User login
Novel antipsychotic ‘encouraging’ for resistant schizophrenia
The topline results from an exploratory study, which were released by the developer Newron Pharmaceuticals, are “very encouraging,” Stephen R. Marder, MD, professor of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said in a company news release.
“The magnitude of the improvements experienced by these TRS patients, not responding to their current antipsychotic, on evenamide was substantial, improved over time, and was likely to be clinically meaningful,” Dr. Marder said.
First 100 patients
The topline results are based on the first 100 patients enrolled in study 014 and randomly assigned to receive evenamide at 7.5 mg, 15 mg, or 30 mg twice daily, as well as patients in the extension arm (study 015) that have completed 30 weeks.
Key findings released by the company included statistically significant improvement over baseline at 30 weeks (P < .001) in Positive and Negative Syndrome Scale (PANSS) scores, with continued improvement over that seen at 6 weeks.
The proportion of patients with clinically meaningful PANSS improvement at 30 weeks more than doubled from 16.5% at 6 weeks.
In addition, results showed statistically significant improvement (P < .001) at week 30 compared with baseline in illness severity as measured by the Clinical Global Impression of Severity (CGI-S), with continued improvement over that seen at 6 weeks.
The proportion of patients whose illness improved by at least one level of severity was 60% at week 6 and increased approximately by an additional 20% at week 30.
The proportion of patients judged to have clinically meaningful improvement, defined as at least “much improved,” on the Clinical Global Impression of Change (CGI-C) was 27% at week 6 – and increased a further 10% at week 30.
Evenamide was also well tolerated, with few adverse effects reported, and 85 of 100 patients remained on treatment at 30 weeks.
New options ‘desperately needed’
Newron plans to present the full results from study 014 at the European Congress of Psychiatry, scheduled for March 25-28 in Paris.
The extension study 015 is ongoing and will provide results on evenamide treatment for up to 1 year by the second quarter of 2023.
The company reported it expects to launch a randomized, placebo-controlled study (study 003) of the drug in TRS this year.
If the current results are confirmed in the randomized controlled trial, “evenamide would be the first medication that could be added to an antipsychotic to improve symptoms in treatment-refractory schizophrenia,” Dr. Marder said.
New therapeutic options for TRS, which occurs in about one-third of patients, are “desperately needed,” Ravi Anand, MD, chief medical officer at Newron, said in the release.
The reported data, comparing the effect of evenamide at 6 weeks vs. 6 months, “suggest that not only was there sustained improvement in the key measures, but the proportion of patients achieving clinically meaningful improvement increased over time,” Dr. Anand added.
A version of this article first appeared on Medscape.com.
The topline results from an exploratory study, which were released by the developer Newron Pharmaceuticals, are “very encouraging,” Stephen R. Marder, MD, professor of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said in a company news release.
“The magnitude of the improvements experienced by these TRS patients, not responding to their current antipsychotic, on evenamide was substantial, improved over time, and was likely to be clinically meaningful,” Dr. Marder said.
First 100 patients
The topline results are based on the first 100 patients enrolled in study 014 and randomly assigned to receive evenamide at 7.5 mg, 15 mg, or 30 mg twice daily, as well as patients in the extension arm (study 015) that have completed 30 weeks.
Key findings released by the company included statistically significant improvement over baseline at 30 weeks (P < .001) in Positive and Negative Syndrome Scale (PANSS) scores, with continued improvement over that seen at 6 weeks.
The proportion of patients with clinically meaningful PANSS improvement at 30 weeks more than doubled from 16.5% at 6 weeks.
In addition, results showed statistically significant improvement (P < .001) at week 30 compared with baseline in illness severity as measured by the Clinical Global Impression of Severity (CGI-S), with continued improvement over that seen at 6 weeks.
The proportion of patients whose illness improved by at least one level of severity was 60% at week 6 and increased approximately by an additional 20% at week 30.
The proportion of patients judged to have clinically meaningful improvement, defined as at least “much improved,” on the Clinical Global Impression of Change (CGI-C) was 27% at week 6 – and increased a further 10% at week 30.
Evenamide was also well tolerated, with few adverse effects reported, and 85 of 100 patients remained on treatment at 30 weeks.
New options ‘desperately needed’
Newron plans to present the full results from study 014 at the European Congress of Psychiatry, scheduled for March 25-28 in Paris.
The extension study 015 is ongoing and will provide results on evenamide treatment for up to 1 year by the second quarter of 2023.
The company reported it expects to launch a randomized, placebo-controlled study (study 003) of the drug in TRS this year.
If the current results are confirmed in the randomized controlled trial, “evenamide would be the first medication that could be added to an antipsychotic to improve symptoms in treatment-refractory schizophrenia,” Dr. Marder said.
New therapeutic options for TRS, which occurs in about one-third of patients, are “desperately needed,” Ravi Anand, MD, chief medical officer at Newron, said in the release.
The reported data, comparing the effect of evenamide at 6 weeks vs. 6 months, “suggest that not only was there sustained improvement in the key measures, but the proportion of patients achieving clinically meaningful improvement increased over time,” Dr. Anand added.
A version of this article first appeared on Medscape.com.
The topline results from an exploratory study, which were released by the developer Newron Pharmaceuticals, are “very encouraging,” Stephen R. Marder, MD, professor of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said in a company news release.
“The magnitude of the improvements experienced by these TRS patients, not responding to their current antipsychotic, on evenamide was substantial, improved over time, and was likely to be clinically meaningful,” Dr. Marder said.
First 100 patients
The topline results are based on the first 100 patients enrolled in study 014 and randomly assigned to receive evenamide at 7.5 mg, 15 mg, or 30 mg twice daily, as well as patients in the extension arm (study 015) that have completed 30 weeks.
Key findings released by the company included statistically significant improvement over baseline at 30 weeks (P < .001) in Positive and Negative Syndrome Scale (PANSS) scores, with continued improvement over that seen at 6 weeks.
The proportion of patients with clinically meaningful PANSS improvement at 30 weeks more than doubled from 16.5% at 6 weeks.
