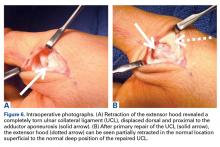
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Open Navicular Dislocation With Midfoot Dissociation in a 45-Year-Old Man
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
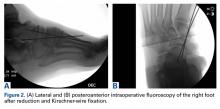
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
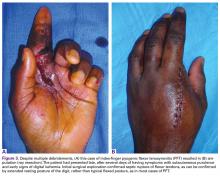
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
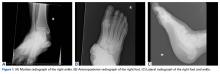
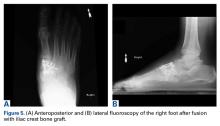
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
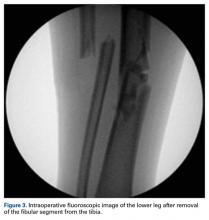
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
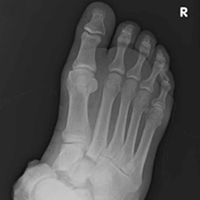
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
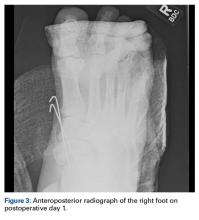
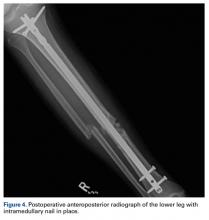
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
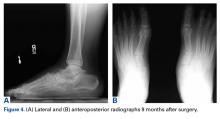
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
Rare Dual Lesion: Extraskeletal Osteosarcoma Developing Within a Simple Lipoma
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
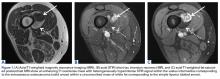
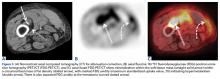
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
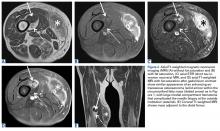
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
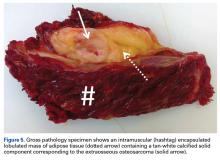
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
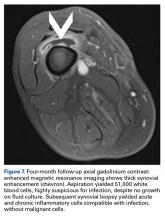
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
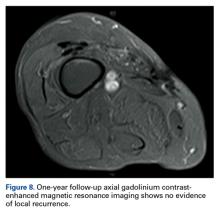
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
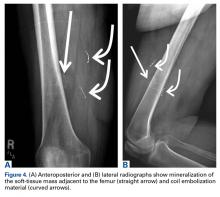
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
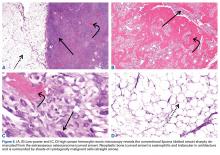
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
Multimodality Approach to a Stener Lesion: Radiographic, Ultrasound, Magnetic Resonance Imaging, and Surgical Correlation
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery?
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
Difficult-to-Detect Low-Grade Infections Responsible for Poor Outcomes in Total Knee Arthroplasty
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Readability of Orthopedic Trauma Patient Education Materials on the Internet
Take-Home Points
- The Flesch-Kincaid Readability Scale is a useful tool in evaluating the readability of PEMs.
- Only 1 article analyzed in our study was below a sixth-grade readability level.
- Coauthorship of PEMs with other subspecialty groups had no effect on readability.
- Poor health literacy has been associated with poor health outcomes.
- Efforts must be undertaken to make PEMs more readable across medical subspecialties.
Patients increasingly turn to the Internet to self-educate about orthopedic conditions.1,2 Accordingly, the Internet has become a valuable tool in maintaining effective physician-patient communication.3-5 Given the Internet’s importance as a medium for conveying patient information, it is important that orthopedic patient education materials (PEMs) on the Internet provide high-quality information that is easily read by the target patient population. Unfortunately, studies have found that many of the Internet’s orthopedic PEMs have been neither of high quality6-8 nor presented such that they are easy for patients to read and comprehend.1,9-12
Readability, which is the reading comprehension level (school grade level) a person must have to understand written materials, is determined by systematic formulae12; readability levels correlate with the ability to comprehend written information.2 Studies have consistently found that orthopedic PEMs are written at readability levels too high for the average patient to understand.1,9,13 The readability of PEMs in orthopedics as a whole9 and within the orthopedic subspecialties of arthroplasty,1 foot and ankle surgery,2 sports medicine,12 and spine surgery13 has been evaluated, but so far there has been no evaluation of PEMs in orthopedic trauma (OT).
We conducted a study to assess the readability of OT-PEMs available online from the American Academy of Orthopaedic Surgeons (AAOS) in conjunction with the Orthopaedic Trauma Association (OTA) and other orthopedic subspecialty societies. We hypothesized the readability levels of these OT-PEMs would be above the level (sixth to eighth grade) recommended by several healthcare organizations, including the Centers for Disease Control and Prevention.9,11,14 We also assessed the effect that orthopedic subspecialty coauthorship has on PEM readability.
Methods
In July 2014, we searched the AAOS online patient education library (Broken Bones & Injuries section, http://orthoinfo.aaos.org/menus/injury.cfm) and the AAOS OrthoPortal website (Trauma section, http://pubsearch.aaos.org/search?q=trauma&client=OrthoInfo&site=PATIENT&output=xml_no_dtd&proxystylesheet=OrthoInfo&filter=0) for all relevant OT-PEMs. Although OTA does not publish its own PEMs on its website, it coauthored several of the articles in the AAOS patient education library. Other subspecialty organizations, including the American Orthopaedic Society for Sports Medicine (AOSSM), the American Society for Surgery of the Hand (ASSH), the Pediatric Orthopaedic Society of North America (POSNA), the American Shoulder and Elbow Surgeons (ASES), the American Association of Hip and Knee Surgeons (AAHKS), and the American Orthopaedic Foot and Ankle Society (AOFAS), coauthored several of these online OT-PEMs as well.
Using the technique described by Badarudeen and Sabharwal,10 we saved all articles to be included in the study as separate Microsoft Word 2011 files. We saved them in plain-text format to remove any HTML tags and any other hidden formatting that might affect readability results. Then we edited them to remove elements that might affect readability result accuracy—deleted article topic–unrelated information (eg, copyright notice, disclaimers, author information) and all numerals, decimal points, bullets, abbreviations, paragraph breaks, colons, semicolons, and dashes.10Mr. Mohan used the Flesch-Kincaid (FK) Readability Scale to calculate grade level for each article. Microsoft Word 2011 was used as described in other investigations of orthopedic PEM readability2,10,12,13: Its readability function is enabled by going to the Tools tab and then to the Spelling & Grammar tool, where the “Show readability statistics” option is selected.10 Readability scores are calculated with the Spelling & Grammar tool; the readability score is displayed after completion of the spelling-and-grammar check. The formula used to calculate FK grade level is15: (0.39 × average number of words per sentence) + (11.8 × average number of syllables per word) – 15.59.
Statistical Analysis
Descriptive statistics, including means and 95% confidence intervals (CIs), were calculated for the FK grade levels. Student t tests were used to compare average FK grade levels of articles written exclusively by AAOS with those of articles coauthored by AAOS and other orthopedic subspecialty societies. A 2-sample unequal-variance t test was used, and significance was set at P < .05. Total number of articles written at or below the sixth- and eighth-grade levels, the reading levels recommended for PEMs, were tabulated.1,9-12 Intraobserver and interobserver reliabilities were calculated with intraclass correlation coefficients (ICCs): Mr. Mohan, who calculated the FK scores earlier, now 1 week later calculated the readability levels of 15 randomly selected articles10,11; in addition, Mr. Mohan and Dr. Yi independently calculated the readability levels of 30 randomly selected articles.10,11 The same method described earlier—edit plain-text files, then use Microsoft Word to obtain FK scores—was again used. ICCs of 0 to 0.24 correspond to poor correlation; 0.25 to 0.49, low correlation; 0.5 to 0.69, fair correlation; 0.7 to 0.89, good correlation; and 0.9 to 1.0, excellent correlation.10,11 All statistical analyses were performed with Microsoft Excel 2011 and VassarStats (http://vassarstats.net/tu.html).
Results
Of the 115 AAOS website articles included in the study and reviewed, 18 were coauthored by OTA, 10 by AOSSM, 14 by POSNA, 2 by ASSH, 2 by ASES, 1 by AAHKS, 3 by AOFAS, 1 by AOSSM and ASES, and 1 by AOFAS and AOSSM.
Mean FK grade level was 9.1 (range, 6.2-12; 95% CI, 8.9-9.3) for all articles reviewed and 9.1 (range, 6.2-12; 95% CI, 8.8-9.4) for articles exclusively written by AAOS. For coauthored articles, mean FK grade level was 9.3 (range, 7.6-11.3; 95% CI, 8.8-9.8) for AAOS-OTA; 8.9 (range, 7.4-10.4; 95% CI, 8.4-9.6) for AAOS-AOSSM; 9.4 (range, 7-11.8; 95% CI, 8.9-10.1) for AAOS-POSNA; 7.8 (range, 7.8-9.1; 95% CI, 7.2-9.8) for AAOS-ASSH; 9 (range, 8.2-9.6; 95% CI, 7.6-10.2) for AAOS-ASES; 9 (range, 7.9-9; 95% CI, 7.9-9.3) for AAOS-AOFAS; 8.1 for the 1 AAOS-AAHKS article; 8.5 for the 1 AAOS-AOSSM-ASES article; and 8 for the 1 AAOS-AOFAS-AOSSM article (Figure).
For FK readability calculations, interobserver reliability (ICC, 0.9982) and intraobserver reliability (ICC, 1) were both excellent.
Discussion
Although increasing numbers of patients are using information from the Internet to inform their healthcare decisions,12 studies have shown that online PEMs are written at a readability level above that of the average patient.1,9,13 In the present study, we also found that OT-PEMs from AAOS are written at a level considerably higher than the recommended sixth-grade reading level,16 potentially impairing patient comprehension and leading to poorer health outcomes.17
The pervasiveness of too-high PEM readability levels has been found across orthopedic subspecialties.2,9,12,13 Following this trend, the OT articles we reviewed had a ninth-grade reading level on average, and only 1 of 115 articles was below the recommended sixth-grade level.10 The issue of too-high PEM readability levels is thus a problem both in OT and in orthopedics in general. Accordingly, efforts to address this problem are warranted, especially as orthopedic PEM readability has not substantially improved over the past several years.18In this study, we also tried to identify any readability differences between articles coauthored by orthopedic societies and articles that were not coauthored by orthopedic societies. We hypothesized that multidisciplinary authorship could improve PEM readability; for example, orthopedic societies could collaborate with other medical specialties (eg, family medicine) that have produced appropriately readable PEMs. One study found that the majority of PEMs from the American Academy of Family Physicians (AAFP) were written below the sixth-grade reading level because of strict organizational regulation of the production of such materials.19 By noting and adopting successful PEM development methods used by groups such as AAFP,19,20 we might be able to improve OT-PEM readability. However, this was not the case in our study, though our observations may have been limited by the small sample of reviewable articles.
One factor contributing to the poor readability of orthopedic PEMs is that orthopedics terminology is complex and includes words that are often difficult to translate into simpler terms without losing their meaning.10 When PEMs are written at a level that is too complex, patients cannot fully comprehend them, which may lead to poor health literacy. This problem may be even more harmful when considering the poor literacy levels of patients at baseline. Kadakia and colleagues16 found that OT patients had poor health literacy; for example, fewer than half knew which bone they fractured. As health literacy is associated with poorer health outcomes and reduced use of healthcare services,21 optimizing patients’ health literacy is of crucial importance to both their education and their outcomes.
Our study should be viewed in light of some important limitations. As OTA does not publish its own PEMs, we assessed only OT-related articles that were available on the AAOS website and were exclusively written by AAOS, or coauthored by AAOS and by OTA and/or another orthopedic subspecialty organization. As these articles represent only a subset of the full spectrum of OT-PEMs available on the Internet, our results may not be generalizable to the entire scope of such materials. However, as AAOS and OTA represent the most authoritative OT organizations, we think these PEMs would be among those most likely to be recommended to patients by their surgeons. In addition, although we used a well-established tool for examining readability—the FK readability scale10-13—this tool has its own inherent limitations, as FK readability grade level is calculated purely on the basis of words per sentence and total syllables per word, and does not take into account other article elements, such as images, which also provide information.1,10 Nevertheless, the FK scale is an inexpensive, easily accessed readability tool that provides a reproducible readability value that is easily comparable to results from earlier studies.10 The final limitation is that we excluded from the study AAOS website articles written in a language other than English. Such articles, however, are important, as a large portion of the patient population speaks English as a second language. Indeed, the readability of Spanish PEMs has been investigated—albeit using a readability measure other than the FK scale—and may be a topic pertinent to orthopedic PEMs.22Most of the literature on the readability of orthopedic PEMs has found their reading levels too high for the average patient to comprehend.1,9-12 The trend continues with our study findings regarding OT-PEMs available online from AAOS. Although the literature on the inadequacies of orthopedic PEMs is vast,1,9-12 more work is needed to improve the quality, accuracy, and readability of these materials. There has been some success in improving PEM readability and producing appropriately readable materials within the medical profession,19,23 so we know that appropriately readable orthopedic PEMs are feasible.
