User login
Guidance for Practicing Primary Care: World Health Organization’s Updated Influenza Guidelines for 2024
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
Pertussis Rates Up Compared With Recent Years
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
New COVID-19 Vaccines That Target KP.2 Variant Available
New COVID-19 vaccines formulated for better protection against the currently circulating variants have been approved by the US Food and Drug Administration.
The COVID vaccines available this fall have been updated to better match the currently circulating COVID strains, said William Schaffner, MD, professor of medicine in the Division of Infectious Diseases at Vanderbilt University, Nashville, Tennessee, in an interview.
“The Pfizer and Moderna vaccines — both mRNA vaccines — target the KP.2 variant, while the Novavax vaccine targets the JN.1 variant, which is a predecessor to KP.2,” said Dr. Schaffner, who also serves as a spokesperson for the National Foundation for Infectious Diseases. “The Novavax vaccine is a protein adjuvant vaccine made in a more traditional fashion and may appeal to those who remain hesitant about receiving an mRNA vaccine,” he explained. However, , he said.
Who Needs It?
“The CDC’s Advisory Committee on Immunization Practices (ACIP) continues to recommend that everyone in the United States who is age 6 months and older receive the updated COVID vaccine this fall, along with influenza vaccine,” Dr. Schaffner said.
“This was not a surprise because COVID will produce a sizable winter outbreak,” he predicted. Although older people and those who have chronic medical conditions such as heart or lung disease, diabetes, or other immunocompromising conditions suffer the most serious impact of COVID, he said. “The virus can strike anyone, even the young and healthy.” The risk for long COVID persists as well, he pointed out.
The ACIP recommendation is endorsed by the American Academy of Pediatrics and other professional organizations, Dr. Shaffner said.
A frequently asked question is whether the COVID and flu vaccines can be given at the same time, and the answer is yes, according to a statement from the Centers for Disease Control and Prevention (CDC).
“The optimal time to be vaccinated is late September and anytime during October in order to get the benefit of protection through the winter,” Dr. Schaffner said.
As with earlier versions of the COVID-19 vaccine, side effects vary from person to person. Reported side effects of the updated vaccine are similar to those seen with earlier versions and may include injection site pain, redness and swelling, fatigue, headache, muscle pain, chills, nausea, and fever, but most of these are short-lived, according to the CDC.
Clinical Guidance
The CDC’s clinical guidance for COVID-19 vaccination outlines more specific guidance for vaccination based on age, vaccination history, and immunocompromised status and will be updated as needed.
A notable difference in the latest guidance is the recommendation of only one shot for adults aged 65 years and older who are NOT moderately or severely immunocompromised. For those who are moderately or severely immunocompromised, the CDC recommends two to three doses of the same brand of vaccine.
Dr. Schaffner strongly encouraged clinicians to recommend the COVID-19 vaccination for all eligible patients. “COVID is a nasty virus that can cause serious disease in anyone,” and protection from previous vaccination or prior infection has likely waned, he said.
Dr. Schaffner also encouraged healthcare professionals and their families to lead by example. “We should all be vaccinated and let our patients know that we are vaccinated and that we want all our patents to be protected,” he said.
The updated COVID-19 vaccination recommendations have become much simpler for clinicians and patients, with a single messenger RNA (mRNA) vaccine required for anyone older than 5 years, said David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
“The recommendations are a bit more complex for children under 5 years old receiving their first vaccination; they require two to three doses depending on the brand,” he said. “It is important to review the latest recommendations to plan the doses with the correct interval timing. Considering the doses may be 3-4 weeks apart, start early,” he advised.
One-Time Dosing
Although the updated mRNA vaccine is currently recommended as a one-time dose, Dr. Cennimo said he can envision a scenario later in the season when a second dose is recommended for the elderly and those at high risk for severe illness. Dr. Cennimo said that he strongly agrees with the recommendations that everyone aged 6 months and older receive an updated COVID-19 vaccine. Older age remains the prime risk factor, but anyone can become infected, he said.
Predicting a prime time to get vaccinated is tricky because no one knows when the expected rise in winter cases will occur, said Dr. Cennimo.
“We know from years of flu vaccine data that some number of people who delay the vaccine will never return and will miss protection,” he said. Therefore, delaying vaccination is not recommended. Dr. Cennimo plans to follow his habit of getting vaccinated in early October. “I anticipate the maximal effectiveness of the vaccine will carry me through the winter,” he said.
Data support the safety and effectiveness for both flu and COVID vaccines if they are given together, and some research on earlier versions of COVID vaccines suggested that receiving flu and COVID vaccines together might increase the antibody response against COVID, but similar studies of the updated version have not been done, Dr. Cennimo said.
Clinicians may have to overcome the barrier of COVID fatigue to encourage vaccination, Dr. Cennimo said. Many people say they “want it to be over,” he said, but SARS-CoV-2, established as a viral respiratory infection, shows no signs of disappearing. In addition, new data continue to show higher mortality associated with COVID-19 than with influenza, he said.
“We need to explain to our patients that COVID-19 is still here and is still dangerous. The yearly influenza vaccination campaigns should have established and normalized the idea of an updated vaccine targeted for the season’s predicated strains is expected,” he emphasized. “We now have years of safety data behind these vaccines, and we need to make a strong recommendation for this protection,” he said.
COVID-19 vaccines are covered by private insurance, as well as by Medicare and Medicaid, according to the CDC. Vaccination for uninsured children is covered through the Vaccines for Children Program.
A version of this article first appeared on Medscape.com.
New COVID-19 vaccines formulated for better protection against the currently circulating variants have been approved by the US Food and Drug Administration.
The COVID vaccines available this fall have been updated to better match the currently circulating COVID strains, said William Schaffner, MD, professor of medicine in the Division of Infectious Diseases at Vanderbilt University, Nashville, Tennessee, in an interview.
“The Pfizer and Moderna vaccines — both mRNA vaccines — target the KP.2 variant, while the Novavax vaccine targets the JN.1 variant, which is a predecessor to KP.2,” said Dr. Schaffner, who also serves as a spokesperson for the National Foundation for Infectious Diseases. “The Novavax vaccine is a protein adjuvant vaccine made in a more traditional fashion and may appeal to those who remain hesitant about receiving an mRNA vaccine,” he explained. However, , he said.
