to be 64.5% at 2 years.6 Long-term follow up of patients undergoing mesh sacrocolpopexy shows a similar anatomic failure rate, with up to one-third of patients meeting the definition of composite failure.3 Unlike mesh-augmented repairs, however, adverse events, including bowel obstruction, mesh exposure, and thromboembolism, are more likely to occur in the mesh sacrocolpopexy group.23
Obliterative procedures have the highest success rates of all prolapse repairs and carry with them low morbidity. However, women must forego the ability for coitus in the future. For all native tissue vaginal repairs, the surgeon and patient must weigh the risks and benefits of concomitant anti-incontinence procedures.
Read about using abdominal sacrocolpopexy for apical prolapse repair.
Abdominal sacrocolpopexy: A tried-and-true approach for apical prolapse repair
Sarah Huber, MD, and Patrick Culligan, MD
Dr. Culligan reports that he is a shareholder in Oragami Surgical LLC and a consultant and speaker for Coloplast and Intuitive Surgical Inc. Dr. Huber reports no financial relationships relevant to this article.
CASE Woman with advanced prolapse desires surgical repair
A 55-year-old woman (G2P2) presents to her gynecologist's office reporting a vaginal bulge and pressure that has been worsening for the past year. She describes a nontender ball of tissue the size of an orange protruding past the introitus that worsens with ambulating and lifting heavy objects. She reports some urinary urgency and increased frequency and at times feels as though her bladder does not empty completely with voiding. She denies any urinary incontinence. The patient has regular bowel movements but does report some difficulty with stool evacuation. She has a history of 2 vaginal deliveries and is sexually active. She is postmenopausal, with the last menses about 4 years ago. She is active and exercises regularly.
The patient's Pap smears, mammograms, and colonoscopy are up to date and test results have been normal. She has no significant medical or surgical history and no significant family history of cancer. On examination, her body mass index is normal, as is the cardiopulmonary exam. Her pelvic organ prolapse quantification system (POP-Q) score is Aa +3, Ba +3, C +4, GH 3, PB 3, TVL 10, Ap +2, Bp +2, and D +2. The patient is interested in surgical management.
What urodynamic tests would be appropriate for this patient, and what treatment options would you recommend?
- Robot-assisted laparoscopic sacrocolpopexy is a safe, effective, and durable treatment for advanced-stage pelvic organ prolapse.
- This procedure can completely correct stage 3 or 4 prolapse when the dissection of the anterior vaginal wall extends to the bladder neck and the dissection of the posterior vaginal wall extends to the perineal body.
- One can avoid the need for concomitant vaginal prolapse repair by gathering up stretched out vaginal epithelium while suturing to the mesh arms.
- Sacral attachment sutures should be placed in the anterior longitudinal ligament distal to the sacral promontory to avoid the L5-S1 disc.
- Unless contraindicated, lightweight macroporous polypropylene mesh is the current implant of choice.
Additional tests needed
Patients with advanced-stage pelvic organ prolapse are at an increased risk for stress urinary incontinence that may be masked by urethral "kinking" due to anatomic distortion of the periurethral support mechanism. Based on recommendations from the American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), we routinely perform a postvoid residual urine volume measurement, urinalysis, urine culture, and a prolapse reduction stress test.24 If the urinalysis is positive for blood, then a preoperative cystoscopy would be indicated.
If stress incontinence is confirmed by reduction stress testing, the patient should be offered an anti-incontinence procedure, such as a mesh midurethral sling.
This patient's overactive bladder symptoms warrant investigation via complex urodynamic testing to allow for comprehensive counseling about her postoperative expectations.
Counseling the patient on the sacrocolpopexy option
Abdominal sacrocolpopexy initially was described in 1962 by Lane as a technique to affix the vaginal apex to the sacral promontory using a graft. Although the procedure has been modified over the years, the principles of using an implanted strengthening material to permanently attach the apex to the anterior longitudinal ligament at the sacrum has proven to be a highly effective and safe treatment, establishing it as the gold standard for apical prolapse repair.25,26
Compared with other methods of apical prolapse repair, sacrocolpopexy via any approach is superior to vaginal surgery in terms of subjective and objective outcomes. In a recent systematic review comparing apical prolapse repairs, patients who underwent a vaginal approach were more likely to report awareness of their prolapse after surgery, undergo repeat surgery, have objective recurrent prolapse, and were at increased risk for postoperative stress urinary incontinence and dyspareunia.26 Prospective studies within our practice have shown 1-year composite subjective and objective cure rates of 94% to 95%.27,28
Selecting a route for sacrocolpopexy
Although sacrocolpopexy can be approached via laparotomy or conventional laparoscopy, we routinely use a robot-assisted approach, as it has been shown to be especially beneficial for complex situations, such as in patients with prior pelvic surgery, a foreshortened vagina, or obesity.29,30
Potential complications
Sacrocolpopexy complications are rare, especially when a minimally invasive approach is used.31 Reported complications of minimally invasive sacrocolpopexy include gastrointestinal or genitourinary injury, bowel obstruction or ileus, incisional hernia, vascular injury, discitis or osteomyelitis, conversion to open procedure, and mesh exposure.
Vaginal mesh exposure is rare following sacrocolpopexy, but it can occur at any time following surgery.31 Some risk factors include mesh material selection (specifically polytetrafluoroethylene [PTFE] mesh), concurrent total hysterectomy, vaginal atrophy, and smoking.32,33 As a result, recent recommendations have advised the use of polypropylene mesh with uterine preservation or supracervical hysterectomy at the time of sacrocolpopexy.34 In fact, supracervical hysterectomy alone appears to cut down or eliminate the risk of mesh exposure in laparoscopic sacrocolpopexy.35
In our practice, avoiding split-thickness vaginal dissection, employing supracervical hysterectomy techniques, and using ultralightweight mesh has resulted in mesh exposure rates approaching zero.28
For atrophic vaginal tissue, one can consider prescribing preoperative vaginal estrogen for 4 to 6 weeks, but this is not essential and should not routinely delay pelvic reconstructive surgery.
What type of implant material is best?
While various materials have been used as the fixation media in sacrocolpopexy, loosely knitted synthetic type I macroporous polypropylene mesh is the best choice due to its efficacy, availability, and low adverse effect profile. We recommend a lightweight mesh with a maximum weight of 25 g/m2. Two such products currently available are the UPsylon Y-Mesh (Boston Scientific, Marlborough, Massachusetts) and Restorelle Y mesh (Coloplast, Minneapolis, Minnesota). Lightweight mesh has been proven to maintain integrity, guaranteeing a successful outcome, while reducing the "mesh load" on the attached tissue.27,28
Comparative studies with fascia lata or cross-linked porcine dermal grafts demonstrated inferior outcomes versus synthetic mesh, and currently the only biologic material on the market indicated for prolapse repair augmentation, ACell Pelvic Floor Matrix (ACell, Columbia, Maryland), has not been extensively tested in sacrocolpopexy.36-38
Vaginal anatomy restored by sacrocolpopexy
Abdominal sacrocolpopexy, specifically via a minimally invasive approach, is an effective and long-lasting treatment that should be offered to women with advanced-stage prolapse.
Using the surgical techniques described below, including attachment of the mesh along the lengths of the anterior and posterior vaginal walls and gathering up excess tissue with mesh attachment, can provide women with adequate support for the entire vagina with restoration of normal vaginal anatomy and caliber.
Step-by-step tips for surgical efficiency
Robotic port placement
- Place the trocars in a "W" layout for the da Vinci Si Surgical System (FIGURE 4, VIDEO 1) or in a linear layout for the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). Both Si and Xi port placement includes a 3- to 5-mm assistant port in the right upper quadrant of the abdomen.
