User login
ASCO 2024: Treating Myeloma Just Got More Complicated
For brevity’s sake, I’ll focus on trials about newly diagnosed MM and myeloma at first relapse. Here’s my take on how to interpret those studies in light of broader evidence, what I view as their key limitations, and how what came out of ASCO 2024 changes my approach.
The Return of Belantamab
Belantamab, a BCMA targeting antibody-drug conjugate, previously had shown a response rate of 34% in a single-arm, heavily pretreated population, albeit with modest progression free survival (PFS), only to fail its confirmatory randomized study against pomalidomide/dexamethasone. Given the ocular toxicity associated with belantamab, many — including myself — had written off this drug (save in exceptional/unique circumstances), especially with the rise of novel immunotherapies targeting BCMA, such as chimeric antigen receptor (CAR T-cell) therapy and bispecific antibodies.
However, this year at ASCO, two key randomized trials were presented with concurrent publications, a trial of belantamab/bortezomib/dexamethasone versus daratumumab/bortezomib/dexamethasone (DVd) (DREAMM-7), and a trial of belantamab/pomalidomide/dexamethasone versus bortezomib/pomalidomide/dexamethasone (DREAMM-8). Both trials evaluated patients with myeloma who had relapsed disease and had received at least one prior line of therapy.
In both trials, the belantamab triplet beat the other triplets for the endpoint of PFS (median PFS 36.6 vs 13 months for DREAMM-7, and 12 months PFS 71% vs 51% for DREAMM-8). We must commend the bold three-versus-three design and a convincing result.
What are the caveats? Some censoring of information happened in DREAMM-7, which helped make the intervention arm look better than reality and the control arm look even worse than reality. To illustrate this point: the control arm of DVd (PFS 13 months) underperformed, compared to the CASTOR trial, where DVd led to a PFS of 16.7 months. The drug remains toxic, with high rates of keratopathy and vision problems in its current dosing schema. (Perhaps the future lies in less frequent dosing.) This toxicity is almost always reversible, but it is a huge problem to deal with, and our current quality-of-life instruments fail miserably at capturing this.
Furthermore, DVd is now emerging as perhaps the weakest daratumumab triplet that exists. Almost all patients in this trial had disease sensitivity to lenalidomide, and daratumumab/lenalidomide/dexamethasone (PFS of 45 months in the POLLUX trial) is unequivocally easier to use and handle (in my opinion) than this belantamab triplet--which is quite literally “an eyesore.” Would belantamab-based triplets beat dara/len/dex for patients with lenalidomide sensitive disease? Or, for that matter, would belantamab combos beat anti-CD38+carfilzomib+dex combinations, or cilta-cel (which is also now approved for first relapse)?
How do I foresee the future of belantamab? Despite these unequivocally positive results, I am not enthused about using it for most patients at first relapse. When trials for bispecifics at first relapse read out, my enthusiasm will likely wane even more. Still, it is useful to have belantamab in the armamentarium. For some patients perceived to be at very high risk of infection, belantamab-based triplets may indeed prove to be a better option than bispecifics. However, I suspect that with better dosing strategies for bispecifics, perhaps even that trend may be mitigated. Since we do not yet have bispecifics available in this line, my suggested algorithm for first relapse is as follows: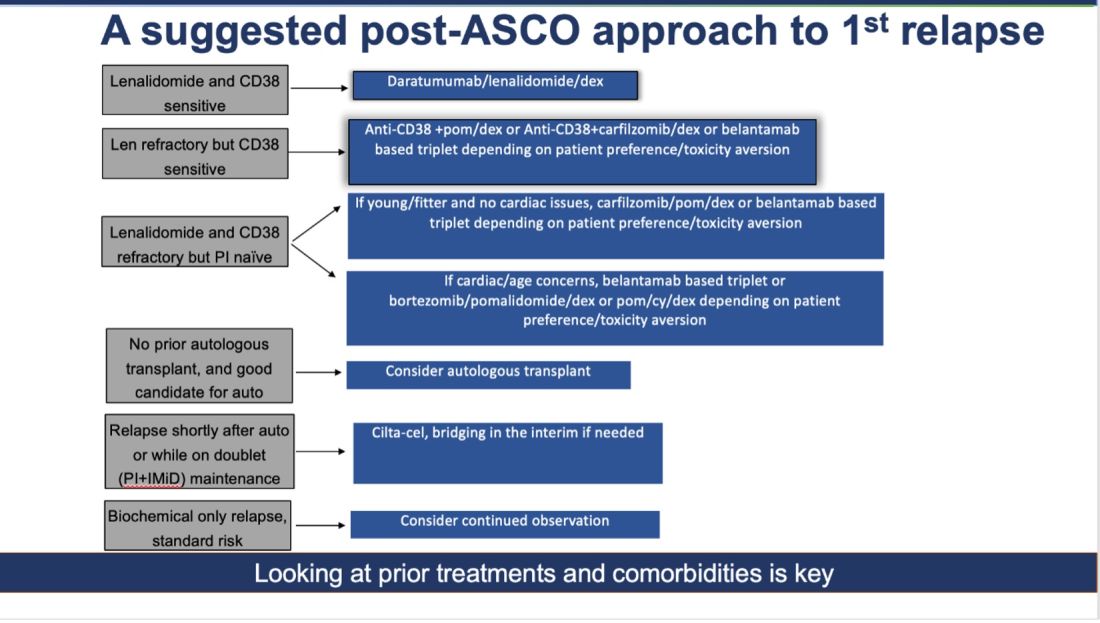
Newly Diagnosed MM: The Era of Quads Solidifies
At ASCO 2024, two key trials with concurrent publications assessed the role of quadruplets (without the use of transplant): the IMROZ trial of a quadruplet of isatuximab/bortezomib/lenalidomide/dexamethasone versus bortezomib/lenalidomide/dexamethasone (VRd), and the BENEFIT trial (isatuximab/lenalidomide/bortezomib/dexamethasone versus isatuximab/lenalidomide/dexamethasone).
The IMROZ trial tested the addition of an anti-CD38 antibody to a triplet backbone, and the results are compelling. The PFS was not reached for the quad vs 54 months for VRd. Unlike in the belantamab trial (where the control arm underperformed), here the control arm really overperformed. In this case, we have never seen such a compelling PFS of 54 months for VRd before. (Based on other trials, VRd PFS has been more in the ballpark of 35-43 months.) This speaks to the fitness and biology of the patients enrolled in this trial, and perhaps to how we will not see such stellar results with this quad recreated in real life.
The addition of isatuximab did not seem to impair quality of life, and although there were more treatment-related deaths with isatuximab, those higher numbers seem to have been driven by longer treatment durations. For this study, the upper age limit was 80 years, and most patients enrolled had an excellent functional status--making it clear that frail patients were greatly underrepresented.
What can we conclude from this study? For fit, older patients (who would have been transplant-eligible in the United States), this study provides excellent proof of concept that very good outcomes can be obtained without the use of transplantation. In treating frail patients, we do not know if quads are safe (or even necessary, compared to gentler sequencing), so these data are not applicable.
High-risk cytogenetics were underrepresented, and although the subgroup analysis for such patients did not show a benefit, it is hard to draw conclusions either way. For me, this trial is further evidence that for many older patients with MM, even if you “can” do a transplant, you probably “shouldn’t, they will experience increasingly better outcomes.
The standard for newly diagnosed MM in older patients for whom transplant is not intended is currently dara/len/dex. Is isa/bort/len/dex better? I do not know. It may give a better PFS, but the addition of bortezomib will lead to more neuropathy: 60% of patients developed neuropathy here, with 7% developing Grade III/IV peripheral neuropathy.
To resolve this issue, highly individualized discussions with patients will be needed. The BENEFIT trial evaluated this question more directly, with a randomized comparison of Isa-VRd versus Isa-Rd (the role of bortezomib being the main variable assessed here) with a primary endpoint of MRD negativity at 10-5 at 18 months. Although MRD negativity allows for a quick read-out, having MRD as an endpoint is a foregone conclusion. Adding another drug will almost certainly lead to deeper responses. But is it worth it?
In the BENEFIT trial, the MRD negativity at 10-5 was 26% versus 53% with the quad. However, peripheral neuropathy rates were much higher with the quad (28% vs 52%). Without longer-term data such as PFS and OS, I do not know whether it is worth the extra risks of neuropathy for older patients. Their priority may not be eradication of cancer cells at all costs. Instead, it may be better quality of life and functioning while preserving survival.
To sum up: Post-ASCO 2024, the approach to newly diagnosed MM just got a lot more complicated. For fit, older patients willing to endure extra toxicities of neuropathy (and acknowledging that we do not know whether survival will be any better with this approach), a quad is a very reasonable option to offer while forgoing transplant, in resource-rich areas of the world, such as the United States. Omitting a transplant now seems very reasonable for most older adults. However, a nuanced and individualized approach remains paramount. And given the speed of new developments, even this suggested approach will be outdated soon!
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
For brevity’s sake, I’ll focus on trials about newly diagnosed MM and myeloma at first relapse. Here’s my take on how to interpret those studies in light of broader evidence, what I view as their key limitations, and how what came out of ASCO 2024 changes my approach.