Who Needs It?
“The CDC’s Advisory Committee on Immunization Practices (ACIP) continues to recommend that everyone in the United States who is age 6 months and older receive the updated COVID vaccine this fall, along with influenza vaccine,” Dr. Schaffner said.
“This was not a surprise because COVID will produce a sizable winter outbreak,” he predicted. Although older people and those who have chronic medical conditions such as heart or lung disease, diabetes, or other immunocompromising conditions suffer the most serious impact of COVID, he said. “The virus can strike anyone, even the young and healthy.” The risk for long COVID persists as well, he pointed out.
The ACIP recommendation is endorsed by the American Academy of Pediatrics and other professional organizations, Dr. Shaffner said.
A frequently asked question is whether the COVID and flu vaccines can be given at the same time, and the answer is yes, according to a statement from the Centers for Disease Control and Prevention (CDC).
“The optimal time to be vaccinated is late September and anytime during October in order to get the benefit of protection through the winter,” Dr. Schaffner said.
As with earlier versions of the COVID-19 vaccine, side effects vary from person to person. Reported side effects of the updated vaccine are similar to those seen with earlier versions and may include injection site pain, redness and swelling, fatigue, headache, muscle pain, chills, nausea, and fever, but most of these are short-lived, according to the CDC.
Clinical Guidance
The CDC’s clinical guidance for COVID-19 vaccination outlines more specific guidance for vaccination based on age, vaccination history, and immunocompromised status and will be updated as needed.
A notable difference in the latest guidance is the recommendation of only one shot for adults aged 65 years and older who are NOT moderately or severely immunocompromised. For those who are moderately or severely immunocompromised, the CDC recommends two to three doses of the same brand of vaccine.
Dr. Schaffner strongly encouraged clinicians to recommend the COVID-19 vaccination for all eligible patients. “COVID is a nasty virus that can cause serious disease in anyone,” and protection from previous vaccination or prior infection has likely waned, he said.
Dr. Schaffner also encouraged healthcare professionals and their families to lead by example. “We should all be vaccinated and let our patients know that we are vaccinated and that we want all our patents to be protected,” he said.
The updated COVID-19 vaccination recommendations have become much simpler for clinicians and patients, with a single messenger RNA (mRNA) vaccine required for anyone older than 5 years, said David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
“The recommendations are a bit more complex for children under 5 years old receiving their first vaccination; they require two to three doses depending on the brand,” he said. “It is important to review the latest recommendations to plan the doses with the correct interval timing. Considering the doses may be 3-4 weeks apart, start early,” he advised.
One-Time Dosing
Although the updated mRNA vaccine is currently recommended as a one-time dose, Dr. Cennimo said he can envision a scenario later in the season when a second dose is recommended for the elderly and those at high risk for severe illness. Dr. Cennimo said that he strongly agrees with the recommendations that everyone aged 6 months and older receive an updated COVID-19 vaccine. Older age remains the prime risk factor, but anyone can become infected, he said.
Predicting a prime time to get vaccinated is tricky because no one knows when the expected rise in winter cases will occur, said Dr. Cennimo.
“We know from years of flu vaccine data that some number of people who delay the vaccine will never return and will miss protection,” he said. Therefore, delaying vaccination is not recommended. Dr. Cennimo plans to follow his habit of getting vaccinated in early October. “I anticipate the maximal effectiveness of the vaccine will carry me through the winter,” he said.
Data support the safety and effectiveness for both flu and COVID vaccines if they are given together, and some research on earlier versions of COVID vaccines suggested that receiving flu and COVID vaccines together might increase the antibody response against COVID, but similar studies of the updated version have not been done, Dr. Cennimo said.
Clinicians may have to overcome the barrier of COVID fatigue to encourage vaccination, Dr. Cennimo said. Many people say they “want it to be over,” he said, but SARS-CoV-2, established as a viral respiratory infection, shows no signs of disappearing. In addition, new data continue to show higher mortality associated with COVID-19 than with influenza, he said.
“We need to explain to our patients that COVID-19 is still here and is still dangerous. The yearly influenza vaccination campaigns should have established and normalized the idea of an updated vaccine targeted for the season’s predicated strains is expected,” he emphasized. “We now have years of safety data behind these vaccines, and we need to make a strong recommendation for this protection,” he said.
COVID-19 vaccines are covered by private insurance, as well as by Medicare and Medicaid, according to the CDC. Vaccination for uninsured children is covered through the Vaccines for Children Program.
A version of this article first appeared on Medscape.com.
New COVID-19 vaccines formulated for better protection against the currently circulating variants have been approved by the US Food and Drug Administration.
The COVID vaccines available this fall have been updated to better match the currently circulating COVID strains, said William Schaffner, MD, professor of medicine in the Division of Infectious Diseases at Vanderbilt University, Nashville, Tennessee, in an interview.
“The Pfizer and Moderna vaccines — both mRNA vaccines — target the KP.2 variant, while the Novavax vaccine targets the JN.1 variant, which is a predecessor to KP.2,” said Dr. Schaffner, who also serves as a spokesperson for the National Foundation for Infectious Diseases. “The Novavax vaccine is a protein adjuvant vaccine made in a more traditional fashion and may appeal to those who remain hesitant about receiving an mRNA vaccine,” he explained. However, , he said.
Who Needs It?
“The CDC’s Advisory Committee on Immunization Practices (ACIP) continues to recommend that everyone in the United States who is age 6 months and older receive the updated COVID vaccine this fall, along with influenza vaccine,” Dr. Schaffner said.
“This was not a surprise because COVID will produce a sizable winter outbreak,” he predicted. Although older people and those who have chronic medical conditions such as heart or lung disease, diabetes, or other immunocompromising conditions suffer the most serious impact of COVID, he said. “The virus can strike anyone, even the young and healthy.” The risk for long COVID persists as well, he pointed out.
The ACIP recommendation is endorsed by the American Academy of Pediatrics and other professional organizations, Dr. Shaffner said.
A frequently asked question is whether the COVID and flu vaccines can be given at the same time, and the answer is yes, according to a statement from the Centers for Disease Control and Prevention (CDC).