Supracervical hysterectomy, if indicated
- Maneuver the uterus with the robotic tenaculum, which obviates the need for a uterine manipulator during the hysterectomy (VIDEO 2).
- Create the bladder flap just above the upper edge of the bladder to facilitate the upcoming anterior wall dissection. This helps to prevent the development of a split-thickness dissection plane.
- 1.5 to 2 cm of cervix should be left in place, and conization should be avoided.
Anterior vaginal wall dissection
- The key to a good full-thickness dissection is sustained tissue traction and countertraction. The bedside assistant pulls the anterior peritoneal cut edge anteriorly for "gross" traction, and further "fine" traction can be created by pulling the areolar tissue with robotic forceps. The cervix is grasped with the tenaculum, which applies a constant midline cephalad countertraction (VIDEO 3).
- Sharp dissection with cold scissors allows for creation of the dissection plane, while cautery is judiciously applied only for hemostasis. If bleeding is encountered, this usually indicates that a split thickness of the vaginal wall has been created, and the surgeon should correct to the proper dissection plane.
- Dissection is made easier by taking down the bladder pillars before advancing down toward the bladder neck.
- The anterior dissection is always carried down to level of the trigone, confirmed by visualization of the Foley bulb (FIGURE 5).
Posterior vaginal wall dissection
- Begin dissection just above the rectal reflection, leaving peritoneum on the posterior cervix (VIDEO 4).
- Extend the incision bilaterally to the uterosacral ligaments only after the correct dissection plane is confirmed by visualization of the areolar tissue.
- Apply cervical traction using the tenaculum in a cephalad midline direction, and place traction on the cut edge of the posterior peritoneum using the bipolar forceps. The tenaculum wrist must be turned away from the working instruments to avoid internal clashing.
- Completely transect the right uterosacral ligament to better facilitate the creation of a contiguous peritoneal opening for burying the mesh. The remainder of the opening will be created later.
- While it is important to avoid split-thickness dissection, the vaginal plane must be "clean" (that is, without fat or adventitia) to allow for robust suturing.
- Dissection at least halfway down the posterior vaginal wall is recommended but proceeding down to the perineal body provides the most optimal support (FIGURE 6).
Sacral dissection
- Use a noncrushing instrument to laterally sweep the bowel to the left side, effectively "plastering" the peritoneum over the sacral promontory (FIGURE 7; VIDEO 5).
- Extend the superficial peritoneal incision down the right paracolic gutter halfway between the ureter and colon until it communicates with the incised posterior peritoneal edge created during the posterior dissection.
- Identify the middle sacral artery to avoid vascular injury, but there is no need to prophylactically coagulate it.
Vaginal mesh attachment
- Cut a lightweight Y-mesh to a length of 6 to 8 cm anteriorly and 8 to 11 cm posteriorly and place it into the surgical field (FIGURE 8; VIDEO 6). The length is determined based on the preoperative office examination and examination under anesthesia prior to starting the procedure.
- Attach the mesh securely and evenly to the anterior and posterior vaginal walls using multiple interrupted monofilament sutures. We aim to place sutures that provide mesh stability without excess vaginal wall incorporation to avoid "through-and-through" suturing.
- The posterior wall suturing is performed first, starting at the perineal body and continuing cephalad (VIDEO 7). We find it easiest to tie the knots between the mesh and the vagina in this space.
- Suture the crotch of the Y-mesh to the cervix so that no gap exists between tissue and mesh.
- For advanced-stage prolapse with significant anterior prolapse, the stretched out vaginal epithelium can be systematically gathered up to reconfigure the tissue to conform to the desired mesh dimensions (VIDEO 8). This tissue remodeling is evident even at the 2- to 4-week postoperative visit.
Peritoneal closure: Step 1
- Reapproximate the cut edges of peritoneum surrounding the vagina and cervix using a continuous purse-string suture of 0 Monocryl (poliglecaprone 25) on an SH needle (Ethicon, Somerville, New Jersey) with a fisherman's knot tied at the end (VIDEO 9). The needle passes are placed close together and close to the incised edge of the cut peritoneum.
- We typically start our peritoneal suture at the 5 o'clock position of the posterior peritoneum, extending in a clockwise direction and ultimately jumping anteriorly around the sacral arm of the mesh.
- Place the mesh within the paracolic peritoneal canal, and secure the needle for later use.
Sacral mesh attachment
- The mesh is tensioned so that a vaginal examination confirms adequate support of all the walls without excess tension or tissue banding. Some laxity of the anterior vaginal wall consistent with a mild cystocele is appropriate.
- Place 2 permanent PTFE sutures along the slope of the sacral promontory into the anterior longitudinal ligament (VIDEO 10). This avoids injury to the disc space that sits at the edge of the promontory. We do not advise the use of bone anchors as they increase the risk for discitis and osteomyelitis.
- Secure the mesh to the anterior longitudinal ligament without any tension. This is facilitated by creating mesh slack via cephalad pressure from a vaginal probe.
Peritoneal closure: Step 2
- Close the remaining paracolic peritoneal incision, completely burying the mesh within the created canal (FIGURE 9).
- At the end of the procedure, perform a repeat vaginal exam, rectal exam, and cystoscopy.
Technique with prior total hysterectomy
- In patients with a prior total hysterectomy, place a 13 x 3.5 cm Breisky vaginal retractor and/or coated nonconductive stent (Marina Medical, Sunrise, Florida) into the vagina to delineate the anterior and posterior walls at the vaginal apex during dissection.
- Some surgeons may opt to retrograde fill the bladder to better identify its location.
- We routinely leave a segment of peritoneum attached to the dome of the vaginal apex for added tissue integrity to prevent erosion.
Read about using transvaginal mesh for POP repair.
Transvaginal mesh: An effective, durable option for POP repair
Vincent R. Lucente, MD, MBA, and Jessica B. Ton, MD
Dr. Lucente reports that he has received grant or research support from Advanced Tactile Imaging, Boston Scientific, Coloplast, and Valencia; is a consultant to Coloplast; is a speaker for Allergan, Boston Scientific, Coloplast, and Shionogi; and serves as an expert witness for American Medical Systems and C.R. Bard. Dr. Ton reports no financial relationships relevant to this article.
As baseline health in the elderly population continues to improve, the number of women in the United States with symptomatic POP will increase by approximately 50% by 2050.39 Unfortunately, after native tissue repair (NTR) the rate of prolapse recurrence is extremely high: approximately 40% regardless of approach, as demonstrated in the OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial by Barber and colleagues.6 The authors of that clinical trial recently revealed that at the 5-year follow-up, these failure rates progressed to 70% for sacrospinous ligament fixation and 61% for uterosacral ligament suspension (data presented at the Society of Gynecologic Surgeons Annual Scientific Meeting 2018, Orlando, Florida). This establishes that NTR is not durable enough to meet the increasing physical demands of this age group and that mesh augmentation must be considered.
For patients at increased risk of prolapse recurrence, using transvaginal mesh (TVM) is the most minimally invasive approach and is an excellent option for mesh augmentation. Avoiding adverse events during placement of TVM depends largely on optimal surgical technique.40 (VIDEO: “Demonstration of an anterior vaginal wall dissection into the true vesicovaginal space”)
- Active advanced age requires a durable reconstructive pelvic surgery for pelvic organ prolapse, and native tissue repair does not meet that demand.
- Mesh augmentation reduces the risk of prolapse recurrence, and vaginal placement of mesh is the most minimally invasive approach.
- Rates of exposure with transvaginal mesh would be minimized with use of a full-thickness vaginal wall dissection.