Am J Orthop. 2017;46(3):E190-E194. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Polishchuk DL, Hashem J, Sabharwal S. Readability of online patient education materials on adult reconstruction web sites. J Arthroplasty. 2012;27(5):716-719.
2. Bluman EM, Foley RP, Chiodo CP. Readability of the patient education section of the AOFAS website. Foot Ankle Int. 2009;30(4):287-291.
3. Hoffmann T, Russell T. Pre-admission orthopaedic occupational therapy home visits conducted using the Internet. J Telemed Telecare. 2008;14(2):83-87.
4. Rider T, Malik M, Chevassut T. Haematology patients and the Internet—the use of on-line health information and the impact on the patient–doctor relationship. Patient Educ Couns. 2014;97(2):223-238.
5. AlGhamdi KM, Moussa NA. Internet use by the public to search for health-related information. Int J Med Inform. 2012;81(6):363-373.
6. Beredjiklian PK, Bozentka DJ, Steinberg DR, Bernstein J. Evaluating the source and content of orthopaedic information on the Internet. The case of carpal tunnel syndrome. J Bone Joint Surg Am. 2000;82(11):1540-1543.
7. Meena S, Palaniswamy A, Chowdhury B. Web-based information on minimally invasive total knee arthroplasty. J Orthop Surg (Hong Kong). 2013;21(3):305-307.
8. Labovitch RS, Bozic KJ, Hansen E. An evaluation of information available on the Internet regarding minimally invasive hip arthroplasty. J Arthroplasty. 2006;21(1):1-5.
9. Badarudeen S, Sabharwal S. Assessing readability of patient education materials: current role in orthopaedics. Clin Orthop Relat Res. 2010;468(10):2572-2580.
10. Badarudeen S, Sabharwal S. Readability of patient education materials from the American Academy of Orthopaedic Surgeons and Pediatric Orthopaedic Society of North America web sites. J Bone Joint Surg Am. 2008;90(1):199-204.
11. Yi PH, Ganta A, Hussein KI, Frank RM, Jawa A. Readability of arthroscopy-related patient education materials from the American Academy of Orthopaedic Surgeons and Arthroscopy Association of North America web sites. Arthroscopy. 2013;29(6):1108-1112.
12. Ganta A, Yi PH, Hussein K, Frank RM. Readability of sports medicine–related patient education materials from the American Academy of Orthopaedic Surgeons and the American Orthopaedic Society for Sports Medicine. Am J Orthop. 2014;43(4):E65-E68.
13. Vives M, Young L, Sabharwal S. Readability of spine-related patient education materials from subspecialty organization and spine practitioner websites. Spine. 2009;34(25):2826-2831.
14. Strategic and Proactive Communication Branch, Division of Communication Services, Office of the Associate Director for Communication, Centers for Disease Control and Prevention, US Department of Health and Human Services. Simply Put: A Guide for Creating Easy-to-Understand Materials. 3rd ed. http://www.cdc.gov/healthliteracy/pdf/Simply_Put.pdf. Published July 2010. Accessed February 7, 2015.
15. Wallace LS, Keenum AJ, DeVoe JE. Evaluation of consumer medical information and oral liquid measuring devices accompanying pediatric prescriptions. Acad Pediatr. 2010;10(4):224-227.
16. Kadakia RJ, Tsahakis JM, Issar NM, et al. Health literacy in an orthopedic trauma patient population: a cross-sectional survey of patient comprehension. J Orthop Trauma. 2013;27(8):467-471.
17. Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695-1701.
18. Feghhi DP, Agarwal N, Hansberry DR, Berberian WS, Sabharwal S. Critical review of patient education materials from the American Academy of Orthopaedic Surgeons. Am J Orthop. 2014;43(8):E168-E174.
19. Schoof ML, Wallace LS. Readability of American Academy of Family Physicians patient education materials. Fam Med. 2014;46(4):291-293.
20. Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: Lippincott; 1996.
21. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
22. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
23. Sheppard ED, Hyde Z, Florence MN, McGwin G, Kirchner JS, Ponce BA. Improving the readability of online foot and ankle patient education materials. Foot Ankle Int. 2014;35(12):1282-1286.
Take-Home Points
- The Flesch-Kincaid Readability Scale is a useful tool in evaluating the readability of PEMs.
- Only 1 article analyzed in our study was below a sixth-grade readability level.
- Coauthorship of PEMs with other subspecialty groups had no effect on readability.
- Poor health literacy has been associated with poor health outcomes.
- Efforts must be undertaken to make PEMs more readable across medical subspecialties.
Patients increasingly turn to the Internet to self-educate about orthopedic conditions.1,2 Accordingly, the Internet has become a valuable tool in maintaining effective physician-patient communication.3-5 Given the Internet’s importance as a medium for conveying patient information, it is important that orthopedic patient education materials (PEMs) on the Internet provide high-quality information that is easily read by the target patient population. Unfortunately, studies have found that many of the Internet’s orthopedic PEMs have been neither of high quality6-8 nor presented such that they are easy for patients to read and comprehend.1,9-12
Readability, which is the reading comprehension level (school grade level) a person must have to understand written materials, is determined by systematic formulae12; readability levels correlate with the ability to comprehend written information.2 Studies have consistently found that orthopedic PEMs are written at readability levels too high for the average patient to understand.1,9,13 The readability of PEMs in orthopedics as a whole9 and within the orthopedic subspecialties of arthroplasty,1 foot and ankle surgery,2 sports medicine,12 and spine surgery13 has been evaluated, but so far there has been no evaluation of PEMs in orthopedic trauma (OT).
We conducted a study to assess the readability of OT-PEMs available online from the American Academy of Orthopaedic Surgeons (AAOS) in conjunction with the Orthopaedic Trauma Association (OTA) and other orthopedic subspecialty societies. We hypothesized the readability levels of these OT-PEMs would be above the level (sixth to eighth grade) recommended by several healthcare organizations, including the Centers for Disease Control and Prevention.9,11,14 We also assessed the effect that orthopedic subspecialty coauthorship has on PEM readability.
Methods
In July 2014, we searched the AAOS online patient education library (Broken Bones & Injuries section, http://orthoinfo.aaos.org/menus/injury.cfm) and the AAOS OrthoPortal website (Trauma section, http://pubsearch.aaos.org/search?q=trauma&client=OrthoInfo&site=PATIENT&output=xml_no_dtd&proxystylesheet=OrthoInfo&filter=0) for all relevant OT-PEMs. Although OTA does not publish its own PEMs on its website, it coauthored several of the articles in the AAOS patient education library. Other subspecialty organizations, including the American Orthopaedic Society for Sports Medicine (AOSSM), the American Society for Surgery of the Hand (ASSH), the Pediatric Orthopaedic Society of North America (POSNA), the American Shoulder and Elbow Surgeons (ASES), the American Association of Hip and Knee Surgeons (AAHKS), and the American Orthopaedic Foot and Ankle Society (AOFAS), coauthored several of these online OT-PEMs as well.
Using the technique described by Badarudeen and Sabharwal,10 we saved all articles to be included in the study as separate Microsoft Word 2011 files. We saved them in plain-text format to remove any HTML tags and any other hidden formatting that might affect readability results. Then we edited them to remove elements that might affect readability result accuracy—deleted article topic–unrelated information (eg, copyright notice, disclaimers, author information) and all numerals, decimal points, bullets, abbreviations, paragraph breaks, colons, semicolons, and dashes.10Mr. Mohan used the Flesch-Kincaid (FK) Readability Scale to calculate grade level for each article. Microsoft Word 2011 was used as described in other investigations of orthopedic PEM readability2,10,12,13: Its readability function is enabled by going to the Tools tab and then to the Spelling & Grammar tool, where the “Show readability statistics” option is selected.10 Readability scores are calculated with the Spelling & Grammar tool; the readability score is displayed after completion of the spelling-and-grammar check. The formula used to calculate FK grade level is15: (0.39 × average number of words per sentence) + (11.8 × average number of syllables per word) – 15.59.
Statistical Analysis
Descriptive statistics, including means and 95% confidence intervals (CIs), were calculated for the FK grade levels. Student t tests were used to compare average FK grade levels of articles written exclusively by AAOS with those of articles coauthored by AAOS and other orthopedic subspecialty societies. A 2-sample unequal-variance t test was used, and significance was set at P < .05. Total number of articles written at or below the sixth- and eighth-grade levels, the reading levels recommended for PEMs, were tabulated.1,9-12 Intraobserver and interobserver reliabilities were calculated with intraclass correlation coefficients (ICCs): Mr. Mohan, who calculated the FK scores earlier, now 1 week later calculated the readability levels of 15 randomly selected articles10,11; in addition, Mr. Mohan and Dr. Yi independently calculated the readability levels of 30 randomly selected articles.10,11 The same method described earlier—edit plain-text files, then use Microsoft Word to obtain FK scores—was again used. ICCs of 0 to 0.24 correspond to poor correlation; 0.25 to 0.49, low correlation; 0.5 to 0.69, fair correlation; 0.7 to 0.89, good correlation; and 0.9 to 1.0, excellent correlation.10,11 All statistical analyses were performed with Microsoft Excel 2011 and VassarStats (http://vassarstats.net/tu.html).
Results
Of the 115 AAOS website articles included in the study and reviewed, 18 were coauthored by OTA, 10 by AOSSM, 14 by POSNA, 2 by ASSH, 2 by ASES, 1 by AAHKS, 3 by AOFAS, 1 by AOSSM and ASES, and 1 by AOFAS and AOSSM.
Mean FK grade level was 9.1 (range, 6.2-12; 95% CI, 8.9-9.3) for all articles reviewed and 9.1 (range, 6.2-12; 95% CI, 8.8-9.4) for articles exclusively written by AAOS. For coauthored articles, mean FK grade level was 9.3 (range, 7.6-11.3; 95% CI, 8.8-9.8) for AAOS-OTA; 8.9 (range, 7.4-10.4; 95% CI, 8.4-9.6) for AAOS-AOSSM; 9.4 (range, 7-11.8; 95% CI, 8.9-10.1) for AAOS-POSNA; 7.8 (range, 7.8-9.1; 95% CI, 7.2-9.8) for AAOS-ASSH; 9 (range, 8.2-9.6; 95% CI, 7.6-10.2) for AAOS-ASES; 9 (range, 7.9-9; 95% CI, 7.9-9.3) for AAOS-AOFAS; 8.1 for the 1 AAOS-AAHKS article; 8.5 for the 1 AAOS-AOSSM-ASES article; and 8 for the 1 AAOS-AOFAS-AOSSM article (Figure).
For FK readability calculations, interobserver reliability (ICC, 0.9982) and intraobserver reliability (ICC, 1) were both excellent.
Discussion
Although increasing numbers of patients are using information from the Internet to inform their healthcare decisions,12 studies have shown that online PEMs are written at a readability level above that of the average patient.1,9,13 In the present study, we also found that OT-PEMs from AAOS are written at a level considerably higher than the recommended sixth-grade reading level,16 potentially impairing patient comprehension and leading to poorer health outcomes.17
The pervasiveness of too-high PEM readability levels has been found across orthopedic subspecialties.2,9,12,13 Following this trend, the OT articles we reviewed had a ninth-grade reading level on average, and only 1 of 115 articles was below the recommended sixth-grade level.10 The issue of too-high PEM readability levels is thus a problem both in OT and in orthopedics in general. Accordingly, efforts to address this problem are warranted, especially as orthopedic PEM readability has not substantially improved over the past several years.18In this study, we also tried to identify any readability differences between articles coauthored by orthopedic societies and articles that were not coauthored by orthopedic societies. We hypothesized that multidisciplinary authorship could improve PEM readability; for example, orthopedic societies could collaborate with other medical specialties (eg, family medicine) that have produced appropriately readable PEMs. One study found that the majority of PEMs from the American Academy of Family Physicians (AAFP) were written below the sixth-grade reading level because of strict organizational regulation of the production of such materials.19 By noting and adopting successful PEM development methods used by groups such as AAFP,19,20 we might be able to improve OT-PEM readability. However, this was not the case in our study, though our observations may have been limited by the small sample of reviewable articles.
One factor contributing to the poor readability of orthopedic PEMs is that orthopedics terminology is complex and includes words that are often difficult to translate into simpler terms without losing their meaning.10 When PEMs are written at a level that is too complex, patients cannot fully comprehend them, which may lead to poor health literacy. This problem may be even more harmful when considering the poor literacy levels of patients at baseline. Kadakia and colleagues16 found that OT patients had poor health literacy; for example, fewer than half knew which bone they fractured. As health literacy is associated with poorer health outcomes and reduced use of healthcare services,21 optimizing patients’ health literacy is of crucial importance to both their education and their outcomes.
