User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Effect of Plate in Close Proximity to Empty External-Fixation Pin Site on Long-Bone Torsional Strength
Take-Home Points
- The location of a bicortical defect in proximity to a tibia plate does not appear to affect the torsional stiffness or torsional failure strength of the bone.
- External fixator pin placement should be based on considerations other than the potential for creating a distal stress riser after definitive fracture management.
A stress riser in cortical bone may be considered any abrupt change in the contour or consistency of the hollow structure, such as a surface defect, that not only weakens the bone but concentrates stresses at that transition point.1 A cortical defect that is 20% of the bone diameter is associated with a 34% decrease in torsional strength, thus representing a “stress riser.”2 High-energy and complex tibia fractures are often provisionally stabilized with external fixation that gives the soft tissues time to recover before definitive fracture fixation. Pin diameter for a medium-size tibia external fixator typically is 5.0 mm, resulting in a 10-mm defect in bicortical placement. Therefore, any tibia with a diameter of <50 mm is at risk for a stress riser fracture.
Although it had been established that sizable cortical defects can decrease the torsional strength of long bone,2 the effect of a plate in close proximity to a defect secondary to an empty external-fixator pin site on torsional strength has not been determined. We conducted a study to evaluate this effect. The null hypothesis was there would be no difference in tibia torsional strength attributable to varying the proximity of a tibia midshaft plate to a 5.0-mm bicortical defect.
Methods
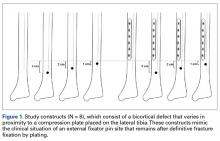
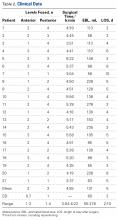
Forty fourth-generation, medium-size left composite tibias (Pacific Research Laboratories) were divided into 8 groups of 5 bones (Figure 1).
Torsion testing to failure was performed for all specimens in a manner similar to that described by Gardner and colleagues.3 Impression molds for the composite tibia constructed from polymethylmethacrylate encased the superior and distal ends, leaving 25.5 cm of exposed midshaft. This allowed the composites to be rigidly clamped into a materials testing system (858 Mini-Bionix; MTS) equipped with a 100.0-Nm torsional load cell (Figure 2).
Results
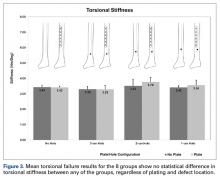
Graphical results for torsional stiffness are presented in Figure 3. R2 for all stiffness calculations was >0.99.
Discussion
Many tibia fractures require provisional stabilization with an external fixator that spans the knee, because of the high-energy nature of the injury or other, higher-priority polytrauma concerns. When the patient or injury is suitable for definitive fixation, the external fixator typically is removed in favor of internal fixation with a plate and screws. Depending on the nature and location of the fracture and the subsequent plate, the empty cortical pin-site defects, often lying at varying distances from the distal end of the plate, can potentially serve as stress risers for fracture.4
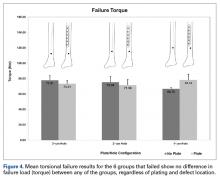
Other studies have evaluated long-bone cortical defects biomechanically1,2,4 and clinically,5-7 and multiple studies have been conducted on the effects of plates on long-bone strength for fracture stabilization.8-13 The present study evaluated the torsional strength of long bones in the presence of a bicortical defect and the proximity of the defect to a plate. There were no differences in stiffness or failure load between any of the groups of plated and unplated fourth-generation composite tibias tested to failure in torsion with varying distal bicortical defects. Hypothetically, one would expect the torsional stiffness of these specimens to increase with the mere addition of a metallic diaphyseal plate. However, this study demonstrated that the addition of a plate did not affect the torsional stiffness or strength of the tibias. Clinically, it is common practice to place external fixator pins as far as possible outside the planned incision site for definitive fracture fixation. Thus, we also hypothesized that the presence of a bicortical pin-site defect and its proximity to the plate would alter the torsional strength of the tibia specimens, and that the distal pin-site defect’s location farthest from the plate would exhibit greater strength, but this did not occur. Although other studies have shown that the presence of bicortical defects decreases the strength of long bones, we were unable to quantify this decrease because the 2 intact groups of composites, plated and unplated, survived failure testing.
This study had several limitations, first being the use of composite tibias as opposed to human cadaver bone. Although fourth-generation composite bone models have been validated as a suitable and accurate biomechanical substitute for cadaver specimens,14 anatomical variations in cadaver tibias may transfer forces differently through plates, screws, and distal pin sites. In order to test plated specimens against the unplated controls, we did not simulate a mid-shaft fracture in any of the tibias. The pin-site defects were intended to reflect the mechanical effects of bicortical defects immediately after pin removal and in the absence of any degree of bone healing. Finally, this study focused on pin-site defects that were distal to a midshaft plate and that may not represent the effects of bicortical pin-site defects proximal to the plate.
Given the results of this biomechanical study in composite tibias, varying the proximity of a bicortical defect to a plate does not affect the torsional stiffness or torsional failure strength of the bone. Placement of an intended bicortical defect should be based on considerations other than the potential for creating a distal stress riser after definitive fracture management.
Am J Orthop. 2017;46(2):E108-E111. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
2. Edgerton BC, An KN, Morrey BF. Torsional strength reduction due to cortical defects in bone. J Orthop Res. 1990;8(6):851-855.
3. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
4. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
5. Burstein AH, Currey J, Frankel VH, Heiple KG, Lunseth P, Vessely JC. Bone strength. The effect of screw holes. J Bone Joint Surg Am. 1972;54(6):1143-1156.
6. Clark CR, Morgan C, Sonstegard DA, Matthews LS. The effect of biopsy-hole shape and size on bone strength. J Bone Joint Surg Am. 1977;59(2):213-217.
7. Evans PE, Thomas WG. Tibial fracture through a traction-pin site. A report of two cases. J Bone Joint Surg Am. 1984;66(9):1475-1476.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Klaue K, Fengels I, Perren SM. Long-term effects of plate osteosynthesis: comparison of four different plates. Injury. 2000;31(suppl 2):B51-B62.
10. Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11(2):118-126.
11. Takemoto RC, Sugi MT, Kummer F, Koval KJ, Egol KA. The effects of locked and unlocked neutralization plates on load bearing of fractures fixed with a lag screw. J Orthop Trauma. 2012;26(9):519-522.
12. Wagner M. General principles for the clinical use of the LCP. Injury. 2003;34(suppl 2):B31-B42.
13. Strauss EJ, Schwarzkopf R, Kummer F, Egol KA. The current status of locked plating: the good, the bad, and the ugly. J Orthop Trauma. 2008;22(7):479-486.
14. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
Take-Home Points
- The location of a bicortical defect in proximity to a tibia plate does not appear to affect the torsional stiffness or torsional failure strength of the bone.
- External fixator pin placement should be based on considerations other than the potential for creating a distal stress riser after definitive fracture management.
A stress riser in cortical bone may be considered any abrupt change in the contour or consistency of the hollow structure, such as a surface defect, that not only weakens the bone but concentrates stresses at that transition point.1 A cortical defect that is 20% of the bone diameter is associated with a 34% decrease in torsional strength, thus representing a “stress riser.”2 High-energy and complex tibia fractures are often provisionally stabilized with external fixation that gives the soft tissues time to recover before definitive fracture fixation. Pin diameter for a medium-size tibia external fixator typically is 5.0 mm, resulting in a 10-mm defect in bicortical placement. Therefore, any tibia with a diameter of <50 mm is at risk for a stress riser fracture.
Although it had been established that sizable cortical defects can decrease the torsional strength of long bone,2 the effect of a plate in close proximity to a defect secondary to an empty external-fixator pin site on torsional strength has not been determined. We conducted a study to evaluate this effect. The null hypothesis was there would be no difference in tibia torsional strength attributable to varying the proximity of a tibia midshaft plate to a 5.0-mm bicortical defect.
Methods
Forty fourth-generation, medium-size left composite tibias (Pacific Research Laboratories) were divided into 8 groups of 5 bones (Figure 1).
Torsion testing to failure was performed for all specimens in a manner similar to that described by Gardner and colleagues.3 Impression molds for the composite tibia constructed from polymethylmethacrylate encased the superior and distal ends, leaving 25.5 cm of exposed midshaft. This allowed the composites to be rigidly clamped into a materials testing system (858 Mini-Bionix; MTS) equipped with a 100.0-Nm torsional load cell (Figure 2).
Results
Graphical results for torsional stiffness are presented in Figure 3. R2 for all stiffness calculations was >0.99.
Discussion
Many tibia fractures require provisional stabilization with an external fixator that spans the knee, because of the high-energy nature of the injury or other, higher-priority polytrauma concerns. When the patient or injury is suitable for definitive fixation, the external fixator typically is removed in favor of internal fixation with a plate and screws. Depending on the nature and location of the fracture and the subsequent plate, the empty cortical pin-site defects, often lying at varying distances from the distal end of the plate, can potentially serve as stress risers for fracture.4
Other studies have evaluated long-bone cortical defects biomechanically1,2,4 and clinically,5-7 and multiple studies have been conducted on the effects of plates on long-bone strength for fracture stabilization.8-13 The present study evaluated the torsional strength of long bones in the presence of a bicortical defect and the proximity of the defect to a plate. There were no differences in stiffness or failure load between any of the groups of plated and unplated fourth-generation composite tibias tested to failure in torsion with varying distal bicortical defects. Hypothetically, one would expect the torsional stiffness of these specimens to increase with the mere addition of a metallic diaphyseal plate. However, this study demonstrated that the addition of a plate did not affect the torsional stiffness or strength of the tibias. Clinically, it is common practice to place external fixator pins as far as possible outside the planned incision site for definitive fracture fixation. Thus, we also hypothesized that the presence of a bicortical pin-site defect and its proximity to the plate would alter the torsional strength of the tibia specimens, and that the distal pin-site defect’s location farthest from the plate would exhibit greater strength, but this did not occur. Although other studies have shown that the presence of bicortical defects decreases the strength of long bones, we were unable to quantify this decrease because the 2 intact groups of composites, plated and unplated, survived failure testing.
This study had several limitations, first being the use of composite tibias as opposed to human cadaver bone. Although fourth-generation composite bone models have been validated as a suitable and accurate biomechanical substitute for cadaver specimens,14 anatomical variations in cadaver tibias may transfer forces differently through plates, screws, and distal pin sites. In order to test plated specimens against the unplated controls, we did not simulate a mid-shaft fracture in any of the tibias. The pin-site defects were intended to reflect the mechanical effects of bicortical defects immediately after pin removal and in the absence of any degree of bone healing. Finally, this study focused on pin-site defects that were distal to a midshaft plate and that may not represent the effects of bicortical pin-site defects proximal to the plate.
Given the results of this biomechanical study in composite tibias, varying the proximity of a bicortical defect to a plate does not affect the torsional stiffness or torsional failure strength of the bone. Placement of an intended bicortical defect should be based on considerations other than the potential for creating a distal stress riser after definitive fracture management.
Am J Orthop. 2017;46(2):E108-E111. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The location of a bicortical defect in proximity to a tibia plate does not appear to affect the torsional stiffness or torsional failure strength of the bone.
- External fixator pin placement should be based on considerations other than the potential for creating a distal stress riser after definitive fracture management.
A stress riser in cortical bone may be considered any abrupt change in the contour or consistency of the hollow structure, such as a surface defect, that not only weakens the bone but concentrates stresses at that transition point.1 A cortical defect that is 20% of the bone diameter is associated with a 34% decrease in torsional strength, thus representing a “stress riser.”2 High-energy and complex tibia fractures are often provisionally stabilized with external fixation that gives the soft tissues time to recover before definitive fracture fixation. Pin diameter for a medium-size tibia external fixator typically is 5.0 mm, resulting in a 10-mm defect in bicortical placement. Therefore, any tibia with a diameter of <50 mm is at risk for a stress riser fracture.
Although it had been established that sizable cortical defects can decrease the torsional strength of long bone,2 the effect of a plate in close proximity to a defect secondary to an empty external-fixator pin site on torsional strength has not been determined. We conducted a study to evaluate this effect. The null hypothesis was there would be no difference in tibia torsional strength attributable to varying the proximity of a tibia midshaft plate to a 5.0-mm bicortical defect.
Methods
Forty fourth-generation, medium-size left composite tibias (Pacific Research Laboratories) were divided into 8 groups of 5 bones (Figure 1).
Torsion testing to failure was performed for all specimens in a manner similar to that described by Gardner and colleagues.3 Impression molds for the composite tibia constructed from polymethylmethacrylate encased the superior and distal ends, leaving 25.5 cm of exposed midshaft. This allowed the composites to be rigidly clamped into a materials testing system (858 Mini-Bionix; MTS) equipped with a 100.0-Nm torsional load cell (Figure 2).
Results
Graphical results for torsional stiffness are presented in Figure 3. R2 for all stiffness calculations was >0.99.
Discussion
Many tibia fractures require provisional stabilization with an external fixator that spans the knee, because of the high-energy nature of the injury or other, higher-priority polytrauma concerns. When the patient or injury is suitable for definitive fixation, the external fixator typically is removed in favor of internal fixation with a plate and screws. Depending on the nature and location of the fracture and the subsequent plate, the empty cortical pin-site defects, often lying at varying distances from the distal end of the plate, can potentially serve as stress risers for fracture.4
Other studies have evaluated long-bone cortical defects biomechanically1,2,4 and clinically,5-7 and multiple studies have been conducted on the effects of plates on long-bone strength for fracture stabilization.8-13 The present study evaluated the torsional strength of long bones in the presence of a bicortical defect and the proximity of the defect to a plate. There were no differences in stiffness or failure load between any of the groups of plated and unplated fourth-generation composite tibias tested to failure in torsion with varying distal bicortical defects. Hypothetically, one would expect the torsional stiffness of these specimens to increase with the mere addition of a metallic diaphyseal plate. However, this study demonstrated that the addition of a plate did not affect the torsional stiffness or strength of the tibias. Clinically, it is common practice to place external fixator pins as far as possible outside the planned incision site for definitive fracture fixation. Thus, we also hypothesized that the presence of a bicortical pin-site defect and its proximity to the plate would alter the torsional strength of the tibia specimens, and that the distal pin-site defect’s location farthest from the plate would exhibit greater strength, but this did not occur. Although other studies have shown that the presence of bicortical defects decreases the strength of long bones, we were unable to quantify this decrease because the 2 intact groups of composites, plated and unplated, survived failure testing.
This study had several limitations, first being the use of composite tibias as opposed to human cadaver bone. Although fourth-generation composite bone models have been validated as a suitable and accurate biomechanical substitute for cadaver specimens,14 anatomical variations in cadaver tibias may transfer forces differently through plates, screws, and distal pin sites. In order to test plated specimens against the unplated controls, we did not simulate a mid-shaft fracture in any of the tibias. The pin-site defects were intended to reflect the mechanical effects of bicortical defects immediately after pin removal and in the absence of any degree of bone healing. Finally, this study focused on pin-site defects that were distal to a midshaft plate and that may not represent the effects of bicortical pin-site defects proximal to the plate.
Given the results of this biomechanical study in composite tibias, varying the proximity of a bicortical defect to a plate does not affect the torsional stiffness or torsional failure strength of the bone. Placement of an intended bicortical defect should be based on considerations other than the potential for creating a distal stress riser after definitive fracture management.
Am J Orthop. 2017;46(2):E108-E111. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
2. Edgerton BC, An KN, Morrey BF. Torsional strength reduction due to cortical defects in bone. J Orthop Res. 1990;8(6):851-855.
3. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
4. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
5. Burstein AH, Currey J, Frankel VH, Heiple KG, Lunseth P, Vessely JC. Bone strength. The effect of screw holes. J Bone Joint Surg Am. 1972;54(6):1143-1156.
6. Clark CR, Morgan C, Sonstegard DA, Matthews LS. The effect of biopsy-hole shape and size on bone strength. J Bone Joint Surg Am. 1977;59(2):213-217.
7. Evans PE, Thomas WG. Tibial fracture through a traction-pin site. A report of two cases. J Bone Joint Surg Am. 1984;66(9):1475-1476.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Klaue K, Fengels I, Perren SM. Long-term effects of plate osteosynthesis: comparison of four different plates. Injury. 2000;31(suppl 2):B51-B62.
10. Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11(2):118-126.
11. Takemoto RC, Sugi MT, Kummer F, Koval KJ, Egol KA. The effects of locked and unlocked neutralization plates on load bearing of fractures fixed with a lag screw. J Orthop Trauma. 2012;26(9):519-522.
12. Wagner M. General principles for the clinical use of the LCP. Injury. 2003;34(suppl 2):B31-B42.
13. Strauss EJ, Schwarzkopf R, Kummer F, Egol KA. The current status of locked plating: the good, the bad, and the ugly. J Orthop Trauma. 2008;22(7):479-486.
14. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
1. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
2. Edgerton BC, An KN, Morrey BF. Torsional strength reduction due to cortical defects in bone. J Orthop Res. 1990;8(6):851-855.
3. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
4. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
5. Burstein AH, Currey J, Frankel VH, Heiple KG, Lunseth P, Vessely JC. Bone strength. The effect of screw holes. J Bone Joint Surg Am. 1972;54(6):1143-1156.
6. Clark CR, Morgan C, Sonstegard DA, Matthews LS. The effect of biopsy-hole shape and size on bone strength. J Bone Joint Surg Am. 1977;59(2):213-217.
7. Evans PE, Thomas WG. Tibial fracture through a traction-pin site. A report of two cases. J Bone Joint Surg Am. 1984;66(9):1475-1476.
8. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11-B19.
9. Klaue K, Fengels I, Perren SM. Long-term effects of plate osteosynthesis: comparison of four different plates. Injury. 2000;31(suppl 2):B51-B62.
10. Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11(2):118-126.
11. Takemoto RC, Sugi MT, Kummer F, Koval KJ, Egol KA. The effects of locked and unlocked neutralization plates on load bearing of fractures fixed with a lag screw. J Orthop Trauma. 2012;26(9):519-522.
12. Wagner M. General principles for the clinical use of the LCP. Injury. 2003;34(suppl 2):B31-B42.
13. Strauss EJ, Schwarzkopf R, Kummer F, Egol KA. The current status of locked plating: the good, the bad, and the ugly. J Orthop Trauma. 2008;22(7):479-486.
14. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
A Rare Case of Spontaneous Fusion of the Knee
Take-Home Points
- Post-infectious or post-inflammatory pathological knee arthrodesis is one of the most challenging complications in orthopedics.
- It can result in significant patient distress with some struggling to maintain any range of motion for functionality.
- TKA for the correction of knee ankylosis is an option, but not without significant morbidity and failure rates.
Spontaneous knee fusion is an unusual and rarely reported phenomenon. Progressive stiffness is commonly experienced by patients with arthritis. However, most patients maintain some range of knee motion, which may be enhanced with medical treatment, rehabilitation with physiotherapy, and ambulation devices. To our knowledge, this article is the first report of a case of spontaneous and progressive bony fusion of a knee joint without a prior diagnosis of inflammatory or septic arthritis or surgical arthrodesis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2015, a 51-year-old woman presented to the orthopedics department with a 13-year history of complete loss of left knee flexion. She denied a history of trauma to or surgical intervention for the knee and denied a medical history of inflammatory or septic arthritis.
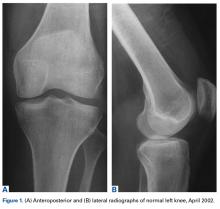
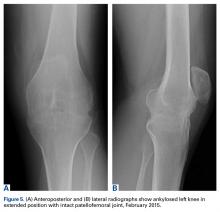
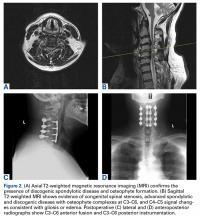
On initial referral to the department, in 2002, the patient, age 38 years at the time, had a 1-year history of progressive left knee stiffness and reduced range of motion (ROM). At the time, she recalled injuring the knee during an aerobics class 2 months prior. A physiotherapy trial (ROM actively and passively assessed 10°-90°) failed. All movement was painful, and 2 crutches were needed for ambulation. The patient was treated nonoperatively with analgesia and was advised to return to physiotherapy. Plain radiographs showed a small effusion but no bony abnormalities or fractures (Figures 1A, 1B).
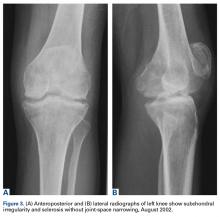
Four months after the initial referral, the patient returned to the outpatient department with persistent knee pain and ROM of 5° to 20°. A repeat radiograph showed extensive left knee joint destruction, cortical irregularity, and narrowing of the joint space (Figures 3A, 3B).
At the latest presentation (2015), the patient had a painless fixed extension deformity of the left knee joint and poor quality of life and wanted surgical intervention.
Discussion
We have reported a rare case of spontaneous knee fusion in a middle-aged patient with no significant predisposing factors and no clear diagnosis. Serologic results were normal and not significant, but imaging was highly suggestive of an inflammatory process and provided a probable diagnosis of an underlying inflammatory condition and/or infection.
In the literature, there are no other reports of similar cases of spontaneous knee joint fusion, though there are some rare cases of the phenomenon in other joints. In 2005, Budoff and Lichtman1 reported a case of spontaneous wrist fusion in an 18-year-old patient with a background of Kienböck disease, which may have predisposed the patient to an underlying synovitis progressing to autofusion of the joint. In 2014, Lui2 described the case of a 64-year-old woman with spontaneous subtalar fusion complicating a subtalar arthroereisis. Although an extensive literature review on the topic is difficult owing to the rarity of the condition, these few cases, unlike our case, appear to describe a predisposing factor or inciting event.
The reversibility of knee arthrodesis remains an issue in our patient’s case and in other cases, and total knee arthroplasty (TKA) may be the most obvious operative intervention. Cameron and Hu3 reported 17 cases of knee fusion take-down with conversion to TKA, and Kim and colleagues4 reported 16 TKAs performed after spontaneous osseous ankylosis and 14 performed after formal knee fusion take-down. Although functional improvements were found in both studies, complication rates were relatively high, at least 53%. Other authors have used TKAs in cases of knee ankylosis after infectious or inflammatory arthritis, but results were suboptimal and unpredictable, and complication rates were 27% and 53.3%.5,6In this difficult scenario, our middle-aged patient’s fixed extension deformity of the knee, likely the result of an idiopathic process, led to severe debilitation and poor quality of life. To perform a TKA in a 51-year-old patient is far from ideal. The reversibility of formally fused and spontaneously fused knees is still in question, and, though there are reports of relatively satisfactory results, most operative options are fraught with complications.
Am J Orthop. 2017;46(2):E83-E85. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Budoff JE, Lichtman DM. Spontaneous wrist fusion: an unusual complication of Kienböck’s disease. J Hand Surg Am. 2005;30(1):59-64.
2. Lui TH. Spontaneous subtalar fusion: an irreversible complication of subtalar arthroereisis. J Foot Ankle Surg. 2014;53(5):652-656.
3. Cameron HU, Hu C. Results of total knee arthroplasty following takedown of formal knee fusion. J Arthroplasty. 1996;11(6):732-737.
4. Kim YH, Kim JS, Cho SH. Total knee arthroplasty after spontaneous osseous ankylosis and takedown of formal knee fusion. J Arthroplasty. 2000;15(4):453-460.
5. Rajgopal A, Ahuja N, Dolai B. Total knee arthroplasty in stiff and ankylosed knees. J Arthroplasty. 2005;20(5):585-590.
6. Kim YH, Cho SH, Kim JS. Total knee arthroplasty in bony ankylosis in gross flexion. J Bone Joint Surg Br. 1999;81(2):296-300.
Take-Home Points
- Post-infectious or post-inflammatory pathological knee arthrodesis is one of the most challenging complications in orthopedics.
- It can result in significant patient distress with some struggling to maintain any range of motion for functionality.
- TKA for the correction of knee ankylosis is an option, but not without significant morbidity and failure rates.
Spontaneous knee fusion is an unusual and rarely reported phenomenon. Progressive stiffness is commonly experienced by patients with arthritis. However, most patients maintain some range of knee motion, which may be enhanced with medical treatment, rehabilitation with physiotherapy, and ambulation devices. To our knowledge, this article is the first report of a case of spontaneous and progressive bony fusion of a knee joint without a prior diagnosis of inflammatory or septic arthritis or surgical arthrodesis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2015, a 51-year-old woman presented to the orthopedics department with a 13-year history of complete loss of left knee flexion. She denied a history of trauma to or surgical intervention for the knee and denied a medical history of inflammatory or septic arthritis.
On initial referral to the department, in 2002, the patient, age 38 years at the time, had a 1-year history of progressive left knee stiffness and reduced range of motion (ROM). At the time, she recalled injuring the knee during an aerobics class 2 months prior. A physiotherapy trial (ROM actively and passively assessed 10°-90°) failed. All movement was painful, and 2 crutches were needed for ambulation. The patient was treated nonoperatively with analgesia and was advised to return to physiotherapy. Plain radiographs showed a small effusion but no bony abnormalities or fractures (Figures 1A, 1B).
Four months after the initial referral, the patient returned to the outpatient department with persistent knee pain and ROM of 5° to 20°. A repeat radiograph showed extensive left knee joint destruction, cortical irregularity, and narrowing of the joint space (Figures 3A, 3B).
At the latest presentation (2015), the patient had a painless fixed extension deformity of the left knee joint and poor quality of life and wanted surgical intervention.
Discussion
We have reported a rare case of spontaneous knee fusion in a middle-aged patient with no significant predisposing factors and no clear diagnosis. Serologic results were normal and not significant, but imaging was highly suggestive of an inflammatory process and provided a probable diagnosis of an underlying inflammatory condition and/or infection.
