User login
FDA Approves First Engineered Cell Therapy for a Solid Tumor
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
The Last 30 Days: How Oncologists’ Choices Affect End-of-Life Cancer Care
TOPLINE:
Patients treated by oncologists in the top quartile for end-of-life prescribing behavior were almost four and a half times more likely to receive end-of-life therapy than those treated by these specialists in the bottom quartile.
METHODOLOGY:
- Researchers analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, focusing on patients who died of cancer between 2012 and 2017.
- A total of 17,609 patients with breast, lung, colorectal, or prostate cancer were included, treated by 960 oncologists across 388 practices.
- Patients were required to have had at least one systemic cancer therapy claim in the last 180 days of life, with the treating oncologist identified on the basis of the therapy claim closest to the time of death.
- The study used multilevel models to estimate oncologists’ rates of providing cancer therapy in the last 30 days of life, adjusting for patient characteristics and practice variation.
- Functional status was assessed on the basis of paid claims for durable medical equipment in the last 60 months of life, with scores categorized as 0, 1, ≥ 2, or unknown.
TAKEAWAY:
- Oncologists in the 95th percentile for high end-of-life prescribing behavior had a 45% adjusted rate of treating patients in the last 30 days of life, compared with 17% among those in the 5th percentile.
- Patients treated by high end-of-life prescribing oncologists had over four times higher odds of receiving systemic therapy in the last 30 days of life (odds ratio [OR], 4.42; 95% CI, 4.00-4.89).
- Higher end-of-life prescribing oncologists also had a higher proportion of patients hospitalized in the last 30 days of life than low prescribers (58% vs 51.9%).
- No significant association was found between oncologist prescribing behavior and patient race or ethnicity, except for Black patients who had lower odds of receiving treatment (OR, 0.77; P < .001).
IN PRACTICE:
“Given calls to rein in overutilization of end-of-life six to eight cancer therapies, our findings highlight an underappreciated area for further research: How treatment discontinuation before death is shaped by oncologists’ unique treatment propensities. Elucidating the reasons for this remarkable variability in oncologist treatment behavior could inform efforts to reduce end-of-life cancer treatment overutilization,” wrote the authors of the study.
SOURCE:
The study was led by Login S. George, PhD, Institute for Health, Health Care Policy and Aging Research, Rutgers University in New Brunswick, New Jersey. It was published online in Cancer.
LIMITATIONS:
The study’s reliance on SEER-Medicare data may limit the generalizability of the findings to patients with Medicare Advantage, private insurance, or Medicaid, as well as younger patients. The lack of data on patient preferences and other health characteristics could confound the results. The study focused on systemic therapies and may not be generalizable to other treatments such as clinical trial drugs, oral therapies, surgery, or radiation. The data from 2012 to 2017 may not reflect more recent trends in cancer treatment.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute and the Rutgers Cancer Institute of New Jersey. George disclosed receiving grants from these organizations. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients treated by oncologists in the top quartile for end-of-life prescribing behavior were almost four and a half times more likely to receive end-of-life therapy than those treated by these specialists in the bottom quartile.
METHODOLOGY:
- Researchers analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, focusing on patients who died of cancer between 2012 and 2017.
- A total of 17,609 patients with breast, lung, colorectal, or prostate cancer were included, treated by 960 oncologists across 388 practices.
- Patients were required to have had at least one systemic cancer therapy claim in the last 180 days of life, with the treating oncologist identified on the basis of the therapy claim closest to the time of death.
- The study used multilevel models to estimate oncologists’ rates of providing cancer therapy in the last 30 days of life, adjusting for patient characteristics and practice variation.
- Functional status was assessed on the basis of paid claims for durable medical equipment in the last 60 months of life, with scores categorized as 0, 1, ≥ 2, or unknown.
TAKEAWAY:
- Oncologists in the 95th percentile for high end-of-life prescribing behavior had a 45% adjusted rate of treating patients in the last 30 days of life, compared with 17% among those in the 5th percentile.