“The optimal time to be vaccinated is late September and anytime during October in order to get the benefit of protection through the winter,” Dr. Schaffner said.
As with earlier versions of the COVID-19 vaccine, side effects vary from person to person. Reported side effects of the updated vaccine are similar to those seen with earlier versions and may include injection site pain, redness and swelling, fatigue, headache, muscle pain, chills, nausea, and fever, but most of these are short-lived, according to the CDC.
Clinical Guidance
The CDC’s clinical guidance for COVID-19 vaccination outlines more specific guidance for vaccination based on age, vaccination history, and immunocompromised status and will be updated as needed.
A notable difference in the latest guidance is the recommendation of only one shot for adults aged 65 years and older who are NOT moderately or severely immunocompromised. For those who are moderately or severely immunocompromised, the CDC recommends two to three doses of the same brand of vaccine.
Dr. Schaffner strongly encouraged clinicians to recommend the COVID-19 vaccination for all eligible patients. “COVID is a nasty virus that can cause serious disease in anyone,” and protection from previous vaccination or prior infection has likely waned, he said.
Dr. Schaffner also encouraged healthcare professionals and their families to lead by example. “We should all be vaccinated and let our patients know that we are vaccinated and that we want all our patents to be protected,” he said.
The updated COVID-19 vaccination recommendations have become much simpler for clinicians and patients, with a single messenger RNA (mRNA) vaccine required for anyone older than 5 years, said David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
“The recommendations are a bit more complex for children under 5 years old receiving their first vaccination; they require two to three doses depending on the brand,” he said. “It is important to review the latest recommendations to plan the doses with the correct interval timing. Considering the doses may be 3-4 weeks apart, start early,” he advised.
One-Time Dosing
Although the updated mRNA vaccine is currently recommended as a one-time dose, Dr. Cennimo said he can envision a scenario later in the season when a second dose is recommended for the elderly and those at high risk for severe illness. Dr. Cennimo said that he strongly agrees with the recommendations that everyone aged 6 months and older receive an updated COVID-19 vaccine. Older age remains the prime risk factor, but anyone can become infected, he said.
Predicting a prime time to get vaccinated is tricky because no one knows when the expected rise in winter cases will occur, said Dr. Cennimo.
“We know from years of flu vaccine data that some number of people who delay the vaccine will never return and will miss protection,” he said. Therefore, delaying vaccination is not recommended. Dr. Cennimo plans to follow his habit of getting vaccinated in early October. “I anticipate the maximal effectiveness of the vaccine will carry me through the winter,” he said.
Data support the safety and effectiveness for both flu and COVID vaccines if they are given together, and some research on earlier versions of COVID vaccines suggested that receiving flu and COVID vaccines together might increase the antibody response against COVID, but similar studies of the updated version have not been done, Dr. Cennimo said.
Clinicians may have to overcome the barrier of COVID fatigue to encourage vaccination, Dr. Cennimo said. Many people say they “want it to be over,” he said, but SARS-CoV-2, established as a viral respiratory infection, shows no signs of disappearing. In addition, new data continue to show higher mortality associated with COVID-19 than with influenza, he said.
“We need to explain to our patients that COVID-19 is still here and is still dangerous. The yearly influenza vaccination campaigns should have established and normalized the idea of an updated vaccine targeted for the season’s predicated strains is expected,” he emphasized. “We now have years of safety data behind these vaccines, and we need to make a strong recommendation for this protection,” he said.
COVID-19 vaccines are covered by private insurance, as well as by Medicare and Medicaid, according to the CDC. Vaccination for uninsured children is covered through the Vaccines for Children Program.
A version of this article first appeared on Medscape.com.
Updated COVID Vaccines: Who Should Get One, and When?
This transcript has been edited for clarity.
Two updated mRNA COVID vaccines, one by Moderna and the other by Pfizer, have been authorized or approved by the US Food and Drug Administration (FDA) for those aged 6 months or older.
Both vaccines target Omicron’s KP.2 strain of the JN.1 lineage. An updated protein-based version by Novavax, also directed at JN.1, has been authorized, but only for those aged 12 years or older.
The Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices recommends a dose of the 2024-2025 updated COVID vaccine for everyone aged 6 months or older. This includes people who have never been vaccinated against COVID, those who have been vaccinated, as well as people with previous COVID infection.
The big question is when, and FDA and CDC have set some parameters. For mRNA updated vaccines, patients should wait at least 2 months after their last dose of any COVID vaccine before getting a dose of the updated vaccine.
If the patient has recently had COVID, the wait time is even longer: Patients can wait 3 months after a COVID infection to be vaccinated, but they don’t have to. FDA’s instructions for the Novavax version are not as straightforward. People can get an updated Novavax dose at least 2 months after their last mRNA COVID vaccine dose, or at least 2 months after completing a Novavax two-dose primary series. Those two Novavax doses should be given at least 3 weeks apart.
Patients can personalize their vaccine plan. They will have the greatest protection in the first few weeks to months after a vaccine, after which antibodies tend to wane. It is a good idea to time vaccination so that protection peaks at big events like weddings and major meetings.
If patients decide to wait, they run the risk of getting a COVID infection. Also keep in mind which variants are circulating and the amount of local activity. Right now, there is a lot of COVID going around, and most of it is related to JN.1, the target of this year’s updated vaccine. If patients decide to wait, they should avoid crowded indoor settings or wear a high-quality mask for some protection.
Here’s the bottom line: Most people (more than 95%) have some degree of COVID protection from previous infection, vaccination, or both. But if they haven’t recently had COVID infection and didn’t get a dose of last year’s vaccine, they are sitting ducks for getting sick without updated protection. The best way to stay well is to get a dose of the updated vaccine as soon as possible. This is especially true for those in high-risk groups or who are near someone who is high risk.
Two thirds of COVID hospitalizations are in those aged 65 or older. Hospitalization rates are highest for those aged 75 or older and among infants under 6 months of age. These babies are too young to be vaccinated, but maternal vaccination during pregnancy and breastfeeding can help protect them.