Our study should be viewed in light of some important limitations. As OTA does not publish its own PEMs, we assessed only OT-related articles that were available on the AAOS website and were exclusively written by AAOS, or coauthored by AAOS and by OTA and/or another orthopedic subspecialty organization. As these articles represent only a subset of the full spectrum of OT-PEMs available on the Internet, our results may not be generalizable to the entire scope of such materials. However, as AAOS and OTA represent the most authoritative OT organizations, we think these PEMs would be among those most likely to be recommended to patients by their surgeons. In addition, although we used a well-established tool for examining readability—the FK readability scale10-13—this tool has its own inherent limitations, as FK readability grade level is calculated purely on the basis of words per sentence and total syllables per word, and does not take into account other article elements, such as images, which also provide information.1,10 Nevertheless, the FK scale is an inexpensive, easily accessed readability tool that provides a reproducible readability value that is easily comparable to results from earlier studies.10 The final limitation is that we excluded from the study AAOS website articles written in a language other than English. Such articles, however, are important, as a large portion of the patient population speaks English as a second language. Indeed, the readability of Spanish PEMs has been investigated—albeit using a readability measure other than the FK scale—and may be a topic pertinent to orthopedic PEMs.22Most of the literature on the readability of orthopedic PEMs has found their reading levels too high for the average patient to comprehend.1,9-12 The trend continues with our study findings regarding OT-PEMs available online from AAOS. Although the literature on the inadequacies of orthopedic PEMs is vast,1,9-12 more work is needed to improve the quality, accuracy, and readability of these materials. There has been some success in improving PEM readability and producing appropriately readable materials within the medical profession,19,23 so we know that appropriately readable orthopedic PEMs are feasible.
Am J Orthop. 2017;46(3):E190-E194. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The Flesch-Kincaid Readability Scale is a useful tool in evaluating the readability of PEMs.
- Only 1 article analyzed in our study was below a sixth-grade readability level.
- Coauthorship of PEMs with other subspecialty groups had no effect on readability.
- Poor health literacy has been associated with poor health outcomes.
- Efforts must be undertaken to make PEMs more readable across medical subspecialties.
Patients increasingly turn to the Internet to self-educate about orthopedic conditions.1,2 Accordingly, the Internet has become a valuable tool in maintaining effective physician-patient communication.3-5 Given the Internet’s importance as a medium for conveying patient information, it is important that orthopedic patient education materials (PEMs) on the Internet provide high-quality information that is easily read by the target patient population. Unfortunately, studies have found that many of the Internet’s orthopedic PEMs have been neither of high quality6-8 nor presented such that they are easy for patients to read and comprehend.1,9-12
Readability, which is the reading comprehension level (school grade level) a person must have to understand written materials, is determined by systematic formulae12; readability levels correlate with the ability to comprehend written information.2 Studies have consistently found that orthopedic PEMs are written at readability levels too high for the average patient to understand.1,9,13 The readability of PEMs in orthopedics as a whole9 and within the orthopedic subspecialties of arthroplasty,1 foot and ankle surgery,2 sports medicine,12 and spine surgery13 has been evaluated, but so far there has been no evaluation of PEMs in orthopedic trauma (OT).
We conducted a study to assess the readability of OT-PEMs available online from the American Academy of Orthopaedic Surgeons (AAOS) in conjunction with the Orthopaedic Trauma Association (OTA) and other orthopedic subspecialty societies. We hypothesized the readability levels of these OT-PEMs would be above the level (sixth to eighth grade) recommended by several healthcare organizations, including the Centers for Disease Control and Prevention.9,11,14 We also assessed the effect that orthopedic subspecialty coauthorship has on PEM readability.
Methods
In July 2014, we searched the AAOS online patient education library (Broken Bones & Injuries section, http://orthoinfo.aaos.org/menus/injury.cfm) and the AAOS OrthoPortal website (Trauma section, http://pubsearch.aaos.org/search?q=trauma&client=OrthoInfo&site=PATIENT&output=xml_no_dtd&proxystylesheet=OrthoInfo&filter=0) for all relevant OT-PEMs. Although OTA does not publish its own PEMs on its website, it coauthored several of the articles in the AAOS patient education library. Other subspecialty organizations, including the American Orthopaedic Society for Sports Medicine (AOSSM), the American Society for Surgery of the Hand (ASSH), the Pediatric Orthopaedic Society of North America (POSNA), the American Shoulder and Elbow Surgeons (ASES), the American Association of Hip and Knee Surgeons (AAHKS), and the American Orthopaedic Foot and Ankle Society (AOFAS), coauthored several of these online OT-PEMs as well.
Using the technique described by Badarudeen and Sabharwal,10 we saved all articles to be included in the study as separate Microsoft Word 2011 files. We saved them in plain-text format to remove any HTML tags and any other hidden formatting that might affect readability results. Then we edited them to remove elements that might affect readability result accuracy—deleted article topic–unrelated information (eg, copyright notice, disclaimers, author information) and all numerals, decimal points, bullets, abbreviations, paragraph breaks, colons, semicolons, and dashes.10Mr. Mohan used the Flesch-Kincaid (FK) Readability Scale to calculate grade level for each article. Microsoft Word 2011 was used as described in other investigations of orthopedic PEM readability2,10,12,13: Its readability function is enabled by going to the Tools tab and then to the Spelling & Grammar tool, where the “Show readability statistics” option is selected.10 Readability scores are calculated with the Spelling & Grammar tool; the readability score is displayed after completion of the spelling-and-grammar check. The formula used to calculate FK grade level is15: (0.39 × average number of words per sentence) + (11.8 × average number of syllables per word) – 15.59.
Statistical Analysis
Descriptive statistics, including means and 95% confidence intervals (CIs), were calculated for the FK grade levels. Student t tests were used to compare average FK grade levels of articles written exclusively by AAOS with those of articles coauthored by AAOS and other orthopedic subspecialty societies. A 2-sample unequal-variance t test was used, and significance was set at P < .05. Total number of articles written at or below the sixth- and eighth-grade levels, the reading levels recommended for PEMs, were tabulated.1,9-12 Intraobserver and interobserver reliabilities were calculated with intraclass correlation coefficients (ICCs): Mr. Mohan, who calculated the FK scores earlier, now 1 week later calculated the readability levels of 15 randomly selected articles10,11; in addition, Mr. Mohan and Dr. Yi independently calculated the readability levels of 30 randomly selected articles.10,11 The same method described earlier—edit plain-text files, then use Microsoft Word to obtain FK scores—was again used. ICCs of 0 to 0.24 correspond to poor correlation; 0.25 to 0.49, low correlation; 0.5 to 0.69, fair correlation; 0.7 to 0.89, good correlation; and 0.9 to 1.0, excellent correlation.10,11 All statistical analyses were performed with Microsoft Excel 2011 and VassarStats (http://vassarstats.net/tu.html).
Results
Of the 115 AAOS website articles included in the study and reviewed, 18 were coauthored by OTA, 10 by AOSSM, 14 by POSNA, 2 by ASSH, 2 by ASES, 1 by AAHKS, 3 by AOFAS, 1 by AOSSM and ASES, and 1 by AOFAS and AOSSM.
Mean FK grade level was 9.1 (range, 6.2-12; 95% CI, 8.9-9.3) for all articles reviewed and 9.1 (range, 6.2-12; 95% CI, 8.8-9.4) for articles exclusively written by AAOS. For coauthored articles, mean FK grade level was 9.3 (range, 7.6-11.3; 95% CI, 8.8-9.8) for AAOS-OTA; 8.9 (range, 7.4-10.4; 95% CI, 8.4-9.6) for AAOS-AOSSM; 9.4 (range, 7-11.8; 95% CI, 8.9-10.1) for AAOS-POSNA; 7.8 (range, 7.8-9.1; 95% CI, 7.2-9.8) for AAOS-ASSH; 9 (range, 8.2-9.6; 95% CI, 7.6-10.2) for AAOS-ASES; 9 (range, 7.9-9; 95% CI, 7.9-9.3) for AAOS-AOFAS; 8.1 for the 1 AAOS-AAHKS article; 8.5 for the 1 AAOS-AOSSM-ASES article; and 8 for the 1 AAOS-AOFAS-AOSSM article (Figure).
For FK readability calculations, interobserver reliability (ICC, 0.9982) and intraobserver reliability (ICC, 1) were both excellent.
Discussion
Although increasing numbers of patients are using information from the Internet to inform their healthcare decisions,12 studies have shown that online PEMs are written at a readability level above that of the average patient.1,9,13 In the present study, we also found that OT-PEMs from AAOS are written at a level considerably higher than the recommended sixth-grade reading level,16 potentially impairing patient comprehension and leading to poorer health outcomes.17
The pervasiveness of too-high PEM readability levels has been found across orthopedic subspecialties.2,9,12,13 Following this trend, the OT articles we reviewed had a ninth-grade reading level on average, and only 1 of 115 articles was below the recommended sixth-grade level.10 The issue of too-high PEM readability levels is thus a problem both in OT and in orthopedics in general. Accordingly, efforts to address this problem are warranted, especially as orthopedic PEM readability has not substantially improved over the past several years.18In this study, we also tried to identify any readability differences between articles coauthored by orthopedic societies and articles that were not coauthored by orthopedic societies. We hypothesized that multidisciplinary authorship could improve PEM readability; for example, orthopedic societies could collaborate with other medical specialties (eg, family medicine) that have produced appropriately readable PEMs. One study found that the majority of PEMs from the American Academy of Family Physicians (AAFP) were written below the sixth-grade reading level because of strict organizational regulation of the production of such materials.19 By noting and adopting successful PEM development methods used by groups such as AAFP,19,20 we might be able to improve OT-PEM readability. However, this was not the case in our study, though our observations may have been limited by the small sample of reviewable articles.
One factor contributing to the poor readability of orthopedic PEMs is that orthopedics terminology is complex and includes words that are often difficult to translate into simpler terms without losing their meaning.10 When PEMs are written at a level that is too complex, patients cannot fully comprehend them, which may lead to poor health literacy. This problem may be even more harmful when considering the poor literacy levels of patients at baseline. Kadakia and colleagues16 found that OT patients had poor health literacy; for example, fewer than half knew which bone they fractured. As health literacy is associated with poorer health outcomes and reduced use of healthcare services,21 optimizing patients’ health literacy is of crucial importance to both their education and their outcomes.
Our study should be viewed in light of some important limitations. As OTA does not publish its own PEMs, we assessed only OT-related articles that were available on the AAOS website and were exclusively written by AAOS, or coauthored by AAOS and by OTA and/or another orthopedic subspecialty organization. As these articles represent only a subset of the full spectrum of OT-PEMs available on the Internet, our results may not be generalizable to the entire scope of such materials. However, as AAOS and OTA represent the most authoritative OT organizations, we think these PEMs would be among those most likely to be recommended to patients by their surgeons. In addition, although we used a well-established tool for examining readability—the FK readability scale10-13—this tool has its own inherent limitations, as FK readability grade level is calculated purely on the basis of words per sentence and total syllables per word, and does not take into account other article elements, such as images, which also provide information.1,10 Nevertheless, the FK scale is an inexpensive, easily accessed readability tool that provides a reproducible readability value that is easily comparable to results from earlier studies.10 The final limitation is that we excluded from the study AAOS website articles written in a language other than English. Such articles, however, are important, as a large portion of the patient population speaks English as a second language. Indeed, the readability of Spanish PEMs has been investigated—albeit using a readability measure other than the FK scale—and may be a topic pertinent to orthopedic PEMs.22Most of the literature on the readability of orthopedic PEMs has found their reading levels too high for the average patient to comprehend.1,9-12 The trend continues with our study findings regarding OT-PEMs available online from AAOS. Although the literature on the inadequacies of orthopedic PEMs is vast,1,9-12 more work is needed to improve the quality, accuracy, and readability of these materials. There has been some success in improving PEM readability and producing appropriately readable materials within the medical profession,19,23 so we know that appropriately readable orthopedic PEMs are feasible.
Am J Orthop. 2017;46(3):E190-E194. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Polishchuk DL, Hashem J, Sabharwal S. Readability of online patient education materials on adult reconstruction web sites. J Arthroplasty. 2012;27(5):716-719.
2. Bluman EM, Foley RP, Chiodo CP. Readability of the patient education section of the AOFAS website. Foot Ankle Int. 2009;30(4):287-291.
3. Hoffmann T, Russell T. Pre-admission orthopaedic occupational therapy home visits conducted using the Internet. J Telemed Telecare. 2008;14(2):83-87.
4. Rider T, Malik M, Chevassut T. Haematology patients and the Internet—the use of on-line health information and the impact on the patient–doctor relationship. Patient Educ Couns. 2014;97(2):223-238.
5. AlGhamdi KM, Moussa NA. Internet use by the public to search for health-related information. Int J Med Inform. 2012;81(6):363-373.
