User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Patellofemoral Pain: An Enigma Explained by Homeostasis and Common Sense
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
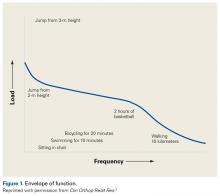
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
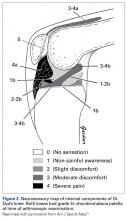
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
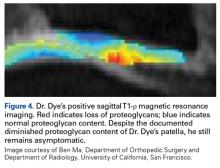
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
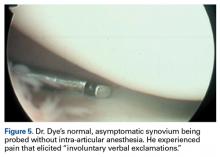
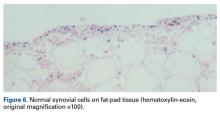
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
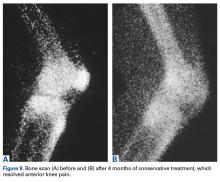
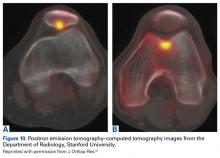
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
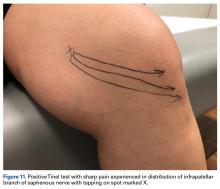
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
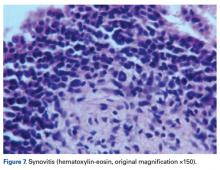
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
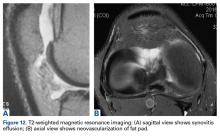
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
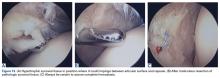
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Loss of tissue homeostasis from overuse or injury produces pain.
- In patients with AKP, treatment should begin with activity modification with the envelope of function; pain-free rehabilitation; an anti-inflammatory program of cold, nonsteroidal anti-inflammatory drugs, and sometimes steroid injection.
- Physical therapy should be done without painful exercise, otherwise it could be counter-productive.
- Patellofemoral syndrome and chondromalacia are not valid clinical diagnoses. A more specific diagnosis based on careful clinical evaluation to determine anatomic origin of pain will better direct treatment.
- Even when lateral retinacular tightness is identified as the probable source of pain, surgery is seldom required.
Symptoms of patellofemoral pain (PFP) without a readily identifiable cause are perhaps the most common yet vexing clinical complaint heard by orthopedic surgeons worldwide. PFP typically occurs over the anterior knee, is often diffuse, and worsens with prolonged knee flexion and the use of stairs. Some prefer the term anterior knee pain (AKP) because we do not always know the pain is patellofemoral in anatomical origin; we know only that it is felt in the anterior knee. Pain is inherently and irreducibly a subjective phenomenon, a function of very discrete central nervous system activity within the sensory area of the contralateral cerebral cortex to the symptomatic knee. Pain is purely subjective and therefore by definition not objectively and consistently measurable between patients. Emotions play a role in pain as well, and somatization resulting in knee pain is a well-known phenomenon, particularly in adolescent women related to stress or even abuse. There is no imaging study that can be used to guide the rational treatment of pain. The best we can do is to ask patients to draw pain diagrams, which provide useful information proven to correlate with areas of tenderness.1
Although many have referred to patients with PFP as having patellofemoral pain syndrome, we reject that term, as it implies a clearly defined syndrome—a consistent set of symptoms, signs, and test results—that does not exist. More complex AKP cases, such as those involving major trauma, complex regional pain syndrome, or multiple operative procedures, are beyond the scope of this article, though many of the principles discussed are applicable. Surprisingly, despite decades of research and clinical experience with a vast number of patients, there still is controversy regarding the underlying etiology of the symptoms and the best, safest treatment.
Primum non nocere. First, do no harm. Let us understand how to reach that noble goal.
Our Hypothesis: Loss of Homeostasis Causes Pain
Homeostasis is a natural process of maintaining relatively stable and asymptomatic physiologic conditions in all organ systems under fluctuating environmental conditions. We hypothesize that pain is the result when load applied to musculoskeletal tissues exceeds the ability to maintain homeostasis. As in other organ systems, in musculoskeletal tissues homeostasis is restored and maintained with appropriate treatment. To illustrate this hypothesis, Dr. Dye coined the term envelope of function (EOF). A combination of magnitude and frequency of load causes loss of homeostasis; with respect to the knee, activity or injury pushes it out of its acceptable EOF in which homeostasis is maintained (Figure 1).2
The therapeutic recommendations that follow from this new biocentric paradigm of joint function are quite different from those associated with hypotheses attributing AKP to chondromalacia and malalignment. This new “common sense” approach, which never encourages treatment that makes symptoms worse, recognizes healing as a complex, rate-limited biological phenomenon that can take time to achieve, especially within a harsh and unforgiving biomechanical environment such as the human patellofemoral joint.
Traditional Explanations and Treatment Strategies
In traditional teaching, 2 causes of AKP have been prominent: chondromalacia patella (CMP) (softening of the articular surface of the patella) and malalignment of the extensor mechanism. Ironically, many of the worst AKP cases are iatrogenic, resulting from surgery to “correct” CMP and/or patellofemoral malalignment or maltracking. Even exercises encouraged by ill-informed physical therapists—such as excessive squats and lunges—can easily worsen AKP symptoms. We think the clinical failure of these traditional methods reflects a profound misunderstanding of the most common cause of AKP.
Chondromalacia Patella—Not the Problem
If chondromalacia is the source of AKP, what is it about conservative treatment that “cures” or even improves structurally softened articular cartilage? How can mere activity modification and exercise result in symptom resolution secondary to improvement in cartilage structure? There is no evidence of this occurring. Nevertheless, patients with this “diagnosis” commonly respond to nonoperative treatment.
Dr. Dye has had personal experience in the possible genesis of AKP in CMP. When he was 46 years old, he allowed his asymptomatic knees to be arthroscopically inspected, without intra-articular anesthesia, so that a neurosensory map of their internal components could be drawn (Figure 2).3
More than 18 years after this neurosensory mapping study, both knees are still asymptomatic, despite substantially reduced proteoglycan content of patellar articular cartilage bilaterally, recently detected with T1-ρ magnetic resonance imaging (MRI), the current favorite of many who use MRI to track early osteoarthritis (Figure 4).
Conversely, during the arthroscopy without intra-articular anesthesia, Dr. Dye discovered quickly and dramatically that the synovium and the fat pad were the most sensitive tissues. Light touch on unanesthetized synovial and fat-pad tissues evoked “involuntary verbal exclamations” (Figure 5).3
When MRI of a patient with AKP shows CMP be cautious not to conclude this structural condition is the direct cause of pain. When overload results in loss of homeostasis, breakdown products of damaged articular cartilage can contribute to symptomatic synovial inflammation. In addition, the damaged articular surfaces may fail to efficiently minimize joint friction and load transmission to subchondral bone. Chondromalacia alone, however, cannot be linked to pain.
Malalignment—Not Often the Problem
That brings us to the historically popular concept of patellofemoral “malalignment/maltracking” as a primary cause for AKP. Although this etiology appeals to many in the orthopedic and physical therapy community,5,6 we and others7-10 reject the notion that it is common. What objective malalignment changes occur when a patient becomes asymptomatic without operative treatment? Imaging measures of malalignment do not change significantly after effective treatment. In studying patients with AKP in the mid 1980’s, Dr. Dye found no difference between 104 adults with PFP and 79 age- and activity-matched controls with respect to 9 objective indicators of malalignment, including quadriceps (Q) angle, congruence angle, sulcus angle, and subchondral sclerosis of the lateral patellar facet.
