User login
Motor function restored in three men after complete paralysis from spinal cord injury
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Cement found in man’s heart after spinal surgery
, according to a new report published in the New England Journal of Medicine.
The 56-year-old man, who was not identified in the report, went to the emergency room after experiencing 2 days of chest pain and shortness of breath. Imaging scans showed that the chest pain was caused by a foreign object, and he was rushed to surgery.
Surgeons then located and removed a thin, sharp, cylindrical piece of cement and repaired the damage to the patient’s heart. The cement had pierced the upper right chamber of his heart and his right lung, according to the report authors from the Yale University School of Medicine.
A week before, the man had undergone a spinal surgery known as kyphoplasty. The procedure treats spine injuries by injecting a special type of medical cement into damaged vertebrae, according to USA Today. The cement had leaked into the patient’s body, hardened, and traveled to his heart.
The man has now “nearly recovered” since the heart surgery and cement removal, which occurred about a month ago, the journal report stated. He experienced no additional complications.
Cement leakage after kyphoplasty can happen but is an extremely rare complication. Less than 2% of patients who undergo the procedure for osteoporosis or brittle bones have complications, according to patient information from the American Association of Neurological Surgeons.
A version of this article first appeared on WebMD.com.
, according to a new report published in the New England Journal of Medicine.
The 56-year-old man, who was not identified in the report, went to the emergency room after experiencing 2 days of chest pain and shortness of breath. Imaging scans showed that the chest pain was caused by a foreign object, and he was rushed to surgery.
Surgeons then located and removed a thin, sharp, cylindrical piece of cement and repaired the damage to the patient’s heart. The cement had pierced the upper right chamber of his heart and his right lung, according to the report authors from the Yale University School of Medicine.
A week before, the man had undergone a spinal surgery known as kyphoplasty. The procedure treats spine injuries by injecting a special type of medical cement into damaged vertebrae, according to USA Today. The cement had leaked into the patient’s body, hardened, and traveled to his heart.
The man has now “nearly recovered” since the heart surgery and cement removal, which occurred about a month ago, the journal report stated. He experienced no additional complications.
Cement leakage after kyphoplasty can happen but is an extremely rare complication. Less than 2% of patients who undergo the procedure for osteoporosis or brittle bones have complications, according to patient information from the American Association of Neurological Surgeons.
A version of this article first appeared on WebMD.com.
, according to a new report published in the New England Journal of Medicine.
The 56-year-old man, who was not identified in the report, went to the emergency room after experiencing 2 days of chest pain and shortness of breath. Imaging scans showed that the chest pain was caused by a foreign object, and he was rushed to surgery.
Surgeons then located and removed a thin, sharp, cylindrical piece of cement and repaired the damage to the patient’s heart. The cement had pierced the upper right chamber of his heart and his right lung, according to the report authors from the Yale University School of Medicine.
A week before, the man had undergone a spinal surgery known as kyphoplasty. The procedure treats spine injuries by injecting a special type of medical cement into damaged vertebrae, according to USA Today. The cement had leaked into the patient’s body, hardened, and traveled to his heart.
The man has now “nearly recovered” since the heart surgery and cement removal, which occurred about a month ago, the journal report stated. He experienced no additional complications.
Cement leakage after kyphoplasty can happen but is an extremely rare complication. Less than 2% of patients who undergo the procedure for osteoporosis or brittle bones have complications, according to patient information from the American Association of Neurological Surgeons.
A version of this article first appeared on WebMD.com.
Near-hanging injuries: Critical care, psychiatric management
Suicide by hanging results in many deaths, and half of those survivors who are admitted later die from cardiac arrest.
Although hanging is a common form of suicide, studies of the clinical outcomes of near-hanging injury are rare. To address this void, Louise de Charentenay, MD, of the Medical-Surgical Intensive Care Unit, Centre Hospitalier de Versailles (France) and colleagues examined the vital and functional outcomes of more than 800 patients with suicidal near-hanging injury over 2 decades. Despite the high in-hospital mortality rate among survivors, those who do survive have an excellent chance of a full neurocognitive recovery. The investigators published their findings in Chest.
New data on near-hanging injuries
Near hanging refers to strangulation or hanging that doesn’t immediately lead to death. Little data have been available on this subject, particularly on the morbidity and mortality of patients admitted to the ICU following near-hanging injuries. In a retrospective analysis spanning 23 years (1992-2014), researchers looked at outcomes and early predictors of hospital deaths in patients with this injury. The study included 886 adult patients who were admitted to 31 university or university-affiliated ICUs in France and Belgium following successful resuscitation of suicidal near-hanging injury.
Investigators used logistic multivariate regression to report vital and functional outcomes at hospital discharge as a primary objective. They also aimed to identify predictors of hospital mortality in these patients.
Among all patients, 450 (50.8%) had hanging-induced cardiac arrest and of these, 371 (95.4%) eventually died. Although the rate of crude hospital deaths decreased over the 23-year period, hanging-induced cardiac arrest emerged as the strongest predictor of hospital mortality, followed by high blood lactate and hyperglycemia at ICU admission. “Hanging-induced cardiac arrest and worse consciousness impairment at ICU admission are directly related to the hanging, whereas higher glycemia and lactate levels at ICU admission represent biochemical markers of physiologic perturbation and injury severity that may suggest avenues for improvement in prehospital care,” wrote the investigators.
More than 56% of the patients survived to discharge, with a majority achieving favorable outcomes (a Glasgow Outcome Scale scores of 4 or 5 at discharge).
‘COVID-lateral’ damage and ICU management
Casey D. Bryant, MD, of the department of anesthesiology and the department of emergency medicine at Wake Forest Baptist Health, Winston-Salem, N.C., has treated these patients in the ICU and is prepared to see more of them in light of the current situation. He said in an interview, “The “COVID-lateral” damage being unleashed on the population as a result of increased isolation, lack of access to resources, higher unemployment, and increased substance abuse was detailed recently in an article by one of my colleagues, Dr. Seth Hawkins (Emerg Med News. 2020 Jun;42[6]:1,31-2). According to the Centers for Disease Control and Prevention, hanging is the second leading cause of suicide in the United States, and one can only assume that with increased mental health crises there will also be an increased number of hanging attempts.”
Dr. Bryant suggested that the first task of doctors who learn that a near-hanging patient has been admitted is to “recover from the gut-punch you feel when you learn that a fellow human has tried to take their own life.” Once one is composed, he said, the first order of business is to come up with a treatment plan, one that typically begins with the airway. “These patients are at a high risk for cervical vertebrae injury (e.g., hangman’s fracture), spinal cord injury, tracheal injury, and neck vessel injury or dissection, so care must be taken to maintain in-line stabilization and limit movement of the neck during intubation while also being prepared for all manner of airway disasters. After airway management, addressing traumatic injuries, and initial stabilization, the focus then shifts to ‘bread and butter’ critical care, including optimization of ventilator management, titration of analgosedation, providing adequate nutrition, and strict avoidance of hypoxia, hypotension, fever, and either hyper- or hypoglycemia.”
Dr. Bryant noted that targeted temperature management prescriptions remain an area of debate in those with comatose state after hanging, but fever should absolutely be avoided. He added: “As the path to recovery begins to be forged, the full gamut of mental health resources should be provided to the patients in order to give them the best chance for success once they leave the ICU, and ultimately the hospital.”
The different hospitals seemed to have varying degrees of success in saving these patients, which is surprising, Mangala Narasimhan, DO, FCCP, regional director of critical care, director of the acute lung injury/ECMO center at Northwell and a professor of medicine at the Hofstra/Northwell School of Medicine, New York, said in an interview. “Usually, the death rate for cardiac arrest is high and the death rate for hanging is high. But here, it was high in some places and low in others.” Different time frames from presenting from hanging and different treatments may explain this, said Dr. Narasimhan.
