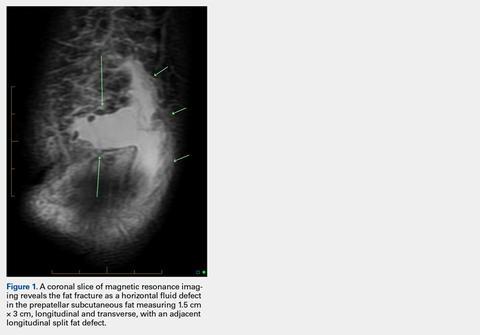
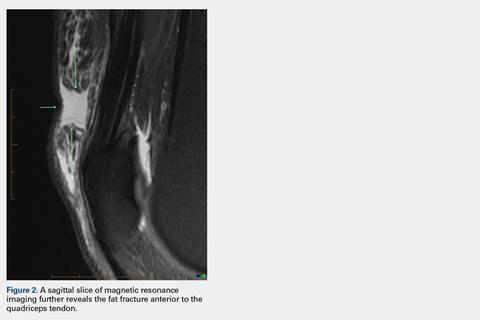
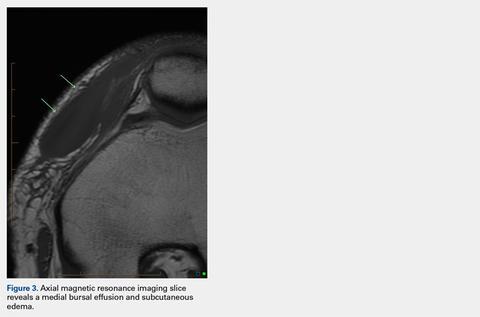
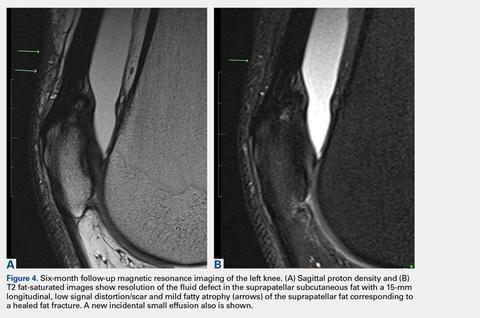
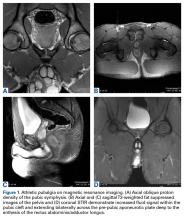
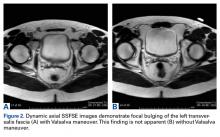
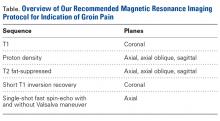
User login
Upper Extremity Injuries in Soccer
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
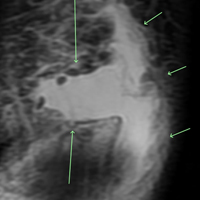
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
TAKE-HOME POINTS
- Upper extremity injuries in soccer are not common, however they can reach up to 18% of all injuries in professional goalkeepers.
- Common injury locations in the upper extremity in soccer are the shoulder/clavicle, hand/finger/thumb, the elbow, and the wrist and most of these injuries are traumatic injuries.
- Mechanism of injury, players’ complaints and presentation, physical examination, and imaging features are all important for a proper evaluation and optimal management.
- Position of play is an important consideration in the management of upper extremity injuries in soccer. Outfield players may be able to return to play before a complete resolution of their injury, with protective accessories.
- Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
Soccer or Football Medicine? Global Sports Medicine for a Global Game
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
The Three H’s: Head, Heart, and Heat Considerations in Soccer
ABSTRACT
Soccer requires significant physical conditioning and endurance, as well as the physicality required for contact play. In order to keep athletes safe, it is important that coaches, medical staff, and the players themselves are educated on the most common dangers to their health that they may encounter on a soccer pitch. This article aims to review the current literature and recommendations on concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer, with a goal of educating all those who help to keep athletes healthy and competing to their full potential.
Continue to: Soccer is one of the most popular sports...
Soccer is one of the most popular sports in the modern world and requires significant physical conditioning and endurance, as well as the physicality required for contact play. This article covers the topics of concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer players. We provide a review of the prevention, recognition, and management required to keep athletes safe on the soccer pitch, both in practice and in competitive play.
HEAD
With an estimated 1.6 to 3.8 million sports-related mild traumatic brain injuries (ie, concussions) occurring annually in the United States,1 there has been an appropriate increase in the focus on prevention and treatment of these injuries. For more than a decade the spotlight has been on concussions that occur in American football, but other sports have also had to examine the prevalence of concussions in their sport. This is certainly true for soccer.
There has been a steady increase in soccer participation in the United States. From 1973 to 2014 there was a 4-fold increase in high school boys and a 35-fold increase in high school girls playing soccer.2 Currently, there are more than 3.7 million youth who play on teams under the supervision of the US Soccer Federation, the sport’s national governing body.3 With the growth of the sport, there has also been an intensified focus on injury prevention in soccer players, including concussive brain injuries.
A recent study examined injury rates in high school soccer players and noted that concussion is the second most common injury (17.9%), after ligament sprains (29.7%).4 The overall injury rate was 2.06 per 1000 athletic exposures (AEs [defined as participation in practice or game play]), but higher in games (4.42 per 1000 AEs) than during practices (1.05 per 1000 AEs). The overall concussion rate was 0.36 per 1000 AEs.4
Most concussions (54.8%) resulted in missing play between 1 to 3 weeks, but a sizeable portion of the athletes (14.9%) were out of play for more than 3 weeks. Additionally, 10.7% of all medical disqualifications were due to concussive injuries. Khodaee and colleagues4 found no statistically significant difference in concussion rates between male and female soccer players over the 9-year period of time that they examined, however previous studies have found higher rates in female athletes.5
In soccer, as in other sports, there is a concern about both the adequate recognition of concussions during practice and play and the underreporting of concussions by athletes. The US Soccer Federation has taken a proactive stance on addressing concussion in youth soccer by developing the “Recognize to Recover” program.6 Recognize to Recover is the US Soccer Federation’s “comprehensive player health and safety program aimed at promoting safe play and reducing injuries in soccer players of all ages.” The website provides an educational video geared toward players, along with links to concussion assessment tools, the US Soccer Federation Concussion Protocol, and US Soccer Federation-Centers for Disease Control fact sheets for athletes, parents, and coaches.6
Continue to: A challenge for all sports...
A challenge for all sports is allowing adequate evaluation of a suspected concussion by properly trained healthcare professionals. The 2017 Berlin Concussion in Sport Group position paper stated, “when a concussion is suspected, the athlete should be removed from the sporting environment and a multimodal assessment should be conducted in standardized fashion (eg, Sport Concussion Assessment Tool- 5th edition). Sporting bodies should allow adequate time to conduct this evaluation.”7 However, the International Federation of Football Association (FIFA) rules limit substitutions to 3 over the course of the game, which can make a thorough evaluation of players difficult as trainers and coaches are under increased pressure to quickly determine whether to use one of their valuable substitutions. Fortunately, the National Collegiate Athletic Association (NCAA) soccer has mitigated this issue by allowing unlimited substitutions during matches, and high school teams generally follow similar rules.
One of the goals of any safety education program is not only to raise the awareness of the signs and symptoms of concussion by all those involved in the sport, but also to increase the number of athletes who self-report their symptoms and decrease those who hide any possible concussions. A study found that a majority (58.6%) of middle school soccer players continued to play while experiencing concussion symptoms.5 However, in a very recent (not yet published) study, 92% of US Soccer Federation players reported that they did seek out a medical evaluation for their concussion.8 This is certainly a positive sign and further research needs to clarify what methods of education or training will maintain this level of self-reporting in soccer players.
There has also been an increased focus on understanding the mechanism of injury of concussions. In soccer, concussions can occur from player-to-player contact, contact with the player surface, contact with playing apparatus (eg, goal posts) and non-contact mechanisms. While there has been a focus on concussions from heading the ball, player-to-player contact is the most common cause of concussions. A 2017 study of 7- to 12-year-old soccer players in 4 European countries found that about 1 out of 10 concussions were caused by heading the ball.9 Comstock and colleageues10 found slightly higher numbers, roughly 25% to 30% (depending on gender), but 70% to 78% (again depending on gender) of those were caused by player-to-player contact rather than contact with the ball.
To date there has not been any meta-analytic review evaluating the cognitive and physical symptoms associated with heading in soccer. A recent review paper stated that the “current evidence seems insufficient to support a ban of heading in children’s football (soccer).”10 However, in December 2015 the US Soccer Federation included age-specific heading limitations. Players ages 10 years and under “shall not engage in heading, either in practices or in games” and players age 11 years and 12 years should have “limited heading in practice; maximum of 30 minutes of heading training per week, with no more than 15-20 headers per player, per week.”11 US Soccer Federation officials acknowledged the limitations in the current science regarding heading in young soccer players but chose to err on the side of caution until further empirical evidence regarding the risks associated with repetitive heading is available.
The US Soccer Federation is also exploring other ways to reduce the incidence rate of concussions, including ensuring that the age-appropriate sized ball is used in practice and play, possible rule changes, evaluation of different playing surfaces, and equipment usage. To date, there is no strong evidence to support the use of mouth guards or helmets to reduce concussions in soccer. Additionally, the current data about the value of head impact sensors in soccer has not supported its widespread use.
Continue to: Finally, the issue of the prevalence...
Finally, the issue of the prevalence of chronic traumatic encephalopathy (CTE) in soccer players is beyond the scope of this article. The expert opinion from the 2017 US Soccer Federation, Major League Soccer (MLS), and National Women’s Soccer League (NWSL) conference concluded, “At present, no data exist that support that soccer participation is a risk factor for the development of neurodegenerative disease. Similarly, at this time, consistent with evidence discussed in the Berlin Concussion in Sport Group (CISG) Consensus Conference, our review suggests no causal relationship has been demonstrated between soccer and CTE pathology.”12
The more we know about concussions, both in general and those sustained during soccer play, the better we are able to diagnose and manage these injuries in our athletes. An important step is creating evidence-based protocols that evolve as our knowledge of concussions does as well. In April 2017, the US Soccer Federation, MLS, and the NWSL held a joint summit entitled, “Head Injury in Soccer: From Science to the Field” to address the current evidence-based science of concussions in soccer.8 An article discussing the findings of this meeting is forthcoming and will undoubtedly guide further development of concussion protocols for soccer players of all ages.