6. Beredjiklian PK, Bozentka DJ, Steinberg DR, Bernstein J. Evaluating the source and content of orthopaedic information on the Internet. The case of carpal tunnel syndrome. J Bone Joint Surg Am. 2000;82(11):1540-1543.
7. Meena S, Palaniswamy A, Chowdhury B. Web-based information on minimally invasive total knee arthroplasty. J Orthop Surg (Hong Kong). 2013;21(3):305-307.
8. Labovitch RS, Bozic KJ, Hansen E. An evaluation of information available on the Internet regarding minimally invasive hip arthroplasty. J Arthroplasty. 2006;21(1):1-5.
9. Badarudeen S, Sabharwal S. Assessing readability of patient education materials: current role in orthopaedics. Clin Orthop Relat Res. 2010;468(10):2572-2580.
10. Badarudeen S, Sabharwal S. Readability of patient education materials from the American Academy of Orthopaedic Surgeons and Pediatric Orthopaedic Society of North America web sites. J Bone Joint Surg Am. 2008;90(1):199-204.
11. Yi PH, Ganta A, Hussein KI, Frank RM, Jawa A. Readability of arthroscopy-related patient education materials from the American Academy of Orthopaedic Surgeons and Arthroscopy Association of North America web sites. Arthroscopy. 2013;29(6):1108-1112.
12. Ganta A, Yi PH, Hussein K, Frank RM. Readability of sports medicine–related patient education materials from the American Academy of Orthopaedic Surgeons and the American Orthopaedic Society for Sports Medicine. Am J Orthop. 2014;43(4):E65-E68.
13. Vives M, Young L, Sabharwal S. Readability of spine-related patient education materials from subspecialty organization and spine practitioner websites. Spine. 2009;34(25):2826-2831.
14. Strategic and Proactive Communication Branch, Division of Communication Services, Office of the Associate Director for Communication, Centers for Disease Control and Prevention, US Department of Health and Human Services. Simply Put: A Guide for Creating Easy-to-Understand Materials. 3rd ed. http://www.cdc.gov/healthliteracy/pdf/Simply_Put.pdf. Published July 2010. Accessed February 7, 2015.
15. Wallace LS, Keenum AJ, DeVoe JE. Evaluation of consumer medical information and oral liquid measuring devices accompanying pediatric prescriptions. Acad Pediatr. 2010;10(4):224-227.
16. Kadakia RJ, Tsahakis JM, Issar NM, et al. Health literacy in an orthopedic trauma patient population: a cross-sectional survey of patient comprehension. J Orthop Trauma. 2013;27(8):467-471.
17. Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695-1701.
18. Feghhi DP, Agarwal N, Hansberry DR, Berberian WS, Sabharwal S. Critical review of patient education materials from the American Academy of Orthopaedic Surgeons. Am J Orthop. 2014;43(8):E168-E174.
19. Schoof ML, Wallace LS. Readability of American Academy of Family Physicians patient education materials. Fam Med. 2014;46(4):291-293.
20. Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: Lippincott; 1996.
21. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
22. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
23. Sheppard ED, Hyde Z, Florence MN, McGwin G, Kirchner JS, Ponce BA. Improving the readability of online foot and ankle patient education materials. Foot Ankle Int. 2014;35(12):1282-1286.
1. Polishchuk DL, Hashem J, Sabharwal S. Readability of online patient education materials on adult reconstruction web sites. J Arthroplasty. 2012;27(5):716-719.
2. Bluman EM, Foley RP, Chiodo CP. Readability of the patient education section of the AOFAS website. Foot Ankle Int. 2009;30(4):287-291.
3. Hoffmann T, Russell T. Pre-admission orthopaedic occupational therapy home visits conducted using the Internet. J Telemed Telecare. 2008;14(2):83-87.
4. Rider T, Malik M, Chevassut T. Haematology patients and the Internet—the use of on-line health information and the impact on the patient–doctor relationship. Patient Educ Couns. 2014;97(2):223-238.
5. AlGhamdi KM, Moussa NA. Internet use by the public to search for health-related information. Int J Med Inform. 2012;81(6):363-373.
6. Beredjiklian PK, Bozentka DJ, Steinberg DR, Bernstein J. Evaluating the source and content of orthopaedic information on the Internet. The case of carpal tunnel syndrome. J Bone Joint Surg Am. 2000;82(11):1540-1543.
7. Meena S, Palaniswamy A, Chowdhury B. Web-based information on minimally invasive total knee arthroplasty. J Orthop Surg (Hong Kong). 2013;21(3):305-307.
8. Labovitch RS, Bozic KJ, Hansen E. An evaluation of information available on the Internet regarding minimally invasive hip arthroplasty. J Arthroplasty. 2006;21(1):1-5.
9. Badarudeen S, Sabharwal S. Assessing readability of patient education materials: current role in orthopaedics. Clin Orthop Relat Res. 2010;468(10):2572-2580.
10. Badarudeen S, Sabharwal S. Readability of patient education materials from the American Academy of Orthopaedic Surgeons and Pediatric Orthopaedic Society of North America web sites. J Bone Joint Surg Am. 2008;90(1):199-204.
11. Yi PH, Ganta A, Hussein KI, Frank RM, Jawa A. Readability of arthroscopy-related patient education materials from the American Academy of Orthopaedic Surgeons and Arthroscopy Association of North America web sites. Arthroscopy. 2013;29(6):1108-1112.
12. Ganta A, Yi PH, Hussein K, Frank RM. Readability of sports medicine–related patient education materials from the American Academy of Orthopaedic Surgeons and the American Orthopaedic Society for Sports Medicine. Am J Orthop. 2014;43(4):E65-E68.
13. Vives M, Young L, Sabharwal S. Readability of spine-related patient education materials from subspecialty organization and spine practitioner websites. Spine. 2009;34(25):2826-2831.
14. Strategic and Proactive Communication Branch, Division of Communication Services, Office of the Associate Director for Communication, Centers for Disease Control and Prevention, US Department of Health and Human Services. Simply Put: A Guide for Creating Easy-to-Understand Materials. 3rd ed. http://www.cdc.gov/healthliteracy/pdf/Simply_Put.pdf. Published July 2010. Accessed February 7, 2015.
15. Wallace LS, Keenum AJ, DeVoe JE. Evaluation of consumer medical information and oral liquid measuring devices accompanying pediatric prescriptions. Acad Pediatr. 2010;10(4):224-227.
16. Kadakia RJ, Tsahakis JM, Issar NM, et al. Health literacy in an orthopedic trauma patient population: a cross-sectional survey of patient comprehension. J Orthop Trauma. 2013;27(8):467-471.
17. Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695-1701.
18. Feghhi DP, Agarwal N, Hansberry DR, Berberian WS, Sabharwal S. Critical review of patient education materials from the American Academy of Orthopaedic Surgeons. Am J Orthop. 2014;43(8):E168-E174.
19. Schoof ML, Wallace LS. Readability of American Academy of Family Physicians patient education materials. Fam Med. 2014;46(4):291-293.
20. Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: Lippincott; 1996.
21. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
22. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
23. Sheppard ED, Hyde Z, Florence MN, McGwin G, Kirchner JS, Ponce BA. Improving the readability of online foot and ankle patient education materials. Foot Ankle Int. 2014;35(12):1282-1286.
Lower Leg Fracture Irreducibility Resulting From Entrapment of the Fibula Within the Tibial Shaft
Take-Home Points
- Preoperative orthogonal radiographs need to be carefully scrutinized in irreducible fractures.
- Open reduction is often necessary when soft-tissue or bone is interposed in a fracture site.
- The fibula’s size and interosseous connection to the tibia can lead to entrapment.
- High-energy mechanisms can lead to significant deformity at time of injury, with spontaneous partial reduction prior to initial assessment.
- Consider intramedullary entrapment of adjacent long bones.
The tibia is the most commonly fractured long bone; each year, almost 500,000 tibia fractures occur in the United States alone.1 Low-energy mechanisms of injury usually result from torsional forces and produce less comminuted fractures. Very high-energy injuries apply direct forces to the shin and are often highly comminuted or open, owing to the limited soft-tissue envelope. The extent of soft-tissue injury occurring with these fractures is the best predictor of the development of a complication, particularly nonunion or infection.1 Low-energy closed fractures with limited comminution and sufficient cortical apposition may be treated with closed reduction and casting, but the most common treatment for tibial shaft fractures is intramedullary nailing.2
In the acute setting, closed reduction allows for temporization of soft tissues and prevention of further damage to neurovascular structures. Whether eventual treatment consists of casting or intramedullary fixation, closed reduction must first be achieved.
In this article, we report a unique case of tibial shaft fracture irreducibility caused by telescoping of the distal fibula within the proximal tibial diaphysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old unrestrained driver of a tow truck rear-ended another vehicle at high speed (~45 mph) and became trapped in the vehicle. Extrication time was prolonged. The driver was brought to the emergency department at a level I trauma center, where he was found to have an obvious closed deformity of the right lower leg but remained neurovascularly intact. Radiographs showed a highly comminuted fracture of the right tibial and fibular midshaft with more than 100% medial displacement of the distal fragment (Figure 1).
The next morning, the patient was taken to the operating room for planned closed intramedullary nailing through a suprapatellar approach. After entry to the tibial medullary canal was obtained, a ball-tipped guide wire was advanced proximal to the fracture site, until significant resistance was met. With the aid of intraoperative fluoroscopic imaging, it was determined that the distal fibula fracture segment was entrapped in the medullary canal of the proximal tibia (Figure 2).
After the fibula was liberated, additional closed manipulative reduction techniques were used on the tibia to restore length, alignment, and rotation, and an appropriately sized nail was advanced distally past the fracture segment and fixed with interlocking screws proximally and distally (Figure 4).
The postoperative course was uncomplicated. At most recent follow-up, radiographs showed near anatomical alignment, and the patient was back to normal activities without use of any pain medication or assistive device.
Discussion
Although other irreducible tibia fracture patterns have been described, midshaft tibia fracture irreducibility caused by entrapment of the fibula within the intramedullary space was a previously unreported difficulty of this very common fracture. More commonly, irreducible tibia fractures are caused by entrapment of soft-tissue structures or fracture fragments.3,4
There are no previous documented cases of fracture patterns in which one long bone telescopes into another. Although not previously reported as occurring traumatically, the fibula was previously used as a vascularized graft in the intramedullary canal of the tibia and elsewhere.
The most well described irreducible fracture-dislocation of the lower leg is the Bosworth type, in which the proximal fragment of the fibula becomes displaced behind the tibia at the ankle joint. In addition, because of the torsional nature of most ankle fractures and the multitude of accompanying soft-tissue structures crossing at the joint, entrapment leading to irreducibility has had several different causes. These soft-tissue entrapment injures are often difficult to distinguish on plain radiographs and require further definition by computed tomography.
The high-energy mechanism of injury we have described is thought to result from lateral translation of the upper portion of the leg with the foot and lower leg fixed in place. We can posit that momentary hypervarus angulation of the tibia and the fibula with subsequent spontaneous partial reduction caused by tissue elasticity could lead to this unique injury pattern.
Although this is the first reported case of entrapment of the fibula within the intramedullary canal of the tibia, the injury should be considered when difficult closed reductions are encountered. It was only after attempted reduction for intramedullary nailing in the operating room that the telescoping fibula and the irreducibility were identified, and open reduction performed. There were no soft-tissue or neurovascular complications, but, had there been, they could have become of urgent concern and altered treatment.
In this case report, we have described a unique fracture pattern that could cause significant morbidity if not appropriately identified and treated in a timely manner.
Am J Orthop. 2017;46(3):E160-E162. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Karladani AH, Granhed H, Kärrholm J, Styf J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch Orthop Trauma Surg. 2001;121(6):325-328.
2. McGanity P. Tibial shaft fractures. In: Heckman JD, Schenck RC Jr, Agarwal A, eds. Current Orthopedic Diagnosis & Treatment. New York, NY: Springer; 2000:184-185.
3. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
4. Green RN, Pullagura MK, Holland JP. Irreducible fracture-dislocation of the knee. Acta Orthop Traumatol Turc. 2014;48(3):363-366.
Take-Home Points
- Preoperative orthogonal radiographs need to be carefully scrutinized in irreducible fractures.
- Open reduction is often necessary when soft-tissue or bone is interposed in a fracture site.
- The fibula’s size and interosseous connection to the tibia can lead to entrapment.
- High-energy mechanisms can lead to significant deformity at time of injury, with spontaneous partial reduction prior to initial assessment.
- Consider intramedullary entrapment of adjacent long bones.