- Optimal surgical technique could be highly reproducible with better surgical training.
The evidence on TVM versus NTR
Several studies have examined whether TVM has a measurable benefit over NTR.
A 2016 Cochrane review by Maher and colleagues included 37 randomized trials (4,023 women) that compared TVM and biologic grafts with NTR.41 Three primary outcomes were defined: awareness of prolapse, recurrence, and repeat surgery. Compared with women treated with NTR, those treated with synthetic nonabsorbable TVM exhibited a greater reduction in awareness of prolapse (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54-0.81), decreased recurrence in the anterior compartment (RR, 0.33; 95% CI, 0.26-0.40), and decreased reoperation for prolapse (RR, 0.53; 95% CI, 0.31-0.88). The overall calculated exposure rate was 12%, with a range of 3.2% to 20.8%.41 As we will discuss, this wide range most likely is attributed to a suboptimal, split-thickness dissection. There were no differences in other key secondary outcomes, including dyspareunia, operating time, and estimated blood loss.41
Longitudinal studies are emerging as almost 2 decades have passed since TVM was introduced. In a study of 5-year follow-up after TVM placement, Meyer and colleagues reported that patients had continued significant improvements in both subjective and objective outcomes.42 The mesh exposure rate was 6%, attributed to severe vaginal atrophy.42 A 10-year observational study by Weintraub and colleagues demonstrated a recurrence rate of only 2.6% in the anterior compartment, 7.6% in the posterior (nonaugmented) compartment, and no exposures or extrusions after anterior TVM placement.43
In contrast to the Cochrane review, in the 2017 multicenter PROSPECT (Prolapse surgery: Pragmatic evaluation and randomized controlled trials) trial, Glazener and colleagues found no difference in desired outcomes with TVM compared with NTR.44 There was an overall 6% to 7% exposure rate over 2 years.44 To reflect "real-world" practice, however, this study was intentionally designed without rigorous standardization of surgical technique. The authors reported that "appropriately experienced surgeons" performed the procedure, but it is unclear how experience was determined given that 20% of the cases were performed by "registrars," the equivalent of US residents or fellows.45
The PROSPECT study protocol described the TVM procedure as "a standard repair with a nonabsorbable mesh inlay to support the stitches," implying that there was no apical attachment of the mesh to the sacrospinous ligament.45 This is a suboptimal use of TVM because it does not address a detachment-type defect common in advanced prolapse. The PROSPECT study reinforces the need for better surgical training and standardization of the TVM procedure.44
How TVM compares with sacrocolpopexy
When comparing the use of TVM with sacrocolpopexy, our experience has been that TVM yields similar outcomes to sacrocolpopexy with additional benefits. We completed a 1-year retrospective cohort study comparing robot-assisted laparoscopic sacrocolpopexy (RALS) with TVM in a total of 86 patients, with both approaches performed by the same surgeon. Both treatment groups showed statistically significant improvements in nearly all functional and quality-of-life measures, including urinary symptoms, sexual function, and POP-Q scores.40 In particular, points Aa and Ba on the POP-Q score were significantly improved with TVM as compared to RALS. This suggests that TVM can achieve both lateral and apical support, where sacrocolpopexy addresses only the apex.40 This has clinical significance when considering DeLancey and colleagues' dynamic magnetic resonance imaging study, which demonstrated advanced prolapse results from both lateral and apical detachment.46 In addition, TVM placement also was considerably faster than RALS by approximately 96 minutes and could be performed using regional anesthesia. Only 1 mesh exposure in each study arm was reported.40
Finally, as with other vaginal procedures, patients who undergo TVM placement require minimal to no pain medication postoperatively and report faster return to daily activities. Almost none of our patients require narcotics, which is a significant benefit in the face of the ongoing national opioid crisis.
Gutman and colleagues compared laparoscopic mesh hysteropexy with TVM; they demonstrated comparable cure rates and, again, significantly longer operative times for the laparoscopic approach (174 vs 64 minutes; P<.0001).47 This multicenter study reported mesh exposure rates of 2.7% for laparoscopy and 6.6% for TVM,47 again likely due to a split-thickness dissection.
Safety of TVM depends on the surgeon factor
Because of the reported complications associated with TVM, in 2011 the US Food and Drug Administration (FDA) issued an update on the safety and efficacy of TVM augmentation and mandated postmarket studies.48 While we do not dispute that the mesh exposure rates were accurate at the time the FDA document was issued, we recognize that exposure has been erroneously attributed to inherent properties of the mesh.
Mesh exposure rates reported in the literature vary widely, ranging from 0% to 30%, even when surgeons used identical mesh products.49 This clearly establishes that the main contributing variable is surgical technique. It is critically important to recognize the "surgeon factor" as a confounder in trials that compare surgical procedures.50 Studies on TVM have shown that low-volume surgeons had significantly higher reoperation rates, while high-volume surgeons achieved a 41% reduction in reoperations.51,52 When TVM is performed by expert surgeons, the reported mesh exposure rates for TVM are noticeably lower.40,42,43,53,54
Decreasing mesh exposure rates would reduce the most common adverse event associated with TVM, thus improving its safety. The critical step to successful TVM placement is the initial dissection. Gynecologists traditionally have performed a split-thickness, colporrhaphy-style dissection to place the mesh within the layers of the vaginal wall.55 Placement within these planes, however, is too superficial and increases the risk of exposure. By contrast, by consistently performing a full-thickness vaginal wall dissection (FIGURE 10) and placing the mesh in the true vesicovaginal space,56 we have achieved a TVM exposure rate as low as 0% to 3%.40,54 If we can standardize the dissection component across our subspecialty, the rate of mesh exposure undoubtedly will decrease.
The PROSPECT investigators readily admitted what the study was not: a trial conducted "exclusively by the most experienced surgeons in the highest volume centres¬with a highly protocolised technique."44 In reality, that is the kind of rigorous study on TVM that our subspecialty demands. We must hold ourselves accountable and ensure that only the most qualified surgeons are placing TVM.
Keep the mesh option available
We support the position of the American Urogynecologic Society in opposing an outright ban of TVM because such a restriction would deny our patients access to an effective, durable, and minimally invasive approach for prolapse repair.57
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201-1206.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-506.
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016-2024.
- American College of Obstetricians and Gynecologists, American Urogynecologic Society. Practice Bulletin No. 185 Summary: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):1170-1172.
- American Urogynecologic Society Best Practice Statement: Evaluation and counseling of patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2017;23(5):281-287.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023-1034.
- Chung CP, Miskimins R, Kuehl TJ, Yandell PM, Shull BL. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23(2):223-227.
- Yazdany T, Yip S, Bhatia NN, Nguyen JN. Suture complications in a teaching institution among patients undergoing uterosacral ligament suspension with permanent braided suture. Int Urogynecol J. 2010;21(7):813-818.
- Toglia MR, Fagan MJ. Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol. 2008;198(5):600.e1-e4.
- Wong MJ, Rezvan A, Bhatia NN, Yazdany T. Uterosacral ligament vaginal vault suspension using delayed absorbable monofilament suture. Int Urogynecol J. 2011;22(11):1389-1394.
- Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- Kapoor S, Sivanesan K, Robertson JA, Veerasingham M, Kapoor V. Sacrospinous hysteropexy: review and meta-analysis of outcomes. Int Urogynecol J. 2017;28(9):1285-1294.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365-371.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
- Buchsbaum GM, Lee TG. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175-183.
- Zebede S, Smith AL, Plowright LN, Hegde A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol. 2013;121(2 pt 1):279-284.
- Bochenska K, Leader-Cramer A, Mueller M, Dave B, Alverdy A, Kenton K. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671-1675.