We’re still seeing racial and ethnic disparities in COVID-related hospitalizations, which are highest among American Indians, Alaska Natives, and Black populations. People with immunocompromising conditions, those with chronic medical conditions, and people living in long-term care facilities are also at greater risk. Unlike last year, additional mRNA doses are not recommended for those aged 65 or older at this time, but that could change.
Since 2020, we’ve come a long way in our fight against COVID, but the battle is still on. In 2023, nearly a million people were hospitalized from COVID. More than 75,000 died. COVID vaccines help protect us from severe disease, hospitalization, and death.
Let’s face it — we all have booster fatigue, but COVID is now endemic. It’s here to stay, and it’s much safer to update antibody protection with vaccination than with infection. Another benefit of getting vaccinated is that it decreases the chance of getting long COVID. The uptake of last year’s COVID vaccine was abysmal; only about 23% of adults and 14% of children received it.
But this is a new year and a new vaccine. Please make sure your patients understand that the virus has changed a lot. Antibodies we built from previous infection and previous vaccination don’t work as well against these new variants. Antibody levels wane over time, so even if they missed the last few vaccine doses without getting sick, they really should consider getting a dose of this new vaccine. The 2024-2025 updated COVID vaccine is the best way to catch up, update their immunity, and keep them protected.
Furthermore, we are now entering respiratory virus season, which means we need to think about, recommend, and administer three shots if indicated: COVID, flu, and RSV. Now is the time. Patients can get all three at the same time, in the same visit, if they choose to do so.
Your recommendation as a physician is powerful. Adults and children who receive a healthcare provider recommendation are more likely to get vaccinated.
Dr. Fryhofer is an adjunct clinical associate professor of medicine, Emory University School of Medicine, Atlanta, Georgia. She reported conflicts of interest with the American Medical Association, the Medical Association of Atlanta, the American College of Physicians, and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two updated mRNA COVID vaccines, one by Moderna and the other by Pfizer, have been authorized or approved by the US Food and Drug Administration (FDA) for those aged 6 months or older.
Both vaccines target Omicron’s KP.2 strain of the JN.1 lineage. An updated protein-based version by Novavax, also directed at JN.1, has been authorized, but only for those aged 12 years or older.
The Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices recommends a dose of the 2024-2025 updated COVID vaccine for everyone aged 6 months or older. This includes people who have never been vaccinated against COVID, those who have been vaccinated, as well as people with previous COVID infection.
The big question is when, and FDA and CDC have set some parameters. For mRNA updated vaccines, patients should wait at least 2 months after their last dose of any COVID vaccine before getting a dose of the updated vaccine.
If the patient has recently had COVID, the wait time is even longer: Patients can wait 3 months after a COVID infection to be vaccinated, but they don’t have to. FDA’s instructions for the Novavax version are not as straightforward. People can get an updated Novavax dose at least 2 months after their last mRNA COVID vaccine dose, or at least 2 months after completing a Novavax two-dose primary series. Those two Novavax doses should be given at least 3 weeks apart.
Patients can personalize their vaccine plan. They will have the greatest protection in the first few weeks to months after a vaccine, after which antibodies tend to wane. It is a good idea to time vaccination so that protection peaks at big events like weddings and major meetings.
If patients decide to wait, they run the risk of getting a COVID infection. Also keep in mind which variants are circulating and the amount of local activity. Right now, there is a lot of COVID going around, and most of it is related to JN.1, the target of this year’s updated vaccine. If patients decide to wait, they should avoid crowded indoor settings or wear a high-quality mask for some protection.
Here’s the bottom line: Most people (more than 95%) have some degree of COVID protection from previous infection, vaccination, or both. But if they haven’t recently had COVID infection and didn’t get a dose of last year’s vaccine, they are sitting ducks for getting sick without updated protection. The best way to stay well is to get a dose of the updated vaccine as soon as possible. This is especially true for those in high-risk groups or who are near someone who is high risk.
Two thirds of COVID hospitalizations are in those aged 65 or older. Hospitalization rates are highest for those aged 75 or older and among infants under 6 months of age. These babies are too young to be vaccinated, but maternal vaccination during pregnancy and breastfeeding can help protect them.
We’re still seeing racial and ethnic disparities in COVID-related hospitalizations, which are highest among American Indians, Alaska Natives, and Black populations. People with immunocompromising conditions, those with chronic medical conditions, and people living in long-term care facilities are also at greater risk. Unlike last year, additional mRNA doses are not recommended for those aged 65 or older at this time, but that could change.
Since 2020, we’ve come a long way in our fight against COVID, but the battle is still on. In 2023, nearly a million people were hospitalized from COVID. More than 75,000 died. COVID vaccines help protect us from severe disease, hospitalization, and death.
Let’s face it — we all have booster fatigue, but COVID is now endemic. It’s here to stay, and it’s much safer to update antibody protection with vaccination than with infection. Another benefit of getting vaccinated is that it decreases the chance of getting long COVID. The uptake of last year’s COVID vaccine was abysmal; only about 23% of adults and 14% of children received it.
But this is a new year and a new vaccine. Please make sure your patients understand that the virus has changed a lot. Antibodies we built from previous infection and previous vaccination don’t work as well against these new variants. Antibody levels wane over time, so even if they missed the last few vaccine doses without getting sick, they really should consider getting a dose of this new vaccine. The 2024-2025 updated COVID vaccine is the best way to catch up, update their immunity, and keep them protected.
Furthermore, we are now entering respiratory virus season, which means we need to think about, recommend, and administer three shots if indicated: COVID, flu, and RSV. Now is the time. Patients can get all three at the same time, in the same visit, if they choose to do so.
Your recommendation as a physician is powerful. Adults and children who receive a healthcare provider recommendation are more likely to get vaccinated.
Dr. Fryhofer is an adjunct clinical associate professor of medicine, Emory University School of Medicine, Atlanta, Georgia. She reported conflicts of interest with the American Medical Association, the Medical Association of Atlanta, the American College of Physicians, and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two updated mRNA COVID vaccines, one by Moderna and the other by Pfizer, have been authorized or approved by the US Food and Drug Administration (FDA) for those aged 6 months or older.
Both vaccines target Omicron’s KP.2 strain of the JN.1 lineage. An updated protein-based version by Novavax, also directed at JN.1, has been authorized, but only for those aged 12 years or older.
The Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices recommends a dose of the 2024-2025 updated COVID vaccine for everyone aged 6 months or older. This includes people who have never been vaccinated against COVID, those who have been vaccinated, as well as people with previous COVID infection.
The big question is when, and FDA and CDC have set some parameters. For mRNA updated vaccines, patients should wait at least 2 months after their last dose of any COVID vaccine before getting a dose of the updated vaccine.
If the patient has recently had COVID, the wait time is even longer: Patients can wait 3 months after a COVID infection to be vaccinated, but they don’t have to. FDA’s instructions for the Novavax version are not as straightforward. People can get an updated Novavax dose at least 2 months after their last mRNA COVID vaccine dose, or at least 2 months after completing a Novavax two-dose primary series. Those two Novavax doses should be given at least 3 weeks apart.
Patients can personalize their vaccine plan. They will have the greatest protection in the first few weeks to months after a vaccine, after which antibodies tend to wane. It is a good idea to time vaccination so that protection peaks at big events like weddings and major meetings.
If patients decide to wait, they run the risk of getting a COVID infection. Also keep in mind which variants are circulating and the amount of local activity. Right now, there is a lot of COVID going around, and most of it is related to JN.1, the target of this year’s updated vaccine. If patients decide to wait, they should avoid crowded indoor settings or wear a high-quality mask for some protection.
Here’s the bottom line: Most people (more than 95%) have some degree of COVID protection from previous infection, vaccination, or both. But if they haven’t recently had COVID infection and didn’t get a dose of last year’s vaccine, they are sitting ducks for getting sick without updated protection. The best way to stay well is to get a dose of the updated vaccine as soon as possible. This is especially true for those in high-risk groups or who are near someone who is high risk.
Two thirds of COVID hospitalizations are in those aged 65 or older. Hospitalization rates are highest for those aged 75 or older and among infants under 6 months of age. These babies are too young to be vaccinated, but maternal vaccination during pregnancy and breastfeeding can help protect them.
We’re still seeing racial and ethnic disparities in COVID-related hospitalizations, which are highest among American Indians, Alaska Natives, and Black populations. People with immunocompromising conditions, those with chronic medical conditions, and people living in long-term care facilities are also at greater risk. Unlike last year, additional mRNA doses are not recommended for those aged 65 or older at this time, but that could change.
Since 2020, we’ve come a long way in our fight against COVID, but the battle is still on. In 2023, nearly a million people were hospitalized from COVID. More than 75,000 died. COVID vaccines help protect us from severe disease, hospitalization, and death.
Let’s face it — we all have booster fatigue, but COVID is now endemic. It’s here to stay, and it’s much safer to update antibody protection with vaccination than with infection. Another benefit of getting vaccinated is that it decreases the chance of getting long COVID. The uptake of last year’s COVID vaccine was abysmal; only about 23% of adults and 14% of children received it.
But this is a new year and a new vaccine. Please make sure your patients understand that the virus has changed a lot. Antibodies we built from previous infection and previous vaccination don’t work as well against these new variants. Antibody levels wane over time, so even if they missed the last few vaccine doses without getting sick, they really should consider getting a dose of this new vaccine. The 2024-2025 updated COVID vaccine is the best way to catch up, update their immunity, and keep them protected.
Furthermore, we are now entering respiratory virus season, which means we need to think about, recommend, and administer three shots if indicated: COVID, flu, and RSV. Now is the time. Patients can get all three at the same time, in the same visit, if they choose to do so.
Your recommendation as a physician is powerful. Adults and children who receive a healthcare provider recommendation are more likely to get vaccinated.
Dr. Fryhofer is an adjunct clinical associate professor of medicine, Emory University School of Medicine, Atlanta, Georgia. She reported conflicts of interest with the American Medical Association, the Medical Association of Atlanta, the American College of Physicians, and Medscape.
A version of this article first appeared on Medscape.com.
As Interest From Families Wanes, Pediatricians Scale Back on COVID Shots
When pediatrician Eric Ball, MD, opened a refrigerator full of childhood vaccines, all the expected shots were there — DTaP, polio, pneumococcal vaccine — except one.
“This is where we usually store our COVID vaccines, but we don’t have any right now because they all expired at the end of last year and we had to dispose of them,” said Dr. Ball, who is part of a pediatric practice in Orange County, California.
“We thought demand would be way higher than it was.”
Providers like Dr. Ball don’t want to waste money ordering doses that won’t be used, but they need enough on hand to vaccinate vulnerable children.
The Centers for Disease Control and Prevention recommends that anyone 6 months or older get the updated COVID vaccination, but in the 2023-24 vaccination season only about 15% of eligible children in the United States got a shot.
Dr. Ball said it was difficult to let vaccines go to waste in 2023. It was the first time the federal government was no longer picking up the tab for the shots, and providers had to pay upfront for the vaccines. Parents would often skip the COVID shot, which can have a very short shelf life, compared with other vaccines.
“Watching it sitting on our shelves expiring every 30 days, that’s like throwing away $150 repeatedly every day, multiple times a month,” Dr. Ball said.
in 2024, Dr. Ball slashed his fall vaccine order to the bare minimum to avoid another costly mistake.
“We took the number of flu vaccines that we order, and then we ordered 5% of that in COVID vaccines,” Dr. Ball said. “It’s a guess.”
That small vaccine order cost more than $63,000, he said.
Pharmacists, pharmacy interns, and techs are allowed to give COVID vaccines only to children age 3 and up, meaning babies and toddlers would need to visit a doctor’s office for inoculation.
It’s difficult to predict how parents will feel about the shots this fall, said Chicago pediatrician Scott Goldstein, MD. Unlike other vaccinations, COVID shots aren’t required for kids to attend school, and parental interest seems to wane with each new formulation. For a physician-owned practice such as Dr. Goldstein’s, the upfront cost of the vaccine can be a gamble.
“The cost of vaccines, that’s far and away our biggest expense. But it’s also the most important thing we do, you could argue, is vaccinating kids,” Dr. Goldstein said.
Insurance doesn’t necessarily cover vaccine storage accidents, which can put the practice at risk of financial ruin.
“We’ve had things happen like a refrigerator gets unplugged. And then we’re all of a sudden out $80,000 overnight,” Dr. Goldstein said.