The tibia is the most commonly fractured long bone; each year, almost 500,000 tibia fractures occur in the United States alone.1 Low-energy mechanisms of injury usually result from torsional forces and produce less comminuted fractures. Very high-energy injuries apply direct forces to the shin and are often highly comminuted or open, owing to the limited soft-tissue envelope. The extent of soft-tissue injury occurring with these fractures is the best predictor of the development of a complication, particularly nonunion or infection.1 Low-energy closed fractures with limited comminution and sufficient cortical apposition may be treated with closed reduction and casting, but the most common treatment for tibial shaft fractures is intramedullary nailing.2
In the acute setting, closed reduction allows for temporization of soft tissues and prevention of further damage to neurovascular structures. Whether eventual treatment consists of casting or intramedullary fixation, closed reduction must first be achieved.
In this article, we report a unique case of tibial shaft fracture irreducibility caused by telescoping of the distal fibula within the proximal tibial diaphysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old unrestrained driver of a tow truck rear-ended another vehicle at high speed (~45 mph) and became trapped in the vehicle. Extrication time was prolonged. The driver was brought to the emergency department at a level I trauma center, where he was found to have an obvious closed deformity of the right lower leg but remained neurovascularly intact. Radiographs showed a highly comminuted fracture of the right tibial and fibular midshaft with more than 100% medial displacement of the distal fragment (Figure 1).
The next morning, the patient was taken to the operating room for planned closed intramedullary nailing through a suprapatellar approach. After entry to the tibial medullary canal was obtained, a ball-tipped guide wire was advanced proximal to the fracture site, until significant resistance was met. With the aid of intraoperative fluoroscopic imaging, it was determined that the distal fibula fracture segment was entrapped in the medullary canal of the proximal tibia (Figure 2).
After the fibula was liberated, additional closed manipulative reduction techniques were used on the tibia to restore length, alignment, and rotation, and an appropriately sized nail was advanced distally past the fracture segment and fixed with interlocking screws proximally and distally (Figure 4).
The postoperative course was uncomplicated. At most recent follow-up, radiographs showed near anatomical alignment, and the patient was back to normal activities without use of any pain medication or assistive device.
Discussion
Although other irreducible tibia fracture patterns have been described, midshaft tibia fracture irreducibility caused by entrapment of the fibula within the intramedullary space was a previously unreported difficulty of this very common fracture. More commonly, irreducible tibia fractures are caused by entrapment of soft-tissue structures or fracture fragments.3,4
There are no previous documented cases of fracture patterns in which one long bone telescopes into another. Although not previously reported as occurring traumatically, the fibula was previously used as a vascularized graft in the intramedullary canal of the tibia and elsewhere.
The most well described irreducible fracture-dislocation of the lower leg is the Bosworth type, in which the proximal fragment of the fibula becomes displaced behind the tibia at the ankle joint. In addition, because of the torsional nature of most ankle fractures and the multitude of accompanying soft-tissue structures crossing at the joint, entrapment leading to irreducibility has had several different causes. These soft-tissue entrapment injures are often difficult to distinguish on plain radiographs and require further definition by computed tomography.
The high-energy mechanism of injury we have described is thought to result from lateral translation of the upper portion of the leg with the foot and lower leg fixed in place. We can posit that momentary hypervarus angulation of the tibia and the fibula with subsequent spontaneous partial reduction caused by tissue elasticity could lead to this unique injury pattern.
Although this is the first reported case of entrapment of the fibula within the intramedullary canal of the tibia, the injury should be considered when difficult closed reductions are encountered. It was only after attempted reduction for intramedullary nailing in the operating room that the telescoping fibula and the irreducibility were identified, and open reduction performed. There were no soft-tissue or neurovascular complications, but, had there been, they could have become of urgent concern and altered treatment.
In this case report, we have described a unique fracture pattern that could cause significant morbidity if not appropriately identified and treated in a timely manner.
Am J Orthop. 2017;46(3):E160-E162. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Preoperative orthogonal radiographs need to be carefully scrutinized in irreducible fractures.
- Open reduction is often necessary when soft-tissue or bone is interposed in a fracture site.
- The fibula’s size and interosseous connection to the tibia can lead to entrapment.
- High-energy mechanisms can lead to significant deformity at time of injury, with spontaneous partial reduction prior to initial assessment.
- Consider intramedullary entrapment of adjacent long bones.
The tibia is the most commonly fractured long bone; each year, almost 500,000 tibia fractures occur in the United States alone.1 Low-energy mechanisms of injury usually result from torsional forces and produce less comminuted fractures. Very high-energy injuries apply direct forces to the shin and are often highly comminuted or open, owing to the limited soft-tissue envelope. The extent of soft-tissue injury occurring with these fractures is the best predictor of the development of a complication, particularly nonunion or infection.1 Low-energy closed fractures with limited comminution and sufficient cortical apposition may be treated with closed reduction and casting, but the most common treatment for tibial shaft fractures is intramedullary nailing.2
In the acute setting, closed reduction allows for temporization of soft tissues and prevention of further damage to neurovascular structures. Whether eventual treatment consists of casting or intramedullary fixation, closed reduction must first be achieved.
In this article, we report a unique case of tibial shaft fracture irreducibility caused by telescoping of the distal fibula within the proximal tibial diaphysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old unrestrained driver of a tow truck rear-ended another vehicle at high speed (~45 mph) and became trapped in the vehicle. Extrication time was prolonged. The driver was brought to the emergency department at a level I trauma center, where he was found to have an obvious closed deformity of the right lower leg but remained neurovascularly intact. Radiographs showed a highly comminuted fracture of the right tibial and fibular midshaft with more than 100% medial displacement of the distal fragment (Figure 1).
The next morning, the patient was taken to the operating room for planned closed intramedullary nailing through a suprapatellar approach. After entry to the tibial medullary canal was obtained, a ball-tipped guide wire was advanced proximal to the fracture site, until significant resistance was met. With the aid of intraoperative fluoroscopic imaging, it was determined that the distal fibula fracture segment was entrapped in the medullary canal of the proximal tibia (Figure 2).
After the fibula was liberated, additional closed manipulative reduction techniques were used on the tibia to restore length, alignment, and rotation, and an appropriately sized nail was advanced distally past the fracture segment and fixed with interlocking screws proximally and distally (Figure 4).
The postoperative course was uncomplicated. At most recent follow-up, radiographs showed near anatomical alignment, and the patient was back to normal activities without use of any pain medication or assistive device.
Discussion
Although other irreducible tibia fracture patterns have been described, midshaft tibia fracture irreducibility caused by entrapment of the fibula within the intramedullary space was a previously unreported difficulty of this very common fracture. More commonly, irreducible tibia fractures are caused by entrapment of soft-tissue structures or fracture fragments.3,4
There are no previous documented cases of fracture patterns in which one long bone telescopes into another. Although not previously reported as occurring traumatically, the fibula was previously used as a vascularized graft in the intramedullary canal of the tibia and elsewhere.
The most well described irreducible fracture-dislocation of the lower leg is the Bosworth type, in which the proximal fragment of the fibula becomes displaced behind the tibia at the ankle joint. In addition, because of the torsional nature of most ankle fractures and the multitude of accompanying soft-tissue structures crossing at the joint, entrapment leading to irreducibility has had several different causes. These soft-tissue entrapment injures are often difficult to distinguish on plain radiographs and require further definition by computed tomography.
The high-energy mechanism of injury we have described is thought to result from lateral translation of the upper portion of the leg with the foot and lower leg fixed in place. We can posit that momentary hypervarus angulation of the tibia and the fibula with subsequent spontaneous partial reduction caused by tissue elasticity could lead to this unique injury pattern.
Although this is the first reported case of entrapment of the fibula within the intramedullary canal of the tibia, the injury should be considered when difficult closed reductions are encountered. It was only after attempted reduction for intramedullary nailing in the operating room that the telescoping fibula and the irreducibility were identified, and open reduction performed. There were no soft-tissue or neurovascular complications, but, had there been, they could have become of urgent concern and altered treatment.
In this case report, we have described a unique fracture pattern that could cause significant morbidity if not appropriately identified and treated in a timely manner.
Am J Orthop. 2017;46(3):E160-E162. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Karladani AH, Granhed H, Kärrholm J, Styf J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch Orthop Trauma Surg. 2001;121(6):325-328.
2. McGanity P. Tibial shaft fractures. In: Heckman JD, Schenck RC Jr, Agarwal A, eds. Current Orthopedic Diagnosis & Treatment. New York, NY: Springer; 2000:184-185.
3. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
4. Green RN, Pullagura MK, Holland JP. Irreducible fracture-dislocation of the knee. Acta Orthop Traumatol Turc. 2014;48(3):363-366.
1. Karladani AH, Granhed H, Kärrholm J, Styf J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch Orthop Trauma Surg. 2001;121(6):325-328.
2. McGanity P. Tibial shaft fractures. In: Heckman JD, Schenck RC Jr, Agarwal A, eds. Current Orthopedic Diagnosis & Treatment. New York, NY: Springer; 2000:184-185.
3. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
4. Green RN, Pullagura MK, Holland JP. Irreducible fracture-dislocation of the knee. Acta Orthop Traumatol Turc. 2014;48(3):363-366.
Electronic Health Record Implementation Is Associated With a Negligible Change in Outpatient Volume and Billing
Take-Home Points
- With EHR implementation there are small changes in the level of billing coding.
- Although these changes may be statistically significant they are relatively minor.
- In the general internal medicine department, level 4 coding increased by 1.2% while level 3 coding decreased by 0.5%.
- In the orthopedics department, level 4 coding increased by 3.3% while level 3 coding decreased by 3.1%.
- Reports in the lay media regarding dramatic up-coding after EHR implementation may be misleading.
The Health Information Technology for Economic and Clinical Health (HITECH) Act, which was signed into law in 2009, mandated that hospitals that care for Medicare patients either begin using electronic health records (EHRs) or pay a nontrivial penalty.1 By now, the majority of orthopedic surgeons have implemented EHRs in their practices.2 Despite ongoing debate in the orthopedic literature,3 EHRs are expected to improve coordination of care, reduce duplicate testing, and reduce costs over the long term as healthcare insurance coverage is extended to millions more Americans.
In early coverage, however, media reported that EHR implementation at some hospitals was correlated with substantial increases in Medicare payments.4 Journalists suggested the billion dollars more paid by Medicare to hospitals in 2010 than in 2005 were partly attributable to up-coding facilitated by EHRs.5 The secretary of the Department of Health and Human Services (DHHS) and the attorney general of the Department of Justice also weighed in on this controversy by expressing their concerns in a letter to the presidents of 5 hospital associations.6 The inspector general of DHHS also published a report critical of Medicare officials’ oversight of EHRs.7Responding to the critical reception of EHR implementations, investigators studied the validity of the early reports and anecdotes. Some initial reports cited the emergency department (ED) as an area at high risk for using the convenience of EHRs to up-code visits.5 The DHHS Office of the Inspector General noted that, between 2001 and 2010, the proportion of claims for lower reimbursement categories of American Medical Association Current Procedural Terminology (CPT) codes decreased while the proportion for higher-paid billing codes increased for all visit types.8 Addressing these concerns, the American Hospital Association9 issued a brief that noted that any observed coding increases were more likely attributable to more ED use by Medicare patients and increased average illness severity. In a thoughtful perspective, Pitts10 conceded that, though utilization and illness severity may explain part of the trend, the trend may also be related to technological innovations and changes in culture and practice style in the ED.
Because these studies and reports variously suggested that EHR implementation affects patient volume and up-coding, and because none of the reports specifically addressed orthopedics, we conducted a study to determine whether any significant up-coding or change in patient volumes occurred around the time of EHR implementation in ambulatory practices at our academic medical center. In a recent national study, Adler-Milstein and Jha11 compared billing data of hospitals that adopted EHRs and hospitals that did not. Although both groups showed increased billing trends, the increases were not significantly different between the EHR adopters and nonadopters. To more effectively control for the confounding differences between groups of EHR adopters and nonadopters, we studied individual departments during EHR implementation at our institution.
Methods
In 2011, our academic medical center began the transition to EHRs (Epic). We examined our center’s trends in patient volumes and billing coding around the time of the transition in the outpatient practice of the general internal medicine (GIM) department (EHR transition, October 2011) and the outpatient practice of the orthopedics department (EHR transition, March 2012). These departments were chosen because they are representative of a GIM practice and a subspecialty practice, and because a recent study found that GIM practitioners and orthopedic surgeons were among those specialists who used EHRs the most.12
After this study was approved by our Human Investigations Committee, we began using CPT codes to identify all outpatient visits (new, consultation, and return) on a monthly basis. We compared the volume of patient visits and the billing coding level in the GIM and orthopedics departments before and after EHR implementation. Pearson χ2 test was used when appropriate, and statistical analyses were performed with SPSS for Windows Version 16.0.
Results
In the GIM department, mean monthly volume of patient visits in the 12 months before EHR implementation was similar to that in the 12 months afterward (613 vs 587; P = .439). Even when normalized for changes in provider availability (maternity leave), the decrease in volume of patient visits after EHR implementation in the GIM department was not significant (6.9%; P = .107). Likewise, in the orthopedics department, mean monthly volume of patient visits in the 17 months before EHR implementation was similar to that in the 7 months afterward (2157 vs 2317; P = .156). In fact, patient volumes remained constant during the EHR transition (Figure 1).