The Return of Belantamab
Belantamab, a BCMA targeting antibody-drug conjugate, previously had shown a response rate of 34% in a single-arm, heavily pretreated population, albeit with modest progression free survival (PFS), only to fail its confirmatory randomized study against pomalidomide/dexamethasone. Given the ocular toxicity associated with belantamab, many — including myself — had written off this drug (save in exceptional/unique circumstances), especially with the rise of novel immunotherapies targeting BCMA, such as chimeric antigen receptor (CAR T-cell) therapy and bispecific antibodies.
However, this year at ASCO, two key randomized trials were presented with concurrent publications, a trial of belantamab/bortezomib/dexamethasone versus daratumumab/bortezomib/dexamethasone (DVd) (DREAMM-7), and a trial of belantamab/pomalidomide/dexamethasone versus bortezomib/pomalidomide/dexamethasone (DREAMM-8). Both trials evaluated patients with myeloma who had relapsed disease and had received at least one prior line of therapy.
In both trials, the belantamab triplet beat the other triplets for the endpoint of PFS (median PFS 36.6 vs 13 months for DREAMM-7, and 12 months PFS 71% vs 51% for DREAMM-8). We must commend the bold three-versus-three design and a convincing result.
What are the caveats? Some censoring of information happened in DREAMM-7, which helped make the intervention arm look better than reality and the control arm look even worse than reality. To illustrate this point: the control arm of DVd (PFS 13 months) underperformed, compared to the CASTOR trial, where DVd led to a PFS of 16.7 months. The drug remains toxic, with high rates of keratopathy and vision problems in its current dosing schema. (Perhaps the future lies in less frequent dosing.) This toxicity is almost always reversible, but it is a huge problem to deal with, and our current quality-of-life instruments fail miserably at capturing this.
Furthermore, DVd is now emerging as perhaps the weakest daratumumab triplet that exists. Almost all patients in this trial had disease sensitivity to lenalidomide, and daratumumab/lenalidomide/dexamethasone (PFS of 45 months in the POLLUX trial) is unequivocally easier to use and handle (in my opinion) than this belantamab triplet--which is quite literally “an eyesore.” Would belantamab-based triplets beat dara/len/dex for patients with lenalidomide sensitive disease? Or, for that matter, would belantamab combos beat anti-CD38+carfilzomib+dex combinations, or cilta-cel (which is also now approved for first relapse)?
How do I foresee the future of belantamab? Despite these unequivocally positive results, I am not enthused about using it for most patients at first relapse. When trials for bispecifics at first relapse read out, my enthusiasm will likely wane even more. Still, it is useful to have belantamab in the armamentarium. For some patients perceived to be at very high risk of infection, belantamab-based triplets may indeed prove to be a better option than bispecifics. However, I suspect that with better dosing strategies for bispecifics, perhaps even that trend may be mitigated. Since we do not yet have bispecifics available in this line, my suggested algorithm for first relapse is as follows:
Newly Diagnosed MM: The Era of Quads Solidifies
At ASCO 2024, two key trials with concurrent publications assessed the role of quadruplets (without the use of transplant): the IMROZ trial of a quadruplet of isatuximab/bortezomib/lenalidomide/dexamethasone versus bortezomib/lenalidomide/dexamethasone (VRd), and the BENEFIT trial (isatuximab/lenalidomide/bortezomib/dexamethasone versus isatuximab/lenalidomide/dexamethasone).
The IMROZ trial tested the addition of an anti-CD38 antibody to a triplet backbone, and the results are compelling. The PFS was not reached for the quad vs 54 months for VRd. Unlike in the belantamab trial (where the control arm underperformed), here the control arm really overperformed. In this case, we have never seen such a compelling PFS of 54 months for VRd before. (Based on other trials, VRd PFS has been more in the ballpark of 35-43 months.) This speaks to the fitness and biology of the patients enrolled in this trial, and perhaps to how we will not see such stellar results with this quad recreated in real life.
The addition of isatuximab did not seem to impair quality of life, and although there were more treatment-related deaths with isatuximab, those higher numbers seem to have been driven by longer treatment durations. For this study, the upper age limit was 80 years, and most patients enrolled had an excellent functional status--making it clear that frail patients were greatly underrepresented.
What can we conclude from this study? For fit, older patients (who would have been transplant-eligible in the United States), this study provides excellent proof of concept that very good outcomes can be obtained without the use of transplantation. In treating frail patients, we do not know if quads are safe (or even necessary, compared to gentler sequencing), so these data are not applicable.
High-risk cytogenetics were underrepresented, and although the subgroup analysis for such patients did not show a benefit, it is hard to draw conclusions either way. For me, this trial is further evidence that for many older patients with MM, even if you “can” do a transplant, you probably “shouldn’t, they will experience increasingly better outcomes.
The standard for newly diagnosed MM in older patients for whom transplant is not intended is currently dara/len/dex. Is isa/bort/len/dex better? I do not know. It may give a better PFS, but the addition of bortezomib will lead to more neuropathy: 60% of patients developed neuropathy here, with 7% developing Grade III/IV peripheral neuropathy.
To resolve this issue, highly individualized discussions with patients will be needed. The BENEFIT trial evaluated this question more directly, with a randomized comparison of Isa-VRd versus Isa-Rd (the role of bortezomib being the main variable assessed here) with a primary endpoint of MRD negativity at 10-5 at 18 months. Although MRD negativity allows for a quick read-out, having MRD as an endpoint is a foregone conclusion. Adding another drug will almost certainly lead to deeper responses. But is it worth it?
In the BENEFIT trial, the MRD negativity at 10-5 was 26% versus 53% with the quad. However, peripheral neuropathy rates were much higher with the quad (28% vs 52%). Without longer-term data such as PFS and OS, I do not know whether it is worth the extra risks of neuropathy for older patients. Their priority may not be eradication of cancer cells at all costs. Instead, it may be better quality of life and functioning while preserving survival.
To sum up: Post-ASCO 2024, the approach to newly diagnosed MM just got a lot more complicated. For fit, older patients willing to endure extra toxicities of neuropathy (and acknowledging that we do not know whether survival will be any better with this approach), a quad is a very reasonable option to offer while forgoing transplant, in resource-rich areas of the world, such as the United States. Omitting a transplant now seems very reasonable for most older adults. However, a nuanced and individualized approach remains paramount. And given the speed of new developments, even this suggested approach will be outdated soon!
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
For brevity’s sake, I’ll focus on trials about newly diagnosed MM and myeloma at first relapse. Here’s my take on how to interpret those studies in light of broader evidence, what I view as their key limitations, and how what came out of ASCO 2024 changes my approach.
The Return of Belantamab
Belantamab, a BCMA targeting antibody-drug conjugate, previously had shown a response rate of 34% in a single-arm, heavily pretreated population, albeit with modest progression free survival (PFS), only to fail its confirmatory randomized study against pomalidomide/dexamethasone. Given the ocular toxicity associated with belantamab, many — including myself — had written off this drug (save in exceptional/unique circumstances), especially with the rise of novel immunotherapies targeting BCMA, such as chimeric antigen receptor (CAR T-cell) therapy and bispecific antibodies.
However, this year at ASCO, two key randomized trials were presented with concurrent publications, a trial of belantamab/bortezomib/dexamethasone versus daratumumab/bortezomib/dexamethasone (DVd) (DREAMM-7), and a trial of belantamab/pomalidomide/dexamethasone versus bortezomib/pomalidomide/dexamethasone (DREAMM-8). Both trials evaluated patients with myeloma who had relapsed disease and had received at least one prior line of therapy.
In both trials, the belantamab triplet beat the other triplets for the endpoint of PFS (median PFS 36.6 vs 13 months for DREAMM-7, and 12 months PFS 71% vs 51% for DREAMM-8). We must commend the bold three-versus-three design and a convincing result.
What are the caveats? Some censoring of information happened in DREAMM-7, which helped make the intervention arm look better than reality and the control arm look even worse than reality. To illustrate this point: the control arm of DVd (PFS 13 months) underperformed, compared to the CASTOR trial, where DVd led to a PFS of 16.7 months. The drug remains toxic, with high rates of keratopathy and vision problems in its current dosing schema. (Perhaps the future lies in less frequent dosing.) This toxicity is almost always reversible, but it is a huge problem to deal with, and our current quality-of-life instruments fail miserably at capturing this.
Furthermore, DVd is now emerging as perhaps the weakest daratumumab triplet that exists. Almost all patients in this trial had disease sensitivity to lenalidomide, and daratumumab/lenalidomide/dexamethasone (PFS of 45 months in the POLLUX trial) is unequivocally easier to use and handle (in my opinion) than this belantamab triplet--which is quite literally “an eyesore.” Would belantamab-based triplets beat dara/len/dex for patients with lenalidomide sensitive disease? Or, for that matter, would belantamab combos beat anti-CD38+carfilzomib+dex combinations, or cilta-cel (which is also now approved for first relapse)?