South Carolina pediatrician Deborah Greenhouse, MD, said she would order more COVID vaccines for older children if the pharmaceutical companies that she buys from had a more forgiving return policy.
“Pfizer is creating that situation. If you’re only going to let us return 30%, we’re not going to buy much,” she said. “We can’t.”
Greenhouse owns her practice, so the remaining 70% of leftover shots would come out of her pocket.
Vaccine maker Pfizer will take back all unused COVID shots for young children, but only 30% of doses for people 12 and older.
Pfizer said in an Aug. 20 emailed statement, “The return policy was instituted as we recognize both the importance and the complexity of pediatric vaccination and wanted to ensure that pediatric offices did not have hurdles to providing vaccine to their young patients.”
Pfizer’s return policy is similar to policies from other drugmakers for pediatric flu vaccines, also recommended during the fall season. Physicians who are worried about unwanted COVID vaccines expiring on the shelves said flu shots cost them about $20 per dose, while COVID shots cost around $150 per dose.
“We run on a very thin margin. If we get stuck holding a ton of vaccine that we cannot return, we can’t absorb that kind of cost,” Dr. Greenhouse said.
Vaccine maker Moderna will accept COVID vaccine returns, but the amount depends on the individual contract with a provider. Novavax will accept the return of only unopened vaccines and doesn’t specify the amount they’ll accept.
Dr. Greenhouse wants to vaccinate as many children as possible but said she can’t afford to stock shots with a short shelf life. Once she runs out of the doses she’s ordered, Dr. Greenhouse plans to tell families to go to a pharmacy to get older children vaccinated. If pediatricians around the country are making the same calculations, doses for very small children could be harder to find at doctors’ offices.
“Frankly, it’s not an ideal situation, but it’s what we have to do to stay in business,” she said.
Dr. Ball worries that parents’ limited interest has caused pediatricians to minimize their vaccine orders, in turn making the newest COVID shots difficult to find once they become available.
“I think there’s just a misperception that it’s less of a big deal to get COVID, but I’m still sending babies to the hospital with COVID,” Dr. Ball said. “We’re still seeing kids with long COVID. This is with us forever.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
When pediatrician Eric Ball, MD, opened a refrigerator full of childhood vaccines, all the expected shots were there — DTaP, polio, pneumococcal vaccine — except one.
“This is where we usually store our COVID vaccines, but we don’t have any right now because they all expired at the end of last year and we had to dispose of them,” said Dr. Ball, who is part of a pediatric practice in Orange County, California.
“We thought demand would be way higher than it was.”
Providers like Dr. Ball don’t want to waste money ordering doses that won’t be used, but they need enough on hand to vaccinate vulnerable children.
The Centers for Disease Control and Prevention recommends that anyone 6 months or older get the updated COVID vaccination, but in the 2023-24 vaccination season only about 15% of eligible children in the United States got a shot.
Dr. Ball said it was difficult to let vaccines go to waste in 2023. It was the first time the federal government was no longer picking up the tab for the shots, and providers had to pay upfront for the vaccines. Parents would often skip the COVID shot, which can have a very short shelf life, compared with other vaccines.
“Watching it sitting on our shelves expiring every 30 days, that’s like throwing away $150 repeatedly every day, multiple times a month,” Dr. Ball said.
in 2024, Dr. Ball slashed his fall vaccine order to the bare minimum to avoid another costly mistake.
“We took the number of flu vaccines that we order, and then we ordered 5% of that in COVID vaccines,” Dr. Ball said. “It’s a guess.”
That small vaccine order cost more than $63,000, he said.
Pharmacists, pharmacy interns, and techs are allowed to give COVID vaccines only to children age 3 and up, meaning babies and toddlers would need to visit a doctor’s office for inoculation.
It’s difficult to predict how parents will feel about the shots this fall, said Chicago pediatrician Scott Goldstein, MD. Unlike other vaccinations, COVID shots aren’t required for kids to attend school, and parental interest seems to wane with each new formulation. For a physician-owned practice such as Dr. Goldstein’s, the upfront cost of the vaccine can be a gamble.
“The cost of vaccines, that’s far and away our biggest expense. But it’s also the most important thing we do, you could argue, is vaccinating kids,” Dr. Goldstein said.
Insurance doesn’t necessarily cover vaccine storage accidents, which can put the practice at risk of financial ruin.
“We’ve had things happen like a refrigerator gets unplugged. And then we’re all of a sudden out $80,000 overnight,” Dr. Goldstein said.
South Carolina pediatrician Deborah Greenhouse, MD, said she would order more COVID vaccines for older children if the pharmaceutical companies that she buys from had a more forgiving return policy.
“Pfizer is creating that situation. If you’re only going to let us return 30%, we’re not going to buy much,” she said. “We can’t.”
Greenhouse owns her practice, so the remaining 70% of leftover shots would come out of her pocket.
Vaccine maker Pfizer will take back all unused COVID shots for young children, but only 30% of doses for people 12 and older.
Pfizer said in an Aug. 20 emailed statement, “The return policy was instituted as we recognize both the importance and the complexity of pediatric vaccination and wanted to ensure that pediatric offices did not have hurdles to providing vaccine to their young patients.”
Pfizer’s return policy is similar to policies from other drugmakers for pediatric flu vaccines, also recommended during the fall season. Physicians who are worried about unwanted COVID vaccines expiring on the shelves said flu shots cost them about $20 per dose, while COVID shots cost around $150 per dose.
“We run on a very thin margin. If we get stuck holding a ton of vaccine that we cannot return, we can’t absorb that kind of cost,” Dr. Greenhouse said.
Vaccine maker Moderna will accept COVID vaccine returns, but the amount depends on the individual contract with a provider. Novavax will accept the return of only unopened vaccines and doesn’t specify the amount they’ll accept.
Dr. Greenhouse wants to vaccinate as many children as possible but said she can’t afford to stock shots with a short shelf life. Once she runs out of the doses she’s ordered, Dr. Greenhouse plans to tell families to go to a pharmacy to get older children vaccinated. If pediatricians around the country are making the same calculations, doses for very small children could be harder to find at doctors’ offices.