The clinical success of McConnell taping, which often produces instant pain relief by using tape to apply loads to the patella and peripatellar soft tissues, is sometimes cited as evidence that maltracking or malalignment is the cause of the pain. We disagree with that conclusion. This pain relief more likely results from relieving pressure and tension on sensitive soft tissues, including synovial, fat-pad, and retinacular tissues—equivalent to, say, using a finger to pull inflamed and swollen bitten cheek tissues away from the teeth, which might repetitively traumatize them. In both cases, healing is not spontaneous; but relieving the sensitive tissue of the exacerbating load is the common principle. We think subtle changes in the tension and impingement of synovial and fat-pad tissues can have profound effects on AKP. Pain relief with McConnell taping no more proves that the source of the pain is malalignment or maltracking than a finger pulling away inflamed and swollen cheek tissues proves that cheek pain is caused by malocclusion.
Patellar Bone Overload—Part of the Problem
Patellar bone has been long assumed to be a source of AKP. To understand this better, Dr. Dye had one of his residents push a 15-gauge needle into the medial facet of his asymptomatic right patella to obtain real-time intraosseous pressure measurements as a control. This was done under local anesthesia, so no pain was felt as the needle entered the patella. However, when an arterial line was connected and flushed prior to pressure measurements, Dr. Dye experienced sharp lancinating pain. Patellar bone is richly innervated, and even mildly increased intraosseous pressure can produce severe symptoms. Dr. Dye’s patella was sore for about 7 months afterward.
Loss and restoration of osseous homeostasis occur often in AKP patients whose positive patellar bone scans (focal or diffuse) show resolution to normal (homeostasis) after symptom dissipation (Figures 9A, 9B).
The Mosaic of Anterior Knee Pain
The densely innervated synovial, fat-pad, and patellar bone tissues are nociceptive sources of AKP in the absence of homeostasis.
Clinical Applications of Homeostasis and Common Sense
Essential points to be covered in the history include overuse, injury, weight gain, systemic illness (which may produce weakness and deconditioning), prior treatment (especially physical therapy) and response to medications or injections. In the case of prior surgery, preoperative and postoperative identification of the patient’s exact symptoms can shed light on the underlying diagnosis and on any symptom changes resulting from treatment.
Sudden pain in the anterior knee can result in pain-mediated reflex quadriceps inhibition and the sensation that the knee is “giving way.” Typically, patients describe the knee collapsing into flexion and when asked if their knee is “unstable” after experiencing such episodes they will readily say yes. However, such a knee is not “unstable” in the sense that there is patholaxity that might require surgery. This is a critical distinction to avoid tragic-ally unnecessary surgery.
Careful evaluation for areas of tenderness may direct treatment to focal pathology, such as patellar or quadriceps tendinitis or tendinosis, pathologic medial parapatellar plica, or postoperative neuroma. Palpation and Tinel testing can uncover a neuroma or neuropathy of the infrapatellar branch of the saphenous nerve (Figure 11) that no other diagnostic tools can.
Poor flexibility, which increases tension and load in peripatellar soft tissues, is very common. In many cases, evaluation of hamstring, prone quadriceps, hip, and gastrocsoleus flexibility with contralateral comparison reveals a need to include stretching in a homeostasis-restoring program.
Insufficient muscular strength and endurance can also result in overload of patellofemoral bony and soft tissues. As all ground reaction force must be absorbed somewhere in the body, and since eccentric muscle contraction absorbs load, other tissues become overloaded if muscle function is insufficient to absorb enough force. Weakness of the hip and core have shown to respond to rehabilitation with resolution to AKP. Proximal weakness screening with step-down or single-leg squat is important.
Joint effusion is an important finding indicative of objective intra-articular pathology and inflammation. Such inflammation may be from overuse resulting in loss of homeostasis (synovitis, cartilage breakdown, symptomatic arthrosis).
Screening examinations for hip and lumbar pathology are mandatory and take only a few minutes.
Treatment Options
Activity Modification
Avoid aggravating the problem. Consider this like a fire. If you are trying to put out a fire (AKP), would you throw sticks (increased activity/aggressive exercise) on it? Of course not. You would turn a hose on it (nonsteroidal anti-inflammatory drug [NSAID] regularly) or perhaps throw a bucket of water (steroid injection) on it. You would not throw gasoline (excessive exercise or activity) on it. Explaining to patients how to remain within their envelope by avoiding any activity that increases symptoms is crucial. No pain no gain is a lie from hell for patients with AKP. Don’t throw sticks on the fire.
We are frustrated that patients with PFP are still often told by well-meaning therapists to perform exercises that end up substantially increasing symptoms. Patients are admonished to push forward with “quad strengthening” by any means necessary, including painful lunges and squats, which can exacerbate synovial and fat-pad impingement and put excessive tension on muscle and tendon tissue, which is ill equipped to absorb the loads. Damaged tissues can usually return to pain-free biological homeostasis if given the opportunity and a reasonable mechanical environment.
Pain-free loading means that each of the hundreds of millions of sensory nerve endings is unperturbed, and is reporting, in effect, “I’m fine in my sector.” Minor discomfort is inevitable, but real pain during activity, and exacerbations after activity, is activity outside the EOF. Strive for patients to have “clinically quiet” knees during activity. This common sense approach is often rewarded with dramatic recovery, over time, even in patients with severe AKP. In long-standing cases, patients may take months or even years to recover, but slow and steady progress should be expected. Later, these may be among your most grateful patients.
Cold Therapy
Cold therapy relieves pain, decreases swelling, slows the metabolic rate, is simple, and has few complications. Many AKP-related tissues are superficial, and the application of cold is logical and effective. However, we should not overdo it, either. Cold applied for 20 minutes once or twice daily is sufficient in most cases, at least initially. If it does not help resolve symptoms, it may be abandoned. Likewise, if a patient does not tolerate cryotherapy, it should not be demanded. Some patients respond better to the application of warmth, which is allowed within reason.
Anti-Inflammatory Medication
Inflammation clearly plays a role in the production of pain and swelling in the soft tissues of the anterior knee (synovium, fat pad, patella and quadriceps tendons/peritenon, and retinacular tissues). Consistent use of oral NSAIDs in the absence of medical contraindications can be valuable, and there are benefits to using mild oral NSAIDs (eg, solubilized ibuprofen 400 mg 2 times daily). Prescription NSAIDs should be used short-term, if possible, to avoid complications; long-term use requires medical supervision and laboratory testing. Oral steroids can be used in similar fashion.
Intra-articular steroids (eg, triamcinolone or methylprednisolone 40 mg with a few cubic centimeters of local anesthetic) can be very helpful in quickly reducing inflammation within synovial and fat-pad tissues. In addition, an intra-articular steroid injection is diagnostic when the pain goes away, even if only for the duration of the local anesthetic; this change indicates the pain must be coming from a structure that is bathed by the intra-articular medication. Longer-term relief provides strong circumstantial evidence of causation related to intra-articular soft-tissue inflammation (loss of homeostasis) and not to chondromalacia or malalignment.
Physical Therapy
Therapy must be performed within the EOF as much as possible. Muscle soreness after a therapeutic workout is acceptable. There can easily be a lag time of 24 hours or more in the production of an activity-induced inflammatory enzyme spike. Therefore, when exercises are being done every other day, the rest days should also be kept well within the EOF. The patient must be essentially pain-free all the time, on exercise days and on rest days. Gentle stretching of tight muscles (especially quadriceps but also hips, hamstrings, and gastrocsoleus) and strengthening of hips and core are encouraged. Gentle stretching on rest days is encouraged as well.
The physical therapist must teach the principles of moderating activities of daily living (ADLs) within the EOF (eg, safe use of stairs, safely getting in and out of chairs and vehicles), for it is in these ADLs that many symptomatic patients experience recurrent overload. Total load in ADLs and in therapy must remain within the EOF to maximize the chance of return to homeostasis. Exercise-induced substantial patellofemoral soreness, effusion, or increased temperature in the knee is not acceptable.