How do I foresee the future of belantamab? Despite these unequivocally positive results, I am not enthused about using it for most patients at first relapse. When trials for bispecifics at first relapse read out, my enthusiasm will likely wane even more. Still, it is useful to have belantamab in the armamentarium. For some patients perceived to be at very high risk of infection, belantamab-based triplets may indeed prove to be a better option than bispecifics. However, I suspect that with better dosing strategies for bispecifics, perhaps even that trend may be mitigated. Since we do not yet have bispecifics available in this line, my suggested algorithm for first relapse is as follows:
Newly Diagnosed MM: The Era of Quads Solidifies
At ASCO 2024, two key trials with concurrent publications assessed the role of quadruplets (without the use of transplant): the IMROZ trial of a quadruplet of isatuximab/bortezomib/lenalidomide/dexamethasone versus bortezomib/lenalidomide/dexamethasone (VRd), and the BENEFIT trial (isatuximab/lenalidomide/bortezomib/dexamethasone versus isatuximab/lenalidomide/dexamethasone).
The IMROZ trial tested the addition of an anti-CD38 antibody to a triplet backbone, and the results are compelling. The PFS was not reached for the quad vs 54 months for VRd. Unlike in the belantamab trial (where the control arm underperformed), here the control arm really overperformed. In this case, we have never seen such a compelling PFS of 54 months for VRd before. (Based on other trials, VRd PFS has been more in the ballpark of 35-43 months.) This speaks to the fitness and biology of the patients enrolled in this trial, and perhaps to how we will not see such stellar results with this quad recreated in real life.
The addition of isatuximab did not seem to impair quality of life, and although there were more treatment-related deaths with isatuximab, those higher numbers seem to have been driven by longer treatment durations. For this study, the upper age limit was 80 years, and most patients enrolled had an excellent functional status--making it clear that frail patients were greatly underrepresented.
What can we conclude from this study? For fit, older patients (who would have been transplant-eligible in the United States), this study provides excellent proof of concept that very good outcomes can be obtained without the use of transplantation. In treating frail patients, we do not know if quads are safe (or even necessary, compared to gentler sequencing), so these data are not applicable.
High-risk cytogenetics were underrepresented, and although the subgroup analysis for such patients did not show a benefit, it is hard to draw conclusions either way. For me, this trial is further evidence that for many older patients with MM, even if you “can” do a transplant, you probably “shouldn’t, they will experience increasingly better outcomes.
The standard for newly diagnosed MM in older patients for whom transplant is not intended is currently dara/len/dex. Is isa/bort/len/dex better? I do not know. It may give a better PFS, but the addition of bortezomib will lead to more neuropathy: 60% of patients developed neuropathy here, with 7% developing Grade III/IV peripheral neuropathy.
To resolve this issue, highly individualized discussions with patients will be needed. The BENEFIT trial evaluated this question more directly, with a randomized comparison of Isa-VRd versus Isa-Rd (the role of bortezomib being the main variable assessed here) with a primary endpoint of MRD negativity at 10-5 at 18 months. Although MRD negativity allows for a quick read-out, having MRD as an endpoint is a foregone conclusion. Adding another drug will almost certainly lead to deeper responses. But is it worth it?
In the BENEFIT trial, the MRD negativity at 10-5 was 26% versus 53% with the quad. However, peripheral neuropathy rates were much higher with the quad (28% vs 52%). Without longer-term data such as PFS and OS, I do not know whether it is worth the extra risks of neuropathy for older patients. Their priority may not be eradication of cancer cells at all costs. Instead, it may be better quality of life and functioning while preserving survival.
To sum up: Post-ASCO 2024, the approach to newly diagnosed MM just got a lot more complicated. For fit, older patients willing to endure extra toxicities of neuropathy (and acknowledging that we do not know whether survival will be any better with this approach), a quad is a very reasonable option to offer while forgoing transplant, in resource-rich areas of the world, such as the United States. Omitting a transplant now seems very reasonable for most older adults. However, a nuanced and individualized approach remains paramount. And given the speed of new developments, even this suggested approach will be outdated soon!
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
Neurofilament Light Chain Detects Early Chemotherapy-Related Neurotoxicity
Investigators found Nfl levels increased in cancer patients following a first infusion of the medication paclitaxel and corresponded to neuropathy severity 6-12 months post-treatment, suggesting the blood protein may provide an early CIPN biomarker.
“Nfl after a single cycle could detect axonal degeneration,” said lead investigator Masarra Joda, a researcher and PhD candidate at the University of Sydney in Australia. She added that “quantification of Nfl may provide a clinically useful marker of emerging neurotoxicity in patients vulnerable to CIPN.”
The findings were presented at the Peripheral Nerve Society (PNS) 2024 annual meeting.
Common, Burdensome Side Effect
A common side effect of chemotherapy, CIPN manifests as sensory neuropathy and causes degeneration of the peripheral axons. A protein biomarker of axonal degeneration, Nfl has previously been investigated as a way of identifying patients at risk of CIPN.
The goal of the current study was to identify the potential link between Nfl with neurophysiological markers of axon degeneration in patients receiving the neurotoxin chemotherapy paclitaxel.
The study included 93 cancer patients. All were assessed at the beginning, middle, and end of treatment. CIPN was assessed using blood samples of Nfl and the Total Neuropathy Score (TNS), the Common Terminology Criteria for Adverse Events (CTCAE) neuropathy scale, and patient-reported measures using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (EORTC-CIPN20).
Axonal degeneration was measured with neurophysiological tests including sural nerve compound sensory action potential (CSAP) for the lower limbs, and sensory median nerve CSAP, as well as stimulus threshold testing, for the upper limbs.
Almost all of study participants (97%) were female. The majority (66%) had breast cancer and 30% had gynecological cancer. Most (73%) were receiving a weekly regimen of paclitaxel, and the remainder were treated with taxanes plus platinum once every 3 weeks. By the end of treatment, 82% of the patients had developed CIPN, which was mild in 44% and moderate/severe in 38%.
Nfl levels increased significantly from baseline to after the first dose of chemotherapy (P < .001), “highlighting that nerve damage occurs from the very beginning of treatment,” senior investigator Susanna Park, PhD, told this news organization.
In addition, “patients with higher Nfl levels after a single paclitaxel treatment had greater neuropathy at the end of treatment (higher EORTC scores [P ≤ .026], and higher TNS scores [P ≤ .00]),” added Dr. Park, who is associate professor at the University of Sydney.
“Importantly, we also looked at long-term outcomes beyond the end of chemotherapy, because chronic neuropathy produces a significant burden in cancer survivors,” said Dr. Park.
“Among a total of 44 patients who completed the 6- to 12-month post-treatment follow-up, NfL levels after a single treatment were linked to severity of nerve damage quantified with neurophysiological tests, and greater Nfl levels at mid-treatment were correlated with worse patient and neurologically graded neuropathy at 6-12 months.”
Dr. Park said the results suggest that NfL may provide a biomarker of long-term axon damage and that Nfl assays “may enable clinicians to evaluate the risk of long-term toxicity early during paclitaxel treatment to hopefully provide clinically significant information to guide better treatment titration.”
Currently, she said, CIPN is a prominent cause of dose reduction and early chemotherapy cessation.
“For example, in early breast cancer around 25% of patients experience a dose reduction due to the severity of neuropathy symptoms.” But, she said, “there is no standardized way of identifying which patients are at risk of long-term neuropathy and therefore, may benefit more from dose reduction. In this setting, a biomarker such as Nfl could provide oncologists with more information about the risk of long-term toxicity and take that into account in dose decision-making.”
For some cancers, she added, there are multiple potential therapy options.
“A biomarker such as NfL could assist in determining risk-benefit profile in terms of switching to alternate therapies. However, further studies will be needed to fully define the utility of NfL as a biomarker of paclitaxel neuropathy.”
Promising Research
Commenting on the research for this news organization, Maryam Lustberg, MD, associate professor, director of the Center for Breast Cancer at Smilow Cancer Hospital and Yale Cancer Center, and chief of Breast Medical Oncology at Yale Cancer Center, in New Haven, Connecticut, said the study “builds on a body of work previously reported by others showing that neurofilament light chains as detected in the blood can be associated with early signs of neurotoxic injury.”
She added that the research “is promising, since existing clinical and patient-reported measures tend to under-detect chemotherapy-induced neuropathy until more permanent injury might have occurred.”
Dr. Lustberg, who is immediate past president of the Multinational Association of Supportive Care in Cancer, said future studies are needed before Nfl testing can be implemented in routine practice, but that “early detection will allow earlier initiation of supportive care strategies such as physical therapy and exercise, as well as dose modifications, which may be helpful for preventing permanent damage and improving quality of life.”
The investigators and Dr. Lustberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Investigators found Nfl levels increased in cancer patients following a first infusion of the medication paclitaxel and corresponded to neuropathy severity 6-12 months post-treatment, suggesting the blood protein may provide an early CIPN biomarker.
“Nfl after a single cycle could detect axonal degeneration,” said lead investigator Masarra Joda, a researcher and PhD candidate at the University of Sydney in Australia. She added that “quantification of Nfl may provide a clinically useful marker of emerging neurotoxicity in patients vulnerable to CIPN.”
The findings were presented at the Peripheral Nerve Society (PNS) 2024 annual meeting.