In addition, results showed statistically significant improvement (P < .001) at week 30 compared with baseline in illness severity as measured by the Clinical Global Impression of Severity (CGI-S), with continued improvement over that seen at 6 weeks.
The proportion of patients whose illness improved by at least one level of severity was 60% at week 6 and increased approximately by an additional 20% at week 30.
The proportion of patients judged to have clinically meaningful improvement, defined as at least “much improved,” on the Clinical Global Impression of Change (CGI-C) was 27% at week 6 – and increased a further 10% at week 30.
Evenamide was also well tolerated, with few adverse effects reported, and 85 of 100 patients remained on treatment at 30 weeks.
New options ‘desperately needed’
Newron plans to present the full results from study 014 at the European Congress of Psychiatry, scheduled for March 25-28 in Paris.
The extension study 015 is ongoing and will provide results on evenamide treatment for up to 1 year by the second quarter of 2023.
The company reported it expects to launch a randomized, placebo-controlled study (study 003) of the drug in TRS this year.
If the current results are confirmed in the randomized controlled trial, “evenamide would be the first medication that could be added to an antipsychotic to improve symptoms in treatment-refractory schizophrenia,” Dr. Marder said.
New therapeutic options for TRS, which occurs in about one-third of patients, are “desperately needed,” Ravi Anand, MD, chief medical officer at Newron, said in the release.
The reported data, comparing the effect of evenamide at 6 weeks vs. 6 months, “suggest that not only was there sustained improvement in the key measures, but the proportion of patients achieving clinically meaningful improvement increased over time,” Dr. Anand added.
A version of this article first appeared on Medscape.com.
‘Affect discrepancies’ may underlie negative symptoms in schizophrenia
Anhedonia is common in schizophrenia patients, but treatments have not been especially successful, possibly because of a lack of understanding the mechanisms behind anhedonia in these patients, Sydney H. James, a PhD candidate at the University of Georgia, Athens, and colleagues wrote.
Although many schizophrenia (SZ) patients exhibit anhedonia on diagnosis in a clinical interview setting, other recent research shows comparable response to pleasant stimuli between schizophrenic patients and healthy controls. The researchers proposed that anhedonia “reflects abnormalities in the valuation of desired affective states in individuals with SZ,” with differences between actual and ideal affect.
In a study published in the Journal of Psychiatric Research, the researchers identified 32 outpatients with schizophrenia and 29 healthy controls. The SZ participants were recruited from community outpatient mental health services in Georgia. All participants completed Structured Clinical Interview for DSM-5 Disorders and the SCID-5 Personality Disorders. Participants then completed the Affect Valuation Index and measures of negative symptom severity. Negative symptom severity was measured using the Negative Symptom Inventory-Self-Report, an 11-item questionnaire assessing three specific experiential and behavioral components (anhedonia, avolition, and asociality) over the past week.
The average age of the SZ patients and controls was approximately 40 years, and 10 SZ patients and 5 controls were male.
Overall, the researchers found a significant main effect of group, a significant main effect of arousal, and a significant group X arousal interaction for positive affect discrepancy scores. For negative affect discrepancy scores, they found a significant main effect on group, nonsignificant main effect of arousal, and significant group X arousal interaction.
Individuals with SZ showed greater positive and negative emotion discrepancy scores, compared with controls, in contrast to the researchers’ hypothesis. “Those diagnosed with SZ were more likely to want to feel less negative than they actually did,” they wrote. The negative affect discrepancy scores were positively associated with negative symptoms. The discrepancies between actual and ideal affect may be impacted by social interactions and the perceived expectations of others for levels of negative affect.
The study findings were limited by the small sample size and inability to test the relationship between ideal and actual affect as related to low-pleasure beliefs, which merits further study, the researchers noted. Other limitations include the focus on an outpatient population with mild to moderate SZ, and the use of a trait format to measure affect rather than experiential emotion knowledge.
However, the results have practical implications for treatment and suggest that, “given the positive associations between negative symptom and affect discrepancy scores, psychosocial treatments could target expectations for future positive and negative emotional experience,” and ecological momentary assessment could be used to track affect through a period of treatment and prompt conversations between SZ patients and therapists about discrepancies, they concluded.
The study participants were compensated by the National Institute of Mental Health through a grant to a corresponding author. Ms. James had no financial conflicts to disclose.
Anhedonia is common in schizophrenia patients, but treatments have not been especially successful, possibly because of a lack of understanding the mechanisms behind anhedonia in these patients, Sydney H. James, a PhD candidate at the University of Georgia, Athens, and colleagues wrote.
Although many schizophrenia (SZ) patients exhibit anhedonia on diagnosis in a clinical interview setting, other recent research shows comparable response to pleasant stimuli between schizophrenic patients and healthy controls. The researchers proposed that anhedonia “reflects abnormalities in the valuation of desired affective states in individuals with SZ,” with differences between actual and ideal affect.
In a study published in the Journal of Psychiatric Research, the researchers identified 32 outpatients with schizophrenia and 29 healthy controls. The SZ participants were recruited from community outpatient mental health services in Georgia. All participants completed Structured Clinical Interview for DSM-5 Disorders and the SCID-5 Personality Disorders. Participants then completed the Affect Valuation Index and measures of negative symptom severity. Negative symptom severity was measured using the Negative Symptom Inventory-Self-Report, an 11-item questionnaire assessing three specific experiential and behavioral components (anhedonia, avolition, and asociality) over the past week.
The average age of the SZ patients and controls was approximately 40 years, and 10 SZ patients and 5 controls were male.
Overall, the researchers found a significant main effect of group, a significant main effect of arousal, and a significant group X arousal interaction for positive affect discrepancy scores. For negative affect discrepancy scores, they found a significant main effect on group, nonsignificant main effect of arousal, and significant group X arousal interaction.
Individuals with SZ showed greater positive and negative emotion discrepancy scores, compared with controls, in contrast to the researchers’ hypothesis. “Those diagnosed with SZ were more likely to want to feel less negative than they actually did,” they wrote. The negative affect discrepancy scores were positively associated with negative symptoms. The discrepancies between actual and ideal affect may be impacted by social interactions and the perceived expectations of others for levels of negative affect.