HEART
The physiologic demands of soccer place considerable stress on the cardiovascular system. Participation in training and competition is characterized by a combination of aerobic and anaerobic physiology with the typical athlete covering approximately 10 km over the course of the 90-minute match. The primary role of the heart and blood vessels is to supply the exercising skeletal muscle with oxygen and energy substrate and to clear the byproducts of metabolism. Among healthy athletes without cardiovascular disease, these processes are typically well tolerated and may be associated with beneficial cardiovascular adaptations over time. However, competitive soccer players are not completely immune to cardiovascular disease. Athletes across the age and competition spectrum may develop symptoms suggestive of underlying cardiovascular disease during play including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope. These athletes require timely clinical evaluation. In extremely rare but high visibility cases, competitive soccer players may succumb to cardiac arrest on the pitch, underscoring the need for comprehensive emergency action plans (EAPs). We provide the practicing clinician with an overview of cardiovascular issues relevant to the competitive soccer athlete.
CARDIOVASCULAR ADAPTATIONS TO SPORT
The pressure (ie, repetitive surges in systemic blood pressure) and volume (ie, sustained increases in high cardiac output) challenges inherent in soccer participation place stress on the cardiovascular system. Healthy athletes across the age spectrum typically tolerate the hemodynamic stressors of participation without issues. Athletes that engage in training and competition over months to years often develop beneficial adaptations of the cardiovascular system that enhance on-field performance and contribute to optimal long-term health. Detailed discussion of how the heart and blood vessels respond to exercise training is beyond the scope of this article, but the interested reader is referred to several prior publications.13,14 In brief, the heart of the healthy soccer athlete demonstrates the balanced mild chamber dilation and wall thickening characteristic of left ventricular eccentric remodeling. This form of exercise-induced cardiac remodeling facilitates maintenance of high stroke volume during exercise with minimal increases in cardiac work. In parallel, routine aerobic exercise training confers favorable changes in the systemic arterial system, which leads to reductions in age-associated ventricular stiffening and maintenance of healthy low blood pressure. It must be emphasized that the healthy heart muscle dilation and thickening that develop in response to sports participation, regardless of age, ethnicity, or gender, are relatively mild and should not be confused with common forms of heart muscle disease that may be seen in athletes at risk for adverse outcomes. In some situations, consultation with an extreme sports cardiologist may be required to differentiate exercise-induced remodeling from over heart muscle pathology.15
Continue to: THE SYMPTOMATIC ATHLETE...
THE SYMPTOMATIC ATHLETE
Any athlete presenting with symptoms suggestive of underlying cardiovascular disease should be withheld from training and competition until a comprehensive clinical evaluation has been completed. Common manifestations of underlying heart disease that occur in soccer players include exertional chest pain/pressure/tightness, shortness of breath that is out of proportion to workload, palpitations or the perception of irregular cardiac activity, and syncope. Chest discomfort, inappropriate shortness of breath, and palpitations that occur during training or competition should be managed with immediate removal from the playing field and prompt medical assessment. In many cases, thorough evaluation will involve collaboration between sports medicine and sports cardiology providers. Evaluations must be individualized on a case-by-case basis as tailored to the athlete’s presenting chief complaint and prior medical history. Most of these assessments will include a detailed medical history and physical examination, a 12-lead electrocardiogram (ECG), provocative exercise testing in a controlled environment, noninvasive cardiac imaging, and in some cases ambulatory rhythm monitoring. Uniformly, the athlete should be withheld from further training and competition until high-risk cardiovascular disease has been excluded. We refer the interested reader to a comprehensive discussion of symptom-based assessment of the athlete with suspected cardiovascular disease.16
Syncope (sudden and abrupt loss of consciousness with spontaneous neurologic recovery) is common among trained athletes. The vast majority of syncope is caused by “neurocardiogenic” mechanisms and carries a benign prognosis. Benign neurally-mediated syncope most often occurs outside of training and competition among athletes with heightened vagal tone and a predisposed susceptibility to triggers including pain, anxiety, emotional stimulation, and sudden postural change. Athletes who experience neurally-mediated syncope outside of training and competition routinely report a pre-event prodrome or aura that permits them to lower themselves to the ground, thereby avoiding injury. A distinct, but similarly benign and common, form of neurally-mediated fainting is post-exertional syncope. Here, fainting occurs within seconds of abrupt termination of exercise due to a rapid reduction cardiac preload and corollary cerebral blood supply. When either form of neurally-mediated syncope is suggested by a comprehensive medical history, normal physical examination, and a normal 12- lead ECG, further evaluation is unnecessary. However, the athlete and their coaching staff should be educated about avoidance tactics including hydration, dietary sodium supplementation, and avoidance of abrupt exercise termination as neutrally-mediated syncope tends to be recurrent without such measures. Fainting episodes that occur during training or competition that are not clearly post-exertional should be considered a medical emergency and should prompt comprehensive evaluation by a qualified cardiovascular specialist. Working closely with team physicians and athletic training staff, it is the responsibility of sports cardiologists to exclude potentially life-threatening forms of electrical, muscular, coronary, and valvular heart disease. Ideally, this evaluation should be conducted rapidly to avoid unnecessary delays in return to play.
CARDIAC ARREST AND SUDDEN DEATH
Numerous high-visibility cases of cardiac arrest on the soccer pitch have alerted the sporting community to the potential for these rare and potentially tragic events. Definitive incidence statistics defining the risk of cardiac arrest among soccer players are lacking. Data from the NCAA database suggest a sudden death incidence rate among collegiate male soccer athletes of approximately 1:24,000 athlete years.8 Similar data documenting incidence among female athletes and among those at lower (ie, youth level and high school) and higher (ie, professional) levels of play are unavailable. Underlying cardiovascular disease in the forms of heart muscle abnormalities (ie, genetic and acquired cardiomyopathy), coronary artery abnormalities (ie, genetic coronary anomalies and atherosclerotic coronary disease), valvular heart problems (ie, congenitally malformed aortic valves), and primary disturbances of the cardiac electrical system (ie, Wolf-Parkinson-White syndrome, long QT syndrome, etc.) explain a substantial percentage of on-pitch cardiac arrest. However, it is increasingly recognized that a significant minority of sudden cardiac deaths among athletes occur in the absence of attributable cardiovascular abnormality.17 Such cases, often referred to as “sudden unexplained death,” present unique challenges in the context of pre-participation screening, as they are undetectable and thus unpredictable.
Reduction of cardiac arrest and sudden death may best be accomplished through a combination of focused pre-participation screening and the development and implementation of a comprehensive EAP. Pre-participation involves the performance of a battery of tests prior to training and competition that are geared toward the detection of occult high-risk cardiovascular disease. Recommendations regarding pre-participation screening vary both across and within countries. Current US recommendations call for a focused medical history and physical examination prior to training and competition with consideration of the addition of a 12-lead ECG on a local level based on expertise and available resources.18 Conversely, current European guidelines including those endorsed by FIFA, suggest routine inclusion of a 12-lead ECG and in some cases, a transthoracic ECG.19 It must be emphasized that no screening approach has been confirmed to reduce the incidence of sudden death, and the decision to extend screening beyond the medical history and physical examination to include a 12-lead ECG or echocardiogram may come at the cost of increased false positive testing. In practice, decisions about how and when to screen are ideally made at a local level after consideration of medical and financial resources.20
Continue to: Even the most comprehensive approach...
Even the most comprehensive approach to screening and evaluation of symptomatic athletes will not completely eliminate on-pitch cardiac arrest. Thus, all stakeholders that engage in the oversight of organized soccer must be committed to the development and implementation of an EAP.21 Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support. Much like screening, emergency action planning involving these key core components must be developed and tailored locally. In the era of contemporary organized athletics, the absence of an EAP at any level of competition, from youth to professional leagues, is unacceptable. Effective EAPs must be developed, documented, and rehearsed at regular intervals. For the health and safety of competitive soccer players, as well as coaching staff and spectators, these steps are of critical importance.
HEAT
Heat-related illnesses can be serious and, at times, even life threatening. It is important for athletic staff and athletes to be well versed in the prevention, signs and symptoms, and treatment of heat-related illnesses in order to prevent serious and lasting injury. We aim to educate physicians about the prevention, recognition, and management of heat-related injury, and stress the importance of similarly educating athletes and coaching staff.
Exertional heat illnesses most often occur at temperatures >86°F, however they can occur at any temperature with heavy exertion.22 Signs and symptoms can be nonspecific early on, including weakness, fatigue, headache, nausea, and dizziness. Later signs can include imbalance, altered mentation, confusion, and behavior that is out of character such as irritability or aggression.23 It is easy to see how the later signs can be confused for concussion in the right context. We cover the recognition and treatment of two common and serious heat-related illnesses: heat exhaustion and exertional heat stroke (EHS).
HEAT EXHAUSTION
Heat exhaustion occurs when an athlete cannot continue to exercise due to weakness and fatigue. While the exact mechanism is not well understood, it has been established that the combined effect of heat and dehydration have been proven to decrease exercise capacity and performance to a greater degree than either alone. The heat created by the body during exercise is 15 to 20 times greater than when at rest, and can increase core body temperature by 1°C every 5 minutes if no heat is lost, such as through sweating.24 Additionally, when fluid deficits reach >3% to 5% of total body water, sweat production and skin blood flow decline, blunting the ability for the body to cool itself and causing progressive elevation of core body temperature if the athlete continues exerting him or herself. When fluid deficits reach 6% to 10%, cardiac output, sweat, and muscle blood flow decrease, likely leading to the symptoms seen with heat exhaustion: weakness, profound fatigue, and occasionally confusion and disorientation. Athletes with suspected heat exhaustion should be moved to a cooler area, laid down with legs elevated, and orally rehydrated. If they do not improve with oral rehydration, they may require intravenous fluids. The diagnosis of heat exhaustion hinges on a rectal temperature of <104°F; if >104°F the athlete should be presumed to have heat stroke, which will be addressed in the following paragraphs. Players can be cleared to return to play in mild cases within 24 to 48 hours with gradual increases in exercise intensity.24
EXERTIONAL HEAT STROKE
EHS occurs when the body can no longer regulate the core body temperature and it rises to upwards of 104°F. In EHS, elevated core body temperature is associated with evidence of end organ dysfunction. The most easily identified on the playing field is likely central nervous system dysfunction, including irritability, confusion, irrational behavior, lethargy, dizziness, confusion, and even loss of consciousness. Temperature should be measured with rectal temperature only, as other methods of measurement have been shown to be consistently inaccurate.22 Heat stroke can be confused with exertional hyponatremia, heat exhaustion, or concussion, especially when core body temperature cannot be determined. However, EHS should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects. Morbidity and mortality are directly related to the length of time required to cool the athlete under 40°C (104°F).24 Cooling should be completed on site prior to transport to a medical facility and is best achieved with submersion in an ice bath (ie, a kiddie pool or soaking tub full of ice and water).22,25 If an ice bath is not available, ice bags should be applied to the neck axilla and groin and exchanged for fresh bags every 2 to 3 minutes.22 Ice bags have been shown to be inferior to whole body cooling, only cooling the athlete .04°C to .08°C/min compared to .15°C to .24°C/min with the ice bath.24 All other tests should be delayed until cooling is achieved, unless they can be completed while cooling the athlete. The athlete can be removed from the ice bath once rectal temperatures reach <101°F to 102°F.23 If the athlete returns to baseline after cooling, transportation to a medical facility may not be necessary. However, they should refrain from physical activity and heat exposure for at least 7 days and should be evaluated by a physician at that time. If all labs are normal and the athlete is asymptomatic, they can start progressive return to play under the direction of an athletic trainer or a sports medicine physician.23
Continue to: HEAT-RELATED ILLNESS...