EHR implementation brought small changes in billing coding levels. In the GIM department, the largest change was a 1.2% increase in level 4 billing coding—an increase accompanied by a 0.5% decrease in level 3 coding.
Discussion
It is remarkable that the volumes of patient visits in the GIM and orthopedics departments at our academic center were not affected by EHR implementation.
Rather than reduce scheduling during the EHR transition, surgeons in our practice either added or lengthened clinic sessions, and the level of ancillary staffing was adjusted accordingly. As staffing costs at any given time are multifactorial and vary widely, estimating the cost of these staffing changes during the EHR transition is difficult. We should note that extending ancillary staff hours during the transition very likely increased costs, and it is unclear whether they were higher or lower than the costs that would have been incurred had we reduced scheduling or tried some combination of these strategies.
Although billing coding levels changed with EHR implementation, the changes were small. In the GIM department, level 4 CPT coded visits as percentages of all visits increased to 59.5% from 58.3%, and level 5 visits increased to 6.2% from 6.0%; in the orthopedics department, level 4 visits increased to 40.2% from 37.1%, and level 5 visits increased to 5.5% from 3.8% (Table). The 1.2% and 0.2% absolute increases in level 4 and level 5 visits in the GIM department represent 2.1% and 3.3% relative increases in level 4 and level 5 visits, and the 3.3% and 1.7% absolute increases in the orthopedics department represent 8.4% and 44.7% relative increases in level 4 and level 5 visits after EHR implementation.
Although the absolute increases in level 4 and level 5 visits were relatively minor, popular media have raised the alarm about 43% and 82% relative increases in level 5 visits after EHR implementation in some hospitals’ EDs.4 Although our orthopedics department showed a 44.7% relative increase in level 5 visits after EHR implementation, this represented an increase of only 1.7% of patient visits overall. Our findings therefore indicate that lay media reports could be misleading. Nevertheless, the small changes we found were statistically significant.
One explanation for these small changes is that EHRs facilitate better documentation of services provided. Therefore, what seem to be billing coding changes could be more accurate reports of high-level care that is the same as before. In addition, because of meaningful use mandates that coincided with the requirement to implement EHRs, additional data elements are now being consistently collected and reviewed (these may not necessarily have been collected and reviewed before). In some patient encounters, these additional data elements may have contributed to higher levels of service, and this effect could be especially apparent in EDs.
Some have suggested a potential for large-scale up-coding during EHR transitions. Others have contended that coding level increases are a consequence of a time-intensive data entry process, collection and review of additional data, and more accurate reporting of services already being provided. We are not convinced that large coding changes are attributable solely to EHR implementation, as the changes at our center have been relatively small.
Nevertheless, minor coding level changes could translate to large changes in healthcare costs when scaled nationally. Although causes may be innocuous, any increases in national healthcare costs are concerning in our time of limited budgets and scrutinized healthcare utilization.
This study had its limitations. First, including billing data from only 2 departments at a single center may limit the generalizability of findings. However, we specifically selected a GIM department and a specialty (orthopedics) department in an attempt to capture a representative sample of practices. Another limitation is that we investigated billing codes over only 2 years, around the implementation of EHRs in these departments, and therefore may have captured only short-term changes. However, as patient volumes and billing are subject to many factors, including staffing changes (eg, new partners, new hires, retirements, other departures), we attempted to limit the effect of confounding variables by limiting the period of analysis.
Overall, changes in patient volume and coded level of service during EHR implementation at our institution were relatively small. Although the trend toward higher billing coding levels was statistically significant, these 0.2% and 1.7% increases in level 5 coding hardly deserve the negative attention from lay media. These small increases are unlikely caused by intentional up-coding, and more likely reflect better documentation of an already high level of care. We hope these findings allay the concern that up-coding increased dramatically with EHR implementation.
Am J Orthop. 2017;46(3):E172-E176. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Centers for Medicare & Medicaid Services. Electronic health records (EHR) incentive programs. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms. Accessed February 5, 2015.
2. American Academy of Orthopaedic Surgeons Practice Management Committee. EMR: A Primer for Orthopaedic Surgeons. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010.
3. Ries MD. Electronic medical records: friends or foes? Clin Orthop Relat Res. 2014;472(1):16-21.
4. Abelson R. Medicare is faulted on shift to electronic records. New York Times. November 29, 2012;B1. http://www.nytimes.com/2012/11/29/business/medicare-is-faulted-in-electronic-medical-records-conversion.html. Accessed February 5, 2015.
5. Abelson R, Creswell J, Palmer G. Medicare bills rise as records turn electronic. New York Times. September 22, 2012;A1. http://www.nytimes.com/2012/09/22/business/medicare-billing-rises-at-hospitals-with-electronic-records.html. Accessed February 5, 2015.
6. Carlson J. Warning bell. Potential for fraud through use of EHRs draws federal scrutiny. Mod Healthc. 2012;42(40):8-9.
7. Levinson DR. Early assessment finds that CMS faces obstacles in overseeing the Medicare EHR Incentive Program. Dept of Health and Human Services, Office of Inspector General website. https://oig.hss.gov/oei/reports/oei-05-11-00250.pdf. Publication OEI-05-11-00250. Published November 2012. Accessed February 5, 2015.
8. Levinson DR. Coding trends of Medicare evaluation and management services. Dept of Health and Human Services, Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-04-10-00180.pdf. Publication OEI-04-10-00180. Published May 2012. Accessed February 5, 2015.
9. American Hospital Association. Sicker, more complex patients are driving up intensity of ED care [issue brief]. http://www.aha.org/content/13/13issuebrief-ed.pdf. Published May 2, 2013. Accessed February 5, 2015.
10. Pitts SR. Higher-complexity ED billing codes—sicker patients, more intensive practice, or improper payments? N Engl J Med. 2012;367(26):2465-2467.
11. Adler-Milstein J, Jha AK. No evidence found that hospitals are using new electronic health records to increase Medicare reimbursements. Health Aff (Millwood). 2014;33(7):1271-1277.
12. Kokkonen EW, Davis SA, Lin HC, Dabade TS, Feldman SR, Fleischer AB Jr. Use of electronic medical records differs by specialty and office settings. J Am Med Inform Assoc. 2013;20(e1):e33-e38.
13. Samaan ZM, Klein MD, Mansour ME, DeWitt TG. The impact of the electronic health record on an academic pediatric primary care center. J Ambul Care Manage. 2009;32(3):180-187.
Take-Home Points
- With EHR implementation there are small changes in the level of billing coding.
- Although these changes may be statistically significant they are relatively minor.
- In the general internal medicine department, level 4 coding increased by 1.2% while level 3 coding decreased by 0.5%.
- In the orthopedics department, level 4 coding increased by 3.3% while level 3 coding decreased by 3.1%.
- Reports in the lay media regarding dramatic up-coding after EHR implementation may be misleading.
The Health Information Technology for Economic and Clinical Health (HITECH) Act, which was signed into law in 2009, mandated that hospitals that care for Medicare patients either begin using electronic health records (EHRs) or pay a nontrivial penalty.1 By now, the majority of orthopedic surgeons have implemented EHRs in their practices.2 Despite ongoing debate in the orthopedic literature,3 EHRs are expected to improve coordination of care, reduce duplicate testing, and reduce costs over the long term as healthcare insurance coverage is extended to millions more Americans.
In early coverage, however, media reported that EHR implementation at some hospitals was correlated with substantial increases in Medicare payments.4 Journalists suggested the billion dollars more paid by Medicare to hospitals in 2010 than in 2005 were partly attributable to up-coding facilitated by EHRs.5 The secretary of the Department of Health and Human Services (DHHS) and the attorney general of the Department of Justice also weighed in on this controversy by expressing their concerns in a letter to the presidents of 5 hospital associations.6 The inspector general of DHHS also published a report critical of Medicare officials’ oversight of EHRs.7Responding to the critical reception of EHR implementations, investigators studied the validity of the early reports and anecdotes. Some initial reports cited the emergency department (ED) as an area at high risk for using the convenience of EHRs to up-code visits.5 The DHHS Office of the Inspector General noted that, between 2001 and 2010, the proportion of claims for lower reimbursement categories of American Medical Association Current Procedural Terminology (CPT) codes decreased while the proportion for higher-paid billing codes increased for all visit types.8 Addressing these concerns, the American Hospital Association9 issued a brief that noted that any observed coding increases were more likely attributable to more ED use by Medicare patients and increased average illness severity. In a thoughtful perspective, Pitts10 conceded that, though utilization and illness severity may explain part of the trend, the trend may also be related to technological innovations and changes in culture and practice style in the ED.
Because these studies and reports variously suggested that EHR implementation affects patient volume and up-coding, and because none of the reports specifically addressed orthopedics, we conducted a study to determine whether any significant up-coding or change in patient volumes occurred around the time of EHR implementation in ambulatory practices at our academic medical center. In a recent national study, Adler-Milstein and Jha11 compared billing data of hospitals that adopted EHRs and hospitals that did not. Although both groups showed increased billing trends, the increases were not significantly different between the EHR adopters and nonadopters. To more effectively control for the confounding differences between groups of EHR adopters and nonadopters, we studied individual departments during EHR implementation at our institution.
Methods
In 2011, our academic medical center began the transition to EHRs (Epic). We examined our center’s trends in patient volumes and billing coding around the time of the transition in the outpatient practice of the general internal medicine (GIM) department (EHR transition, October 2011) and the outpatient practice of the orthopedics department (EHR transition, March 2012). These departments were chosen because they are representative of a GIM practice and a subspecialty practice, and because a recent study found that GIM practitioners and orthopedic surgeons were among those specialists who used EHRs the most.12
After this study was approved by our Human Investigations Committee, we began using CPT codes to identify all outpatient visits (new, consultation, and return) on a monthly basis. We compared the volume of patient visits and the billing coding level in the GIM and orthopedics departments before and after EHR implementation. Pearson χ2 test was used when appropriate, and statistical analyses were performed with SPSS for Windows Version 16.0.
Results
In the GIM department, mean monthly volume of patient visits in the 12 months before EHR implementation was similar to that in the 12 months afterward (613 vs 587; P = .439). Even when normalized for changes in provider availability (maternity leave), the decrease in volume of patient visits after EHR implementation in the GIM department was not significant (6.9%; P = .107). Likewise, in the orthopedics department, mean monthly volume of patient visits in the 17 months before EHR implementation was similar to that in the 7 months afterward (2157 vs 2317; P = .156). In fact, patient volumes remained constant during the EHR transition (Figure 1).
EHR implementation brought small changes in billing coding levels. In the GIM department, the largest change was a 1.2% increase in level 4 billing coding—an increase accompanied by a 0.5% decrease in level 3 coding.
Discussion
It is remarkable that the volumes of patient visits in the GIM and orthopedics departments at our academic center were not affected by EHR implementation.
Rather than reduce scheduling during the EHR transition, surgeons in our practice either added or lengthened clinic sessions, and the level of ancillary staffing was adjusted accordingly. As staffing costs at any given time are multifactorial and vary widely, estimating the cost of these staffing changes during the EHR transition is difficult. We should note that extending ancillary staff hours during the transition very likely increased costs, and it is unclear whether they were higher or lower than the costs that would have been incurred had we reduced scheduling or tried some combination of these strategies.
Although billing coding levels changed with EHR implementation, the changes were small. In the GIM department, level 4 CPT coded visits as percentages of all visits increased to 59.5% from 58.3%, and level 5 visits increased to 6.2% from 6.0%; in the orthopedics department, level 4 visits increased to 40.2% from 37.1%, and level 5 visits increased to 5.5% from 3.8% (Table). The 1.2% and 0.2% absolute increases in level 4 and level 5 visits in the GIM department represent 2.1% and 3.3% relative increases in level 4 and level 5 visits, and the 3.3% and 1.7% absolute increases in the orthopedics department represent 8.4% and 44.7% relative increases in level 4 and level 5 visits after EHR implementation.
Although the absolute increases in level 4 and level 5 visits were relatively minor, popular media have raised the alarm about 43% and 82% relative increases in level 5 visits after EHR implementation in some hospitals’ EDs.4 Although our orthopedics department showed a 44.7% relative increase in level 5 visits after EHR implementation, this represented an increase of only 1.7% of patient visits overall. Our findings therefore indicate that lay media reports could be misleading. Nevertheless, the small changes we found were statistically significant.
One explanation for these small changes is that EHRs facilitate better documentation of services provided. Therefore, what seem to be billing coding changes could be more accurate reports of high-level care that is the same as before. In addition, because of meaningful use mandates that coincided with the requirement to implement EHRs, additional data elements are now being consistently collected and reviewed (these may not necessarily have been collected and reviewed before). In some patient encounters, these additional data elements may have contributed to higher levels of service, and this effect could be especially apparent in EDs.