Patient characteristics
Consistent with previous research, near-hanging patients are predominantly male, have at least one psychiatric diagnosis and a previous suicidal attempt (rarely by hanging), and abuse substances such as an alcohol, Stéphane Legriel, MD, PhD, the study’s corresponding author, said in an interview. Overall, 67.7% of the patients had a diagnosed mental illness and 30% had previously attempted suicide. Most of the hangings took place at home (79%), while some took place in a hospital ward (6%), a correctional facility (7%), or outside (5%).
The study had several limitations: It applied only to near-hanging patients admitted to the ICU, and its long duration may have resulted in heterogeneity of the population and therapeutic interventions, and in some missing data. “However, the multivariate analysis was adjusted for the time period and we carried out a sensitivity analysis after multiple imputation for missing data by means of chained equations, which reinforces confidence in our findings,” Dr. Legriel said. Next steps are to conduct a prospective data collection.
Postdischarge recovery and psychiatric follow-up
Those left to treat survivors of near-hangings are psychiatrists and other mental health clinicians, Eric M. Plakun, MD, said in an interview.
“Some of these survivors will regret they survived and remain high suicide risks. Some will feel their lives are transformed or at least no longer as intensely drawn to suicide as a solution to a life filled with the impact of adversity, trauma, comorbidity, and other struggles – but even these individuals will still have to face the often complex underlying issues that led them to choose suicide as a solution,” said Dr. Plakun, medical director and CEO of the Austen Riggs Center in Stockbridge, Mass.
Patients with medically serious suicide attempts are seen a lot at Austen Riggs, he said, because acute inpatient settings are designed for brief, crisis-focused treatment of those for whom safety is an issue. After the crisis has been stabilized, patients are discharged, and then must begin to achieve recovery as outpatients, he said.
John Kruse, MD, PhD, a psychiatrist who practices in San Francisco, praised the size and the breath of the study. “One limitation was the reliance on hospital records, without an opportunity to directly evaluate or interview the patients involved.”
The authors disclosed no conflicts of interest. The study received grant support from the French public funding agency, Délégation la Recherche Clinique et de l’Innovation in Versailles, France.
SOURCE: de Charentenay L et al. 2020 Aug 3. doi: 10.1016/j.chest.2020.07.064
Suicide by hanging results in many deaths, and half of those survivors who are admitted later die from cardiac arrest.
Although hanging is a common form of suicide, studies of the clinical outcomes of near-hanging injury are rare. To address this void, Louise de Charentenay, MD, of the Medical-Surgical Intensive Care Unit, Centre Hospitalier de Versailles (France) and colleagues examined the vital and functional outcomes of more than 800 patients with suicidal near-hanging injury over 2 decades. Despite the high in-hospital mortality rate among survivors, those who do survive have an excellent chance of a full neurocognitive recovery. The investigators published their findings in Chest.
New data on near-hanging injuries
Near hanging refers to strangulation or hanging that doesn’t immediately lead to death. Little data have been available on this subject, particularly on the morbidity and mortality of patients admitted to the ICU following near-hanging injuries. In a retrospective analysis spanning 23 years (1992-2014), researchers looked at outcomes and early predictors of hospital deaths in patients with this injury. The study included 886 adult patients who were admitted to 31 university or university-affiliated ICUs in France and Belgium following successful resuscitation of suicidal near-hanging injury.
Investigators used logistic multivariate regression to report vital and functional outcomes at hospital discharge as a primary objective. They also aimed to identify predictors of hospital mortality in these patients.
Among all patients, 450 (50.8%) had hanging-induced cardiac arrest and of these, 371 (95.4%) eventually died. Although the rate of crude hospital deaths decreased over the 23-year period, hanging-induced cardiac arrest emerged as the strongest predictor of hospital mortality, followed by high blood lactate and hyperglycemia at ICU admission. “Hanging-induced cardiac arrest and worse consciousness impairment at ICU admission are directly related to the hanging, whereas higher glycemia and lactate levels at ICU admission represent biochemical markers of physiologic perturbation and injury severity that may suggest avenues for improvement in prehospital care,” wrote the investigators.
More than 56% of the patients survived to discharge, with a majority achieving favorable outcomes (a Glasgow Outcome Scale scores of 4 or 5 at discharge).
‘COVID-lateral’ damage and ICU management
Casey D. Bryant, MD, of the department of anesthesiology and the department of emergency medicine at Wake Forest Baptist Health, Winston-Salem, N.C., has treated these patients in the ICU and is prepared to see more of them in light of the current situation. He said in an interview, “The “COVID-lateral” damage being unleashed on the population as a result of increased isolation, lack of access to resources, higher unemployment, and increased substance abuse was detailed recently in an article by one of my colleagues, Dr. Seth Hawkins (Emerg Med News. 2020 Jun;42[6]:1,31-2). According to the Centers for Disease Control and Prevention, hanging is the second leading cause of suicide in the United States, and one can only assume that with increased mental health crises there will also be an increased number of hanging attempts.”
Dr. Bryant suggested that the first task of doctors who learn that a near-hanging patient has been admitted is to “recover from the gut-punch you feel when you learn that a fellow human has tried to take their own life.” Once one is composed, he said, the first order of business is to come up with a treatment plan, one that typically begins with the airway. “These patients are at a high risk for cervical vertebrae injury (e.g., hangman’s fracture), spinal cord injury, tracheal injury, and neck vessel injury or dissection, so care must be taken to maintain in-line stabilization and limit movement of the neck during intubation while also being prepared for all manner of airway disasters. After airway management, addressing traumatic injuries, and initial stabilization, the focus then shifts to ‘bread and butter’ critical care, including optimization of ventilator management, titration of analgosedation, providing adequate nutrition, and strict avoidance of hypoxia, hypotension, fever, and either hyper- or hypoglycemia.”
Dr. Bryant noted that targeted temperature management prescriptions remain an area of debate in those with comatose state after hanging, but fever should absolutely be avoided. He added: “As the path to recovery begins to be forged, the full gamut of mental health resources should be provided to the patients in order to give them the best chance for success once they leave the ICU, and ultimately the hospital.”
The different hospitals seemed to have varying degrees of success in saving these patients, which is surprising, Mangala Narasimhan, DO, FCCP, regional director of critical care, director of the acute lung injury/ECMO center at Northwell and a professor of medicine at the Hofstra/Northwell School of Medicine, New York, said in an interview. “Usually, the death rate for cardiac arrest is high and the death rate for hanging is high. But here, it was high in some places and low in others.” Different time frames from presenting from hanging and different treatments may explain this, said Dr. Narasimhan.
Patient characteristics
Consistent with previous research, near-hanging patients are predominantly male, have at least one psychiatric diagnosis and a previous suicidal attempt (rarely by hanging), and abuse substances such as an alcohol, Stéphane Legriel, MD, PhD, the study’s corresponding author, said in an interview. Overall, 67.7% of the patients had a diagnosed mental illness and 30% had previously attempted suicide. Most of the hangings took place at home (79%), while some took place in a hospital ward (6%), a correctional facility (7%), or outside (5%).
The study had several limitations: It applied only to near-hanging patients admitted to the ICU, and its long duration may have resulted in heterogeneity of the population and therapeutic interventions, and in some missing data. “However, the multivariate analysis was adjusted for the time period and we carried out a sensitivity analysis after multiple imputation for missing data by means of chained equations, which reinforces confidence in our findings,” Dr. Legriel said. Next steps are to conduct a prospective data collection.
