User login
Behavioral treatment tied to lower medical, pharmacy costs
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large retrospective study showed that patients newly diagnosed with a BHC who receive OPBHT following diagnosis incur lower medical and pharmacy costs over roughly the next 1 to 2 years, compared with peers who don’t receive OPBHT.
“Our findings suggest that promoting OPBHT as part of a population health strategy is associated with improved overall medical spending, particularly among adults,” the investigators write.
The study was published online in JAMA Network Open.
Common, undertreated
Nearly a quarter of adults in the United States have a BHC, and they incur greater medical costs than those without a BHC. However, diagnosis of a BHC is often delayed, and most affected individuals receive little to no treatment.
In their cost analysis, Johanna Bellon, PhD, and colleagues with Evernorth Health, St. Louis, analyzed commercial insurance claims data for 203,401 U.S. individuals newly diagnosed with one or more BHCs between 2017 and 2018.
About half of participants had depression and/or anxiety, 11% had substance use or alcohol use disorder, and 6% had a higher-acuity diagnosis, such as bipolar disorder, severe depression, eating disorder, psychotic disorder, or autism spectrum disorder.
About 1 in 5 (22%) had at least one chronic medical condition along with their BHC.
The researchers found that having at least one OPBHT visit was associated with lower medical and pharmacy costs during 15- and 27-month follow-up periods.
Over 15 months, the adjusted mean per member per month (PMPM) medical/pharmacy cost was $686 with no OPBHT visit, compared with $571 with one or more OPBHT visits.
Over 27 months, the adjusted mean PMPM was $464 with no OPBHT, versus $391 with one or more OPBHT visits.
Dose-response effect
In addition, there was a “dose-response” relationship between OPBHT and medical/pharmacy costs, such that estimated cost savings were significantly lower in the treated versus the untreated groups at almost every level of treatment.
“Our findings were also largely age independent, especially over 15 months, suggesting that OPBHT has favorable effects among children, young adults, and adults,” the researchers report.
“This is promising given that disease etiology and progression, treatment paradigms, presence of comorbid medical conditions, and overall medical and pharmacy costs differ among the three groups,” they say.
Notably, the dataset largely encompassed in-person OPBHT, because the study period preceded the transition into virtual care that occurred in 2020.
However, overall use of OPBHT was low – older adults, adults with lower income, individuals with comorbid medical conditions, and persons of racial and ethnic minorities were less likely to receive OPBHT, they found.
“These findings support the cost-effectiveness of practitioner- and insurance-based interventions to increase OPBHT utilization, which is a critical resource as new BHC diagnoses continue to increase,” the researchers say.
“Future research should validate these findings in other populations, including government-insured individuals, and explore data by chronic disease category, over longer time horizons, by type and quality of OPBHT, by type of medical spending, within subpopulations with BHCs, and including virtual and digital behavioral health services,” they suggest.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Does dopamine dysregulation cause schizophrenia?
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Clinical factors drive hospitalization after self-harm
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH
Optimal psychiatric treatment: Target the brain and avoid the body
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.