The study findings were limited by the small sample size and inability to test the relationship between ideal and actual affect as related to low-pleasure beliefs, which merits further study, the researchers noted. Other limitations include the focus on an outpatient population with mild to moderate SZ, and the use of a trait format to measure affect rather than experiential emotion knowledge.
However, the results have practical implications for treatment and suggest that, “given the positive associations between negative symptom and affect discrepancy scores, psychosocial treatments could target expectations for future positive and negative emotional experience,” and ecological momentary assessment could be used to track affect through a period of treatment and prompt conversations between SZ patients and therapists about discrepancies, they concluded.
The study participants were compensated by the National Institute of Mental Health through a grant to a corresponding author. Ms. James had no financial conflicts to disclose.
Anhedonia is common in schizophrenia patients, but treatments have not been especially successful, possibly because of a lack of understanding the mechanisms behind anhedonia in these patients, Sydney H. James, a PhD candidate at the University of Georgia, Athens, and colleagues wrote.
Although many schizophrenia (SZ) patients exhibit anhedonia on diagnosis in a clinical interview setting, other recent research shows comparable response to pleasant stimuli between schizophrenic patients and healthy controls. The researchers proposed that anhedonia “reflects abnormalities in the valuation of desired affective states in individuals with SZ,” with differences between actual and ideal affect.
In a study published in the Journal of Psychiatric Research, the researchers identified 32 outpatients with schizophrenia and 29 healthy controls. The SZ participants were recruited from community outpatient mental health services in Georgia. All participants completed Structured Clinical Interview for DSM-5 Disorders and the SCID-5 Personality Disorders. Participants then completed the Affect Valuation Index and measures of negative symptom severity. Negative symptom severity was measured using the Negative Symptom Inventory-Self-Report, an 11-item questionnaire assessing three specific experiential and behavioral components (anhedonia, avolition, and asociality) over the past week.
The average age of the SZ patients and controls was approximately 40 years, and 10 SZ patients and 5 controls were male.