Some have suggested a potential for large-scale up-coding during EHR transitions. Others have contended that coding level increases are a consequence of a time-intensive data entry process, collection and review of additional data, and more accurate reporting of services already being provided. We are not convinced that large coding changes are attributable solely to EHR implementation, as the changes at our center have been relatively small.
Nevertheless, minor coding level changes could translate to large changes in healthcare costs when scaled nationally. Although causes may be innocuous, any increases in national healthcare costs are concerning in our time of limited budgets and scrutinized healthcare utilization.
This study had its limitations. First, including billing data from only 2 departments at a single center may limit the generalizability of findings. However, we specifically selected a GIM department and a specialty (orthopedics) department in an attempt to capture a representative sample of practices. Another limitation is that we investigated billing codes over only 2 years, around the implementation of EHRs in these departments, and therefore may have captured only short-term changes. However, as patient volumes and billing are subject to many factors, including staffing changes (eg, new partners, new hires, retirements, other departures), we attempted to limit the effect of confounding variables by limiting the period of analysis.
Overall, changes in patient volume and coded level of service during EHR implementation at our institution were relatively small. Although the trend toward higher billing coding levels was statistically significant, these 0.2% and 1.7% increases in level 5 coding hardly deserve the negative attention from lay media. These small increases are unlikely caused by intentional up-coding, and more likely reflect better documentation of an already high level of care. We hope these findings allay the concern that up-coding increased dramatically with EHR implementation.
Am J Orthop. 2017;46(3):E172-E176. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- With EHR implementation there are small changes in the level of billing coding.
- Although these changes may be statistically significant they are relatively minor.
- In the general internal medicine department, level 4 coding increased by 1.2% while level 3 coding decreased by 0.5%.
- In the orthopedics department, level 4 coding increased by 3.3% while level 3 coding decreased by 3.1%.
- Reports in the lay media regarding dramatic up-coding after EHR implementation may be misleading.
The Health Information Technology for Economic and Clinical Health (HITECH) Act, which was signed into law in 2009, mandated that hospitals that care for Medicare patients either begin using electronic health records (EHRs) or pay a nontrivial penalty.1 By now, the majority of orthopedic surgeons have implemented EHRs in their practices.2 Despite ongoing debate in the orthopedic literature,3 EHRs are expected to improve coordination of care, reduce duplicate testing, and reduce costs over the long term as healthcare insurance coverage is extended to millions more Americans.
In early coverage, however, media reported that EHR implementation at some hospitals was correlated with substantial increases in Medicare payments.4 Journalists suggested the billion dollars more paid by Medicare to hospitals in 2010 than in 2005 were partly attributable to up-coding facilitated by EHRs.5 The secretary of the Department of Health and Human Services (DHHS) and the attorney general of the Department of Justice also weighed in on this controversy by expressing their concerns in a letter to the presidents of 5 hospital associations.6 The inspector general of DHHS also published a report critical of Medicare officials’ oversight of EHRs.7Responding to the critical reception of EHR implementations, investigators studied the validity of the early reports and anecdotes. Some initial reports cited the emergency department (ED) as an area at high risk for using the convenience of EHRs to up-code visits.5 The DHHS Office of the Inspector General noted that, between 2001 and 2010, the proportion of claims for lower reimbursement categories of American Medical Association Current Procedural Terminology (CPT) codes decreased while the proportion for higher-paid billing codes increased for all visit types.8 Addressing these concerns, the American Hospital Association9 issued a brief that noted that any observed coding increases were more likely attributable to more ED use by Medicare patients and increased average illness severity. In a thoughtful perspective, Pitts10 conceded that, though utilization and illness severity may explain part of the trend, the trend may also be related to technological innovations and changes in culture and practice style in the ED.
Because these studies and reports variously suggested that EHR implementation affects patient volume and up-coding, and because none of the reports specifically addressed orthopedics, we conducted a study to determine whether any significant up-coding or change in patient volumes occurred around the time of EHR implementation in ambulatory practices at our academic medical center. In a recent national study, Adler-Milstein and Jha11 compared billing data of hospitals that adopted EHRs and hospitals that did not. Although both groups showed increased billing trends, the increases were not significantly different between the EHR adopters and nonadopters. To more effectively control for the confounding differences between groups of EHR adopters and nonadopters, we studied individual departments during EHR implementation at our institution.
Methods
In 2011, our academic medical center began the transition to EHRs (Epic). We examined our center’s trends in patient volumes and billing coding around the time of the transition in the outpatient practice of the general internal medicine (GIM) department (EHR transition, October 2011) and the outpatient practice of the orthopedics department (EHR transition, March 2012). These departments were chosen because they are representative of a GIM practice and a subspecialty practice, and because a recent study found that GIM practitioners and orthopedic surgeons were among those specialists who used EHRs the most.12
After this study was approved by our Human Investigations Committee, we began using CPT codes to identify all outpatient visits (new, consultation, and return) on a monthly basis. We compared the volume of patient visits and the billing coding level in the GIM and orthopedics departments before and after EHR implementation. Pearson χ2 test was used when appropriate, and statistical analyses were performed with SPSS for Windows Version 16.0.
Results
In the GIM department, mean monthly volume of patient visits in the 12 months before EHR implementation was similar to that in the 12 months afterward (613 vs 587; P = .439). Even when normalized for changes in provider availability (maternity leave), the decrease in volume of patient visits after EHR implementation in the GIM department was not significant (6.9%; P = .107). Likewise, in the orthopedics department, mean monthly volume of patient visits in the 17 months before EHR implementation was similar to that in the 7 months afterward (2157 vs 2317; P = .156). In fact, patient volumes remained constant during the EHR transition (Figure 1).
EHR implementation brought small changes in billing coding levels. In the GIM department, the largest change was a 1.2% increase in level 4 billing coding—an increase accompanied by a 0.5% decrease in level 3 coding.
Discussion
It is remarkable that the volumes of patient visits in the GIM and orthopedics departments at our academic center were not affected by EHR implementation.
Rather than reduce scheduling during the EHR transition, surgeons in our practice either added or lengthened clinic sessions, and the level of ancillary staffing was adjusted accordingly. As staffing costs at any given time are multifactorial and vary widely, estimating the cost of these staffing changes during the EHR transition is difficult. We should note that extending ancillary staff hours during the transition very likely increased costs, and it is unclear whether they were higher or lower than the costs that would have been incurred had we reduced scheduling or tried some combination of these strategies.
Although billing coding levels changed with EHR implementation, the changes were small. In the GIM department, level 4 CPT coded visits as percentages of all visits increased to 59.5% from 58.3%, and level 5 visits increased to 6.2% from 6.0%; in the orthopedics department, level 4 visits increased to 40.2% from 37.1%, and level 5 visits increased to 5.5% from 3.8% (Table). The 1.2% and 0.2% absolute increases in level 4 and level 5 visits in the GIM department represent 2.1% and 3.3% relative increases in level 4 and level 5 visits, and the 3.3% and 1.7% absolute increases in the orthopedics department represent 8.4% and 44.7% relative increases in level 4 and level 5 visits after EHR implementation.
Although the absolute increases in level 4 and level 5 visits were relatively minor, popular media have raised the alarm about 43% and 82% relative increases in level 5 visits after EHR implementation in some hospitals’ EDs.4 Although our orthopedics department showed a 44.7% relative increase in level 5 visits after EHR implementation, this represented an increase of only 1.7% of patient visits overall. Our findings therefore indicate that lay media reports could be misleading. Nevertheless, the small changes we found were statistically significant.
One explanation for these small changes is that EHRs facilitate better documentation of services provided. Therefore, what seem to be billing coding changes could be more accurate reports of high-level care that is the same as before. In addition, because of meaningful use mandates that coincided with the requirement to implement EHRs, additional data elements are now being consistently collected and reviewed (these may not necessarily have been collected and reviewed before). In some patient encounters, these additional data elements may have contributed to higher levels of service, and this effect could be especially apparent in EDs.
Some have suggested a potential for large-scale up-coding during EHR transitions. Others have contended that coding level increases are a consequence of a time-intensive data entry process, collection and review of additional data, and more accurate reporting of services already being provided. We are not convinced that large coding changes are attributable solely to EHR implementation, as the changes at our center have been relatively small.
Nevertheless, minor coding level changes could translate to large changes in healthcare costs when scaled nationally. Although causes may be innocuous, any increases in national healthcare costs are concerning in our time of limited budgets and scrutinized healthcare utilization.
This study had its limitations. First, including billing data from only 2 departments at a single center may limit the generalizability of findings. However, we specifically selected a GIM department and a specialty (orthopedics) department in an attempt to capture a representative sample of practices. Another limitation is that we investigated billing codes over only 2 years, around the implementation of EHRs in these departments, and therefore may have captured only short-term changes. However, as patient volumes and billing are subject to many factors, including staffing changes (eg, new partners, new hires, retirements, other departures), we attempted to limit the effect of confounding variables by limiting the period of analysis.
Overall, changes in patient volume and coded level of service during EHR implementation at our institution were relatively small. Although the trend toward higher billing coding levels was statistically significant, these 0.2% and 1.7% increases in level 5 coding hardly deserve the negative attention from lay media. These small increases are unlikely caused by intentional up-coding, and more likely reflect better documentation of an already high level of care. We hope these findings allay the concern that up-coding increased dramatically with EHR implementation.
Am J Orthop. 2017;46(3):E172-E176. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Centers for Medicare & Medicaid Services. Electronic health records (EHR) incentive programs. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms. Accessed February 5, 2015.
2. American Academy of Orthopaedic Surgeons Practice Management Committee. EMR: A Primer for Orthopaedic Surgeons. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010.
3. Ries MD. Electronic medical records: friends or foes? Clin Orthop Relat Res. 2014;472(1):16-21.
4. Abelson R. Medicare is faulted on shift to electronic records. New York Times. November 29, 2012;B1. http://www.nytimes.com/2012/11/29/business/medicare-is-faulted-in-electronic-medical-records-conversion.html. Accessed February 5, 2015.
5. Abelson R, Creswell J, Palmer G. Medicare bills rise as records turn electronic. New York Times. September 22, 2012;A1. http://www.nytimes.com/2012/09/22/business/medicare-billing-rises-at-hospitals-with-electronic-records.html. Accessed February 5, 2015.
6. Carlson J. Warning bell. Potential for fraud through use of EHRs draws federal scrutiny. Mod Healthc. 2012;42(40):8-9.
7. Levinson DR. Early assessment finds that CMS faces obstacles in overseeing the Medicare EHR Incentive Program. Dept of Health and Human Services, Office of Inspector General website. https://oig.hss.gov/oei/reports/oei-05-11-00250.pdf. Publication OEI-05-11-00250. Published November 2012. Accessed February 5, 2015.
8. Levinson DR. Coding trends of Medicare evaluation and management services. Dept of Health and Human Services, Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-04-10-00180.pdf. Publication OEI-04-10-00180. Published May 2012. Accessed February 5, 2015.
9. American Hospital Association. Sicker, more complex patients are driving up intensity of ED care [issue brief]. http://www.aha.org/content/13/13issuebrief-ed.pdf. Published May 2, 2013. Accessed February 5, 2015.
10. Pitts SR. Higher-complexity ED billing codes—sicker patients, more intensive practice, or improper payments? N Engl J Med. 2012;367(26):2465-2467.
11. Adler-Milstein J, Jha AK. No evidence found that hospitals are using new electronic health records to increase Medicare reimbursements. Health Aff (Millwood). 2014;33(7):1271-1277.
12. Kokkonen EW, Davis SA, Lin HC, Dabade TS, Feldman SR, Fleischer AB Jr. Use of electronic medical records differs by specialty and office settings. J Am Med Inform Assoc. 2013;20(e1):e33-e38.
13. Samaan ZM, Klein MD, Mansour ME, DeWitt TG. The impact of the electronic health record on an academic pediatric primary care center. J Ambul Care Manage. 2009;32(3):180-187.
1. Centers for Medicare & Medicaid Services. Electronic health records (EHR) incentive programs. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms. Accessed February 5, 2015.
2. American Academy of Orthopaedic Surgeons Practice Management Committee. EMR: A Primer for Orthopaedic Surgeons. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010.
3. Ries MD. Electronic medical records: friends or foes? Clin Orthop Relat Res. 2014;472(1):16-21.
4. Abelson R. Medicare is faulted on shift to electronic records. New York Times. November 29, 2012;B1. http://www.nytimes.com/2012/11/29/business/medicare-is-faulted-in-electronic-medical-records-conversion.html. Accessed February 5, 2015.
5. Abelson R, Creswell J, Palmer G. Medicare bills rise as records turn electronic. New York Times. September 22, 2012;A1. http://www.nytimes.com/2012/09/22/business/medicare-billing-rises-at-hospitals-with-electronic-records.html. Accessed February 5, 2015.
6. Carlson J. Warning bell. Potential for fraud through use of EHRs draws federal scrutiny. Mod Healthc. 2012;42(40):8-9.
7. Levinson DR. Early assessment finds that CMS faces obstacles in overseeing the Medicare EHR Incentive Program. Dept of Health and Human Services, Office of Inspector General website. https://oig.hss.gov/oei/reports/oei-05-11-00250.pdf. Publication OEI-05-11-00250. Published November 2012. Accessed February 5, 2015.
8. Levinson DR. Coding trends of Medicare evaluation and management services. Dept of Health and Human Services, Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-04-10-00180.pdf. Publication OEI-04-10-00180. Published May 2012. Accessed February 5, 2015.
9. American Hospital Association. Sicker, more complex patients are driving up intensity of ED care [issue brief]. http://www.aha.org/content/13/13issuebrief-ed.pdf. Published May 2, 2013. Accessed February 5, 2015.
10. Pitts SR. Higher-complexity ED billing codes—sicker patients, more intensive practice, or improper payments? N Engl J Med. 2012;367(26):2465-2467.
11. Adler-Milstein J, Jha AK. No evidence found that hospitals are using new electronic health records to increase Medicare reimbursements. Health Aff (Millwood). 2014;33(7):1271-1277.
12. Kokkonen EW, Davis SA, Lin HC, Dabade TS, Feldman SR, Fleischer AB Jr. Use of electronic medical records differs by specialty and office settings. J Am Med Inform Assoc. 2013;20(e1):e33-e38.
13. Samaan ZM, Klein MD, Mansour ME, DeWitt TG. The impact of the electronic health record on an academic pediatric primary care center. J Ambul Care Manage. 2009;32(3):180-187.
5 Points on Pyogenic Flexor Tenosynovitis of the Hand
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.
3. What Are the Timing and Indications for Surgery?
Nonoperative treatment may be appropriate for PFT patients who present early, typically within 48 hours after penetrating trauma to the hand.21 In a 4-patient series, Neviaser and Gunther19 successfully treated PFT nonoperatively, with IV antibiotics, splinting, and elevation. During nonoperative treatment, the affected hand should be regularly examined. If this treatment is to be successful, clinical symptoms should improve within 48 hours; if they do not, surgical irrigation and débridement should be performed.
Regardless of timing and type of irrigation, surgical treatment remains the treatment of choice for the majority of PFT cases. Michon22 developed a 3-tier PFT classification system that is based on intraoperative findings (Table).
4. What Are the Surgical Techniques for PFT Drainage?
Several surgical methods have been developed to decompress and irrigate the flexor sheaths of the hand. However, debates about optimal timing of surgical intervention, surgery type (open surgery or closed catheter irrigation only), and irrigation method continue.
Open Irrigation and Débridement
Open irrigation and débridement procedures were originally described for surgical management of PFT.1 Midaxial and palmar (Bruner zigzag) incisions can be used to expose and open the entire sheath for complete drainage and washout. Both incisions afford good access to the flexor sheath, but the midaxial approach may provide more coverage of the sheath after surgery. Open irrigation and débridement is the treatment of choice for the most advanced cases of PFT and for atypical or chronic tenosynovial infections.4,23,24 The Bruner zigzag incision affords ease of surgical dissection, extension, and more exposure of the flexor tendon sheath at the expense of possible difficulty in closure or flap necrosis in the setting of a swollen digit. Alternatively, the midaxial incision has the advantage of a large, more robust skin flap for more reliable closure.
Closed Tendon Sheath Irrigation
In 1943, Dickson-Wright25 first described catheter irrigation of tendon sheath infections. Later, Neviaser4 described this technique in detail. A proximal incision is made over the metacarpal neck. The tendon sheath is cut transversely at the proximal edge of the A1 pulley. An angiocatheter is inserted 1 cm to 2 cm antegrade into the flexor tendon sheath. Then, a distal midaxial incision is made dorsal to the neurovascular bundle at the level of the distal interphalangeal joint on the ulnar aspect of the finger or the radial aspect of the thumb. The distal edge of the flexor sheath is exposed and resected distal to the distal-most pulley. A Penrose drain can be threaded into the tendon sheath beneath the A4 pulley to keep the wound open and allow for fluid drainage. The sheath is flushed gently in the operating room. After surgery, intermittent bedside irrigation can be continued on the floor.
Neviaser4 reported excellent initial results with this technique; 18 of 20 patients regained complete active and passive range of motion (ROM) by 1 week after surgery. Similarly, Juliano and Eglseder,26 using a similar method, reported 100% excellent results for mild PFT and 88.4% excellent results for more severe infection.
Gutowski and colleagues23 reviewed 47 PFT cases to determine if there is a difference in outcomes between PFT treated with open irrigation and débridement and PFT treated with closed catheter irrigation. Between these groups, they found no significant differences in early postoperative outcomes, including resolution of infection, need for additional surgery, and hospital length of stay.
There are also many differing opinions regarding the best irrigation method. Some authors have asserted that normal saline is sufficient,4,5,23 and others that local antibiotics provide added benefit.27-29 Recently, Draeger and colleagues30 reported promising results with local injection of antibiotics into the tendon sheath and the addition of locally administered corticosteroids in the treatment of PFT in an animal model.
Continuous Closed Irrigation
A continuous closed irrigation system with inlet and outlet tubes has yielded successful results.8,31,32 This system consists of 2 fenestrated tubes placed within the infected space, with the tip of the smaller caliber inlet tube positioned just inside the larger outlet tube. Advantages of this system include the patient’s ability to participate in hand therapy with the system in place and avoidance of pain caused by the high pressures involved in intermittent closed irrigation. Duration of this system has ranged from 2 days to 3 weeks, and results have been good.5,8
Postoperative Irrigation
Use of postoperative irrigation on the floor or at home is controversial, as leaving an indwelling catheter in the tendon sheath can lead to complications. Catheters may increase digital stiffness by decreasing the patient’s ability to participate in therapy or may cause additional injury and irritation to the sheath itself if left in place too long. Lille and colleagues6 retrospectively compared the results of intraoperative closed tendon sheath irrigation alone with those of intraoperative and postoperative closed tendon sheath irrigation. There were no significant differences in mean hospital length of stay, follow-up complication rates, or postoperative ROM—which suggests that postoperative intermittent or continuous irrigation is not necessary.
Our Preferred Technique
We recommend a palmar approach that begins with outlining a Bruner zigzag incision along the entire finger. Then, only the distal-most and proximal-most incision lines are opened, thereby exposing the A5 and A1 pulleys, respectively (Figure 2).
5. What Are the Long-Term Outcomes of PFT?
The principal complication associated with PFT is stiffness with loss of ROM, which can be caused by flexor tendon adhesions, joint capsular thickening, or destruction of the sheath and pulley system.24 In several studies, up to one-fourth of patients with PFT did not obtain full ROM, despite adequate treatment.4-6,27 Therefore, full active ROM exercises should be initiated immediately after surgery to counteract the development of stiffness.
The most severe complication of PFT is amputation of the affected digit (Figures 3A, 3B).
Pang and colleagues2 identified 5 factors associated with increased risk of amputation in patients with PFT: (1) age >43 years; (2) diabetes mellitus, peripheral vascular disease, or renal failure; (3) subcutaneous purulence; (4) signs of digital ischemia at presentation; and (5) growth of more than 1 bacteria species on culture of specimens obtained at time of surgery.
Pang and colleagues2 classified these patients into 3 groups with distinct clinical features and reported each group’s outcomes. The authors based their PFT classification system on increasingly severe clinical presentation, which potentially predicts amputation risk. Patients in stage 1 presented with Kanavel signs of tenosynovitis but no evidence of subcutaneous purulence or ischemia; patients in stage 2 had concurrent localized subcutaneous purulence but no ischemia; and patients in stage 3 had concurrent extensive subcutaneous purulence involving more than 1 phalangeal segment or spreading circumferentially as well as signs of ischemia. These PFT stages were found to correlate with worse patient outcomes. In patients with stage 1 infection, amputation was not required, and average functional return was 80% of total active ROM of the affected digit. In patients with stage 2 infection, the amputation rate was 8%, and return of total active ROM in the remaining digits was 72%. The outcomes for the patients with stage 3 infection were the worst. The amputation rate for patients with all 3 classification criteria (Kanavel signs, subcutaneous purulence, digital ischemia) was 59%, and return of total active ROM in the remaining digits was only 49%. Use of this clinical classification system makes it possible to guide treatment and predict outcome and return to function.
Conclusion
PFT is a common hand infection that can cause significant morbidity. Early treatment is crucial: this requires use of IV antibiotics, or surgical irrigation and débridement in more advanced cases. However, despite prompt and thorough treatment, severe infection can lead to long-term impaired function and even amputation of the affected digit. More research is needed to determine optimal timing and technique for surgical intervention and to elucidate the role of local antibiotics and corticosteroids in treating this infection and potentially preventing the morbid outcomes we currently see.
Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.
1. Kanavel AB. The symptoms, signs, and diagnosis of tenosynovitis and major fascial-space abscesses. In: Kanavel AB, ed. Infections of the Hand. 6th ed. Philadelphia, PA: Lea & Febiger; 1933:364-395.
2. Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2007;89(8):1742-1748.
3. Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. J Hand Surg Am. 1983;8(5 pt 1):553-559.
4. Neviaser RJ. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J Hand Surg Am. 1978;3(5):462-466.
5. Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. J Hand Surg Br. 1999;24(3):328-333.
6. Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. J Hand Surg Br. 2000;25(3):304-307.
7. Boles SD, Schmidt CC. Pyogenic flexor tenosynovitis. Hand Clin. 1998;14(4):567-578.
8. Nemoto K, Yanagida M, Nemoto T. Closed continuous irrigation as a treatment for infection in the hand. J Hand Surg Br. 1993;18(6):783-789.
9. Dailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. J Hand Surg Eur Vol. 2008;33(3):280-285.
10. Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19(4):991-1005, xi.
11. Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. J Hand Surg Br. 2006;31(3):350-352.
12. Fowler JR Greenhill D, Schaffer AA, Thoder JJ, Ilyas AM. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800.
13. Aubert JP, Stein A, Raoult D, Magalon G. Flexor tenosynovitis in the hand: an unusual aetiology. J Hand Surg Br. 1995;20(4):509-510.
14. Wright TW, Linscheid RL, O’Duffy JD. Acute flexor tenosynovitis in association with Clostridium difficile infection: a case report. J Hand Surg Am. 1996;21(2):304-306.
15. Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis: a case report and literature review. Clin Orthop Relat Res. 1992;(281):212-215.
16. Mamane W, Falcone MO, Doursounian L, Nourissat G. Isolated gonococcal tenosynovitis. Case report and review of literature [in French]. Chir Main. 2010;29(5):335-337.
17. Regnard PJ, Barry P, Isselin J. Mycobacterial tenosynovitis of the flexor tendons of the hand. A report of five cases. J Hand Surg Br. 1996;21(3):351-354.
18. Abrams RA, Botte MJ. Hand infections: treatment recommendations for specific types. J Am Acad Orthop Surg. 1996;4(4):219-230.
19. Neviaser RJ, Gunther SF. Tenosynovial infections in the hand: diagnosis and management. Instr Course Lect. 1980;29:108-128.
20. Szabo R, Palumbo C. Infections of the hand. In: Chapman M, ed. Chapman’s Orthopedic Surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1989-2008.
21. Neviaser R. Acute infections. In: Green D, Hotchkiss R, Pederson W, eds. Green’s Operative Hand Surgery. 4th ed. New York, NY: Churchill Livingstone; 1999:1033-1047.
22. Michon J. Phlegmon of the tendon sheaths [in French]. Ann Chir. 1974;28(4):277-280.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plast Surg. 2002;49(4):350-354.
24. Stern PJ. Selected acute infections. Instr Course Lect. 1990;39:539-546.
25. Dickson-Wright A. Tendon sheath infection. Proc R Soc Med. 1943-1944;37:504-505.
26. Juliano PJ, Eglseder WA. Limited open-tendon-sheath irrigation in the treatment of pyogenic flexor tenosynovitis. Orthop Rev. 1991;20(12):1065-1069.
27. Pollen AG. Acute infection of the tendon sheaths. Hand. 1974;6(1):21-25.
28. Besser MI. Digital flexor tendon irrigation. Hand. 1976;8(1):72.
29. Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Ann Surg. 1966;163(4):645-650.
30. Draeger RW, Singh B, Bynum DK, Dahners LE. Corticosteroids as an adjunct to antibiotics and surgical drainage for the treatment of pyogenic flexor tenosynovitis. J Bone Joint Surg Am. 2010;92(16):2653-2662.
31. Delsignore JL, Ritland D, Becker DR, Watson HK. Continuous catheter irrigation for the treatment of suppurative flexor synovitis. Conn Med. 1986;50(8):503-506.
32. Gosain AK, Markisson RE. Catheter irrigation for treatment of pyogenic closed space infections of the hand. Br J Plast Surg. 1991;44(4):270-273.