Postdischarge recovery and psychiatric follow-up
Those left to treat survivors of near-hangings are psychiatrists and other mental health clinicians, Eric M. Plakun, MD, said in an interview.
“Some of these survivors will regret they survived and remain high suicide risks. Some will feel their lives are transformed or at least no longer as intensely drawn to suicide as a solution to a life filled with the impact of adversity, trauma, comorbidity, and other struggles – but even these individuals will still have to face the often complex underlying issues that led them to choose suicide as a solution,” said Dr. Plakun, medical director and CEO of the Austen Riggs Center in Stockbridge, Mass.
Patients with medically serious suicide attempts are seen a lot at Austen Riggs, he said, because acute inpatient settings are designed for brief, crisis-focused treatment of those for whom safety is an issue. After the crisis has been stabilized, patients are discharged, and then must begin to achieve recovery as outpatients, he said.
John Kruse, MD, PhD, a psychiatrist who practices in San Francisco, praised the size and the breath of the study. “One limitation was the reliance on hospital records, without an opportunity to directly evaluate or interview the patients involved.”
The authors disclosed no conflicts of interest. The study received grant support from the French public funding agency, Délégation la Recherche Clinique et de l’Innovation in Versailles, France.
SOURCE: de Charentenay L et al. 2020 Aug 3. doi: 10.1016/j.chest.2020.07.064
Suicide by hanging results in many deaths, and half of those survivors who are admitted later die from cardiac arrest.
Although hanging is a common form of suicide, studies of the clinical outcomes of near-hanging injury are rare. To address this void, Louise de Charentenay, MD, of the Medical-Surgical Intensive Care Unit, Centre Hospitalier de Versailles (France) and colleagues examined the vital and functional outcomes of more than 800 patients with suicidal near-hanging injury over 2 decades. Despite the high in-hospital mortality rate among survivors, those who do survive have an excellent chance of a full neurocognitive recovery. The investigators published their findings in Chest.
New data on near-hanging injuries
Near hanging refers to strangulation or hanging that doesn’t immediately lead to death. Little data have been available on this subject, particularly on the morbidity and mortality of patients admitted to the ICU following near-hanging injuries. In a retrospective analysis spanning 23 years (1992-2014), researchers looked at outcomes and early predictors of hospital deaths in patients with this injury. The study included 886 adult patients who were admitted to 31 university or university-affiliated ICUs in France and Belgium following successful resuscitation of suicidal near-hanging injury.
Investigators used logistic multivariate regression to report vital and functional outcomes at hospital discharge as a primary objective. They also aimed to identify predictors of hospital mortality in these patients.
Among all patients, 450 (50.8%) had hanging-induced cardiac arrest and of these, 371 (95.4%) eventually died. Although the rate of crude hospital deaths decreased over the 23-year period, hanging-induced cardiac arrest emerged as the strongest predictor of hospital mortality, followed by high blood lactate and hyperglycemia at ICU admission. “Hanging-induced cardiac arrest and worse consciousness impairment at ICU admission are directly related to the hanging, whereas higher glycemia and lactate levels at ICU admission represent biochemical markers of physiologic perturbation and injury severity that may suggest avenues for improvement in prehospital care,” wrote the investigators.
More than 56% of the patients survived to discharge, with a majority achieving favorable outcomes (a Glasgow Outcome Scale scores of 4 or 5 at discharge).
‘COVID-lateral’ damage and ICU management
Casey D. Bryant, MD, of the department of anesthesiology and the department of emergency medicine at Wake Forest Baptist Health, Winston-Salem, N.C., has treated these patients in the ICU and is prepared to see more of them in light of the current situation. He said in an interview, “The “COVID-lateral” damage being unleashed on the population as a result of increased isolation, lack of access to resources, higher unemployment, and increased substance abuse was detailed recently in an article by one of my colleagues, Dr. Seth Hawkins (Emerg Med News. 2020 Jun;42[6]:1,31-2). According to the Centers for Disease Control and Prevention, hanging is the second leading cause of suicide in the United States, and one can only assume that with increased mental health crises there will also be an increased number of hanging attempts.”
Dr. Bryant suggested that the first task of doctors who learn that a near-hanging patient has been admitted is to “recover from the gut-punch you feel when you learn that a fellow human has tried to take their own life.” Once one is composed, he said, the first order of business is to come up with a treatment plan, one that typically begins with the airway. “These patients are at a high risk for cervical vertebrae injury (e.g., hangman’s fracture), spinal cord injury, tracheal injury, and neck vessel injury or dissection, so care must be taken to maintain in-line stabilization and limit movement of the neck during intubation while also being prepared for all manner of airway disasters. After airway management, addressing traumatic injuries, and initial stabilization, the focus then shifts to ‘bread and butter’ critical care, including optimization of ventilator management, titration of analgosedation, providing adequate nutrition, and strict avoidance of hypoxia, hypotension, fever, and either hyper- or hypoglycemia.”
Dr. Bryant noted that targeted temperature management prescriptions remain an area of debate in those with comatose state after hanging, but fever should absolutely be avoided. He added: “As the path to recovery begins to be forged, the full gamut of mental health resources should be provided to the patients in order to give them the best chance for success once they leave the ICU, and ultimately the hospital.”
The different hospitals seemed to have varying degrees of success in saving these patients, which is surprising, Mangala Narasimhan, DO, FCCP, regional director of critical care, director of the acute lung injury/ECMO center at Northwell and a professor of medicine at the Hofstra/Northwell School of Medicine, New York, said in an interview. “Usually, the death rate for cardiac arrest is high and the death rate for hanging is high. But here, it was high in some places and low in others.” Different time frames from presenting from hanging and different treatments may explain this, said Dr. Narasimhan.
Patient characteristics
Consistent with previous research, near-hanging patients are predominantly male, have at least one psychiatric diagnosis and a previous suicidal attempt (rarely by hanging), and abuse substances such as an alcohol, Stéphane Legriel, MD, PhD, the study’s corresponding author, said in an interview. Overall, 67.7% of the patients had a diagnosed mental illness and 30% had previously attempted suicide. Most of the hangings took place at home (79%), while some took place in a hospital ward (6%), a correctional facility (7%), or outside (5%).
The study had several limitations: It applied only to near-hanging patients admitted to the ICU, and its long duration may have resulted in heterogeneity of the population and therapeutic interventions, and in some missing data. “However, the multivariate analysis was adjusted for the time period and we carried out a sensitivity analysis after multiple imputation for missing data by means of chained equations, which reinforces confidence in our findings,” Dr. Legriel said. Next steps are to conduct a prospective data collection.
Postdischarge recovery and psychiatric follow-up
Those left to treat survivors of near-hangings are psychiatrists and other mental health clinicians, Eric M. Plakun, MD, said in an interview.
“Some of these survivors will regret they survived and remain high suicide risks. Some will feel their lives are transformed or at least no longer as intensely drawn to suicide as a solution to a life filled with the impact of adversity, trauma, comorbidity, and other struggles – but even these individuals will still have to face the often complex underlying issues that led them to choose suicide as a solution,” said Dr. Plakun, medical director and CEO of the Austen Riggs Center in Stockbridge, Mass.
Patients with medically serious suicide attempts are seen a lot at Austen Riggs, he said, because acute inpatient settings are designed for brief, crisis-focused treatment of those for whom safety is an issue. After the crisis has been stabilized, patients are discharged, and then must begin to achieve recovery as outpatients, he said.