Common, Burdensome Side Effect
A common side effect of chemotherapy, CIPN manifests as sensory neuropathy and causes degeneration of the peripheral axons. A protein biomarker of axonal degeneration, Nfl has previously been investigated as a way of identifying patients at risk of CIPN.
The goal of the current study was to identify the potential link between Nfl with neurophysiological markers of axon degeneration in patients receiving the neurotoxin chemotherapy paclitaxel.
The study included 93 cancer patients. All were assessed at the beginning, middle, and end of treatment. CIPN was assessed using blood samples of Nfl and the Total Neuropathy Score (TNS), the Common Terminology Criteria for Adverse Events (CTCAE) neuropathy scale, and patient-reported measures using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (EORTC-CIPN20).
Axonal degeneration was measured with neurophysiological tests including sural nerve compound sensory action potential (CSAP) for the lower limbs, and sensory median nerve CSAP, as well as stimulus threshold testing, for the upper limbs.
Almost all of study participants (97%) were female. The majority (66%) had breast cancer and 30% had gynecological cancer. Most (73%) were receiving a weekly regimen of paclitaxel, and the remainder were treated with taxanes plus platinum once every 3 weeks. By the end of treatment, 82% of the patients had developed CIPN, which was mild in 44% and moderate/severe in 38%.
Nfl levels increased significantly from baseline to after the first dose of chemotherapy (P < .001), “highlighting that nerve damage occurs from the very beginning of treatment,” senior investigator Susanna Park, PhD, told this news organization.
In addition, “patients with higher Nfl levels after a single paclitaxel treatment had greater neuropathy at the end of treatment (higher EORTC scores [P ≤ .026], and higher TNS scores [P ≤ .00]),” added Dr. Park, who is associate professor at the University of Sydney.
“Importantly, we also looked at long-term outcomes beyond the end of chemotherapy, because chronic neuropathy produces a significant burden in cancer survivors,” said Dr. Park.
“Among a total of 44 patients who completed the 6- to 12-month post-treatment follow-up, NfL levels after a single treatment were linked to severity of nerve damage quantified with neurophysiological tests, and greater Nfl levels at mid-treatment were correlated with worse patient and neurologically graded neuropathy at 6-12 months.”
Dr. Park said the results suggest that NfL may provide a biomarker of long-term axon damage and that Nfl assays “may enable clinicians to evaluate the risk of long-term toxicity early during paclitaxel treatment to hopefully provide clinically significant information to guide better treatment titration.”
Currently, she said, CIPN is a prominent cause of dose reduction and early chemotherapy cessation.
“For example, in early breast cancer around 25% of patients experience a dose reduction due to the severity of neuropathy symptoms.” But, she said, “there is no standardized way of identifying which patients are at risk of long-term neuropathy and therefore, may benefit more from dose reduction. In this setting, a biomarker such as Nfl could provide oncologists with more information about the risk of long-term toxicity and take that into account in dose decision-making.”
For some cancers, she added, there are multiple potential therapy options.
“A biomarker such as NfL could assist in determining risk-benefit profile in terms of switching to alternate therapies. However, further studies will be needed to fully define the utility of NfL as a biomarker of paclitaxel neuropathy.”
Promising Research
Commenting on the research for this news organization, Maryam Lustberg, MD, associate professor, director of the Center for Breast Cancer at Smilow Cancer Hospital and Yale Cancer Center, and chief of Breast Medical Oncology at Yale Cancer Center, in New Haven, Connecticut, said the study “builds on a body of work previously reported by others showing that neurofilament light chains as detected in the blood can be associated with early signs of neurotoxic injury.”
She added that the research “is promising, since existing clinical and patient-reported measures tend to under-detect chemotherapy-induced neuropathy until more permanent injury might have occurred.”
Dr. Lustberg, who is immediate past president of the Multinational Association of Supportive Care in Cancer, said future studies are needed before Nfl testing can be implemented in routine practice, but that “early detection will allow earlier initiation of supportive care strategies such as physical therapy and exercise, as well as dose modifications, which may be helpful for preventing permanent damage and improving quality of life.”
The investigators and Dr. Lustberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Investigators found Nfl levels increased in cancer patients following a first infusion of the medication paclitaxel and corresponded to neuropathy severity 6-12 months post-treatment, suggesting the blood protein may provide an early CIPN biomarker.
“Nfl after a single cycle could detect axonal degeneration,” said lead investigator Masarra Joda, a researcher and PhD candidate at the University of Sydney in Australia. She added that “quantification of Nfl may provide a clinically useful marker of emerging neurotoxicity in patients vulnerable to CIPN.”
The findings were presented at the Peripheral Nerve Society (PNS) 2024 annual meeting.
Common, Burdensome Side Effect
A common side effect of chemotherapy, CIPN manifests as sensory neuropathy and causes degeneration of the peripheral axons. A protein biomarker of axonal degeneration, Nfl has previously been investigated as a way of identifying patients at risk of CIPN.
The goal of the current study was to identify the potential link between Nfl with neurophysiological markers of axon degeneration in patients receiving the neurotoxin chemotherapy paclitaxel.
The study included 93 cancer patients. All were assessed at the beginning, middle, and end of treatment. CIPN was assessed using blood samples of Nfl and the Total Neuropathy Score (TNS), the Common Terminology Criteria for Adverse Events (CTCAE) neuropathy scale, and patient-reported measures using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy Module (EORTC-CIPN20).
Axonal degeneration was measured with neurophysiological tests including sural nerve compound sensory action potential (CSAP) for the lower limbs, and sensory median nerve CSAP, as well as stimulus threshold testing, for the upper limbs.
Almost all of study participants (97%) were female. The majority (66%) had breast cancer and 30% had gynecological cancer. Most (73%) were receiving a weekly regimen of paclitaxel, and the remainder were treated with taxanes plus platinum once every 3 weeks. By the end of treatment, 82% of the patients had developed CIPN, which was mild in 44% and moderate/severe in 38%.
Nfl levels increased significantly from baseline to after the first dose of chemotherapy (P < .001), “highlighting that nerve damage occurs from the very beginning of treatment,” senior investigator Susanna Park, PhD, told this news organization.
In addition, “patients with higher Nfl levels after a single paclitaxel treatment had greater neuropathy at the end of treatment (higher EORTC scores [P ≤ .026], and higher TNS scores [P ≤ .00]),” added Dr. Park, who is associate professor at the University of Sydney.
“Importantly, we also looked at long-term outcomes beyond the end of chemotherapy, because chronic neuropathy produces a significant burden in cancer survivors,” said Dr. Park.
“Among a total of 44 patients who completed the 6- to 12-month post-treatment follow-up, NfL levels after a single treatment were linked to severity of nerve damage quantified with neurophysiological tests, and greater Nfl levels at mid-treatment were correlated with worse patient and neurologically graded neuropathy at 6-12 months.”
Dr. Park said the results suggest that NfL may provide a biomarker of long-term axon damage and that Nfl assays “may enable clinicians to evaluate the risk of long-term toxicity early during paclitaxel treatment to hopefully provide clinically significant information to guide better treatment titration.”
Currently, she said, CIPN is a prominent cause of dose reduction and early chemotherapy cessation.
“For example, in early breast cancer around 25% of patients experience a dose reduction due to the severity of neuropathy symptoms.” But, she said, “there is no standardized way of identifying which patients are at risk of long-term neuropathy and therefore, may benefit more from dose reduction. In this setting, a biomarker such as Nfl could provide oncologists with more information about the risk of long-term toxicity and take that into account in dose decision-making.”
For some cancers, she added, there are multiple potential therapy options.
“A biomarker such as NfL could assist in determining risk-benefit profile in terms of switching to alternate therapies. However, further studies will be needed to fully define the utility of NfL as a biomarker of paclitaxel neuropathy.”
Promising Research
Commenting on the research for this news organization, Maryam Lustberg, MD, associate professor, director of the Center for Breast Cancer at Smilow Cancer Hospital and Yale Cancer Center, and chief of Breast Medical Oncology at Yale Cancer Center, in New Haven, Connecticut, said the study “builds on a body of work previously reported by others showing that neurofilament light chains as detected in the blood can be associated with early signs of neurotoxic injury.”
She added that the research “is promising, since existing clinical and patient-reported measures tend to under-detect chemotherapy-induced neuropathy until more permanent injury might have occurred.”
Dr. Lustberg, who is immediate past president of the Multinational Association of Supportive Care in Cancer, said future studies are needed before Nfl testing can be implemented in routine practice, but that “early detection will allow earlier initiation of supportive care strategies such as physical therapy and exercise, as well as dose modifications, which may be helpful for preventing permanent damage and improving quality of life.”
The investigators and Dr. Lustberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT PNS 2024
Oncology Mergers Are on the Rise. How Can Independent Practices Survive?
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
Doctors Endorsing Products on X May Not Disclose Company Ties
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
One Patient Changed This Oncologist’s View of Hope. Here’s How.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
FROM ASCO 2024
Myeloma: VRd Plus Isatuximab Improves Outcomes
Patients who took isatuximab (Sarclisa) plus bortezomib, lenalidomide, and dexamethasone (VRd) reached higher estimated progression-free survival at a median 59.7 months vs. those who took VRd alone (63.2% vs. 45.2%, respectively, 98.5% CI, hazard ratio [HR] = 0.60, P < .001), reported Thierry Facon, MD, professor of hematology at Lille University Hospital, France, and colleagues at the annual meeting of the American Society of Clinical Oncology in Chicago. The study was simultaneously published in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” Dr. Facon said in an interview. The findings demonstrated the VRd-isatuximab’s potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients,” he said.