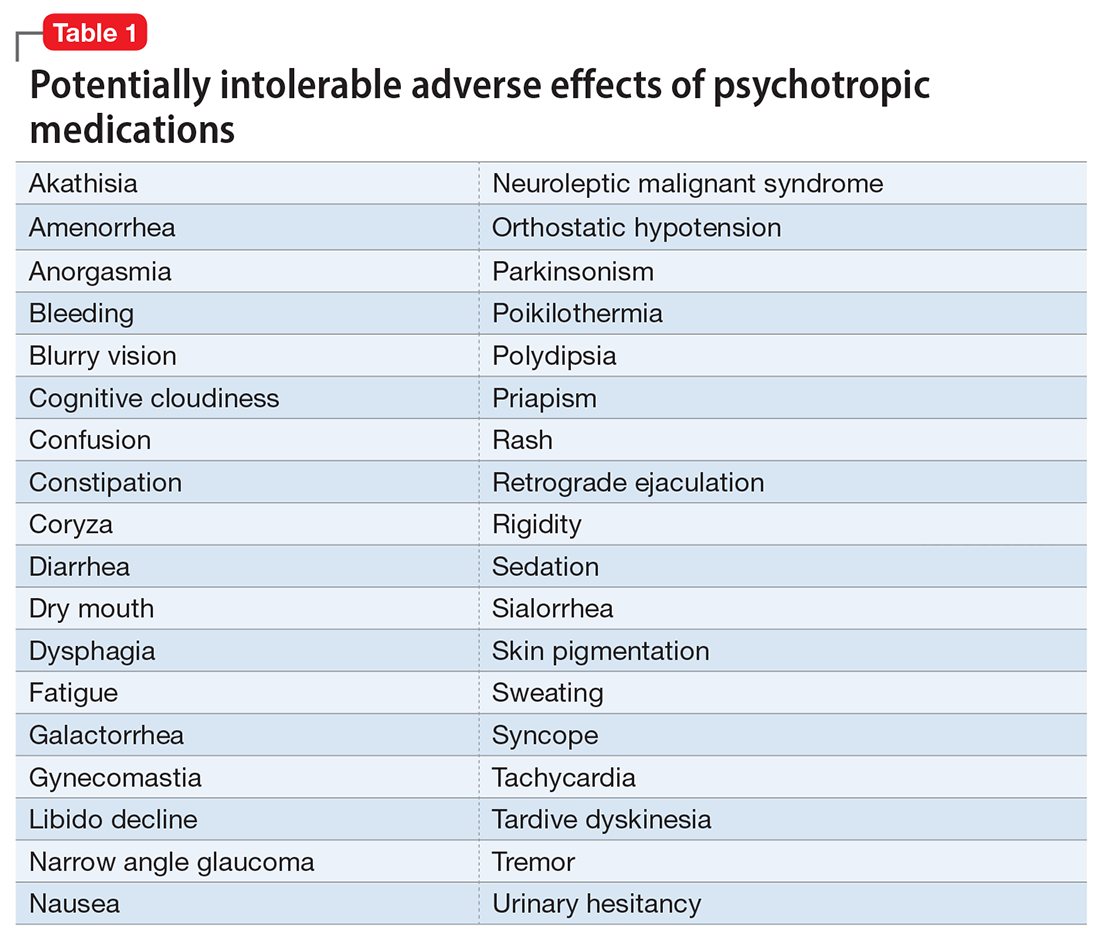
However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.
Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
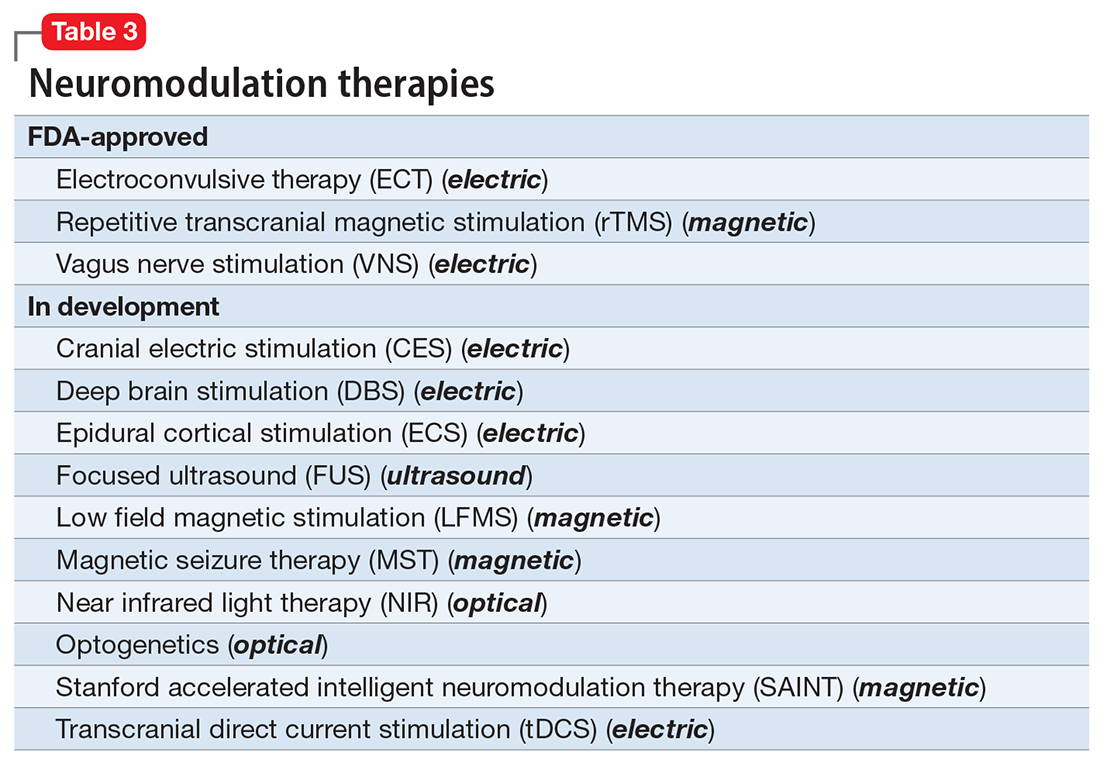
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17
A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

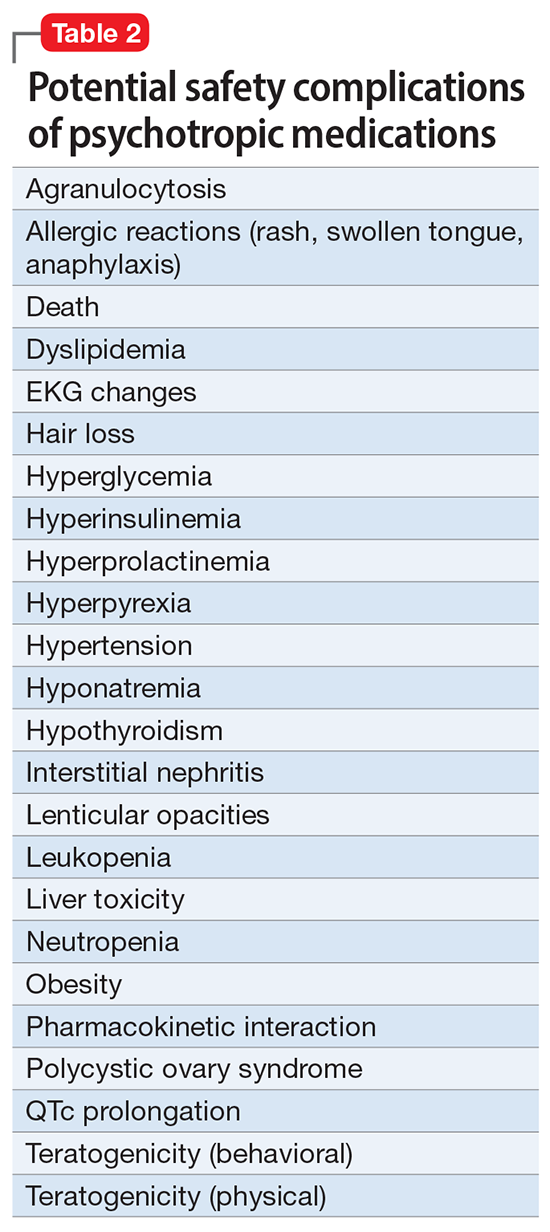
However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
Pharmacotherapy for psychiatric disorders is a mixed blessing. The advent of psychotropic medications since the 1950s (antipsychotics, antidepressants, anxiolytics, mood stabilizers) has revolutionized the treatment of serious psychiatric brain disorders, allowing certain patients to be discharged to the community after a lifetime of institutionalization.

However, like all medications, psychotropic agents are often associated with various potentially intolerable symptoms (Table 1) or safety complications (Table 2) because they interact with every organ in the body besides their intended target, the brain, and its neurochemical circuitry.