Imaging
Advanced imaging in AKP can be a contentious subject. It is too easy to assume images hold the answers. A finding of CMP or alignment abnormality must be viewed with caution, as usually it is not an indication for patellofemoral surgery. You are treating a patient, not a picture. You must be responsible to integrate all available data (history, physical examination, imaging, response to treatment, etc) to make an accurate diagnosis. Always inspect all the imaging data yourself. Do not “push in the mental clutch” but rather do the challenging work of putting all the clinical pieces of the puzzle together to reach the right answer. Do not let the radiologist make the diagnosis!
Radiographs
It is imperative to obtain good-quality radiographs, including axial radiographs of the patella in early flexion, to check for evidence of arthrosis and other joint pathology that may be producing pain. Dr. Post always obtains bilateral knee radiographs to help understand the degree of any arthrosis or malalignment in the contralateral asymptomatic knee. The information in bilateral radiographs is also instructive for patients. Knowing that the contralateral knee shows the same radiographic changes, or even more, helps them understand that the structural factors as imaged do not dictate symptoms. More advanced or extensive imaging is not needed unless appropriate and patient therapy reaches a stalemate.
Bone Scans
In recalcitrant patients with persistent pain, a bone scan provides sensitive imaging of osseous metabolic activity and thereby clarifies the etiology of the pain. A negative scan rules out the bone as a significant cause, freeing the clinician to concentrate solely on the soft tissues. In a way that MRI can miss, a positive bone scan identifies specific regions that have lost osseous homeostasis and are being overloaded. Microscopically, these regions’ changes are very similar to the abnormal bone remodeling that occurs in early-stage stress fractures. Whether focal or diffuse, a positive bone scan means symptoms likely will take longer to reverse than is the case with a negative scan. Often, the stark findings of a positive bone scan can grab the patient’s attention and improve understanding and compliance. Focal inferior pole uptake is the most difficult pattern to reverse, perhaps because it may represent the most extreme biomechanical environment of the patellofemoral joint. In Dr. Dye’s experience, patients with this pattern may often require drilling of the inferior pole to achieve restoration of tissue homeostasis.
Magnetic Resonance Imaging
MRI can be useful, though scans are commonly read as normal. In some cases, MRI evidence of tendinopathy and other intra-articular pathology can direct both operative and nonoperative treatment of AKP. Carefully look for evidence of soft-tissue impingement—such as mild synovial swelling, low-grade effusion, and neovascularization of the fat pad—as in many cases it exists, and has been missed by the radiologist (Figures 12A, 12B).
When Surgery Is Needed: General Principles
Although the majority of patients with AKP do not need surgery, some do. Think of surgery as a tool used to create an environment in which homeostasis may be restored. Arthroscopy and meticulous débridement may be used to treat recalcitrant focal synovitis or fat-pad hypertrophy—or focal chondral pathology (eg, unstable flap of articular cartilage) that has produced mechanical symptoms with secondary inflammation. A well-localized area of patellar tendinosis may respond to either arthroscopic or open débridement. A true mechanical alignment abnormality may produce focal overload to such a degree that the most complete nonoperative programs cannot overcome the loss of homeostasis. In such a case, imaging studies that precisely document overloaded areas and associated malalignment must make sense given the clinical picture, and then must be used in developing a rational surgical plan for unloading bone and soft-tissue pathology to create a mechanical and biological environment for healing and return to homeostasis. At times, the articular damage may be so severe that patellofemoral arthroplasty is the best choice. The exact indications for these procedures are well described elsewhere.13
Surgery for Patients With PFP Caused by Recalcitrant Synovitis
As this type of surgery is not often covered in the literature, we offer some treatment pearls here. Arthroscopy for persistent focal synovitis should not be approached lightly; though the mechanics of removing abnormal inflamed synovial tissue may be straightforward, perioperative management and long-term postoperative management are not. The patient must be mentally prepared for the process; blood-thinning agents, fish oil, and turmeric must be discontinued; and hemostasis must be meticulous (Figures 13A-13C).
Conclusion
The history of medicine has included many misunderstandings of cause and effect. Trephination was used for headaches, leeches for fever, and, more recently, antacids for Helicobacter pylori caused duodenal ulcers. Stimulated by the enigma of AKP, we think our common sense way of thinking about tissue homeostasis in the musculoskeletal system represents an emerging orthopedic biological paradigm that is applicable to the entire body. We should let the remarkable capacity of vertebrate biology do the “heavy lifting” of healing. The traditional orthopedic emphasis on structure and alignment has a role, but we see it as complementary and secondary to the biological paradigm and find that the evidence presented herein supports our contention. The answer is seen only when one looks beyond the viewbox.
Primum non nocere. Your patients will be most grateful.
Am J Orthop. 2017;46(2):92-100. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
1. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
2. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;(325):10-18.
3. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777.
4. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
5. Grelsamer RP. Patellar malalignment. J Bone Joint Surg Am. 2000;82-A(11):1639-1650.
6. Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639-646.
7. Sanchis-Alfonso V. Anterior Knee Pain and Patellar Stability. London, England: Springer-Verlag; 2006.
8. Post WR. Anterior knee pain: diagnosis and treatment. J Am Acad Orthop Surg. 2005;13(8):534-543.
9. Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264-272.
10. Dye SF, Staubli HU, Beidert RM, Vaupel GL. The mosaic of pathophysiology causing patellofemoral pain: therapeutic implications. Oper Tech Sports Med. 1999;7:46-54.
11. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453-469.
12. Draper CE, Fredericson M, Gold GE, et al. Patients with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. J Orthop Res. 2012;30(2):209-213.
13. Post WR, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
A Practical Guide to Understanding and Treating Patellofemoral Pain
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
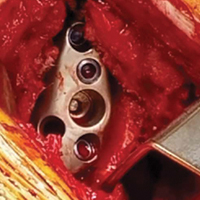
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Anterior knee pain is common, particularly in young females.
- For most patients, activity modification and rest will control the pain; continuing to engage in painful activity only prolongs symptoms.
- In physical therapy, core stability, weight loss, and hip strengthening are essential.
- Surgery is required only in a very small subset of patients with anterior knee pain.
- Traumatic and overload- related chondral defects that have resisted a reasonable amount of conservative (nonoperative) treatment may be arthroscopically assessed and treated when documented to cause persistent pain.
Anterior knee pain is common (AKP), particularly in young females. Understanding the biomechanics of a rapidly growing young female knee, whose pelvis is relatively wider than her male counterpart, helps greatly in understanding origins of AKP.1
Compared with males of similar weight and size, females often walk and run with increased valgus at the knee and internal rotation of the hip on heel strike. The patella contacts the lateral edge of the trochlea with more focal load on the distal lateral patella for a longer time in a female than in a male of similar stature because of the increased lateral force vector. Add the rigors of athletics, excessive body weight, use of high heels, or a predisposing structural anomaly, and painful focal overload can develop—resulting in pain on stairs, inability to run, and a visit to your office. Some male patients also develop AKP, often related to patellofemoral dysplasia or activity-related overload leading to a similar pattern and need for care. Fortunately, most young patients improve when they reduce physical activity, attain stable musculoskeletal maturity, or both.
In addition to focal articular overload occurring, retinacular structures about the anterior knee can be stressed by the structural imbalance resulting from the excessive and sudden internal rotation of the hip that occurs even during normal gait and often is related to female lower extremity function. Small nerve damage in the stressed retinaculum is an important cause of peripatellar pain2 and is best identified by clinical examination. Additionally, the infrapatellar fat pad may become pinched, causing synovial inflammation.
With these patients, reassurance can go a long way, and resting, taping, bracing, and anti-inflammatory medications are helpful. Dye3 has emphasized nonoperative treatmentand allowing patients to re-establish homeostatic balance of the patellofemoral joint. Establishing normal body weight plays a key role in the process, and focusing on lower extremity core stability, starting with increased strength in the hip external rotators, is important.4 In the majority of patients, these measures are all that is needed.