According to Dr. Facon, more than 180,000 people worldwide are diagnosed with MM each year, he said, making it the second-most common hematologic malignancy.
“There is a need for new frontline therapeutic options for all MM patients,” he said. “Effective frontline therapy has the potential to modify the course of the disease, which is a key outcome for transplant-ineligible patients who often face high rates of attrition in later lines of therapy.”
For the industry-funded IMROZ study, researchers recruited patients aged 18-80 at 93 sites in 21 nations from 2017-2019. All were ineligible for transplant due to comorbidities or being aged 65 or older. Exclusions included Eastern Cooperative Oncology Group (ECOG) performance status scores of more than 2.
The subjects were randomly assigned in a 3-to-2 ratio to isatuximab-VRd (n = 265) or VRd alone (n = 181) and received four induction cycles (6 weeks per cycle) followed by 4-week cycles of continuous treatment until disease progression, unacceptable adverse event, or other criteria for discontinuation. If progression occurred, patients could be switched from the VRd-only group to the isatuximab-VRd group.
The median age in both the isatuximab-VRd and VRd groups was 72. The percentages of women were 46.0% and 48.1%, respectively, and 72.5% and 72.4%, respectively, were White. The next largest race/ethnic group was Asian (11.7% and 9.4%, respectively). Almost all had ECOG status of 0 or 1 (88.7% and 89.5%, respectively).
At study cut-off in September 2023, the percentages of subjects in the isatuximab-VRd and VRd groups who were still receiving treatment were 47.2% and 24.3%, respectively.
An intention-to-treat analysis found that the two groups had similar rates of overall response (91.3% for isatuximab-VRd vs. 92.3% for VRd), but the isatuximab-VRd group had higher complete or better response (74.7% vs. 64.1%, P = .01).
The percentage of patients who were minimal residual disease (MRD)-negative and had a complete response was also higher in the VRd-isatuximab group vs. the VRd group (55.5% vs. 40.9%, respectively, P = .003). A total of 26.0% of patients in the VRd-isatuximab group died vs. 32.6% in the VRd group; the estimated overall survival rates at 60 months were 72.3% and 66.3%, respectively, HR = 0.78, 99.97% CI).
As for adverse events, grade 5 events were more common in the VRd-isatuximab group (11.0% vs. 5.5%), as were deaths within the first 60 days of treatment (1.5% vs. 0.6%). “The difference was driven in part by different treatment exposures,” the researchers reported. Treatment-emergent events led to treatment discontinuation in 22.8% and 26.0% of patients, respectively.
“The safety and tolerability of Sarclisa observed was consistent with the established safety profile of Sarclisa and VRd with no new safety signals observed,” Dr. Facon said.
In an interview, Zandra Klippel, MD, global product head for multiple myeloma at Sanofi — the maker of isatuximab and funder of the study — said the Food and Drug Administration has accepted a priority review application for the investigational use of isatuximab in combination with VRd for the treatment of patients with transplant-ineligible, newly diagnosed MM.
“Our FDA approval date is expected on September 27, 2024,” Dr. Klippel said. “If all goes well, we anticipate launching as early as 2024 in the US and rolling out in other key countries starting in 2025 and continuing through 2026.”
Dr. Klippel added that isatuximab “continues to be evaluated in multiple ongoing phase 3 clinical trials in combination with current standard treatments across the MM treatment continuum.”
In an interview, Sagar Lonial, MD, chair and professor of hematology and medical oncology and chief medical officer at Winship Cancer Institute at Emory University in Atlanta, said the study is “important.”
However, Dr. Lonial, who is familiar with the findings but didn’t take part in the study, said it’s difficult to understand the impact of the treatment on frail patients. It appears that the combination treatment may be good for frail patients, he said, “but I need to better understand the magnitude of the benefit in that subset a little more.”
As for adverse events, he said “they are what would be expected for a trial like this.”
Pneumonia and COVID-19 infections were higher in the VRd-isatuximab group, he said, and “we know in general that vaccine responses are blocked by CD38 antibodies.” This can be managed, he said, via intravenous immunoglobulin support.
Manni Mohyuddin, MD, assistant professor at Huntsman Cancer Institute in Utah, said in an interview that the findings suggest that in older, fit patients, “you can get fairly good outcomes without use of transplant.”
In the United States, many more patients in the cohort would have been considered transplant-eligible, he said, and not eliminated from consideration for transplant due to age over 65. However, as patients age, “you get more diminishing returns for transplants,” said Dr. Mohyuddin, who is familiar with the study findings but didn’t take part in the research.
All the drugs in the new combination are FDA approved, he said, although the combination isn’t. “I suspect this will make it to our guidelines very soon and then be reimbursed by insurance companies and Medicare.”
The study was funded by Sanofi and an M.D. Anderson Cancer Center support grant. Dr. Facon has no disclosures. Other study authors report multiple ties relationships with various drug makers. Dr. Lonial disclosed ties with Takeda, Amgen, Novartis, BMS, GSK, AbbVie, Genentech, Pfizer, Regeneron, Janssen, AstraZeneca, and TG Therapeutics). Dr. Mohyuddin disclosed a relationship with Janssen.
Patients who took isatuximab (Sarclisa) plus bortezomib, lenalidomide, and dexamethasone (VRd) reached higher estimated progression-free survival at a median 59.7 months vs. those who took VRd alone (63.2% vs. 45.2%, respectively, 98.5% CI, hazard ratio [HR] = 0.60, P < .001), reported Thierry Facon, MD, professor of hematology at Lille University Hospital, France, and colleagues at the annual meeting of the American Society of Clinical Oncology in Chicago. The study was simultaneously published in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” Dr. Facon said in an interview. The findings demonstrated the VRd-isatuximab’s potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients,” he said.
According to Dr. Facon, more than 180,000 people worldwide are diagnosed with MM each year, he said, making it the second-most common hematologic malignancy.
“There is a need for new frontline therapeutic options for all MM patients,” he said. “Effective frontline therapy has the potential to modify the course of the disease, which is a key outcome for transplant-ineligible patients who often face high rates of attrition in later lines of therapy.”
For the industry-funded IMROZ study, researchers recruited patients aged 18-80 at 93 sites in 21 nations from 2017-2019. All were ineligible for transplant due to comorbidities or being aged 65 or older. Exclusions included Eastern Cooperative Oncology Group (ECOG) performance status scores of more than 2.
The subjects were randomly assigned in a 3-to-2 ratio to isatuximab-VRd (n = 265) or VRd alone (n = 181) and received four induction cycles (6 weeks per cycle) followed by 4-week cycles of continuous treatment until disease progression, unacceptable adverse event, or other criteria for discontinuation. If progression occurred, patients could be switched from the VRd-only group to the isatuximab-VRd group.
The median age in both the isatuximab-VRd and VRd groups was 72. The percentages of women were 46.0% and 48.1%, respectively, and 72.5% and 72.4%, respectively, were White. The next largest race/ethnic group was Asian (11.7% and 9.4%, respectively). Almost all had ECOG status of 0 or 1 (88.7% and 89.5%, respectively).
At study cut-off in September 2023, the percentages of subjects in the isatuximab-VRd and VRd groups who were still receiving treatment were 47.2% and 24.3%, respectively.
An intention-to-treat analysis found that the two groups had similar rates of overall response (91.3% for isatuximab-VRd vs. 92.3% for VRd), but the isatuximab-VRd group had higher complete or better response (74.7% vs. 64.1%, P = .01).
The percentage of patients who were minimal residual disease (MRD)-negative and had a complete response was also higher in the VRd-isatuximab group vs. the VRd group (55.5% vs. 40.9%, respectively, P = .003). A total of 26.0% of patients in the VRd-isatuximab group died vs. 32.6% in the VRd group; the estimated overall survival rates at 60 months were 72.3% and 66.3%, respectively, HR = 0.78, 99.97% CI).
As for adverse events, grade 5 events were more common in the VRd-isatuximab group (11.0% vs. 5.5%), as were deaths within the first 60 days of treatment (1.5% vs. 0.6%). “The difference was driven in part by different treatment exposures,” the researchers reported. Treatment-emergent events led to treatment discontinuation in 22.8% and 26.0% of patients, respectively.
“The safety and tolerability of Sarclisa observed was consistent with the established safety profile of Sarclisa and VRd with no new safety signals observed,” Dr. Facon said.
In an interview, Zandra Klippel, MD, global product head for multiple myeloma at Sanofi — the maker of isatuximab and funder of the study — said the Food and Drug Administration has accepted a priority review application for the investigational use of isatuximab in combination with VRd for the treatment of patients with transplant-ineligible, newly diagnosed MM.
“Our FDA approval date is expected on September 27, 2024,” Dr. Klippel said. “If all goes well, we anticipate launching as early as 2024 in the US and rolling out in other key countries starting in 2025 and continuing through 2026.”