Imagine if we could treat our psychiatric patients while bypassing the body and achieve response, remission, and ultimately recovery without any systemic adverse effects. Adherence would dramatically improve, our patients’ quality of life would be enhanced, and the overall effectiveness (defined as the complex package of efficacy, safety, and tolerability) would be superior to current pharmacotherapies. This is important because most psychiatric medications must be taken daily for years, even a lifetime, to avoid a relapse of the illness. Psychiatrists frequently must manage adverse effects or switch the patient to a different medication if a tolerability or safety issue emerges, which is very common in psychiatric practice. A significant part of psychopharmacologic management includes ordering various laboratory tests to monitor adverse reactions in major organs, especially the liver, kidney, and heart. Additionally, psychiatric physicians must be constantly cognizant of medications prescribed by other clinicians for comorbid medical conditions to successfully navigate the turbulent seas of pharmacokinetic interactions.
I am sure you have noticed that whenever you watch a direct-to-consumer commercial for any medication, 90% of the advertisement is a background voice listing the various tolerability and safety complications of the medication as required by the FDA. Interestingly, these ads frequently contain colorful scenery and joyful clips, which I suspect are cleverly designed to distract the audience from focusing on the list of adverse effects.
Benefits of nonpharmacologic treatments
No wonder I am a fan of psychotherapy, a well-established psychiatric treatment modality that completely avoids body tissues. It directly targets the brain without needlessly interacting with any other organ. Psychotherapy’s many benefits (improving insight, enhancing adherence, improving self-esteem, reducing risky behaviors, guiding stress management and coping skills, modifying unhealthy beliefs, and ultimately relieving symptoms such as anxiety and depression) are achieved without any somatic adverse effects! Psychotherapy has also been shown to induce neuroplasticity and reduce inflammatory biomarkers.1 Unlike FDA-approved medications, psychotherapy does not include a “package insert,” 10 to 20 pages (in small print) that mostly focus on warnings, precautions, and sundry physical adverse effects. Even the dosing of psychotherapy is left entirely up to the treating clinician!
Although I have had many gratifying results with pharmacotherapy in my practice, especially in combination with psychotherapy,2 I also have observed excellent outcomes with nonpharmacologic approaches, especially neuromodulation therapies. The best antidepressant I have ever used since my residency training days is electroconvulsive therapy (ECT). My experience is consistent with a large meta-analysis3showing a huge effect size (Cohen d = .91) in contrast to the usual effect size of .3 to .5 for standard antidepressants (except IV ketamine). A recent study showed ECT is even better than the vaunted rapid-acting ketamine,4 which is further evidence of its remarkable efficacy in depression. Neuroimaging studies report that ECT rapidly increases the volume of the hippocampus,5,6 which shrinks in size in patients with unipolar or bipolar depression.
Neuromodulation may very well be the future of psychiatric therapeutics. It targets the brain and avoids the body, thus achieving efficacy with minimal systemic tolerability (ie, patient complaints) (Table 1) or safety (abnormal laboratory test results) issues (Table 2). This sounds ideal, and it is arguably an optimal approach to repairing the brain and healing the mind.
Continue to: ECT is the oldest...
ECT is the oldest neuromodulation technique (developed almost 100 years ago and significantly refined since then). Newer FDA-approved neuromodulation therapies include repetitive transcranial magnetic stimulation (rTMS), which was approved for depression in 2013, obsessive-compulsive disorder (OCD) in 2018, smoking cessation in 2020, and anxious depression in 2021.7 Vagus nerve stimulation (VNS) is used for drug-resistant epilepsy and was later approved for treatment-resistant depression,8,9 but some studies report it can be helpful for fear and anxiety in autism spectrum disorder10 and primary insomnia.11
There are many other neuromodulation therapies in development12 that have not yet been FDA approved (Table 3). The most prominent of these is deep brain stimulation (DBS), which is approved for Parkinson disease and has been reported in many studies to improve treatment-resistant depression13,14 and OCD.15 Another promising neuromodulation therapy is transcranial direct current stimulation (tDCS), which has promising results in schizophrenia16 similar to ECT’s effects in treatment-resistant schizophrenia.17

A particularly exciting neuromodulation approach published by Stanford University researchers is Stanford accelerated intelligent neuromodulation therapy (SAINT),18 which uses intermittent theta-burst stimulation (iTBS) daily for 5 days, targeted at the subgenual anterior cingulate gyrus (Brodman area 25). Remarkably, efficacy was rapid, with a very high remission rate (absence of symptoms) in approximately 90% of patients with severe depression.18
The future is bright for neuromodulation therapies, and for a good reason. Why send a chemical agent to every cell and organ in the body when the brain can be targeted directly? As psychiatric neuroscience advances to a point where we can localize the abnormal neurologic circuit in a specific brain region for each psychiatric disorder, it will be possible to treat almost all psychiatric disorders without burdening patients with the intolerable symptoms or safety adverse effects of medications. Psychiatrists should modulate their perspective about the future of psychiatric treatments. And finally, I propose that psychotherapy should be reclassified as a “verbal neuromodulation” technique.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
1. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
2. Nasrallah HA. Bipolar disorder: clinical questions beg for answers. Current Psychiatry. 2006;5(12):11-12.
3. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
4. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022:e223352. doi:10.1001/jamapsychiatry.2022.3352
5. Nuninga JO, Mandl RCW, Boks MP, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 2020;25(7):1559-1568.
6. Joshi SH, Espinoza RT, Pirnia T, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
7. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715.
8. Hilz MJ. Transcutaneous vagus nerve stimulation - a brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
9. Pigato G, Rosson S, Bresolin N, et al. Vagus nerve stimulation in treatment-resistant depression: a case series of long-term follow-up. J ECT. 2022. doi:10.1097/YCT.0000000000000869
10. Shivaswamy T, Souza RR, Engineer CT, et al. Vagus nerve stimulation as a treatment for fear and anxiety in individuals with autism spectrum disorder. J Psychiatr Brain Sci. 2022;7(4):e220007. doi:10.20900/jpbs.20220007
11. Wu Y, Song L, Wang X, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. doi:10.3390/brainsci12101296
12. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
14. Choi KS, Mayberg H. Connectomic DBS in major depression. In: Horn A, ed. Connectomic Deep Brain Stimulation. Academic Press; 2022:433-447.
15. Cruz S, Gutiérrez-Rojas L, González-Domenech P, et al. Deep brain stimulation in obsessive-compulsive disorder: results from meta-analysis. Psychiatry Res. 2022;317:114869. doi:10.1016/j.psychres.2022.114869
16. Lisoni J, Baldacci G, Nibbio G, et al. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J Psychiatr Res. 2022;155:430-442.
17. Sinclair DJ, Zhao S, Qi F, et al. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3(3):CD011847. doi:10.1002/14651858.CD011847.pub2
18. Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726.
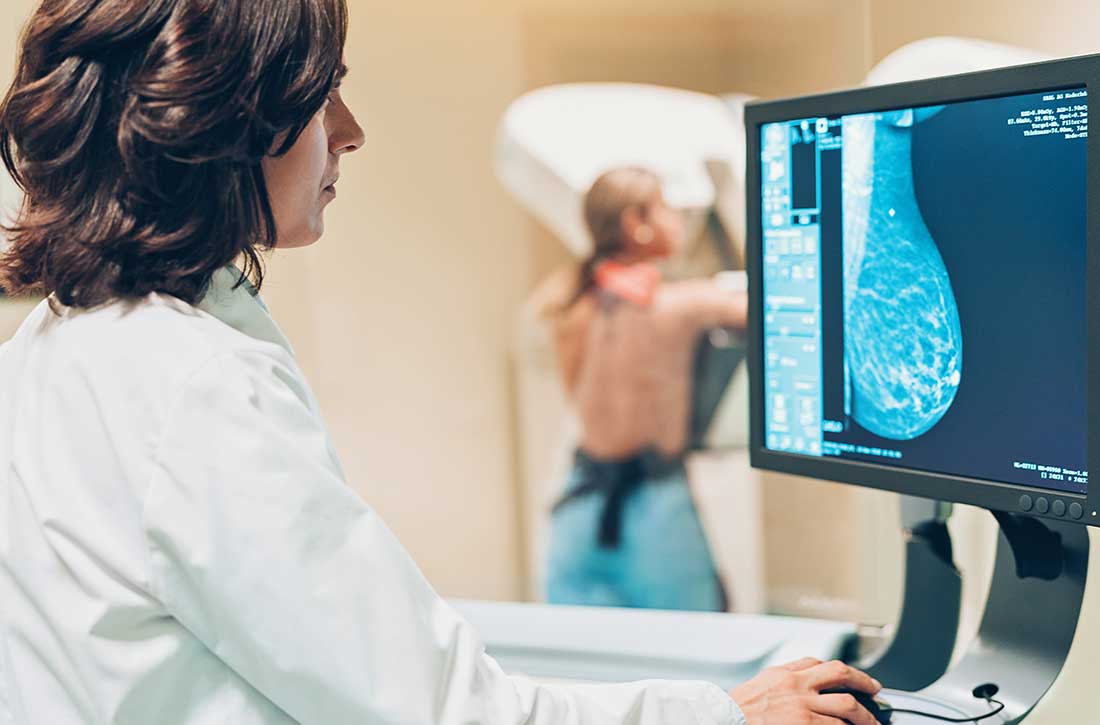
Breast cancer screening in women receiving antipsychotics
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
Women with severe mental illness (SMI) are more likely to develop breast cancer and often have more advanced stages of breast cancer when it is detected.1 Antipsychotics have a wide variety of FDA-approved indications and many important life-saving properties. However, patients treated with antipsychotic medications that increase prolactin levels require special consideration with regards to referral for breast cancer screening. Although no clear causal link between antipsychotic use and breast cancer has been established, antipsychotics that raise serum prolactin levels (haloperidol, iloperidone, lurasidone, olanzapine, paliperidone, risperidone) are associated with a higher risk of breast cancer than antipsychotics that produce smaller increases in prolactin levels (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, quetiapine, and ziprasidone).2,3 Risperidone and paliperidone have the highest propensities to increase prolactin (45 to >100 ng/mL), whereas other second-generation antipsychotics are associated with only modest elevations.4 Prolonged exposure to high serum prolactin levels should be avoided in women due to the increased risk for breast cancer.2,3 Although there are no clear rules regarding which number or cluster of personal risk factors necessitates a further risk assessment for breast cancer, women receiving antipsychotics (especially those age ≥40) can be referred for further assessment. An individualized, patient-centered approach should be used.