Overall, the researchers found a significant main effect of group, a significant main effect of arousal, and a significant group X arousal interaction for positive affect discrepancy scores. For negative affect discrepancy scores, they found a significant main effect on group, nonsignificant main effect of arousal, and significant group X arousal interaction.
Individuals with SZ showed greater positive and negative emotion discrepancy scores, compared with controls, in contrast to the researchers’ hypothesis. “Those diagnosed with SZ were more likely to want to feel less negative than they actually did,” they wrote. The negative affect discrepancy scores were positively associated with negative symptoms. The discrepancies between actual and ideal affect may be impacted by social interactions and the perceived expectations of others for levels of negative affect.
The study findings were limited by the small sample size and inability to test the relationship between ideal and actual affect as related to low-pleasure beliefs, which merits further study, the researchers noted. Other limitations include the focus on an outpatient population with mild to moderate SZ, and the use of a trait format to measure affect rather than experiential emotion knowledge.
However, the results have practical implications for treatment and suggest that, “given the positive associations between negative symptom and affect discrepancy scores, psychosocial treatments could target expectations for future positive and negative emotional experience,” and ecological momentary assessment could be used to track affect through a period of treatment and prompt conversations between SZ patients and therapists about discrepancies, they concluded.
The study participants were compensated by the National Institute of Mental Health through a grant to a corresponding author. Ms. James had no financial conflicts to disclose.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
One in four cardiologists worldwide report mental health issues
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Contemporary psychiatry: A SWOT analysis
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
From debate to stalemate and hate: An epidemic of intellectual constipation
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
GLP-1 agonists for weight loss: What you need to know
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.
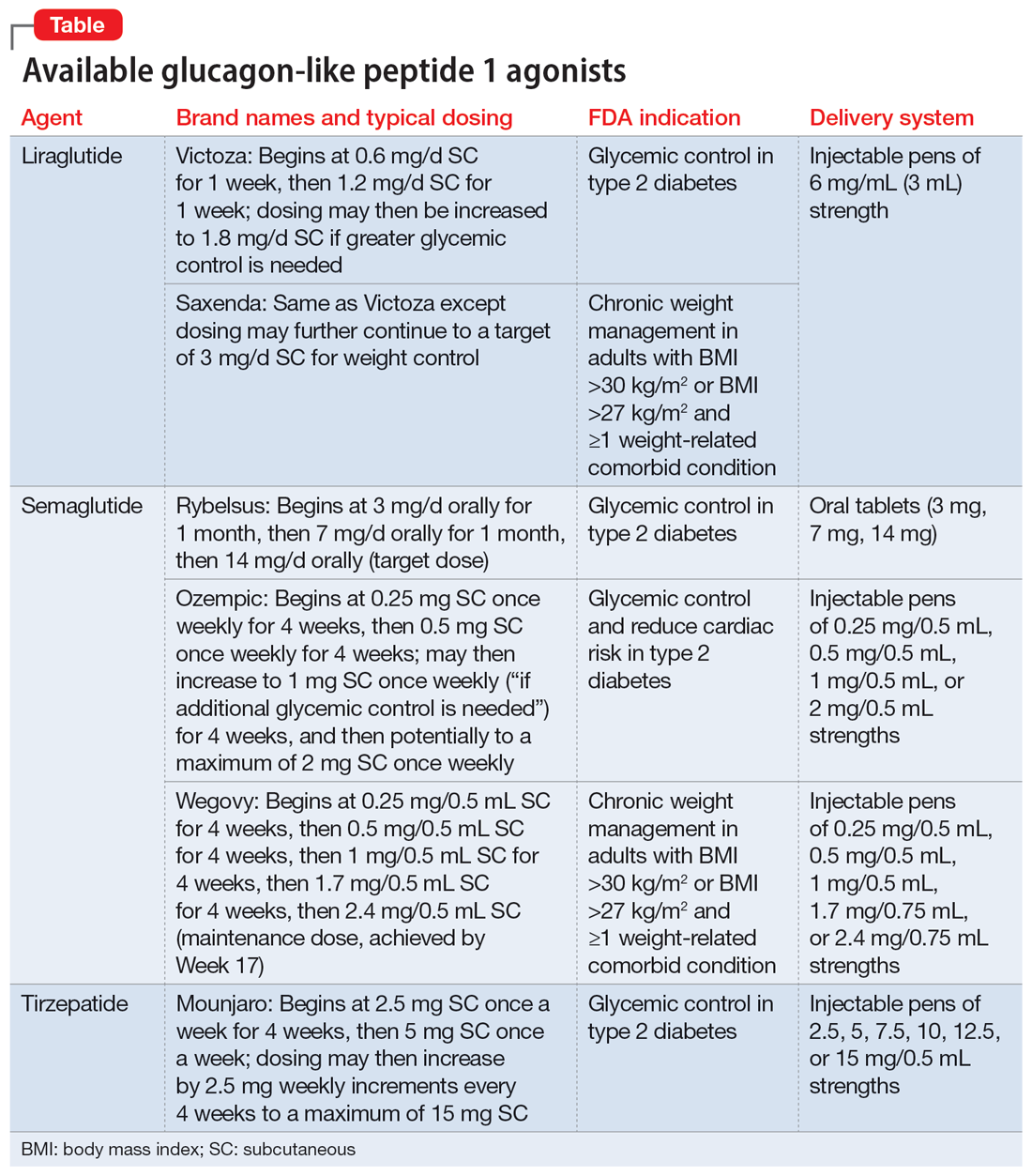
What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9
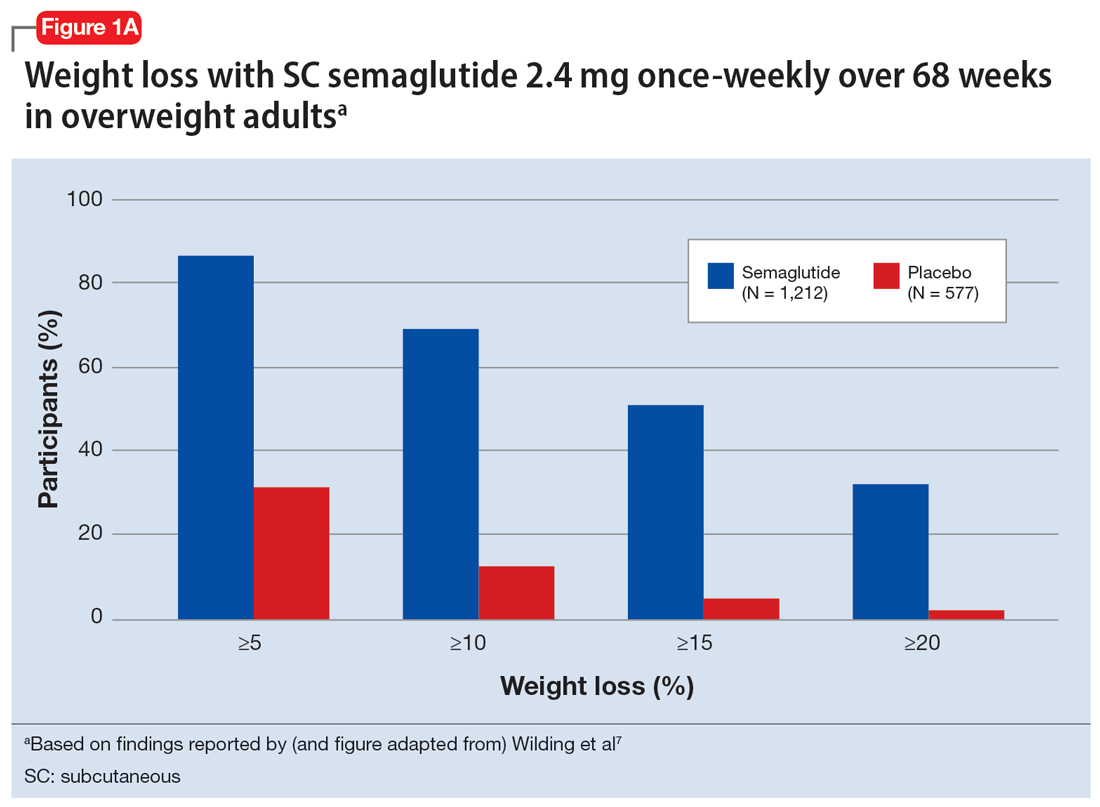
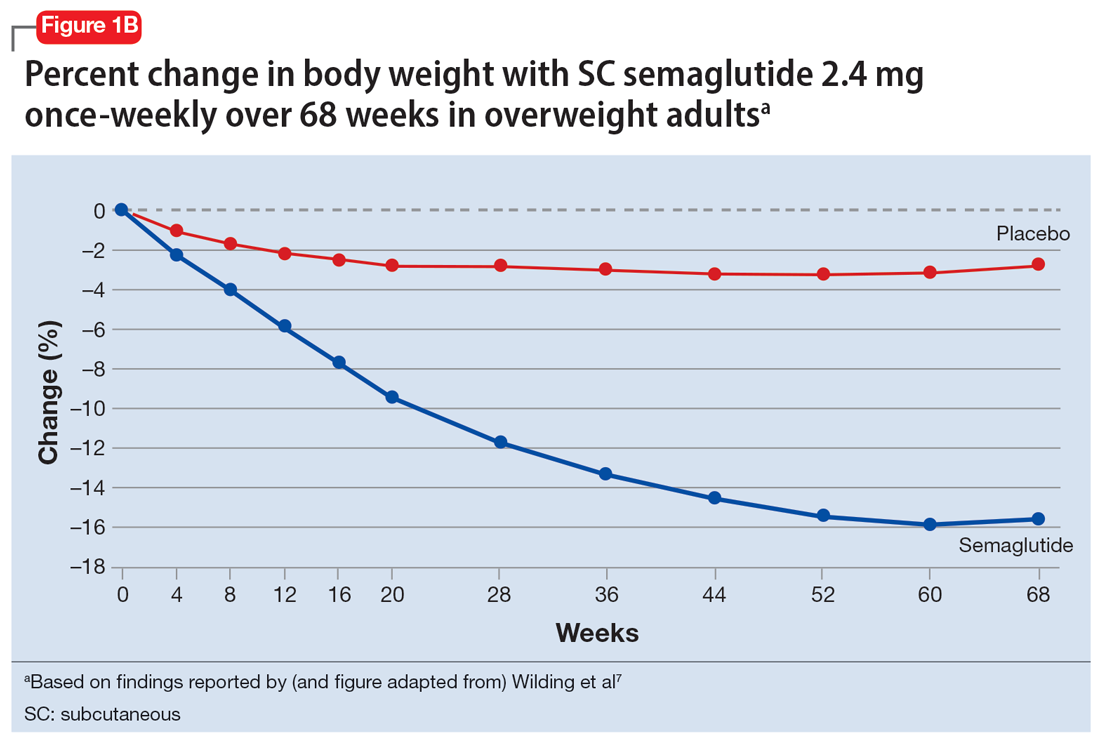
In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.