Traumatic Anterior Knee Pain
Direct trauma to the anterior knee causes an entirely different sort of pain. Knee pain may be retinacular, neuronal, synovial, bony, or articular. Nothing replaces careful, detailed clinical history taking and physical examination in determining the source of this pain. Much AKP, particularly in its early stages, is very focal. A specific injection of an anesthetic into a suspected retinacular pain location may solve the diagnostic dilemma. With many patients, paying attention to the specific degree of knee flexion in which the injury occurred helps in localizing the lesion. A flexed-knee impact injury (dashboard or fall directly onto anterior knee) is a common cause of articular damage on the mid or proximal patella and distal medial femoral condyle. Identifying this cause is particularly important in worker’s compensation cases, as the pattern is diagnostic of a direct blow to the knee and may confirm the patient’s history.
Treating painful patellofemoral lesions related to direct trauma can be difficult. Once they are identified and correlated with the physical examination and magnetic resonance imaging (MRI) findings, a treatment plan can be developed.
Examination, Testing, Imaging
Knowing how AKP started is important. Asking a patient to point to the origin of pain is essential. A pain diagram (having the patient draw a picture of the pain location) is also very helpful.5 Spontaneous onset suggests an underlying structural and/or functional problem rather than a traumatic event. Examination should include palpation of all structures and the retinaculum about the knee; careful appraisal of patella tracking, location of pain, and crepitus (angle of knee flexion), and evidence of possible pain referred from the back or hip; gait analysis for functional aberrations; assessment of patellar mobility; and standard radiographs, including a perfect lateral radiograph and a knee-flexion axial radiograph of no more than 30° to 45°. Computed tomography, radionuclide scintigraphy,3 and MRI can be very useful in select patients, but such imaging generally is not necessary in the management of routine AKP. However, these studies can be extremely helpful in patients with resistant pain.
Resistant Anterior Knee Pain
When nonoperative measures (rest, bracing, taping, physical therapy, activity modification) fail to relieve pain, more aggressive treatment may be warranted. The clinician must take extra time to listen to the patient, look for the precise source of the pain, and address it directly. Treatment depends on the specific source of pain. A chronically painful retinacular lesion or neuroma usually responds to release of the painful segment. After a retinacular source of pain has been identified and temporarily eliminated with injection of a local anesthetic, the pain source can be accurately resected and the patient quickly cured. When the chronically painful locus is an injured fat pad, resection provides complete relief.
For most orthopedic surgeons, the greatest dilemma is how to address a young person’s persistent pain in the setting of minimal objective evidence. In my experience with hundreds of arthroscopies, distinct distal lateral patella articular softening is common. In some cases, the degree of articular softening can be extreme, extending toward the central ridge or even across the center of the patella and involving 40% to 50% of the patella articular surface. This spongy, soft cartilage does not resist load normally, and in many cases pain is disabling. Most important is to acknowledge the problem, as many of these patients have been living with articular lesion pain for a year or more. As quality of life can be severely diminished by chronic patellofemoral pain, it behooves us to find answers and provide appropriate treatment. Although patients with this degree of articular softening and breakdown represent a small percentage of all patients with patellofemoral pain, identifying these cases is essential.
However benign-appearing, a resistant, painful patella articular lesion can be disabling. The key to treating a young person with a patella articular lesion objectively proved with imaging or arthroscopy is to inform the patient and family of the resistant nature of some lesions. In a referral patellofemoral practice, I see many patients who are disabled and depressed about the results of articular breakdown related to focal overload. Once the problem is identified, there is hope.
Prolonged rest and activity withdrawal usually help, but in some cases pain with stairs and daily activities continues. Running is usually impossible, which can be devastating for many young people.
My approach is to exhaust the nonoperative measures, which include focusing intensely on core stability training. The physical therapist must understand the importance of this treatment component; the patient must understand the importance of strengthening the hip external rotators and the vastus medialis oblique, modifying gait, avoiding pain-inducing activities, controlling weight, using proper footwear, and being patient. Applying heavy resistance to the quadriceps during rehabilitation will likely perpetuate or exacerbate the problem. The goals are to limit loading of the articular lesion and improve lower extremity function emphasizing reduction and balanced distribution of load.
Other Causes of Anterior Knee Pain
The possibility of an unusual source of pain should always be considered. Some causes (osteochondral lesion, bipartite patella, patella baja, radiographic evidence of focal overload) are apparent only on imaging. MRI may provide evidence of hypertrophic synovium, thickened fat pad, or patellar tendonitis. The physical examination is important in determining unusual sources of pain, such as those related to trauma or retinacular neuronal injury from direct impact. Pain referred from the hip or back can also cause AKP. As kinesiophobia may also play a role, it should be considered whenever an objective cause of the pain cannot be identified.
Surgery for Anterior Knee Pain
Surgery should be considered only after prolonged rest and healing have failed to resolve the pain caused by sustained direct trauma to the anterior knee. Physical therapy typically is not useful in direct trauma. If a painful traumatic articular lesion persists, then direct treatment—removing loose articular fragments and resurfacing or unloading a damaged articular surface—may be appropriate. In most cases, 6 to 12 months should be allowed before considering surgery. Meanwhile, rest, bracing, anti-inflammatory measures, reassurance, and work modification are the cornerstones of treatment.
After all conservative measures have failed in a patient with spontaneous-onset AKP related to repetitive focal overload, and disability caused by an objectively proven articular lesion related to mechanical dysfunction or dysplasia, diagnostic arthroscopy may be appropriate. Quantitation and characterization of the lesion with images and measurements are imperative in forming an optimal surgical plan. Remember that not all problems can be cured with surgery, and there is no patellofemoral problem that cannot potentially be made worse with improper surgery.
Am J Orthop. 2017;46(2):101-103. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
1. Sanchis-Alfonso V, Dye SF. How to deal with anterior knee pain in the active young patient. Sports Health. 2016 Dec 5. [Epub ahead of print]
2. Fulkerson JP, Tennant R, Jaivin JS, Grunnet M. Histologic evidence of retinacular nerve injury associated with patellofemoral malalignment. Clin Orthop Relat Res. 1985;(197):196-205.
3. Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-110.
4. Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12-19.
5. Post WR, Fulkerson J. Knee pain diagrams: correlation with physical examination findings in patients with anterior knee pain. Arthroscopy. 1994;10(6):618-623.
Novel Solution for Massive Glenoid Defects in Shoulder Arthroplasty: A Patient-Specific Glenoid Vault Reconstruction System
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
High-Resolution Wireless Ultrasound
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
Robotic-Assisted Total Knee Arthroplasty
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Management of Proximal Biceps Pathology in Overhead Athletes: What Is the Role of Biceps Tenodesis?
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Precision and Accuracy of Identification of Anatomical Surface Landmarks by 30 Expert Hip Arthroscopists
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
How Should the Treatment Costs of Distal Radius Fractures Be Measured?
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
Safety of Superior Labrum Anterior and Posterior (SLAP) Repair Posterior to Biceps Tendon Is Improved With a Percutaneous Approach
Take-Home Points
- Anchors placed posterior to the biceps during SLAP repair are at risk for glenoid vault penetration and/or suprascapular nerve (SSN) injury.
- Vault penetration and SSN injury are avoided by using a Port of Wilmington (PW) portal instead of an anterior portal.
- A percutaneous PW portal is safe and passes through the rotator cuff muscle only.
Since being classified by Snyder and colleagues,1 various arthroscopic techniques have been used to repair superior labrum anterior and posterior (SLAP) tears, particularly type II tears. Despite being commonly performed, repairs of SLAP lesions remain challenging. There is high variability in the rate of good/excellent functional outcomes and athletes’ return to previous level of play after SLAP repairs.2,3 Furthermore, the rate of complications after SLAP repair is as high as 5%.4
One of the most common complications of repair of a type II SLAP tear is nerve injury.4 In particular, suprascapular nerve (SSN) injury has occurred after arthroscopic repair of SLAP tears.5,6 Three cadaveric studies have demonstrated that glenoid vault penetration is common during placement of knotted anchors for SLAP repair and that the SSN is at risk during placement of these anchors.7-9 However, 2 of the 3 studies used only an anterior portal in their evaluation of anchor placement. Safety of anchor placement posterior to the biceps tendon may be improved with a percutaneous approach using a Port of Wilmington (PW) portal.10,11 No studies have evaluated the risk of glenoid vault penetration and SSN injury with shorter knotless anchors.
We conducted a study to compare a standard anterosuperolateral (ASL) portal with a percutaneous PW portal for knotless anchors placed posterior to the biceps tendon during repair of SLAP tears. We hypothesized that anchors placed through the PW portal would be less likely to penetrate the glenoid vault and would be farther from the SSN in the event of bone penetration.
Materials and Methods
Six matched pairs of fresh human cadaveric shoulders were used in this study. Each specimen included the scapula, the clavicle, and the humerus. All 6 specimens were male, and their mean age was 41.2 years (range, 23-59 years). Shoulder arthroscopy was performed for placement of SLAP anchors, and open dissection followed.
Anchor Placement
The scapula was clamped and the shoulder placed in the lateral decubitus position with 30° of abduction, 20° of forward flexion, and neutral rotation.10 A standard posterior glenohumeral viewing portal was established and a 30° arthroscope inserted. Both shoulders of each matched pair were randomly assigned to anchor placement through either an ASL portal or a PW portal. Two anchors were placed in the superior glenoid to simulate repair of a posterior SLAP tear.11 Each was a 2.9-mm short (12.5-mm) knotless anchor (BioComposite PushLock; Arthrex) that included a polyetheretherketone (PEEK) eyelet for threading sutures before anchor placement. A drill guide was inserted according to manufacturer guidelines, and a 2.9-mm drill was used to make a bone socket 18 mm deep. The anchor eyelet was loaded with suture tape (Labral Tape; Arthrex), and the anchor and suture were inserted into the socket. The sutures were left uncut to aid in anchor visualization during open dissection. On a right shoulder, the first anchor was placed just posterior to the biceps tendon, at 11 o’clock, and the second anchor about 1 cm posterior to the first, at 10 o’clock. All anchors were placed by an arthroscopy fellowship–trained shoulder surgeon. Before placement, anchor location was confirmed by another arthroscopy fellowship–trained shoulder surgeon.
The ASL portal was created, with an 18-gauge spinal needle and an outside-in technique, about 1 cm lateral to the anterolateral corner of the acromion.
In the opposite shoulder, the PW portal was created, with a percutaneous technique, about 1 cm anterior and 1 cm lateral to the posterolateral corner of the acromion. An 18-gauge spinal needle was inserted to allow a 45° angle of approach to the posterosuperior glenoid.11
Cadaveric Dissection
After anchor placement, another shoulder surgeon performed the dissection. Skin, subcutaneous tissue, deltoid, and clavicle were removed. In the percutaneous specimens, PW portal location relative to rotator cuff was recorded before cuff removal. After overlying soft tissues were removed from a specimen, the anchors were examined for glenoid vault penetration. In the setting of vault penetration, digital calipers were used to measure the shortest distance from anchor to SSN.
Results
In the ASL portal group, 8 (66.7%) of 12 anchors (4/6 at 11 o’clock, 4/6 at 10 o’clock) penetrated the medial glenoid vault.
In the PW portal group, 2 (16.7%) of 12 anchors (1/6 at 11 o’clock, 1/6 at 10 o’clock, both from a single specimen) penetrated the medial glenoid vault. Actually, in each case the eyelet and not the anchor penetrated the vault. In the penetration cases, distance to SSN was 20 mm for the 11 o’clock anchor and 8 mm for the 10 o’clock anchor (Table). Of the 6 portals, 3 passed through the supraspinatus muscle, 2 through the infraspinatus musculotendinous junction, and 1 through the infraspinatus muscle.
Discussion
Our study findings support the hypothesis that SLAP repair anchors placed posterior to the biceps tendon are more likely to remain in bone with use of a percutaneous approach relative to an ASL approach. Our findings also support the growing body of evidence that such anchors placed with an anterior approach increase the risk for SSN injury.
Three other cadaveric studies have evaluated anchor placement for SLAP repair. Chan and colleagues7 evaluated drill penetration during bone socket preparation for SLAP repair in 21 matched pairs of formalin-embalmed cadavers. A 20-mm drill was used for correspondence to a 14.5-mm anchor, though no anchors were inserted, and sockets were created in an open manner. Through a mimicked ASL portal, 1 socket was made anterior to the biceps tendon, at 1 o’clock; then, through a mimicked PW portal, 2 sockets were made posterior to the tendon, at 11 o’clock and 9 to 10 o’clock. Glenoid vault penetration occurred in 29% of the 42 anterior sockets, but only 1 anchor (2.4%) touched the SSN. Penetration did not occur with the 11 o’clock anchors. The 9 to 10 o’clock anchor was at highest risk for SSN injury (9.5%, 4 cases). The study was limited by lack of anchor placement and open creation of bone sockets in embalmed cadavers.
Koh and colleagues8 evaluated arthroscopic placement of anterior SLAP anchors in 6 matched pairs of fresh-frozen cadavers. Through an ASL portal, each 14.5-mm knotted anchor was placed anterior to the biceps tendon, at 1 o’clock. As in the study by Chan and colleagues,7 drill depth was 20 mm. Notably, anchors were seated 2 mm beyond manufacturer recommendations, and the cadavers were of Asian origin, likely indicating smaller glenoids compared to specimens from North America or Europe. All 12 anchors penetrated the glenoid vault; mean distance to SSN was 3.1 mm.
Morgan and colleagues9 compared anterior and ASL portals created for SLAP repairs in 10 matched-pair cadavers. Anchors were placed at 1 o’clock, 11 o’clock, and 10 o’clock. As in the studies by Chan and colleagues7 and Koh and colleagues,8 14.5-mm knotted anchors were used. One anterior anchor (10%) placed through an ASL portal penetrated the cortex by 1 mm, and 2 anterior anchors (20%) placed through anterior portals penetrated the cortex (1 was completely out of the bone). Overall, 65% of 11 o’clock anchors and 100% of 10 o’clock anchors violated the glenoid vault. With the 11 o’clock anchors, mean distance to SSN was 6 mm for ASL portals and 4.2 mm for anterior portals; with the 10 o’clock anchors, mean distance to SSN was 8 mm for ASL portals and 2.1 mm for anterior portals.
Overall, the results of these 3 studies suggest that, with use of ASL portals, placement of SLAP anchors anterior to the biceps tendon is safe. Using the same portals, however, anchors placed posterior to the tendon are at higher risk for glenoid vault penetration. Supporting these findings are our study’s penetration rates: 66.7% for anchors placed through ASL portals and 16.7% for anchors placed through percutaneous PW portals. The different rates are not surprising given that the coracoid process projects anterior to the glenoid and provides additional bone stock for placement of anchors anteriorly vs posteriorly. Therefore, with percutaneous PW portals, the approach angle directs the anchor toward the bone of the coracoid base. Furthermore, the SSN passes nearest the posterior aspect of the glenoid. In a study by Shishido and Kikuchi,12 the distance from the posterior rim of the glenoid to the SSN was 18 mm, and from the superior rim was 29 mm. Therefore, anchors placed with an anterior approach naturally are directed toward the SSN.
In addition to portal placement and approach angle, anchor length likely affects the risks for glenoid vault penetration and SSN injury.
One limitation of this study was the small number of cadavers, all of which were male. Female cadavers and cadavers of other ethnic origins likely have smaller glenoid vaults, and thus their inclusion would have altered our results. This issue was well described in studies mentioned in this article, and our goal was simply to compare ASL portals with percutaneous PW portals, so we think it does not change the fact that the risks for glenoid vault penetration and SSN injury are reduced with use of PW portals for anchors placed posterior to the biceps tendon.
Conclusion
This study was the first to examine glenoid vault penetration and SSN proximity with short anchors for SLAP repair. The risk for glenoid vault penetration during repair of SLAP tears posterior to the biceps tendon was reduced by anchor placement with a percutaneous posterior approach. The percutaneous posterior approach also directs the anchor away from the SSN.
Am J Orthop. 2017;46(1):E60-E64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
2. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
3. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
4. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
5. Kim SH, Koh YG, Sung CH, Moon HK, Park YS. Iatrogenic suprascapular nerve injury after repair of type II SLAP lesion. Arthroscopy. 2010;26(7):1005-1008.
6. Yoo JC, Lee YS, Ahn JH, Park JH, Kang HJ, Koh KH. Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg. 2009;18(4):e27-e29.
7. Chan H, Beaupre LA, Bouliane MJ. Injury of the suprascapular nerve during arthroscopic repair of superior labral tears: an anatomic study. J Shoulder Elbow Surg. 2010;19(5):709-715.
8. Koh KH, Park WH, Lim TK, Yoo JC. Medial perforation of the glenoid neck following SLAP repair places the suprascapular nerve at risk: a cadaveric study. J Shoulder Elbow Surg. 2011;20(2):245-250.
9. Morgan RT, Henn RF 3rd, Paryavi E, Dreese J. Injury to the suprascapular nerve during superior labrum anterior and posterior repair: is a rotator interval portal safer than an anterosuperior portal? Arthroscopy. 2014;30(11):1418-1423.
10. Lo IK, Lind CC, Burkhart SS. Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy. 2004;20(6):596-602.
11. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
12. Shishido H, Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):372-376.
13. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
14. Kim SH, Crater RB, Hargens AR. Movement-induced knot migration after anterior stabilization in the shoulder. Arthroscopy. 2013;29(3):485-490.
Take-Home Points
- Anchors placed posterior to the biceps during SLAP repair are at risk for glenoid vault penetration and/or suprascapular nerve (SSN) injury.
- Vault penetration and SSN injury are avoided by using a Port of Wilmington (PW) portal instead of an anterior portal.
- A percutaneous PW portal is safe and passes through the rotator cuff muscle only.
Since being classified by Snyder and colleagues,1 various arthroscopic techniques have been used to repair superior labrum anterior and posterior (SLAP) tears, particularly type II tears. Despite being commonly performed, repairs of SLAP lesions remain challenging. There is high variability in the rate of good/excellent functional outcomes and athletes’ return to previous level of play after SLAP repairs.2,3 Furthermore, the rate of complications after SLAP repair is as high as 5%.4
One of the most common complications of repair of a type II SLAP tear is nerve injury.4 In particular, suprascapular nerve (SSN) injury has occurred after arthroscopic repair of SLAP tears.5,6 Three cadaveric studies have demonstrated that glenoid vault penetration is common during placement of knotted anchors for SLAP repair and that the SSN is at risk during placement of these anchors.7-9 However, 2 of the 3 studies used only an anterior portal in their evaluation of anchor placement. Safety of anchor placement posterior to the biceps tendon may be improved with a percutaneous approach using a Port of Wilmington (PW) portal.10,11 No studies have evaluated the risk of glenoid vault penetration and SSN injury with shorter knotless anchors.
We conducted a study to compare a standard anterosuperolateral (ASL) portal with a percutaneous PW portal for knotless anchors placed posterior to the biceps tendon during repair of SLAP tears. We hypothesized that anchors placed through the PW portal would be less likely to penetrate the glenoid vault and would be farther from the SSN in the event of bone penetration.
Materials and Methods
Six matched pairs of fresh human cadaveric shoulders were used in this study. Each specimen included the scapula, the clavicle, and the humerus. All 6 specimens were male, and their mean age was 41.2 years (range, 23-59 years). Shoulder arthroscopy was performed for placement of SLAP anchors, and open dissection followed.
Anchor Placement
The scapula was clamped and the shoulder placed in the lateral decubitus position with 30° of abduction, 20° of forward flexion, and neutral rotation.10 A standard posterior glenohumeral viewing portal was established and a 30° arthroscope inserted. Both shoulders of each matched pair were randomly assigned to anchor placement through either an ASL portal or a PW portal. Two anchors were placed in the superior glenoid to simulate repair of a posterior SLAP tear.11 Each was a 2.9-mm short (12.5-mm) knotless anchor (BioComposite PushLock; Arthrex) that included a polyetheretherketone (PEEK) eyelet for threading sutures before anchor placement. A drill guide was inserted according to manufacturer guidelines, and a 2.9-mm drill was used to make a bone socket 18 mm deep. The anchor eyelet was loaded with suture tape (Labral Tape; Arthrex), and the anchor and suture were inserted into the socket. The sutures were left uncut to aid in anchor visualization during open dissection. On a right shoulder, the first anchor was placed just posterior to the biceps tendon, at 11 o’clock, and the second anchor about 1 cm posterior to the first, at 10 o’clock. All anchors were placed by an arthroscopy fellowship–trained shoulder surgeon. Before placement, anchor location was confirmed by another arthroscopy fellowship–trained shoulder surgeon.
The ASL portal was created, with an 18-gauge spinal needle and an outside-in technique, about 1 cm lateral to the anterolateral corner of the acromion.
In the opposite shoulder, the PW portal was created, with a percutaneous technique, about 1 cm anterior and 1 cm lateral to the posterolateral corner of the acromion. An 18-gauge spinal needle was inserted to allow a 45° angle of approach to the posterosuperior glenoid.11
Cadaveric Dissection
After anchor placement, another shoulder surgeon performed the dissection. Skin, subcutaneous tissue, deltoid, and clavicle were removed. In the percutaneous specimens, PW portal location relative to rotator cuff was recorded before cuff removal. After overlying soft tissues were removed from a specimen, the anchors were examined for glenoid vault penetration. In the setting of vault penetration, digital calipers were used to measure the shortest distance from anchor to SSN.
Results
In the ASL portal group, 8 (66.7%) of 12 anchors (4/6 at 11 o’clock, 4/6 at 10 o’clock) penetrated the medial glenoid vault.
In the PW portal group, 2 (16.7%) of 12 anchors (1/6 at 11 o’clock, 1/6 at 10 o’clock, both from a single specimen) penetrated the medial glenoid vault. Actually, in each case the eyelet and not the anchor penetrated the vault. In the penetration cases, distance to SSN was 20 mm for the 11 o’clock anchor and 8 mm for the 10 o’clock anchor (Table). Of the 6 portals, 3 passed through the supraspinatus muscle, 2 through the infraspinatus musculotendinous junction, and 1 through the infraspinatus muscle.
Discussion
Our study findings support the hypothesis that SLAP repair anchors placed posterior to the biceps tendon are more likely to remain in bone with use of a percutaneous approach relative to an ASL approach. Our findings also support the growing body of evidence that such anchors placed with an anterior approach increase the risk for SSN injury.
Three other cadaveric studies have evaluated anchor placement for SLAP repair. Chan and colleagues7 evaluated drill penetration during bone socket preparation for SLAP repair in 21 matched pairs of formalin-embalmed cadavers. A 20-mm drill was used for correspondence to a 14.5-mm anchor, though no anchors were inserted, and sockets were created in an open manner. Through a mimicked ASL portal, 1 socket was made anterior to the biceps tendon, at 1 o’clock; then, through a mimicked PW portal, 2 sockets were made posterior to the tendon, at 11 o’clock and 9 to 10 o’clock. Glenoid vault penetration occurred in 29% of the 42 anterior sockets, but only 1 anchor (2.4%) touched the SSN. Penetration did not occur with the 11 o’clock anchors. The 9 to 10 o’clock anchor was at highest risk for SSN injury (9.5%, 4 cases). The study was limited by lack of anchor placement and open creation of bone sockets in embalmed cadavers.
Koh and colleagues8 evaluated arthroscopic placement of anterior SLAP anchors in 6 matched pairs of fresh-frozen cadavers. Through an ASL portal, each 14.5-mm knotted anchor was placed anterior to the biceps tendon, at 1 o’clock. As in the study by Chan and colleagues,7 drill depth was 20 mm. Notably, anchors were seated 2 mm beyond manufacturer recommendations, and the cadavers were of Asian origin, likely indicating smaller glenoids compared to specimens from North America or Europe. All 12 anchors penetrated the glenoid vault; mean distance to SSN was 3.1 mm.
Morgan and colleagues9 compared anterior and ASL portals created for SLAP repairs in 10 matched-pair cadavers. Anchors were placed at 1 o’clock, 11 o’clock, and 10 o’clock. As in the studies by Chan and colleagues7 and Koh and colleagues,8 14.5-mm knotted anchors were used. One anterior anchor (10%) placed through an ASL portal penetrated the cortex by 1 mm, and 2 anterior anchors (20%) placed through anterior portals penetrated the cortex (1 was completely out of the bone). Overall, 65% of 11 o’clock anchors and 100% of 10 o’clock anchors violated the glenoid vault. With the 11 o’clock anchors, mean distance to SSN was 6 mm for ASL portals and 4.2 mm for anterior portals; with the 10 o’clock anchors, mean distance to SSN was 8 mm for ASL portals and 2.1 mm for anterior portals.
Overall, the results of these 3 studies suggest that, with use of ASL portals, placement of SLAP anchors anterior to the biceps tendon is safe. Using the same portals, however, anchors placed posterior to the tendon are at higher risk for glenoid vault penetration. Supporting these findings are our study’s penetration rates: 66.7% for anchors placed through ASL portals and 16.7% for anchors placed through percutaneous PW portals. The different rates are not surprising given that the coracoid process projects anterior to the glenoid and provides additional bone stock for placement of anchors anteriorly vs posteriorly. Therefore, with percutaneous PW portals, the approach angle directs the anchor toward the bone of the coracoid base. Furthermore, the SSN passes nearest the posterior aspect of the glenoid. In a study by Shishido and Kikuchi,12 the distance from the posterior rim of the glenoid to the SSN was 18 mm, and from the superior rim was 29 mm. Therefore, anchors placed with an anterior approach naturally are directed toward the SSN.
In addition to portal placement and approach angle, anchor length likely affects the risks for glenoid vault penetration and SSN injury.
One limitation of this study was the small number of cadavers, all of which were male. Female cadavers and cadavers of other ethnic origins likely have smaller glenoid vaults, and thus their inclusion would have altered our results. This issue was well described in studies mentioned in this article, and our goal was simply to compare ASL portals with percutaneous PW portals, so we think it does not change the fact that the risks for glenoid vault penetration and SSN injury are reduced with use of PW portals for anchors placed posterior to the biceps tendon.
Conclusion
This study was the first to examine glenoid vault penetration and SSN proximity with short anchors for SLAP repair. The risk for glenoid vault penetration during repair of SLAP tears posterior to the biceps tendon was reduced by anchor placement with a percutaneous posterior approach. The percutaneous posterior approach also directs the anchor away from the SSN.
Am J Orthop. 2017;46(1):E60-E64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Anchors placed posterior to the biceps during SLAP repair are at risk for glenoid vault penetration and/or suprascapular nerve (SSN) injury.
- Vault penetration and SSN injury are avoided by using a Port of Wilmington (PW) portal instead of an anterior portal.
- A percutaneous PW portal is safe and passes through the rotator cuff muscle only.
Since being classified by Snyder and colleagues,1 various arthroscopic techniques have been used to repair superior labrum anterior and posterior (SLAP) tears, particularly type II tears. Despite being commonly performed, repairs of SLAP lesions remain challenging. There is high variability in the rate of good/excellent functional outcomes and athletes’ return to previous level of play after SLAP repairs.2,3 Furthermore, the rate of complications after SLAP repair is as high as 5%.4
One of the most common complications of repair of a type II SLAP tear is nerve injury.4 In particular, suprascapular nerve (SSN) injury has occurred after arthroscopic repair of SLAP tears.5,6 Three cadaveric studies have demonstrated that glenoid vault penetration is common during placement of knotted anchors for SLAP repair and that the SSN is at risk during placement of these anchors.7-9 However, 2 of the 3 studies used only an anterior portal in their evaluation of anchor placement. Safety of anchor placement posterior to the biceps tendon may be improved with a percutaneous approach using a Port of Wilmington (PW) portal.10,11 No studies have evaluated the risk of glenoid vault penetration and SSN injury with shorter knotless anchors.
We conducted a study to compare a standard anterosuperolateral (ASL) portal with a percutaneous PW portal for knotless anchors placed posterior to the biceps tendon during repair of SLAP tears. We hypothesized that anchors placed through the PW portal would be less likely to penetrate the glenoid vault and would be farther from the SSN in the event of bone penetration.
Materials and Methods
Six matched pairs of fresh human cadaveric shoulders were used in this study. Each specimen included the scapula, the clavicle, and the humerus. All 6 specimens were male, and their mean age was 41.2 years (range, 23-59 years). Shoulder arthroscopy was performed for placement of SLAP anchors, and open dissection followed.
Anchor Placement
The scapula was clamped and the shoulder placed in the lateral decubitus position with 30° of abduction, 20° of forward flexion, and neutral rotation.10 A standard posterior glenohumeral viewing portal was established and a 30° arthroscope inserted. Both shoulders of each matched pair were randomly assigned to anchor placement through either an ASL portal or a PW portal. Two anchors were placed in the superior glenoid to simulate repair of a posterior SLAP tear.11 Each was a 2.9-mm short (12.5-mm) knotless anchor (BioComposite PushLock; Arthrex) that included a polyetheretherketone (PEEK) eyelet for threading sutures before anchor placement. A drill guide was inserted according to manufacturer guidelines, and a 2.9-mm drill was used to make a bone socket 18 mm deep. The anchor eyelet was loaded with suture tape (Labral Tape; Arthrex), and the anchor and suture were inserted into the socket. The sutures were left uncut to aid in anchor visualization during open dissection. On a right shoulder, the first anchor was placed just posterior to the biceps tendon, at 11 o’clock, and the second anchor about 1 cm posterior to the first, at 10 o’clock. All anchors were placed by an arthroscopy fellowship–trained shoulder surgeon. Before placement, anchor location was confirmed by another arthroscopy fellowship–trained shoulder surgeon.
The ASL portal was created, with an 18-gauge spinal needle and an outside-in technique, about 1 cm lateral to the anterolateral corner of the acromion.
In the opposite shoulder, the PW portal was created, with a percutaneous technique, about 1 cm anterior and 1 cm lateral to the posterolateral corner of the acromion. An 18-gauge spinal needle was inserted to allow a 45° angle of approach to the posterosuperior glenoid.11
Cadaveric Dissection
After anchor placement, another shoulder surgeon performed the dissection. Skin, subcutaneous tissue, deltoid, and clavicle were removed. In the percutaneous specimens, PW portal location relative to rotator cuff was recorded before cuff removal. After overlying soft tissues were removed from a specimen, the anchors were examined for glenoid vault penetration. In the setting of vault penetration, digital calipers were used to measure the shortest distance from anchor to SSN.
Results
In the ASL portal group, 8 (66.7%) of 12 anchors (4/6 at 11 o’clock, 4/6 at 10 o’clock) penetrated the medial glenoid vault.