Dr. Klippel added that isatuximab “continues to be evaluated in multiple ongoing phase 3 clinical trials in combination with current standard treatments across the MM treatment continuum.”
In an interview, Sagar Lonial, MD, chair and professor of hematology and medical oncology and chief medical officer at Winship Cancer Institute at Emory University in Atlanta, said the study is “important.”
However, Dr. Lonial, who is familiar with the findings but didn’t take part in the study, said it’s difficult to understand the impact of the treatment on frail patients. It appears that the combination treatment may be good for frail patients, he said, “but I need to better understand the magnitude of the benefit in that subset a little more.”
As for adverse events, he said “they are what would be expected for a trial like this.”
Pneumonia and COVID-19 infections were higher in the VRd-isatuximab group, he said, and “we know in general that vaccine responses are blocked by CD38 antibodies.” This can be managed, he said, via intravenous immunoglobulin support.
Manni Mohyuddin, MD, assistant professor at Huntsman Cancer Institute in Utah, said in an interview that the findings suggest that in older, fit patients, “you can get fairly good outcomes without use of transplant.”
In the United States, many more patients in the cohort would have been considered transplant-eligible, he said, and not eliminated from consideration for transplant due to age over 65. However, as patients age, “you get more diminishing returns for transplants,” said Dr. Mohyuddin, who is familiar with the study findings but didn’t take part in the research.
All the drugs in the new combination are FDA approved, he said, although the combination isn’t. “I suspect this will make it to our guidelines very soon and then be reimbursed by insurance companies and Medicare.”
The study was funded by Sanofi and an M.D. Anderson Cancer Center support grant. Dr. Facon has no disclosures. Other study authors report multiple ties relationships with various drug makers. Dr. Lonial disclosed ties with Takeda, Amgen, Novartis, BMS, GSK, AbbVie, Genentech, Pfizer, Regeneron, Janssen, AstraZeneca, and TG Therapeutics). Dr. Mohyuddin disclosed a relationship with Janssen.
Patients who took isatuximab (Sarclisa) plus bortezomib, lenalidomide, and dexamethasone (VRd) reached higher estimated progression-free survival at a median 59.7 months vs. those who took VRd alone (63.2% vs. 45.2%, respectively, 98.5% CI, hazard ratio [HR] = 0.60, P < .001), reported Thierry Facon, MD, professor of hematology at Lille University Hospital, France, and colleagues at the annual meeting of the American Society of Clinical Oncology in Chicago. The study was simultaneously published in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” Dr. Facon said in an interview. The findings demonstrated the VRd-isatuximab’s potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients,” he said.
According to Dr. Facon, more than 180,000 people worldwide are diagnosed with MM each year, he said, making it the second-most common hematologic malignancy.
“There is a need for new frontline therapeutic options for all MM patients,” he said. “Effective frontline therapy has the potential to modify the course of the disease, which is a key outcome for transplant-ineligible patients who often face high rates of attrition in later lines of therapy.”
For the industry-funded IMROZ study, researchers recruited patients aged 18-80 at 93 sites in 21 nations from 2017-2019. All were ineligible for transplant due to comorbidities or being aged 65 or older. Exclusions included Eastern Cooperative Oncology Group (ECOG) performance status scores of more than 2.
The subjects were randomly assigned in a 3-to-2 ratio to isatuximab-VRd (n = 265) or VRd alone (n = 181) and received four induction cycles (6 weeks per cycle) followed by 4-week cycles of continuous treatment until disease progression, unacceptable adverse event, or other criteria for discontinuation. If progression occurred, patients could be switched from the VRd-only group to the isatuximab-VRd group.
The median age in both the isatuximab-VRd and VRd groups was 72. The percentages of women were 46.0% and 48.1%, respectively, and 72.5% and 72.4%, respectively, were White. The next largest race/ethnic group was Asian (11.7% and 9.4%, respectively). Almost all had ECOG status of 0 or 1 (88.7% and 89.5%, respectively).
At study cut-off in September 2023, the percentages of subjects in the isatuximab-VRd and VRd groups who were still receiving treatment were 47.2% and 24.3%, respectively.
An intention-to-treat analysis found that the two groups had similar rates of overall response (91.3% for isatuximab-VRd vs. 92.3% for VRd), but the isatuximab-VRd group had higher complete or better response (74.7% vs. 64.1%, P = .01).
The percentage of patients who were minimal residual disease (MRD)-negative and had a complete response was also higher in the VRd-isatuximab group vs. the VRd group (55.5% vs. 40.9%, respectively, P = .003). A total of 26.0% of patients in the VRd-isatuximab group died vs. 32.6% in the VRd group; the estimated overall survival rates at 60 months were 72.3% and 66.3%, respectively, HR = 0.78, 99.97% CI).
As for adverse events, grade 5 events were more common in the VRd-isatuximab group (11.0% vs. 5.5%), as were deaths within the first 60 days of treatment (1.5% vs. 0.6%). “The difference was driven in part by different treatment exposures,” the researchers reported. Treatment-emergent events led to treatment discontinuation in 22.8% and 26.0% of patients, respectively.
“The safety and tolerability of Sarclisa observed was consistent with the established safety profile of Sarclisa and VRd with no new safety signals observed,” Dr. Facon said.
In an interview, Zandra Klippel, MD, global product head for multiple myeloma at Sanofi — the maker of isatuximab and funder of the study — said the Food and Drug Administration has accepted a priority review application for the investigational use of isatuximab in combination with VRd for the treatment of patients with transplant-ineligible, newly diagnosed MM.
“Our FDA approval date is expected on September 27, 2024,” Dr. Klippel said. “If all goes well, we anticipate launching as early as 2024 in the US and rolling out in other key countries starting in 2025 and continuing through 2026.”
Dr. Klippel added that isatuximab “continues to be evaluated in multiple ongoing phase 3 clinical trials in combination with current standard treatments across the MM treatment continuum.”
In an interview, Sagar Lonial, MD, chair and professor of hematology and medical oncology and chief medical officer at Winship Cancer Institute at Emory University in Atlanta, said the study is “important.”
However, Dr. Lonial, who is familiar with the findings but didn’t take part in the study, said it’s difficult to understand the impact of the treatment on frail patients. It appears that the combination treatment may be good for frail patients, he said, “but I need to better understand the magnitude of the benefit in that subset a little more.”
As for adverse events, he said “they are what would be expected for a trial like this.”
Pneumonia and COVID-19 infections were higher in the VRd-isatuximab group, he said, and “we know in general that vaccine responses are blocked by CD38 antibodies.” This can be managed, he said, via intravenous immunoglobulin support.
Manni Mohyuddin, MD, assistant professor at Huntsman Cancer Institute in Utah, said in an interview that the findings suggest that in older, fit patients, “you can get fairly good outcomes without use of transplant.”
In the United States, many more patients in the cohort would have been considered transplant-eligible, he said, and not eliminated from consideration for transplant due to age over 65. However, as patients age, “you get more diminishing returns for transplants,” said Dr. Mohyuddin, who is familiar with the study findings but didn’t take part in the research.
All the drugs in the new combination are FDA approved, he said, although the combination isn’t. “I suspect this will make it to our guidelines very soon and then be reimbursed by insurance companies and Medicare.”
The study was funded by Sanofi and an M.D. Anderson Cancer Center support grant. Dr. Facon has no disclosures. Other study authors report multiple ties relationships with various drug makers. Dr. Lonial disclosed ties with Takeda, Amgen, Novartis, BMS, GSK, AbbVie, Genentech, Pfizer, Regeneron, Janssen, AstraZeneca, and TG Therapeutics). Dr. Mohyuddin disclosed a relationship with Janssen.
FROM ASCO 2024
Are Children Born Through ART at Higher Risk for Cancer?
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Myeloma: First-In-Class ADC Regimen Yields Key Benefits
“Taken together with results from the [previous] DREAMM-7 trial, these data highlight the potential of belantamab mafodotin-containing triplets to address an unmet need for novel regimens to treat patients with multiple myeloma at the first relapse,” senior author Suzanne Trudel, MD, of the department of medical oncology and hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada, said in presenting the late-breaking findings in a press briefing at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
The results, published concurrently in The New England Journal of Medicine, are from an interim analysis of the ongoing phase 3, global open-label DREAMM-8 trial, involving 302 patients with lenalidomide-refractory multiple myeloma who were randomized to treatment with either belantamab mafodotin (n = 155) or bortezomib (n=147), each in addition to the pom-dex combination.
The study met its primary endpoint of PFS at a median follow-up of 21.8 months, with the median PFS in the belantamab mafodotin group not met, and the rate 12.7 months for bortezomib (HR 0.52; P < .001).
The 12-month rate of PFS was significantly higher with belantamab mafodotin compared with the bortezomib group (71% versus 51%).
The overall response rates between the 2 groups were similar (77% versus 72%), however, the belantamab mafodotin group had an improved rate of complete response of 40% versus 16% in the bortezomib group.
The median duration of response was not reached with belantamab mafodotin versus 17.5 months with bortezomib.
While a positive trend for median overall survival favored belantamab mafodotin for median overall survival (HR .77), the authors note that survival data still need to mature.