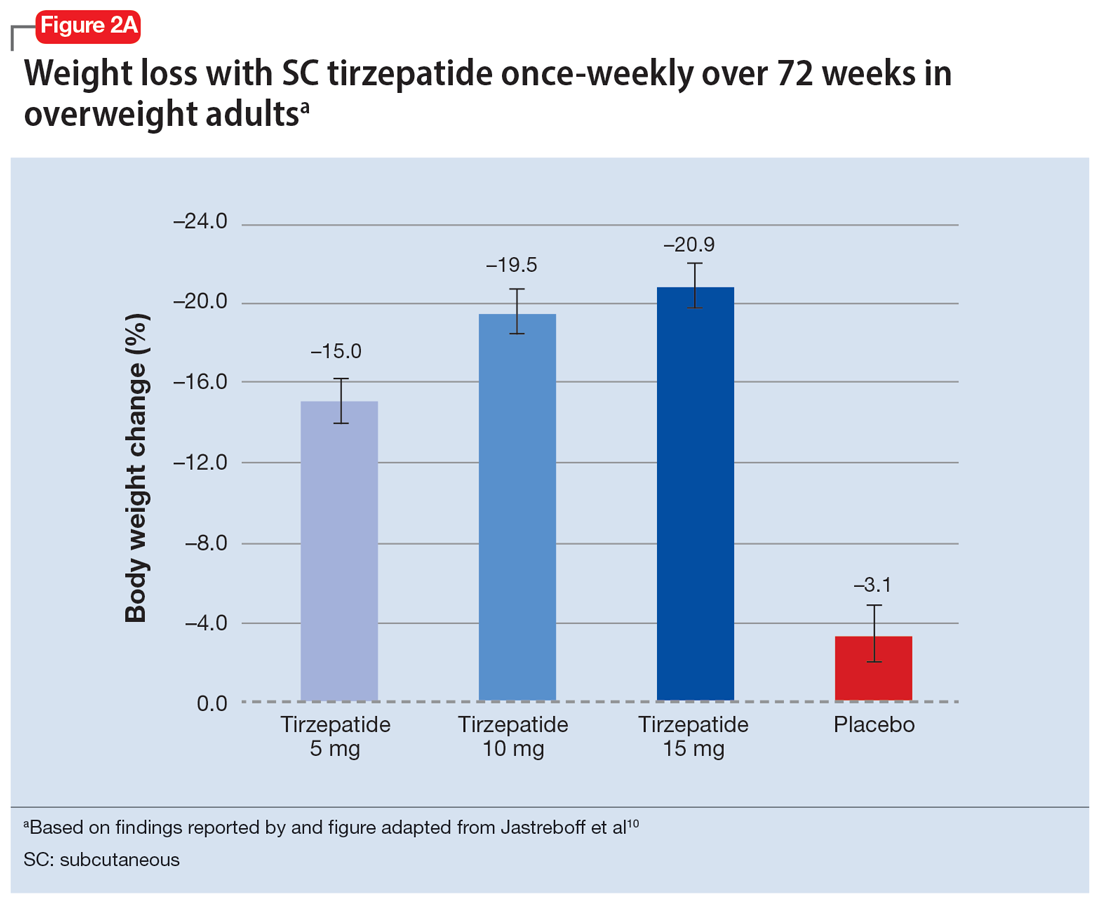
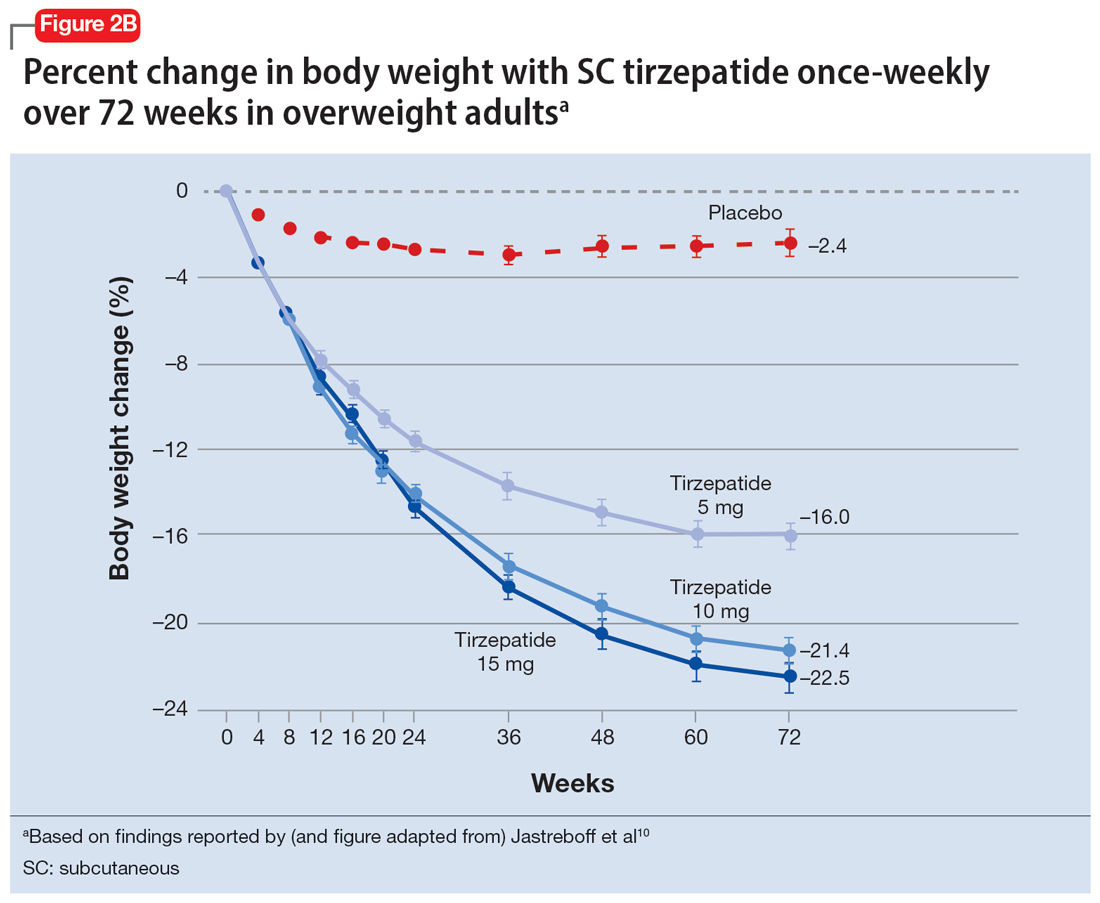
Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.