John Kruse, MD, PhD, a psychiatrist who practices in San Francisco, praised the size and the breath of the study. “One limitation was the reliance on hospital records, without an opportunity to directly evaluate or interview the patients involved.”
The authors disclosed no conflicts of interest. The study received grant support from the French public funding agency, Délégation la Recherche Clinique et de l’Innovation in Versailles, France.
SOURCE: de Charentenay L et al. 2020 Aug 3. doi: 10.1016/j.chest.2020.07.064
TBI deaths from falls on the rise
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.
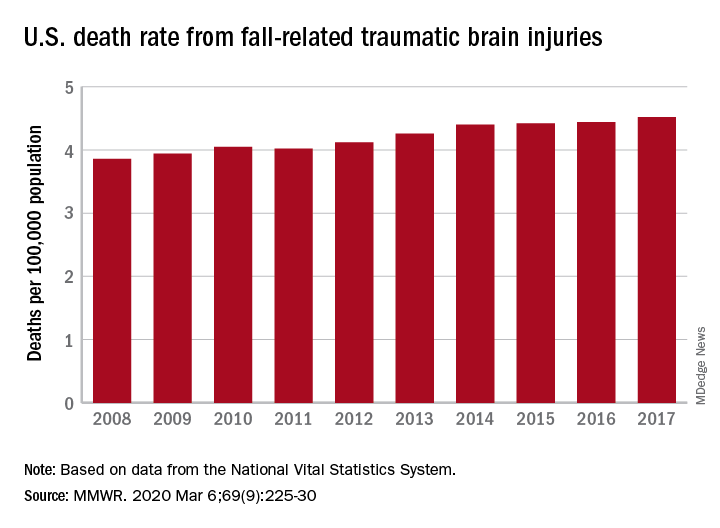
Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
FROM MMWR
Staged hemispheric embolization: How to treat hemimegalencephaly within days of birth
BALTIMORE – About one in 4,000 children are born with hemimegalencephaly, meaning one brain hemisphere is abnormally formed and larger than the other.
The abnormal hemisphere causes seizures, and when they become intractable, the standard of care is to remove it as soon as possible; the longer the abnormal hemisphere is left in, the worse children do developmentally, and the less likely hemispherectomy will stop the seizures.
A problem comes up, however, when children become intractable before they’re 3 months old: “Neurosurgeons won’t touch them,” said Taeun Chang, MD, a neonatal neurointensivist at Children’s National Medical Center in Washington.
Newborns’ coagulation systems aren’t fully developed, and the risk of fatal hemorrhage is too high, she explained.
Out of what she said was a sense of “desperation” to address the situation, Dr. Chang has spearheaded a new approach for newborns at Children’s National, serial glue embolization to induce targeted strokes in the affected hemisphere. She reported on the first five cases at the American Epilepsy Society annual meeting.
At this point, “I feel like we’ve pretty much figured out the technique in terms of minimizing the complications. There’s no reason to wait anymore” for surgery as newborns get worse and worse, she said.
The technique
In two or three stages over several days, the major branches of the affected hemisphere’s anterior, middle, and posterior cerebral arteries are embolized. “You have to glue a long area and put in a lot of glue and glue up the secondary branches because [newborns] are so good at forming collaterals,” Dr. Chang said.
Fresh frozen plasma is given before and after each embolization session to boost coagulation proteins. Nicardipine is given during the procedure to prevent vasospasms. The one death in the series, case four, was in an 11-day old girl who vasospasmed, ruptured an artery over the tip of the guidewire, and hemorrhaged.
After the procedure, body temperature is kept at 36° C to prevent fever; sodium is kept high, and ins and outs are matched, to reduce brain edema; and blood pressure is tightly controlled. Children are kept on EEG during embolization and for days afterwards, and seizures, if any, are treated. The next embolization comes after peak swelling has passed in about 48-72 hours.
“The reason we can get away with this without herniation is that newborns’ skulls are soft, and their sutures are open,” so cerebral edema is manageable, Dr. Chang said.
Learning curve and outcomes
“What we learned in the first two cases” – a 23-day-old boy and 49-day-old girl – “was to create effective strokes. That’s not something any of us are taught to do,” she said.
“We were not trying to destroy the whole hemisphere, just the area that was seizing on EEG.” That was a mistake, she said: Adjacent areas began seizing and both children went on to anatomical hemispherectomies and needed shunts.
They are 5 years old now, and both on four seizure medications. The boy is in a wheelchair, fed by a G-tube, and has fewer than 20 words. The girl has a gait trainer, is fed mostly by G-tube, and has more than 50 words.
The third patient had her middle and posterior cerebral arteries embolized beginning when she was 43 days old. She was seizure free when she left the NICU, but eventually had a functional hemispherectomy. She’s 2 years old now, eating by mouth, in a gait trainer, and speaks in one- or two-word sentences. She’s on three seizure medications.
Outcomes have been best for patient five. Her posterior, middle, and anterior cerebral arteries were embolized starting at 14 days. She’s 1 year old now, seizure free on three medications, eating by G-tube and mouth, and has three-five words.
Dr. Chang said that newborns with hemimegalencephaly at Children’s National aren’t lingering as long on failing drug regimens these days. “We go to intervention now that we have this option” after they fail just two or three medications.
Given that the fifth patient, treated at 2 weeks old, is the only one who has been seizure free, she suspects it’s probably best to do embolization sooner rather than later, just as with anatomical hemispherectomy in older children. “We’ve got the sense that even a couple of weeks makes a difference. People need to come to us sooner,” Dr. Chang said.
It’s possible embolization could be a sound alternative to surgery even after 3 months of age. Focal embolization might also be a viable alternative to surgery to knock out epileptogenic lesions in children with tuberous sclerosis. Dr. Chang and her colleagues are interested in those and other possibilities, and plan to continue to develop the approach, she said.
There was no funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Chang T et al. AES 2019, Abstract 1.225.
BALTIMORE – About one in 4,000 children are born with hemimegalencephaly, meaning one brain hemisphere is abnormally formed and larger than the other.
The abnormal hemisphere causes seizures, and when they become intractable, the standard of care is to remove it as soon as possible; the longer the abnormal hemisphere is left in, the worse children do developmentally, and the less likely hemispherectomy will stop the seizures.
A problem comes up, however, when children become intractable before they’re 3 months old: “Neurosurgeons won’t touch them,” said Taeun Chang, MD, a neonatal neurointensivist at Children’s National Medical Center in Washington.
Newborns’ coagulation systems aren’t fully developed, and the risk of fatal hemorrhage is too high, she explained.
Out of what she said was a sense of “desperation” to address the situation, Dr. Chang has spearheaded a new approach for newborns at Children’s National, serial glue embolization to induce targeted strokes in the affected hemisphere. She reported on the first five cases at the American Epilepsy Society annual meeting.
At this point, “I feel like we’ve pretty much figured out the technique in terms of minimizing the complications. There’s no reason to wait anymore” for surgery as newborns get worse and worse, she said.
The technique
In two or three stages over several days, the major branches of the affected hemisphere’s anterior, middle, and posterior cerebral arteries are embolized. “You have to glue a long area and put in a lot of glue and glue up the secondary branches because [newborns] are so good at forming collaterals,” Dr. Chang said.
Fresh frozen plasma is given before and after each embolization session to boost coagulation proteins. Nicardipine is given during the procedure to prevent vasospasms. The one death in the series, case four, was in an 11-day old girl who vasospasmed, ruptured an artery over the tip of the guidewire, and hemorrhaged.
After the procedure, body temperature is kept at 36° C to prevent fever; sodium is kept high, and ins and outs are matched, to reduce brain edema; and blood pressure is tightly controlled. Children are kept on EEG during embolization and for days afterwards, and seizures, if any, are treated. The next embolization comes after peak swelling has passed in about 48-72 hours.