HEAT-RELATED ILLNESS
It is impossible to predict exactly which athletes will be most at risk for heat-related illness, so it is important to have a high degree of suspicion when environmental conditions are right. Athletes with recent illness, fever, or lack of sleep are at higher risk. Additional intrinsic risk factors include low fitness level, obesity, and inadequate hydration. Athletes who are highly competitive or motivated can be more likely to push through the early signs of illness or be reluctant to report symptoms.23 Those with a history of exertional heat illness are more at risk for developing it again in the future.23
The extrinsic risk factors for the development of heat-related illness are much easier to identify and modify in order to keep athletes safe. High temperature and high humidity conditions, heavy sun exposure, and exposure to similar conditions the preceding day put athletes at risk for exertional heat illness. Risks are even greater when the exercise is prolonged or intense with few breaks and access to hydration is limited.23 Therefore, prevention of exertional heat illness is centered on these external risk factors.
Each team should have a heat policy as part of their EAP aimed at prevention and early recognition of heat-related illness. This policy should be shared with all athletes and coaches. The plan should be centered on acclimatization, activity modification, and early recognition and management as previously discussed. The US Soccer Federation “Recognize to Recover” Heat Guidelines suggest a 3-step process for appropriate activity modification:22
1. Find the wet bulb globe temperature, either using a wet bulb globe thermometer or the temperature and humidity (Figure 1).
2. Find your regional weather category on the map (Figure 2).
3. Find your alert level and work to rest ratio recommendations (Figure 3).
Scheduled hydration breaks should be given as listed in Figure 3. Breaks of 4 minutes should be given for each 30 minutes of continuous practice or play. In a regulation 90-minute match, a hydration break should be given at 30 and 75 minutes (with half time at 45 minutes) at minimum. Athletes should be educated about where hydration can be accessed, and given unlimited access to hydration even outside of planned breaks.22
Acclimatization to conditions is another integral part of preventing heat-related illness. It allows the body time to adapt to exercising in heat gradually, with a measured progression of exertion over the course of 10 to 14 days. The “Recognize to Recover” Heat Guidelines also provide guidance on acclimatization, and specifics can be found on the website.1 Generally speaking, the warmest part of the day, usually between 11 AM and 4 PM, should be avoided for all training sessions, and length of practice and exertion should be gradually increased over 2 weeks.22
In summary, appropriate acclimatization, hydration, activity modification, and education of athletes and staff are essential for the prevention of heat-related illness. Athletes and staff should understand the signs and symptoms of heat-related illness so that it can be recognized early and treated appropriately. If an athlete is altered in the heat and rectal temperature is >104°F or rectal temperature cannot be obtained, rapid cooling using an ice bath or ice bags is essential to prevent the morbidity and mortality associated with EHS. Above all, teams should have an explicit plan that includes protocols for acclimatization, activity modification, and all necessary equipment to prevent and treat heat-related illnesses should they occur, and ultimately keep athletes safe and healthy.
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain Injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
2. The National Federation of State High School Associations. 2013-14 high school athletics participation survey. http://www.nfhs.org/ParticipationStatics/PDF/2013-14_Participation_Survey_PDF.pdf. Accessed August 6, 2018.
3. Youth Council. US Soccer Federation Web site. https://www.ussoccer.com/about/affiliates/youth-council. Accessed July 31, 2018.
4. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
5. Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314-1320. doi:10.2106/JBJS.16.01573.
6. US Soccer Federation. U.S. Soccer’s comprehensive player health and safety program. Recognize to Recover Web site. http://www.recognizetorecover.org/#us-soccers-comprehensive-player-health-and-safety-program. Accessed July 31, 2018.
7. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi:10.1136/bjsports-2017-097699.
8. Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600. doi:10.1161/CIRCULATIONAHA.110.004622.
9. Faude O, Rössler R, Junge A, et al. Head injuries in children’s football-results from two prospective cohort studies in four European countries. Scand J Med Sci Sports. 2017;27(12):1986-1992. doi:10.1111/sms.12839.
10. Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169(9):830-837. doi:10.1001/jamapediatrics.2015.1062.
11. Tarnutzer AA. Should heading be forbidden in children’s football? Sci Med Football. 2018;2(1):75-79.
12. US Soccer Federation. US Soccer, NWSL and MLS to host “head injury in soccer; science to field”. https://www.ussoccer.com/stories/2017/04/18/17/35/20170418-news-us-soccer-nwsl-mls-host-head-injury-in-soccer-science-to-field. Published April 18, 2017. Accessed August 6, 2018.
13. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54(5):380-386. doi:10.1016/j.pcad.2012.01.006.
14. Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi:10.1161/CIRCULATIONAHA.110.981571.
15. Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the "Grey Zone" of clinical uncertainty. Can J Cardiol. 2016;32(4):429-437. doi:10.1016/j.cjca.2015.11.025.
16. Baggish AL, Battle RW, Beckerman JG, et al; ACC’s Sports and Exercise Council Leadership Group. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70(15):1902-1918. doi:10.1016/j.jacc.2017.08.055.
17. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10-19. doi:10.1161/CIRCULATIONAHA.115.015431.
18. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356-2361. doi:10.1016/j.jacc.2015.09.034.
19. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
20. Baggish AL, Kovacs RJ. Preparticipation cardiovascular screening: clinical partnership is the only certainty. Br J Sports Med. 2017;51(3):150-151. doi:10.1136/bjsports-2016-096954.
21. Hainline B, Drezner J, Baggish A, et al. Interassociation consensus statement on cardiovascular care of college student-athletes. J Athl Train. 2016;51(4):344-357. doi:10.4085/j.jacc.2016.03.527.
22. US Soccer Federation. Environmental conditions. Recognize to Recover Web site. http://www.recognizetorecover.org/environmental/#environmental-conditions. Accessed April 15, 2018.
23. Korey Stringer Institute. Emergency conditions: heat illnesses. University of Connecticut Web site. https://ksi.uconn.edu/. Accessed April 15, 2018.
24. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
25. Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392-397. doi:10.1080/10903127.2017.1392666.
ABSTRACT
Soccer requires significant physical conditioning and endurance, as well as the physicality required for contact play. In order to keep athletes safe, it is important that coaches, medical staff, and the players themselves are educated on the most common dangers to their health that they may encounter on a soccer pitch. This article aims to review the current literature and recommendations on concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer, with a goal of educating all those who help to keep athletes healthy and competing to their full potential.
Continue to: Soccer is one of the most popular sports...
Soccer is one of the most popular sports in the modern world and requires significant physical conditioning and endurance, as well as the physicality required for contact play. This article covers the topics of concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer players. We provide a review of the prevention, recognition, and management required to keep athletes safe on the soccer pitch, both in practice and in competitive play.
HEAD
With an estimated 1.6 to 3.8 million sports-related mild traumatic brain injuries (ie, concussions) occurring annually in the United States,1 there has been an appropriate increase in the focus on prevention and treatment of these injuries. For more than a decade the spotlight has been on concussions that occur in American football, but other sports have also had to examine the prevalence of concussions in their sport. This is certainly true for soccer.
There has been a steady increase in soccer participation in the United States. From 1973 to 2014 there was a 4-fold increase in high school boys and a 35-fold increase in high school girls playing soccer.2 Currently, there are more than 3.7 million youth who play on teams under the supervision of the US Soccer Federation, the sport’s national governing body.3 With the growth of the sport, there has also been an intensified focus on injury prevention in soccer players, including concussive brain injuries.
A recent study examined injury rates in high school soccer players and noted that concussion is the second most common injury (17.9%), after ligament sprains (29.7%).4 The overall injury rate was 2.06 per 1000 athletic exposures (AEs [defined as participation in practice or game play]), but higher in games (4.42 per 1000 AEs) than during practices (1.05 per 1000 AEs). The overall concussion rate was 0.36 per 1000 AEs.4
Most concussions (54.8%) resulted in missing play between 1 to 3 weeks, but a sizeable portion of the athletes (14.9%) were out of play for more than 3 weeks. Additionally, 10.7% of all medical disqualifications were due to concussive injuries. Khodaee and colleagues4 found no statistically significant difference in concussion rates between male and female soccer players over the 9-year period of time that they examined, however previous studies have found higher rates in female athletes.5
In soccer, as in other sports, there is a concern about both the adequate recognition of concussions during practice and play and the underreporting of concussions by athletes. The US Soccer Federation has taken a proactive stance on addressing concussion in youth soccer by developing the “Recognize to Recover” program.6 Recognize to Recover is the US Soccer Federation’s “comprehensive player health and safety program aimed at promoting safe play and reducing injuries in soccer players of all ages.” The website provides an educational video geared toward players, along with links to concussion assessment tools, the US Soccer Federation Concussion Protocol, and US Soccer Federation-Centers for Disease Control fact sheets for athletes, parents, and coaches.6
Continue to: A challenge for all sports...