- Jones K, Wang G, Romano R, St Marie P, Harmanli O. Colpocleisis: a survey of current practice patterns. Female Pelvic Med Reconstr Surg. 2017;23(4):276-280.
- Jones KA, Zhuo Y, Solak S, Harmanli O. Hysterectomy at the time of colpocleisis: a decision analysis. Int Urogynecol J. 2016;27(5):805-810.
- Kandadai P, Flynn M, Zweizig S, Patterson D. Cost-utility of routine endometrial evaluation before le fort colpocleisis. Female Pelvic Med Reconstr Surg. 2014;20(3):168-173.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97(1):31-34.
- Oliphant SS, Shepherd JP, Lowder JL. Midurethral sling for treatment of occult stress urinary incontinence at the time of colpocleisis: a decision analysis. Female Pelvic Med Reconstr Surg. 2012;18(4):216-220.
- Siddiqui NY, Grimes CL, Casiano ER, et al; Society of Gynecologic Surgeons Systematic Review Group. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):44-55.
- Winters JC, Dmochowski RR, Goldman HB, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2464-2472.
- Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24(11):1815-1833.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376.
- Salamon CG, Lewis C, Priestley J, Gurshumov E, Culligan PJ. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371-1375.
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J. 2014;25(6):731-735.
- Eddib A, Danakas A, Hughes S, et al. Influence of morbid obesity on surgical outcomes in robotic-assisted gynecologic surgery. J Gynecol Surg. 2014;30(2):81-86.
- Gallo T, Kashani S, Patel DA, Elsahwi K, Silasi D-A, Azodi M. Robotic-assisted laparoscopic hysterectomy: outcomes in obese and morbidly obese patients. JSLS. 2012;16(3):421-427.
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303-318.
- Cundiff GW, Varner E, Visco AG, et al; Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1-e5.
- Wu JM, Wells EC, Hundley AF, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
- Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60-65.
- Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205-212.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol. 2013;121(1):143-151.
- Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137-143.
- Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
- ACOG Committee on Practice Bulletins-Gynecology, American Urogynecologic Society. ACOG Practice Bulletin No. 185: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):e234-e250.
- Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015;21(2):87-92.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database System Rev. 2016:CD012079.
- Meyer I, McGwin G, Swain T, Alvarez MD, Ellington DR, Richter HE. Synthetic graft augmentation in vaginal prolapse surgery: long-term objective and subjective outcomes. J Minim Invasive Gynecol. 2016;23(4):614-621.
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long‐term functional outcomes following mesh‐augmented posterior vaginal prolapse repair. Int J Gynecol Obstet. 2016;135(1):107-111.
- Glazener CM, Breeman S, Elders A, et al; PROSPECT Study Group. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381-392.
- Clinical and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomized controlled trials within Comprehensive Cohort Study. PROSPECT study protocol. The National Institute for Health Research. https://www.journalslibrary.nihr.ac.uk/programmes/hta/076018. Accessed January 17, 2018.
- Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural failure sites in anterior vaginal wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853-862.
- Gutman RE, Rardin CR, Sokol ER, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1-e11.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf. Published July 2011. Accessed January 9, 2017.
- Murphy M, Holzberg A, van Raalte H, et al; Pelvic Surgeons Network. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication: "Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse." Int Urogynecol J. 2012;23(1):5-9.
- Roman H, Marpeau L, Hulsey TC. Surgeons' experience and interaction effect in randomized controlled trials regarding new surgical procedures. Am J Obstet Gynecol. 2008;199(2):108.e1-e6.
- Eilber KS, Alperin M, Khan A, et al. The role of the surgeon on outcomes of vaginal prolapse surgery with mesh. Female Pelvic Med Reconstr Surg. 2017;23 (5):293-296.
- Kelly EC, Winick-Ng J, Welk B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet Gynecol. 2016;128(1):65-72.
- Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364(19):1826-1836.
- van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008:199(6):694.e1-e6.
- Iyer S, Botros SM. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J. 2017;28(4):527-535.
- Ting M, Gonzalez A, Ephraim S, Murphy M, Lucente V. The importance of a full thickness vaginal wall dissection. Comment on "Transvaginal mesh: a historical review and update of the current state of affairs in the United States." Int Urogynecol J. 2017;28(10):1609-1610.
- American Urogynecologic Society. Position statement on restriction of surgical options for pelvic floor disorders. https://www.augs.org/assets/1/6/Position_Statement_Surgical_Options_for_PFDs.pdf. Published March 26, 2013. Accessed January 9, 2017.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201-1206.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-506.
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016-2024.
- American College of Obstetricians and Gynecologists, American Urogynecologic Society. Practice Bulletin No. 185 Summary: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):1170-1172.
- American Urogynecologic Society Best Practice Statement: Evaluation and counseling of patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2017;23(5):281-287.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023-1034.
- Chung CP, Miskimins R, Kuehl TJ, Yandell PM, Shull BL. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23(2):223-227.
- Yazdany T, Yip S, Bhatia NN, Nguyen JN. Suture complications in a teaching institution among patients undergoing uterosacral ligament suspension with permanent braided suture. Int Urogynecol J. 2010;21(7):813-818.
- Toglia MR, Fagan MJ. Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol. 2008;198(5):600.e1-e4.
- Wong MJ, Rezvan A, Bhatia NN, Yazdany T. Uterosacral ligament vaginal vault suspension using delayed absorbable monofilament suture. Int Urogynecol J. 2011;22(11):1389-1394.
- Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- Kapoor S, Sivanesan K, Robertson JA, Veerasingham M, Kapoor V. Sacrospinous hysteropexy: review and meta-analysis of outcomes. Int Urogynecol J. 2017;28(9):1285-1294.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365-371.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
- Buchsbaum GM, Lee TG. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175-183.
- Zebede S, Smith AL, Plowright LN, Hegde A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol. 2013;121(2 pt 1):279-284.
- Bochenska K, Leader-Cramer A, Mueller M, Dave B, Alverdy A, Kenton K. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671-1675.
- Jones K, Wang G, Romano R, St Marie P, Harmanli O. Colpocleisis: a survey of current practice patterns. Female Pelvic Med Reconstr Surg. 2017;23(4):276-280.
- Jones KA, Zhuo Y, Solak S, Harmanli O. Hysterectomy at the time of colpocleisis: a decision analysis. Int Urogynecol J. 2016;27(5):805-810.
- Kandadai P, Flynn M, Zweizig S, Patterson D. Cost-utility of routine endometrial evaluation before le fort colpocleisis. Female Pelvic Med Reconstr Surg. 2014;20(3):168-173.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97(1):31-34.
- Oliphant SS, Shepherd JP, Lowder JL. Midurethral sling for treatment of occult stress urinary incontinence at the time of colpocleisis: a decision analysis. Female Pelvic Med Reconstr Surg. 2012;18(4):216-220.
- Siddiqui NY, Grimes CL, Casiano ER, et al; Society of Gynecologic Surgeons Systematic Review Group. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):44-55.
- Winters JC, Dmochowski RR, Goldman HB, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2464-2472.
- Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24(11):1815-1833.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376.
- Salamon CG, Lewis C, Priestley J, Gurshumov E, Culligan PJ. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371-1375.
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J. 2014;25(6):731-735.
- Eddib A, Danakas A, Hughes S, et al. Influence of morbid obesity on surgical outcomes in robotic-assisted gynecologic surgery. J Gynecol Surg. 2014;30(2):81-86.
- Gallo T, Kashani S, Patel DA, Elsahwi K, Silasi D-A, Azodi M. Robotic-assisted laparoscopic hysterectomy: outcomes in obese and morbidly obese patients. JSLS. 2012;16(3):421-427.