“The reason we can get away with this without herniation is that newborns’ skulls are soft, and their sutures are open,” so cerebral edema is manageable, Dr. Chang said.
Learning curve and outcomes
“What we learned in the first two cases” – a 23-day-old boy and 49-day-old girl – “was to create effective strokes. That’s not something any of us are taught to do,” she said.
“We were not trying to destroy the whole hemisphere, just the area that was seizing on EEG.” That was a mistake, she said: Adjacent areas began seizing and both children went on to anatomical hemispherectomies and needed shunts.
They are 5 years old now, and both on four seizure medications. The boy is in a wheelchair, fed by a G-tube, and has fewer than 20 words. The girl has a gait trainer, is fed mostly by G-tube, and has more than 50 words.
The third patient had her middle and posterior cerebral arteries embolized beginning when she was 43 days old. She was seizure free when she left the NICU, but eventually had a functional hemispherectomy. She’s 2 years old now, eating by mouth, in a gait trainer, and speaks in one- or two-word sentences. She’s on three seizure medications.
Outcomes have been best for patient five. Her posterior, middle, and anterior cerebral arteries were embolized starting at 14 days. She’s 1 year old now, seizure free on three medications, eating by G-tube and mouth, and has three-five words.
Dr. Chang said that newborns with hemimegalencephaly at Children’s National aren’t lingering as long on failing drug regimens these days. “We go to intervention now that we have this option” after they fail just two or three medications.
Given that the fifth patient, treated at 2 weeks old, is the only one who has been seizure free, she suspects it’s probably best to do embolization sooner rather than later, just as with anatomical hemispherectomy in older children. “We’ve got the sense that even a couple of weeks makes a difference. People need to come to us sooner,” Dr. Chang said.
It’s possible embolization could be a sound alternative to surgery even after 3 months of age. Focal embolization might also be a viable alternative to surgery to knock out epileptogenic lesions in children with tuberous sclerosis. Dr. Chang and her colleagues are interested in those and other possibilities, and plan to continue to develop the approach, she said.
There was no funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Chang T et al. AES 2019, Abstract 1.225.
BALTIMORE – About one in 4,000 children are born with hemimegalencephaly, meaning one brain hemisphere is abnormally formed and larger than the other.
The abnormal hemisphere causes seizures, and when they become intractable, the standard of care is to remove it as soon as possible; the longer the abnormal hemisphere is left in, the worse children do developmentally, and the less likely hemispherectomy will stop the seizures.
A problem comes up, however, when children become intractable before they’re 3 months old: “Neurosurgeons won’t touch them,” said Taeun Chang, MD, a neonatal neurointensivist at Children’s National Medical Center in Washington.
Newborns’ coagulation systems aren’t fully developed, and the risk of fatal hemorrhage is too high, she explained.
Out of what she said was a sense of “desperation” to address the situation, Dr. Chang has spearheaded a new approach for newborns at Children’s National, serial glue embolization to induce targeted strokes in the affected hemisphere. She reported on the first five cases at the American Epilepsy Society annual meeting.
At this point, “I feel like we’ve pretty much figured out the technique in terms of minimizing the complications. There’s no reason to wait anymore” for surgery as newborns get worse and worse, she said.
The technique
In two or three stages over several days, the major branches of the affected hemisphere’s anterior, middle, and posterior cerebral arteries are embolized. “You have to glue a long area and put in a lot of glue and glue up the secondary branches because [newborns] are so good at forming collaterals,” Dr. Chang said.
Fresh frozen plasma is given before and after each embolization session to boost coagulation proteins. Nicardipine is given during the procedure to prevent vasospasms. The one death in the series, case four, was in an 11-day old girl who vasospasmed, ruptured an artery over the tip of the guidewire, and hemorrhaged.
After the procedure, body temperature is kept at 36° C to prevent fever; sodium is kept high, and ins and outs are matched, to reduce brain edema; and blood pressure is tightly controlled. Children are kept on EEG during embolization and for days afterwards, and seizures, if any, are treated. The next embolization comes after peak swelling has passed in about 48-72 hours.
“The reason we can get away with this without herniation is that newborns’ skulls are soft, and their sutures are open,” so cerebral edema is manageable, Dr. Chang said.
Learning curve and outcomes
“What we learned in the first two cases” – a 23-day-old boy and 49-day-old girl – “was to create effective strokes. That’s not something any of us are taught to do,” she said.
“We were not trying to destroy the whole hemisphere, just the area that was seizing on EEG.” That was a mistake, she said: Adjacent areas began seizing and both children went on to anatomical hemispherectomies and needed shunts.
They are 5 years old now, and both on four seizure medications. The boy is in a wheelchair, fed by a G-tube, and has fewer than 20 words. The girl has a gait trainer, is fed mostly by G-tube, and has more than 50 words.
The third patient had her middle and posterior cerebral arteries embolized beginning when she was 43 days old. She was seizure free when she left the NICU, but eventually had a functional hemispherectomy. She’s 2 years old now, eating by mouth, in a gait trainer, and speaks in one- or two-word sentences. She’s on three seizure medications.
Outcomes have been best for patient five. Her posterior, middle, and anterior cerebral arteries were embolized starting at 14 days. She’s 1 year old now, seizure free on three medications, eating by G-tube and mouth, and has three-five words.
Dr. Chang said that newborns with hemimegalencephaly at Children’s National aren’t lingering as long on failing drug regimens these days. “We go to intervention now that we have this option” after they fail just two or three medications.
Given that the fifth patient, treated at 2 weeks old, is the only one who has been seizure free, she suspects it’s probably best to do embolization sooner rather than later, just as with anatomical hemispherectomy in older children. “We’ve got the sense that even a couple of weeks makes a difference. People need to come to us sooner,” Dr. Chang said.
It’s possible embolization could be a sound alternative to surgery even after 3 months of age. Focal embolization might also be a viable alternative to surgery to knock out epileptogenic lesions in children with tuberous sclerosis. Dr. Chang and her colleagues are interested in those and other possibilities, and plan to continue to develop the approach, she said.
There was no funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Chang T et al. AES 2019, Abstract 1.225.