A challenge for all sports is allowing adequate evaluation of a suspected concussion by properly trained healthcare professionals. The 2017 Berlin Concussion in Sport Group position paper stated, “when a concussion is suspected, the athlete should be removed from the sporting environment and a multimodal assessment should be conducted in standardized fashion (eg, Sport Concussion Assessment Tool- 5th edition). Sporting bodies should allow adequate time to conduct this evaluation.”7 However, the International Federation of Football Association (FIFA) rules limit substitutions to 3 over the course of the game, which can make a thorough evaluation of players difficult as trainers and coaches are under increased pressure to quickly determine whether to use one of their valuable substitutions. Fortunately, the National Collegiate Athletic Association (NCAA) soccer has mitigated this issue by allowing unlimited substitutions during matches, and high school teams generally follow similar rules.
One of the goals of any safety education program is not only to raise the awareness of the signs and symptoms of concussion by all those involved in the sport, but also to increase the number of athletes who self-report their symptoms and decrease those who hide any possible concussions. A study found that a majority (58.6%) of middle school soccer players continued to play while experiencing concussion symptoms.5 However, in a very recent (not yet published) study, 92% of US Soccer Federation players reported that they did seek out a medical evaluation for their concussion.8 This is certainly a positive sign and further research needs to clarify what methods of education or training will maintain this level of self-reporting in soccer players.
There has also been an increased focus on understanding the mechanism of injury of concussions. In soccer, concussions can occur from player-to-player contact, contact with the player surface, contact with playing apparatus (eg, goal posts) and non-contact mechanisms. While there has been a focus on concussions from heading the ball, player-to-player contact is the most common cause of concussions. A 2017 study of 7- to 12-year-old soccer players in 4 European countries found that about 1 out of 10 concussions were caused by heading the ball.9 Comstock and colleageues10 found slightly higher numbers, roughly 25% to 30% (depending on gender), but 70% to 78% (again depending on gender) of those were caused by player-to-player contact rather than contact with the ball.
To date there has not been any meta-analytic review evaluating the cognitive and physical symptoms associated with heading in soccer. A recent review paper stated that the “current evidence seems insufficient to support a ban of heading in children’s football (soccer).”10 However, in December 2015 the US Soccer Federation included age-specific heading limitations. Players ages 10 years and under “shall not engage in heading, either in practices or in games” and players age 11 years and 12 years should have “limited heading in practice; maximum of 30 minutes of heading training per week, with no more than 15-20 headers per player, per week.”11 US Soccer Federation officials acknowledged the limitations in the current science regarding heading in young soccer players but chose to err on the side of caution until further empirical evidence regarding the risks associated with repetitive heading is available.
The US Soccer Federation is also exploring other ways to reduce the incidence rate of concussions, including ensuring that the age-appropriate sized ball is used in practice and play, possible rule changes, evaluation of different playing surfaces, and equipment usage. To date, there is no strong evidence to support the use of mouth guards or helmets to reduce concussions in soccer. Additionally, the current data about the value of head impact sensors in soccer has not supported its widespread use.
Continue to: Finally, the issue of the prevalence...
Finally, the issue of the prevalence of chronic traumatic encephalopathy (CTE) in soccer players is beyond the scope of this article. The expert opinion from the 2017 US Soccer Federation, Major League Soccer (MLS), and National Women’s Soccer League (NWSL) conference concluded, “At present, no data exist that support that soccer participation is a risk factor for the development of neurodegenerative disease. Similarly, at this time, consistent with evidence discussed in the Berlin Concussion in Sport Group (CISG) Consensus Conference, our review suggests no causal relationship has been demonstrated between soccer and CTE pathology.”12
The more we know about concussions, both in general and those sustained during soccer play, the better we are able to diagnose and manage these injuries in our athletes. An important step is creating evidence-based protocols that evolve as our knowledge of concussions does as well. In April 2017, the US Soccer Federation, MLS, and the NWSL held a joint summit entitled, “Head Injury in Soccer: From Science to the Field” to address the current evidence-based science of concussions in soccer.8 An article discussing the findings of this meeting is forthcoming and will undoubtedly guide further development of concussion protocols for soccer players of all ages.
HEART
The physiologic demands of soccer place considerable stress on the cardiovascular system. Participation in training and competition is characterized by a combination of aerobic and anaerobic physiology with the typical athlete covering approximately 10 km over the course of the 90-minute match. The primary role of the heart and blood vessels is to supply the exercising skeletal muscle with oxygen and energy substrate and to clear the byproducts of metabolism. Among healthy athletes without cardiovascular disease, these processes are typically well tolerated and may be associated with beneficial cardiovascular adaptations over time. However, competitive soccer players are not completely immune to cardiovascular disease. Athletes across the age and competition spectrum may develop symptoms suggestive of underlying cardiovascular disease during play including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope. These athletes require timely clinical evaluation. In extremely rare but high visibility cases, competitive soccer players may succumb to cardiac arrest on the pitch, underscoring the need for comprehensive emergency action plans (EAPs). We provide the practicing clinician with an overview of cardiovascular issues relevant to the competitive soccer athlete.
CARDIOVASCULAR ADAPTATIONS TO SPORT
The pressure (ie, repetitive surges in systemic blood pressure) and volume (ie, sustained increases in high cardiac output) challenges inherent in soccer participation place stress on the cardiovascular system. Healthy athletes across the age spectrum typically tolerate the hemodynamic stressors of participation without issues. Athletes that engage in training and competition over months to years often develop beneficial adaptations of the cardiovascular system that enhance on-field performance and contribute to optimal long-term health. Detailed discussion of how the heart and blood vessels respond to exercise training is beyond the scope of this article, but the interested reader is referred to several prior publications.13,14 In brief, the heart of the healthy soccer athlete demonstrates the balanced mild chamber dilation and wall thickening characteristic of left ventricular eccentric remodeling. This form of exercise-induced cardiac remodeling facilitates maintenance of high stroke volume during exercise with minimal increases in cardiac work. In parallel, routine aerobic exercise training confers favorable changes in the systemic arterial system, which leads to reductions in age-associated ventricular stiffening and maintenance of healthy low blood pressure. It must be emphasized that the healthy heart muscle dilation and thickening that develop in response to sports participation, regardless of age, ethnicity, or gender, are relatively mild and should not be confused with common forms of heart muscle disease that may be seen in athletes at risk for adverse outcomes. In some situations, consultation with an extreme sports cardiologist may be required to differentiate exercise-induced remodeling from over heart muscle pathology.15
Continue to: THE SYMPTOMATIC ATHLETE...
THE SYMPTOMATIC ATHLETE
Any athlete presenting with symptoms suggestive of underlying cardiovascular disease should be withheld from training and competition until a comprehensive clinical evaluation has been completed. Common manifestations of underlying heart disease that occur in soccer players include exertional chest pain/pressure/tightness, shortness of breath that is out of proportion to workload, palpitations or the perception of irregular cardiac activity, and syncope. Chest discomfort, inappropriate shortness of breath, and palpitations that occur during training or competition should be managed with immediate removal from the playing field and prompt medical assessment. In many cases, thorough evaluation will involve collaboration between sports medicine and sports cardiology providers. Evaluations must be individualized on a case-by-case basis as tailored to the athlete’s presenting chief complaint and prior medical history. Most of these assessments will include a detailed medical history and physical examination, a 12-lead electrocardiogram (ECG), provocative exercise testing in a controlled environment, noninvasive cardiac imaging, and in some cases ambulatory rhythm monitoring. Uniformly, the athlete should be withheld from further training and competition until high-risk cardiovascular disease has been excluded. We refer the interested reader to a comprehensive discussion of symptom-based assessment of the athlete with suspected cardiovascular disease.16
Syncope (sudden and abrupt loss of consciousness with spontaneous neurologic recovery) is common among trained athletes. The vast majority of syncope is caused by “neurocardiogenic” mechanisms and carries a benign prognosis. Benign neurally-mediated syncope most often occurs outside of training and competition among athletes with heightened vagal tone and a predisposed susceptibility to triggers including pain, anxiety, emotional stimulation, and sudden postural change. Athletes who experience neurally-mediated syncope outside of training and competition routinely report a pre-event prodrome or aura that permits them to lower themselves to the ground, thereby avoiding injury. A distinct, but similarly benign and common, form of neurally-mediated fainting is post-exertional syncope. Here, fainting occurs within seconds of abrupt termination of exercise due to a rapid reduction cardiac preload and corollary cerebral blood supply. When either form of neurally-mediated syncope is suggested by a comprehensive medical history, normal physical examination, and a normal 12- lead ECG, further evaluation is unnecessary. However, the athlete and their coaching staff should be educated about avoidance tactics including hydration, dietary sodium supplementation, and avoidance of abrupt exercise termination as neutrally-mediated syncope tends to be recurrent without such measures. Fainting episodes that occur during training or competition that are not clearly post-exertional should be considered a medical emergency and should prompt comprehensive evaluation by a qualified cardiovascular specialist. Working closely with team physicians and athletic training staff, it is the responsibility of sports cardiologists to exclude potentially life-threatening forms of electrical, muscular, coronary, and valvular heart disease. Ideally, this evaluation should be conducted rapidly to avoid unnecessary delays in return to play.
CARDIAC ARREST AND SUDDEN DEATH
Numerous high-visibility cases of cardiac arrest on the soccer pitch have alerted the sporting community to the potential for these rare and potentially tragic events. Definitive incidence statistics defining the risk of cardiac arrest among soccer players are lacking. Data from the NCAA database suggest a sudden death incidence rate among collegiate male soccer athletes of approximately 1:24,000 athlete years.8 Similar data documenting incidence among female athletes and among those at lower (ie, youth level and high school) and higher (ie, professional) levels of play are unavailable. Underlying cardiovascular disease in the forms of heart muscle abnormalities (ie, genetic and acquired cardiomyopathy), coronary artery abnormalities (ie, genetic coronary anomalies and atherosclerotic coronary disease), valvular heart problems (ie, congenitally malformed aortic valves), and primary disturbances of the cardiac electrical system (ie, Wolf-Parkinson-White syndrome, long QT syndrome, etc.) explain a substantial percentage of on-pitch cardiac arrest. However, it is increasingly recognized that a significant minority of sudden cardiac deaths among athletes occur in the absence of attributable cardiovascular abnormality.17 Such cases, often referred to as “sudden unexplained death,” present unique challenges in the context of pre-participation screening, as they are undetectable and thus unpredictable.