What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9


In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.


Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.

What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9


In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.


Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
Managing excited catatonia: A suggested approach
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3
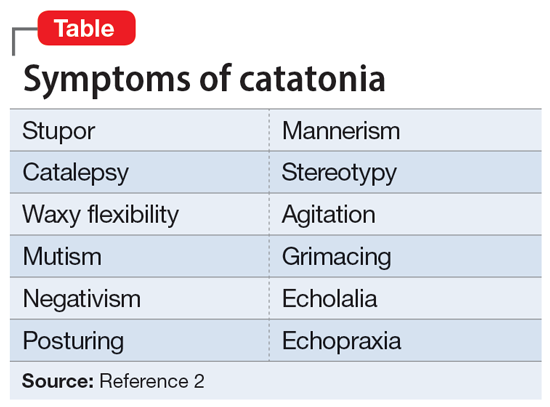
A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).
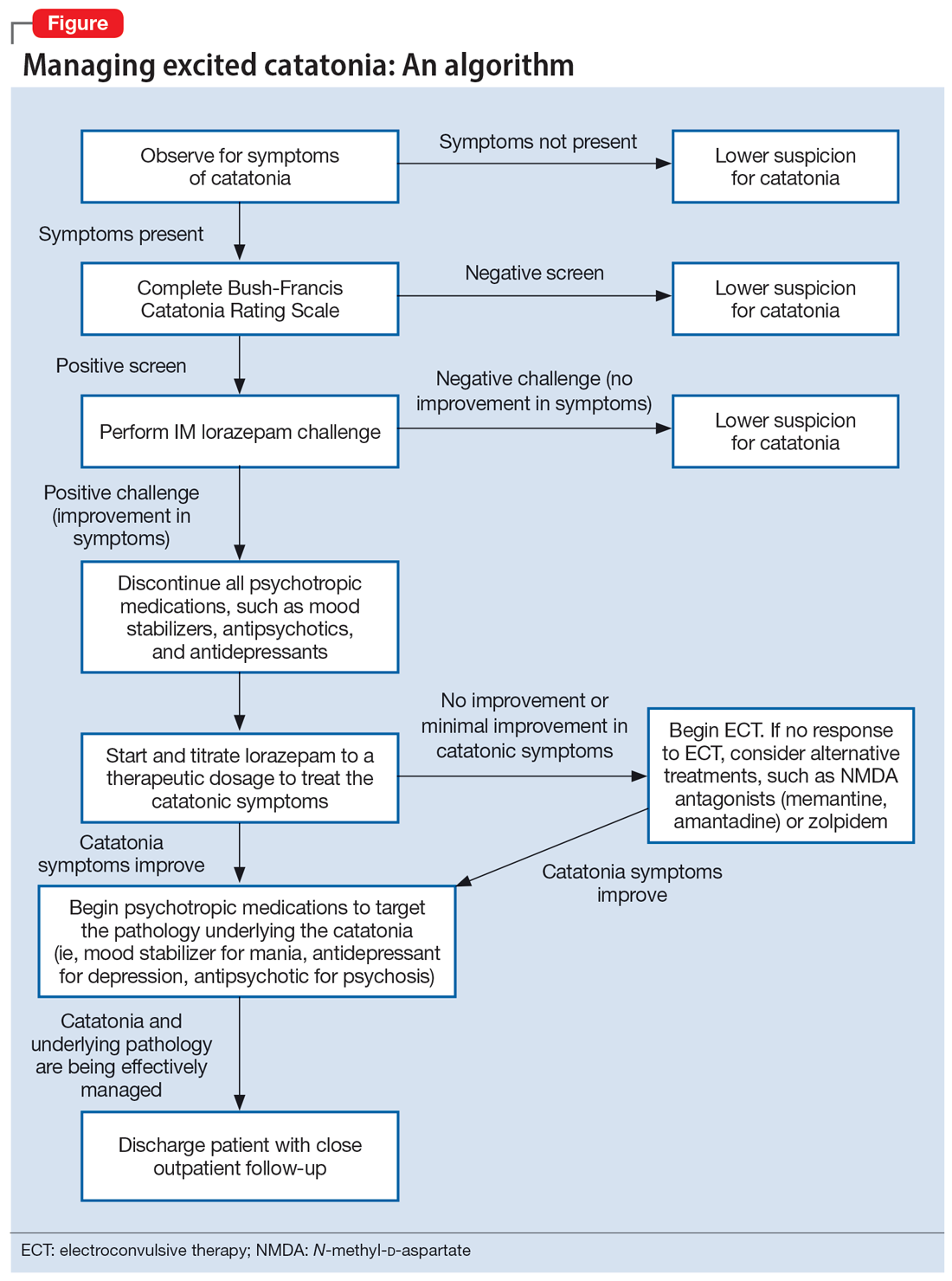
Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3

A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).

Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
Catatonia is often difficult to identify and treat. The excited catatonia subtype can be particularly challenging to diagnose because it can present with symptoms similar to those seen in mania or psychosis. In this article, we present 3 cases of excited catatonia that illustrate how to identify it, how to treat the catatonia as well as the underlying pathology, and factors to consider during this process to mitigate the risk of adverse outcomes. We also outline a treatment algorithm we used for the 3 cases. Although we describe using this approach for patients with excited catatonia, it is generalizable to other types of catatonia.
Many causes, varying presentations
Catatonia is a psychomotor syndrome characterized by mutism, negativism, stereotypy, waxy flexibility, and other symptoms.1 It is defined by the presence of ≥3 of the 12 symptoms listed in the Table.2 Causes of catatonia include metabolic abnormalities, endocrine disorders, drug intoxication, neurodevelopmental disorders, medication adverse effects, psychosis, and mood disorders.1,3

A subtype of this syndrome, excited catatonia, can present with restlessness, agitation, emotional lability, poor sleep, and altered mental status in addition to the more typical symptoms.1,4 Because excited catatonia can resemble mania or psychosis, it is particularly challenging to identify the underlying disorder causing it and appropriate treatment. Fink et al4 discussed how clinicians have interpreted the different presentations of excited catatonia to gain insight into the underlying diagnosis. If the patient’s thought process appears disorganized, psychosis may be suspected.4 If the patient is delusional and grandiose, they may be manic, and when altered mental status dominates the presentation, delirium may be the culprit.4
Regardless of the underlying cause, the first step is to treat the catatonia. Benzodiazepines and electroconvulsive therapy (ECT) are the most well validated treatments for catatonia and have been used to treat excited catatonia.1 Excited catatonia is often misdiagnosed and subsequently mistreated. In the following 3 cases, excited catatonia was successfully identified and treated using the same approach (Figure).

Case 1
Mr. A, age 27, has a history of bipolar I disorder. He was brought to the hospital by ambulance after being found to be yelling and acting belligerently, and he was admitted to the inpatient psychiatry unit for manic decompensation due to medication nonadherence. He was started on divalproex sodium 500 mg twice a day for mood stabilization, risperidone 1 mg twice a day for adjunct mood stabilization and psychosis, and lorazepam 1 mg 3 times a day for agitation. Mr. A exhibited odd behavior; he would take off his clothes in the hallway, run around the unit, and randomly yell at staff or to himself. At other times, he would stay silent, repeat the same statements, or oddly posture in the hallway for minutes at a time. These behaviors were seen primarily in the hour or 2 preceding lorazepam administration and improved after he received lorazepam.
Mr. A’s treating team completed the Bush-Francis Catatonia Rating Scale (BFCRS), which yielded a positive catatonia screen of 7/14. As a result, divalproex sodium and risperidone were held, and lorazepam was increased to 2 mg twice a day.
After several days, Mr. A was no longer acting oddly and was able to speak more spontaneously; however, he began to exhibit overt signs of mania. He would speak rapidly and make grandiose claims about managing millions of dollars as the CEO of a famous company. Divalproex sodium was restarted at 500 mg twice a day and increased to 500 mg 3 times a day for mood stabilization. Mr. A continued to receive lorazepam 2 mg 3 times a day for catatonia, and risperidone was restarted at 1 mg twice a day to more effectively target his manic symptoms. Risperidone was increased to 2 mg twice a day. After this change, Mr. A’s grandiosity dissipated, his speech normalized, and his thought process became organized. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium 500 mg 3 times a day, and risperidone 2 mg twice a day. Mr. A’s length of stay (LOS) for this admission was 11 days.
Continue to: Case 2
Case 2
Mr. B, age 49, presented with irritability and odd posturing. He has a history of schizoaffective disorder, bipolar type for which he was receiving a maintenance regimen of lithium 600 mg/d at bedtime and risperidone 2 mg/d at bedtime. He had multiple previous psychiatric admissions for catatonia. On this admission, Mr. B was irritable and difficult to redirect. He yelled at staff members and had a stiff gait. The BFCRS yielded a positive screening score of 3/14 and a severity score of 8/23. As a result, the treatment team conducted a lorazepam challenge.
After Mr. B received lorazepam 1 mg IM, his thought organization and irritability improved, which allowed him to have a coherent conversation with the interviewer. His gait stiffness also improved. His risperidone and lithium were held, and oral lorazepam 1 mg 3 times a day was started for catatonia. Lorazepam was gradually increased to 4 mg 3 times a day. Mr. B became euthymic and redirectable, and had an improved gait. However, he was also tangential and hyperverbal; these symptoms were indicative of the underlying mania that precipitated his catatonia.
Divalproex sodium extended release (ER) was started and increased to 1,500 mg/d at bedtime for mood stabilization. Lithium was restarted and increased to 300 mg twice a day for adjunct mood stabilization. Risperidone was not restarted. Toward the end of his admission, Mr. B was noted to be overly sedated, so the lorazepam dosage was decreased. He was discharged on lorazepam 2 mg 3 times a day, divalproex sodium ER 1,500 mg/d at bedtime, and lithium 300 mg twice a day. At discharge, Mr. B was calm and euthymic, with a linear thought process. His LOS was 25 days.
Case 3
Mr. C, age 62, presented to the emergency department (ED) because he had exhibited erratic behavior and had not slept for the past week. He has a history of bipolar I disorder, hypothyroidism, diabetes, and hypertension. For many years, he had been stable on divalproex sodium ER 2,500 mg/d at bedtime for mood stabilization and clozapine 100 mg/d at bedtime for adjunct mood stabilization and psychosis. In the ED, Mr. C was irritable, distractible, and tangential. On admission, he was speaking slowly with increased speech latency in response to questions, exhibiting stereotypy, repeating statements over and over, and walking very slowly.