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303-318.
- Cundiff GW, Varner E, Visco AG, et al; Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1-e5.
- Wu JM, Wells EC, Hundley AF, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
- Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60-65.
- Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205-212.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol. 2013;121(1):143-151.
- Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137-143.
- Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
- ACOG Committee on Practice Bulletins-Gynecology, American Urogynecologic Society. ACOG Practice Bulletin No. 185: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):e234-e250.
- Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015;21(2):87-92.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database System Rev. 2016:CD012079.
- Meyer I, McGwin G, Swain T, Alvarez MD, Ellington DR, Richter HE. Synthetic graft augmentation in vaginal prolapse surgery: long-term objective and subjective outcomes. J Minim Invasive Gynecol. 2016;23(4):614-621.
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long‐term functional outcomes following mesh‐augmented posterior vaginal prolapse repair. Int J Gynecol Obstet. 2016;135(1):107-111.
- Glazener CM, Breeman S, Elders A, et al; PROSPECT Study Group. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381-392.
- Clinical and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomized controlled trials within Comprehensive Cohort Study. PROSPECT study protocol. The National Institute for Health Research. https://www.journalslibrary.nihr.ac.uk/programmes/hta/076018. Accessed January 17, 2018.
- Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural failure sites in anterior vaginal wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853-862.
- Gutman RE, Rardin CR, Sokol ER, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1-e11.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf. Published July 2011. Accessed January 9, 2017.
- Murphy M, Holzberg A, van Raalte H, et al; Pelvic Surgeons Network. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication: "Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse." Int Urogynecol J. 2012;23(1):5-9.
- Roman H, Marpeau L, Hulsey TC. Surgeons' experience and interaction effect in randomized controlled trials regarding new surgical procedures. Am J Obstet Gynecol. 2008;199(2):108.e1-e6.
- Eilber KS, Alperin M, Khan A, et al. The role of the surgeon on outcomes of vaginal prolapse surgery with mesh. Female Pelvic Med Reconstr Surg. 2017;23 (5):293-296.
- Kelly EC, Winick-Ng J, Welk B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet Gynecol. 2016;128(1):65-72.
- Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364(19):1826-1836.
- van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008:199(6):694.e1-e6.
- Iyer S, Botros SM. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J. 2017;28(4):527-535.
- Ting M, Gonzalez A, Ephraim S, Murphy M, Lucente V. The importance of a full thickness vaginal wall dissection. Comment on "Transvaginal mesh: a historical review and update of the current state of affairs in the United States." Int Urogynecol J. 2017;28(10):1609-1610.
- American Urogynecologic Society. Position statement on restriction of surgical options for pelvic floor disorders. https://www.augs.org/assets/1/6/Position_Statement_Surgical_Options_for_PFDs.pdf. Published March 26, 2013. Accessed January 9, 2017.
Teach your adolescent patients about normal menses, so they know when it’s abnormal
CHICAGO – , according to S. Paige Hertweck, MD, chief of gynecology at Norton Children’s Hospital in Louisville, Ky.
“Remember to use the menstrual cycle as a vital sign,” Dr. Hertweck told attendees at the American Academy of Pediatrics annual meeting. “Even within the first year of menarche, most girls have a period at least every 90 days, so work up those who don’t.”
The median age of menarche is 12.4 years, typically beginning within 2-3 years of breast budding at Tanner Stage 4 breast development, she said. By 15 years of age, 98% of girls have begun menstruation.
Girls’ cycles typically last 21-45 days, an average of 32.2 days during their first year of menstruation, with flow for 7 days or less, requiring an average of 3-6 pads and/or tampons per day. Dr. Hertweck recommends you write down these features of normal menstruation so that your patients can tell you when their cycle is abnormal or menses doesn’t return.
“Cycle length is more variable for teens versus women 20-40 years old,” she said. However, “it’s not true that ‘anything goes’ for cycle length” in teens, she added. “Cycles that are consistently outside the range of 21-45 days are statistically uncommon.” Hence the need to evaluate causes of amenorrhea in girls whose cycles exceed 90 days.
Possible causes of amenorrhea include pregnancy, polycystic ovary syndrome, thyroid abnormalities, hyperprolactinemia, primary ovarian insufficiency, or hypogonadal amenorrhea, typically stimulated by the first instance of anorexia, Crohn’s disease, celiac disease, or a gluten intolerance.
Primary amenorrhea
Dr. Hertweck listed five benchmarks that indicate primary amenorrhea requiring evaluation. Those indicators include girls who have no menarche by age 15 years or within 3 years of breast budding, no breast development by age 13 years, or no menses by age 14 years with hirsutism or with a history of excessive exercise or of an eating disorder.
You can start by examining what normal menstruation relies on: an intact central nervous system with a functioning pituitary, an ovarian response, and a normal uterus, cervix, and vagina. You should check the patient’s follicle-stimulating hormone, thyroid-stimulating hormone, and prolactin levels to assess CNS functioning, and estradiol levels to assess ovarian response. A genital exam with a pelvic ultrasound can reveal any possible defects in the uterus, cervix, or vagina.
The presence of breasts without a uterus indicates normal estrogen production, so the missing uterus could be a congenital defect or result from androgen insensitivity, Dr. Hertweck explained. In those without breasts, gonadal dysgenesis or gonadal enzymatic deficiency may explain no estrogen production. If the patient has both breasts and a uterus, you should rule out pregnancy first and then track CNS changes via FSH, TSH, and prolactin levels.
Premature ovarian insufficiency
Approximately 1% of females experience premature ovarian insufficiency, which can be diagnosed as early as age 14 years and should be suspected in a patient with a uterus but without breasts who has low estradiol levels, CNS failure identified by a high FSH level, and gonadal failure.
Formal diagnosis requires two separate instances of FSH elevation, and chromosomal testing should be done to rule out gonadal dysgenesis. You also should test the serum anti-Müllerian hormone biomarker (readings above 8 are concerning) and look for two possible causes. The FMR1 (Fragile X) premutation carrier status could be a cause, or presence of 21-hydroxylase and/or adrenal antibodies indicate autoimmune polyglandular syndrome.
Catching premature ovarian insufficiency early enough may allow patients to preserve some fertility if they still have oocytes present. Aside from this, girls will need hormone replacement therapy to fulfill developmental emotional and physical needs, such as bone growth and overall health. Despite a history of treating teens with premature ovarian insufficiency like adults, you should follow the practice guidelines specific to adolescents by the American College of Obstetricians and Gynecologists committee opinion statement (Obstet Gynecol. 2014;123:193-7).
Menorrhagia: heavy menstrual bleeding
Even though average blood loss is estimated at 30 mL per period, that number means little in clinical practice because patients cannot measure the actual amount of menses. Better indicators of abnormally greater flow include flow lasting longer than 7 days, finding clots larger than a quarter, changing menstrual products every 1-2 hours, leaking onto clothing such that patients need to take extra clothes to school, and any heavy periods that occur with easy bruising or with a family history of bleeding disorders.
First-line treatment for heavy menstrual bleeding in teens is hormonal contraception, either combination oral contraceptive pills, the transdermal patch, or the intravaginal ring, which can be combined with other therapies.
An alternative for those under age 18 (per Food and Drug Administration labeling) is oral tranexamic acid, found in a crossover trial with an oral contraceptive pill to be just as effective at reducing average blood loss and improving quality of life, but with fewer side effects and better compliance. Before prescribing anything for heavy menstrual bleeding, however, you must consider possible causes and rule some out that require different management.