REPORTING FROM AES 2019
Hyperphosphorylated tau visible in TBI survivors decades after brain injury
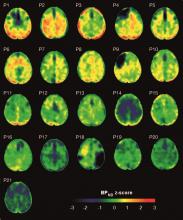
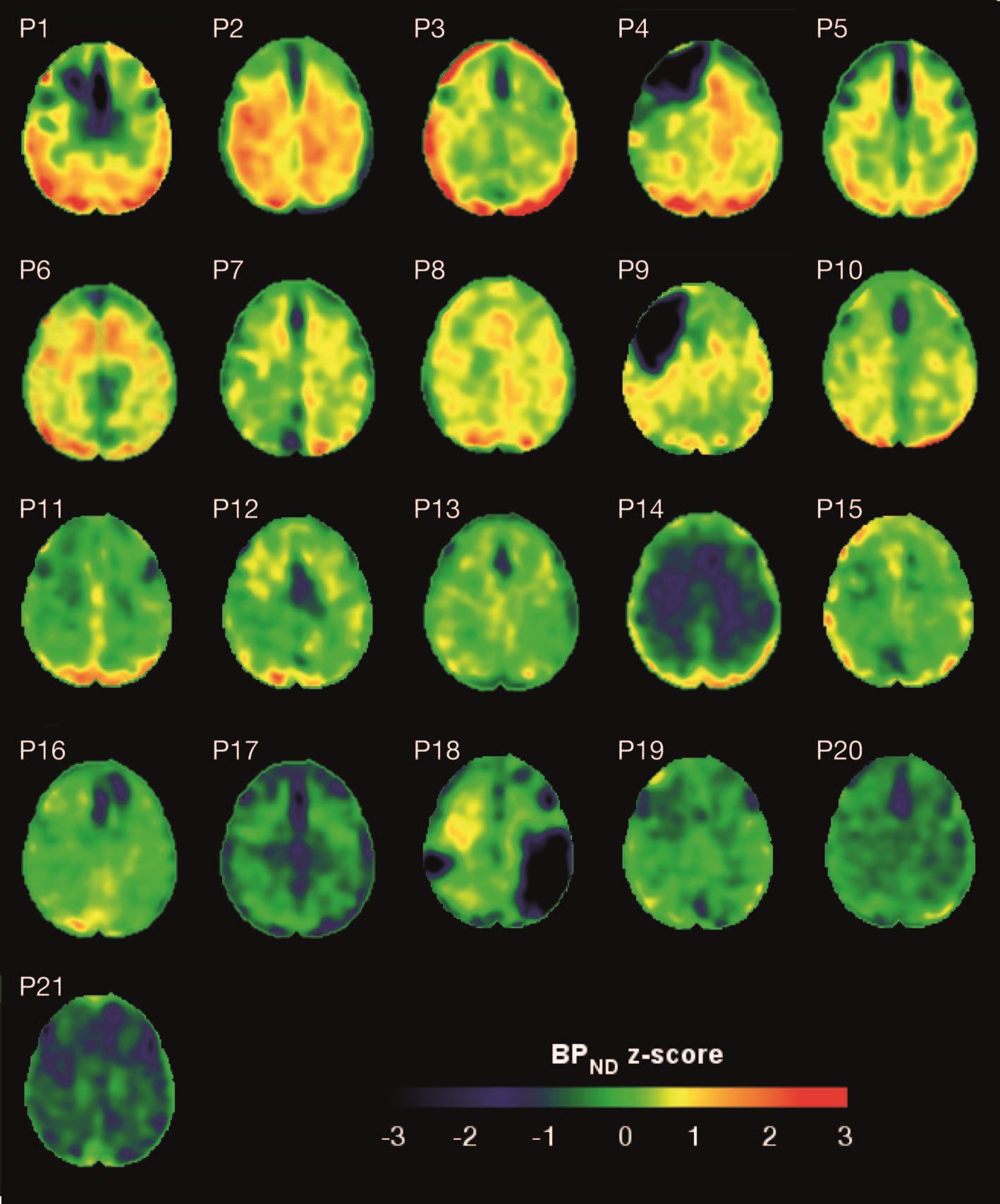
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
FROM SCIENCE TRANSLATIONAL MEDICINE
Epilepsy surgery outcome prediction seeks to gain ground
BANGKOK –
She and her colleagues have created and validated an online risk prediction tool that clinicians can use to predict a patient’s individualized likelihood of complete freedom from seizures 2 and 5 years after undergoing resective brain surgery for drug-resistant epilepsy. The risk predictor, known as the Epilepsy Surgery Nomogram, uses a handful of simple clinical characteristics – patient gender, pathologic cause of the seizures, the proposed type of epilepsy surgery, the presence or absence of generalized tonic-clonic seizures, epilepsy duration, and preoperative seizure frequency – and spits out the patient’s predicted seizure outcome, she explained at the congress, sponsored by the International League Against Epilepsy.
“The point here is that every patient is an individual. And to give people predictions based on 500- or 600-patient Kaplan-Meier-derived curves that just provide the average outcome for the whole cohort isn’t really going to give them what they need as far as their individualized chance of becoming seizure free,” said Dr. Jehi, a neurologist at the Cleveland Clinic.
Similarly, reliance solely upon clinical judgment is a minefield. Multiple biases prevent physicians from making objective medical predictions, she continued.
“We think of the process of medical decision-making and outcome prediction as being a process that is logical and rational, where the accumulation of knowledge improves the decisions that we make, and where past experience improves judgment, and where collective decisions are more reliable. This is what intuitively we all think. That’s why we think we are invincible as physicians. And to that I say, really? There is a wealth of literature that actually disproves each one of these points,” Dr. Jehi declared.
Outcomes of brain surgery for drug-resistant epilepsy have remained static for more than half a century: Ten years after surgery, roughly half of treated patients remain completely seizure free. The inability of clinicians to use advanced statistics to inform potential surgical candidates about their individualized chance of becoming seizure free has probably contributed to underutilization of epilepsy surgery, she added.
The Epilepsy Surgery Nomogram was developed through detailed analysis of the records of 846 patients who underwent epilepsy surgery at the Cleveland Clinic. The resultant nomogram was then validated in a cohort of 604 patients who had resective surgery at the Mayo Clinic and epilepsy surgery centers in Brazil, Italy, and France. In the development cohort, the rate of complete freedom from seizures was 57% at 2 years and 40% at 5 years. In the validation study, the nomogram had a concordance statistic of 0.60 for complete freedom from seizures, which is considered better than chance, but well below the 0.80 threshold defined as strong concordance (Lancet Neurol. 2015 Mar;14[3]:283-90).
However, in an era when personalized medicine has become a catch phrase, the Epilepsy Surgery Nomogram has captured the attention of officials at the National Institutes of Health. Indeed, Dr. Jehi and her coworkers have received a $3.4 million, 5-year grant from the NIH to improve their risk prediction model by incorporating additional variables, including EEG data, MRI findings, family history, and genetic information. The enhanced risk calculator also will include a predictor of the likelihood that an individual will experience clinically meaningful improvement in quality of life in response to epilepsy surgery, since that’s an important outcome even in the absence of 100% freedom from seizures.
Recently, Dr. Jehi and coworkers have developed and then externally validated nomograms to predict the individualized risk of clinically relevant postoperative naming decline after temporal lobe epilepsy surgery in adults. A model based upon five variables – side of surgery, sex, education, age at epilepsy onset, and age at epilepsy surgery – performed very well, with a concordance statistic of 0.81. Moreover, a second nomogram predicting moderate to severe postoperative naming decline on the basis of just three variables – side of surgery, age at epilepsy onset, and preoperative score on the Boston Naming Test – had a concordance statistic of 0.84 (Neurology. 2018 Dec 4;91[23]:e2144-e2152. doi: 10.1212/WNL.0000000000006629).
“Our future hopefully is one where there will always be room for gut feelings and intuition because we definitely need them. We want to honor them. But hopefully it is one where algorithms can help our guesses be more educated and where the science of algorithms and predictive modeling can help inform our outcome predictions and decision-making process,” she said.
The original Epilepsy Surgery Nomogram project was funded by the Cleveland Clinic Epilepsy Center. The postoperative naming decline nomograms project was funded by the NIH.
BANGKOK –
She and her colleagues have created and validated an online risk prediction tool that clinicians can use to predict a patient’s individualized likelihood of complete freedom from seizures 2 and 5 years after undergoing resective brain surgery for drug-resistant epilepsy. The risk predictor, known as the Epilepsy Surgery Nomogram, uses a handful of simple clinical characteristics – patient gender, pathologic cause of the seizures, the proposed type of epilepsy surgery, the presence or absence of generalized tonic-clonic seizures, epilepsy duration, and preoperative seizure frequency – and spits out the patient’s predicted seizure outcome, she explained at the congress, sponsored by the International League Against Epilepsy.