Reduction of cardiac arrest and sudden death may best be accomplished through a combination of focused pre-participation screening and the development and implementation of a comprehensive EAP. Pre-participation involves the performance of a battery of tests prior to training and competition that are geared toward the detection of occult high-risk cardiovascular disease. Recommendations regarding pre-participation screening vary both across and within countries. Current US recommendations call for a focused medical history and physical examination prior to training and competition with consideration of the addition of a 12-lead ECG on a local level based on expertise and available resources.18 Conversely, current European guidelines including those endorsed by FIFA, suggest routine inclusion of a 12-lead ECG and in some cases, a transthoracic ECG.19 It must be emphasized that no screening approach has been confirmed to reduce the incidence of sudden death, and the decision to extend screening beyond the medical history and physical examination to include a 12-lead ECG or echocardiogram may come at the cost of increased false positive testing. In practice, decisions about how and when to screen are ideally made at a local level after consideration of medical and financial resources.20
Continue to: Even the most comprehensive approach...
Even the most comprehensive approach to screening and evaluation of symptomatic athletes will not completely eliminate on-pitch cardiac arrest. Thus, all stakeholders that engage in the oversight of organized soccer must be committed to the development and implementation of an EAP.21 Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support. Much like screening, emergency action planning involving these key core components must be developed and tailored locally. In the era of contemporary organized athletics, the absence of an EAP at any level of competition, from youth to professional leagues, is unacceptable. Effective EAPs must be developed, documented, and rehearsed at regular intervals. For the health and safety of competitive soccer players, as well as coaching staff and spectators, these steps are of critical importance.
HEAT
Heat-related illnesses can be serious and, at times, even life threatening. It is important for athletic staff and athletes to be well versed in the prevention, signs and symptoms, and treatment of heat-related illnesses in order to prevent serious and lasting injury. We aim to educate physicians about the prevention, recognition, and management of heat-related injury, and stress the importance of similarly educating athletes and coaching staff.
Exertional heat illnesses most often occur at temperatures >86°F, however they can occur at any temperature with heavy exertion.22 Signs and symptoms can be nonspecific early on, including weakness, fatigue, headache, nausea, and dizziness. Later signs can include imbalance, altered mentation, confusion, and behavior that is out of character such as irritability or aggression.23 It is easy to see how the later signs can be confused for concussion in the right context. We cover the recognition and treatment of two common and serious heat-related illnesses: heat exhaustion and exertional heat stroke (EHS).
HEAT EXHAUSTION
Heat exhaustion occurs when an athlete cannot continue to exercise due to weakness and fatigue. While the exact mechanism is not well understood, it has been established that the combined effect of heat and dehydration have been proven to decrease exercise capacity and performance to a greater degree than either alone. The heat created by the body during exercise is 15 to 20 times greater than when at rest, and can increase core body temperature by 1°C every 5 minutes if no heat is lost, such as through sweating.24 Additionally, when fluid deficits reach >3% to 5% of total body water, sweat production and skin blood flow decline, blunting the ability for the body to cool itself and causing progressive elevation of core body temperature if the athlete continues exerting him or herself. When fluid deficits reach 6% to 10%, cardiac output, sweat, and muscle blood flow decrease, likely leading to the symptoms seen with heat exhaustion: weakness, profound fatigue, and occasionally confusion and disorientation. Athletes with suspected heat exhaustion should be moved to a cooler area, laid down with legs elevated, and orally rehydrated. If they do not improve with oral rehydration, they may require intravenous fluids. The diagnosis of heat exhaustion hinges on a rectal temperature of <104°F; if >104°F the athlete should be presumed to have heat stroke, which will be addressed in the following paragraphs. Players can be cleared to return to play in mild cases within 24 to 48 hours with gradual increases in exercise intensity.24
EXERTIONAL HEAT STROKE
EHS occurs when the body can no longer regulate the core body temperature and it rises to upwards of 104°F. In EHS, elevated core body temperature is associated with evidence of end organ dysfunction. The most easily identified on the playing field is likely central nervous system dysfunction, including irritability, confusion, irrational behavior, lethargy, dizziness, confusion, and even loss of consciousness. Temperature should be measured with rectal temperature only, as other methods of measurement have been shown to be consistently inaccurate.22 Heat stroke can be confused with exertional hyponatremia, heat exhaustion, or concussion, especially when core body temperature cannot be determined. However, EHS should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects. Morbidity and mortality are directly related to the length of time required to cool the athlete under 40°C (104°F).24 Cooling should be completed on site prior to transport to a medical facility and is best achieved with submersion in an ice bath (ie, a kiddie pool or soaking tub full of ice and water).22,25 If an ice bath is not available, ice bags should be applied to the neck axilla and groin and exchanged for fresh bags every 2 to 3 minutes.22 Ice bags have been shown to be inferior to whole body cooling, only cooling the athlete .04°C to .08°C/min compared to .15°C to .24°C/min with the ice bath.24 All other tests should be delayed until cooling is achieved, unless they can be completed while cooling the athlete. The athlete can be removed from the ice bath once rectal temperatures reach <101°F to 102°F.23 If the athlete returns to baseline after cooling, transportation to a medical facility may not be necessary. However, they should refrain from physical activity and heat exposure for at least 7 days and should be evaluated by a physician at that time. If all labs are normal and the athlete is asymptomatic, they can start progressive return to play under the direction of an athletic trainer or a sports medicine physician.23
Continue to: HEAT-RELATED ILLNESS...
HEAT-RELATED ILLNESS
It is impossible to predict exactly which athletes will be most at risk for heat-related illness, so it is important to have a high degree of suspicion when environmental conditions are right. Athletes with recent illness, fever, or lack of sleep are at higher risk. Additional intrinsic risk factors include low fitness level, obesity, and inadequate hydration. Athletes who are highly competitive or motivated can be more likely to push through the early signs of illness or be reluctant to report symptoms.23 Those with a history of exertional heat illness are more at risk for developing it again in the future.23
The extrinsic risk factors for the development of heat-related illness are much easier to identify and modify in order to keep athletes safe. High temperature and high humidity conditions, heavy sun exposure, and exposure to similar conditions the preceding day put athletes at risk for exertional heat illness. Risks are even greater when the exercise is prolonged or intense with few breaks and access to hydration is limited.23 Therefore, prevention of exertional heat illness is centered on these external risk factors.
Each team should have a heat policy as part of their EAP aimed at prevention and early recognition of heat-related illness. This policy should be shared with all athletes and coaches. The plan should be centered on acclimatization, activity modification, and early recognition and management as previously discussed. The US Soccer Federation “Recognize to Recover” Heat Guidelines suggest a 3-step process for appropriate activity modification:22
1. Find the wet bulb globe temperature, either using a wet bulb globe thermometer or the temperature and humidity (Figure 1).
2. Find your regional weather category on the map (Figure 2).
3. Find your alert level and work to rest ratio recommendations (Figure 3).
Scheduled hydration breaks should be given as listed in Figure 3. Breaks of 4 minutes should be given for each 30 minutes of continuous practice or play. In a regulation 90-minute match, a hydration break should be given at 30 and 75 minutes (with half time at 45 minutes) at minimum. Athletes should be educated about where hydration can be accessed, and given unlimited access to hydration even outside of planned breaks.22
Acclimatization to conditions is another integral part of preventing heat-related illness. It allows the body time to adapt to exercising in heat gradually, with a measured progression of exertion over the course of 10 to 14 days. The “Recognize to Recover” Heat Guidelines also provide guidance on acclimatization, and specifics can be found on the website.1 Generally speaking, the warmest part of the day, usually between 11 AM and 4 PM, should be avoided for all training sessions, and length of practice and exertion should be gradually increased over 2 weeks.22
In summary, appropriate acclimatization, hydration, activity modification, and education of athletes and staff are essential for the prevention of heat-related illness. Athletes and staff should understand the signs and symptoms of heat-related illness so that it can be recognized early and treated appropriately. If an athlete is altered in the heat and rectal temperature is >104°F or rectal temperature cannot be obtained, rapid cooling using an ice bath or ice bags is essential to prevent the morbidity and mortality associated with EHS. Above all, teams should have an explicit plan that includes protocols for acclimatization, activity modification, and all necessary equipment to prevent and treat heat-related illnesses should they occur, and ultimately keep athletes safe and healthy.
ABSTRACT
Soccer requires significant physical conditioning and endurance, as well as the physicality required for contact play. In order to keep athletes safe, it is important that coaches, medical staff, and the players themselves are educated on the most common dangers to their health that they may encounter on a soccer pitch. This article aims to review the current literature and recommendations on concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer, with a goal of educating all those who help to keep athletes healthy and competing to their full potential.
Continue to: Soccer is one of the most popular sports...
Soccer is one of the most popular sports in the modern world and requires significant physical conditioning and endurance, as well as the physicality required for contact play. This article covers the topics of concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer players. We provide a review of the prevention, recognition, and management required to keep athletes safe on the soccer pitch, both in practice and in competitive play.
HEAD
With an estimated 1.6 to 3.8 million sports-related mild traumatic brain injuries (ie, concussions) occurring annually in the United States,1 there has been an appropriate increase in the focus on prevention and treatment of these injuries. For more than a decade the spotlight has been on concussions that occur in American football, but other sports have also had to examine the prevalence of concussions in their sport. This is certainly true for soccer.
There has been a steady increase in soccer participation in the United States. From 1973 to 2014 there was a 4-fold increase in high school boys and a 35-fold increase in high school girls playing soccer.2 Currently, there are more than 3.7 million youth who play on teams under the supervision of the US Soccer Federation, the sport’s national governing body.3 With the growth of the sport, there has also been an intensified focus on injury prevention in soccer players, including concussive brain injuries.
A recent study examined injury rates in high school soccer players and noted that concussion is the second most common injury (17.9%), after ligament sprains (29.7%).4 The overall injury rate was 2.06 per 1000 athletic exposures (AEs [defined as participation in practice or game play]), but higher in games (4.42 per 1000 AEs) than during practices (1.05 per 1000 AEs). The overall concussion rate was 0.36 per 1000 AEs.4
Most concussions (54.8%) resulted in missing play between 1 to 3 weeks, but a sizeable portion of the athletes (14.9%) were out of play for more than 3 weeks. Additionally, 10.7% of all medical disqualifications were due to concussive injuries. Khodaee and colleagues4 found no statistically significant difference in concussion rates between male and female soccer players over the 9-year period of time that they examined, however previous studies have found higher rates in female athletes.5
In soccer, as in other sports, there is a concern about both the adequate recognition of concussions during practice and play and the underreporting of concussions by athletes. The US Soccer Federation has taken a proactive stance on addressing concussion in youth soccer by developing the “Recognize to Recover” program.6 Recognize to Recover is the US Soccer Federation’s “comprehensive player health and safety program aimed at promoting safe play and reducing injuries in soccer players of all ages.” The website provides an educational video geared toward players, along with links to concussion assessment tools, the US Soccer Federation Concussion Protocol, and US Soccer Federation-Centers for Disease Control fact sheets for athletes, parents, and coaches.6
Continue to: A challenge for all sports...