Aside from pregnancy, one potential cause of menorrhagia is infection such as chlamydia or gonorrhea, which should be considered even in those with a negative sexual history, Dr. Hertweck said. Other possible causes include an immature hypothalamic-pituitary-ovarian axis, polycystic ovary syndrome (even with low hemoglobin), malignancy with a hormone-producing tumor, hypothalamic dysfunction (often stimulated by eating disorders, obesity, rapid weight loss, or gluten intolerance), or coagulopathy.
“Teens with menorrhagia may need to be screened for a bleeding disorder,” Dr. Hertweck said. At a minimum, she recommends checking complete blood count, ferritin, and TSH. “The most common bleeding disorders associated with heavy menstrual bleeding include platelet function disorders and von Willebrand.”
Up to half of teen girls with menorrhagia who visit a hematologist or multidisciplinary clinic receive a diagnosis of a bleeding disorder, Dr. Hertweck said. And up to half of those with menorrhagia at menarche may have von Willebrand, as do one in six adolescents who go to the emergency department because of heavy menstrual bleeding.
Von Willebrand syndrome
Von Willebrand syndrome is a deficiency or dysfunction of von Willebrand factor (vWF), a protein with binding sites for platelets, collagen, and factor VIII that “serves as a bridge between platelets and injury sites in vessel walls” and “protects factor VIII from rapid proteolytic degradation,” Dr. Hertweck said. Von Willebrand syndrome is the most common inherited congenital bleeding disorder. Although acquired von Willebrand syndrome is rare, it has grown in incidence among those with complex cardiovascular, hematologic, or immunologic disorders.
“Correct diagnosis is complex and not always straightforward,” Dr. Hertweck said, but “a positive response to questions in four categories is highly sensitive.” They are as follows:
• Menses lasting at least 7 days and interfering with a person’s daily activities.
• “History of treatment for anemia.
• Family history of a diagnosed bleeding disorder.
• History of excessive bleeding after tooth extraction, delivery, miscarriage, or surgery.
Diagnostic assays include platelet concentration of vWF antigen, an activity test of vWF-platelet binding, and factor VIII activity. However, you often need to repeat diagnostic testing because vWF antigens vary according to race, blood type, age, acute phase response, and menstrual cycle timing, Dr. Hertweck said.
“Remember to draw von Willebrand testing only during the first 3 days of the menstrual cycle when estrogen levels are at the nadir,” she said.
Because estrogen increases vWF, treatment for von Willebrand syndrome should be progestin only, either oral pills, medroxyprogesterone acetate (MPA, or Depo-Provera injections), or an etonogestrel implant.
Dr. Hertweck presented several cases of abnormal menstruation and extreme conditions such as severe menorrhagia. Outside of von Willebrand in such patients, possible platelet disorders could include Glanzmann thrombasthenia (a platelet function disorder that is caused by an abnormality in the genes for glycoproteins IIb/IIIa) and platelet storage pool disorder, both of which should be diagnosed by a hematologist.
Dr. Hertweck reported having a research grant from Merck related to contraceptive implants in adolescents.
CHICAGO – , according to S. Paige Hertweck, MD, chief of gynecology at Norton Children’s Hospital in Louisville, Ky.
“Remember to use the menstrual cycle as a vital sign,” Dr. Hertweck told attendees at the American Academy of Pediatrics annual meeting. “Even within the first year of menarche, most girls have a period at least every 90 days, so work up those who don’t.”
The median age of menarche is 12.4 years, typically beginning within 2-3 years of breast budding at Tanner Stage 4 breast development, she said. By 15 years of age, 98% of girls have begun menstruation.
Girls’ cycles typically last 21-45 days, an average of 32.2 days during their first year of menstruation, with flow for 7 days or less, requiring an average of 3-6 pads and/or tampons per day. Dr. Hertweck recommends you write down these features of normal menstruation so that your patients can tell you when their cycle is abnormal or menses doesn’t return.
“Cycle length is more variable for teens versus women 20-40 years old,” she said. However, “it’s not true that ‘anything goes’ for cycle length” in teens, she added. “Cycles that are consistently outside the range of 21-45 days are statistically uncommon.” Hence the need to evaluate causes of amenorrhea in girls whose cycles exceed 90 days.
Possible causes of amenorrhea include pregnancy, polycystic ovary syndrome, thyroid abnormalities, hyperprolactinemia, primary ovarian insufficiency, or hypogonadal amenorrhea, typically stimulated by the first instance of anorexia, Crohn’s disease, celiac disease, or a gluten intolerance.
Primary amenorrhea
Dr. Hertweck listed five benchmarks that indicate primary amenorrhea requiring evaluation. Those indicators include girls who have no menarche by age 15 years or within 3 years of breast budding, no breast development by age 13 years, or no menses by age 14 years with hirsutism or with a history of excessive exercise or of an eating disorder.
You can start by examining what normal menstruation relies on: an intact central nervous system with a functioning pituitary, an ovarian response, and a normal uterus, cervix, and vagina. You should check the patient’s follicle-stimulating hormone, thyroid-stimulating hormone, and prolactin levels to assess CNS functioning, and estradiol levels to assess ovarian response. A genital exam with a pelvic ultrasound can reveal any possible defects in the uterus, cervix, or vagina.
The presence of breasts without a uterus indicates normal estrogen production, so the missing uterus could be a congenital defect or result from androgen insensitivity, Dr. Hertweck explained. In those without breasts, gonadal dysgenesis or gonadal enzymatic deficiency may explain no estrogen production. If the patient has both breasts and a uterus, you should rule out pregnancy first and then track CNS changes via FSH, TSH, and prolactin levels.
Premature ovarian insufficiency
Approximately 1% of females experience premature ovarian insufficiency, which can be diagnosed as early as age 14 years and should be suspected in a patient with a uterus but without breasts who has low estradiol levels, CNS failure identified by a high FSH level, and gonadal failure.
Formal diagnosis requires two separate instances of FSH elevation, and chromosomal testing should be done to rule out gonadal dysgenesis. You also should test the serum anti-Müllerian hormone biomarker (readings above 8 are concerning) and look for two possible causes. The FMR1 (Fragile X) premutation carrier status could be a cause, or presence of 21-hydroxylase and/or adrenal antibodies indicate autoimmune polyglandular syndrome.
Catching premature ovarian insufficiency early enough may allow patients to preserve some fertility if they still have oocytes present. Aside from this, girls will need hormone replacement therapy to fulfill developmental emotional and physical needs, such as bone growth and overall health. Despite a history of treating teens with premature ovarian insufficiency like adults, you should follow the practice guidelines specific to adolescents by the American College of Obstetricians and Gynecologists committee opinion statement (Obstet Gynecol. 2014;123:193-7).
Menorrhagia: heavy menstrual bleeding
Even though average blood loss is estimated at 30 mL per period, that number means little in clinical practice because patients cannot measure the actual amount of menses. Better indicators of abnormally greater flow include flow lasting longer than 7 days, finding clots larger than a quarter, changing menstrual products every 1-2 hours, leaking onto clothing such that patients need to take extra clothes to school, and any heavy periods that occur with easy bruising or with a family history of bleeding disorders.
First-line treatment for heavy menstrual bleeding in teens is hormonal contraception, either combination oral contraceptive pills, the transdermal patch, or the intravaginal ring, which can be combined with other therapies.
An alternative for those under age 18 (per Food and Drug Administration labeling) is oral tranexamic acid, found in a crossover trial with an oral contraceptive pill to be just as effective at reducing average blood loss and improving quality of life, but with fewer side effects and better compliance. Before prescribing anything for heavy menstrual bleeding, however, you must consider possible causes and rule some out that require different management.