“The point here is that every patient is an individual. And to give people predictions based on 500- or 600-patient Kaplan-Meier-derived curves that just provide the average outcome for the whole cohort isn’t really going to give them what they need as far as their individualized chance of becoming seizure free,” said Dr. Jehi, a neurologist at the Cleveland Clinic.
Similarly, reliance solely upon clinical judgment is a minefield. Multiple biases prevent physicians from making objective medical predictions, she continued.
“We think of the process of medical decision-making and outcome prediction as being a process that is logical and rational, where the accumulation of knowledge improves the decisions that we make, and where past experience improves judgment, and where collective decisions are more reliable. This is what intuitively we all think. That’s why we think we are invincible as physicians. And to that I say, really? There is a wealth of literature that actually disproves each one of these points,” Dr. Jehi declared.
Outcomes of brain surgery for drug-resistant epilepsy have remained static for more than half a century: Ten years after surgery, roughly half of treated patients remain completely seizure free. The inability of clinicians to use advanced statistics to inform potential surgical candidates about their individualized chance of becoming seizure free has probably contributed to underutilization of epilepsy surgery, she added.
The Epilepsy Surgery Nomogram was developed through detailed analysis of the records of 846 patients who underwent epilepsy surgery at the Cleveland Clinic. The resultant nomogram was then validated in a cohort of 604 patients who had resective surgery at the Mayo Clinic and epilepsy surgery centers in Brazil, Italy, and France. In the development cohort, the rate of complete freedom from seizures was 57% at 2 years and 40% at 5 years. In the validation study, the nomogram had a concordance statistic of 0.60 for complete freedom from seizures, which is considered better than chance, but well below the 0.80 threshold defined as strong concordance (Lancet Neurol. 2015 Mar;14[3]:283-90).
However, in an era when personalized medicine has become a catch phrase, the Epilepsy Surgery Nomogram has captured the attention of officials at the National Institutes of Health. Indeed, Dr. Jehi and her coworkers have received a $3.4 million, 5-year grant from the NIH to improve their risk prediction model by incorporating additional variables, including EEG data, MRI findings, family history, and genetic information. The enhanced risk calculator also will include a predictor of the likelihood that an individual will experience clinically meaningful improvement in quality of life in response to epilepsy surgery, since that’s an important outcome even in the absence of 100% freedom from seizures.
Recently, Dr. Jehi and coworkers have developed and then externally validated nomograms to predict the individualized risk of clinically relevant postoperative naming decline after temporal lobe epilepsy surgery in adults. A model based upon five variables – side of surgery, sex, education, age at epilepsy onset, and age at epilepsy surgery – performed very well, with a concordance statistic of 0.81. Moreover, a second nomogram predicting moderate to severe postoperative naming decline on the basis of just three variables – side of surgery, age at epilepsy onset, and preoperative score on the Boston Naming Test – had a concordance statistic of 0.84 (Neurology. 2018 Dec 4;91[23]:e2144-e2152. doi: 10.1212/WNL.0000000000006629).
“Our future hopefully is one where there will always be room for gut feelings and intuition because we definitely need them. We want to honor them. But hopefully it is one where algorithms can help our guesses be more educated and where the science of algorithms and predictive modeling can help inform our outcome predictions and decision-making process,” she said.
The original Epilepsy Surgery Nomogram project was funded by the Cleveland Clinic Epilepsy Center. The postoperative naming decline nomograms project was funded by the NIH.
BANGKOK –
She and her colleagues have created and validated an online risk prediction tool that clinicians can use to predict a patient’s individualized likelihood of complete freedom from seizures 2 and 5 years after undergoing resective brain surgery for drug-resistant epilepsy. The risk predictor, known as the Epilepsy Surgery Nomogram, uses a handful of simple clinical characteristics – patient gender, pathologic cause of the seizures, the proposed type of epilepsy surgery, the presence or absence of generalized tonic-clonic seizures, epilepsy duration, and preoperative seizure frequency – and spits out the patient’s predicted seizure outcome, she explained at the congress, sponsored by the International League Against Epilepsy.
“The point here is that every patient is an individual. And to give people predictions based on 500- or 600-patient Kaplan-Meier-derived curves that just provide the average outcome for the whole cohort isn’t really going to give them what they need as far as their individualized chance of becoming seizure free,” said Dr. Jehi, a neurologist at the Cleveland Clinic.
Similarly, reliance solely upon clinical judgment is a minefield. Multiple biases prevent physicians from making objective medical predictions, she continued.
“We think of the process of medical decision-making and outcome prediction as being a process that is logical and rational, where the accumulation of knowledge improves the decisions that we make, and where past experience improves judgment, and where collective decisions are more reliable. This is what intuitively we all think. That’s why we think we are invincible as physicians. And to that I say, really? There is a wealth of literature that actually disproves each one of these points,” Dr. Jehi declared.
Outcomes of brain surgery for drug-resistant epilepsy have remained static for more than half a century: Ten years after surgery, roughly half of treated patients remain completely seizure free. The inability of clinicians to use advanced statistics to inform potential surgical candidates about their individualized chance of becoming seizure free has probably contributed to underutilization of epilepsy surgery, she added.
The Epilepsy Surgery Nomogram was developed through detailed analysis of the records of 846 patients who underwent epilepsy surgery at the Cleveland Clinic. The resultant nomogram was then validated in a cohort of 604 patients who had resective surgery at the Mayo Clinic and epilepsy surgery centers in Brazil, Italy, and France. In the development cohort, the rate of complete freedom from seizures was 57% at 2 years and 40% at 5 years. In the validation study, the nomogram had a concordance statistic of 0.60 for complete freedom from seizures, which is considered better than chance, but well below the 0.80 threshold defined as strong concordance (Lancet Neurol. 2015 Mar;14[3]:283-90).
However, in an era when personalized medicine has become a catch phrase, the Epilepsy Surgery Nomogram has captured the attention of officials at the National Institutes of Health. Indeed, Dr. Jehi and her coworkers have received a $3.4 million, 5-year grant from the NIH to improve their risk prediction model by incorporating additional variables, including EEG data, MRI findings, family history, and genetic information. The enhanced risk calculator also will include a predictor of the likelihood that an individual will experience clinically meaningful improvement in quality of life in response to epilepsy surgery, since that’s an important outcome even in the absence of 100% freedom from seizures.
Recently, Dr. Jehi and coworkers have developed and then externally validated nomograms to predict the individualized risk of clinically relevant postoperative naming decline after temporal lobe epilepsy surgery in adults. A model based upon five variables – side of surgery, sex, education, age at epilepsy onset, and age at epilepsy surgery – performed very well, with a concordance statistic of 0.81. Moreover, a second nomogram predicting moderate to severe postoperative naming decline on the basis of just three variables – side of surgery, age at epilepsy onset, and preoperative score on the Boston Naming Test – had a concordance statistic of 0.84 (Neurology. 2018 Dec 4;91[23]:e2144-e2152. doi: 10.1212/WNL.0000000000006629).
“Our future hopefully is one where there will always be room for gut feelings and intuition because we definitely need them. We want to honor them. But hopefully it is one where algorithms can help our guesses be more educated and where the science of algorithms and predictive modeling can help inform our outcome predictions and decision-making process,” she said.
The original Epilepsy Surgery Nomogram project was funded by the Cleveland Clinic Epilepsy Center. The postoperative naming decline nomograms project was funded by the NIH.
REPORTING FROM IEC 2019
Researchers examine potential causes of dementia in CTE
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
FROM JAMA NEUROLOGY
Nerve transfer improves function after spinal cord injury
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
FROM LANCET
‘Trigger zone’ resection ups seizure-free odds in temporal lobe epilepsy
Resection of a brain area implicated in seizure modulation improves the odds of being seizure free in patients with temporal lobe epilepsy, according to results of a recent multicenter analysis.