A challenge for all sports is allowing adequate evaluation of a suspected concussion by properly trained healthcare professionals. The 2017 Berlin Concussion in Sport Group position paper stated, “when a concussion is suspected, the athlete should be removed from the sporting environment and a multimodal assessment should be conducted in standardized fashion (eg, Sport Concussion Assessment Tool- 5th edition). Sporting bodies should allow adequate time to conduct this evaluation.”7 However, the International Federation of Football Association (FIFA) rules limit substitutions to 3 over the course of the game, which can make a thorough evaluation of players difficult as trainers and coaches are under increased pressure to quickly determine whether to use one of their valuable substitutions. Fortunately, the National Collegiate Athletic Association (NCAA) soccer has mitigated this issue by allowing unlimited substitutions during matches, and high school teams generally follow similar rules.
One of the goals of any safety education program is not only to raise the awareness of the signs and symptoms of concussion by all those involved in the sport, but also to increase the number of athletes who self-report their symptoms and decrease those who hide any possible concussions. A study found that a majority (58.6%) of middle school soccer players continued to play while experiencing concussion symptoms.5 However, in a very recent (not yet published) study, 92% of US Soccer Federation players reported that they did seek out a medical evaluation for their concussion.8 This is certainly a positive sign and further research needs to clarify what methods of education or training will maintain this level of self-reporting in soccer players.
There has also been an increased focus on understanding the mechanism of injury of concussions. In soccer, concussions can occur from player-to-player contact, contact with the player surface, contact with playing apparatus (eg, goal posts) and non-contact mechanisms. While there has been a focus on concussions from heading the ball, player-to-player contact is the most common cause of concussions. A 2017 study of 7- to 12-year-old soccer players in 4 European countries found that about 1 out of 10 concussions were caused by heading the ball.9 Comstock and colleageues10 found slightly higher numbers, roughly 25% to 30% (depending on gender), but 70% to 78% (again depending on gender) of those were caused by player-to-player contact rather than contact with the ball.
To date there has not been any meta-analytic review evaluating the cognitive and physical symptoms associated with heading in soccer. A recent review paper stated that the “current evidence seems insufficient to support a ban of heading in children’s football (soccer).”10 However, in December 2015 the US Soccer Federation included age-specific heading limitations. Players ages 10 years and under “shall not engage in heading, either in practices or in games” and players age 11 years and 12 years should have “limited heading in practice; maximum of 30 minutes of heading training per week, with no more than 15-20 headers per player, per week.”11 US Soccer Federation officials acknowledged the limitations in the current science regarding heading in young soccer players but chose to err on the side of caution until further empirical evidence regarding the risks associated with repetitive heading is available.
The US Soccer Federation is also exploring other ways to reduce the incidence rate of concussions, including ensuring that the age-appropriate sized ball is used in practice and play, possible rule changes, evaluation of different playing surfaces, and equipment usage. To date, there is no strong evidence to support the use of mouth guards or helmets to reduce concussions in soccer. Additionally, the current data about the value of head impact sensors in soccer has not supported its widespread use.
Continue to: Finally, the issue of the prevalence...
Finally, the issue of the prevalence of chronic traumatic encephalopathy (CTE) in soccer players is beyond the scope of this article. The expert opinion from the 2017 US Soccer Federation, Major League Soccer (MLS), and National Women’s Soccer League (NWSL) conference concluded, “At present, no data exist that support that soccer participation is a risk factor for the development of neurodegenerative disease. Similarly, at this time, consistent with evidence discussed in the Berlin Concussion in Sport Group (CISG) Consensus Conference, our review suggests no causal relationship has been demonstrated between soccer and CTE pathology.”12
The more we know about concussions, both in general and those sustained during soccer play, the better we are able to diagnose and manage these injuries in our athletes. An important step is creating evidence-based protocols that evolve as our knowledge of concussions does as well. In April 2017, the US Soccer Federation, MLS, and the NWSL held a joint summit entitled, “Head Injury in Soccer: From Science to the Field” to address the current evidence-based science of concussions in soccer.8 An article discussing the findings of this meeting is forthcoming and will undoubtedly guide further development of concussion protocols for soccer players of all ages.
HEART
The physiologic demands of soccer place considerable stress on the cardiovascular system. Participation in training and competition is characterized by a combination of aerobic and anaerobic physiology with the typical athlete covering approximately 10 km over the course of the 90-minute match. The primary role of the heart and blood vessels is to supply the exercising skeletal muscle with oxygen and energy substrate and to clear the byproducts of metabolism. Among healthy athletes without cardiovascular disease, these processes are typically well tolerated and may be associated with beneficial cardiovascular adaptations over time. However, competitive soccer players are not completely immune to cardiovascular disease. Athletes across the age and competition spectrum may develop symptoms suggestive of underlying cardiovascular disease during play including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope. These athletes require timely clinical evaluation. In extremely rare but high visibility cases, competitive soccer players may succumb to cardiac arrest on the pitch, underscoring the need for comprehensive emergency action plans (EAPs). We provide the practicing clinician with an overview of cardiovascular issues relevant to the competitive soccer athlete.
CARDIOVASCULAR ADAPTATIONS TO SPORT
The pressure (ie, repetitive surges in systemic blood pressure) and volume (ie, sustained increases in high cardiac output) challenges inherent in soccer participation place stress on the cardiovascular system. Healthy athletes across the age spectrum typically tolerate the hemodynamic stressors of participation without issues. Athletes that engage in training and competition over months to years often develop beneficial adaptations of the cardiovascular system that enhance on-field performance and contribute to optimal long-term health. Detailed discussion of how the heart and blood vessels respond to exercise training is beyond the scope of this article, but the interested reader is referred to several prior publications.13,14 In brief, the heart of the healthy soccer athlete demonstrates the balanced mild chamber dilation and wall thickening characteristic of left ventricular eccentric remodeling. This form of exercise-induced cardiac remodeling facilitates maintenance of high stroke volume during exercise with minimal increases in cardiac work. In parallel, routine aerobic exercise training confers favorable changes in the systemic arterial system, which leads to reductions in age-associated ventricular stiffening and maintenance of healthy low blood pressure. It must be emphasized that the healthy heart muscle dilation and thickening that develop in response to sports participation, regardless of age, ethnicity, or gender, are relatively mild and should not be confused with common forms of heart muscle disease that may be seen in athletes at risk for adverse outcomes. In some situations, consultation with an extreme sports cardiologist may be required to differentiate exercise-induced remodeling from over heart muscle pathology.15
Continue to: THE SYMPTOMATIC ATHLETE...
THE SYMPTOMATIC ATHLETE
Any athlete presenting with symptoms suggestive of underlying cardiovascular disease should be withheld from training and competition until a comprehensive clinical evaluation has been completed. Common manifestations of underlying heart disease that occur in soccer players include exertional chest pain/pressure/tightness, shortness of breath that is out of proportion to workload, palpitations or the perception of irregular cardiac activity, and syncope. Chest discomfort, inappropriate shortness of breath, and palpitations that occur during training or competition should be managed with immediate removal from the playing field and prompt medical assessment. In many cases, thorough evaluation will involve collaboration between sports medicine and sports cardiology providers. Evaluations must be individualized on a case-by-case basis as tailored to the athlete’s presenting chief complaint and prior medical history. Most of these assessments will include a detailed medical history and physical examination, a 12-lead electrocardiogram (ECG), provocative exercise testing in a controlled environment, noninvasive cardiac imaging, and in some cases ambulatory rhythm monitoring. Uniformly, the athlete should be withheld from further training and competition until high-risk cardiovascular disease has been excluded. We refer the interested reader to a comprehensive discussion of symptom-based assessment of the athlete with suspected cardiovascular disease.16
Syncope (sudden and abrupt loss of consciousness with spontaneous neurologic recovery) is common among trained athletes. The vast majority of syncope is caused by “neurocardiogenic” mechanisms and carries a benign prognosis. Benign neurally-mediated syncope most often occurs outside of training and competition among athletes with heightened vagal tone and a predisposed susceptibility to triggers including pain, anxiety, emotional stimulation, and sudden postural change. Athletes who experience neurally-mediated syncope outside of training and competition routinely report a pre-event prodrome or aura that permits them to lower themselves to the ground, thereby avoiding injury. A distinct, but similarly benign and common, form of neurally-mediated fainting is post-exertional syncope. Here, fainting occurs within seconds of abrupt termination of exercise due to a rapid reduction cardiac preload and corollary cerebral blood supply. When either form of neurally-mediated syncope is suggested by a comprehensive medical history, normal physical examination, and a normal 12- lead ECG, further evaluation is unnecessary. However, the athlete and their coaching staff should be educated about avoidance tactics including hydration, dietary sodium supplementation, and avoidance of abrupt exercise termination as neutrally-mediated syncope tends to be recurrent without such measures. Fainting episodes that occur during training or competition that are not clearly post-exertional should be considered a medical emergency and should prompt comprehensive evaluation by a qualified cardiovascular specialist. Working closely with team physicians and athletic training staff, it is the responsibility of sports cardiologists to exclude potentially life-threatening forms of electrical, muscular, coronary, and valvular heart disease. Ideally, this evaluation should be conducted rapidly to avoid unnecessary delays in return to play.