- Patients treated by high end-of-life prescribing oncologists had over four times higher odds of receiving systemic therapy in the last 30 days of life (odds ratio [OR], 4.42; 95% CI, 4.00-4.89).
- Higher end-of-life prescribing oncologists also had a higher proportion of patients hospitalized in the last 30 days of life than low prescribers (58% vs 51.9%).
- No significant association was found between oncologist prescribing behavior and patient race or ethnicity, except for Black patients who had lower odds of receiving treatment (OR, 0.77; P < .001).
IN PRACTICE:
“Given calls to rein in overutilization of end-of-life six to eight cancer therapies, our findings highlight an underappreciated area for further research: How treatment discontinuation before death is shaped by oncologists’ unique treatment propensities. Elucidating the reasons for this remarkable variability in oncologist treatment behavior could inform efforts to reduce end-of-life cancer treatment overutilization,” wrote the authors of the study.
SOURCE:
The study was led by Login S. George, PhD, Institute for Health, Health Care Policy and Aging Research, Rutgers University in New Brunswick, New Jersey. It was published online in Cancer.
LIMITATIONS:
The study’s reliance on SEER-Medicare data may limit the generalizability of the findings to patients with Medicare Advantage, private insurance, or Medicaid, as well as younger patients. The lack of data on patient preferences and other health characteristics could confound the results. The study focused on systemic therapies and may not be generalizable to other treatments such as clinical trial drugs, oral therapies, surgery, or radiation. The data from 2012 to 2017 may not reflect more recent trends in cancer treatment.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute and the Rutgers Cancer Institute of New Jersey. George disclosed receiving grants from these organizations. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients treated by oncologists in the top quartile for end-of-life prescribing behavior were almost four and a half times more likely to receive end-of-life therapy than those treated by these specialists in the bottom quartile.
METHODOLOGY:
- Researchers analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, focusing on patients who died of cancer between 2012 and 2017.
- A total of 17,609 patients with breast, lung, colorectal, or prostate cancer were included, treated by 960 oncologists across 388 practices.
- Patients were required to have had at least one systemic cancer therapy claim in the last 180 days of life, with the treating oncologist identified on the basis of the therapy claim closest to the time of death.
- The study used multilevel models to estimate oncologists’ rates of providing cancer therapy in the last 30 days of life, adjusting for patient characteristics and practice variation.
- Functional status was assessed on the basis of paid claims for durable medical equipment in the last 60 months of life, with scores categorized as 0, 1, ≥ 2, or unknown.
TAKEAWAY:
- Oncologists in the 95th percentile for high end-of-life prescribing behavior had a 45% adjusted rate of treating patients in the last 30 days of life, compared with 17% among those in the 5th percentile.
- Patients treated by high end-of-life prescribing oncologists had over four times higher odds of receiving systemic therapy in the last 30 days of life (odds ratio [OR], 4.42; 95% CI, 4.00-4.89).
- Higher end-of-life prescribing oncologists also had a higher proportion of patients hospitalized in the last 30 days of life than low prescribers (58% vs 51.9%).
- No significant association was found between oncologist prescribing behavior and patient race or ethnicity, except for Black patients who had lower odds of receiving treatment (OR, 0.77; P < .001).
IN PRACTICE:
“Given calls to rein in overutilization of end-of-life six to eight cancer therapies, our findings highlight an underappreciated area for further research: How treatment discontinuation before death is shaped by oncologists’ unique treatment propensities. Elucidating the reasons for this remarkable variability in oncologist treatment behavior could inform efforts to reduce end-of-life cancer treatment overutilization,” wrote the authors of the study.
SOURCE:
The study was led by Login S. George, PhD, Institute for Health, Health Care Policy and Aging Research, Rutgers University in New Brunswick, New Jersey. It was published online in Cancer.