Further analyses showed early and sustained separation in favor of belantamab mafodotin for PFS in all prespecified subgroups, including those with high-risk cytogenetics, and those refractory to lenalidomide and anti-CD38s.
In terms of safety, grade 3 or higher adverse events (AEs) occurred among 91% of those in the belantamab mafodotin group compared with 73% in the bortezomib group, however, when the researchers adjusted for time on treatment, the belantamab mafodotin group had similar or lower rates of AEs.
Discontinuation rates for fatal or AEs of any cause were similar in both arms.
The most prominent side effects of belantamab mafodotin are the ocular AEs that affect the majority of patients. In the DREAMM-8 study, the ocular events affected 89% of patients, with events that were grade 3 or higher occurring among 43% (grade 3, 42%; grade 4, 1%).
The ocular events, which included blurred vision, dry eye, and a foreign body sensation in the eyes, were generally reversible and managed with treatment delays and dose modifications.
As of the time of the analysis, the first occurrence of the ocular events had improved in 92% of patients and resolved in 85%, with a median time to resolution of 57 days.
The AEs resulted in treatment discontinuation for 9% of patients.
The ocular events were managed with a protocol-recommended modification of the belantamab mafodotin dose, which included dose delays until the KVA grade improved to 1 or lower, as well as reductions in the frequency of administration from every 4 weeks to every 8 weeks.
“Ocular AEs are seen in the majority of patients, and the best strategies to mitigate things at this time that we know of are dose holds for grade 2 ocular events, which allow for full recovery and minimize cumulative toxicity, and then prolonging dosing intervals for subsequent doses,” Dr. Trudel said in an interview.
Previous FDA Approval Withdrawn
Of note, belantamab mafodotin previously generated high interest for relapsed/refractory multiple melanoma, with early clinical results earning the therapy accelerated approval from the US Food and Drug Administration (FDA).
However, the FDA approval was subsequently revoked when the DREAMM-3 trial filed to achieve its primary outcome of superior PFS.
Dr. Trudel explained in an interview that since then, key changes have included combinations to improve responses, “overcome early progression and allow patients to benefit from the long duration of response that is achieved with belantamab mafodotin once they respond.”
While the ocular toxicities are common, Dr. Trudel underscored that they are “reversible and manageable.”
Antibody-Drug Conjugates: Less is More?
The ocular AEs observed with belantamab mafodotin are among the variety of unique side effects that are reported with the emerging antibody-drug conjugates, which, with precision targeting, deliver highly potent cytotoxic ‘payloads’ that bind to cells, earning the drugs nicknames such as “smart bombs” and “biologic missiles.”
In the case of belantamab mafodotin, the target is the protein B-cell maturation antigen (BCMA).
In a commentary on the DREAMM-8 study presented at the meeting, Sagar Lonial, MD, chair of the department of hematology and medical oncology at the Winship Cancer Institute of Emory University in Atlanta, noted the importance of BCMA: “In describing it to fellows, I explain that everything bad that a myeloma cell wants to do is mediated through BCMA.”
He underscored, however, the need to consider strategic dosing reductions, evoking iconic architect Ludwig Mies van der Rohe’s adage “less is more.”
“These results show belantamab mafodotin is clearly effective, but the question is how do we most effectively deliver it,” he said. “The idea that more is better is not necessarily the case when we’re talking about antibody drug conjugates,” he said.
“We need to use less [drug], less frequently, and do it in a way that preserves patient function,” Dr. Lonial said. “Missed doses may actually result in better safety profiles and maintain the efficacy of the treatment,” he said.
That being said, Dr. Lonial emphasized that the DREAMM-8 study is important, showing “the longest PFS in a pom-dex combination that we’ve seen in multiple myeloma.”
And “less ocular toxicity with similar efficacy are big wins,” he added.
“Future studies should take less frequent dosing into account as they are planned and as they’re executed.”
Other Therapies
In addition to the bortezomib, pom-dex regimen, other currently approved triplet regimens used at the first relapse in multiple myeloma include selinexor-bortezomib-dexamethasone, however that regimen is associated with adverse events that can pose challenges.
Furthermore, two chimeric antigen receptor (CAR) T-cell therapies — ciltacabtagene autoleucel and idecabtagene vicleucel, have emerged and been approved for multiple myeloma patients who have received at least one and at least two previous lines of therapy, respectively.
While those CAR T-cell therapies show important improvements in PFS benefit and quality of life compared with standard triplet regimens, access is a significant stumbling block, and safety issues, including the potential for cytokine release syndrome and neurotoxic effects are also a concern.
“Each regimen for myeloma comes with unique toxicities. Thus, it is beneficial for physicians and patients to have access to multiple treatment regimens to individualize to the patient, based on patient characteristics [and] drug related factors,” Dr. Trudel said.
The current DREAMM-8 regimen represents a convenient, “off-the-shelf option that can be given in the community,” she added.
The trial was sponsored by GSK. Dr. Trudel disclosed relationships with Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Jansen Biotech, Pfizer, Roche, and Sanofi. Dr. Lonial reported ties with Takeda, Amgen, Novartis, BMS, GSK, ABBVIE, Genentech, Pfizer, Regeneron, Janssen, and TG Therapeutics.
“Taken together with results from the [previous] DREAMM-7 trial, these data highlight the potential of belantamab mafodotin-containing triplets to address an unmet need for novel regimens to treat patients with multiple myeloma at the first relapse,” senior author Suzanne Trudel, MD, of the department of medical oncology and hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada, said in presenting the late-breaking findings in a press briefing at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
The results, published concurrently in The New England Journal of Medicine, are from an interim analysis of the ongoing phase 3, global open-label DREAMM-8 trial, involving 302 patients with lenalidomide-refractory multiple myeloma who were randomized to treatment with either belantamab mafodotin (n = 155) or bortezomib (n=147), each in addition to the pom-dex combination.
The study met its primary endpoint of PFS at a median follow-up of 21.8 months, with the median PFS in the belantamab mafodotin group not met, and the rate 12.7 months for bortezomib (HR 0.52; P < .001).
The 12-month rate of PFS was significantly higher with belantamab mafodotin compared with the bortezomib group (71% versus 51%).
The overall response rates between the 2 groups were similar (77% versus 72%), however, the belantamab mafodotin group had an improved rate of complete response of 40% versus 16% in the bortezomib group.
The median duration of response was not reached with belantamab mafodotin versus 17.5 months with bortezomib.
While a positive trend for median overall survival favored belantamab mafodotin for median overall survival (HR .77), the authors note that survival data still need to mature.
Further analyses showed early and sustained separation in favor of belantamab mafodotin for PFS in all prespecified subgroups, including those with high-risk cytogenetics, and those refractory to lenalidomide and anti-CD38s.
In terms of safety, grade 3 or higher adverse events (AEs) occurred among 91% of those in the belantamab mafodotin group compared with 73% in the bortezomib group, however, when the researchers adjusted for time on treatment, the belantamab mafodotin group had similar or lower rates of AEs.
Discontinuation rates for fatal or AEs of any cause were similar in both arms.
The most prominent side effects of belantamab mafodotin are the ocular AEs that affect the majority of patients. In the DREAMM-8 study, the ocular events affected 89% of patients, with events that were grade 3 or higher occurring among 43% (grade 3, 42%; grade 4, 1%).
The ocular events, which included blurred vision, dry eye, and a foreign body sensation in the eyes, were generally reversible and managed with treatment delays and dose modifications.
As of the time of the analysis, the first occurrence of the ocular events had improved in 92% of patients and resolved in 85%, with a median time to resolution of 57 days.
The AEs resulted in treatment discontinuation for 9% of patients.
The ocular events were managed with a protocol-recommended modification of the belantamab mafodotin dose, which included dose delays until the KVA grade improved to 1 or lower, as well as reductions in the frequency of administration from every 4 weeks to every 8 weeks.
“Ocular AEs are seen in the majority of patients, and the best strategies to mitigate things at this time that we know of are dose holds for grade 2 ocular events, which allow for full recovery and minimize cumulative toxicity, and then prolonging dosing intervals for subsequent doses,” Dr. Trudel said in an interview.
Previous FDA Approval Withdrawn
Of note, belantamab mafodotin previously generated high interest for relapsed/refractory multiple melanoma, with early clinical results earning the therapy accelerated approval from the US Food and Drug Administration (FDA).
However, the FDA approval was subsequently revoked when the DREAMM-3 trial filed to achieve its primary outcome of superior PFS.
Dr. Trudel explained in an interview that since then, key changes have included combinations to improve responses, “overcome early progression and allow patients to benefit from the long duration of response that is achieved with belantamab mafodotin once they respond.”
While the ocular toxicities are common, Dr. Trudel underscored that they are “reversible and manageable.”
Antibody-Drug Conjugates: Less is More?
The ocular AEs observed with belantamab mafodotin are among the variety of unique side effects that are reported with the emerging antibody-drug conjugates, which, with precision targeting, deliver highly potent cytotoxic ‘payloads’ that bind to cells, earning the drugs nicknames such as “smart bombs” and “biologic missiles.”
In the case of belantamab mafodotin, the target is the protein B-cell maturation antigen (BCMA).