CARDIAC ARREST AND SUDDEN DEATH
Numerous high-visibility cases of cardiac arrest on the soccer pitch have alerted the sporting community to the potential for these rare and potentially tragic events. Definitive incidence statistics defining the risk of cardiac arrest among soccer players are lacking. Data from the NCAA database suggest a sudden death incidence rate among collegiate male soccer athletes of approximately 1:24,000 athlete years.8 Similar data documenting incidence among female athletes and among those at lower (ie, youth level and high school) and higher (ie, professional) levels of play are unavailable. Underlying cardiovascular disease in the forms of heart muscle abnormalities (ie, genetic and acquired cardiomyopathy), coronary artery abnormalities (ie, genetic coronary anomalies and atherosclerotic coronary disease), valvular heart problems (ie, congenitally malformed aortic valves), and primary disturbances of the cardiac electrical system (ie, Wolf-Parkinson-White syndrome, long QT syndrome, etc.) explain a substantial percentage of on-pitch cardiac arrest. However, it is increasingly recognized that a significant minority of sudden cardiac deaths among athletes occur in the absence of attributable cardiovascular abnormality.17 Such cases, often referred to as “sudden unexplained death,” present unique challenges in the context of pre-participation screening, as they are undetectable and thus unpredictable.
Reduction of cardiac arrest and sudden death may best be accomplished through a combination of focused pre-participation screening and the development and implementation of a comprehensive EAP. Pre-participation involves the performance of a battery of tests prior to training and competition that are geared toward the detection of occult high-risk cardiovascular disease. Recommendations regarding pre-participation screening vary both across and within countries. Current US recommendations call for a focused medical history and physical examination prior to training and competition with consideration of the addition of a 12-lead ECG on a local level based on expertise and available resources.18 Conversely, current European guidelines including those endorsed by FIFA, suggest routine inclusion of a 12-lead ECG and in some cases, a transthoracic ECG.19 It must be emphasized that no screening approach has been confirmed to reduce the incidence of sudden death, and the decision to extend screening beyond the medical history and physical examination to include a 12-lead ECG or echocardiogram may come at the cost of increased false positive testing. In practice, decisions about how and when to screen are ideally made at a local level after consideration of medical and financial resources.20
Continue to: Even the most comprehensive approach...
Even the most comprehensive approach to screening and evaluation of symptomatic athletes will not completely eliminate on-pitch cardiac arrest. Thus, all stakeholders that engage in the oversight of organized soccer must be committed to the development and implementation of an EAP.21 Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support. Much like screening, emergency action planning involving these key core components must be developed and tailored locally. In the era of contemporary organized athletics, the absence of an EAP at any level of competition, from youth to professional leagues, is unacceptable. Effective EAPs must be developed, documented, and rehearsed at regular intervals. For the health and safety of competitive soccer players, as well as coaching staff and spectators, these steps are of critical importance.
HEAT
Heat-related illnesses can be serious and, at times, even life threatening. It is important for athletic staff and athletes to be well versed in the prevention, signs and symptoms, and treatment of heat-related illnesses in order to prevent serious and lasting injury. We aim to educate physicians about the prevention, recognition, and management of heat-related injury, and stress the importance of similarly educating athletes and coaching staff.
Exertional heat illnesses most often occur at temperatures >86°F, however they can occur at any temperature with heavy exertion.22 Signs and symptoms can be nonspecific early on, including weakness, fatigue, headache, nausea, and dizziness. Later signs can include imbalance, altered mentation, confusion, and behavior that is out of character such as irritability or aggression.23 It is easy to see how the later signs can be confused for concussion in the right context. We cover the recognition and treatment of two common and serious heat-related illnesses: heat exhaustion and exertional heat stroke (EHS).
HEAT EXHAUSTION
Heat exhaustion occurs when an athlete cannot continue to exercise due to weakness and fatigue. While the exact mechanism is not well understood, it has been established that the combined effect of heat and dehydration have been proven to decrease exercise capacity and performance to a greater degree than either alone. The heat created by the body during exercise is 15 to 20 times greater than when at rest, and can increase core body temperature by 1°C every 5 minutes if no heat is lost, such as through sweating.24 Additionally, when fluid deficits reach >3% to 5% of total body water, sweat production and skin blood flow decline, blunting the ability for the body to cool itself and causing progressive elevation of core body temperature if the athlete continues exerting him or herself. When fluid deficits reach 6% to 10%, cardiac output, sweat, and muscle blood flow decrease, likely leading to the symptoms seen with heat exhaustion: weakness, profound fatigue, and occasionally confusion and disorientation. Athletes with suspected heat exhaustion should be moved to a cooler area, laid down with legs elevated, and orally rehydrated. If they do not improve with oral rehydration, they may require intravenous fluids. The diagnosis of heat exhaustion hinges on a rectal temperature of <104°F; if >104°F the athlete should be presumed to have heat stroke, which will be addressed in the following paragraphs. Players can be cleared to return to play in mild cases within 24 to 48 hours with gradual increases in exercise intensity.24
EXERTIONAL HEAT STROKE
EHS occurs when the body can no longer regulate the core body temperature and it rises to upwards of 104°F. In EHS, elevated core body temperature is associated with evidence of end organ dysfunction. The most easily identified on the playing field is likely central nervous system dysfunction, including irritability, confusion, irrational behavior, lethargy, dizziness, confusion, and even loss of consciousness. Temperature should be measured with rectal temperature only, as other methods of measurement have been shown to be consistently inaccurate.22 Heat stroke can be confused with exertional hyponatremia, heat exhaustion, or concussion, especially when core body temperature cannot be determined. However, EHS should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects. Morbidity and mortality are directly related to the length of time required to cool the athlete under 40°C (104°F).24 Cooling should be completed on site prior to transport to a medical facility and is best achieved with submersion in an ice bath (ie, a kiddie pool or soaking tub full of ice and water).22,25 If an ice bath is not available, ice bags should be applied to the neck axilla and groin and exchanged for fresh bags every 2 to 3 minutes.22 Ice bags have been shown to be inferior to whole body cooling, only cooling the athlete .04°C to .08°C/min compared to .15°C to .24°C/min with the ice bath.24 All other tests should be delayed until cooling is achieved, unless they can be completed while cooling the athlete. The athlete can be removed from the ice bath once rectal temperatures reach <101°F to 102°F.23 If the athlete returns to baseline after cooling, transportation to a medical facility may not be necessary. However, they should refrain from physical activity and heat exposure for at least 7 days and should be evaluated by a physician at that time. If all labs are normal and the athlete is asymptomatic, they can start progressive return to play under the direction of an athletic trainer or a sports medicine physician.23
Continue to: HEAT-RELATED ILLNESS...
HEAT-RELATED ILLNESS
It is impossible to predict exactly which athletes will be most at risk for heat-related illness, so it is important to have a high degree of suspicion when environmental conditions are right. Athletes with recent illness, fever, or lack of sleep are at higher risk. Additional intrinsic risk factors include low fitness level, obesity, and inadequate hydration. Athletes who are highly competitive or motivated can be more likely to push through the early signs of illness or be reluctant to report symptoms.23 Those with a history of exertional heat illness are more at risk for developing it again in the future.23
The extrinsic risk factors for the development of heat-related illness are much easier to identify and modify in order to keep athletes safe. High temperature and high humidity conditions, heavy sun exposure, and exposure to similar conditions the preceding day put athletes at risk for exertional heat illness. Risks are even greater when the exercise is prolonged or intense with few breaks and access to hydration is limited.23 Therefore, prevention of exertional heat illness is centered on these external risk factors.
Each team should have a heat policy as part of their EAP aimed at prevention and early recognition of heat-related illness. This policy should be shared with all athletes and coaches. The plan should be centered on acclimatization, activity modification, and early recognition and management as previously discussed. The US Soccer Federation “Recognize to Recover” Heat Guidelines suggest a 3-step process for appropriate activity modification:22
1. Find the wet bulb globe temperature, either using a wet bulb globe thermometer or the temperature and humidity (Figure 1).
2. Find your regional weather category on the map (Figure 2).
3. Find your alert level and work to rest ratio recommendations (Figure 3).
Scheduled hydration breaks should be given as listed in Figure 3. Breaks of 4 minutes should be given for each 30 minutes of continuous practice or play. In a regulation 90-minute match, a hydration break should be given at 30 and 75 minutes (with half time at 45 minutes) at minimum. Athletes should be educated about where hydration can be accessed, and given unlimited access to hydration even outside of planned breaks.22
Acclimatization to conditions is another integral part of preventing heat-related illness. It allows the body time to adapt to exercising in heat gradually, with a measured progression of exertion over the course of 10 to 14 days. The “Recognize to Recover” Heat Guidelines also provide guidance on acclimatization, and specifics can be found on the website.1 Generally speaking, the warmest part of the day, usually between 11 AM and 4 PM, should be avoided for all training sessions, and length of practice and exertion should be gradually increased over 2 weeks.22
In summary, appropriate acclimatization, hydration, activity modification, and education of athletes and staff are essential for the prevention of heat-related illness. Athletes and staff should understand the signs and symptoms of heat-related illness so that it can be recognized early and treated appropriately. If an athlete is altered in the heat and rectal temperature is >104°F or rectal temperature cannot be obtained, rapid cooling using an ice bath or ice bags is essential to prevent the morbidity and mortality associated with EHS. Above all, teams should have an explicit plan that includes protocols for acclimatization, activity modification, and all necessary equipment to prevent and treat heat-related illnesses should they occur, and ultimately keep athletes safe and healthy.
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain Injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
2. The National Federation of State High School Associations. 2013-14 high school athletics participation survey. http://www.nfhs.org/ParticipationStatics/PDF/2013-14_Participation_Survey_PDF.pdf. Accessed August 6, 2018.
3. Youth Council. US Soccer Federation Web site. https://www.ussoccer.com/about/affiliates/youth-council. Accessed July 31, 2018.
4. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
5. Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314-1320. doi:10.2106/JBJS.16.01573.
6. US Soccer Federation. U.S. Soccer’s comprehensive player health and safety program. Recognize to Recover Web site. http://www.recognizetorecover.org/#us-soccers-comprehensive-player-health-and-safety-program. Accessed July 31, 2018.
7. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi:10.1136/bjsports-2017-097699.
8. Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600. doi:10.1161/CIRCULATIONAHA.110.004622.
9. Faude O, Rössler R, Junge A, et al. Head injuries in children’s football-results from two prospective cohort studies in four European countries. Scand J Med Sci Sports. 2017;27(12):1986-1992. doi:10.1111/sms.12839.
10. Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169(9):830-837. doi:10.1001/jamapediatrics.2015.1062.
11. Tarnutzer AA. Should heading be forbidden in children’s football? Sci Med Football. 2018;2(1):75-79.
12. US Soccer Federation. US Soccer, NWSL and MLS to host “head injury in soccer; science to field”. https://www.ussoccer.com/stories/2017/04/18/17/35/20170418-news-us-soccer-nwsl-mls-host-head-injury-in-soccer-science-to-field. Published April 18, 2017. Accessed August 6, 2018.
13. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54(5):380-386. doi:10.1016/j.pcad.2012.01.006.
14. Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi:10.1161/CIRCULATIONAHA.110.981571.
15. Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the "Grey Zone" of clinical uncertainty. Can J Cardiol. 2016;32(4):429-437. doi:10.1016/j.cjca.2015.11.025.
16. Baggish AL, Battle RW, Beckerman JG, et al; ACC’s Sports and Exercise Council Leadership Group. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70(15):1902-1918. doi:10.1016/j.jacc.2017.08.055.
17. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10-19. doi:10.1161/CIRCULATIONAHA.115.015431.
18. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356-2361. doi:10.1016/j.jacc.2015.09.034.
19. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
20. Baggish AL, Kovacs RJ. Preparticipation cardiovascular screening: clinical partnership is the only certainty. Br J Sports Med. 2017;51(3):150-151. doi:10.1136/bjsports-2016-096954.
21. Hainline B, Drezner J, Baggish A, et al. Interassociation consensus statement on cardiovascular care of college student-athletes. J Athl Train. 2016;51(4):344-357. doi:10.4085/j.jacc.2016.03.527.
22. US Soccer Federation. Environmental conditions. Recognize to Recover Web site. http://www.recognizetorecover.org/environmental/#environmental-conditions. Accessed April 15, 2018.
23. Korey Stringer Institute. Emergency conditions: heat illnesses. University of Connecticut Web site. https://ksi.uconn.edu/. Accessed April 15, 2018.
24. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
25. Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392-397. doi:10.1080/10903127.2017.1392666.
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain Injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
2. The National Federation of State High School Associations. 2013-14 high school athletics participation survey. http://www.nfhs.org/ParticipationStatics/PDF/2013-14_Participation_Survey_PDF.pdf. Accessed August 6, 2018.
3. Youth Council. US Soccer Federation Web site. https://www.ussoccer.com/about/affiliates/youth-council. Accessed July 31, 2018.
4. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
5. Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314-1320. doi:10.2106/JBJS.16.01573.
6. US Soccer Federation. U.S. Soccer’s comprehensive player health and safety program. Recognize to Recover Web site. http://www.recognizetorecover.org/#us-soccers-comprehensive-player-health-and-safety-program. Accessed July 31, 2018.
7. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi:10.1136/bjsports-2017-097699.
8. Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600. doi:10.1161/CIRCULATIONAHA.110.004622.
9. Faude O, Rössler R, Junge A, et al. Head injuries in children’s football-results from two prospective cohort studies in four European countries. Scand J Med Sci Sports. 2017;27(12):1986-1992. doi:10.1111/sms.12839.
10. Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169(9):830-837. doi:10.1001/jamapediatrics.2015.1062.
11. Tarnutzer AA. Should heading be forbidden in children’s football? Sci Med Football. 2018;2(1):75-79.
12. US Soccer Federation. US Soccer, NWSL and MLS to host “head injury in soccer; science to field”. https://www.ussoccer.com/stories/2017/04/18/17/35/20170418-news-us-soccer-nwsl-mls-host-head-injury-in-soccer-science-to-field. Published April 18, 2017. Accessed August 6, 2018.
13. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54(5):380-386. doi:10.1016/j.pcad.2012.01.006.
14. Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi:10.1161/CIRCULATIONAHA.110.981571.
15. Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the "Grey Zone" of clinical uncertainty. Can J Cardiol. 2016;32(4):429-437. doi:10.1016/j.cjca.2015.11.025.
16. Baggish AL, Battle RW, Beckerman JG, et al; ACC’s Sports and Exercise Council Leadership Group. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70(15):1902-1918. doi:10.1016/j.jacc.2017.08.055.
17. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10-19. doi:10.1161/CIRCULATIONAHA.115.015431.
18. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356-2361. doi:10.1016/j.jacc.2015.09.034.
19. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
20. Baggish AL, Kovacs RJ. Preparticipation cardiovascular screening: clinical partnership is the only certainty. Br J Sports Med. 2017;51(3):150-151. doi:10.1136/bjsports-2016-096954.
21. Hainline B, Drezner J, Baggish A, et al. Interassociation consensus statement on cardiovascular care of college student-athletes. J Athl Train. 2016;51(4):344-357. doi:10.4085/j.jacc.2016.03.527.
22. US Soccer Federation. Environmental conditions. Recognize to Recover Web site. http://www.recognizetorecover.org/environmental/#environmental-conditions. Accessed April 15, 2018.
23. Korey Stringer Institute. Emergency conditions: heat illnesses. University of Connecticut Web site. https://ksi.uconn.edu/. Accessed April 15, 2018.
24. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
25. Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392-397. doi:10.1080/10903127.2017.1392666.
TAKE-HOME POINTS
- Current concussion education programs such as “Recognize to Recover” aim to increase self-reporting of concussion symptoms by players, and recognition and appropriate evaluation by medical and coaching staff.
- Athletes who develop symptoms suggestive of underlying cardiovascular disease during play, including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope should be withheld from play until they can be evaluated by a qualified medical professional.
- Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support.
- Exertional heat stroke should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects.
- Prevention of exertional heat illness should center around appropriate acclimatization, access to adequate hydration and scheduled hydration breaks, and avoiding exertion all together when conditions are too dangerous.
Knee Injuries in Elite Level Soccer Players
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.
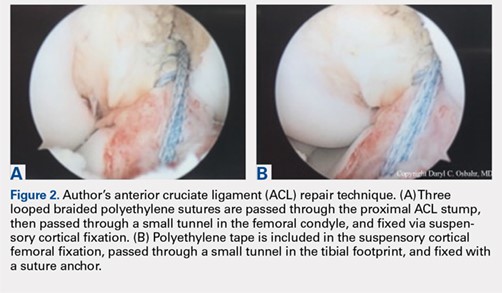
In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.
Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.

In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.

Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.

In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.

Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
TAKE-HOME POINTS
- Soccer is one of the most popular sports in the world and has a high incidence of resultant knee injuries.
- Significant, identifiable risk factors put soccer players at risk for serious knee injuries, such as ACL ruptures; age, female sex, and position played influence injury susceptibility.
- ACL injury most commonly occurs via non-contact mechanisms, and female players are at a significantly higher risk of ACL injury than male counterparts.
- The prevalence of osteoarthritis in retired soccer players is high, underscoring the need to be familiar with meniscal and cartilage repair/restoration techniques and associated outcomes.
- The FIFA11+ program reduces injury by 30%, with reported relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of this warm-up program.
How to manage difficult dislocations
SAN DIEGO – Being prepared to manage difficult dislocations is key to maintaining the flow of patient care in any emergency department.
In a video interview at the annual meeting of the American College of Emergency Physicians, Danielle D. Campagne, MD, FACEP, shared her clinical pearls for managing dislocations of the jaw, hip, ankle, and shoulder.
Dr. Campagne is an emergency medicine physician who practices at Community Regional Medical Center in Fresno, Calif. She is also vice chief of emergency medicine at University of California, San Francisco, Fresno. She reported having no financial disclosures.
SAN DIEGO – Being prepared to manage difficult dislocations is key to maintaining the flow of patient care in any emergency department.
In a video interview at the annual meeting of the American College of Emergency Physicians, Danielle D. Campagne, MD, FACEP, shared her clinical pearls for managing dislocations of the jaw, hip, ankle, and shoulder.
Dr. Campagne is an emergency medicine physician who practices at Community Regional Medical Center in Fresno, Calif. She is also vice chief of emergency medicine at University of California, San Francisco, Fresno. She reported having no financial disclosures.
SAN DIEGO – Being prepared to manage difficult dislocations is key to maintaining the flow of patient care in any emergency department.
In a video interview at the annual meeting of the American College of Emergency Physicians, Danielle D. Campagne, MD, FACEP, shared her clinical pearls for managing dislocations of the jaw, hip, ankle, and shoulder.
Dr. Campagne is an emergency medicine physician who practices at Community Regional Medical Center in Fresno, Calif. She is also vice chief of emergency medicine at University of California, San Francisco, Fresno. She reported having no financial disclosures.
REPORTING FROM ACEP18
The Cold, Hard Facts of Cryotherapy in Orthopedics
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
TAKE-HOME POINTS
- Cryotherapy is often used in postoperative orthopedic care but there is limited literature demonstrating its efficacy.
- Postoperative cryotherapy has been used to reduce visual analog scale pain scores, analgesic consumption, and to increase range of motion.
- There is no consensus on the advantages of postoperative cryotherapy vs traditional ice application.
- Adverse outcomes from postoperative cryotherapy use include frostbite/skin loss, compartment syndrome, and perniosis.
- Future studies, including a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery.
Treatment of Grade III Acromioclavicular Separations in Professional Baseball Pitchers: A Survey of Major League Baseball Team Physicians
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
TAKE-HOME POINTS
- There was no difference in return to previous level of play between professional pitchers treated nonoperatively and operatively for grade III AC separation.
- MLB team physicians prefer nonoperative management for acute grade III AC joint separation in professional pitchers.
- The majority of MLB physicians do not use injections for nonoperative treatment of grade III AC separations; however, use of orthobiologics (eg, PRP) is becoming more commonplace.
- Persistent functional limitations and pain are the most common surgical indications for treatment of grade III AC separation in high level throwing athletes.
- If operative intervention is indicated for grade III AC separation, open coracoclavicular reconstruction and adjunct distal clavicle excision are preferred by most MLB team physicians.
Fat Fracture: A Rare Cause of Anterior and Medial Knee Pain in a Professional Baseball Player
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
TAKE-HOME POINTS
- A fat fracture should be considered in the setting of a blunt injury to the anterior knee when a palpable soft-tissue defect is observed and the extensor mechanism is clinically intact.
- An ultrasound or MRI can assist in making the diagnosis, which can aid in guiding the patient with management and in determining the expected duration of symptoms.
- Injuries to the anterior knee that may present as contusions but have a prolonged course of symptoms should not be overlooked.
Radial Shaft Stress Fracture in a Major League Pitcher
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Referral Patterns for Chronic Groin Pain and Athletic Pubalgia/Sports Hernia: Magnetic Resonance Imaging Findings, Treatment, and Outcomes
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.