LIMITATIONS:
The study’s reliance on SEER-Medicare data may limit the generalizability of the findings to patients with Medicare Advantage, private insurance, or Medicaid, as well as younger patients. The lack of data on patient preferences and other health characteristics could confound the results. The study focused on systemic therapies and may not be generalizable to other treatments such as clinical trial drugs, oral therapies, surgery, or radiation. The data from 2012 to 2017 may not reflect more recent trends in cancer treatment.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute and the Rutgers Cancer Institute of New Jersey. George disclosed receiving grants from these organizations. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Ancient Viruses in Our DNA Hold Clues to Cancer Treatment
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Pilot Study Finds Experimental CBD Cream Decreases UVA Skin Damage
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Cyclosporine for Recalcitrant Bullous Pemphigoid Induced by Nivolumab Therapy for Malignant Melanoma
To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
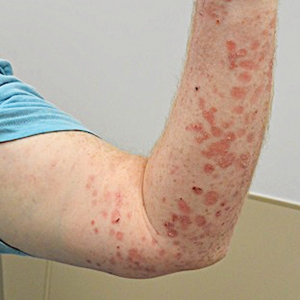
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.
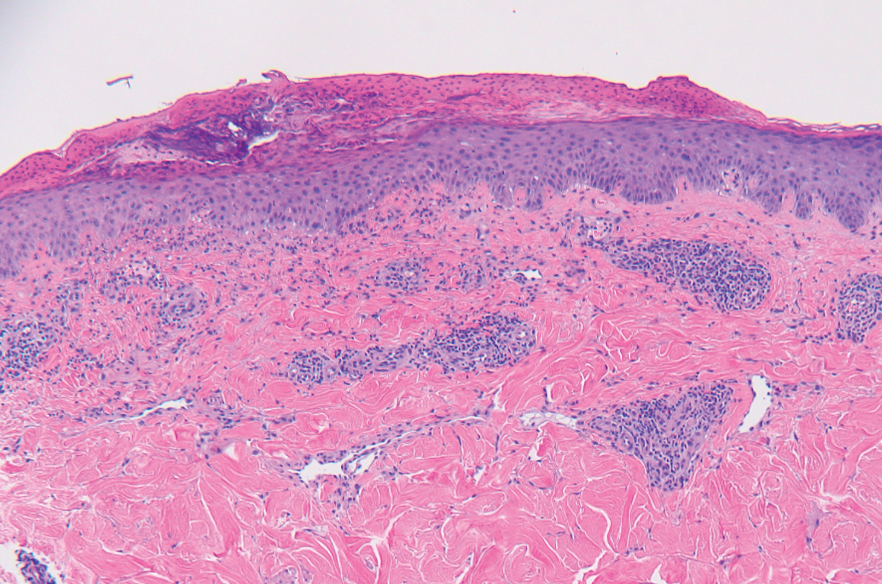
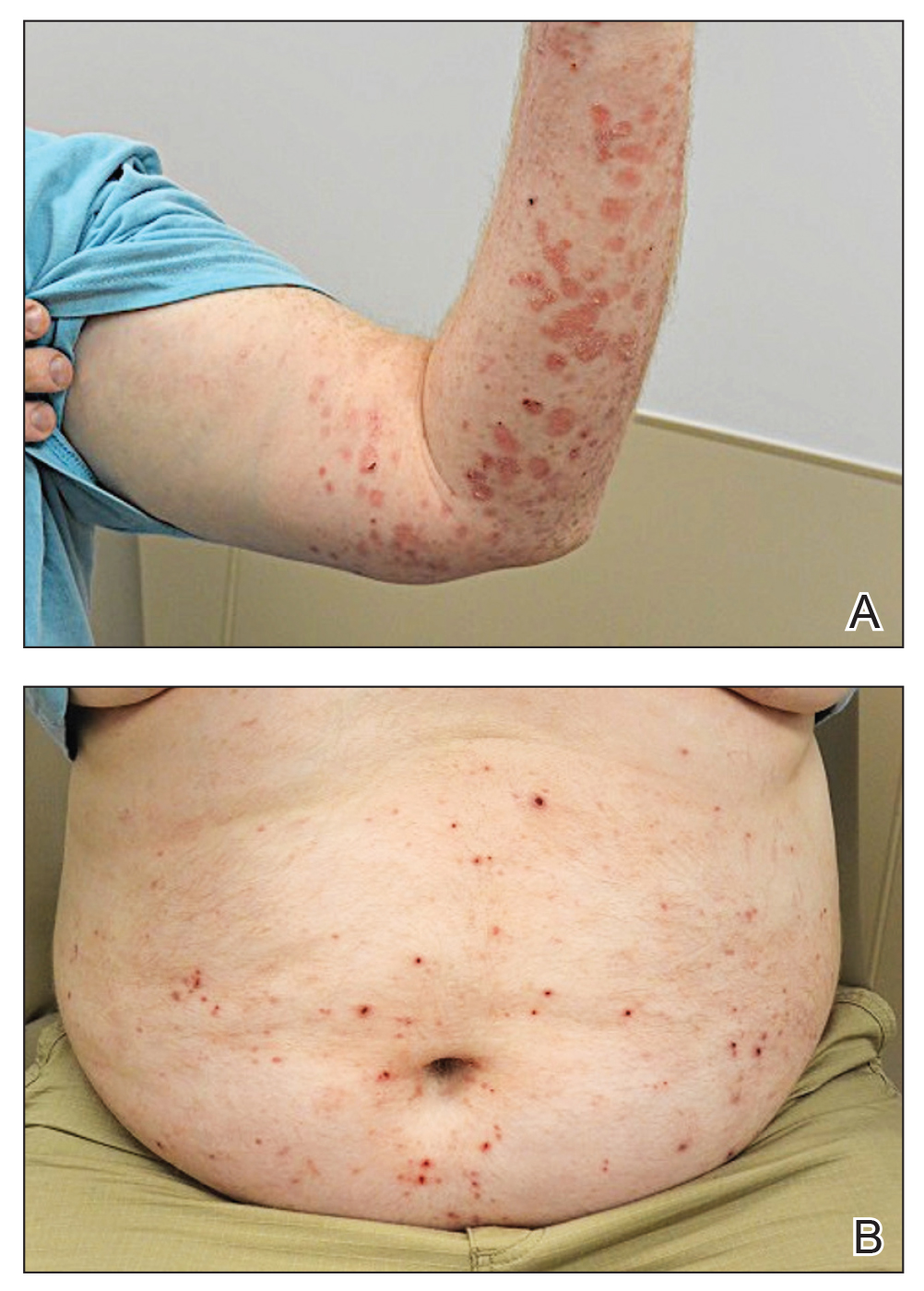