Recognize risk factors
Patients with SMI often need to take a regimen of medications, including antipsychotics, for weeks or months to stabilize their symptoms. Once a woman with SMI is stabilized, consider referral to a clinic that can comprehensively assess for breast cancer risk. Nonmodifiable risk factors include older age, certain genetic mutations (BRCA1 and BRCA2), early menarche, late menopause, high breast tissue density as detected by mammography, a family history of breast cancer, and exposure to radiation.5,6 Modifiable risk factors include physical inactivity, being overweight or obese, hormonal exposure, drinking alcohol, and the presence of certain factors in the patient’s reproductive history (first pregnancy after age 30, not breastfeeding, and never having a full-term pregnancy).2,3 When making such referrals, it is important to avoid making the patient feel alarmed or frightened of antipsychotics. Instead, explain that a referral for breast cancer screening is routine.
When to refer
All women age ≥40 should be offered a referral to a clinic that can provide screening mammography. If a woman has pain, detects a lump in her breast, has a bloody discharge from the nipple, or has changes in the shape or texture of the nipple or breast, a more urgent referral should be made.4 The most important thing to remember is that early breast lesion detection can be life-saving and can avert the need for more invasive surgeries as well as exposure to chemotherapy and radiation.
What to do when prolactin is elevated
Ongoing monitoring of serum prolactin levels can help ensure that the patient’s levels remain in a normal range (<25 ng/mL).2,3,5,6 If hyperprolactinemia is detected, consider switching to an antipsychotic less likely to increase prolactin. Alternatively, the addition of aripiprazole/brexpiprazole or a dopamine agonist as combination therapy can be considered to rapidly restore normal prolactin levels.2 Such changes should be carefully considered because patients may decompensate if antipsychotics are abruptly switched. An individualized risk vs benefit analysis is necessary for any patient in this situation. Risks include not only the recurrence of psychiatric symptoms but also a potential loss of their current level of functioning. Patients may need to continue to take an antipsychotic that is more likely to increase prolactin, in which case close monitoring is advised as well as collaboration with other physicians and members of the patient’s care team. Involving the patient’s support system is helpful.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
1. Weinstein LC, Stefancic A, Cunningham AT, et al. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):134-151.
2. Rahman T, Sahrmann JM, Olsen MA, et al. Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol. 2022;42(1):7-16.
3. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
4. Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421-453.
5. Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. Breast cancer. Accessed June 1, 2022. https://www.cdc.gov/cancer/breast/index.htm
6. Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 2008;78(12):1361-1366.
What my Grandma’s schizophrenia taught me
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Grandma was sitting in her chair in the corner of the living room, and her eyes were wide, filled with fear and suspicion as she glanced between me, Mom, and Papa. “They are out to get me,” she said, slightly frantic. She glanced down at her right hand, fixated on a spot on the dorsum. Gingerly lifting her arm, she angled her hand toward my mom’s face. “You see that? They have been conducting experiments on me. I AM THE QUEEN,” she sobbed, “and you are planning together” she said, directing her attention to Papa and me. In that moment, Grandma was convinced Papa and I were conspiring to assassinate her. It hurt to see my grandmother look at me with genuine fear in her eyes. It was overwhelming to watch her deteriorate from the person I had been accustomed to for most of my life to the paranoid individual shaking in front of me.
This was the first time I had really observed my grandmother experiencing acute psychosis. My mom explained to me at a young age that my grandmother had an illness in her mind. I noticed that compared to other people in my life, my grandmother seemed to express less emotion and changed topics in conversations frequently, but by having an understanding provided by my mother, my brother and I didn’t think much of it; that was just Grandma. She would occasionally talk about her experiences with hearing voices or people on the television talking about her. For the most part, though, she was stable; she was able to carry out cleaning, cooking, and watching her favorite shows.
That was until she turned 65 and started on Medicare for insurance. The government required her to trial a less expensive medication and wanted her family practitioner to adjust the medications she had been on for years. This decision was made by people unfamiliar with my grandmother and her story. As a result, my family struggled alongside Grandma for over a month as she battled hallucinations and labile emotions. Living in rural Ohio, she had no access to a psychiatrist or other mental health professional during this period. The adjustments to her medications, changes in her insurance coverage, and lack of consistent psychiatric care led to a deterioration of her stability. This was the only time in my life that I saw Grandma at a place where she would have needed to be hospitalized if the symptoms lasted much longer. I spent evenings sitting with her in that dark and scary place, listening, sympathizing, and challenging her distortions of reality. This experience laid the foundation for my growing passion for providing care and advocating for people experiencing mental illness. I observed firsthand how the absence of consistent, compassionate, and informed care could lead to psychiatric hospitalization.
In the past, my grandfather hid my grandmother’s diagnosis from those around them. This approach prevented my uncle from disclosing the same information to my cousins. I observed how they would look at her with confusion and sometimes fear, which was rooted in a lack of understanding. This desire to hide Grandma’s schizophrenia stemmed from the marginalization society imposed upon her. There were sneers, comments regarding lack of religious faith, and expressions that she was not trying hard enough. My grandparents decided together to inform their church of my grandmother’s illness. The results were astounding. People looked at my grandmother not with confusion but with sympathy and would go out of their way to check on her. Knowledge is power, and awareness can break down stigma. Seeing the difference knowledge could have on a church community further solidified my desire to educate not only patients and their family members but also communities.
Access is another huge barrier my grandmother has faced. There is a lack of referring and awareness as well as large geographic disparities of psychiatrists around my hometown. My grandmother has also had struggles with being able to pay for services, medication, and therapy. This shows the desperate need for more mental health professionals who are competent and knowledgeable in how social determinants of health impact outcomes. These factors contributed to my decision to pursue a Master of Public Health degree. I aspire to use this background to prevent what happened to my Grandma from happening to other patients and to be an advocate for enhanced access to services, improving community mental health and awareness, and promoting continuity of care to increase treatment compliance. That is what my Grandma has fostered in me as a future psychiatrist.
Clozapine underutilized in treatment-resistant schizophrenia
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
FDA puts REMS requirements on hold to ensure continuity of care
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
Patients with schizophrenia may be twice as likely to develop dementia
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL MEDICINE
Menopause an independent risk factor for schizophrenia relapse
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCHIZOPHRENIA BULLETIN