In a commentary on the DREAMM-8 study presented at the meeting, Sagar Lonial, MD, chair of the department of hematology and medical oncology at the Winship Cancer Institute of Emory University in Atlanta, noted the importance of BCMA: “In describing it to fellows, I explain that everything bad that a myeloma cell wants to do is mediated through BCMA.”
He underscored, however, the need to consider strategic dosing reductions, evoking iconic architect Ludwig Mies van der Rohe’s adage “less is more.”
“These results show belantamab mafodotin is clearly effective, but the question is how do we most effectively deliver it,” he said. “The idea that more is better is not necessarily the case when we’re talking about antibody drug conjugates,” he said.
“We need to use less [drug], less frequently, and do it in a way that preserves patient function,” Dr. Lonial said. “Missed doses may actually result in better safety profiles and maintain the efficacy of the treatment,” he said.
That being said, Dr. Lonial emphasized that the DREAMM-8 study is important, showing “the longest PFS in a pom-dex combination that we’ve seen in multiple myeloma.”
And “less ocular toxicity with similar efficacy are big wins,” he added.
“Future studies should take less frequent dosing into account as they are planned and as they’re executed.”
Other Therapies
In addition to the bortezomib, pom-dex regimen, other currently approved triplet regimens used at the first relapse in multiple myeloma include selinexor-bortezomib-dexamethasone, however that regimen is associated with adverse events that can pose challenges.
Furthermore, two chimeric antigen receptor (CAR) T-cell therapies — ciltacabtagene autoleucel and idecabtagene vicleucel, have emerged and been approved for multiple myeloma patients who have received at least one and at least two previous lines of therapy, respectively.
While those CAR T-cell therapies show important improvements in PFS benefit and quality of life compared with standard triplet regimens, access is a significant stumbling block, and safety issues, including the potential for cytokine release syndrome and neurotoxic effects are also a concern.
“Each regimen for myeloma comes with unique toxicities. Thus, it is beneficial for physicians and patients to have access to multiple treatment regimens to individualize to the patient, based on patient characteristics [and] drug related factors,” Dr. Trudel said.
The current DREAMM-8 regimen represents a convenient, “off-the-shelf option that can be given in the community,” she added.
The trial was sponsored by GSK. Dr. Trudel disclosed relationships with Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Jansen Biotech, Pfizer, Roche, and Sanofi. Dr. Lonial reported ties with Takeda, Amgen, Novartis, BMS, GSK, ABBVIE, Genentech, Pfizer, Regeneron, Janssen, and TG Therapeutics.
“Taken together with results from the [previous] DREAMM-7 trial, these data highlight the potential of belantamab mafodotin-containing triplets to address an unmet need for novel regimens to treat patients with multiple myeloma at the first relapse,” senior author Suzanne Trudel, MD, of the department of medical oncology and hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada, said in presenting the late-breaking findings in a press briefing at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
The results, published concurrently in The New England Journal of Medicine, are from an interim analysis of the ongoing phase 3, global open-label DREAMM-8 trial, involving 302 patients with lenalidomide-refractory multiple myeloma who were randomized to treatment with either belantamab mafodotin (n = 155) or bortezomib (n=147), each in addition to the pom-dex combination.
The study met its primary endpoint of PFS at a median follow-up of 21.8 months, with the median PFS in the belantamab mafodotin group not met, and the rate 12.7 months for bortezomib (HR 0.52; P < .001).
The 12-month rate of PFS was significantly higher with belantamab mafodotin compared with the bortezomib group (71% versus 51%).
The overall response rates between the 2 groups were similar (77% versus 72%), however, the belantamab mafodotin group had an improved rate of complete response of 40% versus 16% in the bortezomib group.
The median duration of response was not reached with belantamab mafodotin versus 17.5 months with bortezomib.
While a positive trend for median overall survival favored belantamab mafodotin for median overall survival (HR .77), the authors note that survival data still need to mature.
Further analyses showed early and sustained separation in favor of belantamab mafodotin for PFS in all prespecified subgroups, including those with high-risk cytogenetics, and those refractory to lenalidomide and anti-CD38s.
In terms of safety, grade 3 or higher adverse events (AEs) occurred among 91% of those in the belantamab mafodotin group compared with 73% in the bortezomib group, however, when the researchers adjusted for time on treatment, the belantamab mafodotin group had similar or lower rates of AEs.
Discontinuation rates for fatal or AEs of any cause were similar in both arms.
The most prominent side effects of belantamab mafodotin are the ocular AEs that affect the majority of patients. In the DREAMM-8 study, the ocular events affected 89% of patients, with events that were grade 3 or higher occurring among 43% (grade 3, 42%; grade 4, 1%).
The ocular events, which included blurred vision, dry eye, and a foreign body sensation in the eyes, were generally reversible and managed with treatment delays and dose modifications.
As of the time of the analysis, the first occurrence of the ocular events had improved in 92% of patients and resolved in 85%, with a median time to resolution of 57 days.
The AEs resulted in treatment discontinuation for 9% of patients.
The ocular events were managed with a protocol-recommended modification of the belantamab mafodotin dose, which included dose delays until the KVA grade improved to 1 or lower, as well as reductions in the frequency of administration from every 4 weeks to every 8 weeks.
“Ocular AEs are seen in the majority of patients, and the best strategies to mitigate things at this time that we know of are dose holds for grade 2 ocular events, which allow for full recovery and minimize cumulative toxicity, and then prolonging dosing intervals for subsequent doses,” Dr. Trudel said in an interview.
Previous FDA Approval Withdrawn
Of note, belantamab mafodotin previously generated high interest for relapsed/refractory multiple melanoma, with early clinical results earning the therapy accelerated approval from the US Food and Drug Administration (FDA).
However, the FDA approval was subsequently revoked when the DREAMM-3 trial filed to achieve its primary outcome of superior PFS.
Dr. Trudel explained in an interview that since then, key changes have included combinations to improve responses, “overcome early progression and allow patients to benefit from the long duration of response that is achieved with belantamab mafodotin once they respond.”
While the ocular toxicities are common, Dr. Trudel underscored that they are “reversible and manageable.”
Antibody-Drug Conjugates: Less is More?
The ocular AEs observed with belantamab mafodotin are among the variety of unique side effects that are reported with the emerging antibody-drug conjugates, which, with precision targeting, deliver highly potent cytotoxic ‘payloads’ that bind to cells, earning the drugs nicknames such as “smart bombs” and “biologic missiles.”
In the case of belantamab mafodotin, the target is the protein B-cell maturation antigen (BCMA).
In a commentary on the DREAMM-8 study presented at the meeting, Sagar Lonial, MD, chair of the department of hematology and medical oncology at the Winship Cancer Institute of Emory University in Atlanta, noted the importance of BCMA: “In describing it to fellows, I explain that everything bad that a myeloma cell wants to do is mediated through BCMA.”
He underscored, however, the need to consider strategic dosing reductions, evoking iconic architect Ludwig Mies van der Rohe’s adage “less is more.”
“These results show belantamab mafodotin is clearly effective, but the question is how do we most effectively deliver it,” he said. “The idea that more is better is not necessarily the case when we’re talking about antibody drug conjugates,” he said.
“We need to use less [drug], less frequently, and do it in a way that preserves patient function,” Dr. Lonial said. “Missed doses may actually result in better safety profiles and maintain the efficacy of the treatment,” he said.
That being said, Dr. Lonial emphasized that the DREAMM-8 study is important, showing “the longest PFS in a pom-dex combination that we’ve seen in multiple myeloma.”
And “less ocular toxicity with similar efficacy are big wins,” he added.
“Future studies should take less frequent dosing into account as they are planned and as they’re executed.”
Other Therapies
In addition to the bortezomib, pom-dex regimen, other currently approved triplet regimens used at the first relapse in multiple myeloma include selinexor-bortezomib-dexamethasone, however that regimen is associated with adverse events that can pose challenges.
Furthermore, two chimeric antigen receptor (CAR) T-cell therapies — ciltacabtagene autoleucel and idecabtagene vicleucel, have emerged and been approved for multiple myeloma patients who have received at least one and at least two previous lines of therapy, respectively.
While those CAR T-cell therapies show important improvements in PFS benefit and quality of life compared with standard triplet regimens, access is a significant stumbling block, and safety issues, including the potential for cytokine release syndrome and neurotoxic effects are also a concern.
“Each regimen for myeloma comes with unique toxicities. Thus, it is beneficial for physicians and patients to have access to multiple treatment regimens to individualize to the patient, based on patient characteristics [and] drug related factors,” Dr. Trudel said.
The current DREAMM-8 regimen represents a convenient, “off-the-shelf option that can be given in the community,” she added.
The trial was sponsored by GSK. Dr. Trudel disclosed relationships with Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Jansen Biotech, Pfizer, Roche, and Sanofi. Dr. Lonial reported ties with Takeda, Amgen, Novartis, BMS, GSK, ABBVIE, Genentech, Pfizer, Regeneron, Janssen, and TG Therapeutics.
FROM ASCO 2024
The ASCO Annual Meeting Starts This Week
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
ASTRO Releases New EBRT Guideline for Symptomatic Bone Mets
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
FROM PRACTICAL RADIATION ONCOLOGY