Aside from pregnancy, one potential cause of menorrhagia is infection such as chlamydia or gonorrhea, which should be considered even in those with a negative sexual history, Dr. Hertweck said. Other possible causes include an immature hypothalamic-pituitary-ovarian axis, polycystic ovary syndrome (even with low hemoglobin), malignancy with a hormone-producing tumor, hypothalamic dysfunction (often stimulated by eating disorders, obesity, rapid weight loss, or gluten intolerance), or coagulopathy.
“Teens with menorrhagia may need to be screened for a bleeding disorder,” Dr. Hertweck said. At a minimum, she recommends checking complete blood count, ferritin, and TSH. “The most common bleeding disorders associated with heavy menstrual bleeding include platelet function disorders and von Willebrand.”
Up to half of teen girls with menorrhagia who visit a hematologist or multidisciplinary clinic receive a diagnosis of a bleeding disorder, Dr. Hertweck said. And up to half of those with menorrhagia at menarche may have von Willebrand, as do one in six adolescents who go to the emergency department because of heavy menstrual bleeding.
Von Willebrand syndrome
Von Willebrand syndrome is a deficiency or dysfunction of von Willebrand factor (vWF), a protein with binding sites for platelets, collagen, and factor VIII that “serves as a bridge between platelets and injury sites in vessel walls” and “protects factor VIII from rapid proteolytic degradation,” Dr. Hertweck said. Von Willebrand syndrome is the most common inherited congenital bleeding disorder. Although acquired von Willebrand syndrome is rare, it has grown in incidence among those with complex cardiovascular, hematologic, or immunologic disorders.
“Correct diagnosis is complex and not always straightforward,” Dr. Hertweck said, but “a positive response to questions in four categories is highly sensitive.” They are as follows:
• Menses lasting at least 7 days and interfering with a person’s daily activities.
• “History of treatment for anemia.
• Family history of a diagnosed bleeding disorder.
• History of excessive bleeding after tooth extraction, delivery, miscarriage, or surgery.
Diagnostic assays include platelet concentration of vWF antigen, an activity test of vWF-platelet binding, and factor VIII activity. However, you often need to repeat diagnostic testing because vWF antigens vary according to race, blood type, age, acute phase response, and menstrual cycle timing, Dr. Hertweck said.
“Remember to draw von Willebrand testing only during the first 3 days of the menstrual cycle when estrogen levels are at the nadir,” she said.
Because estrogen increases vWF, treatment for von Willebrand syndrome should be progestin only, either oral pills, medroxyprogesterone acetate (MPA, or Depo-Provera injections), or an etonogestrel implant.
Dr. Hertweck presented several cases of abnormal menstruation and extreme conditions such as severe menorrhagia. Outside of von Willebrand in such patients, possible platelet disorders could include Glanzmann thrombasthenia (a platelet function disorder that is caused by an abnormality in the genes for glycoproteins IIb/IIIa) and platelet storage pool disorder, both of which should be diagnosed by a hematologist.
Dr. Hertweck reported having a research grant from Merck related to contraceptive implants in adolescents.
CHICAGO – , according to S. Paige Hertweck, MD, chief of gynecology at Norton Children’s Hospital in Louisville, Ky.
“Remember to use the menstrual cycle as a vital sign,” Dr. Hertweck told attendees at the American Academy of Pediatrics annual meeting. “Even within the first year of menarche, most girls have a period at least every 90 days, so work up those who don’t.”
The median age of menarche is 12.4 years, typically beginning within 2-3 years of breast budding at Tanner Stage 4 breast development, she said. By 15 years of age, 98% of girls have begun menstruation.
Girls’ cycles typically last 21-45 days, an average of 32.2 days during their first year of menstruation, with flow for 7 days or less, requiring an average of 3-6 pads and/or tampons per day. Dr. Hertweck recommends you write down these features of normal menstruation so that your patients can tell you when their cycle is abnormal or menses doesn’t return.
“Cycle length is more variable for teens versus women 20-40 years old,” she said. However, “it’s not true that ‘anything goes’ for cycle length” in teens, she added. “Cycles that are consistently outside the range of 21-45 days are statistically uncommon.” Hence the need to evaluate causes of amenorrhea in girls whose cycles exceed 90 days.
Possible causes of amenorrhea include pregnancy, polycystic ovary syndrome, thyroid abnormalities, hyperprolactinemia, primary ovarian insufficiency, or hypogonadal amenorrhea, typically stimulated by the first instance of anorexia, Crohn’s disease, celiac disease, or a gluten intolerance.
Primary amenorrhea
Dr. Hertweck listed five benchmarks that indicate primary amenorrhea requiring evaluation. Those indicators include girls who have no menarche by age 15 years or within 3 years of breast budding, no breast development by age 13 years, or no menses by age 14 years with hirsutism or with a history of excessive exercise or of an eating disorder.
You can start by examining what normal menstruation relies on: an intact central nervous system with a functioning pituitary, an ovarian response, and a normal uterus, cervix, and vagina. You should check the patient’s follicle-stimulating hormone, thyroid-stimulating hormone, and prolactin levels to assess CNS functioning, and estradiol levels to assess ovarian response. A genital exam with a pelvic ultrasound can reveal any possible defects in the uterus, cervix, or vagina.
The presence of breasts without a uterus indicates normal estrogen production, so the missing uterus could be a congenital defect or result from androgen insensitivity, Dr. Hertweck explained. In those without breasts, gonadal dysgenesis or gonadal enzymatic deficiency may explain no estrogen production. If the patient has both breasts and a uterus, you should rule out pregnancy first and then track CNS changes via FSH, TSH, and prolactin levels.
Premature ovarian insufficiency
Approximately 1% of females experience premature ovarian insufficiency, which can be diagnosed as early as age 14 years and should be suspected in a patient with a uterus but without breasts who has low estradiol levels, CNS failure identified by a high FSH level, and gonadal failure.
Formal diagnosis requires two separate instances of FSH elevation, and chromosomal testing should be done to rule out gonadal dysgenesis. You also should test the serum anti-Müllerian hormone biomarker (readings above 8 are concerning) and look for two possible causes. The FMR1 (Fragile X) premutation carrier status could be a cause, or presence of 21-hydroxylase and/or adrenal antibodies indicate autoimmune polyglandular syndrome.
Catching premature ovarian insufficiency early enough may allow patients to preserve some fertility if they still have oocytes present. Aside from this, girls will need hormone replacement therapy to fulfill developmental emotional and physical needs, such as bone growth and overall health. Despite a history of treating teens with premature ovarian insufficiency like adults, you should follow the practice guidelines specific to adolescents by the American College of Obstetricians and Gynecologists committee opinion statement (Obstet Gynecol. 2014;123:193-7).
Menorrhagia: heavy menstrual bleeding
Even though average blood loss is estimated at 30 mL per period, that number means little in clinical practice because patients cannot measure the actual amount of menses. Better indicators of abnormally greater flow include flow lasting longer than 7 days, finding clots larger than a quarter, changing menstrual products every 1-2 hours, leaking onto clothing such that patients need to take extra clothes to school, and any heavy periods that occur with easy bruising or with a family history of bleeding disorders.
First-line treatment for heavy menstrual bleeding in teens is hormonal contraception, either combination oral contraceptive pills, the transdermal patch, or the intravaginal ring, which can be combined with other therapies.
An alternative for those under age 18 (per Food and Drug Administration labeling) is oral tranexamic acid, found in a crossover trial with an oral contraceptive pill to be just as effective at reducing average blood loss and improving quality of life, but with fewer side effects and better compliance. Before prescribing anything for heavy menstrual bleeding, however, you must consider possible causes and rule some out that require different management.