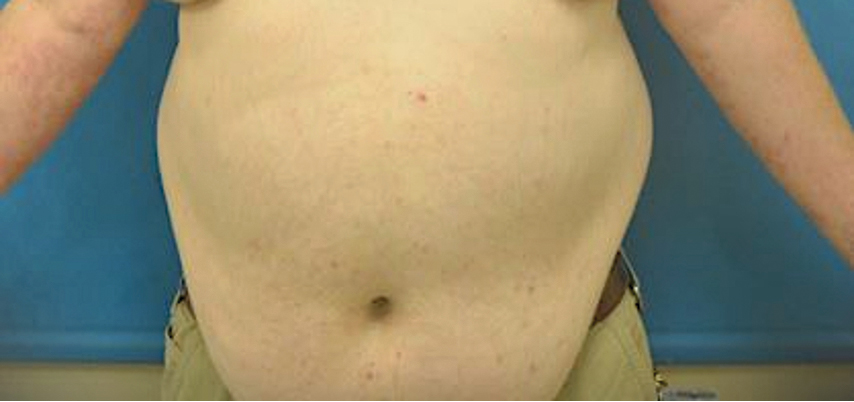
Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
- Puzanov I, Diab A, Abdallah K, et al; . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. doi:10.3978/j.issn.2218-6751.2015.06.06
- Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal reactivity and treatment recommendations in immune checkpoint inhibitor-induced bullous pemphigoid—a retrospective, monocentric study. Front Immunol. 2022;13:953546. doi:10.3389/fimmu.2022.953546
- Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:adv00377. doi:10.2340/00015555-3740
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669. doi:10.1111/ijd.13984
- Yang H, Yao Z, Zhou X, et al. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377. doi:10.1016/j.clim.2020.108377
- Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 suppl 3):16-22. doi:10.1016/0049-0172(92)90009-3
- Ahmed AR, Shetty S, Kaveri S, et al. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-708.e3. doi:10.1016/j.jaad.2015.11.030
- Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-946. doi:10.1016/j.jaad.2010.02.063
- Schmidt E, Hunzelmann N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. 2006;31:503-508. doi:10.1111/j.1365-2230.2006.02151.x
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65:552-558.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.



Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.
To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.



Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
- Puzanov I, Diab A, Abdallah K, et al; . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. doi:10.3978/j.issn.2218-6751.2015.06.06
- Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal reactivity and treatment recommendations in immune checkpoint inhibitor-induced bullous pemphigoid—a retrospective, monocentric study. Front Immunol. 2022;13:953546. doi:10.3389/fimmu.2022.953546
- Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:adv00377. doi:10.2340/00015555-3740
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669. doi:10.1111/ijd.13984
- Yang H, Yao Z, Zhou X, et al. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377. doi:10.1016/j.clim.2020.108377
- Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 suppl 3):16-22. doi:10.1016/0049-0172(92)90009-3
- Ahmed AR, Shetty S, Kaveri S, et al. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-708.e3. doi:10.1016/j.jaad.2015.11.030
- Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-946. doi:10.1016/j.jaad.2010.02.063
- Schmidt E, Hunzelmann N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. 2006;31:503-508. doi:10.1111/j.1365-2230.2006.02151.x
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65:552-558.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
- Puzanov I, Diab A, Abdallah K, et al; . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. doi:10.3978/j.issn.2218-6751.2015.06.06
- Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal reactivity and treatment recommendations in immune checkpoint inhibitor-induced bullous pemphigoid—a retrospective, monocentric study. Front Immunol. 2022;13:953546. doi:10.3389/fimmu.2022.953546
- Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:adv00377. doi:10.2340/00015555-3740
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669. doi:10.1111/ijd.13984
- Yang H, Yao Z, Zhou X, et al. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377. doi:10.1016/j.clim.2020.108377
- Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 suppl 3):16-22. doi:10.1016/0049-0172(92)90009-3
- Ahmed AR, Shetty S, Kaveri S, et al. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-708.e3. doi:10.1016/j.jaad.2015.11.030
- Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-946. doi:10.1016/j.jaad.2010.02.063
- Schmidt E, Hunzelmann N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. 2006;31:503-508. doi:10.1111/j.1365-2230.2006.02151.x
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65:552-558.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
Practice Points
- Bullous pemphigoid is a rare dermatologic immune-related adverse event that can occur secondary to anti–programmed death receptor 1 therapy.
- For cases of immune checkpoint inhibitor–induced bullous pemphigoid that are recalcitrant to corticosteroids and rituximab, cyclosporine might be an effective alternative.
Study Finds Varying Skin Cancer Rates Based on Sexual Orientation
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Greater Transparency of Oncologists’ Pharma Relationships Needed
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
FROM ASCO 2024
Should Cancer Trial Eligibility Become More Inclusive?
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
Weight Loss Drugs Cut Cancer Risk in Diabetes Patients
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Urticaria Linked to Higher Cancer Risk, Study Finds
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.