In the literature, there are no other reports of similar cases of spontaneous knee joint fusion, though there are some rare cases of the phenomenon in other joints. In 2005, Budoff and Lichtman1 reported a case of spontaneous wrist fusion in an 18-year-old patient with a background of Kienböck disease, which may have predisposed the patient to an underlying synovitis progressing to autofusion of the joint. In 2014, Lui2 described the case of a 64-year-old woman with spontaneous subtalar fusion complicating a subtalar arthroereisis. Although an extensive literature review on the topic is difficult owing to the rarity of the condition, these few cases, unlike our case, appear to describe a predisposing factor or inciting event.
The reversibility of knee arthrodesis remains an issue in our patient’s case and in other cases, and total knee arthroplasty (TKA) may be the most obvious operative intervention. Cameron and Hu3 reported 17 cases of knee fusion take-down with conversion to TKA, and Kim and colleagues4 reported 16 TKAs performed after spontaneous osseous ankylosis and 14 performed after formal knee fusion take-down. Although functional improvements were found in both studies, complication rates were relatively high, at least 53%. Other authors have used TKAs in cases of knee ankylosis after infectious or inflammatory arthritis, but results were suboptimal and unpredictable, and complication rates were 27% and 53.3%.5,6In this difficult scenario, our middle-aged patient’s fixed extension deformity of the knee, likely the result of an idiopathic process, led to severe debilitation and poor quality of life. To perform a TKA in a 51-year-old patient is far from ideal. The reversibility of formally fused and spontaneously fused knees is still in question, and, though there are reports of relatively satisfactory results, most operative options are fraught with complications.
Am J Orthop. 2017;46(2):E83-E85. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Post-infectious or post-inflammatory pathological knee arthrodesis is one of the most challenging complications in orthopedics.
- It can result in significant patient distress with some struggling to maintain any range of motion for functionality.
- TKA for the correction of knee ankylosis is an option, but not without significant morbidity and failure rates.
Spontaneous knee fusion is an unusual and rarely reported phenomenon. Progressive stiffness is commonly experienced by patients with arthritis. However, most patients maintain some range of knee motion, which may be enhanced with medical treatment, rehabilitation with physiotherapy, and ambulation devices. To our knowledge, this article is the first report of a case of spontaneous and progressive bony fusion of a knee joint without a prior diagnosis of inflammatory or septic arthritis or surgical arthrodesis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2015, a 51-year-old woman presented to the orthopedics department with a 13-year history of complete loss of left knee flexion. She denied a history of trauma to or surgical intervention for the knee and denied a medical history of inflammatory or septic arthritis.
On initial referral to the department, in 2002, the patient, age 38 years at the time, had a 1-year history of progressive left knee stiffness and reduced range of motion (ROM). At the time, she recalled injuring the knee during an aerobics class 2 months prior. A physiotherapy trial (ROM actively and passively assessed 10°-90°) failed. All movement was painful, and 2 crutches were needed for ambulation. The patient was treated nonoperatively with analgesia and was advised to return to physiotherapy. Plain radiographs showed a small effusion but no bony abnormalities or fractures (Figures 1A, 1B).
Four months after the initial referral, the patient returned to the outpatient department with persistent knee pain and ROM of 5° to 20°. A repeat radiograph showed extensive left knee joint destruction, cortical irregularity, and narrowing of the joint space (Figures 3A, 3B).
At the latest presentation (2015), the patient had a painless fixed extension deformity of the left knee joint and poor quality of life and wanted surgical intervention.
Discussion
We have reported a rare case of spontaneous knee fusion in a middle-aged patient with no significant predisposing factors and no clear diagnosis. Serologic results were normal and not significant, but imaging was highly suggestive of an inflammatory process and provided a probable diagnosis of an underlying inflammatory condition and/or infection.
In the literature, there are no other reports of similar cases of spontaneous knee joint fusion, though there are some rare cases of the phenomenon in other joints. In 2005, Budoff and Lichtman1 reported a case of spontaneous wrist fusion in an 18-year-old patient with a background of Kienböck disease, which may have predisposed the patient to an underlying synovitis progressing to autofusion of the joint. In 2014, Lui2 described the case of a 64-year-old woman with spontaneous subtalar fusion complicating a subtalar arthroereisis. Although an extensive literature review on the topic is difficult owing to the rarity of the condition, these few cases, unlike our case, appear to describe a predisposing factor or inciting event.
The reversibility of knee arthrodesis remains an issue in our patient’s case and in other cases, and total knee arthroplasty (TKA) may be the most obvious operative intervention. Cameron and Hu3 reported 17 cases of knee fusion take-down with conversion to TKA, and Kim and colleagues4 reported 16 TKAs performed after spontaneous osseous ankylosis and 14 performed after formal knee fusion take-down. Although functional improvements were found in both studies, complication rates were relatively high, at least 53%. Other authors have used TKAs in cases of knee ankylosis after infectious or inflammatory arthritis, but results were suboptimal and unpredictable, and complication rates were 27% and 53.3%.5,6In this difficult scenario, our middle-aged patient’s fixed extension deformity of the knee, likely the result of an idiopathic process, led to severe debilitation and poor quality of life. To perform a TKA in a 51-year-old patient is far from ideal. The reversibility of formally fused and spontaneously fused knees is still in question, and, though there are reports of relatively satisfactory results, most operative options are fraught with complications.
Am J Orthop. 2017;46(2):E83-E85. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Budoff JE, Lichtman DM. Spontaneous wrist fusion: an unusual complication of Kienböck’s disease. J Hand Surg Am. 2005;30(1):59-64.
2. Lui TH. Spontaneous subtalar fusion: an irreversible complication of subtalar arthroereisis. J Foot Ankle Surg. 2014;53(5):652-656.
3. Cameron HU, Hu C. Results of total knee arthroplasty following takedown of formal knee fusion. J Arthroplasty. 1996;11(6):732-737.
4. Kim YH, Kim JS, Cho SH. Total knee arthroplasty after spontaneous osseous ankylosis and takedown of formal knee fusion. J Arthroplasty. 2000;15(4):453-460.
5. Rajgopal A, Ahuja N, Dolai B. Total knee arthroplasty in stiff and ankylosed knees. J Arthroplasty. 2005;20(5):585-590.
6. Kim YH, Cho SH, Kim JS. Total knee arthroplasty in bony ankylosis in gross flexion. J Bone Joint Surg Br. 1999;81(2):296-300.
1. Budoff JE, Lichtman DM. Spontaneous wrist fusion: an unusual complication of Kienböck’s disease. J Hand Surg Am. 2005;30(1):59-64.
2. Lui TH. Spontaneous subtalar fusion: an irreversible complication of subtalar arthroereisis. J Foot Ankle Surg. 2014;53(5):652-656.
3. Cameron HU, Hu C. Results of total knee arthroplasty following takedown of formal knee fusion. J Arthroplasty. 1996;11(6):732-737.
4. Kim YH, Kim JS, Cho SH. Total knee arthroplasty after spontaneous osseous ankylosis and takedown of formal knee fusion. J Arthroplasty. 2000;15(4):453-460.
5. Rajgopal A, Ahuja N, Dolai B. Total knee arthroplasty in stiff and ankylosed knees. J Arthroplasty. 2005;20(5):585-590.
6. Kim YH, Cho SH, Kim JS. Total knee arthroplasty in bony ankylosis in gross flexion. J Bone Joint Surg Br. 1999;81(2):296-300.
Pronator Teres Myotendinous Tear
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
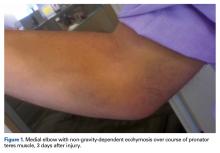
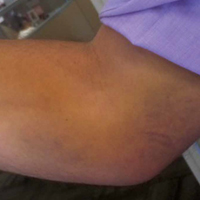
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
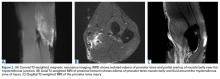
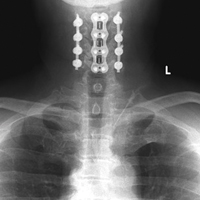
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Guidelines for Treatment of Lateral Patella Dislocations in Skeletally Mature Patients
Take-Home Points
- Lateral patella dislocation is sufficiently treated with modern versions of patellofemoral surgery.
- Comprehensive assessment for underlying osseous pathology is paramount (torsional abnormalities of the femur or tibia, trochlea dysplasia, patella alta, etc).
- In such cases, isolated medial patellofemoral ligament reconstructions will fail. Instead, the underlying osseous abnormalities must be addressed during concomitant procedures (derotational osteotomy, tibial tubercle transfer, trochleoplasty, etc).
The incidence of patellar instability is high, particularly in young females. In principle, cases of patellar instability can be classified as traumatic (dislocation is caused by external, often direct forces) or nontraumatic (anatomy predisposes to instability).1-4
Anatomy Predisposing to Patella Dislocation
Most patients present with specific anatomical factors that predispose to patellar instability (isolated or combined).
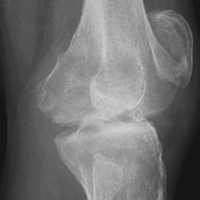
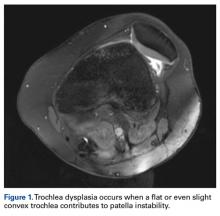
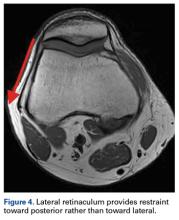
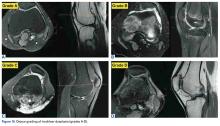
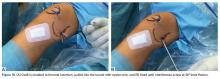
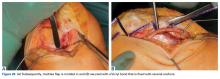
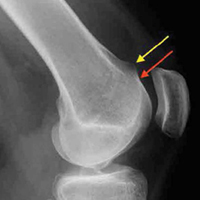
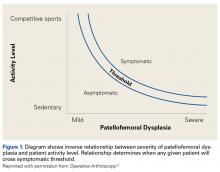
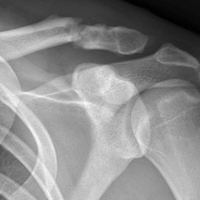
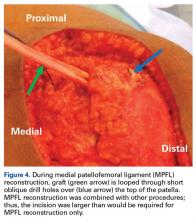
Of the osteochondral factors, dysplasia of the femoral trochlea (trochlea groove [TG]) is most important. In healthy patients, the concave trochlea stabilizes the patella in knee flexion angles above 20°. In particular, the lateral facet of the trochlea plays a key role in withstanding the lateralizing quadriceps vector. The dysplastic trochlea, which has a flat or even a convex surface, destabilizes the patella (Figure 1). Moreover, patella alta is a pivotal factor in the development of LPD.
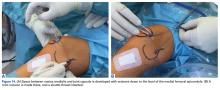
The anteromedial soft tissue of the knee (retinaculum) has 3 layers, the second of which contains the
Diagnostics
Physical Examination
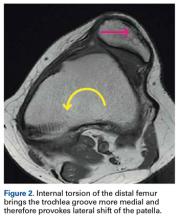
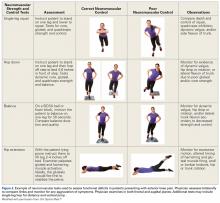
It is recommended that the physician starts the examination by assessing the walking and standing patient while focusing on torsional malalignment of the lower extremities (increased antetorsion of the femur, increased external torsion of the tibia), which is often indicated by squinting patellae.8,27,28
Imaging
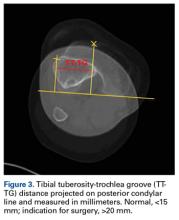
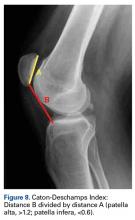
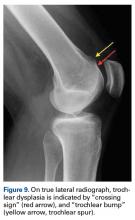
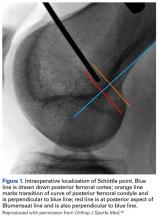
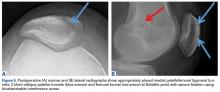
Radiographs are the basis for each patient’s imaging analysis. For a patient with valgus or varus clinical appearance, a weight-bearing whole-leg radiograph is used to precisely assess the degree of deformity in the frontal plane. A true lateral radiograph (congruent posterior condyles) provides information about patellar height (patella alta/infera). Most indices that quantify patellar height use the tibia as reference (eg, tuberosity, anterior aspect of articulation surface).
MRI is the gold standard for LPD diagnosis—it can be used to easily identify soft-tissue lesions and establish their patellar or femoral location (eg, MPFL rupture). MRI also provides information on potential pathologies of quadriceps tendon, patella tendon, and infrapatellar fat pad. Compared with radiographs, MRI is more sensitive in detecting osteochondral lesions in LPD.
Treatment
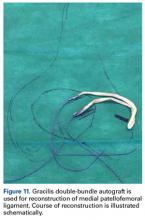
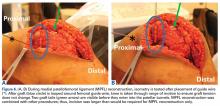
MPFL Reconstruction
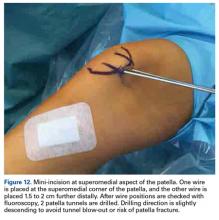
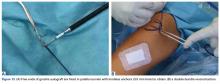
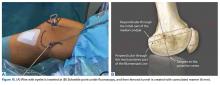
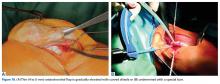
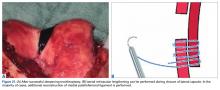
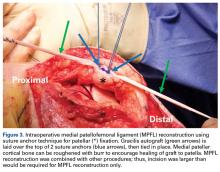
Isolated MPFL reconstruction is commonly regarded as a standard, straightforward procedure.
Trochleoplasty
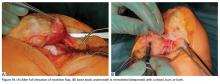
In cases of recurrent LPD or a flat or convex trochlea (Dejour type B, C, or D dysplasia), deepening trochleoplasty should be considered.
Osteotomy
The most popular type of osteotomy in the setting of LPD is the transfer of the TT (TTT).
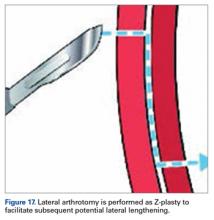
Derotational osteotomies of the femur (externally rotating) provide good outcomes in patients with LPD and associated torsional deformities,61-63 though the literature is incongruent with respect to whether rotational osteotomies of the femur should be performed at the proximal or distal aspect.64-67 In the majority of our LPD cases, we combine femoral derotation with MPFL reconstruction.
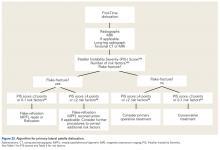
Treatment Algorithms
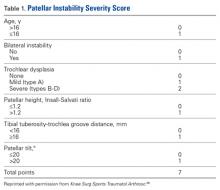
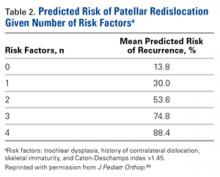
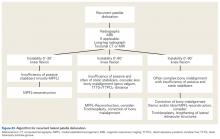
We suggest using different algorithms for primary LPD (Figure 22, Tables 1-2) and recurrent LPD (Figure 23).
Conclusion
In skeletally mature patients, LPD is sufficiently treated with modern versions of patellofemoral surgery. Comprehensive assessment for underlying pathology is paramount as preparation for developing an appropriate surgical plan for the patient.
Am J Orthop. 2017;46(2):E86-E96. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
3. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
4. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
5. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
6. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
7. Biedert RM. Osteotomies [in German]. Orthopade. 2008;37(9):872, 874-876, 878-880 passim.
8. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
9. Lee TQ, Anzel SH, Bennett KA, Pang D, Kim WC. The influence of fixed rotational deformities of the femur on the patellofemoral contact pressures in human cadaver knees. Clin Orthop Relat Res. 1994;(302):69-74.
10. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthroscopy. 2007;23(5):542-553.
11. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546, x.
12. Elias DA, White LM, Fithian DC. Acute lateral patellar dislocation at MR imaging: injury patterns of medial patellar soft-tissue restraints and osteochondral injuries of the inferomedial patella. Radiology. 2002;225(3):736-743.
13. Warren LA, Marshall JL, Girgis F. The prime static stabilizer of the medical side of the knee. J Bone Joint Surg Am. 1974;56(4):665-674.
14. Amis AA. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc. 2007;15(2):48-56.
15. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
16. Conlan T, Garth WP Jr, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
17. Tuxøe JI, Teir M, Winge S, Nielsen PL. The medial patellofemoral ligament: a dissection study. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):138-140.
18. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
19. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
20. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
21. Burks RT, Desio SM, Bachus KN, Tyson L, Springer K. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11(1):24-31.
22. Muneta T, Sekiya I, Tsuchiya M, Shinomiya K. A technique for reconstruction of the medial patellofemoral ligament. Clin Orthop Relat Res. 1999;(359):151-155.
23. Nomura E, Inoue M, Osada N. Augmented repair of avulsion-tear type medial patellofemoral ligament injury in acute patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(5):346-351.
24. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
25. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
26. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):547-554.
27. Scuderi GR. Surgical treatment for patellar instability. Orthop Clin North Am. 1992;23(4):619-630.
28. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
29. Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33(11):677-685.
30. Loudon JK, Wiesner D, Goist-Foley HL, Asjes C, Loudon KL. Intrarater reliability of functional performance tests for subjects with patellofemoral pain syndrome. J Athl Train. 2002;37(3):256-261.
31. Kolowich PA, Paulos LE, Rosenberg TD, Farnsworth S. Lateral release of the patella: indications and contraindications. Am J Sports Med. 1990;18(4):359-365.
32. Fairbank HA. Internal derangement of the knee in children and adolescents: (Section of Orthopaedics). Proc R Soc Med. 1937;30(4):427-432.
33. Hughston JC. Subluxation of the patella. J Bone Joint Surg Am. 1968;50(5):1003-1026.
34. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
35. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
36. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923.
37. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
38. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
39. Mackay ND, Smith NA, Parsons N, Spalding T, Thompson P, Sprowson AP. Medial patellofemoral ligament reconstruction for patellar dislocation: a systematic review. Orthop J Sports Med. 2014;2(8):2325967114544021.
40. Stupay KL, Swart E, Shubin Stein BE. Widespread implementation of medial patellofemoral ligament reconstruction for recurrent patellar instability maintains functional outcomes at midterm to long-term follow-up while decreasing complication rates: a systematic review. Arthroscopy. 2015;31(7):1372-1380.
41. Neumann MV, Stalder M, Schuster AJ. Reconstructive surgery for patellofemoral joint incongruency. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):873-878.
42. Banke IJ, Kohn LM, Meidinger G, et al. Combined trochleoplasty and MPFL reconstruction for treatment of chronic patellofemoral instability: a prospective minimum 2-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2014;22(11):2591-2598.
43. Dejour D, Byn P, Ntagiopoulos PG. The Lyon’s sulcus-deepening trochleoplasty in previous unsuccessful patellofemoral surgery. Int Orthop. 2013;37(3):433-439.
44. Thaunat M, Bessiere C, Pujol N, Boisrenoult P, Beaufils P. Recession wedge trochleoplasty as an additional procedure in the surgical treatment of patellar instability with major trochlear dysplasia: early results. Orthop Traumatol Surg Res. 2011;97(8):833-845.
45. Utting MR, Mulford JS, Eldridge JD. A prospective evaluation of trochleoplasty for the treatment of patellofemoral dislocation and instability. J Bone Joint Surg Br. 2008;90(2):180-185.
46. Blønd L, Haugegaard M. Combined arthroscopic deepening trochleoplasty and reconstruction of the medial patellofemoral ligament for patients with recurrent patella dislocation and trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2484-2490.
47. Nelitz M, Dreyhaupt J, Lippacher S. Combined trochleoplasty and medial patellofemoral ligament reconstruction for recurrent patellar dislocations in severe trochlear dysplasia: a minimum 2-year follow-up study. Am J Sports Med. 2013;41(5):1005-1012.
48. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
49. Biedert R. Trochleoplasty—simple or tricky? Knee. 2014;21(6):1297-1298.
50. Ntagiopoulos PG, Dejour D. Current concepts on trochleoplasty procedures for the surgical treatment of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2531-2539.
51. Nelitz M, Theile M, Dornacher D, Wölfle J, Reichel H, Lippacher S. Analysis of failed surgery for patellar instability in children with open growth plates. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):822-828.
52. Schöttle PB, Fucentese SF, Pfirrmann C, Bereiter H, Romero J. Trochleaplasty for patellar instability due to trochlear dysplasia: a minimum 2-year clinical and radiological follow-up of 19 knees. Acta Orthop. 2005;76(5):693-698.
53. Longo UG, Rizzello G, Ciuffreda M, et al. Elmslie-Trillat, Maquet, Fulkerson, Roux Goldthwait, and other distal realignment procedures for the management of patellar dislocation: systematic review and quantitative synthesis of the literature. Arthroscopy. 2016;32(5):929-943.
54. Barber FA, McGarry JE. Elmslie-Trillat procedure for the treatment of recurrent patellar instability. Arthroscopy. 2008;24(1):77-81.
55. Karataglis D, Green MA, Learmonth DJ. Functional outcome following modified Elmslie-Trillat procedure. Knee. 2006;13(6):464-468.
56. Kumar A, Jones S, Bickerstaff DR, Smith TW. A functional evaluation of the modified Elmslie-Trillat procedure for patello-femoral dysfunction. Knee. 2001;8(4):287-292.
57. Nakagawa K, Wada Y, Minamide M, Tsuchiya A, Moriya H. Deterioration of long-term clinical results after the Elmslie-Trillat procedure for dislocation of the patella. J Bone Joint Surg Br. 2002;84(6):861-864.
58. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
59. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
60. Burnham JM, Howard JS, Hayes CB, Lattermann C. Medial patellofemoral ligament reconstruction with concomitant tibial tubercle transfer: a systematic review of outcomes and complications. Arthroscopy. 2016;32(6):1185-1195.
61. Dickschas J, Harrer J, Pfefferkorn R, Strecker W. Operative treatment of patellofemoral maltracking with torsional osteotomy. Arch Orthop Trauma Surg. 2012;132(3):289-298.
62. Nelitz M, Dreyhaupt J, Williams SR, Dornacher D. Combined supracondylar femoral derotation osteotomy and patellofemoral ligament reconstruction for recurrent patellar dislocation and severe femoral anteversion syndrome: surgical technique and clinical outcome. Int Orthop. 2015;39(12):2355-2362.
63. Strecker W, Dickschas J. Torsional osteotomy: operative treatment of patellofemoral maltracking [in German]. Oper Orthop Traumatol. 2015;27(6):505-524.
64. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
65. Delgado ED, Schoenecker PL, Rich MM, Capelli AM. Treatment of severe torsional malalignment syndrome. J Pediatr Orthop. 1996;16(4):484-488.
66. Dickschas J, Harrer J, Reuter B, Schwitulla J, Strecker W. Torsional osteotomies of the femur. J Orthop Res. 2015;33(3):318-324.
67. Stevens PM, Gililland JM, Anderson LA, Mickelson JB, Nielson J, Klatt JW. Success of torsional correction surgery after failed surgeries for patellofemoral pain and instability. Strategies Trauma Limb Reconstr. 2014;9(1):5-12.
68. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2308-2314.
69. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation [published online October 21, 2015]. J Pediatr Orthop. doi:10.1097/BPO.0000000000000674.
Take-Home Points
- Lateral patella dislocation is sufficiently treated with modern versions of patellofemoral surgery.
- Comprehensive assessment for underlying osseous pathology is paramount (torsional abnormalities of the femur or tibia, trochlea dysplasia, patella alta, etc).
- In such cases, isolated medial patellofemoral ligament reconstructions will fail. Instead, the underlying osseous abnormalities must be addressed during concomitant procedures (derotational osteotomy, tibial tubercle transfer, trochleoplasty, etc).
The incidence of patellar instability is high, particularly in young females. In principle, cases of patellar instability can be classified as traumatic (dislocation is caused by external, often direct forces) or nontraumatic (anatomy predisposes to instability).1-4
Anatomy Predisposing to Patella Dislocation
Most patients present with specific anatomical factors that predispose to patellar instability (isolated or combined).
Of the osteochondral factors, dysplasia of the femoral trochlea (trochlea groove [TG]) is most important. In healthy patients, the concave trochlea stabilizes the patella in knee flexion angles above 20°. In particular, the lateral facet of the trochlea plays a key role in withstanding the lateralizing quadriceps vector. The dysplastic trochlea, which has a flat or even a convex surface, destabilizes the patella (Figure 1). Moreover, patella alta is a pivotal factor in the development of LPD.
The anteromedial soft tissue of the knee (retinaculum) has 3 layers, the second of which contains the
Diagnostics
Physical Examination
It is recommended that the physician starts the examination by assessing the walking and standing patient while focusing on torsional malalignment of the lower extremities (increased antetorsion of the femur, increased external torsion of the tibia), which is often indicated by squinting patellae.8,27,28
Imaging
Radiographs are the basis for each patient’s imaging analysis. For a patient with valgus or varus clinical appearance, a weight-bearing whole-leg radiograph is used to precisely assess the degree of deformity in the frontal plane. A true lateral radiograph (congruent posterior condyles) provides information about patellar height (patella alta/infera). Most indices that quantify patellar height use the tibia as reference (eg, tuberosity, anterior aspect of articulation surface).
MRI is the gold standard for LPD diagnosis—it can be used to easily identify soft-tissue lesions and establish their patellar or femoral location (eg, MPFL rupture). MRI also provides information on potential pathologies of quadriceps tendon, patella tendon, and infrapatellar fat pad. Compared with radiographs, MRI is more sensitive in detecting osteochondral lesions in LPD.
Treatment
MPFL Reconstruction
Isolated MPFL reconstruction is commonly regarded as a standard, straightforward procedure.
Trochleoplasty
In cases of recurrent LPD or a flat or convex trochlea (Dejour type B, C, or D dysplasia), deepening trochleoplasty should be considered.
Osteotomy
The most popular type of osteotomy in the setting of LPD is the transfer of the TT (TTT).
Derotational osteotomies of the femur (externally rotating) provide good outcomes in patients with LPD and associated torsional deformities,61-63 though the literature is incongruent with respect to whether rotational osteotomies of the femur should be performed at the proximal or distal aspect.64-67 In the majority of our LPD cases, we combine femoral derotation with MPFL reconstruction.
Treatment Algorithms
We suggest using different algorithms for primary LPD (Figure 22, Tables 1-2) and recurrent LPD (Figure 23).
Conclusion
In skeletally mature patients, LPD is sufficiently treated with modern versions of patellofemoral surgery. Comprehensive assessment for underlying pathology is paramount as preparation for developing an appropriate surgical plan for the patient.
Am J Orthop. 2017;46(2):E86-E96. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Lateral patella dislocation is sufficiently treated with modern versions of patellofemoral surgery.
- Comprehensive assessment for underlying osseous pathology is paramount (torsional abnormalities of the femur or tibia, trochlea dysplasia, patella alta, etc).
- In such cases, isolated medial patellofemoral ligament reconstructions will fail. Instead, the underlying osseous abnormalities must be addressed during concomitant procedures (derotational osteotomy, tibial tubercle transfer, trochleoplasty, etc).
The incidence of patellar instability is high, particularly in young females. In principle, cases of patellar instability can be classified as traumatic (dislocation is caused by external, often direct forces) or nontraumatic (anatomy predisposes to instability).1-4
Anatomy Predisposing to Patella Dislocation
Most patients present with specific anatomical factors that predispose to patellar instability (isolated or combined).
Of the osteochondral factors, dysplasia of the femoral trochlea (trochlea groove [TG]) is most important. In healthy patients, the concave trochlea stabilizes the patella in knee flexion angles above 20°. In particular, the lateral facet of the trochlea plays a key role in withstanding the lateralizing quadriceps vector. The dysplastic trochlea, which has a flat or even a convex surface, destabilizes the patella (Figure 1). Moreover, patella alta is a pivotal factor in the development of LPD.
The anteromedial soft tissue of the knee (retinaculum) has 3 layers, the second of which contains the
Diagnostics
Physical Examination
It is recommended that the physician starts the examination by assessing the walking and standing patient while focusing on torsional malalignment of the lower extremities (increased antetorsion of the femur, increased external torsion of the tibia), which is often indicated by squinting patellae.8,27,28
Imaging
Radiographs are the basis for each patient’s imaging analysis. For a patient with valgus or varus clinical appearance, a weight-bearing whole-leg radiograph is used to precisely assess the degree of deformity in the frontal plane. A true lateral radiograph (congruent posterior condyles) provides information about patellar height (patella alta/infera). Most indices that quantify patellar height use the tibia as reference (eg, tuberosity, anterior aspect of articulation surface).
MRI is the gold standard for LPD diagnosis—it can be used to easily identify soft-tissue lesions and establish their patellar or femoral location (eg, MPFL rupture). MRI also provides information on potential pathologies of quadriceps tendon, patella tendon, and infrapatellar fat pad. Compared with radiographs, MRI is more sensitive in detecting osteochondral lesions in LPD.
Treatment
MPFL Reconstruction
Isolated MPFL reconstruction is commonly regarded as a standard, straightforward procedure.
Trochleoplasty
In cases of recurrent LPD or a flat or convex trochlea (Dejour type B, C, or D dysplasia), deepening trochleoplasty should be considered.
Osteotomy
The most popular type of osteotomy in the setting of LPD is the transfer of the TT (TTT).
Derotational osteotomies of the femur (externally rotating) provide good outcomes in patients with LPD and associated torsional deformities,61-63 though the literature is incongruent with respect to whether rotational osteotomies of the femur should be performed at the proximal or distal aspect.64-67 In the majority of our LPD cases, we combine femoral derotation with MPFL reconstruction.
Treatment Algorithms
We suggest using different algorithms for primary LPD (Figure 22, Tables 1-2) and recurrent LPD (Figure 23).
Conclusion
In skeletally mature patients, LPD is sufficiently treated with modern versions of patellofemoral surgery. Comprehensive assessment for underlying pathology is paramount as preparation for developing an appropriate surgical plan for the patient.
Am J Orthop. 2017;46(2):E86-E96. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
3. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
4. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
5. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
6. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
7. Biedert RM. Osteotomies [in German]. Orthopade. 2008;37(9):872, 874-876, 878-880 passim.
8. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
9. Lee TQ, Anzel SH, Bennett KA, Pang D, Kim WC. The influence of fixed rotational deformities of the femur on the patellofemoral contact pressures in human cadaver knees. Clin Orthop Relat Res. 1994;(302):69-74.
10. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthroscopy. 2007;23(5):542-553.
11. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546, x.
12. Elias DA, White LM, Fithian DC. Acute lateral patellar dislocation at MR imaging: injury patterns of medial patellar soft-tissue restraints and osteochondral injuries of the inferomedial patella. Radiology. 2002;225(3):736-743.
13. Warren LA, Marshall JL, Girgis F. The prime static stabilizer of the medical side of the knee. J Bone Joint Surg Am. 1974;56(4):665-674.
14. Amis AA. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc. 2007;15(2):48-56.
15. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
16. Conlan T, Garth WP Jr, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
17. Tuxøe JI, Teir M, Winge S, Nielsen PL. The medial patellofemoral ligament: a dissection study. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):138-140.
18. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
19. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
20. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
21. Burks RT, Desio SM, Bachus KN, Tyson L, Springer K. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11(1):24-31.
22. Muneta T, Sekiya I, Tsuchiya M, Shinomiya K. A technique for reconstruction of the medial patellofemoral ligament. Clin Orthop Relat Res. 1999;(359):151-155.
23. Nomura E, Inoue M, Osada N. Augmented repair of avulsion-tear type medial patellofemoral ligament injury in acute patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(5):346-351.
24. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
25. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
26. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):547-554.
27. Scuderi GR. Surgical treatment for patellar instability. Orthop Clin North Am. 1992;23(4):619-630.
28. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
29. Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33(11):677-685.
30. Loudon JK, Wiesner D, Goist-Foley HL, Asjes C, Loudon KL. Intrarater reliability of functional performance tests for subjects with patellofemoral pain syndrome. J Athl Train. 2002;37(3):256-261.
31. Kolowich PA, Paulos LE, Rosenberg TD, Farnsworth S. Lateral release of the patella: indications and contraindications. Am J Sports Med. 1990;18(4):359-365.
32. Fairbank HA. Internal derangement of the knee in children and adolescents: (Section of Orthopaedics). Proc R Soc Med. 1937;30(4):427-432.
33. Hughston JC. Subluxation of the patella. J Bone Joint Surg Am. 1968;50(5):1003-1026.
34. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
35. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
36. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923.
37. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
38. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
39. Mackay ND, Smith NA, Parsons N, Spalding T, Thompson P, Sprowson AP. Medial patellofemoral ligament reconstruction for patellar dislocation: a systematic review. Orthop J Sports Med. 2014;2(8):2325967114544021.
40. Stupay KL, Swart E, Shubin Stein BE. Widespread implementation of medial patellofemoral ligament reconstruction for recurrent patellar instability maintains functional outcomes at midterm to long-term follow-up while decreasing complication rates: a systematic review. Arthroscopy. 2015;31(7):1372-1380.
41. Neumann MV, Stalder M, Schuster AJ. Reconstructive surgery for patellofemoral joint incongruency. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):873-878.
42. Banke IJ, Kohn LM, Meidinger G, et al. Combined trochleoplasty and MPFL reconstruction for treatment of chronic patellofemoral instability: a prospective minimum 2-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2014;22(11):2591-2598.
43. Dejour D, Byn P, Ntagiopoulos PG. The Lyon’s sulcus-deepening trochleoplasty in previous unsuccessful patellofemoral surgery. Int Orthop. 2013;37(3):433-439.
44. Thaunat M, Bessiere C, Pujol N, Boisrenoult P, Beaufils P. Recession wedge trochleoplasty as an additional procedure in the surgical treatment of patellar instability with major trochlear dysplasia: early results. Orthop Traumatol Surg Res. 2011;97(8):833-845.
45. Utting MR, Mulford JS, Eldridge JD. A prospective evaluation of trochleoplasty for the treatment of patellofemoral dislocation and instability. J Bone Joint Surg Br. 2008;90(2):180-185.
46. Blønd L, Haugegaard M. Combined arthroscopic deepening trochleoplasty and reconstruction of the medial patellofemoral ligament for patients with recurrent patella dislocation and trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2484-2490.
47. Nelitz M, Dreyhaupt J, Lippacher S. Combined trochleoplasty and medial patellofemoral ligament reconstruction for recurrent patellar dislocations in severe trochlear dysplasia: a minimum 2-year follow-up study. Am J Sports Med. 2013;41(5):1005-1012.
48. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
49. Biedert R. Trochleoplasty—simple or tricky? Knee. 2014;21(6):1297-1298.
50. Ntagiopoulos PG, Dejour D. Current concepts on trochleoplasty procedures for the surgical treatment of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2531-2539.
51. Nelitz M, Theile M, Dornacher D, Wölfle J, Reichel H, Lippacher S. Analysis of failed surgery for patellar instability in children with open growth plates. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):822-828.
52. Schöttle PB, Fucentese SF, Pfirrmann C, Bereiter H, Romero J. Trochleaplasty for patellar instability due to trochlear dysplasia: a minimum 2-year clinical and radiological follow-up of 19 knees. Acta Orthop. 2005;76(5):693-698.
53. Longo UG, Rizzello G, Ciuffreda M, et al. Elmslie-Trillat, Maquet, Fulkerson, Roux Goldthwait, and other distal realignment procedures for the management of patellar dislocation: systematic review and quantitative synthesis of the literature. Arthroscopy. 2016;32(5):929-943.
54. Barber FA, McGarry JE. Elmslie-Trillat procedure for the treatment of recurrent patellar instability. Arthroscopy. 2008;24(1):77-81.
55. Karataglis D, Green MA, Learmonth DJ. Functional outcome following modified Elmslie-Trillat procedure. Knee. 2006;13(6):464-468.
56. Kumar A, Jones S, Bickerstaff DR, Smith TW. A functional evaluation of the modified Elmslie-Trillat procedure for patello-femoral dysfunction. Knee. 2001;8(4):287-292.
57. Nakagawa K, Wada Y, Minamide M, Tsuchiya A, Moriya H. Deterioration of long-term clinical results after the Elmslie-Trillat procedure for dislocation of the patella. J Bone Joint Surg Br. 2002;84(6):861-864.
58. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
59. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
60. Burnham JM, Howard JS, Hayes CB, Lattermann C. Medial patellofemoral ligament reconstruction with concomitant tibial tubercle transfer: a systematic review of outcomes and complications. Arthroscopy. 2016;32(6):1185-1195.
61. Dickschas J, Harrer J, Pfefferkorn R, Strecker W. Operative treatment of patellofemoral maltracking with torsional osteotomy. Arch Orthop Trauma Surg. 2012;132(3):289-298.
62. Nelitz M, Dreyhaupt J, Williams SR, Dornacher D. Combined supracondylar femoral derotation osteotomy and patellofemoral ligament reconstruction for recurrent patellar dislocation and severe femoral anteversion syndrome: surgical technique and clinical outcome. Int Orthop. 2015;39(12):2355-2362.
63. Strecker W, Dickschas J. Torsional osteotomy: operative treatment of patellofemoral maltracking [in German]. Oper Orthop Traumatol. 2015;27(6):505-524.
64. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
65. Delgado ED, Schoenecker PL, Rich MM, Capelli AM. Treatment of severe torsional malalignment syndrome. J Pediatr Orthop. 1996;16(4):484-488.
66. Dickschas J, Harrer J, Reuter B, Schwitulla J, Strecker W. Torsional osteotomies of the femur. J Orthop Res. 2015;33(3):318-324.
67. Stevens PM, Gililland JM, Anderson LA, Mickelson JB, Nielson J, Klatt JW. Success of torsional correction surgery after failed surgeries for patellofemoral pain and instability. Strategies Trauma Limb Reconstr. 2014;9(1):5-12.
68. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2308-2314.
69. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation [published online October 21, 2015]. J Pediatr Orthop. doi:10.1097/BPO.0000000000000674.
1. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
3. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
4. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
5. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
6. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
7. Biedert RM. Osteotomies [in German]. Orthopade. 2008;37(9):872, 874-876, 878-880 passim.
8. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
9. Lee TQ, Anzel SH, Bennett KA, Pang D, Kim WC. The influence of fixed rotational deformities of the femur on the patellofemoral contact pressures in human cadaver knees. Clin Orthop Relat Res. 1994;(302):69-74.
10. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthroscopy. 2007;23(5):542-553.
11. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546, x.
12. Elias DA, White LM, Fithian DC. Acute lateral patellar dislocation at MR imaging: injury patterns of medial patellar soft-tissue restraints and osteochondral injuries of the inferomedial patella. Radiology. 2002;225(3):736-743.
13. Warren LA, Marshall JL, Girgis F. The prime static stabilizer of the medical side of the knee. J Bone Joint Surg Am. 1974;56(4):665-674.
14. Amis AA. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc. 2007;15(2):48-56.
15. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
16. Conlan T, Garth WP Jr, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
17. Tuxøe JI, Teir M, Winge S, Nielsen PL. The medial patellofemoral ligament: a dissection study. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):138-140.
18. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
19. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
20. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
21. Burks RT, Desio SM, Bachus KN, Tyson L, Springer K. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11(1):24-31.
22. Muneta T, Sekiya I, Tsuchiya M, Shinomiya K. A technique for reconstruction of the medial patellofemoral ligament. Clin Orthop Relat Res. 1999;(359):151-155.
23. Nomura E, Inoue M, Osada N. Augmented repair of avulsion-tear type medial patellofemoral ligament injury in acute patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(5):346-351.
24. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
25. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
26. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):547-554.
27. Scuderi GR. Surgical treatment for patellar instability. Orthop Clin North Am. 1992;23(4):619-630.
28. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
29. Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33(11):677-685.
30. Loudon JK, Wiesner D, Goist-Foley HL, Asjes C, Loudon KL. Intrarater reliability of functional performance tests for subjects with patellofemoral pain syndrome. J Athl Train. 2002;37(3):256-261.
31. Kolowich PA, Paulos LE, Rosenberg TD, Farnsworth S. Lateral release of the patella: indications and contraindications. Am J Sports Med. 1990;18(4):359-365.
32. Fairbank HA. Internal derangement of the knee in children and adolescents: (Section of Orthopaedics). Proc R Soc Med. 1937;30(4):427-432.
33. Hughston JC. Subluxation of the patella. J Bone Joint Surg Am. 1968;50(5):1003-1026.
34. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
35. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
36. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923.
37. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
38. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
39. Mackay ND, Smith NA, Parsons N, Spalding T, Thompson P, Sprowson AP. Medial patellofemoral ligament reconstruction for patellar dislocation: a systematic review. Orthop J Sports Med. 2014;2(8):2325967114544021.
40. Stupay KL, Swart E, Shubin Stein BE. Widespread implementation of medial patellofemoral ligament reconstruction for recurrent patellar instability maintains functional outcomes at midterm to long-term follow-up while decreasing complication rates: a systematic review. Arthroscopy. 2015;31(7):1372-1380.
41. Neumann MV, Stalder M, Schuster AJ. Reconstructive surgery for patellofemoral joint incongruency. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):873-878.
42. Banke IJ, Kohn LM, Meidinger G, et al. Combined trochleoplasty and MPFL reconstruction for treatment of chronic patellofemoral instability: a prospective minimum 2-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2014;22(11):2591-2598.
43. Dejour D, Byn P, Ntagiopoulos PG. The Lyon’s sulcus-deepening trochleoplasty in previous unsuccessful patellofemoral surgery. Int Orthop. 2013;37(3):433-439.
44. Thaunat M, Bessiere C, Pujol N, Boisrenoult P, Beaufils P. Recession wedge trochleoplasty as an additional procedure in the surgical treatment of patellar instability with major trochlear dysplasia: early results. Orthop Traumatol Surg Res. 2011;97(8):833-845.
45. Utting MR, Mulford JS, Eldridge JD. A prospective evaluation of trochleoplasty for the treatment of patellofemoral dislocation and instability. J Bone Joint Surg Br. 2008;90(2):180-185.
46. Blønd L, Haugegaard M. Combined arthroscopic deepening trochleoplasty and reconstruction of the medial patellofemoral ligament for patients with recurrent patella dislocation and trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2484-2490.
47. Nelitz M, Dreyhaupt J, Lippacher S. Combined trochleoplasty and medial patellofemoral ligament reconstruction for recurrent patellar dislocations in severe trochlear dysplasia: a minimum 2-year follow-up study. Am J Sports Med. 2013;41(5):1005-1012.
48. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
49. Biedert R. Trochleoplasty—simple or tricky? Knee. 2014;21(6):1297-1298.
50. Ntagiopoulos PG, Dejour D. Current concepts on trochleoplasty procedures for the surgical treatment of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2531-2539.
51. Nelitz M, Theile M, Dornacher D, Wölfle J, Reichel H, Lippacher S. Analysis of failed surgery for patellar instability in children with open growth plates. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):822-828.
52. Schöttle PB, Fucentese SF, Pfirrmann C, Bereiter H, Romero J. Trochleaplasty for patellar instability due to trochlear dysplasia: a minimum 2-year clinical and radiological follow-up of 19 knees. Acta Orthop. 2005;76(5):693-698.
53. Longo UG, Rizzello G, Ciuffreda M, et al. Elmslie-Trillat, Maquet, Fulkerson, Roux Goldthwait, and other distal realignment procedures for the management of patellar dislocation: systematic review and quantitative synthesis of the literature. Arthroscopy. 2016;32(5):929-943.
54. Barber FA, McGarry JE. Elmslie-Trillat procedure for the treatment of recurrent patellar instability. Arthroscopy. 2008;24(1):77-81.
55. Karataglis D, Green MA, Learmonth DJ. Functional outcome following modified Elmslie-Trillat procedure. Knee. 2006;13(6):464-468.
56. Kumar A, Jones S, Bickerstaff DR, Smith TW. A functional evaluation of the modified Elmslie-Trillat procedure for patello-femoral dysfunction. Knee. 2001;8(4):287-292.
57. Nakagawa K, Wada Y, Minamide M, Tsuchiya A, Moriya H. Deterioration of long-term clinical results after the Elmslie-Trillat procedure for dislocation of the patella. J Bone Joint Surg Br. 2002;84(6):861-864.
58. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
59. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
60. Burnham JM, Howard JS, Hayes CB, Lattermann C. Medial patellofemoral ligament reconstruction with concomitant tibial tubercle transfer: a systematic review of outcomes and complications. Arthroscopy. 2016;32(6):1185-1195.
61. Dickschas J, Harrer J, Pfefferkorn R, Strecker W. Operative treatment of patellofemoral maltracking with torsional osteotomy. Arch Orthop Trauma Surg. 2012;132(3):289-298.
62. Nelitz M, Dreyhaupt J, Williams SR, Dornacher D. Combined supracondylar femoral derotation osteotomy and patellofemoral ligament reconstruction for recurrent patellar dislocation and severe femoral anteversion syndrome: surgical technique and clinical outcome. Int Orthop. 2015;39(12):2355-2362.
63. Strecker W, Dickschas J. Torsional osteotomy: operative treatment of patellofemoral maltracking [in German]. Oper Orthop Traumatol. 2015;27(6):505-524.
64. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
65. Delgado ED, Schoenecker PL, Rich MM, Capelli AM. Treatment of severe torsional malalignment syndrome. J Pediatr Orthop. 1996;16(4):484-488.
66. Dickschas J, Harrer J, Reuter B, Schwitulla J, Strecker W. Torsional osteotomies of the femur. J Orthop Res. 2015;33(3):318-324.
67. Stevens PM, Gililland JM, Anderson LA, Mickelson JB, Nielson J, Klatt JW. Success of torsional correction surgery after failed surgeries for patellofemoral pain and instability. Strategies Trauma Limb Reconstr. 2014;9(1):5-12.
68. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2308-2314.
69. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation [published online October 21, 2015]. J Pediatr Orthop. doi:10.1097/BPO.0000000000000674.
Combined Anterior-Posterior Decompression and Fusion for Cervical Spondylotic Myelopathy
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
The Patellofemoral Compartment: Making Sense of It
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
The Diagnosis and Initial Treatment of Patellofemoral Disorders
Take-Home Points
- Patellofemoral disorders should be classified and diagnosed according to specific diagnostic categories (eg, lateral patellar compression syndrome) based on etiology rather than nondescriptive terminology (eg, internal derangement, patellofemoral pain syndrome).
- Patellofemoral dysplasia defines a spectrum of abnormalities ranging from the mild lateral patellar compression syndrome to the severe recurrent patellar dislocation.
- There is an inverse relationship between patient activity level and underlying patellofemoral dysplasia. This relationship determines threshold levels for each patient becoming symptomatic.
- Patients should be examined for 7 physical abnormalities, and if present, in what severity. These 7 are: vastus medialis obliquus deficiency, medial patellofemoral ligament laxity, lateral retinaculum tightness, increased quadriceps angle, hip abductor weakness, patella alta, and trochlear dysplasia.
- Advanced imaging is rarely, if ever, needed to make a diagnosis or to formulate an initial treatment plan for these common patellofemoral disorders.
To diagnose any disease or disorder implies an understanding of the condition’s cause(s), which should then lead to a logical treatment plan. For all too long, however, the diagnosis and treatment of patellofemoral disorders have been hampered by diagnoses that lack specific definitions based on etiology. A few of these are: internal derangement, chondromalacia patellae, patellar maltracking, and patellofemoral pain syndrome.
To simplify the diagnosis of patellofemoral disorders, we use a clinical classification based on etiology. This system’s defined diagnostic categories are useful in identifying probable cause(s), which can be appropriately evaluated and treated (Table).1 In simple terms, the philosophy of this approach is to try to find out what’s wrong, and try to fix it!
This clinical classification provides a framework for common patellofemoral conditions that are more easily diagnosed, yet is intentionally incomplete omitting rare conditions (eg, tumors, metabolic bone disease, neurologic conditions).
Patellofemoral Dysplasia
Patellofemoral dysplasia (or extensor mechanism malfunction) is a cluster of physical abnormalities relating to the patellofemoral joint that vary from mild to severe and affect the normal function of that joint. As such, patellofemoral dysplasia itself should be considered on a continuum of mild to severe. To simplify the diagnosis, the clinician should systematically identify these factors and their severity. Armed with this information, the clinician can make the diagnosis and formulate a logical treatment plan for each individual patient.
This article focuses on 7 physical abnormalities that are most likely developmental and that can be identified through physical and radiologic examination. When and how each patient with patellofemoral dysplasia becomes symptomatic are determined by 2 key factors: patellofemoral dysplasia severity and activity level (sedentary to strenuous), in an inverse relationship (Figure 1).2
Seven Key Patellofemoral Physical Abnormalities
Of the 7 commonly identified physical abnormalities that affect the normal functioning of the patellofemoral joint, 5 are discovered by physical examination and 2 by radiography; CT and MRI are seldom needed in the initial evaluation. The most accurate and objective method should be used to assess the presence and severity of each abnormality.
The 7 abnormalities are vastus medialis obliquus (VMO) deficiency, medial patellofemoral ligament (MPFL) laxity, lateral retinaculum (LR) tightness, increased quadriceps (Q) angle, hip abductor weakness, patella alta, and trochlear dysplasia. We list these not in order of importance but in the order in which they are usually encountered during initial evaluation. We advocate for examining both knees including axial patellofemoral radiographs because patellofemoral disorders are frequently bilateral. It is helpful to use an abnormality checklist so none are forgotten. Also useful is a simple shorthand for findings: 0 = normal (no abnormality), 1 = mild abnormality, 2 = moderate abnormality, 3 = severe abnormality, with the right knee always recorded first (R/L). For example, severe left MPFL laxity is recorded as 0/3. Numerical values (eg, Q angles) can be directly recorded in this manner: 14°/23°.
1. Vastus Medialis Obliquus Deficiency
VMO deficiency is best seen as the sitting patient actively maintains the unsupported foot and leg at 30° knee flexion. Normally, the VMO inserts into the upper half or third of the medial edge of the patella; a deficient VMO inserts higher into the medial edge of the quadriceps tendon, or it is absent and leaves a characteristic hollow at the medial edge of the patella (Figure 2).4
2. Medial Patellofemoral Ligament Laxity
MPFL laxity is assessed with the lateral glide test. Again, the patient sits, but with quadriceps relaxed and foot and leg supported at 30° knee flexion. With the clinician mentally dividing the patella into vertical quadrants and pushing the patella laterally, the normal patella moves about 1 quadrant or 1 fingerbreadth. Severe MPFL laxity often elicits a positive apprehension response during the test. (Tip: Many patients are unable to relax the quadriceps while sitting; therefore, examine them supine and lift the knee into 30° flexion.) Such laxity usually means the MPFL was torn in a previous dislocation and remains elongated, leaving the patella vulnerable to repeated dislocations. The clinician should be alert to the possibility of hyperelastosis (Ehlers-Danlos syndrome) and a hyper-mobile patella. The opposite limb should be evaluated for asymmetric laxity.
3. Lateral Retinaculum Tightness
LR tightness is assessed with the medial glide test, again with the quadriceps relaxed and the knee supported at 30° flexion. With a normal LR, the patella can be pushed medially about 1 quadrant or 1 fingerbreadth. Some clinicians prefer the lateral tilt-up test, in which the lateral edge of the patella is lifted up, but this method is more difficult to quantify, is affected by the cross-sectional shape of the patella, and lacks consistency.
4. Increased Quadriceps Angle
The Q angle is one of the most important factors in the normal functioning of the patellofemoral joint. For more than a century,8 multiple operations have been used successfully to move the tibial tubercle (TT) and patellar ligament from a lateral position to a medial position thereby decreasing the Q angle. It is only logical to measure this angle at every knee examination to check for an abnormal increase, and the degree. The term quadriceps angle, or Q angle, was first used in 1964 by Brattström,7 who defined it as the “supplemental angle” to the valgus angle formed by the “quadriceps’ resultant” (line of force or vector) “+ patella + ligamentum patellae”. This might be called the dynamic Q angle. With there being no clinical method of measuring the “quadriceps line of force”, or quadriceps vector, clinicians used a line from the anterior superior iliac spine to the center of the patella, yet still called it the Q angle. By convention, this anatomical Q angle has been accepted as the Q angle.
Because the Q angle is the only clinical measurement of TT lateralization at initial evaluation, its measurement should be standardized, accurate, and simple to perform. Placing the patient supine with the lower limb in neutral rotation (patella anterior) and the knee in full extension standardizes the position. In full extension, the tibia reaches its maximum external rotation owing to the terminal “screw home” mechanism. The clinician should center the patella to the trochlear groove (TG) while measuring the Q angle, as it is the relationship of the TT to the trochlea, not to the patella, that is important. If LR tightness prevents the patella from centering, that fact should be recorded during the medial glide test for LR tightness.
Despite the importance of measuring the Q angle, there has been no standard technique. Multiple authors have attempted to define the “normal” Q angle. In 1999, Post9 reviewed 7 articles on the topic and found no agreement. Mean normal Q angles varied widely, from 5° to 23° (SD range, 0.08°-5°). Grelsamer and colleagues,10 using a long-armed goniometer and standard technique, found a mean Q angle of 15.7° for women and 13.3° for men; the small, 2.4° difference between them disappeared when the measurements were corrected for height. Men and women of similar height have similar Q angles. These findings disproved the common misattribution of the differences to the wider female pelvis.
Given this confusion and the lack of accuracy in measuring the Q angle, many, if not most, surgeons turned to special CT and MRI scanning techniques to measure the distance of lateralization from TT to TG (TT-TG distance). This technique, by necessity, enforced a standardization not found in the earlier Q angles studies. Patients were positioned supine with the knee fully extended, and patellar position was ignored in favor of the TG. However, recent articles11-14have called into question the accuracy and usefulness of TT-TG distance as an assessment of TT lateralization. As such, standardized measurement of the Q angle remains a simple, inexpensive, and clinically relevant method of assessing TT lateralization.
The possible causes of an increased Q angle are valgus limb alignment, internal femoral torsion, external tibial torsion, combined internal femoral and external tibial torsion with foot pronation (the “miserable malalignment” of James and colleagues15), and a TT-lateralizing proximal tibial malformation.
5. Hip Abductor Weakness
The step-down test is easily performed in the office by having the patient stand on a short stool or stair and then slowly step down with the opposite limb to just touch the heel and slowly arise again. A positive test is indicated by the Trendelenburg sign, with the pelvis dropping down and away from the symptomatic supporting limb, the flexing knee collapsing into valgus, and the patient tending to wobble and lack stability (Figure 4).16
6. Patella Alta
Patella alta not only allows the patella to escape the confines of the trochlea earlier during active knee extension increasing the risk of patellar dislocation, but also decreases the contact footprint with the trochlea, increasing the patellofemoral joint reaction force and potentially causing patellofemoral pain and even secondary chondrosis. The simplest way to assess patellar height is with a lateral radiograph of the knee. The 3 popular methods (Insall-Salvati, Caton-Deschamps, Blackburn-Peel) all put the normal patellar height ratio at approximately 1:1, ± 20%. Berg and colleagues18 compared radiologic techniques for measuring patellar height ratio and found that Blackburn-Peel was the most accurate, reliable, and reproducible method.
7. Trochlear Dysplasia
Trochlear dysplasia, most simply a flattening of the TG, is perhaps the most important factor effecting normal patellofemoral function. However, it remains the most difficult to correctly address surgically. Senavongse and Amis19 conducted a cadaveric study demonstrating the prime importance of the TG. They found patellar stability was reduced 30% by releasing the VMO, 49% by cutting the MPFL in full knee extension, and 70% by flattening the trochlea. The most common, successful operations for correcting patellar instability depend on changing other factors that guide patellar excursion to compensate for this trochlear flattening.
The simplest way to assess trochlear dysplasia is to measure the sulcus angle on an accurate axial view radiograph of the knee at 45° flexion (Merchant view).20 Dejour and colleagues21 popularized a technique of assessing and classifying trochlear dysplasia from a true lateral radiograph of the knee, which has the advantage of showing the trochlear at its proximal extent. Davies and colleagues22 evaluated the Dejour technique, along with patellar tilt, patellar height, and sulcus angle, to identify a rapid and reproducible radiologic feature that would indicate the need for further analysis by other imaging studies (eg, CT, MRI). They found that, if the sulcus angle was normal, analysis of other radiologic features was unlikely to reveal additional useful information. They also showed a correlation of increasing sulcus angle and severity of those other dysplasia features. Merchant and colleagues20 found a mean normal sulcus angle of 138º (SD, 6º; range, 126º-150º), and Aglietti and colleagues23 confirmed those findings with nearly identical values (mean, 137º; SD, 6º; range, 116º-151º).
Diagnosis and Initial Treatment Plan
Patellofemoral disorders generally are divided into patellofemoral pain and instability, but these 2 diagnostic categories are too broad to be useful. Patellofemoral pain is a symptom. Patellofemoral pain syndrome should never be used as a diagnosis because there is no accepted definition for the cluster of findings that customarily defines a syndrome. At initial evaluation, after the easily diagnosed causes of anterior knee pain (eg, prepatellar bursitis, TT apophysitis, patellar and quadriceps tendinitis) have been ruled out, the clinician should consider types of patellofemoral dysplasia for a presumptive diagnosis, which will then lead to a logical treatment program for each identified disorder. With a presumptive diagnosis established, almost all patients suffering from chronic anterior knee pain without history of injury are treated initially with rest, ice, and nonsteroidal anti-inflammatory drugs to restore joint homeostasis.3
Lateral Patellar Compression Syndrome
In 1975, Ficat and colleagues24 described features of what they called syndrome d’hyperpression externe de la rotule. Two years later, Ficat and Hungerford25 defined the syndrome as one “in which the patella is well centered in the trochlear sulcus and stable, but in which there is a functional lateralization onto a physiologically and often anatomically predominant lateral facet.” Using the tools we have described here, the clinician usually finds the cause(s) of this “functional lateralization.” Four abnormalities—VMO deficiency, LR tightness, increased standardized Q angle, and hip abductor weakness—can cause functional lateralization either alone when severe or in combination when mild or moderate.
For a presumptive diagnosis of LPCS, initial treatment is nonoperative, and successful in about 90% of patients. It should be obvious that most patients with chronic anterior knee pain have quadriceps atrophy. Physical therapy should be specifically focused on quadriceps strengthening, with absolutely no stress placed on the patellofemoral joint in flexion initially, and on hip abductor strengthening. Progressive resistive isometric quadriceps exercises can be performed with a weight-bench technique (Figures 5A-5D).26
Chronic Subluxation of Patella
With the use of axial patellofemoral radiographs (Merchant views),20 the clinician can determine if the “patella is well centered in the trochlear sulcus and stable” (an important part of the definition of LPCS). If the patient has no symptoms of recurrent instability or patellar dislocation, and these radiographs show a laterally subluxed patella (one not well centered in the trochlea), the diagnosis is most likely CSP, a moderate form of patellofemoral dysplasia (section II of the Table). In addition to the 4 abnormalities used in the diagnosis of LPCS (mentioned earlier), trochlear dysplasia also comes into play in the diagnosis of CSP. Just as the other abnormalities can vary from mild to severe, trochlear dysplasia can vary from mild (slightly shallow sulcus angle) to severe (flat or even convex sulcus angle). As the sulcus becomes shallower, the patella slides more laterally, increasing the likelihood of patellar dislocation.
As the patient with CSP gives no history of episodic patellar instability, treatment for CSP is almost identical to that for LPCS, with the primary focus on isometric quadriceps strengthening (DeHaven isometric exercises)27 and hip abductor muscle strengthening. In the presence of CSP radiographically, it is important to use McConnell taping and/or patellar bracing during muscular strengthening. A patient who achieves 20-lb isometric quadriceps strength, demonstrates a normal step-down test, and is assumed to be asymptomatic can be allowed to return to sports activities with use of a patellar brace. The patient should be counseled that there is an increased risk for patellar dislocation because of this chronic subluxation and the shallower sulcus.
As in LPCS, CSP symptoms that persist after dynamic strength is regained may require surgical intervention. The severity of identified abnormal factors (tight LR, increased Q angle, trochlear dysplasia) guides the surgeon in selecting appropriate corrective technique(s).
Recurrent Dislocation of Patella
Admittedly, given the number and subtlety of abnormal factors, the diagnosis of LPCS as a cause of patellofemoral pain can be challenging. However, RDP is at the opposite end of the spectrum. A history of prior patellar dislocation(s) almost always makes the diagnosis of RDP easier. The patient occasionally complains of a recurrent symptom, the knee “going out” or “giving way,” indicating that the diagnosis might be RDP. By carefully asking what the patient was doing and what happened when the knee “went out”, the clinician may be able to determine if the injury stemmed from sudden patellar pain causing reflex inhibition of the quadriceps or was a true dislocation. Both may be described as “going out” or “giving way”.
Assessment for the same 7 abnormalities helps establish the diagnosis, a logical treatment plan, and a guide for indicated surgery. The diagnostic focus is MPFL laxity and trochlear dysplasia. Prior lateral dislocation of the patella almost always requires rupture of the normal MPFL. The infrequent exception is a patient with hyper-elasticity of the skin and multiple joints (Ehlers-Danlos syndrome). Trochlear dysplasia is a significant risk factor for patellar dislocation. If the trochlea is normal and there is no MPFL laxity, the diagnosis of RDP should be questioned.
If surgery is indicated, the surgeon uses a list of the patient’s abnormalities and their severity as a guide in selecting reconstructive techniques. The more abnormalities found and the greater the severity of each, the more techniques are needed to achieve success. Preoperative exercises help speed postoperative recovery by addressing quadriceps and hip abductor weakness. In addition, an active exercise program gives the surgeon insight into the patient’s desire for and commitment to recovery. Other physical abnormalities to be considered in preoperative planning include MPFL laxity, LR tightness, increased Q angle, patella alta, and trochlear dysplasia.
Surgical tips: 1. When releasing the LR, never cut the vastus lateralis tendon, as this has a high likelihood of causing iatrogenic medial patellar subluxation.29 2. When medializing the TT, consider compensating for a shallow trochlea by “over-correcting” the Q angle to 5° to 10° measured with a surgical goniometer intraoperatively.
Summary
Basing clinical classification of disorders on etiology is a simple and effective way to diagnose common patellofemoral conditions. Identifying and rating the severity of patellofemoral dysplasia, using 7 commonly found physical abnormalities, guide the physician to a proper diagnosis and down logical treatment pathways. These principles should be incorporated into the routine evaluation of patellofemoral disorders to optimize diagnosis, formulate a treatment plan, and improve patient outcomes. After all, this is what our patients are asking us to do: Try to find what’s wrong, and then try to fix it!
Am J Orthop. 2017;46(2):68-75. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Merchant AC. Classification of patellofemoral disorders. Arthroscopy. 1988;4(4):235-240.
2. Merchant AC. Patellofemoral disorders: biomechanics, diagnosis, and nonoperative treatment. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, eds. Operative Arthroscopy. New York, NY: Raven Press; 1991:261-275.
3. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
4. Merchant AC. A philosophy of the patellofemoral joint: a logical clinical approach. In: Sanchis-Alfonso V, ed. Anterior Knee Pain and Patellar Instability. 2nd ed. London, England: Springer; 2011:519-530.
5. Jan MH, Lin DH, Lin JJ, Lin CH, Cheng CK, Lin YF. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37(9):1743-1749.
6. Pattyn E, Verdonk P, Steyaert A, et al. Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med. 2011;39(7):1450-1455.
7. Brattström H. Shape of the intercondylar groove normally and in recurrent dislocation of the patella. A clinical and x-ray anatomical investigation. Acta Orthop Scand Suppl. 1964;68:1-147.
8. Roux D. Luxation habituelle de la rotule: traitement operatoire. Rev Chir Orthop Reparatrice Appar Mot. 1888;8:682-689.
9. Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy. 1999;15(8):841-851.
10. Grelsamer RP, Dubey A, Weinstein CH. Men and women have similar Q angles: a clinical and trigonometric evaluation. J Bone Joint Surg Br. 2005;87(11):1498-1501.
11. Skelley N, Friedman M, McGinnis M, Smith C, Hillen T, Matava M. Inter- and intraobserver reliability in the MRI measurement of the tibial tubercle-trochlear groove distance and trochlea dysplasia. Am J Sports Med. 2015;43(4):873-878.
12. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity-trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am. 2015;97(17):1441-1448.
13. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
14. Ridley TJ, Hinckel BB, Kruckeberg BM, Agel J, Arendt EA. Anatomical patella instability risk factors on MRI show sensitivity without specificity in patients with patellofemoral instability: a systematic review. JISAKOS. 2016;1(3):141-152.
15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
16. Powers CM, Souza RB, Fulkerson JP. Patellofemoral joint. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. St. Louis, MO: Saunders Elsevier; 2008:601-636.
17. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22-29.
18. Berg EE, Mason SL, Lucas MJ. Patellar height ratios. A comparison of four measurement methods. Am J Sports Med. 1996;24(2):218-221.
19. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(4):577-582.
20. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391-1396.
21. Dejour H, Neyret P, Walch G. Factors in patellar instability. In: Aichroth PM, Cannon WD Jr, Patel DV, eds. Knee Surgery: Current Practice. London, England: Martin Dunitz; 1992.
22. Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. J Bone Joint Surg Br. 2000;82(8):1162-1166.
23. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
24. Ficat P, Ficat C, Bailieaux A. External hypertension syndrome of the patella. Its significance in the recognition of arthrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1975;61(1):39-59.
25. Ficat P, Hungerford DS. Disorders of the Patellofemoral Joint. Baltimore, MD: Williams & Wilkins; 1977.
26. Merchant AC. The lateral compression syndrome. In: Fox JM, Del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:157-175.
27. DeHaven KE, Dolan WA, Mayer PJ. Chondromalacia patellae in athletes. Clinical presentation and conservative management. Am J Sports Med. 1979;7(1):5-11.
28. Merchant AC. Patellofemoral joint disorders. In: Chapman MW, ed. Operative Orthopedics. Vol 3. Philadelphia, PA: Lippincott; 1988:2321-2366.
29. Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628-1632.
Take-Home Points
- Patellofemoral disorders should be classified and diagnosed according to specific diagnostic categories (eg, lateral patellar compression syndrome) based on etiology rather than nondescriptive terminology (eg, internal derangement, patellofemoral pain syndrome).
- Patellofemoral dysplasia defines a spectrum of abnormalities ranging from the mild lateral patellar compression syndrome to the severe recurrent patellar dislocation.
- There is an inverse relationship between patient activity level and underlying patellofemoral dysplasia. This relationship determines threshold levels for each patient becoming symptomatic.
- Patients should be examined for 7 physical abnormalities, and if present, in what severity. These 7 are: vastus medialis obliquus deficiency, medial patellofemoral ligament laxity, lateral retinaculum tightness, increased quadriceps angle, hip abductor weakness, patella alta, and trochlear dysplasia.
- Advanced imaging is rarely, if ever, needed to make a diagnosis or to formulate an initial treatment plan for these common patellofemoral disorders.
To diagnose any disease or disorder implies an understanding of the condition’s cause(s), which should then lead to a logical treatment plan. For all too long, however, the diagnosis and treatment of patellofemoral disorders have been hampered by diagnoses that lack specific definitions based on etiology. A few of these are: internal derangement, chondromalacia patellae, patellar maltracking, and patellofemoral pain syndrome.
To simplify the diagnosis of patellofemoral disorders, we use a clinical classification based on etiology. This system’s defined diagnostic categories are useful in identifying probable cause(s), which can be appropriately evaluated and treated (Table).1 In simple terms, the philosophy of this approach is to try to find out what’s wrong, and try to fix it!
This clinical classification provides a framework for common patellofemoral conditions that are more easily diagnosed, yet is intentionally incomplete omitting rare conditions (eg, tumors, metabolic bone disease, neurologic conditions).
Patellofemoral Dysplasia
Patellofemoral dysplasia (or extensor mechanism malfunction) is a cluster of physical abnormalities relating to the patellofemoral joint that vary from mild to severe and affect the normal function of that joint. As such, patellofemoral dysplasia itself should be considered on a continuum of mild to severe. To simplify the diagnosis, the clinician should systematically identify these factors and their severity. Armed with this information, the clinician can make the diagnosis and formulate a logical treatment plan for each individual patient.
This article focuses on 7 physical abnormalities that are most likely developmental and that can be identified through physical and radiologic examination. When and how each patient with patellofemoral dysplasia becomes symptomatic are determined by 2 key factors: patellofemoral dysplasia severity and activity level (sedentary to strenuous), in an inverse relationship (Figure 1).2
Seven Key Patellofemoral Physical Abnormalities
Of the 7 commonly identified physical abnormalities that affect the normal functioning of the patellofemoral joint, 5 are discovered by physical examination and 2 by radiography; CT and MRI are seldom needed in the initial evaluation. The most accurate and objective method should be used to assess the presence and severity of each abnormality.
The 7 abnormalities are vastus medialis obliquus (VMO) deficiency, medial patellofemoral ligament (MPFL) laxity, lateral retinaculum (LR) tightness, increased quadriceps (Q) angle, hip abductor weakness, patella alta, and trochlear dysplasia. We list these not in order of importance but in the order in which they are usually encountered during initial evaluation. We advocate for examining both knees including axial patellofemoral radiographs because patellofemoral disorders are frequently bilateral. It is helpful to use an abnormality checklist so none are forgotten. Also useful is a simple shorthand for findings: 0 = normal (no abnormality), 1 = mild abnormality, 2 = moderate abnormality, 3 = severe abnormality, with the right knee always recorded first (R/L). For example, severe left MPFL laxity is recorded as 0/3. Numerical values (eg, Q angles) can be directly recorded in this manner: 14°/23°.
1. Vastus Medialis Obliquus Deficiency
VMO deficiency is best seen as the sitting patient actively maintains the unsupported foot and leg at 30° knee flexion. Normally, the VMO inserts into the upper half or third of the medial edge of the patella; a deficient VMO inserts higher into the medial edge of the quadriceps tendon, or it is absent and leaves a characteristic hollow at the medial edge of the patella (Figure 2).4
2. Medial Patellofemoral Ligament Laxity
MPFL laxity is assessed with the lateral glide test. Again, the patient sits, but with quadriceps relaxed and foot and leg supported at 30° knee flexion. With the clinician mentally dividing the patella into vertical quadrants and pushing the patella laterally, the normal patella moves about 1 quadrant or 1 fingerbreadth. Severe MPFL laxity often elicits a positive apprehension response during the test. (Tip: Many patients are unable to relax the quadriceps while sitting; therefore, examine them supine and lift the knee into 30° flexion.) Such laxity usually means the MPFL was torn in a previous dislocation and remains elongated, leaving the patella vulnerable to repeated dislocations. The clinician should be alert to the possibility of hyperelastosis (Ehlers-Danlos syndrome) and a hyper-mobile patella. The opposite limb should be evaluated for asymmetric laxity.
3. Lateral Retinaculum Tightness
LR tightness is assessed with the medial glide test, again with the quadriceps relaxed and the knee supported at 30° flexion. With a normal LR, the patella can be pushed medially about 1 quadrant or 1 fingerbreadth. Some clinicians prefer the lateral tilt-up test, in which the lateral edge of the patella is lifted up, but this method is more difficult to quantify, is affected by the cross-sectional shape of the patella, and lacks consistency.
4. Increased Quadriceps Angle
The Q angle is one of the most important factors in the normal functioning of the patellofemoral joint. For more than a century,8 multiple operations have been used successfully to move the tibial tubercle (TT) and patellar ligament from a lateral position to a medial position thereby decreasing the Q angle. It is only logical to measure this angle at every knee examination to check for an abnormal increase, and the degree. The term quadriceps angle, or Q angle, was first used in 1964 by Brattström,7 who defined it as the “supplemental angle” to the valgus angle formed by the “quadriceps’ resultant” (line of force or vector) “+ patella + ligamentum patellae”. This might be called the dynamic Q angle. With there being no clinical method of measuring the “quadriceps line of force”, or quadriceps vector, clinicians used a line from the anterior superior iliac spine to the center of the patella, yet still called it the Q angle. By convention, this anatomical Q angle has been accepted as the Q angle.
Because the Q angle is the only clinical measurement of TT lateralization at initial evaluation, its measurement should be standardized, accurate, and simple to perform. Placing the patient supine with the lower limb in neutral rotation (patella anterior) and the knee in full extension standardizes the position. In full extension, the tibia reaches its maximum external rotation owing to the terminal “screw home” mechanism. The clinician should center the patella to the trochlear groove (TG) while measuring the Q angle, as it is the relationship of the TT to the trochlea, not to the patella, that is important. If LR tightness prevents the patella from centering, that fact should be recorded during the medial glide test for LR tightness.
Despite the importance of measuring the Q angle, there has been no standard technique. Multiple authors have attempted to define the “normal” Q angle. In 1999, Post9 reviewed 7 articles on the topic and found no agreement. Mean normal Q angles varied widely, from 5° to 23° (SD range, 0.08°-5°). Grelsamer and colleagues,10 using a long-armed goniometer and standard technique, found a mean Q angle of 15.7° for women and 13.3° for men; the small, 2.4° difference between them disappeared when the measurements were corrected for height. Men and women of similar height have similar Q angles. These findings disproved the common misattribution of the differences to the wider female pelvis.
Given this confusion and the lack of accuracy in measuring the Q angle, many, if not most, surgeons turned to special CT and MRI scanning techniques to measure the distance of lateralization from TT to TG (TT-TG distance). This technique, by necessity, enforced a standardization not found in the earlier Q angles studies. Patients were positioned supine with the knee fully extended, and patellar position was ignored in favor of the TG. However, recent articles11-14have called into question the accuracy and usefulness of TT-TG distance as an assessment of TT lateralization. As such, standardized measurement of the Q angle remains a simple, inexpensive, and clinically relevant method of assessing TT lateralization.
The possible causes of an increased Q angle are valgus limb alignment, internal femoral torsion, external tibial torsion, combined internal femoral and external tibial torsion with foot pronation (the “miserable malalignment” of James and colleagues15), and a TT-lateralizing proximal tibial malformation.
5. Hip Abductor Weakness
The step-down test is easily performed in the office by having the patient stand on a short stool or stair and then slowly step down with the opposite limb to just touch the heel and slowly arise again. A positive test is indicated by the Trendelenburg sign, with the pelvis dropping down and away from the symptomatic supporting limb, the flexing knee collapsing into valgus, and the patient tending to wobble and lack stability (Figure 4).16
6. Patella Alta
Patella alta not only allows the patella to escape the confines of the trochlea earlier during active knee extension increasing the risk of patellar dislocation, but also decreases the contact footprint with the trochlea, increasing the patellofemoral joint reaction force and potentially causing patellofemoral pain and even secondary chondrosis. The simplest way to assess patellar height is with a lateral radiograph of the knee. The 3 popular methods (Insall-Salvati, Caton-Deschamps, Blackburn-Peel) all put the normal patellar height ratio at approximately 1:1, ± 20%. Berg and colleagues18 compared radiologic techniques for measuring patellar height ratio and found that Blackburn-Peel was the most accurate, reliable, and reproducible method.
7. Trochlear Dysplasia
Trochlear dysplasia, most simply a flattening of the TG, is perhaps the most important factor effecting normal patellofemoral function. However, it remains the most difficult to correctly address surgically. Senavongse and Amis19 conducted a cadaveric study demonstrating the prime importance of the TG. They found patellar stability was reduced 30% by releasing the VMO, 49% by cutting the MPFL in full knee extension, and 70% by flattening the trochlea. The most common, successful operations for correcting patellar instability depend on changing other factors that guide patellar excursion to compensate for this trochlear flattening.
The simplest way to assess trochlear dysplasia is to measure the sulcus angle on an accurate axial view radiograph of the knee at 45° flexion (Merchant view).20 Dejour and colleagues21 popularized a technique of assessing and classifying trochlear dysplasia from a true lateral radiograph of the knee, which has the advantage of showing the trochlear at its proximal extent. Davies and colleagues22 evaluated the Dejour technique, along with patellar tilt, patellar height, and sulcus angle, to identify a rapid and reproducible radiologic feature that would indicate the need for further analysis by other imaging studies (eg, CT, MRI). They found that, if the sulcus angle was normal, analysis of other radiologic features was unlikely to reveal additional useful information. They also showed a correlation of increasing sulcus angle and severity of those other dysplasia features. Merchant and colleagues20 found a mean normal sulcus angle of 138º (SD, 6º; range, 126º-150º), and Aglietti and colleagues23 confirmed those findings with nearly identical values (mean, 137º; SD, 6º; range, 116º-151º).
Diagnosis and Initial Treatment Plan
Patellofemoral disorders generally are divided into patellofemoral pain and instability, but these 2 diagnostic categories are too broad to be useful. Patellofemoral pain is a symptom. Patellofemoral pain syndrome should never be used as a diagnosis because there is no accepted definition for the cluster of findings that customarily defines a syndrome. At initial evaluation, after the easily diagnosed causes of anterior knee pain (eg, prepatellar bursitis, TT apophysitis, patellar and quadriceps tendinitis) have been ruled out, the clinician should consider types of patellofemoral dysplasia for a presumptive diagnosis, which will then lead to a logical treatment program for each identified disorder. With a presumptive diagnosis established, almost all patients suffering from chronic anterior knee pain without history of injury are treated initially with rest, ice, and nonsteroidal anti-inflammatory drugs to restore joint homeostasis.3
Lateral Patellar Compression Syndrome
In 1975, Ficat and colleagues24 described features of what they called syndrome d’hyperpression externe de la rotule. Two years later, Ficat and Hungerford25 defined the syndrome as one “in which the patella is well centered in the trochlear sulcus and stable, but in which there is a functional lateralization onto a physiologically and often anatomically predominant lateral facet.” Using the tools we have described here, the clinician usually finds the cause(s) of this “functional lateralization.” Four abnormalities—VMO deficiency, LR tightness, increased standardized Q angle, and hip abductor weakness—can cause functional lateralization either alone when severe or in combination when mild or moderate.
For a presumptive diagnosis of LPCS, initial treatment is nonoperative, and successful in about 90% of patients. It should be obvious that most patients with chronic anterior knee pain have quadriceps atrophy. Physical therapy should be specifically focused on quadriceps strengthening, with absolutely no stress placed on the patellofemoral joint in flexion initially, and on hip abductor strengthening. Progressive resistive isometric quadriceps exercises can be performed with a weight-bench technique (Figures 5A-5D).26
Chronic Subluxation of Patella
With the use of axial patellofemoral radiographs (Merchant views),20 the clinician can determine if the “patella is well centered in the trochlear sulcus and stable” (an important part of the definition of LPCS). If the patient has no symptoms of recurrent instability or patellar dislocation, and these radiographs show a laterally subluxed patella (one not well centered in the trochlea), the diagnosis is most likely CSP, a moderate form of patellofemoral dysplasia (section II of the Table). In addition to the 4 abnormalities used in the diagnosis of LPCS (mentioned earlier), trochlear dysplasia also comes into play in the diagnosis of CSP. Just as the other abnormalities can vary from mild to severe, trochlear dysplasia can vary from mild (slightly shallow sulcus angle) to severe (flat or even convex sulcus angle). As the sulcus becomes shallower, the patella slides more laterally, increasing the likelihood of patellar dislocation.
As the patient with CSP gives no history of episodic patellar instability, treatment for CSP is almost identical to that for LPCS, with the primary focus on isometric quadriceps strengthening (DeHaven isometric exercises)27 and hip abductor muscle strengthening. In the presence of CSP radiographically, it is important to use McConnell taping and/or patellar bracing during muscular strengthening. A patient who achieves 20-lb isometric quadriceps strength, demonstrates a normal step-down test, and is assumed to be asymptomatic can be allowed to return to sports activities with use of a patellar brace. The patient should be counseled that there is an increased risk for patellar dislocation because of this chronic subluxation and the shallower sulcus.
As in LPCS, CSP symptoms that persist after dynamic strength is regained may require surgical intervention. The severity of identified abnormal factors (tight LR, increased Q angle, trochlear dysplasia) guides the surgeon in selecting appropriate corrective technique(s).
Recurrent Dislocation of Patella
Admittedly, given the number and subtlety of abnormal factors, the diagnosis of LPCS as a cause of patellofemoral pain can be challenging. However, RDP is at the opposite end of the spectrum. A history of prior patellar dislocation(s) almost always makes the diagnosis of RDP easier. The patient occasionally complains of a recurrent symptom, the knee “going out” or “giving way,” indicating that the diagnosis might be RDP. By carefully asking what the patient was doing and what happened when the knee “went out”, the clinician may be able to determine if the injury stemmed from sudden patellar pain causing reflex inhibition of the quadriceps or was a true dislocation. Both may be described as “going out” or “giving way”.
Assessment for the same 7 abnormalities helps establish the diagnosis, a logical treatment plan, and a guide for indicated surgery. The diagnostic focus is MPFL laxity and trochlear dysplasia. Prior lateral dislocation of the patella almost always requires rupture of the normal MPFL. The infrequent exception is a patient with hyper-elasticity of the skin and multiple joints (Ehlers-Danlos syndrome). Trochlear dysplasia is a significant risk factor for patellar dislocation. If the trochlea is normal and there is no MPFL laxity, the diagnosis of RDP should be questioned.
If surgery is indicated, the surgeon uses a list of the patient’s abnormalities and their severity as a guide in selecting reconstructive techniques. The more abnormalities found and the greater the severity of each, the more techniques are needed to achieve success. Preoperative exercises help speed postoperative recovery by addressing quadriceps and hip abductor weakness. In addition, an active exercise program gives the surgeon insight into the patient’s desire for and commitment to recovery. Other physical abnormalities to be considered in preoperative planning include MPFL laxity, LR tightness, increased Q angle, patella alta, and trochlear dysplasia.
Surgical tips: 1. When releasing the LR, never cut the vastus lateralis tendon, as this has a high likelihood of causing iatrogenic medial patellar subluxation.29 2. When medializing the TT, consider compensating for a shallow trochlea by “over-correcting” the Q angle to 5° to 10° measured with a surgical goniometer intraoperatively.
Summary
Basing clinical classification of disorders on etiology is a simple and effective way to diagnose common patellofemoral conditions. Identifying and rating the severity of patellofemoral dysplasia, using 7 commonly found physical abnormalities, guide the physician to a proper diagnosis and down logical treatment pathways. These principles should be incorporated into the routine evaluation of patellofemoral disorders to optimize diagnosis, formulate a treatment plan, and improve patient outcomes. After all, this is what our patients are asking us to do: Try to find what’s wrong, and then try to fix it!
Am J Orthop. 2017;46(2):68-75. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patellofemoral disorders should be classified and diagnosed according to specific diagnostic categories (eg, lateral patellar compression syndrome) based on etiology rather than nondescriptive terminology (eg, internal derangement, patellofemoral pain syndrome).
- Patellofemoral dysplasia defines a spectrum of abnormalities ranging from the mild lateral patellar compression syndrome to the severe recurrent patellar dislocation.
- There is an inverse relationship between patient activity level and underlying patellofemoral dysplasia. This relationship determines threshold levels for each patient becoming symptomatic.
- Patients should be examined for 7 physical abnormalities, and if present, in what severity. These 7 are: vastus medialis obliquus deficiency, medial patellofemoral ligament laxity, lateral retinaculum tightness, increased quadriceps angle, hip abductor weakness, patella alta, and trochlear dysplasia.
- Advanced imaging is rarely, if ever, needed to make a diagnosis or to formulate an initial treatment plan for these common patellofemoral disorders.
To diagnose any disease or disorder implies an understanding of the condition’s cause(s), which should then lead to a logical treatment plan. For all too long, however, the diagnosis and treatment of patellofemoral disorders have been hampered by diagnoses that lack specific definitions based on etiology. A few of these are: internal derangement, chondromalacia patellae, patellar maltracking, and patellofemoral pain syndrome.
To simplify the diagnosis of patellofemoral disorders, we use a clinical classification based on etiology. This system’s defined diagnostic categories are useful in identifying probable cause(s), which can be appropriately evaluated and treated (Table).1 In simple terms, the philosophy of this approach is to try to find out what’s wrong, and try to fix it!
This clinical classification provides a framework for common patellofemoral conditions that are more easily diagnosed, yet is intentionally incomplete omitting rare conditions (eg, tumors, metabolic bone disease, neurologic conditions).
Patellofemoral Dysplasia
Patellofemoral dysplasia (or extensor mechanism malfunction) is a cluster of physical abnormalities relating to the patellofemoral joint that vary from mild to severe and affect the normal function of that joint. As such, patellofemoral dysplasia itself should be considered on a continuum of mild to severe. To simplify the diagnosis, the clinician should systematically identify these factors and their severity. Armed with this information, the clinician can make the diagnosis and formulate a logical treatment plan for each individual patient.
This article focuses on 7 physical abnormalities that are most likely developmental and that can be identified through physical and radiologic examination. When and how each patient with patellofemoral dysplasia becomes symptomatic are determined by 2 key factors: patellofemoral dysplasia severity and activity level (sedentary to strenuous), in an inverse relationship (Figure 1).2
Seven Key Patellofemoral Physical Abnormalities
Of the 7 commonly identified physical abnormalities that affect the normal functioning of the patellofemoral joint, 5 are discovered by physical examination and 2 by radiography; CT and MRI are seldom needed in the initial evaluation. The most accurate and objective method should be used to assess the presence and severity of each abnormality.
The 7 abnormalities are vastus medialis obliquus (VMO) deficiency, medial patellofemoral ligament (MPFL) laxity, lateral retinaculum (LR) tightness, increased quadriceps (Q) angle, hip abductor weakness, patella alta, and trochlear dysplasia. We list these not in order of importance but in the order in which they are usually encountered during initial evaluation. We advocate for examining both knees including axial patellofemoral radiographs because patellofemoral disorders are frequently bilateral. It is helpful to use an abnormality checklist so none are forgotten. Also useful is a simple shorthand for findings: 0 = normal (no abnormality), 1 = mild abnormality, 2 = moderate abnormality, 3 = severe abnormality, with the right knee always recorded first (R/L). For example, severe left MPFL laxity is recorded as 0/3. Numerical values (eg, Q angles) can be directly recorded in this manner: 14°/23°.
1. Vastus Medialis Obliquus Deficiency
VMO deficiency is best seen as the sitting patient actively maintains the unsupported foot and leg at 30° knee flexion. Normally, the VMO inserts into the upper half or third of the medial edge of the patella; a deficient VMO inserts higher into the medial edge of the quadriceps tendon, or it is absent and leaves a characteristic hollow at the medial edge of the patella (Figure 2).4
2. Medial Patellofemoral Ligament Laxity
MPFL laxity is assessed with the lateral glide test. Again, the patient sits, but with quadriceps relaxed and foot and leg supported at 30° knee flexion. With the clinician mentally dividing the patella into vertical quadrants and pushing the patella laterally, the normal patella moves about 1 quadrant or 1 fingerbreadth. Severe MPFL laxity often elicits a positive apprehension response during the test. (Tip: Many patients are unable to relax the quadriceps while sitting; therefore, examine them supine and lift the knee into 30° flexion.) Such laxity usually means the MPFL was torn in a previous dislocation and remains elongated, leaving the patella vulnerable to repeated dislocations. The clinician should be alert to the possibility of hyperelastosis (Ehlers-Danlos syndrome) and a hyper-mobile patella. The opposite limb should be evaluated for asymmetric laxity.
3. Lateral Retinaculum Tightness
LR tightness is assessed with the medial glide test, again with the quadriceps relaxed and the knee supported at 30° flexion. With a normal LR, the patella can be pushed medially about 1 quadrant or 1 fingerbreadth. Some clinicians prefer the lateral tilt-up test, in which the lateral edge of the patella is lifted up, but this method is more difficult to quantify, is affected by the cross-sectional shape of the patella, and lacks consistency.
4. Increased Quadriceps Angle
The Q angle is one of the most important factors in the normal functioning of the patellofemoral joint. For more than a century,8 multiple operations have been used successfully to move the tibial tubercle (TT) and patellar ligament from a lateral position to a medial position thereby decreasing the Q angle. It is only logical to measure this angle at every knee examination to check for an abnormal increase, and the degree. The term quadriceps angle, or Q angle, was first used in 1964 by Brattström,7 who defined it as the “supplemental angle” to the valgus angle formed by the “quadriceps’ resultant” (line of force or vector) “+ patella + ligamentum patellae”. This might be called the dynamic Q angle. With there being no clinical method of measuring the “quadriceps line of force”, or quadriceps vector, clinicians used a line from the anterior superior iliac spine to the center of the patella, yet still called it the Q angle. By convention, this anatomical Q angle has been accepted as the Q angle.
Because the Q angle is the only clinical measurement of TT lateralization at initial evaluation, its measurement should be standardized, accurate, and simple to perform. Placing the patient supine with the lower limb in neutral rotation (patella anterior) and the knee in full extension standardizes the position. In full extension, the tibia reaches its maximum external rotation owing to the terminal “screw home” mechanism. The clinician should center the patella to the trochlear groove (TG) while measuring the Q angle, as it is the relationship of the TT to the trochlea, not to the patella, that is important. If LR tightness prevents the patella from centering, that fact should be recorded during the medial glide test for LR tightness.
Despite the importance of measuring the Q angle, there has been no standard technique. Multiple authors have attempted to define the “normal” Q angle. In 1999, Post9 reviewed 7 articles on the topic and found no agreement. Mean normal Q angles varied widely, from 5° to 23° (SD range, 0.08°-5°). Grelsamer and colleagues,10 using a long-armed goniometer and standard technique, found a mean Q angle of 15.7° for women and 13.3° for men; the small, 2.4° difference between them disappeared when the measurements were corrected for height. Men and women of similar height have similar Q angles. These findings disproved the common misattribution of the differences to the wider female pelvis.
Given this confusion and the lack of accuracy in measuring the Q angle, many, if not most, surgeons turned to special CT and MRI scanning techniques to measure the distance of lateralization from TT to TG (TT-TG distance). This technique, by necessity, enforced a standardization not found in the earlier Q angles studies. Patients were positioned supine with the knee fully extended, and patellar position was ignored in favor of the TG. However, recent articles11-14have called into question the accuracy and usefulness of TT-TG distance as an assessment of TT lateralization. As such, standardized measurement of the Q angle remains a simple, inexpensive, and clinically relevant method of assessing TT lateralization.
The possible causes of an increased Q angle are valgus limb alignment, internal femoral torsion, external tibial torsion, combined internal femoral and external tibial torsion with foot pronation (the “miserable malalignment” of James and colleagues15), and a TT-lateralizing proximal tibial malformation.
5. Hip Abductor Weakness
The step-down test is easily performed in the office by having the patient stand on a short stool or stair and then slowly step down with the opposite limb to just touch the heel and slowly arise again. A positive test is indicated by the Trendelenburg sign, with the pelvis dropping down and away from the symptomatic supporting limb, the flexing knee collapsing into valgus, and the patient tending to wobble and lack stability (Figure 4).16
6. Patella Alta
Patella alta not only allows the patella to escape the confines of the trochlea earlier during active knee extension increasing the risk of patellar dislocation, but also decreases the contact footprint with the trochlea, increasing the patellofemoral joint reaction force and potentially causing patellofemoral pain and even secondary chondrosis. The simplest way to assess patellar height is with a lateral radiograph of the knee. The 3 popular methods (Insall-Salvati, Caton-Deschamps, Blackburn-Peel) all put the normal patellar height ratio at approximately 1:1, ± 20%. Berg and colleagues18 compared radiologic techniques for measuring patellar height ratio and found that Blackburn-Peel was the most accurate, reliable, and reproducible method.
7. Trochlear Dysplasia
Trochlear dysplasia, most simply a flattening of the TG, is perhaps the most important factor effecting normal patellofemoral function. However, it remains the most difficult to correctly address surgically. Senavongse and Amis19 conducted a cadaveric study demonstrating the prime importance of the TG. They found patellar stability was reduced 30% by releasing the VMO, 49% by cutting the MPFL in full knee extension, and 70% by flattening the trochlea. The most common, successful operations for correcting patellar instability depend on changing other factors that guide patellar excursion to compensate for this trochlear flattening.
The simplest way to assess trochlear dysplasia is to measure the sulcus angle on an accurate axial view radiograph of the knee at 45° flexion (Merchant view).20 Dejour and colleagues21 popularized a technique of assessing and classifying trochlear dysplasia from a true lateral radiograph of the knee, which has the advantage of showing the trochlear at its proximal extent. Davies and colleagues22 evaluated the Dejour technique, along with patellar tilt, patellar height, and sulcus angle, to identify a rapid and reproducible radiologic feature that would indicate the need for further analysis by other imaging studies (eg, CT, MRI). They found that, if the sulcus angle was normal, analysis of other radiologic features was unlikely to reveal additional useful information. They also showed a correlation of increasing sulcus angle and severity of those other dysplasia features. Merchant and colleagues20 found a mean normal sulcus angle of 138º (SD, 6º; range, 126º-150º), and Aglietti and colleagues23 confirmed those findings with nearly identical values (mean, 137º; SD, 6º; range, 116º-151º).
Diagnosis and Initial Treatment Plan
Patellofemoral disorders generally are divided into patellofemoral pain and instability, but these 2 diagnostic categories are too broad to be useful. Patellofemoral pain is a symptom. Patellofemoral pain syndrome should never be used as a diagnosis because there is no accepted definition for the cluster of findings that customarily defines a syndrome. At initial evaluation, after the easily diagnosed causes of anterior knee pain (eg, prepatellar bursitis, TT apophysitis, patellar and quadriceps tendinitis) have been ruled out, the clinician should consider types of patellofemoral dysplasia for a presumptive diagnosis, which will then lead to a logical treatment program for each identified disorder. With a presumptive diagnosis established, almost all patients suffering from chronic anterior knee pain without history of injury are treated initially with rest, ice, and nonsteroidal anti-inflammatory drugs to restore joint homeostasis.3
Lateral Patellar Compression Syndrome
In 1975, Ficat and colleagues24 described features of what they called syndrome d’hyperpression externe de la rotule. Two years later, Ficat and Hungerford25 defined the syndrome as one “in which the patella is well centered in the trochlear sulcus and stable, but in which there is a functional lateralization onto a physiologically and often anatomically predominant lateral facet.” Using the tools we have described here, the clinician usually finds the cause(s) of this “functional lateralization.” Four abnormalities—VMO deficiency, LR tightness, increased standardized Q angle, and hip abductor weakness—can cause functional lateralization either alone when severe or in combination when mild or moderate.
For a presumptive diagnosis of LPCS, initial treatment is nonoperative, and successful in about 90% of patients. It should be obvious that most patients with chronic anterior knee pain have quadriceps atrophy. Physical therapy should be specifically focused on quadriceps strengthening, with absolutely no stress placed on the patellofemoral joint in flexion initially, and on hip abductor strengthening. Progressive resistive isometric quadriceps exercises can be performed with a weight-bench technique (Figures 5A-5D).26
Chronic Subluxation of Patella
With the use of axial patellofemoral radiographs (Merchant views),20 the clinician can determine if the “patella is well centered in the trochlear sulcus and stable” (an important part of the definition of LPCS). If the patient has no symptoms of recurrent instability or patellar dislocation, and these radiographs show a laterally subluxed patella (one not well centered in the trochlea), the diagnosis is most likely CSP, a moderate form of patellofemoral dysplasia (section II of the Table). In addition to the 4 abnormalities used in the diagnosis of LPCS (mentioned earlier), trochlear dysplasia also comes into play in the diagnosis of CSP. Just as the other abnormalities can vary from mild to severe, trochlear dysplasia can vary from mild (slightly shallow sulcus angle) to severe (flat or even convex sulcus angle). As the sulcus becomes shallower, the patella slides more laterally, increasing the likelihood of patellar dislocation.
As the patient with CSP gives no history of episodic patellar instability, treatment for CSP is almost identical to that for LPCS, with the primary focus on isometric quadriceps strengthening (DeHaven isometric exercises)27 and hip abductor muscle strengthening. In the presence of CSP radiographically, it is important to use McConnell taping and/or patellar bracing during muscular strengthening. A patient who achieves 20-lb isometric quadriceps strength, demonstrates a normal step-down test, and is assumed to be asymptomatic can be allowed to return to sports activities with use of a patellar brace. The patient should be counseled that there is an increased risk for patellar dislocation because of this chronic subluxation and the shallower sulcus.
As in LPCS, CSP symptoms that persist after dynamic strength is regained may require surgical intervention. The severity of identified abnormal factors (tight LR, increased Q angle, trochlear dysplasia) guides the surgeon in selecting appropriate corrective technique(s).
Recurrent Dislocation of Patella
Admittedly, given the number and subtlety of abnormal factors, the diagnosis of LPCS as a cause of patellofemoral pain can be challenging. However, RDP is at the opposite end of the spectrum. A history of prior patellar dislocation(s) almost always makes the diagnosis of RDP easier. The patient occasionally complains of a recurrent symptom, the knee “going out” or “giving way,” indicating that the diagnosis might be RDP. By carefully asking what the patient was doing and what happened when the knee “went out”, the clinician may be able to determine if the injury stemmed from sudden patellar pain causing reflex inhibition of the quadriceps or was a true dislocation. Both may be described as “going out” or “giving way”.
Assessment for the same 7 abnormalities helps establish the diagnosis, a logical treatment plan, and a guide for indicated surgery. The diagnostic focus is MPFL laxity and trochlear dysplasia. Prior lateral dislocation of the patella almost always requires rupture of the normal MPFL. The infrequent exception is a patient with hyper-elasticity of the skin and multiple joints (Ehlers-Danlos syndrome). Trochlear dysplasia is a significant risk factor for patellar dislocation. If the trochlea is normal and there is no MPFL laxity, the diagnosis of RDP should be questioned.
If surgery is indicated, the surgeon uses a list of the patient’s abnormalities and their severity as a guide in selecting reconstructive techniques. The more abnormalities found and the greater the severity of each, the more techniques are needed to achieve success. Preoperative exercises help speed postoperative recovery by addressing quadriceps and hip abductor weakness. In addition, an active exercise program gives the surgeon insight into the patient’s desire for and commitment to recovery. Other physical abnormalities to be considered in preoperative planning include MPFL laxity, LR tightness, increased Q angle, patella alta, and trochlear dysplasia.
Surgical tips: 1. When releasing the LR, never cut the vastus lateralis tendon, as this has a high likelihood of causing iatrogenic medial patellar subluxation.29 2. When medializing the TT, consider compensating for a shallow trochlea by “over-correcting” the Q angle to 5° to 10° measured with a surgical goniometer intraoperatively.
Summary
Basing clinical classification of disorders on etiology is a simple and effective way to diagnose common patellofemoral conditions. Identifying and rating the severity of patellofemoral dysplasia, using 7 commonly found physical abnormalities, guide the physician to a proper diagnosis and down logical treatment pathways. These principles should be incorporated into the routine evaluation of patellofemoral disorders to optimize diagnosis, formulate a treatment plan, and improve patient outcomes. After all, this is what our patients are asking us to do: Try to find what’s wrong, and then try to fix it!
Am J Orthop. 2017;46(2):68-75. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Merchant AC. Classification of patellofemoral disorders. Arthroscopy. 1988;4(4):235-240.
2. Merchant AC. Patellofemoral disorders: biomechanics, diagnosis, and nonoperative treatment. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, eds. Operative Arthroscopy. New York, NY: Raven Press; 1991:261-275.
3. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
4. Merchant AC. A philosophy of the patellofemoral joint: a logical clinical approach. In: Sanchis-Alfonso V, ed. Anterior Knee Pain and Patellar Instability. 2nd ed. London, England: Springer; 2011:519-530.
5. Jan MH, Lin DH, Lin JJ, Lin CH, Cheng CK, Lin YF. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37(9):1743-1749.
6. Pattyn E, Verdonk P, Steyaert A, et al. Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med. 2011;39(7):1450-1455.
7. Brattström H. Shape of the intercondylar groove normally and in recurrent dislocation of the patella. A clinical and x-ray anatomical investigation. Acta Orthop Scand Suppl. 1964;68:1-147.
8. Roux D. Luxation habituelle de la rotule: traitement operatoire. Rev Chir Orthop Reparatrice Appar Mot. 1888;8:682-689.
9. Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy. 1999;15(8):841-851.
10. Grelsamer RP, Dubey A, Weinstein CH. Men and women have similar Q angles: a clinical and trigonometric evaluation. J Bone Joint Surg Br. 2005;87(11):1498-1501.
11. Skelley N, Friedman M, McGinnis M, Smith C, Hillen T, Matava M. Inter- and intraobserver reliability in the MRI measurement of the tibial tubercle-trochlear groove distance and trochlea dysplasia. Am J Sports Med. 2015;43(4):873-878.
12. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity-trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am. 2015;97(17):1441-1448.
13. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
14. Ridley TJ, Hinckel BB, Kruckeberg BM, Agel J, Arendt EA. Anatomical patella instability risk factors on MRI show sensitivity without specificity in patients with patellofemoral instability: a systematic review. JISAKOS. 2016;1(3):141-152.
15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
16. Powers CM, Souza RB, Fulkerson JP. Patellofemoral joint. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. St. Louis, MO: Saunders Elsevier; 2008:601-636.
17. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22-29.
18. Berg EE, Mason SL, Lucas MJ. Patellar height ratios. A comparison of four measurement methods. Am J Sports Med. 1996;24(2):218-221.
19. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(4):577-582.
20. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391-1396.
21. Dejour H, Neyret P, Walch G. Factors in patellar instability. In: Aichroth PM, Cannon WD Jr, Patel DV, eds. Knee Surgery: Current Practice. London, England: Martin Dunitz; 1992.
22. Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. J Bone Joint Surg Br. 2000;82(8):1162-1166.
23. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
24. Ficat P, Ficat C, Bailieaux A. External hypertension syndrome of the patella. Its significance in the recognition of arthrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1975;61(1):39-59.
25. Ficat P, Hungerford DS. Disorders of the Patellofemoral Joint. Baltimore, MD: Williams & Wilkins; 1977.
26. Merchant AC. The lateral compression syndrome. In: Fox JM, Del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:157-175.
27. DeHaven KE, Dolan WA, Mayer PJ. Chondromalacia patellae in athletes. Clinical presentation and conservative management. Am J Sports Med. 1979;7(1):5-11.
28. Merchant AC. Patellofemoral joint disorders. In: Chapman MW, ed. Operative Orthopedics. Vol 3. Philadelphia, PA: Lippincott; 1988:2321-2366.
29. Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628-1632.
1. Merchant AC. Classification of patellofemoral disorders. Arthroscopy. 1988;4(4):235-240.
2. Merchant AC. Patellofemoral disorders: biomechanics, diagnosis, and nonoperative treatment. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, eds. Operative Arthroscopy. New York, NY: Raven Press; 1991:261-275.
3. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
4. Merchant AC. A philosophy of the patellofemoral joint: a logical clinical approach. In: Sanchis-Alfonso V, ed. Anterior Knee Pain and Patellar Instability. 2nd ed. London, England: Springer; 2011:519-530.
5. Jan MH, Lin DH, Lin JJ, Lin CH, Cheng CK, Lin YF. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37(9):1743-1749.
6. Pattyn E, Verdonk P, Steyaert A, et al. Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med. 2011;39(7):1450-1455.
7. Brattström H. Shape of the intercondylar groove normally and in recurrent dislocation of the patella. A clinical and x-ray anatomical investigation. Acta Orthop Scand Suppl. 1964;68:1-147.
8. Roux D. Luxation habituelle de la rotule: traitement operatoire. Rev Chir Orthop Reparatrice Appar Mot. 1888;8:682-689.
9. Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy. 1999;15(8):841-851.
10. Grelsamer RP, Dubey A, Weinstein CH. Men and women have similar Q angles: a clinical and trigonometric evaluation. J Bone Joint Surg Br. 2005;87(11):1498-1501.
11. Skelley N, Friedman M, McGinnis M, Smith C, Hillen T, Matava M. Inter- and intraobserver reliability in the MRI measurement of the tibial tubercle-trochlear groove distance and trochlea dysplasia. Am J Sports Med. 2015;43(4):873-878.
12. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity-trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am. 2015;97(17):1441-1448.
13. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
14. Ridley TJ, Hinckel BB, Kruckeberg BM, Agel J, Arendt EA. Anatomical patella instability risk factors on MRI show sensitivity without specificity in patients with patellofemoral instability: a systematic review. JISAKOS. 2016;1(3):141-152.
15. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
16. Powers CM, Souza RB, Fulkerson JP. Patellofemoral joint. In: Magee DJ, Zachazewski JE, Quillen WS, eds. Pathology and Intervention in Musculoskeletal Rehabilitation. St. Louis, MO: Saunders Elsevier; 2008:601-636.
17. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22-29.
18. Berg EE, Mason SL, Lucas MJ. Patellar height ratios. A comparison of four measurement methods. Am J Sports Med. 1996;24(2):218-221.
19. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br. 2005;87(4):577-582.
20. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391-1396.
21. Dejour H, Neyret P, Walch G. Factors in patellar instability. In: Aichroth PM, Cannon WD Jr, Patel DV, eds. Knee Surgery: Current Practice. London, England: Martin Dunitz; 1992.
22. Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. J Bone Joint Surg Br. 2000;82(8):1162-1166.
23. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
24. Ficat P, Ficat C, Bailieaux A. External hypertension syndrome of the patella. Its significance in the recognition of arthrosis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1975;61(1):39-59.
25. Ficat P, Hungerford DS. Disorders of the Patellofemoral Joint. Baltimore, MD: Williams & Wilkins; 1977.
26. Merchant AC. The lateral compression syndrome. In: Fox JM, Del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:157-175.
27. DeHaven KE, Dolan WA, Mayer PJ. Chondromalacia patellae in athletes. Clinical presentation and conservative management. Am J Sports Med. 1979;7(1):5-11.
28. Merchant AC. Patellofemoral joint disorders. In: Chapman MW, ed. Operative Orthopedics. Vol 3. Philadelphia, PA: Lippincott; 1988:2321-2366.
29. Sanchis-Alfonso V, Merchant AC. Iatrogenic medial patellar instability: an avoidable injury. Arthroscopy. 2015;31(8):1628-1632.
Correct Positioning of the Medial Patellofemoral Ligament: Troubleshooting in the Operating Room
Take-Home Points
- Use fluoroscopy, isometry, or both to double-check the femoral attachment point. Failure to do so can lead to an overtensioned or undertensioned graft caused by anisometric graft placement.
- To minimize the risk of fracture, avoid drilling transverse tunnels across the patella.
- Do not “pre-tension” the medial patellofemoral ligament graft. There should be little or no tension in the graft when the patella is centered in the groove, regardless of the angle of knee flexion.
- The angle of knee flexion during securing of the graft may be important for inaccurate femoral tunnel placement. Before final fixation of the graft, always range the knee fully to make sure full passive motion will be possible once the graft is secured.
- Understanding the anatomy of the MPFL is key before considering reconstructing: That is, fluoroscopy only suggests a “cloud” to begin assessment of the femoral attachment site and is secondary to anatomic references and check of length changes between the attachment point through range of motion. New studies demonstrate the patellar attachment is broad and extends proximally from the historical patellar attachment site to an equal distance along the distal quadriceps.
The medial patellofemoral ligament (MPFL), which is essential in preventing lateral patellar instability, becomes torn in almost 100% of dislocation events.1 Therefore, in cases of failed nonoperative management, this important constraint should be reconstructed. Reconstruction is technically challenging, precision is needed to avoid postoperative complications, and a thorough understanding of the native MPFL anatomy is paramount.
As a thickening of the medial patellar retinaculum, the MPFL connects the medial patella to the medial femur. The femoral insertion has been described a few ways. In a cadaveric study, LaPrade and colleagues2 noted that it inserts 1.9 mm anterior and 3.2 mm distal to the adductor tubercle. Radiographically, the attachment has been described by Schöttle and colleagues3 and Stephen and colleagues.4 These techniques are discussed in more detail later.
The MPFL is a static restraint to lateral patellar translation—it acts only as a checkrein. It functions mainly in 0° to 30° of knee flexion because once the patella engages the trochlear groove, the bony articulation guides the patella during the rest of knee flexion.5 Most authors agree that the native MPFL is mostly isometric, and the re-created ligament should replicate it.6,7 Using cadaveric specimens, Steensen and colleagues6 found that, from 0° to 90° of knee flexion, the distance from the inferior patellar attachment to the superior femoral attachment changed only 1.1 mm.
Biomechanical studies have shown that a MPFL graft with excessive tension predisposes to postoperative abnormal patellofemoral contact pressures, which cause anterior knee pain, loss of knee flexion, and patellofemoral chondrosis.8-10 Furthermore, an overtensioned graft can cause iatrogenic medial patellar subluxation, and an undertensioned graft may still allow for pathologic lateral patellar translation.
Anatomical Bony Insertions
Femoral Insertion
Precise localization of the proper anatomical femoral attachment of the MPFL is a crucial step in reconstruction.11 Small errors in femoral location have resulted in significant loss of graft isometry, increased patellofemoral contact pressures in cadaveric models,4,7 and increased rates of failure after both MPFL repair12 and reconstruction.13 Several methods for confirming proper femoral location during surgery have been described; these methods help obviate the need for large formal dissection of the medial knee.
In a cadaveric study, Schöttle and colleagues3 described a reproducible radiographic point that precisely identifies the appropriate femoral location for MPFL graft placement. The point is located on a standard true lateral radiograph of the distal femur. First, a line is drawn extending the posterior cortex of the femur distally. Next, 2 lines are drawn perpendicular to the first: one intersecting the posterior point of the Blumensaat line, the other intersecting the transition between the posterior femoral condyle and the posterior femoral cortex3 (Figure 1).
Another radiographic method for intraoperatively identifying the anatomical MPFL femoral attachment was described by Stephen and colleagues.4 They used a cadaveric model to confirm radiographic findings and found that the femoral attachment point, taking the anterior-to-posterior medial femoral condyle distance to be 100%, was identified 40% from the posterior border of the medial femoral condyle, 50% from the distal border, and 60% from the anterior border. This simple “40%–50%–60%” normalizing rule for radiographically defining the femoral attachment point is another helpful intraoperative adjunct for templating the appropriate location for graft placement, but calculation in a sterile operative environment can be difficult.
Both of these techniques depend on a perfect lateral radiograph of the knee, as even minor variations in a radiograph can have a dramatic effect on the appearance of the starting point.
Palpation of bony landmarks is another method for preliminarily identifying the appropriate location for femoral pin placement. If done properly, palpation helps obviate the need for corrections when confirming location using isometry or radiography. The center of the femoral attachment of the MPFL can be located in a groove midway between the medial epicondyle and the adductor tubercle.4 Fujino and colleagues15 conducted a cadaveric study of 31 knees in an effort to relate osseous landmarks with the femoral attachment of the MPFL. In all knees, the adductor tubercle was a reliable osseous landmark. The anatomical MPFL attachment was 10.6 mm distal to the apex of the adductor tubercle and was consistent between knees.
Although all these options offer the best available and most reproducible methods for establishing an anatomical femoral graft insertion site, it is important to note that they are based on cadaveric specimens without recurrent patellar instability. Most knees with chronic patellar instability have associated anatomical abnormalities that are not present in nondysplastic cadaveric specimens, which may alter the relationship of osseous landmarks such as the medial epicondyle and adductor tubercle.16 In a recent study of 30 patients with chronic lateral patellar instability, Sanchis-Alfonso and colleagues16 used 3-dimensional computed tomography with these radiographic landmarks and simulated femoral graft attachment sites. They found that the methods of Schöttle and colleagues3 and Stephen and colleagues4 did not provide precise anatomical femoral placement. Ziegler and colleagues14 correlated the anatomical femoral location of the MPFL with the Schöttle point and found the radiographic site to be 4 mm, on average, off the anatomical location. The location of an appropriate anatomical femoral attachment should be confirmed using multiple methods, including palpation of known osseous landmarks, intraoperative fluoroscopy, and, most important, assessment of graft isometry through full range of motion (ROM).
Patellar Insertion
The patellar attachment of the MPFL has received considerably less attention than the femoral attachment.11 Anatomical studies have shown that the MPFL inserts on the superomedial half to third of the patella, in addition to a portion inserting on the undersurface of the vastus medialis.17
Troubleshooting
It is essential to check graft tension through full knee ROM and observe how the graft behaves in order to prevent iatrogenic complications11 (Figures 6A, 6B).
If the graft is secured in high degrees of knee flexion, and the femoral location is not anatomical, a different phenomenon occurs when the knee is brought back into extension. For proximal femoral tunnels, the graft loosens in knee extension and may lead to continued lateral patellar instability. On the other hand, a distal femoral tunnel may result in iatrogenic medial patellar subluxation as the graft becomes too tight in extension.
Correct Amount of Graft Tension
Overtightening the MPFL during fixation is an easy but avoidable mistake. Unlike the anterior cruciate ligament, the MPFL should not be secured while applying maximum tension. Stephen and colleagues7 and Beck and colleagues8 found that tension of only 2 N (~0.5 lb) is needed to accurately re-create the biomechanics of the native graft.
The amount of tension may inadvertently be increased by an interference screw, which tends to pull the graft into the femoral tunnel during insertion. Attention should be given to watching and palpating the graft as the screw is inserted, especially during the last few turns. Turning the screw half a turn backwards after full insertion can release this increased tension and help avoid overtensioning.
Correct Amount of Knee Flexion
This is probably the least studied aspect of MPFL reconstruction. Recommendations range from 0° to 90° of knee flexion during fixation.7,25-30 Most recommendations are surgeon preference, or are based on a sound rationale that lacks supporting research. Tensioning in full extension has been advocated for assessing for the appropriate amount of lateral patellar translation.27 Authors who endorse deeper knee flexion (60°-90°) think that, because the patella engages a deeper trochlear groove in increased flexion, the bony articulation can be used to establish graft length.30,31
Our cadaveric study showed that lower degrees of knee flexion are safest for minimizing the effect of a malpositioned femoral tunnel.26 If femoral tunnel location is not exactly anatomical, any errors are magnified (with even worse graft mechanics) the deeper in flexion the graft is fixed. Once the patella engages the trochlear groove, at about 30° of knee flexion, this can assist in establishing correct graft length. Therefore, we recommend fixation of the graft in 30° to 45° of knee flexion. Our study results also showed that, if femoral tunnel location is anatomical, the graft will be mostly isometric through knee ROM, and, therefore, amount of initial knee flexion does not affect graft behavior.
Regardless of knee flexion chosen, it is imperative to take the knee through full ROM after fixation to ensure the graft does not excessively loosen or tighten in flexion or extension.
Conclusion
MPFL reconstruction is fraught with errors and technical nuances that may be underappreciated. Accurately locating the femoral insertion is crucial to a biomechanically sound graft, and this location should be scrutinized during surgery with accurate radiographs or bony landmarks and verified with knee ROM. Although there is no clear gold standard for fixation and graft options, the graft should be secured while pulling very little tension (2 N) and with the knee in 30° to 45° of flexion to minimize the effect of any inaccuracies in femoral location. Overall, most patients do well after MPFL reconstruction, and attention to surgical technical detail helps maximize the chances of a satisfactory outcome.
Am J Orthop. 2017;46(2):76-81. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
2. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
3. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
4. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879.
5. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
6. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
7. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364-372.
8. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
9. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159.
10. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485.
11. Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175-182.
12. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
13. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
14. Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
15. Fujino K, Tajima G, Yan J, et al. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998-1003.
16. Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838-2844.
17. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
18. Tateishi T, Tsuchiya M, Motosugi N, et al. Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):400-407.
19. Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628-633.
20. Schöttle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147-151.
21. Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Pässler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542-1544.
22. Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431-2437.
23. Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):2325967115569198.
24. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483.
25. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
26. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location [published online April 16, 2016]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4111-4.
27. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
28. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
29. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63.
30. Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211-215.
31. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261.
Take-Home Points
- Use fluoroscopy, isometry, or both to double-check the femoral attachment point. Failure to do so can lead to an overtensioned or undertensioned graft caused by anisometric graft placement.
- To minimize the risk of fracture, avoid drilling transverse tunnels across the patella.
- Do not “pre-tension” the medial patellofemoral ligament graft. There should be little or no tension in the graft when the patella is centered in the groove, regardless of the angle of knee flexion.
- The angle of knee flexion during securing of the graft may be important for inaccurate femoral tunnel placement. Before final fixation of the graft, always range the knee fully to make sure full passive motion will be possible once the graft is secured.
- Understanding the anatomy of the MPFL is key before considering reconstructing: That is, fluoroscopy only suggests a “cloud” to begin assessment of the femoral attachment site and is secondary to anatomic references and check of length changes between the attachment point through range of motion. New studies demonstrate the patellar attachment is broad and extends proximally from the historical patellar attachment site to an equal distance along the distal quadriceps.
The medial patellofemoral ligament (MPFL), which is essential in preventing lateral patellar instability, becomes torn in almost 100% of dislocation events.1 Therefore, in cases of failed nonoperative management, this important constraint should be reconstructed. Reconstruction is technically challenging, precision is needed to avoid postoperative complications, and a thorough understanding of the native MPFL anatomy is paramount.
As a thickening of the medial patellar retinaculum, the MPFL connects the medial patella to the medial femur. The femoral insertion has been described a few ways. In a cadaveric study, LaPrade and colleagues2 noted that it inserts 1.9 mm anterior and 3.2 mm distal to the adductor tubercle. Radiographically, the attachment has been described by Schöttle and colleagues3 and Stephen and colleagues.4 These techniques are discussed in more detail later.
The MPFL is a static restraint to lateral patellar translation—it acts only as a checkrein. It functions mainly in 0° to 30° of knee flexion because once the patella engages the trochlear groove, the bony articulation guides the patella during the rest of knee flexion.5 Most authors agree that the native MPFL is mostly isometric, and the re-created ligament should replicate it.6,7 Using cadaveric specimens, Steensen and colleagues6 found that, from 0° to 90° of knee flexion, the distance from the inferior patellar attachment to the superior femoral attachment changed only 1.1 mm.
Biomechanical studies have shown that a MPFL graft with excessive tension predisposes to postoperative abnormal patellofemoral contact pressures, which cause anterior knee pain, loss of knee flexion, and patellofemoral chondrosis.8-10 Furthermore, an overtensioned graft can cause iatrogenic medial patellar subluxation, and an undertensioned graft may still allow for pathologic lateral patellar translation.
Anatomical Bony Insertions
Femoral Insertion
Precise localization of the proper anatomical femoral attachment of the MPFL is a crucial step in reconstruction.11 Small errors in femoral location have resulted in significant loss of graft isometry, increased patellofemoral contact pressures in cadaveric models,4,7 and increased rates of failure after both MPFL repair12 and reconstruction.13 Several methods for confirming proper femoral location during surgery have been described; these methods help obviate the need for large formal dissection of the medial knee.
In a cadaveric study, Schöttle and colleagues3 described a reproducible radiographic point that precisely identifies the appropriate femoral location for MPFL graft placement. The point is located on a standard true lateral radiograph of the distal femur. First, a line is drawn extending the posterior cortex of the femur distally. Next, 2 lines are drawn perpendicular to the first: one intersecting the posterior point of the Blumensaat line, the other intersecting the transition between the posterior femoral condyle and the posterior femoral cortex3 (Figure 1).
Another radiographic method for intraoperatively identifying the anatomical MPFL femoral attachment was described by Stephen and colleagues.4 They used a cadaveric model to confirm radiographic findings and found that the femoral attachment point, taking the anterior-to-posterior medial femoral condyle distance to be 100%, was identified 40% from the posterior border of the medial femoral condyle, 50% from the distal border, and 60% from the anterior border. This simple “40%–50%–60%” normalizing rule for radiographically defining the femoral attachment point is another helpful intraoperative adjunct for templating the appropriate location for graft placement, but calculation in a sterile operative environment can be difficult.
Both of these techniques depend on a perfect lateral radiograph of the knee, as even minor variations in a radiograph can have a dramatic effect on the appearance of the starting point.
Palpation of bony landmarks is another method for preliminarily identifying the appropriate location for femoral pin placement. If done properly, palpation helps obviate the need for corrections when confirming location using isometry or radiography. The center of the femoral attachment of the MPFL can be located in a groove midway between the medial epicondyle and the adductor tubercle.4 Fujino and colleagues15 conducted a cadaveric study of 31 knees in an effort to relate osseous landmarks with the femoral attachment of the MPFL. In all knees, the adductor tubercle was a reliable osseous landmark. The anatomical MPFL attachment was 10.6 mm distal to the apex of the adductor tubercle and was consistent between knees.
Although all these options offer the best available and most reproducible methods for establishing an anatomical femoral graft insertion site, it is important to note that they are based on cadaveric specimens without recurrent patellar instability. Most knees with chronic patellar instability have associated anatomical abnormalities that are not present in nondysplastic cadaveric specimens, which may alter the relationship of osseous landmarks such as the medial epicondyle and adductor tubercle.16 In a recent study of 30 patients with chronic lateral patellar instability, Sanchis-Alfonso and colleagues16 used 3-dimensional computed tomography with these radiographic landmarks and simulated femoral graft attachment sites. They found that the methods of Schöttle and colleagues3 and Stephen and colleagues4 did not provide precise anatomical femoral placement. Ziegler and colleagues14 correlated the anatomical femoral location of the MPFL with the Schöttle point and found the radiographic site to be 4 mm, on average, off the anatomical location. The location of an appropriate anatomical femoral attachment should be confirmed using multiple methods, including palpation of known osseous landmarks, intraoperative fluoroscopy, and, most important, assessment of graft isometry through full range of motion (ROM).
Patellar Insertion
The patellar attachment of the MPFL has received considerably less attention than the femoral attachment.11 Anatomical studies have shown that the MPFL inserts on the superomedial half to third of the patella, in addition to a portion inserting on the undersurface of the vastus medialis.17
Troubleshooting
It is essential to check graft tension through full knee ROM and observe how the graft behaves in order to prevent iatrogenic complications11 (Figures 6A, 6B).
If the graft is secured in high degrees of knee flexion, and the femoral location is not anatomical, a different phenomenon occurs when the knee is brought back into extension. For proximal femoral tunnels, the graft loosens in knee extension and may lead to continued lateral patellar instability. On the other hand, a distal femoral tunnel may result in iatrogenic medial patellar subluxation as the graft becomes too tight in extension.
Correct Amount of Graft Tension
Overtightening the MPFL during fixation is an easy but avoidable mistake. Unlike the anterior cruciate ligament, the MPFL should not be secured while applying maximum tension. Stephen and colleagues7 and Beck and colleagues8 found that tension of only 2 N (~0.5 lb) is needed to accurately re-create the biomechanics of the native graft.
The amount of tension may inadvertently be increased by an interference screw, which tends to pull the graft into the femoral tunnel during insertion. Attention should be given to watching and palpating the graft as the screw is inserted, especially during the last few turns. Turning the screw half a turn backwards after full insertion can release this increased tension and help avoid overtensioning.
Correct Amount of Knee Flexion
This is probably the least studied aspect of MPFL reconstruction. Recommendations range from 0° to 90° of knee flexion during fixation.7,25-30 Most recommendations are surgeon preference, or are based on a sound rationale that lacks supporting research. Tensioning in full extension has been advocated for assessing for the appropriate amount of lateral patellar translation.27 Authors who endorse deeper knee flexion (60°-90°) think that, because the patella engages a deeper trochlear groove in increased flexion, the bony articulation can be used to establish graft length.30,31
Our cadaveric study showed that lower degrees of knee flexion are safest for minimizing the effect of a malpositioned femoral tunnel.26 If femoral tunnel location is not exactly anatomical, any errors are magnified (with even worse graft mechanics) the deeper in flexion the graft is fixed. Once the patella engages the trochlear groove, at about 30° of knee flexion, this can assist in establishing correct graft length. Therefore, we recommend fixation of the graft in 30° to 45° of knee flexion. Our study results also showed that, if femoral tunnel location is anatomical, the graft will be mostly isometric through knee ROM, and, therefore, amount of initial knee flexion does not affect graft behavior.
Regardless of knee flexion chosen, it is imperative to take the knee through full ROM after fixation to ensure the graft does not excessively loosen or tighten in flexion or extension.
Conclusion
MPFL reconstruction is fraught with errors and technical nuances that may be underappreciated. Accurately locating the femoral insertion is crucial to a biomechanically sound graft, and this location should be scrutinized during surgery with accurate radiographs or bony landmarks and verified with knee ROM. Although there is no clear gold standard for fixation and graft options, the graft should be secured while pulling very little tension (2 N) and with the knee in 30° to 45° of flexion to minimize the effect of any inaccuracies in femoral location. Overall, most patients do well after MPFL reconstruction, and attention to surgical technical detail helps maximize the chances of a satisfactory outcome.
Am J Orthop. 2017;46(2):76-81. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Use fluoroscopy, isometry, or both to double-check the femoral attachment point. Failure to do so can lead to an overtensioned or undertensioned graft caused by anisometric graft placement.
- To minimize the risk of fracture, avoid drilling transverse tunnels across the patella.
- Do not “pre-tension” the medial patellofemoral ligament graft. There should be little or no tension in the graft when the patella is centered in the groove, regardless of the angle of knee flexion.
- The angle of knee flexion during securing of the graft may be important for inaccurate femoral tunnel placement. Before final fixation of the graft, always range the knee fully to make sure full passive motion will be possible once the graft is secured.
- Understanding the anatomy of the MPFL is key before considering reconstructing: That is, fluoroscopy only suggests a “cloud” to begin assessment of the femoral attachment site and is secondary to anatomic references and check of length changes between the attachment point through range of motion. New studies demonstrate the patellar attachment is broad and extends proximally from the historical patellar attachment site to an equal distance along the distal quadriceps.
The medial patellofemoral ligament (MPFL), which is essential in preventing lateral patellar instability, becomes torn in almost 100% of dislocation events.1 Therefore, in cases of failed nonoperative management, this important constraint should be reconstructed. Reconstruction is technically challenging, precision is needed to avoid postoperative complications, and a thorough understanding of the native MPFL anatomy is paramount.
As a thickening of the medial patellar retinaculum, the MPFL connects the medial patella to the medial femur. The femoral insertion has been described a few ways. In a cadaveric study, LaPrade and colleagues2 noted that it inserts 1.9 mm anterior and 3.2 mm distal to the adductor tubercle. Radiographically, the attachment has been described by Schöttle and colleagues3 and Stephen and colleagues.4 These techniques are discussed in more detail later.
The MPFL is a static restraint to lateral patellar translation—it acts only as a checkrein. It functions mainly in 0° to 30° of knee flexion because once the patella engages the trochlear groove, the bony articulation guides the patella during the rest of knee flexion.5 Most authors agree that the native MPFL is mostly isometric, and the re-created ligament should replicate it.6,7 Using cadaveric specimens, Steensen and colleagues6 found that, from 0° to 90° of knee flexion, the distance from the inferior patellar attachment to the superior femoral attachment changed only 1.1 mm.
Biomechanical studies have shown that a MPFL graft with excessive tension predisposes to postoperative abnormal patellofemoral contact pressures, which cause anterior knee pain, loss of knee flexion, and patellofemoral chondrosis.8-10 Furthermore, an overtensioned graft can cause iatrogenic medial patellar subluxation, and an undertensioned graft may still allow for pathologic lateral patellar translation.
Anatomical Bony Insertions
Femoral Insertion
Precise localization of the proper anatomical femoral attachment of the MPFL is a crucial step in reconstruction.11 Small errors in femoral location have resulted in significant loss of graft isometry, increased patellofemoral contact pressures in cadaveric models,4,7 and increased rates of failure after both MPFL repair12 and reconstruction.13 Several methods for confirming proper femoral location during surgery have been described; these methods help obviate the need for large formal dissection of the medial knee.
In a cadaveric study, Schöttle and colleagues3 described a reproducible radiographic point that precisely identifies the appropriate femoral location for MPFL graft placement. The point is located on a standard true lateral radiograph of the distal femur. First, a line is drawn extending the posterior cortex of the femur distally. Next, 2 lines are drawn perpendicular to the first: one intersecting the posterior point of the Blumensaat line, the other intersecting the transition between the posterior femoral condyle and the posterior femoral cortex3 (Figure 1).
Another radiographic method for intraoperatively identifying the anatomical MPFL femoral attachment was described by Stephen and colleagues.4 They used a cadaveric model to confirm radiographic findings and found that the femoral attachment point, taking the anterior-to-posterior medial femoral condyle distance to be 100%, was identified 40% from the posterior border of the medial femoral condyle, 50% from the distal border, and 60% from the anterior border. This simple “40%–50%–60%” normalizing rule for radiographically defining the femoral attachment point is another helpful intraoperative adjunct for templating the appropriate location for graft placement, but calculation in a sterile operative environment can be difficult.
Both of these techniques depend on a perfect lateral radiograph of the knee, as even minor variations in a radiograph can have a dramatic effect on the appearance of the starting point.
Palpation of bony landmarks is another method for preliminarily identifying the appropriate location for femoral pin placement. If done properly, palpation helps obviate the need for corrections when confirming location using isometry or radiography. The center of the femoral attachment of the MPFL can be located in a groove midway between the medial epicondyle and the adductor tubercle.4 Fujino and colleagues15 conducted a cadaveric study of 31 knees in an effort to relate osseous landmarks with the femoral attachment of the MPFL. In all knees, the adductor tubercle was a reliable osseous landmark. The anatomical MPFL attachment was 10.6 mm distal to the apex of the adductor tubercle and was consistent between knees.
Although all these options offer the best available and most reproducible methods for establishing an anatomical femoral graft insertion site, it is important to note that they are based on cadaveric specimens without recurrent patellar instability. Most knees with chronic patellar instability have associated anatomical abnormalities that are not present in nondysplastic cadaveric specimens, which may alter the relationship of osseous landmarks such as the medial epicondyle and adductor tubercle.16 In a recent study of 30 patients with chronic lateral patellar instability, Sanchis-Alfonso and colleagues16 used 3-dimensional computed tomography with these radiographic landmarks and simulated femoral graft attachment sites. They found that the methods of Schöttle and colleagues3 and Stephen and colleagues4 did not provide precise anatomical femoral placement. Ziegler and colleagues14 correlated the anatomical femoral location of the MPFL with the Schöttle point and found the radiographic site to be 4 mm, on average, off the anatomical location. The location of an appropriate anatomical femoral attachment should be confirmed using multiple methods, including palpation of known osseous landmarks, intraoperative fluoroscopy, and, most important, assessment of graft isometry through full range of motion (ROM).
Patellar Insertion
The patellar attachment of the MPFL has received considerably less attention than the femoral attachment.11 Anatomical studies have shown that the MPFL inserts on the superomedial half to third of the patella, in addition to a portion inserting on the undersurface of the vastus medialis.17
Troubleshooting
It is essential to check graft tension through full knee ROM and observe how the graft behaves in order to prevent iatrogenic complications11 (Figures 6A, 6B).
If the graft is secured in high degrees of knee flexion, and the femoral location is not anatomical, a different phenomenon occurs when the knee is brought back into extension. For proximal femoral tunnels, the graft loosens in knee extension and may lead to continued lateral patellar instability. On the other hand, a distal femoral tunnel may result in iatrogenic medial patellar subluxation as the graft becomes too tight in extension.
Correct Amount of Graft Tension
Overtightening the MPFL during fixation is an easy but avoidable mistake. Unlike the anterior cruciate ligament, the MPFL should not be secured while applying maximum tension. Stephen and colleagues7 and Beck and colleagues8 found that tension of only 2 N (~0.5 lb) is needed to accurately re-create the biomechanics of the native graft.
The amount of tension may inadvertently be increased by an interference screw, which tends to pull the graft into the femoral tunnel during insertion. Attention should be given to watching and palpating the graft as the screw is inserted, especially during the last few turns. Turning the screw half a turn backwards after full insertion can release this increased tension and help avoid overtensioning.
Correct Amount of Knee Flexion
This is probably the least studied aspect of MPFL reconstruction. Recommendations range from 0° to 90° of knee flexion during fixation.7,25-30 Most recommendations are surgeon preference, or are based on a sound rationale that lacks supporting research. Tensioning in full extension has been advocated for assessing for the appropriate amount of lateral patellar translation.27 Authors who endorse deeper knee flexion (60°-90°) think that, because the patella engages a deeper trochlear groove in increased flexion, the bony articulation can be used to establish graft length.30,31
Our cadaveric study showed that lower degrees of knee flexion are safest for minimizing the effect of a malpositioned femoral tunnel.26 If femoral tunnel location is not exactly anatomical, any errors are magnified (with even worse graft mechanics) the deeper in flexion the graft is fixed. Once the patella engages the trochlear groove, at about 30° of knee flexion, this can assist in establishing correct graft length. Therefore, we recommend fixation of the graft in 30° to 45° of knee flexion. Our study results also showed that, if femoral tunnel location is anatomical, the graft will be mostly isometric through knee ROM, and, therefore, amount of initial knee flexion does not affect graft behavior.
Regardless of knee flexion chosen, it is imperative to take the knee through full ROM after fixation to ensure the graft does not excessively loosen or tighten in flexion or extension.
Conclusion
MPFL reconstruction is fraught with errors and technical nuances that may be underappreciated. Accurately locating the femoral insertion is crucial to a biomechanically sound graft, and this location should be scrutinized during surgery with accurate radiographs or bony landmarks and verified with knee ROM. Although there is no clear gold standard for fixation and graft options, the graft should be secured while pulling very little tension (2 N) and with the knee in 30° to 45° of flexion to minimize the effect of any inaccuracies in femoral location. Overall, most patients do well after MPFL reconstruction, and attention to surgical technical detail helps maximize the chances of a satisfactory outcome.
Am J Orthop. 2017;46(2):76-81. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
2. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
3. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
4. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879.
5. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
6. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
7. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364-372.
8. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
9. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159.
10. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485.
11. Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175-182.
12. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
13. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
14. Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
15. Fujino K, Tajima G, Yan J, et al. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998-1003.
16. Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838-2844.
17. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
18. Tateishi T, Tsuchiya M, Motosugi N, et al. Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):400-407.
19. Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628-633.
20. Schöttle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147-151.
21. Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Pässler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542-1544.
22. Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431-2437.
23. Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):2325967115569198.
24. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483.
25. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
26. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location [published online April 16, 2016]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4111-4.
27. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
28. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
29. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63.
30. Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211-215.
31. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261.
1. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
2. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
3. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
4. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879.
5. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
6. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513.
7. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364-372.
8. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
9. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159.
10. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485.
11. Sanchis-Alfonso V. Guidelines for medial patellofemoral ligament reconstruction in chronic lateral patellar instability. J Am Acad Orthop Surg. 2014;22(3):175-182.
12. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
13. Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med. 2014;42(3):716-722.
14. Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
15. Fujino K, Tajima G, Yan J, et al. Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):998-1003.
16. Sanchis-Alfonso V, Ramirez-Fuentes C, Montesinos-Berry E, Aparisi-Rodriguez F, Martí-Bonmatí L. Does radiographic location ensure precise anatomic location of the femoral fixation site in medial patellofemoral ligament surgery? Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2838-2844.
17. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
18. Tateishi T, Tsuchiya M, Motosugi N, et al. Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):400-407.
19. Mariani PP, Liguori L, Cerullo G, Iannella G, Floris L. Arthroscopic patellar reinsertion of the MPFL in acute patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):628-633.
20. Schöttle PB, Hensler D, Imhoff AB. Anatomical double-bundle MPFL reconstruction with an aperture fixation. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):147-151.
21. Siebold R, Chikale S, Sartory N, Hariri N, Feil S, Pässler HH. Hamstring graft fixation in MPFL reconstruction at the patella using a transosseous suture technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1542-1544.
22. Song SY, Kim IS, Chang HG, Shin JH, Kim HJ, Seo YJ. Anatomic medial patellofemoral ligament reconstruction using patellar suture anchor fixation for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2431-2437.
23. Burrus MT, Werner BC, Conte EJ, Diduch DR. Troubleshooting the femoral attachment during medial patellofemoral ligament reconstruction: location, location, location. Orthop J Sports Med. 2015;3(1):2325967115569198.
24. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483.
25. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
26. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location [published online April 16, 2016]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4111-4.
27. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
28. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
29. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63.
30. Nomura E, Horiuchi Y, Kihara M. A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee. 2000;7(4):211-215.
31. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261.
Clinical Rehabilitation of Anterior Knee Pain: Current Concepts
Take-Home Points
- Ensure that relative rest and activity modification allow the knee to stay within the available “envelope of function” of the joint.
- Careful physical examination is imperative to assess strength, flexibility, and altered movement patterns, which in many cases are all part of the etiology of AKP.
- Patience and perseverance are paramount. Patients need to clearly understand the goals of rehabilitation as well as the concepts related to “envelope of function” so they can continue to keep themselves within this envelope. This education is crucial to their success.
- Only once a patient has been brought into the pain-free functional envelope can rehabilitation be redirected to expanding the envelope toward the patient’s particular goals.
- Quantity does not equal quality. To create an appropriate care plan, the physician must assess the adequacy of the patient’s rehabilitation thus far—ask specific questions about the types of exercises the patient is doing in physical therapy and quickly assess strength with a few simple in-office tests.
Anterior knee pain (AKP) is a common presentation. Although the exact etiology and nature of AKP continue to be poorly understood, overuse principles can be useful in directing treatment. In overuse injury, repetitive submaximal or subclinical trauma results in macroscopic trauma, microscopic trauma, or both. The structural tissue unit is damaged or its clinical responsiveness is exceeded, which can lead to pain or movement dysfunction. Overuse injuries commonly have an endogenous source, mechanical circumstances in which the musculoskeletal tissue is subjected to more tensile force or stress than the tissue can tolerate. The approach to treatment and rehabilitation of AKP is best facilitated with a thorough understanding of the concept of tissue homeostasis and the “envelope of function.”
Although the cause of AKP is multifactorial, the contributions of muscle strength deficits, diminished neuromuscular control, and altered muscle firing patterns to the development and severity of AKP are well established.1-5 The hallmark of nonoperative management of AKP is physiotherapy that re-establishes strength, neuromuscular control, muscle activation, and optimal biomechanics during daily activities, advancing to graded levels of sporting activities.
The purpose of this paper is to discuss the factors associated with the diminished neuromuscular control observed in AKP and to review appropriate rehabilitation concepts for patients with AKP. Practical tools are provided to aid the surgeon to identify neuromuscular deficits in the clinic setting, along with assessing the adequacy of prior therapy and the need for further rehabilitation.
Common Neuromuscular Deficits in AKP
Weakness of the knee extensor muscles has long been implicated as the main issue in AKP, and therefore the focus of rehabilitation has been on muscle strengthening, especially of the vastus medialis obliquus. Research has found that knee extensor weakness is not only a characteristic of patients with AKP but a risk factor for developing AKP.4 Restoration of knee extensor strength and function is essential for recovery.6 Another issue in AKP may be incorrect firing of the knee extensor muscles. Altered vastus medialis obliquus response time and a motor control deficit of the quadriceps musculature have been demonstrated.7,8 Restoration of knee extensor strength, though important, is too often the sole focus of some rehabilitation programs.
Hip muscle weakness has also been implicated as an important component of AKP.9-12 Impaired gluteal muscle function can lead to increased hip joint adduction and internal rotation during activities such as stair climbing, squatting, and sports.9,10,13 In a systematic review, Meira and Brumitt12 concluded that hip strength and position are linked to AKP and that patients with AKP present with a common deficit once symptomatic. The dysfunction in neuromuscular control in AKP may also stem from disordered firing sequences in the muscles. A systematic review of hip electromyographic studies found moderate to strong evidence that gluteus medius muscle activity is delayed and of shorter duration during stair ascent and descent in patients with AKP.11 The study also found some evidence that this activity is delayed and of shorter duration during running and that gluteus maximus muscle activity is increased during stair descent. The authors recommended that interventions focused on correcting these deficits—such as hip strengthening, biofeedback, and gait retraining—should be included in AKP treatment and research.
In recent AKP research, the core, including hip and abdominal muscles, demonstrated decreased strength and altered recruitment patterns during functional movement.14,15 The authors recommended including core strengthening and core stability exercises in AKP management. In combination, these knee extensor, hip, and core strength deficits in patients with AKP lead to altered movement patterns during functional activities and may in turn exacerbate symptoms. Addressing both the strength deficits and the recruitment patterns of these core and lower extremity muscles is essential for optimizing rehabilitation and limiting recurrence of AKP symptoms.
Stretching to improve muscle tendon length is another component of AKP treatment. Reduced quadriceps muscle length has been implicated as a cause of AKP and is a common finding in symptomatic patients.16 In addition, a recent randomized controlled trial found decreased hip flexibility in patients with AKP.17 It is important to assess the flexibility of the gastrocnemius, soleus, quadriceps, and hamstrings muscles and the iliotibial band, as well as the hip flexors, extensors, and rotators, so that rehabilitation can be designed to address any specific deficits in range of motion (ROM).16-23 In patients with AKP, it is also important to address muscle tendon length deficits and strengthening simultaneously to avoid exceeding the available envelope of function. Gaining full ROM at joints can facilitate increasing strength gains24 and potentially improve the synergy of muscle contractions during functional activities.
Appropriate Rehabilitation in AKP
Appropriate rehabilitation addresses all identified strength and flexibility deficits in order to improve functional biomechanics and normalize altered body movement patterns during daily activities (eg, walking, squatting, stair climbing). Often, if part of the kinetic chain is weak or injured, the body engages in an activity by “working around” the injured body part. This change often results in faulty body mechanics or altered movement patterns. In AKP, these modified biomechanics can result in pain centered on the patella and associated soft-tissue structures. In developing ways to compensate for strength and ROM deficits, patients with AKP exacerbate their symptoms. In long-standing AKP, these compensatory strategies are most often unintentional and ingrained.
The main role of physical therapists is to identify any faulty movement patterns, dissect the underlying neuromuscular causes of these deficits, and build an individualized rehabilitation program. Physical therapy should be customized to the patient’s level of strength and fitness and whenever possible should be made challenging (and fun!) for the patient. The exercises should be increased in intensity and duration as the patient improves strength, endurance, and control in the activities. The patient’s response to each intervention will help guide exercise progression and define the need for further treatments.
Patients should be assessed for overuse patterns. Overuse can occur with repetitive exercise activity, such as running, or with repetitive work activity that involves lifting, squatting, or stair climbing. It is important to modify or reduce such activity to ensure that a patient with AKP remains within an envelope of pain-free function. Once the patient is functioning in this envelope, rehabilitation can be redirected to expand it, while improving strength, coordination, balance, and overall dynamic control of the core and lower limbs.
The purpose of any rehabilitation program is to build strength through the entire kinetic chain, focusing on hip and core strength initially, and then adding concentric and eccentric lower limb strength. Having a strong base from which to initiate lower limb movements makes correct lower limb form more likely to follow. Corrected muscle firing patterns allow for appropriate sequencing of the muscle activation needed for proper movements. Corrected muscle tendon lengths allow for optimal firing of the muscles controlling the lower limb, and for the flexibility needed for everyday ROM and biomechanics. Patients with AKP require re-education of movements that occur during daily functional activities, including gait. Once correct movement patterns are established in daily activities, it is important to address sporting or work-related activities. This is one important reason to ensure that physiotherapy visits are distributed over time and that patient-centered goals are addressed during each visit. In addition, during therapy, it is essential to reexamine body movement patterns to identify any relapse to prior dysfunction as the intensity or frequency of activity increases.
In AKP management, the dosage and duration of exercise prescriptions are challenging, and patience and perseverance are paramount. The initial goal of therapy is to increase strength and ROM to enable practice of correct motion in daily activities (eg, stair climbing, sitting, and walking). The physical therapist’s challenge is to teach correct motion within the envelope of function, as described by Dye.25 Pain is not gain, and all exercises must be performed without pain to avoid flaring symptoms. The patient and the therapist must collaborate to complete a pain free rehabilitation program, and must operate within that zone. Providing prescriptions with specific goals may be helpful. Example goals are, “Increase core and lower extremity strength to achieve squatting without medial collapse of knee,” “Hip and core strengthening and endurance,” “Equal quadriceps strength and girth,” and “Functional movement retraining.”
Assessing Adequacy of Rehabilitation in AKP
When a patient presents with a diagnosis of AKP, it can be difficult to establish whether a prior rehabilitation program was appropriate. The fact that a patient attended physiotherapy says nothing about the quality of the therapy provided. Neither does the number of sessions attended. To assess the quality of the rehabilitation and determine if there are any major deficits in neuromuscular function, the physician can perform a simple battery of screening tests (Figure 1).26
More advanced tests can be used to better understand the neuromuscular function of the patient with AKP and tease out specific deficits. Figure 226 describes some of these tests and the typical compensatory motions seen in patients with altered movement patterns.
Am J Orthop. 2017;46(2):82-86. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296.
2. Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil. 2006;85(3):234-243.
3. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4(2):85-100.
4. Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193-206.
5. Smith TO, McNamara I, Donell ST. The contemporary management of anterior knee pain and patellofemoral instability. Knee. 2013;20(suppl 1):S3-S15.
6. Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572-1577.
7. Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-311.
8. Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med. 1991;19(2):131-137.
9. Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55(1):9-15.
10. Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736-742.
11. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214.
12. Meira EP, Brumitt J. Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3(5):455-465.
13. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51.
14. Biabanimoghadam M, Motealleh A, Cowan SM. Core muscle recruitment pattern during voluntary heel raises is different between patients with patellofemoral pain and healthy individuals. Knee. 2016;23(3):382-386.
15. Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43(8):584-588.
16. Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480-489.
17. Hamstra-Wright KL, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome [published online June 22, 2016]. Clin J Sport Med. doi:10.1097/JSM.0000000000000307.
18. Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801.
19. White LC, Dolphin P, Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28.
20. Waryasz GR, McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med. 2008;7:9.
21. Hudson Z, Darthuy E. Iliotibial band tightness and patellofemoral pain syndrome: a case–control study. Man Ther. 2009;14(2):147-151.
22. Winslow J, Yoder E. Patellofemoral pain in female ballet dancers: correlation with iliotibial band tightness and tibial external rotation. J Orthop Sports Phys Ther. 1995;22(1):18-21.
23. Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630-636.
24. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014;28(1):245-255.
25. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
26. Hiemstra LA, Kerslake S, Irving C. Anterior knee pain in the athlete. Clin Sports Med. 2014;33(3):437-459
Take-Home Points
- Ensure that relative rest and activity modification allow the knee to stay within the available “envelope of function” of the joint.
- Careful physical examination is imperative to assess strength, flexibility, and altered movement patterns, which in many cases are all part of the etiology of AKP.
- Patience and perseverance are paramount. Patients need to clearly understand the goals of rehabilitation as well as the concepts related to “envelope of function” so they can continue to keep themselves within this envelope. This education is crucial to their success.
- Only once a patient has been brought into the pain-free functional envelope can rehabilitation be redirected to expanding the envelope toward the patient’s particular goals.
- Quantity does not equal quality. To create an appropriate care plan, the physician must assess the adequacy of the patient’s rehabilitation thus far—ask specific questions about the types of exercises the patient is doing in physical therapy and quickly assess strength with a few simple in-office tests.
Anterior knee pain (AKP) is a common presentation. Although the exact etiology and nature of AKP continue to be poorly understood, overuse principles can be useful in directing treatment. In overuse injury, repetitive submaximal or subclinical trauma results in macroscopic trauma, microscopic trauma, or both. The structural tissue unit is damaged or its clinical responsiveness is exceeded, which can lead to pain or movement dysfunction. Overuse injuries commonly have an endogenous source, mechanical circumstances in which the musculoskeletal tissue is subjected to more tensile force or stress than the tissue can tolerate. The approach to treatment and rehabilitation of AKP is best facilitated with a thorough understanding of the concept of tissue homeostasis and the “envelope of function.”
Although the cause of AKP is multifactorial, the contributions of muscle strength deficits, diminished neuromuscular control, and altered muscle firing patterns to the development and severity of AKP are well established.1-5 The hallmark of nonoperative management of AKP is physiotherapy that re-establishes strength, neuromuscular control, muscle activation, and optimal biomechanics during daily activities, advancing to graded levels of sporting activities.
The purpose of this paper is to discuss the factors associated with the diminished neuromuscular control observed in AKP and to review appropriate rehabilitation concepts for patients with AKP. Practical tools are provided to aid the surgeon to identify neuromuscular deficits in the clinic setting, along with assessing the adequacy of prior therapy and the need for further rehabilitation.
Common Neuromuscular Deficits in AKP
Weakness of the knee extensor muscles has long been implicated as the main issue in AKP, and therefore the focus of rehabilitation has been on muscle strengthening, especially of the vastus medialis obliquus. Research has found that knee extensor weakness is not only a characteristic of patients with AKP but a risk factor for developing AKP.4 Restoration of knee extensor strength and function is essential for recovery.6 Another issue in AKP may be incorrect firing of the knee extensor muscles. Altered vastus medialis obliquus response time and a motor control deficit of the quadriceps musculature have been demonstrated.7,8 Restoration of knee extensor strength, though important, is too often the sole focus of some rehabilitation programs.
Hip muscle weakness has also been implicated as an important component of AKP.9-12 Impaired gluteal muscle function can lead to increased hip joint adduction and internal rotation during activities such as stair climbing, squatting, and sports.9,10,13 In a systematic review, Meira and Brumitt12 concluded that hip strength and position are linked to AKP and that patients with AKP present with a common deficit once symptomatic. The dysfunction in neuromuscular control in AKP may also stem from disordered firing sequences in the muscles. A systematic review of hip electromyographic studies found moderate to strong evidence that gluteus medius muscle activity is delayed and of shorter duration during stair ascent and descent in patients with AKP.11 The study also found some evidence that this activity is delayed and of shorter duration during running and that gluteus maximus muscle activity is increased during stair descent. The authors recommended that interventions focused on correcting these deficits—such as hip strengthening, biofeedback, and gait retraining—should be included in AKP treatment and research.
In recent AKP research, the core, including hip and abdominal muscles, demonstrated decreased strength and altered recruitment patterns during functional movement.14,15 The authors recommended including core strengthening and core stability exercises in AKP management. In combination, these knee extensor, hip, and core strength deficits in patients with AKP lead to altered movement patterns during functional activities and may in turn exacerbate symptoms. Addressing both the strength deficits and the recruitment patterns of these core and lower extremity muscles is essential for optimizing rehabilitation and limiting recurrence of AKP symptoms.
Stretching to improve muscle tendon length is another component of AKP treatment. Reduced quadriceps muscle length has been implicated as a cause of AKP and is a common finding in symptomatic patients.16 In addition, a recent randomized controlled trial found decreased hip flexibility in patients with AKP.17 It is important to assess the flexibility of the gastrocnemius, soleus, quadriceps, and hamstrings muscles and the iliotibial band, as well as the hip flexors, extensors, and rotators, so that rehabilitation can be designed to address any specific deficits in range of motion (ROM).16-23 In patients with AKP, it is also important to address muscle tendon length deficits and strengthening simultaneously to avoid exceeding the available envelope of function. Gaining full ROM at joints can facilitate increasing strength gains24 and potentially improve the synergy of muscle contractions during functional activities.
Appropriate Rehabilitation in AKP
Appropriate rehabilitation addresses all identified strength and flexibility deficits in order to improve functional biomechanics and normalize altered body movement patterns during daily activities (eg, walking, squatting, stair climbing). Often, if part of the kinetic chain is weak or injured, the body engages in an activity by “working around” the injured body part. This change often results in faulty body mechanics or altered movement patterns. In AKP, these modified biomechanics can result in pain centered on the patella and associated soft-tissue structures. In developing ways to compensate for strength and ROM deficits, patients with AKP exacerbate their symptoms. In long-standing AKP, these compensatory strategies are most often unintentional and ingrained.
The main role of physical therapists is to identify any faulty movement patterns, dissect the underlying neuromuscular causes of these deficits, and build an individualized rehabilitation program. Physical therapy should be customized to the patient’s level of strength and fitness and whenever possible should be made challenging (and fun!) for the patient. The exercises should be increased in intensity and duration as the patient improves strength, endurance, and control in the activities. The patient’s response to each intervention will help guide exercise progression and define the need for further treatments.
Patients should be assessed for overuse patterns. Overuse can occur with repetitive exercise activity, such as running, or with repetitive work activity that involves lifting, squatting, or stair climbing. It is important to modify or reduce such activity to ensure that a patient with AKP remains within an envelope of pain-free function. Once the patient is functioning in this envelope, rehabilitation can be redirected to expand it, while improving strength, coordination, balance, and overall dynamic control of the core and lower limbs.
The purpose of any rehabilitation program is to build strength through the entire kinetic chain, focusing on hip and core strength initially, and then adding concentric and eccentric lower limb strength. Having a strong base from which to initiate lower limb movements makes correct lower limb form more likely to follow. Corrected muscle firing patterns allow for appropriate sequencing of the muscle activation needed for proper movements. Corrected muscle tendon lengths allow for optimal firing of the muscles controlling the lower limb, and for the flexibility needed for everyday ROM and biomechanics. Patients with AKP require re-education of movements that occur during daily functional activities, including gait. Once correct movement patterns are established in daily activities, it is important to address sporting or work-related activities. This is one important reason to ensure that physiotherapy visits are distributed over time and that patient-centered goals are addressed during each visit. In addition, during therapy, it is essential to reexamine body movement patterns to identify any relapse to prior dysfunction as the intensity or frequency of activity increases.
In AKP management, the dosage and duration of exercise prescriptions are challenging, and patience and perseverance are paramount. The initial goal of therapy is to increase strength and ROM to enable practice of correct motion in daily activities (eg, stair climbing, sitting, and walking). The physical therapist’s challenge is to teach correct motion within the envelope of function, as described by Dye.25 Pain is not gain, and all exercises must be performed without pain to avoid flaring symptoms. The patient and the therapist must collaborate to complete a pain free rehabilitation program, and must operate within that zone. Providing prescriptions with specific goals may be helpful. Example goals are, “Increase core and lower extremity strength to achieve squatting without medial collapse of knee,” “Hip and core strengthening and endurance,” “Equal quadriceps strength and girth,” and “Functional movement retraining.”
Assessing Adequacy of Rehabilitation in AKP
When a patient presents with a diagnosis of AKP, it can be difficult to establish whether a prior rehabilitation program was appropriate. The fact that a patient attended physiotherapy says nothing about the quality of the therapy provided. Neither does the number of sessions attended. To assess the quality of the rehabilitation and determine if there are any major deficits in neuromuscular function, the physician can perform a simple battery of screening tests (Figure 1).26
More advanced tests can be used to better understand the neuromuscular function of the patient with AKP and tease out specific deficits. Figure 226 describes some of these tests and the typical compensatory motions seen in patients with altered movement patterns.
Am J Orthop. 2017;46(2):82-86. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Ensure that relative rest and activity modification allow the knee to stay within the available “envelope of function” of the joint.
- Careful physical examination is imperative to assess strength, flexibility, and altered movement patterns, which in many cases are all part of the etiology of AKP.
- Patience and perseverance are paramount. Patients need to clearly understand the goals of rehabilitation as well as the concepts related to “envelope of function” so they can continue to keep themselves within this envelope. This education is crucial to their success.
- Only once a patient has been brought into the pain-free functional envelope can rehabilitation be redirected to expanding the envelope toward the patient’s particular goals.
- Quantity does not equal quality. To create an appropriate care plan, the physician must assess the adequacy of the patient’s rehabilitation thus far—ask specific questions about the types of exercises the patient is doing in physical therapy and quickly assess strength with a few simple in-office tests.
Anterior knee pain (AKP) is a common presentation. Although the exact etiology and nature of AKP continue to be poorly understood, overuse principles can be useful in directing treatment. In overuse injury, repetitive submaximal or subclinical trauma results in macroscopic trauma, microscopic trauma, or both. The structural tissue unit is damaged or its clinical responsiveness is exceeded, which can lead to pain or movement dysfunction. Overuse injuries commonly have an endogenous source, mechanical circumstances in which the musculoskeletal tissue is subjected to more tensile force or stress than the tissue can tolerate. The approach to treatment and rehabilitation of AKP is best facilitated with a thorough understanding of the concept of tissue homeostasis and the “envelope of function.”
Although the cause of AKP is multifactorial, the contributions of muscle strength deficits, diminished neuromuscular control, and altered muscle firing patterns to the development and severity of AKP are well established.1-5 The hallmark of nonoperative management of AKP is physiotherapy that re-establishes strength, neuromuscular control, muscle activation, and optimal biomechanics during daily activities, advancing to graded levels of sporting activities.
The purpose of this paper is to discuss the factors associated with the diminished neuromuscular control observed in AKP and to review appropriate rehabilitation concepts for patients with AKP. Practical tools are provided to aid the surgeon to identify neuromuscular deficits in the clinic setting, along with assessing the adequacy of prior therapy and the need for further rehabilitation.
Common Neuromuscular Deficits in AKP
Weakness of the knee extensor muscles has long been implicated as the main issue in AKP, and therefore the focus of rehabilitation has been on muscle strengthening, especially of the vastus medialis obliquus. Research has found that knee extensor weakness is not only a characteristic of patients with AKP but a risk factor for developing AKP.4 Restoration of knee extensor strength and function is essential for recovery.6 Another issue in AKP may be incorrect firing of the knee extensor muscles. Altered vastus medialis obliquus response time and a motor control deficit of the quadriceps musculature have been demonstrated.7,8 Restoration of knee extensor strength, though important, is too often the sole focus of some rehabilitation programs.
Hip muscle weakness has also been implicated as an important component of AKP.9-12 Impaired gluteal muscle function can lead to increased hip joint adduction and internal rotation during activities such as stair climbing, squatting, and sports.9,10,13 In a systematic review, Meira and Brumitt12 concluded that hip strength and position are linked to AKP and that patients with AKP present with a common deficit once symptomatic. The dysfunction in neuromuscular control in AKP may also stem from disordered firing sequences in the muscles. A systematic review of hip electromyographic studies found moderate to strong evidence that gluteus medius muscle activity is delayed and of shorter duration during stair ascent and descent in patients with AKP.11 The study also found some evidence that this activity is delayed and of shorter duration during running and that gluteus maximus muscle activity is increased during stair descent. The authors recommended that interventions focused on correcting these deficits—such as hip strengthening, biofeedback, and gait retraining—should be included in AKP treatment and research.
In recent AKP research, the core, including hip and abdominal muscles, demonstrated decreased strength and altered recruitment patterns during functional movement.14,15 The authors recommended including core strengthening and core stability exercises in AKP management. In combination, these knee extensor, hip, and core strength deficits in patients with AKP lead to altered movement patterns during functional activities and may in turn exacerbate symptoms. Addressing both the strength deficits and the recruitment patterns of these core and lower extremity muscles is essential for optimizing rehabilitation and limiting recurrence of AKP symptoms.
Stretching to improve muscle tendon length is another component of AKP treatment. Reduced quadriceps muscle length has been implicated as a cause of AKP and is a common finding in symptomatic patients.16 In addition, a recent randomized controlled trial found decreased hip flexibility in patients with AKP.17 It is important to assess the flexibility of the gastrocnemius, soleus, quadriceps, and hamstrings muscles and the iliotibial band, as well as the hip flexors, extensors, and rotators, so that rehabilitation can be designed to address any specific deficits in range of motion (ROM).16-23 In patients with AKP, it is also important to address muscle tendon length deficits and strengthening simultaneously to avoid exceeding the available envelope of function. Gaining full ROM at joints can facilitate increasing strength gains24 and potentially improve the synergy of muscle contractions during functional activities.
Appropriate Rehabilitation in AKP
Appropriate rehabilitation addresses all identified strength and flexibility deficits in order to improve functional biomechanics and normalize altered body movement patterns during daily activities (eg, walking, squatting, stair climbing). Often, if part of the kinetic chain is weak or injured, the body engages in an activity by “working around” the injured body part. This change often results in faulty body mechanics or altered movement patterns. In AKP, these modified biomechanics can result in pain centered on the patella and associated soft-tissue structures. In developing ways to compensate for strength and ROM deficits, patients with AKP exacerbate their symptoms. In long-standing AKP, these compensatory strategies are most often unintentional and ingrained.
The main role of physical therapists is to identify any faulty movement patterns, dissect the underlying neuromuscular causes of these deficits, and build an individualized rehabilitation program. Physical therapy should be customized to the patient’s level of strength and fitness and whenever possible should be made challenging (and fun!) for the patient. The exercises should be increased in intensity and duration as the patient improves strength, endurance, and control in the activities. The patient’s response to each intervention will help guide exercise progression and define the need for further treatments.
Patients should be assessed for overuse patterns. Overuse can occur with repetitive exercise activity, such as running, or with repetitive work activity that involves lifting, squatting, or stair climbing. It is important to modify or reduce such activity to ensure that a patient with AKP remains within an envelope of pain-free function. Once the patient is functioning in this envelope, rehabilitation can be redirected to expand it, while improving strength, coordination, balance, and overall dynamic control of the core and lower limbs.
The purpose of any rehabilitation program is to build strength through the entire kinetic chain, focusing on hip and core strength initially, and then adding concentric and eccentric lower limb strength. Having a strong base from which to initiate lower limb movements makes correct lower limb form more likely to follow. Corrected muscle firing patterns allow for appropriate sequencing of the muscle activation needed for proper movements. Corrected muscle tendon lengths allow for optimal firing of the muscles controlling the lower limb, and for the flexibility needed for everyday ROM and biomechanics. Patients with AKP require re-education of movements that occur during daily functional activities, including gait. Once correct movement patterns are established in daily activities, it is important to address sporting or work-related activities. This is one important reason to ensure that physiotherapy visits are distributed over time and that patient-centered goals are addressed during each visit. In addition, during therapy, it is essential to reexamine body movement patterns to identify any relapse to prior dysfunction as the intensity or frequency of activity increases.
In AKP management, the dosage and duration of exercise prescriptions are challenging, and patience and perseverance are paramount. The initial goal of therapy is to increase strength and ROM to enable practice of correct motion in daily activities (eg, stair climbing, sitting, and walking). The physical therapist’s challenge is to teach correct motion within the envelope of function, as described by Dye.25 Pain is not gain, and all exercises must be performed without pain to avoid flaring symptoms. The patient and the therapist must collaborate to complete a pain free rehabilitation program, and must operate within that zone. Providing prescriptions with specific goals may be helpful. Example goals are, “Increase core and lower extremity strength to achieve squatting without medial collapse of knee,” “Hip and core strengthening and endurance,” “Equal quadriceps strength and girth,” and “Functional movement retraining.”
Assessing Adequacy of Rehabilitation in AKP
When a patient presents with a diagnosis of AKP, it can be difficult to establish whether a prior rehabilitation program was appropriate. The fact that a patient attended physiotherapy says nothing about the quality of the therapy provided. Neither does the number of sessions attended. To assess the quality of the rehabilitation and determine if there are any major deficits in neuromuscular function, the physician can perform a simple battery of screening tests (Figure 1).26
More advanced tests can be used to better understand the neuromuscular function of the patient with AKP and tease out specific deficits. Figure 226 describes some of these tests and the typical compensatory motions seen in patients with altered movement patterns.
Am J Orthop. 2017;46(2):82-86. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296.
2. Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil. 2006;85(3):234-243.
3. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4(2):85-100.
4. Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193-206.
5. Smith TO, McNamara I, Donell ST. The contemporary management of anterior knee pain and patellofemoral instability. Knee. 2013;20(suppl 1):S3-S15.
6. Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572-1577.
7. Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-311.
8. Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med. 1991;19(2):131-137.
9. Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55(1):9-15.
10. Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736-742.
11. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214.
12. Meira EP, Brumitt J. Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3(5):455-465.
13. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51.
14. Biabanimoghadam M, Motealleh A, Cowan SM. Core muscle recruitment pattern during voluntary heel raises is different between patients with patellofemoral pain and healthy individuals. Knee. 2016;23(3):382-386.
15. Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43(8):584-588.
16. Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480-489.
17. Hamstra-Wright KL, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome [published online June 22, 2016]. Clin J Sport Med. doi:10.1097/JSM.0000000000000307.
18. Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801.
19. White LC, Dolphin P, Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28.
20. Waryasz GR, McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med. 2008;7:9.
21. Hudson Z, Darthuy E. Iliotibial band tightness and patellofemoral pain syndrome: a case–control study. Man Ther. 2009;14(2):147-151.
22. Winslow J, Yoder E. Patellofemoral pain in female ballet dancers: correlation with iliotibial band tightness and tibial external rotation. J Orthop Sports Phys Ther. 1995;22(1):18-21.
23. Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630-636.
24. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014;28(1):245-255.
25. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
26. Hiemstra LA, Kerslake S, Irving C. Anterior knee pain in the athlete. Clin Sports Med. 2014;33(3):437-459
1. Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296.
2. Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil. 2006;85(3):234-243.
3. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4(2):85-100.
4. Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193-206.
5. Smith TO, McNamara I, Donell ST. The contemporary management of anterior knee pain and patellofemoral instability. Knee. 2013;20(suppl 1):S3-S15.
6. Natri A, Kannus P, Järvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30(11):1572-1577.
7. Witvrouw E, Bellemans J, Verdonk R, Cambier D, Coorevits P, Almqvist F. Patellar tendon vs. doubled semitendinosus and gracilis tendon for anterior cruciate ligament reconstruction. Int Orthop. 2001;25(5):308-311.
8. Voight ML, Wieder DL. Comparative reflex response times of vastus medialis obliquus and vastus lateralis in normal subjects and subjects with extensor mechanism dysfunction. An electromyographic study. Am J Sports Med. 1991;19(2):131-137.
9. Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55(1):9-15.
10. Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736-742.
11. Barton CJ, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214.
12. Meira EP, Brumitt J. Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3(5):455-465.
13. Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51.
14. Biabanimoghadam M, Motealleh A, Cowan SM. Core muscle recruitment pattern during voluntary heel raises is different between patients with patellofemoral pain and healthy individuals. Knee. 2016;23(3):382-386.
15. Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43(8):584-588.
16. Witvrouw E, Lysens R, Bellemans J, Cambier D, Vanderstraeten G. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two-year prospective study. Am J Sports Med. 2000;28(4):480-489.
17. Hamstra-Wright KL, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome [published online June 22, 2016]. Clin J Sport Med. doi:10.1097/JSM.0000000000000307.
18. Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801.
19. White LC, Dolphin P, Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28.
20. Waryasz GR, McDermott AY. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn Med. 2008;7:9.
21. Hudson Z, Darthuy E. Iliotibial band tightness and patellofemoral pain syndrome: a case–control study. Man Ther. 2009;14(2):147-151.
22. Winslow J, Yoder E. Patellofemoral pain in female ballet dancers: correlation with iliotibial band tightness and tibial external rotation. J Orthop Sports Phys Ther. 1995;22(1):18-21.
23. Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630-636.
24. McMahon GE, Morse CI, Burden A, Winwood K, Onambélé GL. Impact of range of motion during ecologically valid resistance training protocols on muscle size, subcutaneous fat, and strength. J Strength Cond Res. 2014;28(1):245-255.
25. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
26. Hiemstra LA, Kerslake S, Irving C. Anterior knee pain in the athlete. Clin Sports Med. 2014;33(3):437-459
The Role of Medial Patellofemoral Ligament Repair and Imbrication
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MPFL repair has the best results with isolated ligament avulsions in first-time dislocations. This can be demonstrated on MRI and verified at the time of arthroscopy.
- Recurrent dislocations, even if acute, have a higher failure rate with MPFL repair. In this setting, MPFL reconstruction provides more consistent outcomes.
- In cases of chronic lateral patellar dislocation, imbrication may be enough when other associated procedures have sufficiently stabilized the patella without the need for a strong soft-tissue checkrein.
- Femoral-sided repairs are more challenging due to the need to optimize the insertion point on the femur, as small changes in positioning can cause increased stress on the repaired tissue and lead to failure.
- If a repair is to have a chance to work, it must be performed at the site of the tear. Thus, preoperative planning and intraoperative inspection is important to precisely identify the site, which can involve intrasubstance and multifocal injuries as well as the femoral and patellar complex attachments.
The medial patellofemoral ligament (MPFL) is the primary soft-tissue restraint to lateral patellar translation.1 In cases of first-time acute lateral patellar dislocation, injury to the MPFL is described as the essential lesion, occurring in almost 100% of cases.2-4 Because of the relatively high frequency of recurrent instability after first-time acute lateral patellar dislocation,5-7 much research has been focused on MPFL repair and reconstruction.8-11 Although the clinical results of isolated MPFL repair are highly variable, this variability is likely secondary to relatively inconsistent clinical indications for repair, with repair described for patients with acute as well as chronic or recurrent instability.10-13 From these early successes and failures, much has been learned about the appropriate indications for MPFL repair as well as medial retinacular “reefing” or imbrication in the chronic setting.
Relevant Anatomy
The MPFL is an extracapsular thickening of the medial retinacular structures and can be most consistently identified just distal to the vastus medialis obliquus, running within layer 2 of the medial side of the knee (using the often-referenced layer system popularized by Warren and Marshall14). The MPFL origin on the medial aspect of the femur falls within a well-defined saddle between the adductor tubercle and the medial epicondyle.15 From this relatively narrow origin, the MPFL broadens before attaching to the proximal one-third of the medial aspect of the patella.
Over the past 2 decades, the osseous anatomy surrounding the femoral origin of the MPFL has been of much interest in large part because of the increasing popularity of MPFL reconstruction. Although useful for MPFL reconstruction, the vast amount of literature and our improved understanding of this anatomical region can be extrapolated to MPFL repair. The radiographic landmarks described by Schöttle and colleagues16 have advanced our knowledge of the femoral origin of the MPFL, with fluoroscopic guidance allowing for more limited dissection and increased accuracy of repair for femoral-sided MPFL injuries.
Location of MPFL Injury
Understanding and appreciating the specific location of the MPFL injury are paramount to successful MPFL repair. Unfortunately, the location and pattern of MPFL injury cannot be consistently predicted. Although early surgical dissections described femoral-sided injuries as the most common injury site,4 more recent studies using magnetic resonance imaging (MRI) have described a more even distribution of MPFL injury patterns, which include patella-based ruptures, femoral-based ruptures, intrasubstance ruptures, and multifocal injuries.17 In addition, age and skeletal maturity likely play a role in the MPFL injury location, as skeletally immature patients more often have patella-based ruptures.2,18,19 In acute MPFL repair, MRI appears to be the most accurate imaging modality for determining the patella- or femoral-based injuries most amenable to repair and for identifying clinically significant osteochondral lesions, which are not uncommon after first-time patellar dislocation.20,21
Medial Reefing, Imbrication, and Advancement
Medial reefing, imbrication, and advancement, collectively referred to as proximal realignment procedures, describe a variety of techniques that essentially shorten or tighten the medial retinacular structures.22-24 Although the terms cover a variety of similar surgical techniques and are often used interchangeably in the literature, imbrication, or overlapping of adjacent edges, is the single most accurate term used to define this spectrum of procedures. These procedures historically were performed in the setting of chronic or recurrent patellar instability, with the primary goal being to imbricate the attenuated medial retinaculum, which includes the MPFL. However, the procedure has had good clinical outcomes when performed in isolation for patients with normal bony anatomy.25 Such anatomy is rare in chronic or recurrent dislocators, and these proximal soft-tissue procedures are often combined with other osseous realignment procedures, including distal realignment, trochleoplasty, and distal femoral osteotomy.26
Discussion
MPFL Repair: Indications and Surgical Technique
Although optimal management of first-time patellar dislocation continues to be a topic for debate, the frequency of recurrent instability,7,27 particularly in young patients, has led some to advocate early surgical management.9,28 A clear indication for early operative intervention is the presence of a large osteochondral lesion that can undergo fixation or is causing persistent mechanical symptoms with recurrent effusion (Figures 1A, 1B).
Numerous open and arthroscopic MPFL repair techniques have been described.10,30-33 Nevertheless, comparative studies are limited, and the greatest debate about MPFL repair continues to be appropriate indications. Arthroscopic MPFL repair can be technically demanding and can fully visualize only patella-based injuries. In addition, all-arthroscopic repair techniques may place suture material in the joint, which causes concern regarding suture irritation. As a result, the majority of MPFL repair techniques described in the literature use an open approach, which typically includes a 4-cm to 5-cm longitudinal incision along the medial aspect of the patella. Sharp dissection is carried down through the medial retinaculum to the underlying joint capsule. The plane between the medial retinaculum and the underlying joint capsule is bluntly developed posteriorly until the medial epicondyle and the adductor tubercle are palpated. For a patella-based rupture, the MPFL is defined within layer 2, and 2 suture anchors are placed within the superior third of the patella. Although there are other patellar fixation methods, suture anchors provide adequate fixation with minimal risk of iatrogenic patellar fracture. With anchors in place, horizontal mattress sutures are placed in the stump of the MPFL. For femoral-based ruptures, the same surgical exposure is used to identify the MPFL. However, depending on the size of the incision and the mobility of the tissue, a second incision can be made posterior and parallel to the first—best achieved using a spinal needle to fluoroscopically localize Schöttle’s point.16 An incision is made in line with the spinal needle, and dissection is continued down to the previously developed extracapsular plane. Under fluoroscopic guidance (Figure 3), 1 or 2 suture anchors are placed at Schöttle point, and horizontal mattress sutures are placed through the avulsed MPFL femoral origin.
MPFL Imbrication: Indications and Surgical Technique
MPFL reconstruction is the technique of choice in recurrent patellofemoral instability when no other procedures are required. When combined with distal realignment procedures, distal femoral osteotomy, open patellofemoral cartilage resurfacing procedures, or trochleoplasty, MPFL imbrication can be considered in place of MPFL reconstruction. Recurrent patellofemoral instability is influenced by various factors, including static soft-tissue restraints, dynamic muscle action, and bony anatomy, only one of which is directly addressed with MPFL imbrication. Relying on native tissues without a graft increases the risk for recurrent instability because of concern that the already attenuated native tissues will stretch out further, particularly in the presence of hyperlaxity. Although the significance of trochlear dysplasia in patellofemoral instability was first noted by Dejour and colleagues,34 the presence of trochlear dysplasia has been shown to negatively influence outcomes of isolated MPFL imbrication.35 Because of the relative frequency of trochlear dysplasia and axial or coronal plane malalignment in patients with chronic or recurrent patellar instability, MPFL imbrication typically is not performed on its own, and it is best used in conjunction with a distal realignment procedure or distal femoral osteotomy. MPFL reconstruction should be performed instead of MPFL imbrication in patients with severe trochlear dysplasia, in patients with hyperlaxity signs, and in young patients who participate in cutting or pivoting sports.
When distal realignment procedures are performed for axial alignment, or distal femoral osteotomy is performed for severe genu valgum, patellofemoral laxity is tested after the bony correction is completed. If the patella is still dislocatable, MPFL reconstruction provides the most predictable outcome. If laxity is increased, but the patella remains in the trochlea, typically MPFL imbrication is adequate.
Similar to MPFL repair, both open and arthroscopic techniques have been described in the literature.36-38 As MPFL imbrication is most commonly performed in conjunction with large open procedures, this procedure can often be incorporated with other open incisions. In addition, open MPFL imbrication allows for precise control and tensioning of the medial retinacular structures, which is not always easily achieved by arthroscopic methods.
If a separate incision is required, a 4-cm to 5-cm longitudinal incision is made along the medial border of the patella, just as described for MPFL repair. The medial retinacular tissue, including the MPFL, is identified and isolated extracapsularly. Imbrication can be performed with sutures only (using a cuff of tissue along the medial border of the patella and placing pants-over-vest sutures in the adjacent tissue) or with sutures and anchors (more similar to MPFL repair described earlier). In either scenario, adequately tensioning the MPFL and associated medial retinaculum is essential in order to restore the checkrein function of the attenuated MPFL. Although typically described in the setting of MPFL reconstruction, the MPFL can easily be overtensioned during MPFL imbrication. This potential pitfall can be avoided by recognizing that forces over 2 N will overtension medial structures and thereby increase contact pressures at the medial patellar facet.39 The complication can easily be prevented simply by placing the knee in 30° flexion and centering the patella in the trochlear groove while performing the MPFL imbrication.
Conclusion
Careful patient selection is the most important element for successful MPFL repair or imbrication. MPFL repair is most reliably used in patients with clear patella- or femoral-sided avulsions and in patients with a first-time patellar dislocation and a clear surgical indication, such as a large osteochondral fragment. Proximal realignment procedures, which include MPFL reefing, imbrication, and advancement, typically are not performed in isolation, as other osseous procedures are often needed concomitantly in order to preserve the checkrein effect provided by proximal realignment procedures. As is the case with MPFL reconstruction, understanding the relevant anatomy and avoiding overtensioning of the medial structures during MPFL repair or proximal realignment procedures are crucial.
Am J Orthop. 2017;46(2):87-91. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.
1. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
2. Askenberger M, Arendt EA, Ekström W, Voss U, Finnbogason T, Janarv PM. Medial patellofemoral ligament injuries in children with first-time lateral patellar dislocations: a magnetic resonance imaging and arthroscopic study. Am J Sports Med. 2016;44(1):152-158.
3. Felus J, Kowalczyk B. Age-related differences in medial patellofemoral ligament injury patterns in traumatic patellar dislocation: case series of 50 surgically treated children and adolescents. Am J Sports Med. 2012;40(10):2357-2364.
4. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60.
5. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
6. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120.
7. Mäenpää H, Huhtala H, Lento MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
8. Apostolovic M, Vukomanovic B, Slavkovic N, et al. Acute patellar dislocation in adolescents: operative versus nonoperative treatment. Int Orthop. 2011;35(10):1483-1487.
9. Camanho GL, Viegas Ade C, Bitar AC, Demange MK, Hernandez AJ. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25(6):620-625.
10. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254.
11. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025.
12. Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am J Sports Med. 2000;28(6):804-810.
13. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914.
14. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56-62.
15. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
16. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804.
17. Petri M, von Falck C, Broese M, et al. Influence of rupture patterns of the medial patellofemoral ligament (MPFL) on the outcome after operative treatment of traumatic patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):683-689.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449.
19. Seeley M, Bowman KF, Walsh C, Sabb BJ, Vanderhave KL. Magnetic resonance imaging of acute patellar dislocation in children: patterns of injury and risk factors for recurrence. J Pediatr Orthop. 2012;32(2):145-155.
20. Balcarek P, Walde TA, Frosch S, Schüttrumpf JP, Wachowski MM, Stürmer KM. MRI but not arthroscopy accurately diagnoses femoral MPFL injury in first-time patellar dislocations. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1575-1580.
21. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518.
22. Lee CH, Wu CC, Pan RY, Lu HT, Shen HC. Medial retinacular flap advancement and arthroscopic lateral release for symptomatic chronic patellar lateral subluxation with tilting. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2499-2504.
23. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P Jr. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629.
24. Xu H, Zhang C, Pei G, Zhu Q, Han Y. Arthroscopic medial retinacular imbrication for the treatment of recurrent patellar instability: a simple and all-inside technique. Orthopedics. 2011;34(7):524-529.
25. Boddula MR, Adamson GJ, Pink MM. Medial reefing without lateral release for recurrent patellar instability: midterm and long-term outcomes. Am J Sports Med. 2013;42(1):216-224.
26. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
27. Garth WP Jr, Pomphrey M Jr, Merrill K. Functional treatment of patellar dislocation in an athletic population. Am J Sports Med. 1996;24(6):785-791.
28. Sillanpää PJ, Mattila VM, Mäenpää H, Kiuru M, Visuri T, Pihlajamäki H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91(2):263-273.
29. Kuroda Y, Matsushita T, Matsumoto T, Kawakami Y, Kurosaka M, Kuroda R. Bilateral medial patellofemoral ligament reconstruction in high-level athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2465-2469.
30. Christiansen SE, Jakobsen BW, Lund B, Lind M. Isolated repair of the medial patellofemoral ligament in primary dislocation of the patella: a prospective randomized study. Arthroscopy. 2008;24(8):881-887.
31. Dodson CC, Shindle MK, Dines JS, Altchek DW. Arthroscopic suture anchor repair for lateral patellar instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):143-146.
32. Fukushima K, Horaguchi T, Okano T, Yoshimatsu T, Saito A, Ryu J. Patellar dislocation: arthroscopic patellar stabilization with anchor sutures. Arthroscopy. 2004;20(7):761-764.
33. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36(12):2301-2309.
34. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
35. Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44(6):1515-1523.
36. Halbrecht JL. Arthroscopic patella realignment: an all-inside technique. Arthroscopy. 2001;17(9):940-945.
37. Henry JE, Pflum FA Jr. Arthroscopic proximal patella realignment and stabilization. Arthroscopy. 1995;11(4):424-425.
38. Nam EK, Karzel RP. Mini-open medial reefing and arthroscopic lateral release for the treatment of recurrent patellar dislocation: a medium-term follow-up. Am J Sports Med. 2005;33(2):220-230.
39. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(9):1557-1563.