Aside from pregnancy, one potential cause of menorrhagia is infection such as chlamydia or gonorrhea, which should be considered even in those with a negative sexual history, Dr. Hertweck said. Other possible causes include an immature hypothalamic-pituitary-ovarian axis, polycystic ovary syndrome (even with low hemoglobin), malignancy with a hormone-producing tumor, hypothalamic dysfunction (often stimulated by eating disorders, obesity, rapid weight loss, or gluten intolerance), or coagulopathy.
“Teens with menorrhagia may need to be screened for a bleeding disorder,” Dr. Hertweck said. At a minimum, she recommends checking complete blood count, ferritin, and TSH. “The most common bleeding disorders associated with heavy menstrual bleeding include platelet function disorders and von Willebrand.”
Up to half of teen girls with menorrhagia who visit a hematologist or multidisciplinary clinic receive a diagnosis of a bleeding disorder, Dr. Hertweck said. And up to half of those with menorrhagia at menarche may have von Willebrand, as do one in six adolescents who go to the emergency department because of heavy menstrual bleeding.
Von Willebrand syndrome
Von Willebrand syndrome is a deficiency or dysfunction of von Willebrand factor (vWF), a protein with binding sites for platelets, collagen, and factor VIII that “serves as a bridge between platelets and injury sites in vessel walls” and “protects factor VIII from rapid proteolytic degradation,” Dr. Hertweck said. Von Willebrand syndrome is the most common inherited congenital bleeding disorder. Although acquired von Willebrand syndrome is rare, it has grown in incidence among those with complex cardiovascular, hematologic, or immunologic disorders.
“Correct diagnosis is complex and not always straightforward,” Dr. Hertweck said, but “a positive response to questions in four categories is highly sensitive.” They are as follows:
• Menses lasting at least 7 days and interfering with a person’s daily activities.
• “History of treatment for anemia.
• Family history of a diagnosed bleeding disorder.
• History of excessive bleeding after tooth extraction, delivery, miscarriage, or surgery.
Diagnostic assays include platelet concentration of vWF antigen, an activity test of vWF-platelet binding, and factor VIII activity. However, you often need to repeat diagnostic testing because vWF antigens vary according to race, blood type, age, acute phase response, and menstrual cycle timing, Dr. Hertweck said.
“Remember to draw von Willebrand testing only during the first 3 days of the menstrual cycle when estrogen levels are at the nadir,” she said.
Because estrogen increases vWF, treatment for von Willebrand syndrome should be progestin only, either oral pills, medroxyprogesterone acetate (MPA, or Depo-Provera injections), or an etonogestrel implant.
Dr. Hertweck presented several cases of abnormal menstruation and extreme conditions such as severe menorrhagia. Outside of von Willebrand in such patients, possible platelet disorders could include Glanzmann thrombasthenia (a platelet function disorder that is caused by an abnormality in the genes for glycoproteins IIb/IIIa) and platelet storage pool disorder, both of which should be diagnosed by a hematologist.
Dr. Hertweck reported having a research grant from Merck related to contraceptive implants in adolescents.
EXPERT ANALYSIS FROM AAP 2017
2017 Update on female sexual dysfunction
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Fast Tracks
- Although not fully understood how, estradiol can improve sexual desire, progesterone tends to dampen sexual desire, and testosterone has a neutral effect in premenopausal women
- Newly available since July 2017, prasterone is a once-daily intravaginal agent that treats moderate to severe dyspareunia and has costs similar to topical estrogens
- Estrogen therapy may be considered in a breast cancer mutation carrier who has undergone prophylactic mastectomies and bilateral salpingo-oophorectomy
2016 Update on pelvic floor dysfunction
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6
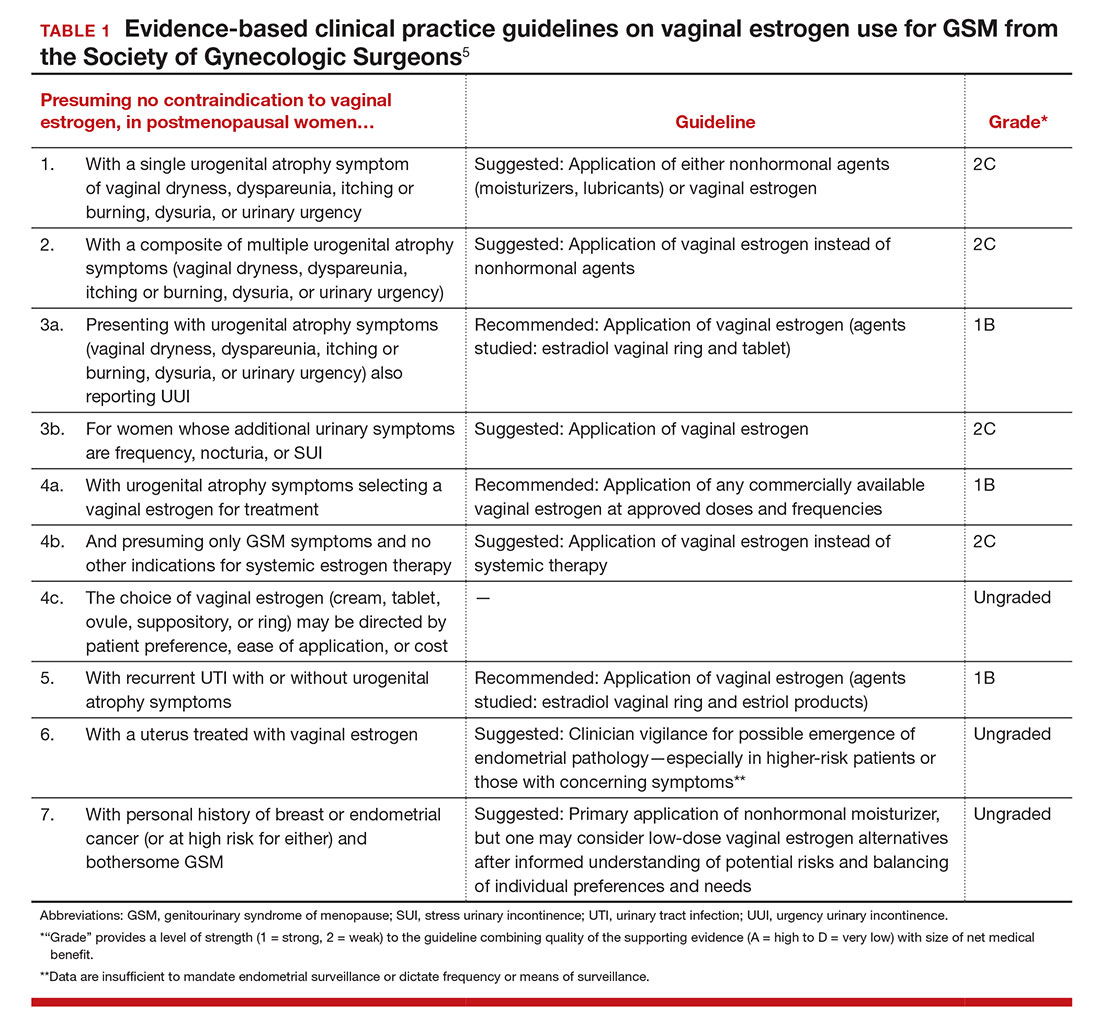

Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
In this Article
- Low-dose estrogen vaginal cream
- Ospemifene therapy
- Fractional CO2 laser treatment
2016 Update on female sexual dysfunction
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.
Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
In This Article
- This roundtable's expert panel
- Dyspareunia and low sexual desire in a breast cancer survivor
- Laser treatment and vaginal health
How do you break the ice with patients to ask about their sexual health?
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
In this Article
- Conversation icebreakers
- The sexual status examination
- When to refer