Patients with long-term postoperative freedom from seizures had a larger proportion of the piriform cortex resected versus patients who were not seizure free. Removing at least half the piriform cortex was associated with a 1500% increase in odds of seizure freedom, first author Marian Galovic, MD, of the department of clinical and experimental epilepsy at the University College London’s Queen Square Institute of Neurology and his colleagues reported in JAMA Neurology.
“If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection,” Dr. Galovic and his coinvestigators wrote.
The area tempestas in the piriform cortex has been identified as an epileptic trigger zone in animal studies, but to date, evidence of a human epileptic trigger zone in this area remain limited, according to the investigators.
To evaluate the impact of resection in this area, Dr. Galovic and his colleagues evaluated 107 patients with temporal lobe epilepsy from an ongoing, single-center, prospective study, and validated their findings with 31 patients from two other independent cohorts.
Of the 107 patients in the main cohort, 46% were completely seizure free for a median of 5 years after epilepsy surgery, with results of voxel-based morphometry showing that those patients had a more pronounced loss of gray matter in the ipsilateral piriform, compared with non–seizure-free patients.
The seizure-free patients had a median of 83% of the piriform cortex resected, compared with 52% for the non–seizure-free patients (P less than .001), results of a volumetric analysis confirmed.
Anxiety or psychosis outcomes were not influenced by the extent of piriform cortex resection, the investigators wrote, adding that poor verbal memory outcome was linked to the extent of resection of other brain regions, but not the piriform cortex.
The investigators confirmed these findings in the 31 patients of the validation cohort, with significant associations between extent of piriform cortex resection and postsurgical outcomes.
Resecting at least half of the region increased odds of being seizure free by a factor of 16 (95% CI, 5-47; P less than .001), Dr. Galovic and his colleagues added.
“Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe,” they wrote in a discussion of their results.
The findings, if confirmed, could have implications not only for surgical practice, they wrote, but also for the understanding of the mechanisms underlying epileptic networks, which could lead to new drug and nondrug interventions to mitigate seizure activity.
Dr. Galovic reported receiving a grant from the Medical Research Council. His coauthors reported disclosures with the Medical Research Council, Wellcome Trust, Medtronic, Neuropace, Nevro, Eisai, UCB, and Mallinckrodt, among other entities.
SOURCE: Galovic M et al. JAMA Neurol. 2019 Mar 11. doi: 10.1001/jamaneurol.2019.0204.
Resection of a brain area implicated in seizure modulation improves the odds of being seizure free in patients with temporal lobe epilepsy, according to results of a recent multicenter analysis.
Patients with long-term postoperative freedom from seizures had a larger proportion of the piriform cortex resected versus patients who were not seizure free. Removing at least half the piriform cortex was associated with a 1500% increase in odds of seizure freedom, first author Marian Galovic, MD, of the department of clinical and experimental epilepsy at the University College London’s Queen Square Institute of Neurology and his colleagues reported in JAMA Neurology.
“If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection,” Dr. Galovic and his coinvestigators wrote.
The area tempestas in the piriform cortex has been identified as an epileptic trigger zone in animal studies, but to date, evidence of a human epileptic trigger zone in this area remain limited, according to the investigators.
To evaluate the impact of resection in this area, Dr. Galovic and his colleagues evaluated 107 patients with temporal lobe epilepsy from an ongoing, single-center, prospective study, and validated their findings with 31 patients from two other independent cohorts.
Of the 107 patients in the main cohort, 46% were completely seizure free for a median of 5 years after epilepsy surgery, with results of voxel-based morphometry showing that those patients had a more pronounced loss of gray matter in the ipsilateral piriform, compared with non–seizure-free patients.
The seizure-free patients had a median of 83% of the piriform cortex resected, compared with 52% for the non–seizure-free patients (P less than .001), results of a volumetric analysis confirmed.
Anxiety or psychosis outcomes were not influenced by the extent of piriform cortex resection, the investigators wrote, adding that poor verbal memory outcome was linked to the extent of resection of other brain regions, but not the piriform cortex.
The investigators confirmed these findings in the 31 patients of the validation cohort, with significant associations between extent of piriform cortex resection and postsurgical outcomes.
Resecting at least half of the region increased odds of being seizure free by a factor of 16 (95% CI, 5-47; P less than .001), Dr. Galovic and his colleagues added.
“Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe,” they wrote in a discussion of their results.
The findings, if confirmed, could have implications not only for surgical practice, they wrote, but also for the understanding of the mechanisms underlying epileptic networks, which could lead to new drug and nondrug interventions to mitigate seizure activity.
Dr. Galovic reported receiving a grant from the Medical Research Council. His coauthors reported disclosures with the Medical Research Council, Wellcome Trust, Medtronic, Neuropace, Nevro, Eisai, UCB, and Mallinckrodt, among other entities.
SOURCE: Galovic M et al. JAMA Neurol. 2019 Mar 11. doi: 10.1001/jamaneurol.2019.0204.
Resection of a brain area implicated in seizure modulation improves the odds of being seizure free in patients with temporal lobe epilepsy, according to results of a recent multicenter analysis.
Patients with long-term postoperative freedom from seizures had a larger proportion of the piriform cortex resected versus patients who were not seizure free. Removing at least half the piriform cortex was associated with a 1500% increase in odds of seizure freedom, first author Marian Galovic, MD, of the department of clinical and experimental epilepsy at the University College London’s Queen Square Institute of Neurology and his colleagues reported in JAMA Neurology.
“If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection,” Dr. Galovic and his coinvestigators wrote.
The area tempestas in the piriform cortex has been identified as an epileptic trigger zone in animal studies, but to date, evidence of a human epileptic trigger zone in this area remain limited, according to the investigators.
To evaluate the impact of resection in this area, Dr. Galovic and his colleagues evaluated 107 patients with temporal lobe epilepsy from an ongoing, single-center, prospective study, and validated their findings with 31 patients from two other independent cohorts.
Of the 107 patients in the main cohort, 46% were completely seizure free for a median of 5 years after epilepsy surgery, with results of voxel-based morphometry showing that those patients had a more pronounced loss of gray matter in the ipsilateral piriform, compared with non–seizure-free patients.
The seizure-free patients had a median of 83% of the piriform cortex resected, compared with 52% for the non–seizure-free patients (P less than .001), results of a volumetric analysis confirmed.
Anxiety or psychosis outcomes were not influenced by the extent of piriform cortex resection, the investigators wrote, adding that poor verbal memory outcome was linked to the extent of resection of other brain regions, but not the piriform cortex.
The investigators confirmed these findings in the 31 patients of the validation cohort, with significant associations between extent of piriform cortex resection and postsurgical outcomes.
Resecting at least half of the region increased odds of being seizure free by a factor of 16 (95% CI, 5-47; P less than .001), Dr. Galovic and his colleagues added.
“Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe,” they wrote in a discussion of their results.
The findings, if confirmed, could have implications not only for surgical practice, they wrote, but also for the understanding of the mechanisms underlying epileptic networks, which could lead to new drug and nondrug interventions to mitigate seizure activity.
Dr. Galovic reported receiving a grant from the Medical Research Council. His coauthors reported disclosures with the Medical Research Council, Wellcome Trust, Medtronic, Neuropace, Nevro, Eisai, UCB, and Mallinckrodt, among other entities.
SOURCE: Galovic M et al. JAMA Neurol. 2019 Mar 11. doi: 10.1001/jamaneurol.2019.0204.
FROM JAMA NEUROLOGY