The BFCRS yielded a positive screening score of 5/14 and a severity score of 10/23. Lorazepam 1 mg IM was administered. After 15 minutes, Mr. C’s speech, gait, and distractibility improved. As a result, clozapine and divalproex sodium were held, and he was started on oral lorazepam 1 mg 3 times a day. After several days, Mr. C was speaking fluently and no longer exhibiting stereotypy or having outbursts where he would make repetitive statements. However, he was tangential and irritable at times, which were signs of his underlying mania. Divalproex sodium ER was restarted at 250 mg/d at bedtime for mood stabilization and gradually increased to 2,500 mg/d at bedtime. Clozapine was also restarted at 25 mg/d at bedtime and gradually increased to 200 mg/d at bedtime. The lorazepam was gradually tapered and discontinued over the course of 3 weeks due to oversedation.
Continue to: At discharge...
At discharge, Mr. C was euthymic, calm, linear, and goal-directed. He was discharged on divalproex sodium ER 2,500 mg/d at bedtime and clozapine 200 mg/d at bedtime. His LOS for this admission was 22 days.
A stepwise approach can improve outcomes
The Figure outlines the method we used to manage excited catatonia in these 3 cases. Each of these patients exhibited signs of excited catatonia, but because those symptoms were nearly identical to those of mania, it was initially difficult to identify catatonia. Excited catatonia was suspected after more typical catatonic symptoms—such as a stiff gait, slowed speech, and stereotypy—were observed. The BFCRS was completed to get an objective measure of the likelihood that the patient was catatonic. In all 3 cases, the BFCRS resulted in a positive screen for catatonia. Following this, the patients described in Case 2 and Case 3 received a lorazepam challenge, which confirmed their catatonia. No lorazepam challenge was performed in Case 1 because the patient was already receiving lorazepam when the BFCRS was completed. Although most catatonic patients will respond to a lorazepam challenge, not all will. Therefore, clinicians should maintain some degree of suspicion for catatonia if a patient has a positive screen on the BFCRS but a negative lorazepam challenge.
In all 3 cases, after catatonia was confirmed, the patient’s psychotropic medications were discontinued. In all 3 cases, the antipsychotic was held to prevent progression to neuroleptic malignant syndrome (NMS) or malignant catatonia. Rasmussen et al3 found that 3.6% of the catatonic patients in their sample who were treated with antipsychotics developed NMS. A review of prospective studies looking at patients treated with antipsychotics found the incidence of NMS was .07% to 1.8%.5 Because NMS is often clinically indistinguishable from malignant catatonia,4,6 this incidence of NMS may have represented an increased incidence in malignant catatonia.
In all 3 cases, the mood stabilizer was held to prevent it from complicating the clinical picture. Discontinuing the mood stabilizer and focusing on treating the catatonia before targeting the underlying mania increased the likelihood of differentiating the patient’s catatonic symptoms from manic symptoms. This resulted in more precise medication selection and titration by allowing us to identify the specific symptoms that were being targeted by each medication.
Oral lorazepam was prescribed to target catatonia in all 3 cases, and the dosage was gradually increased until symptoms began to resolve. As the catatonia resolved, the manic symptoms became more easily identifiable, and at this point a mood stabilizer was started and titrated to a therapeutic dose to target the mania. In Case 1 and Case 3, the antipsychotic was restarted to treat the mania more effectively. It was not restarted in Case 2 because the patient’s mania was effectively being managed by 2 mood stabilizers. The risks and benefits of starting an antipsychotic in a catatonic or recently catatonic patient should be carefully considered. In the 2 cases where the antipsychotic was restarted, the patients were closely monitored, and there were no signs of NMS or malignant catatonia.
Continue to: As discharge approached...
As discharge approached, the dosages of oral lorazepam were reevaluated. Catatonic patients can typically tolerate high doses of benzodiazepines without becoming overly sedated, but each patient has a different threshold at which the dosage causes oversedation. In all 3 patients, lorazepam was initially titrated to a dose that treated their catatonic symptoms without causing intolerable sedation. In Case 2 and Case 3, as the catatonia began to resolve, the patients became increasingly sedated on their existing lorazepam dosage, so it was decreased. Because the patient in Case 1 did not become overly sedated, his lorazepam dosage did not need to be reduced.
For 2 of these patients, our approach resulted in a shorter LOS compared to their previous hospitalizations. The LOS in Case 2 was 25 days; 5 years earlier, he had a 49-day LOS for mania and catatonia. During the past admission, the identification and treatment of the catatonia was delayed, which resulted in the patient requiring multiple transfers to the medical unit for unstable vital signs. The LOS in Case 3 was 22 days; 6 months prior to this admission, the patient had 2 psychiatric admissions that totaled 37 days. Although the patient’s presentation in the 2 previous admissions was similar to his presentation as described in Case 3, catatonia had not been identified or treated in either admission. Since his catatonia and mania were treated in Case 3, he has not required a readmission. The patient in Case 1 was previously hospitalized, but information about the LOS of these admissions was not available. These results suggest that early identification and treatment of catatonia via the approach we used can improve patient outcomes.
Bottom Line
Excited catatonia can be challenging to diagnose and treat because it can present with symptoms similar to those seen in mania or psychosis. We describe 3 cases in which we used a stepwise approach to optimize treatment and improve outcomes for patients with excited catatonia. This approach may work equally well for other catatonia subtypes.
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: how to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Crouse EL, Joel B. Moran JB. Catatonia: recognition, management, and prevention of complications. Current Psychiatry. 2018;17(12):45-49.
Drug Brand Names
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Risperidone • Risperdal
Divalproex sodium • Depakote
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
1. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):8-13.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:119-121.
3. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
4. Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003.
5. Adityanjee, Aderibigbe YA, Matthews T. Epidemiology of neuroleptic malignant syndrome. Clin Neuropharmacol. 1999;22(3):151-158.
6. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
Behavioral treatment tied to lower medical, pharmacy costs
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Does dopamine dysregulation cause schizophrenia?
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Clinical factors drive hospitalization after self-harm
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH





