User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
In-Office Diagnostic Needle Arthroscopy
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
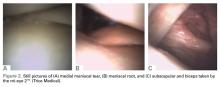
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
Editorial Board Biographies
Matthew J. Matava, MD
Associate Editor for Professional Sports
Dr. Matava is a professor of Orthopedic Surgery and Physical Therapy, Chief of the Sports Medicine Service, and the Head Team Physician for the varsity athletic program at Washington University in St. Louis. He is also a team physician for the National Hockey League’s St. Louis Blues. Formerly, he was the Head Team Physician for the St. Louis Rams, and was President of the National Football League Physicians Society (NFLPS) from 2013-2015. Dr. Matava earned his Medical Degree from the University of Missouri-Kansas City. He completed his internship and orthopedic surgery residency at Emory University in Atlanta, GA, followed by a fellowship in sports medicine and arthroscopic surgery at the Cincinnati Sports Medicine and Orthopedic Center. He is the recipient of several research awards from Emory University, is a member of the Alpha Omega Medical Honor Society, and received the Palma Chironis Award for Excellence in Teaching from the Washington University Department of Orthopedic Surgery in 2012. Dr. Matava has been listed as a “Best Doctor in America” since 2005, and was recently hailed by Orthopedics This Week as one of the top 28 sports knee surgeons in the nation.
Jeffrey Sawyer, MD
Associate Editor for Pediatrics
Dr. Sawyer is a professor of Orthopaedic Surgery and the Pediatric Orthopaedic Fellowship Director at the University of Tennessee-Campbell Clinic. He also serves as a reviewer/editor for the Journal of Pediatric Orthopaedics and Orthopedic Clinics of North America. He graduated from the University of Rochester School of Medicine and completed his residency at the University of Pennsylvania, prior to completing his Pediatric Orthopaedic Fellowship at the University of Tennessee-Campbell Clinic. Dr. Sawyer has held numerous leadership positions in the Pediatric Orthopaedic Society of North America (POSNA). He also was a POSNA Traveling Fellow and won the POSNA Special Achievement Award for his work on the Pediatric Orthopaedic Workforce. He is a national authority on pediatric orthopedic trauma, and is on the Executive Committee of the Children’s Spine Foundation.
Brian K. Vickaryous, MD
Associate Editor for Trauma
Dr. Vickaryous is a specialist in orthopedic traumatology at the Florida Hospital Orthopedic Institute in Orlando, Florida, and has an additional subspecialty board certification in sports medicine. He attended the University of Miami, Florida through the combined degree Medical
Honors Program and completed his residency at the William Beaumont Army Medical Center/Texas Tech University of the Health Sciences. Dr. Vickaryous has also deployed overseas as Commander of the Trauma Unit, the 8th Forward Surgical Team, in Iraq in support of Operation Iraqi Freedom. He currently is a member of the American Academy of Orthopaedic
Surgeons (AAOS) and the Orthopaedic Trauma Association (OTA).
Michael B. Gerhardt, MD
Associate Editor for Sports Medicine
Dr. Gerhardt is a sports medicine specialist at the Kerlan-Jobe Institute and Santa Monica Orthopaedic Group in Los Angeles, CA. He also serves as faculty in the Department of Orthopaedic Surgery at Cedars-Sinai Medical Center. Dr. Gerhardt earned his undergraduate degree from UC San Diego and graduated medical school with honors from the Medical College of Pennsylvania. He received the Leonard Marmur Award for excellence in research and education during his orthopedic residency at the University of Southern California, prior to completing a Sports Medicine Fellowship in 2003. He received further training in hip arthroscopy at the Nashville Orthopaedic Sports Medicine and Orthopaedic Clinic, and maintains a leadership role in the area of sports medicine and hip preservation on a national and international level. Currently, he serves as Team Physician for the US Soccer Men’s National Team, the Los Angeles Galaxy, and Pepperdine University.
Matthew J. Matava, MD
Associate Editor for Professional Sports
Dr. Matava is a professor of Orthopedic Surgery and Physical Therapy, Chief of the Sports Medicine Service, and the Head Team Physician for the varsity athletic program at Washington University in St. Louis. He is also a team physician for the National Hockey League’s St. Louis Blues. Formerly, he was the Head Team Physician for the St. Louis Rams, and was President of the National Football League Physicians Society (NFLPS) from 2013-2015. Dr. Matava earned his Medical Degree from the University of Missouri-Kansas City. He completed his internship and orthopedic surgery residency at Emory University in Atlanta, GA, followed by a fellowship in sports medicine and arthroscopic surgery at the Cincinnati Sports Medicine and Orthopedic Center. He is the recipient of several research awards from Emory University, is a member of the Alpha Omega Medical Honor Society, and received the Palma Chironis Award for Excellence in Teaching from the Washington University Department of Orthopedic Surgery in 2012. Dr. Matava has been listed as a “Best Doctor in America” since 2005, and was recently hailed by Orthopedics This Week as one of the top 28 sports knee surgeons in the nation.
Jeffrey Sawyer, MD
Associate Editor for Pediatrics
Dr. Sawyer is a professor of Orthopaedic Surgery and the Pediatric Orthopaedic Fellowship Director at the University of Tennessee-Campbell Clinic. He also serves as a reviewer/editor for the Journal of Pediatric Orthopaedics and Orthopedic Clinics of North America. He graduated from the University of Rochester School of Medicine and completed his residency at the University of Pennsylvania, prior to completing his Pediatric Orthopaedic Fellowship at the University of Tennessee-Campbell Clinic. Dr. Sawyer has held numerous leadership positions in the Pediatric Orthopaedic Society of North America (POSNA). He also was a POSNA Traveling Fellow and won the POSNA Special Achievement Award for his work on the Pediatric Orthopaedic Workforce. He is a national authority on pediatric orthopedic trauma, and is on the Executive Committee of the Children’s Spine Foundation.
Brian K. Vickaryous, MD
Associate Editor for Trauma
Dr. Vickaryous is a specialist in orthopedic traumatology at the Florida Hospital Orthopedic Institute in Orlando, Florida, and has an additional subspecialty board certification in sports medicine. He attended the University of Miami, Florida through the combined degree Medical
Honors Program and completed his residency at the William Beaumont Army Medical Center/Texas Tech University of the Health Sciences. Dr. Vickaryous has also deployed overseas as Commander of the Trauma Unit, the 8th Forward Surgical Team, in Iraq in support of Operation Iraqi Freedom. He currently is a member of the American Academy of Orthopaedic
Surgeons (AAOS) and the Orthopaedic Trauma Association (OTA).
Michael B. Gerhardt, MD
Associate Editor for Sports Medicine
Dr. Gerhardt is a sports medicine specialist at the Kerlan-Jobe Institute and Santa Monica Orthopaedic Group in Los Angeles, CA. He also serves as faculty in the Department of Orthopaedic Surgery at Cedars-Sinai Medical Center. Dr. Gerhardt earned his undergraduate degree from UC San Diego and graduated medical school with honors from the Medical College of Pennsylvania. He received the Leonard Marmur Award for excellence in research and education during his orthopedic residency at the University of Southern California, prior to completing a Sports Medicine Fellowship in 2003. He received further training in hip arthroscopy at the Nashville Orthopaedic Sports Medicine and Orthopaedic Clinic, and maintains a leadership role in the area of sports medicine and hip preservation on a national and international level. Currently, he serves as Team Physician for the US Soccer Men’s National Team, the Los Angeles Galaxy, and Pepperdine University.
Matthew J. Matava, MD
Associate Editor for Professional Sports
Dr. Matava is a professor of Orthopedic Surgery and Physical Therapy, Chief of the Sports Medicine Service, and the Head Team Physician for the varsity athletic program at Washington University in St. Louis. He is also a team physician for the National Hockey League’s St. Louis Blues. Formerly, he was the Head Team Physician for the St. Louis Rams, and was President of the National Football League Physicians Society (NFLPS) from 2013-2015. Dr. Matava earned his Medical Degree from the University of Missouri-Kansas City. He completed his internship and orthopedic surgery residency at Emory University in Atlanta, GA, followed by a fellowship in sports medicine and arthroscopic surgery at the Cincinnati Sports Medicine and Orthopedic Center. He is the recipient of several research awards from Emory University, is a member of the Alpha Omega Medical Honor Society, and received the Palma Chironis Award for Excellence in Teaching from the Washington University Department of Orthopedic Surgery in 2012. Dr. Matava has been listed as a “Best Doctor in America” since 2005, and was recently hailed by Orthopedics This Week as one of the top 28 sports knee surgeons in the nation.
Jeffrey Sawyer, MD
Associate Editor for Pediatrics
Dr. Sawyer is a professor of Orthopaedic Surgery and the Pediatric Orthopaedic Fellowship Director at the University of Tennessee-Campbell Clinic. He also serves as a reviewer/editor for the Journal of Pediatric Orthopaedics and Orthopedic Clinics of North America. He graduated from the University of Rochester School of Medicine and completed his residency at the University of Pennsylvania, prior to completing his Pediatric Orthopaedic Fellowship at the University of Tennessee-Campbell Clinic. Dr. Sawyer has held numerous leadership positions in the Pediatric Orthopaedic Society of North America (POSNA). He also was a POSNA Traveling Fellow and won the POSNA Special Achievement Award for his work on the Pediatric Orthopaedic Workforce. He is a national authority on pediatric orthopedic trauma, and is on the Executive Committee of the Children’s Spine Foundation.
Brian K. Vickaryous, MD
Associate Editor for Trauma
Dr. Vickaryous is a specialist in orthopedic traumatology at the Florida Hospital Orthopedic Institute in Orlando, Florida, and has an additional subspecialty board certification in sports medicine. He attended the University of Miami, Florida through the combined degree Medical
Honors Program and completed his residency at the William Beaumont Army Medical Center/Texas Tech University of the Health Sciences. Dr. Vickaryous has also deployed overseas as Commander of the Trauma Unit, the 8th Forward Surgical Team, in Iraq in support of Operation Iraqi Freedom. He currently is a member of the American Academy of Orthopaedic
Surgeons (AAOS) and the Orthopaedic Trauma Association (OTA).
Michael B. Gerhardt, MD
Associate Editor for Sports Medicine
Dr. Gerhardt is a sports medicine specialist at the Kerlan-Jobe Institute and Santa Monica Orthopaedic Group in Los Angeles, CA. He also serves as faculty in the Department of Orthopaedic Surgery at Cedars-Sinai Medical Center. Dr. Gerhardt earned his undergraduate degree from UC San Diego and graduated medical school with honors from the Medical College of Pennsylvania. He received the Leonard Marmur Award for excellence in research and education during his orthopedic residency at the University of Southern California, prior to completing a Sports Medicine Fellowship in 2003. He received further training in hip arthroscopy at the Nashville Orthopaedic Sports Medicine and Orthopaedic Clinic, and maintains a leadership role in the area of sports medicine and hip preservation on a national and international level. Currently, he serves as Team Physician for the US Soccer Men’s National Team, the Los Angeles Galaxy, and Pepperdine University.
Presentation of the 2016 Resident Writer’s Award
Darla Conrad (left), Senior Director, North America Education Solutions, Johnson & Johnson Medical Devices, presents Kalpit N. Shah, MD (right) with his plaque for the second-place Resident Writer’s Award, and Christopher Rice, MD (center) with his plaque for the third-place Resident Writer’s Award at the 2017 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) in San Diego.
Winners of the 2016 Resident Writer’s Award
First-Place Award
An Original Study
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Dr. Kusnezov is a senior resident, completing his orthopedic surgery residency training, at the Texas Tech University Health Sciences Center/William Beaumont Army Medical Center joint military-civilian program in El Paso, Texas. Prior to residency, he completed both his undergraduate education and medical school at the University of California, Los Angeles, graduating Summa Cum Laude and AOA, respectively. Dr. Kusnezov is currently engaged in a multitude of ongoing projects with over 50 peer-reviewed publications to date. His research interests include trauma and limb salvage, complex total joint reconstruction, and interdisciplinary system improvement.
Second-Place Award
An Original Study
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Dr. Shah is currently in his third year of orthopedic surgery residency training at Brown University in Providence, Rhode Island. Prior to residency, he completed undergraduate education at the University of California, Berkeley, and medical school at the University of California, Irvine. He hopes to pursue a hand and upper extremity fellowship after residency. His research interests include upper extremity trauma and surgical complications, as well as technology and its implications on orthopedic surgery.
Third-Place Award
An Original Study
Treating Tibia Fractures With Far Cortical Locking Implants
Dr. Rice is an orthopedic surgery resident at the University of Wisconsin, Madison. He received his medical degree from the University of Utah and attended Brigham Young University for his undergraduate studies. He has a special interest in disorders of the hand and upper extremity trauma.
Darla Conrad (left), Senior Director, North America Education Solutions, Johnson & Johnson Medical Devices, presents Kalpit N. Shah, MD (right) with his plaque for the second-place Resident Writer’s Award, and Christopher Rice, MD (center) with his plaque for the third-place Resident Writer’s Award at the 2017 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) in San Diego.
Winners of the 2016 Resident Writer’s Award
First-Place Award
An Original Study
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Dr. Kusnezov is a senior resident, completing his orthopedic surgery residency training, at the Texas Tech University Health Sciences Center/William Beaumont Army Medical Center joint military-civilian program in El Paso, Texas. Prior to residency, he completed both his undergraduate education and medical school at the University of California, Los Angeles, graduating Summa Cum Laude and AOA, respectively. Dr. Kusnezov is currently engaged in a multitude of ongoing projects with over 50 peer-reviewed publications to date. His research interests include trauma and limb salvage, complex total joint reconstruction, and interdisciplinary system improvement.
Second-Place Award
An Original Study
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Dr. Shah is currently in his third year of orthopedic surgery residency training at Brown University in Providence, Rhode Island. Prior to residency, he completed undergraduate education at the University of California, Berkeley, and medical school at the University of California, Irvine. He hopes to pursue a hand and upper extremity fellowship after residency. His research interests include upper extremity trauma and surgical complications, as well as technology and its implications on orthopedic surgery.
Third-Place Award
An Original Study
Treating Tibia Fractures With Far Cortical Locking Implants
Dr. Rice is an orthopedic surgery resident at the University of Wisconsin, Madison. He received his medical degree from the University of Utah and attended Brigham Young University for his undergraduate studies. He has a special interest in disorders of the hand and upper extremity trauma.
Darla Conrad (left), Senior Director, North America Education Solutions, Johnson & Johnson Medical Devices, presents Kalpit N. Shah, MD (right) with his plaque for the second-place Resident Writer’s Award, and Christopher Rice, MD (center) with his plaque for the third-place Resident Writer’s Award at the 2017 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) in San Diego.
Winners of the 2016 Resident Writer’s Award
First-Place Award
An Original Study
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Dr. Kusnezov is a senior resident, completing his orthopedic surgery residency training, at the Texas Tech University Health Sciences Center/William Beaumont Army Medical Center joint military-civilian program in El Paso, Texas. Prior to residency, he completed both his undergraduate education and medical school at the University of California, Los Angeles, graduating Summa Cum Laude and AOA, respectively. Dr. Kusnezov is currently engaged in a multitude of ongoing projects with over 50 peer-reviewed publications to date. His research interests include trauma and limb salvage, complex total joint reconstruction, and interdisciplinary system improvement.
Second-Place Award
An Original Study
Patient-Reported Outcome Measures: How Do Digital Tablets Stack Up to Paper Forms? A Randomized, Controlled Study
Dr. Shah is currently in his third year of orthopedic surgery residency training at Brown University in Providence, Rhode Island. Prior to residency, he completed undergraduate education at the University of California, Berkeley, and medical school at the University of California, Irvine. He hopes to pursue a hand and upper extremity fellowship after residency. His research interests include upper extremity trauma and surgical complications, as well as technology and its implications on orthopedic surgery.
Third-Place Award
An Original Study
Treating Tibia Fractures With Far Cortical Locking Implants
Dr. Rice is an orthopedic surgery resident at the University of Wisconsin, Madison. He received his medical degree from the University of Utah and attended Brigham Young University for his undergraduate studies. He has a special interest in disorders of the hand and upper extremity trauma.
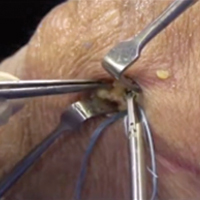
Medial Patellofemoral Ligament Repair
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Video, Part 1. Femoral Attachment
1. Ultrasound is used to identify femoral and patellar attachments of medial patellofemoral ligament (MPFL).
2. MPFL is followed from patella to its attachment near adductor tubercle.
3. In-plane ultrasound guidance is used to place needle anterior and distal to tubercle.
4. Percutaneous incision is made down to needle tip. Spear is placed at needle tip for anatomical placement of socket.
5. Socket is drilled.
6. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed.
7. Leading edge of torn MPFL is identified.
8. Suture passer (Labral FastPass Scorpion; Arthrex) is used to pass sutures through leading edge of torn MPFL to create horizontal mattress.
9. Sutures are tied.
10. Repair is complete.
Video, Part 2. Patellar Attachment
1. Ultrasound is used to scan patella to identify ideal or exact location of tear. In-plane ultrasound guidance is used to place spinal needle at desired socket location.
2. After spinal needle is positioned, in-line percutaneous incision is made, and needle is palpated at patella.
3. Spear is then placed at spinal needle tip for anatomical positioning of socket.
4. Socket is drilled.
5. 3.0-mm suture anchor (BioComposite Knotless SutureTak; Arthrex) is placed in socket.
6. Leading edge of torn medial patellofemoral ligament (MPFL) is identified.
7. Suture passer (Labral Past Pass Scorpion; Arthrex) is used to pass suture from anchor in horizontal mattress fashion through leading edge of torn MPFL.
8. Wire with loop (FiberSnare; Arthrex) is used as part of knotless technology to pull suture back through anchor to create knotless fixation.
9. Suture is pulled for appropriate tensioning of tissue.
10. Ultrasound is used to visualize construct to confirm that MPFL tissue abuts anchor and that repair is complete.
Treatment of Unstable Trochanteric Femur Fractures: Proximal Femur Nail Versus Proximal Femur Locking Compression Plate
Take-Home Points
- Both PFN and PFLCP are effective treatments for unstable trochanteric femur fractures.
- PFN is superior to PFLCP only in terms of shorter incisions and shorter time to full weight-bearing.
- Both devices have good long-term functional outcomes.
- Complication rates in unstable trochanteric fractures treated with both implants are comparable.
- Larger randomized controlled multicenter studies are needed to further evaluate and compare both implants in displaced unstable trochanteric femur fractures.
Trochanteric fractures are among the most widely treated orthopedic injuries, occurring mainly as low-energy injuries in elderly patients and high-energy injuries in younger patients.1,2 About half of these injuries are unstable.3 According to the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) system, trochanteric fractures can be classified stable (AO/OTA 31.A1-1 to 31.A2-1) or unstable (AO/OTA 31.A2-2 to 31.A3.3).4,5 For surgical fixation of trochanteric femur fractures, various internal fixation devices have been used, either extramedullary (EM) or intramedullary (IM).6 The dynamic hip screw (DHS) is the implant of choice in the treatment of stable trochanteric femur fractures (AO/OTA 31-A1), as it provides secure fixation and controlled impaction.7 Mechanical and technical failures continue to occur in up to 6% to 18% of cases of unstable trochanteric fractures treated with DHS.8 Excessive sliding of the lag screw within the plate barrel results in limb shortening and distal fragment medialization, which are the main causes of these failures.9,10 Dissatisfaction with DHS use in unstable fractures led to the use of IM nails. The various IM devices available are condylocephalic (Ender) nails and cephalomedullary nails, such as gamma nails; IM hip screws; trochanteric antegrade nails; proximal femoral nails (PFNs); and trochanteric fixation nails.11,12 Unstable trochanteric fractures treated with these IM fixation devices have had good results.12-14 Because of their central location and shorter lever arm, IM nails decrease the tensile strain on the implant and thereby reduce the risk of implant failure and provide more efficient load transfer while maintaining the advantage of controlled fracture impaction, as in DHS.15,16 According to some authors, IM nail insertion theoretically requires less operative time and less soft-tissue dissection, potentially resulting in decreased overall morbidity.15,16 PFN is one of the most effective fixation methods used to treat unstable trochanteric femur fractures.17 However, it is associated with various technical problems and failures, such as anterior femoral cortex penetration (caused by mismatch of nail curvature and intact femur), lag screw prominence in the lateral thigh, creation of a large hole in the greater trochanter (leading to abductors weakness), and potential for the Z-effect.18,19 Studies have compared PFN with the Less Invasive Stabilization System-Distal Femur (LISS-DF) in the treatment of proximal femur fracture, and the clinical results are encouraging.20,21 Recently, the proximal femoral locking compression plate (PFLCP) was introduced as a new implant that allows for angular-stable plating in the treatment of complex comminuted and osteoporotic intertrochanteric fractures.22,23
To our knowledge, our study is the first to compare functional outcomes and complications of unstable trochanteric fractures treated with PFN and those treated with PFLCP. We hypothesized that both PFN and PFLCP would provide good functional outcomes with acceptable and comparable complications in the treatment of unstable trochanteric fractures.
Materials and Methods
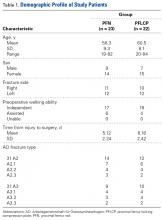
The protocol for this prospective comparative study was approved by the Institutional Review Board at Mayo Institute of Medical Sciences. Informed consent was provided by all patients. A power analysis with power of 90% to detect a Harris Hip Score (HHS) difference of 10 as being significant at the 5% level, and with a 10% to 15% dropout rate, determined that a sample size of 50 patients was needed. Each group (PFN, PFLCP) required at least 25 participants. From April 2009 to June 2011, 74 patients with unilateral closed unstable trochanteric fractures were admitted to our hospital. Of these patients, 48 met our inclusion criteria and were included in the study. A sealed envelope method was used to randomly assign 24 of these patients to PFN treatment and the other 24 to PFLCP treatment. One patient died of causes unrelated to an implant during the study, and 2 were lost to follow-up (telephone numbers changed). The remaining 45 patients (23 PFN, 22 PFLCP) reached 2-year follow-up.
Inclusion criteria were unilateral, closed unstable trochanteric fractures, and age over 18 years. Exclusion criteria were bilateral fractures, polytrauma, pathologic fractures, open fractures (American Society of Anesthesiologists [ASA] grade 4 or 5),24 and associated hip osteoarthritis (Kellgren-Lawrence grade 3 or 4).25 We collected data on demographics, operative time, incision length, intraoperative blood loss (measured by gravimetric method), hospital length of stay (LOS), and time to full weight-bearing. Mean (SD) age was 58.3 (9.3) years for the PFN group (range, 19-82 years) and 60.5 (8.1) years for the PFLCP group (range, 20-84 years).
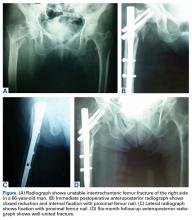
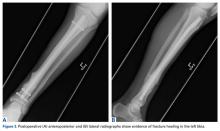
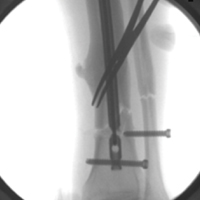
Before surgery, each patient’s standard plain radiographs (1 anteroposterior [AP], 1 lateral) were evaluated. Patients underwent surgery as soon as their general medical condition allowed. Surgery was performed through a lateral approach with the patient supine and in traction on a fracture table. PFN patients received 2 femoral neck screws (DePuy Synthes) (Figures A-D), and PFLCP patients received PFLCP (DePuy Synthes) in a fashion similar to that described in AO internal fixation manuals.
Absolute values of differences were used for statistical analysis. For categorical outcome variables (eg, reoperation reason and type), Pearson χ2 test was used; for continuous variables (eg, pain, HHS), Student t test was used. Statistical significance was set at P = .05 (2-sided).
Results
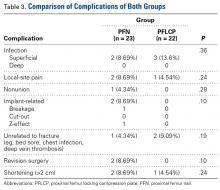
Intraoperative blood loss (P = .02) and incision length (P = .008) were significantly less in the PFN group than in the PFLCP group. No significant difference was found between the groups in terms of operative time (P = .08), reduction quality (P = .82), radiologic exposure time (P = .18), LOS (P = .32), union rate (P = .42), and time to union (P = .68).
Two PFN patients and 3 PFLCP patients developed a superficial infection (P = .36); all 5 infections were controlled with oral antibiotics. There was 1 nonunion in the PFN group but none in the PFLCP group (P = .28). The nonunion patient, who also had a broken implant without any history of fresh trauma, was treated with implant removal and bipolar hemiarthroplasty.
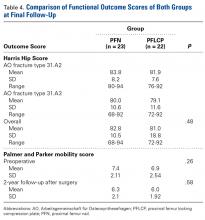
There was no significant difference between the groups in terms of functional outcome (HHS) at final follow-up (P = .48).
Discussion
The goal in managing proximal femoral fractures is to achieve near anatomical reduction with stable fracture fixation. Over the years, EM and IM devices have been used to treat trochanteric fractures; each has its merits and demerits.29,30 However, unstable trochanteric fractures treated with EM devices (eg, DHS, dynamic condylar screw) have high complication rates (6%-18%).8,31 Excessive sliding of the lag screw within the plate barrel may result in limb shortening and distal fragment medialization. EM devices cannot adequately prevent secondary limb shortening after weight-bearing, owing to medialization of the distal fragment.32,33 Varus collapse and implant failure (eg, cut-out of the femoral head screw) are also common.29 These complications led to the development of IM hip screw devices, such as PFN, which has several potential advantages, including a shorter lever arm (decreases tensile strain on implant) and efficient load transfer capacity. PFN has been found to have increased fracture stability, with no difference in operative time or intraoperative complication rates, but some studies have reported implant failure and other complications (3%-17%) in PFN-treated unstable trochanteric fractures.29,34,35
We conducted the present study to compare PFN and PFLCP, new treatment options for unstable and highly comminuted trochanteric fractures. The characteristics of the patients in this study are very different from those in most hip fracture studies. Our PFN and PFLCP groups’ mean ages were lower relative to other studies.14,15,36 In addition, time from injury to surgery was longer for both our groups than for groups in other studies, though some studies36 have reported comparable times. Moreover, our groups showed no statistically significant differences in operative time, radiologic exposure time, LOS, union rate, or time to union. Our PFN patients had significantly shorter incisions and less time to full weight-bearing.
Wang and colleagues37 compared the clinical outcomes of DHS, IM fixation (IMF), and PFLCP in the treatment of trochanteric fractures in elderly patients. Incision length and operative time were shorter for the IMF group than for DHS and PFLCP, but there were no significant differences between DHS and PFLCP. Intraoperative blood loss, rehabilitation, and time to healing were less for the IMF and PFLCP groups than for DHS, but there were no significant differences between IMF and PFLCP. Functional recovery was better for the IMF and PFLCP groups than for DHS, and there were significant differences among the 3 groups. There were fewer complications in the PFLCP group than in IMF and DHS.
Yao and colleagues38 compared reverse LISS and PFN treatment of intertrochanteric fractures and reported no significant differences in operative time, intraoperative blood loss, or functional outcome. Regarding complications, the PFN group had none, and the LISS group had 3 (1 nonunion with locking screw breakage, 2 varus unions).
Haq and colleagues39 compared PFN and contralateral reverse distal femoral locking compression plate (reverse DFLCP) in the management of unstable intertrochanteric fractures with compromised lateral wall and reported better intraoperative variables, better functional outcomes, and lower failure rates in the PFN group than in the reverse DFLCP group.
Zha and colleagues22 followed up 110 patients with intertrochanteric and subtrochanteric fractures treated with PFLCP fixation and reported a 100% union rate at 1-year follow-up. Mean operative time was 35.5minutes, and mean bleeding amount was 150mL, which included operative blood loss and wound drainage. Mean radiologic exposure time was 5minutes, and mean incision length was 9cm. There was 1 case of implant breakage.
Strohm and colleagues40 reported good results in children with trochanteric fractures treated with conventional locking compression plate.
Brett and colleagues41 compared blade plate and PFLCP with and without a kickstand screw in a composite femur subtrochanteric fracture gap model. In their biomechanical study, the PFLCP with a kickstand screw provided higher axial but less torsional stiffness than the blade plate. The authors concluded that, though the devices are biomechanically equivalent, PFLCP may allow percutaneous insertion that avoids the potential morbidity associated with the blade plate’s extensile approach.
Our PFN group’s mean (SD) time to healing was 4.2 (1.3) months. In other studies, mean healing time for IMF-treated unstable trochanteric fractures was 3 to 4 months. Some authors have reported even longer healing times, up to 17 months,42 for PFN-treated trochanteric fractures. Many of the studies indicated that gradual weight-bearing was allowed around 6 weeks, when callus formation was adequate.43 Our treatment protocol differed in that its protected weight-bearing period was prolonged, and controlled weight-bearing was delayed until around 6 weeks, when callus formation was adequate.
The better PFLCP outcomes in our study, relative to most other studies, can be attributed to the relatively younger age of our PFN and PFLCP groups. In a study of 19 patients with trochanteric fractures treated with open reduction and internal fixation using PFLCP, Wirtz and colleagues44 reported 4 cases of secondary varus collapse, 2 cut-outs of the proximal fragment, and 1 implant failure caused by a broken proximal screw. Eight patients experienced persistent trochanteric pain, and 3 underwent hardware removal.
Streubel and colleagues45 retrospectively analyzed 29 patients with 30 OTA 31.A3 fractures treated with PFLCP and reported 11 failures (37%) at 20-month follow-up. The most frequent failure mode (5 cases) was varus collapse with screw cut-out. Presence of a kickstand screw and medial cortical reduction were not significantly different between cases that failed and those that did not.
Glassner and Tejwani46 retrospectively studied 10 patients with trochanteric fractures treated with open reduction and internal fixation with PFLCP. Failure modes were implant fracture (4 cases) and fixation loss (3 cases) resulting from varus collapse and implant cutout.
One of our PFN patients had a lower neck screw back out by 9-month follow-up. As the fracture had consolidated well, the patient underwent screw removal. Another PFN patient had a broken implant and fracture nonunion at 1-year follow-up. Various complications have been reported in the literature,13,14,47,48 but none occurred in our study. There were no implant-related complications in our PFLCP group, possibly because of the mechanical advantage of 3-dimensional and angular-stable fixation with PFLCP in unstable trochanteric fractures.
Gadegone and Salphale49 analyzed 100 cases of PFN-treated trochanteric fractures and reported femoral head cut-through (4.8%), intraoperative femoral shaft fracture (0.8%), implant breakage (0.8%), wound-healing impairment (9.7%), and false placement of osteosynthesis materials (0.8%). The 19% reoperation rate in their study mainly involved cephalic screw removal for lateral protrusion at the proximal thigh. Our PFN reoperation rate was 8.7%; none of our PFLCP patients required revision surgery.
Tyllianakis and colleagues50 analyzed 45 cases of PFN-treated unstable trochanteric fractures and concluded technical or mechanical complications were related more to fracture type, surgical technique, and time to weight-bearing than to the implant itself. Our postoperative wound complication rate was similar to that of other studies.14,47,51 Regarding functional outcomes, our groups’ HHSs were good and comparable at final follow-up, as were their PPM scores.
This study was limited in that it was a small prospective comparative single-center study with a small number of patients. Larger randomized controlled multicenter studies are needed to evaluate and compare both implants in displaced unstable trochanteric femur fractures.
This study found that both PFN and PFLCP were effective treatments for unstable trochanteric femur fractures. PFN is superior to PFLCP only in terms of shorter incisions and shorter time to full weight-bearing. Both devices can be used in unstable trochanteric fractures, and both have good functional outcomes and acceptable complication rates.
Am J Orthop. 2017;46(2):E116-E123. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;(252):163-166.
2. Kyle RF, Cabanela ME, Russell TA, et al. Fractures of the proximal part of the femur. Instr Course Lect. 1995;44:227-253.
3. Koval KJ, Aharonoff GB, Rokito AS, Lyon T, Zuckerman JD. Patients with femoral neck and intertrochanteric fractures. Are they the same? Clin Orthop Relat Res. 1996;(330):166-172.
4. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
5. Lindskog D, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
6. Kokoroghiannis C, Aktselis I, Deligeorgis A, Fragkomichalos E, Papadimas D, Pappadas I. Evolving concepts of stability and intramedullary fixation of intertrochanteric fractures—a review. Injury. 2012;43(6):686-693.
7. Larsson S, Friberg S, Hansson LI. Trochanteric fractures. Influence of reduction and implant position on impaction and complications. Clin Orthop Relat Res. 1990;(259):130-139.
8. Simpson AH, Varty K, Dodd CA. Sliding hip screws: modes of failure. Injury. 1989;20(4):227-231.
9. Rha JD, Kim YH, Yoon SI, Park TS, Lee MH. Factors affecting sliding of the lag screw in intertrochanteric fractures. Int Orthop. 1993;17(5):320-324.
10. Baixauli F, Vicent V, Baixauli E, et al. A reinforced rigid fixation device for unstable intertrochanteric fractures. Clin Orthop Relat Res. 1999;(361):205-215.
11. Harrington P, Nihal A, Singhania AK, Howell FR. Intramedullary hip screw versus sliding hip screw for unstable intertrochanteric femoral fractures in the elderly. Injury. 2002;33(1):23-28.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Pajarinen J, Lindahl J, Michelsson O, Savolainen V, Hirvensalo E. Pertrochanteric femoral fractures treated with a dynamic hip screw or a proximal femoral nail. A randomised study comparing postoperative rehabilitation. J Bone Joint Surg Br. 2005;87(1):76-81.
14. Papasimos S, Koutsojannis CM, Panagopoulos A, Megas P, Lambiris E. A randomised comparison of AMBI, TGN and PFN for treatment of unstable trochanteric fractures. Arch Orthop Trauma Surg. 2005;125(7):462-468.
15. Saudan M, Lübbeke A, Sadowski C, Riand N, Stern R, Hoffmeyer P. Pertrochanteric fractures: is there an advantage to an intramedullary nail? A randomized, prospective study of 206 patients comparing the dynamic hip screw and proximal femoral nail. J Orthop Trauma. 2002;16(6):386-393.
16. Schipper IB, Steyerberg EW, Castelein RM, et al. Treatment of unstable trochanteric fractures. Randomised comparison of the gamma nail and the proximal femoral nail. J Bone Joint Surg Br. 2004;86(1):86-94.
17. Gardenbroek TJ, Segers MJ, Simmermacher RK, Hammacher ER. The proximal femur nail antirotation: an identifiable improvement in the treatment of unstable pertrochanteric fractures? J Trauma. 2011;71(1):169-174.
18. Egol KA, Chang EY, Cvitkovic J, Kummer FJ, Koval KJ. Mismatch of current intramedullary nails with the anterior bow of the femur. J Orthop Trauma. 2004;18(7):410-415.
19. Werner-Tutschku W, Lajtai G, Schmiedhuber G, Lang T, Pirkl C, Orthner E. Intra- and perioperative complications in the stabilization of per- and subtrochanteric femoral fractures by means of PFN [in German]. Unfallchirurg. 2002;105(10):881-885.
20. Ma CH, Tu YK, Yu SW, Yen CY, Yeh JH, Wu CH. Reverse LISS plates for unstable proximal femoral fractures. Injury. 2010;41(8):827-833.
21. Pryce Lewis JR, Ashcroft GP. Reverse LISS plating for proximal segmental femoral fractures in the polytrauma patient: a case report. Injury. 2007;38(2):235-239.
22. Zha GC, Chen ZL, Qi XB, Sun JY. Treatment of pertrochanteric fractures with a proximal femur locking compression plate. Injury. 2011;42(11):1294-1299.
23. Oh CW, Kim JJ, Byun YS, et al. Minimally invasive plate osteosynthesis of subtrochanteric femur fractures with a locking plate: a prospective series of 20 fractures. Arch Orthop Trauma Surg. 2009;129(12):1659-1665.
24. American Society of Anesthesiologists new classification of physical status. Anesthesiology. 1963;24:111-114.
25. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
26. Vidyadhara S, Rao SK. One and two femoral neck screws with intramedullary nails for unstable trochanteric fractures of femur in the elderly—randomised clinical trial. Injury. 2007;38(7):806-814.
27. Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br. 1993;75(5):797-798.
28. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
29. Sadowski C, Lübbeke A, Saudan M, Riand N, Stern R, Hoffmeyer P. Treatment of reverse oblique and transverse intertrochanteric fractures with use of an intramedullary nail or a 95 degrees screw-plate: a prospective, randomized study. J Bone Joint Surg Am. 2002;84(3):372-381.
30. Suckel AA, Dietz K, Wuelker N, Helwig P. Evaluation of complications of three different types of proximal extra-articular femur fractures: differences in complications, age, sex and surviving rates. Int Orthop. 2007;31(5):689-695.
31. Nuber S, Schönweiss T, Rüter A. Stabilisation of unstable trochanteric femoral fractures. Dynamic hip screw (DHS) with trochanteric stabilisation plate vs. proximal femur nail (PFN) [in German]. Unfallchirurg. 2003;106(1):39-47.
32. Klinger HM, Baums MH, Eckert M, Neugebauer R. A comparative study of unstable per- and intertrochanteric femoral fractures treated with dynamic hip screw (DHS) and trochanteric butt-press plate vs. proximal femoral nail (PFN) [in German]. Zentralbl Chir. 2005;130(4):301-306.
33. Bridle SH, Patel AD, Bircher M, Calvert PT. Fixation of intertrochanteric fractures of the femur. A randomised prospective comparison of the gamma nail and the dynamic hip screw. J Bone Joint Surg Br. 1991;73(2):330-334.
34. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric gamma nail and compression hip screw for trochanteric fractures: a randomized, prospective, comparative study in 210 elderly patients with a new design of the gamma nail. J Orthop Trauma. 2005;19(4):229-233.
35. Lenich A, Mayr E, Rüter A, Möckl CH, Füchtmeier B. First results with the trochanter fixation nail (TFN): a report on 120 cases. Arch Orthop Trauma Surg. 2006;126(10):706-712.
36. Tao R, Lu Y, Xu H, Zhou ZY, Wang YH, Liu F. Internal fixation of intertrochanteric hip fractures: a clinical comparison of two implant designs. ScientificWorldJournal. 2013;2013:834825.
37. Wang Y, Yang YY, Yu ZH, Li CQ, Wu YS, Zheng XX. Comparative study of intertrochanteric fractures treated with proximal femur locking compress plate in aged [in Chinese]. Zhongguo Gu Shang. 2011;24(5):370-373.
38. Yao C, Zhang CQ, Jin DX, Chen YF. Early results of reverse less invasive stabilization system plating in treating elderly intertrochanteric fractures: a prospective study compared to proximal femoral nail. Chin Med J (Engl). 2011;124(14):2150-2157.
39. Haq RU, Manhas V, Pankaj A, Srivastava A, Dhammi IK, Jain AK. Proximal femoral nails compared with reverse distal femoral locking plates in intertrochanteric fractures with a compromised lateral wall; a randomised controlled trial. Int Orthop. 2014;38(7):1443-1449.
40. Strohm PC, Schmal H, Kuminack K, Reising K, Südkamp NP. Intertrochanteric femoral fractures in children [in German]. Unfallchirurg. 2006;109(5):425-430.
41. Brett CD, Lee MA, Khalafi AK, Hazelwood SJ. A comparison of percutaneous versus traditional open plate fixation in a subtrochanteric fracture gap model. In: Proceedings of the Annual Meeting of the Orthopaedic Trauma Association (OTA); October 5-7, 2006; Phoenix, AZ. Basic science poster 71 (abstract).
42. Park SY, Yang KH, Yoo JH, Yoon HK, Park HW. The treatment of reverse obliquity intertrochanteric fractures with the intramedullary hip nail. J Trauma. 2008;65(4):852-857.
43. Habernek H, Wallner T, Aschauer E, Schmid L. Comparison of Ender nails, dynamic hip screws, and gamma nails in the treatment of peritrochanteric femoral fractures. Orthopedics. 2000;23(2):121-127.
44. Wirtz C, Abbassi F, Evangelopoulos DS, Kohl S, Siebenrock KA, Krüger A. High failure rate of trochanteric fracture osteosynthesis with proximal femoral locking compression plate. Injury. 2013;44(6):751-756.
45. Streubel PN, Moustoukas MJ, Obremskey WT. Mechanical failure after locking plate fixation of unstable intertrochanteric femur fractures. J Orthop Trauma. 2013;27(1):22-28.
46. Glassner PJ, Tejwani NC. Failure of proximal femoral locking compression plate: a case series. J Orthop Trauma. 2011;25(2):76-83.
47. Ekström W, Karlsson-Thur C, Larsson S, Ragnarsson B, Alberts KA. Functional outcome in treatment of unstable trochanteric and subtrochanteric fractures with the proximal femoral nail and the Medoff sliding plate. J Orthop Trauma. 2007;21(1):18-25.
48. Boldin C, Seibert FJ, Fankhauser F, Peicha G, Grechenig W, Szyszkowitz R. The proximal femoral nail (PFN)—a minimal invasive treatment of unstable proximal femoral fractures: a prospective study of 55 patients with a follow-up of 15 months. Acta Orthop Scand. 2003;74(1):53-58.
49. Gadegone WM, Salphale YS. Proximal femoral nail—an analysis of 100 cases of proximal femoral fractures with an average follow up of 1 year. Int Orthop. 2007;31(3):403-408.
50. Tyllianakis M, Panagopoulos A, Papadopoulos A, Papasimos S, Mousafiris K. Treatment of extracapsular hip fractures with the proximal femoral nail (PFN): long term results in 45 patients. Acta Orthop Belg. 2004;70(5):444-454.
51. Morihara T, Arai Y, Tokugawa S, Fujita S, Chatani K, Kubo T. Proximal femoral nail for treatment of trochanteric femoral fractures. J Orthop Surg (Hong Kong). 2007;15(3):273-277.
Take-Home Points
- Both PFN and PFLCP are effective treatments for unstable trochanteric femur fractures.
- PFN is superior to PFLCP only in terms of shorter incisions and shorter time to full weight-bearing.
- Both devices have good long-term functional outcomes.
- Complication rates in unstable trochanteric fractures treated with both implants are comparable.
- Larger randomized controlled multicenter studies are needed to further evaluate and compare both implants in displaced unstable trochanteric femur fractures.
Trochanteric fractures are among the most widely treated orthopedic injuries, occurring mainly as low-energy injuries in elderly patients and high-energy injuries in younger patients.1,2 About half of these injuries are unstable.3 According to the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) system, trochanteric fractures can be classified stable (AO/OTA 31.A1-1 to 31.A2-1) or unstable (AO/OTA 31.A2-2 to 31.A3.3).4,5 For surgical fixation of trochanteric femur fractures, various internal fixation devices have been used, either extramedullary (EM) or intramedullary (IM).6 The dynamic hip screw (DHS) is the implant of choice in the treatment of stable trochanteric femur fractures (AO/OTA 31-A1), as it provides secure fixation and controlled impaction.7 Mechanical and technical failures continue to occur in up to 6% to 18% of cases of unstable trochanteric fractures treated with DHS.8 Excessive sliding of the lag screw within the plate barrel results in limb shortening and distal fragment medialization, which are the main causes of these failures.9,10 Dissatisfaction with DHS use in unstable fractures led to the use of IM nails. The various IM devices available are condylocephalic (Ender) nails and cephalomedullary nails, such as gamma nails; IM hip screws; trochanteric antegrade nails; proximal femoral nails (PFNs); and trochanteric fixation nails.11,12 Unstable trochanteric fractures treated with these IM fixation devices have had good results.12-14 Because of their central location and shorter lever arm, IM nails decrease the tensile strain on the implant and thereby reduce the risk of implant failure and provide more efficient load transfer while maintaining the advantage of controlled fracture impaction, as in DHS.15,16 According to some authors, IM nail insertion theoretically requires less operative time and less soft-tissue dissection, potentially resulting in decreased overall morbidity.15,16 PFN is one of the most effective fixation methods used to treat unstable trochanteric femur fractures.17 However, it is associated with various technical problems and failures, such as anterior femoral cortex penetration (caused by mismatch of nail curvature and intact femur), lag screw prominence in the lateral thigh, creation of a large hole in the greater trochanter (leading to abductors weakness), and potential for the Z-effect.18,19 Studies have compared PFN with the Less Invasive Stabilization System-Distal Femur (LISS-DF) in the treatment of proximal femur fracture, and the clinical results are encouraging.20,21 Recently, the proximal femoral locking compression plate (PFLCP) was introduced as a new implant that allows for angular-stable plating in the treatment of complex comminuted and osteoporotic intertrochanteric fractures.22,23
To our knowledge, our study is the first to compare functional outcomes and complications of unstable trochanteric fractures treated with PFN and those treated with PFLCP. We hypothesized that both PFN and PFLCP would provide good functional outcomes with acceptable and comparable complications in the treatment of unstable trochanteric fractures.