In the PW portal group, 2 (16.7%) of 12 anchors (1/6 at 11 o’clock, 1/6 at 10 o’clock, both from a single specimen) penetrated the medial glenoid vault. Actually, in each case the eyelet and not the anchor penetrated the vault. In the penetration cases, distance to SSN was 20 mm for the 11 o’clock anchor and 8 mm for the 10 o’clock anchor (Table). Of the 6 portals, 3 passed through the supraspinatus muscle, 2 through the infraspinatus musculotendinous junction, and 1 through the infraspinatus muscle.
Discussion
Our study findings support the hypothesis that SLAP repair anchors placed posterior to the biceps tendon are more likely to remain in bone with use of a percutaneous approach relative to an ASL approach. Our findings also support the growing body of evidence that such anchors placed with an anterior approach increase the risk for SSN injury.
Three other cadaveric studies have evaluated anchor placement for SLAP repair. Chan and colleagues7 evaluated drill penetration during bone socket preparation for SLAP repair in 21 matched pairs of formalin-embalmed cadavers. A 20-mm drill was used for correspondence to a 14.5-mm anchor, though no anchors were inserted, and sockets were created in an open manner. Through a mimicked ASL portal, 1 socket was made anterior to the biceps tendon, at 1 o’clock; then, through a mimicked PW portal, 2 sockets were made posterior to the tendon, at 11 o’clock and 9 to 10 o’clock. Glenoid vault penetration occurred in 29% of the 42 anterior sockets, but only 1 anchor (2.4%) touched the SSN. Penetration did not occur with the 11 o’clock anchors. The 9 to 10 o’clock anchor was at highest risk for SSN injury (9.5%, 4 cases). The study was limited by lack of anchor placement and open creation of bone sockets in embalmed cadavers.
Koh and colleagues8 evaluated arthroscopic placement of anterior SLAP anchors in 6 matched pairs of fresh-frozen cadavers. Through an ASL portal, each 14.5-mm knotted anchor was placed anterior to the biceps tendon, at 1 o’clock. As in the study by Chan and colleagues,7 drill depth was 20 mm. Notably, anchors were seated 2 mm beyond manufacturer recommendations, and the cadavers were of Asian origin, likely indicating smaller glenoids compared to specimens from North America or Europe. All 12 anchors penetrated the glenoid vault; mean distance to SSN was 3.1 mm.
Morgan and colleagues9 compared anterior and ASL portals created for SLAP repairs in 10 matched-pair cadavers. Anchors were placed at 1 o’clock, 11 o’clock, and 10 o’clock. As in the studies by Chan and colleagues7 and Koh and colleagues,8 14.5-mm knotted anchors were used. One anterior anchor (10%) placed through an ASL portal penetrated the cortex by 1 mm, and 2 anterior anchors (20%) placed through anterior portals penetrated the cortex (1 was completely out of the bone). Overall, 65% of 11 o’clock anchors and 100% of 10 o’clock anchors violated the glenoid vault. With the 11 o’clock anchors, mean distance to SSN was 6 mm for ASL portals and 4.2 mm for anterior portals; with the 10 o’clock anchors, mean distance to SSN was 8 mm for ASL portals and 2.1 mm for anterior portals.
Overall, the results of these 3 studies suggest that, with use of ASL portals, placement of SLAP anchors anterior to the biceps tendon is safe. Using the same portals, however, anchors placed posterior to the tendon are at higher risk for glenoid vault penetration. Supporting these findings are our study’s penetration rates: 66.7% for anchors placed through ASL portals and 16.7% for anchors placed through percutaneous PW portals. The different rates are not surprising given that the coracoid process projects anterior to the glenoid and provides additional bone stock for placement of anchors anteriorly vs posteriorly. Therefore, with percutaneous PW portals, the approach angle directs the anchor toward the bone of the coracoid base. Furthermore, the SSN passes nearest the posterior aspect of the glenoid. In a study by Shishido and Kikuchi,12 the distance from the posterior rim of the glenoid to the SSN was 18 mm, and from the superior rim was 29 mm. Therefore, anchors placed with an anterior approach naturally are directed toward the SSN.
In addition to portal placement and approach angle, anchor length likely affects the risks for glenoid vault penetration and SSN injury.
One limitation of this study was the small number of cadavers, all of which were male. Female cadavers and cadavers of other ethnic origins likely have smaller glenoid vaults, and thus their inclusion would have altered our results. This issue was well described in studies mentioned in this article, and our goal was simply to compare ASL portals with percutaneous PW portals, so we think it does not change the fact that the risks for glenoid vault penetration and SSN injury are reduced with use of PW portals for anchors placed posterior to the biceps tendon.
Conclusion
This study was the first to examine glenoid vault penetration and SSN proximity with short anchors for SLAP repair. The risk for glenoid vault penetration during repair of SLAP tears posterior to the biceps tendon was reduced by anchor placement with a percutaneous posterior approach. The percutaneous posterior approach also directs the anchor away from the SSN.
Am J Orthop. 2017;46(1):E60-E64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
2. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
3. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
4. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
5. Kim SH, Koh YG, Sung CH, Moon HK, Park YS. Iatrogenic suprascapular nerve injury after repair of type II SLAP lesion. Arthroscopy. 2010;26(7):1005-1008.
6. Yoo JC, Lee YS, Ahn JH, Park JH, Kang HJ, Koh KH. Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg. 2009;18(4):e27-e29.
7. Chan H, Beaupre LA, Bouliane MJ. Injury of the suprascapular nerve during arthroscopic repair of superior labral tears: an anatomic study. J Shoulder Elbow Surg. 2010;19(5):709-715.
8. Koh KH, Park WH, Lim TK, Yoo JC. Medial perforation of the glenoid neck following SLAP repair places the suprascapular nerve at risk: a cadaveric study. J Shoulder Elbow Surg. 2011;20(2):245-250.
9. Morgan RT, Henn RF 3rd, Paryavi E, Dreese J. Injury to the suprascapular nerve during superior labrum anterior and posterior repair: is a rotator interval portal safer than an anterosuperior portal? Arthroscopy. 2014;30(11):1418-1423.
10. Lo IK, Lind CC, Burkhart SS. Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy. 2004;20(6):596-602.
11. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
12. Shishido H, Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):372-376.
13. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
14. Kim SH, Crater RB, Hargens AR. Movement-induced knot migration after anterior stabilization in the shoulder. Arthroscopy. 2013;29(3):485-490.
1. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
2. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
3. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
4. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
5. Kim SH, Koh YG, Sung CH, Moon HK, Park YS. Iatrogenic suprascapular nerve injury after repair of type II SLAP lesion. Arthroscopy. 2010;26(7):1005-1008.
6. Yoo JC, Lee YS, Ahn JH, Park JH, Kang HJ, Koh KH. Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg. 2009;18(4):e27-e29.
7. Chan H, Beaupre LA, Bouliane MJ. Injury of the suprascapular nerve during arthroscopic repair of superior labral tears: an anatomic study. J Shoulder Elbow Surg. 2010;19(5):709-715.
8. Koh KH, Park WH, Lim TK, Yoo JC. Medial perforation of the glenoid neck following SLAP repair places the suprascapular nerve at risk: a cadaveric study. J Shoulder Elbow Surg. 2011;20(2):245-250.
9. Morgan RT, Henn RF 3rd, Paryavi E, Dreese J. Injury to the suprascapular nerve during superior labrum anterior and posterior repair: is a rotator interval portal safer than an anterosuperior portal? Arthroscopy. 2014;30(11):1418-1423.
10. Lo IK, Lind CC, Burkhart SS. Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy. 2004;20(6):596-602.
11. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
12. Shishido H, Kikuchi S. Injury of the suprascapular nerve in shoulder surgery: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):372-376.
13. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
14. Kim SH, Crater RB, Hargens AR. Movement-induced knot migration after anterior stabilization in the shoulder. Arthroscopy. 2013;29(3):485-490.
Prospective Evaluation of Opioid Consumption After Distal Radius Fracture Repair Surgery
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.