“Frankly, it’s not an ideal situation, but it’s what we have to do to stay in business,” she said.
Dr. Ball worries that parents’ limited interest has caused pediatricians to minimize their vaccine orders, in turn making the newest COVID shots difficult to find once they become available.
“I think there’s just a misperception that it’s less of a big deal to get COVID, but I’m still sending babies to the hospital with COVID,” Dr. Ball said. “We’re still seeing kids with long COVID. This is with us forever.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
When pediatrician Eric Ball, MD, opened a refrigerator full of childhood vaccines, all the expected shots were there — DTaP, polio, pneumococcal vaccine — except one.
“This is where we usually store our COVID vaccines, but we don’t have any right now because they all expired at the end of last year and we had to dispose of them,” said Dr. Ball, who is part of a pediatric practice in Orange County, California.
“We thought demand would be way higher than it was.”
Providers like Dr. Ball don’t want to waste money ordering doses that won’t be used, but they need enough on hand to vaccinate vulnerable children.
The Centers for Disease Control and Prevention recommends that anyone 6 months or older get the updated COVID vaccination, but in the 2023-24 vaccination season only about 15% of eligible children in the United States got a shot.
Dr. Ball said it was difficult to let vaccines go to waste in 2023. It was the first time the federal government was no longer picking up the tab for the shots, and providers had to pay upfront for the vaccines. Parents would often skip the COVID shot, which can have a very short shelf life, compared with other vaccines.
“Watching it sitting on our shelves expiring every 30 days, that’s like throwing away $150 repeatedly every day, multiple times a month,” Dr. Ball said.
in 2024, Dr. Ball slashed his fall vaccine order to the bare minimum to avoid another costly mistake.
“We took the number of flu vaccines that we order, and then we ordered 5% of that in COVID vaccines,” Dr. Ball said. “It’s a guess.”
That small vaccine order cost more than $63,000, he said.
Pharmacists, pharmacy interns, and techs are allowed to give COVID vaccines only to children age 3 and up, meaning babies and toddlers would need to visit a doctor’s office for inoculation.
It’s difficult to predict how parents will feel about the shots this fall, said Chicago pediatrician Scott Goldstein, MD. Unlike other vaccinations, COVID shots aren’t required for kids to attend school, and parental interest seems to wane with each new formulation. For a physician-owned practice such as Dr. Goldstein’s, the upfront cost of the vaccine can be a gamble.
“The cost of vaccines, that’s far and away our biggest expense. But it’s also the most important thing we do, you could argue, is vaccinating kids,” Dr. Goldstein said.
Insurance doesn’t necessarily cover vaccine storage accidents, which can put the practice at risk of financial ruin.
“We’ve had things happen like a refrigerator gets unplugged. And then we’re all of a sudden out $80,000 overnight,” Dr. Goldstein said.
South Carolina pediatrician Deborah Greenhouse, MD, said she would order more COVID vaccines for older children if the pharmaceutical companies that she buys from had a more forgiving return policy.
“Pfizer is creating that situation. If you’re only going to let us return 30%, we’re not going to buy much,” she said. “We can’t.”
Greenhouse owns her practice, so the remaining 70% of leftover shots would come out of her pocket.
Vaccine maker Pfizer will take back all unused COVID shots for young children, but only 30% of doses for people 12 and older.
Pfizer said in an Aug. 20 emailed statement, “The return policy was instituted as we recognize both the importance and the complexity of pediatric vaccination and wanted to ensure that pediatric offices did not have hurdles to providing vaccine to their young patients.”
Pfizer’s return policy is similar to policies from other drugmakers for pediatric flu vaccines, also recommended during the fall season. Physicians who are worried about unwanted COVID vaccines expiring on the shelves said flu shots cost them about $20 per dose, while COVID shots cost around $150 per dose.
“We run on a very thin margin. If we get stuck holding a ton of vaccine that we cannot return, we can’t absorb that kind of cost,” Dr. Greenhouse said.
Vaccine maker Moderna will accept COVID vaccine returns, but the amount depends on the individual contract with a provider. Novavax will accept the return of only unopened vaccines and doesn’t specify the amount they’ll accept.
Dr. Greenhouse wants to vaccinate as many children as possible but said she can’t afford to stock shots with a short shelf life. Once she runs out of the doses she’s ordered, Dr. Greenhouse plans to tell families to go to a pharmacy to get older children vaccinated. If pediatricians around the country are making the same calculations, doses for very small children could be harder to find at doctors’ offices.
“Frankly, it’s not an ideal situation, but it’s what we have to do to stay in business,” she said.
Dr. Ball worries that parents’ limited interest has caused pediatricians to minimize their vaccine orders, in turn making the newest COVID shots difficult to find once they become available.
“I think there’s just a misperception that it’s less of a big deal to get COVID, but I’m still sending babies to the hospital with COVID,” Dr. Ball said. “We’re still seeing kids with long COVID. This is with us forever.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
Part of Taking a Good (Human) Patient History Includes Asking About Pet Vaccinations
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
The New Formula for Stronger, Longer-Lasting Vaccines
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6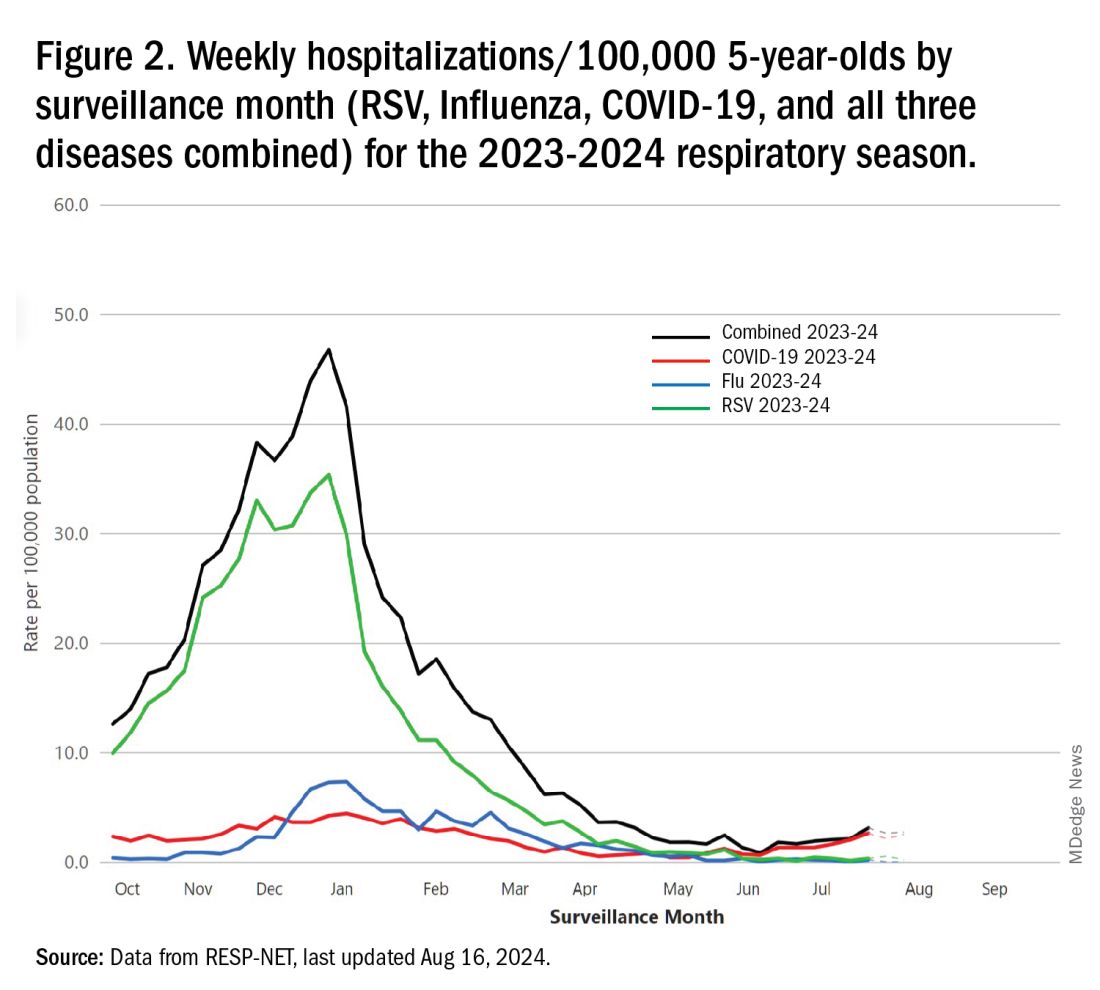
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 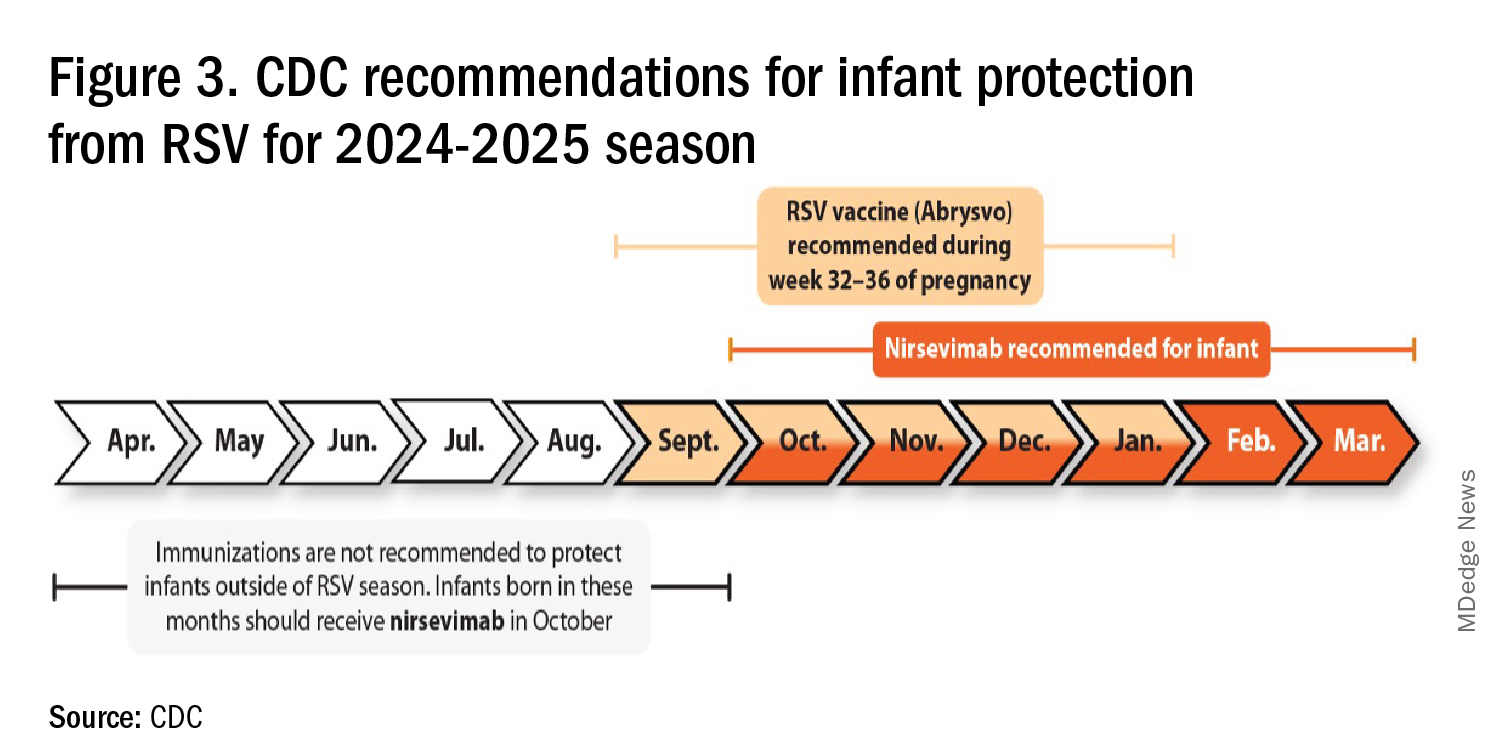
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
Whooping Cough Likely on Pace for a 5-Year High
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.