Materials and Methods
The protocol for this prospective comparative study was approved by the Institutional Review Board at Mayo Institute of Medical Sciences. Informed consent was provided by all patients. A power analysis with power of 90% to detect a Harris Hip Score (HHS) difference of 10 as being significant at the 5% level, and with a 10% to 15% dropout rate, determined that a sample size of 50 patients was needed. Each group (PFN, PFLCP) required at least 25 participants. From April 2009 to June 2011, 74 patients with unilateral closed unstable trochanteric fractures were admitted to our hospital. Of these patients, 48 met our inclusion criteria and were included in the study. A sealed envelope method was used to randomly assign 24 of these patients to PFN treatment and the other 24 to PFLCP treatment. One patient died of causes unrelated to an implant during the study, and 2 were lost to follow-up (telephone numbers changed). The remaining 45 patients (23 PFN, 22 PFLCP) reached 2-year follow-up.
Inclusion criteria were unilateral, closed unstable trochanteric fractures, and age over 18 years. Exclusion criteria were bilateral fractures, polytrauma, pathologic fractures, open fractures (American Society of Anesthesiologists [ASA] grade 4 or 5),24 and associated hip osteoarthritis (Kellgren-Lawrence grade 3 or 4).25 We collected data on demographics, operative time, incision length, intraoperative blood loss (measured by gravimetric method), hospital length of stay (LOS), and time to full weight-bearing. Mean (SD) age was 58.3 (9.3) years for the PFN group (range, 19-82 years) and 60.5 (8.1) years for the PFLCP group (range, 20-84 years).
Before surgery, each patient’s standard plain radiographs (1 anteroposterior [AP], 1 lateral) were evaluated. Patients underwent surgery as soon as their general medical condition allowed. Surgery was performed through a lateral approach with the patient supine and in traction on a fracture table. PFN patients received 2 femoral neck screws (DePuy Synthes) (Figures A-D), and PFLCP patients received PFLCP (DePuy Synthes) in a fashion similar to that described in AO internal fixation manuals.
Absolute values of differences were used for statistical analysis. For categorical outcome variables (eg, reoperation reason and type), Pearson χ2 test was used; for continuous variables (eg, pain, HHS), Student t test was used. Statistical significance was set at P = .05 (2-sided).
Results
Intraoperative blood loss (P = .02) and incision length (P = .008) were significantly less in the PFN group than in the PFLCP group. No significant difference was found between the groups in terms of operative time (P = .08), reduction quality (P = .82), radiologic exposure time (P = .18), LOS (P = .32), union rate (P = .42), and time to union (P = .68).
Two PFN patients and 3 PFLCP patients developed a superficial infection (P = .36); all 5 infections were controlled with oral antibiotics. There was 1 nonunion in the PFN group but none in the PFLCP group (P = .28). The nonunion patient, who also had a broken implant without any history of fresh trauma, was treated with implant removal and bipolar hemiarthroplasty.
There was no significant difference between the groups in terms of functional outcome (HHS) at final follow-up (P = .48).
Discussion
The goal in managing proximal femoral fractures is to achieve near anatomical reduction with stable fracture fixation. Over the years, EM and IM devices have been used to treat trochanteric fractures; each has its merits and demerits.29,30 However, unstable trochanteric fractures treated with EM devices (eg, DHS, dynamic condylar screw) have high complication rates (6%-18%).8,31 Excessive sliding of the lag screw within the plate barrel may result in limb shortening and distal fragment medialization. EM devices cannot adequately prevent secondary limb shortening after weight-bearing, owing to medialization of the distal fragment.32,33 Varus collapse and implant failure (eg, cut-out of the femoral head screw) are also common.29 These complications led to the development of IM hip screw devices, such as PFN, which has several potential advantages, including a shorter lever arm (decreases tensile strain on implant) and efficient load transfer capacity. PFN has been found to have increased fracture stability, with no difference in operative time or intraoperative complication rates, but some studies have reported implant failure and other complications (3%-17%) in PFN-treated unstable trochanteric fractures.29,34,35
We conducted the present study to compare PFN and PFLCP, new treatment options for unstable and highly comminuted trochanteric fractures. The characteristics of the patients in this study are very different from those in most hip fracture studies. Our PFN and PFLCP groups’ mean ages were lower relative to other studies.14,15,36 In addition, time from injury to surgery was longer for both our groups than for groups in other studies, though some studies36 have reported comparable times. Moreover, our groups showed no statistically significant differences in operative time, radiologic exposure time, LOS, union rate, or time to union. Our PFN patients had significantly shorter incisions and less time to full weight-bearing.
Wang and colleagues37 compared the clinical outcomes of DHS, IM fixation (IMF), and PFLCP in the treatment of trochanteric fractures in elderly patients. Incision length and operative time were shorter for the IMF group than for DHS and PFLCP, but there were no significant differences between DHS and PFLCP. Intraoperative blood loss, rehabilitation, and time to healing were less for the IMF and PFLCP groups than for DHS, but there were no significant differences between IMF and PFLCP. Functional recovery was better for the IMF and PFLCP groups than for DHS, and there were significant differences among the 3 groups. There were fewer complications in the PFLCP group than in IMF and DHS.
Yao and colleagues38 compared reverse LISS and PFN treatment of intertrochanteric fractures and reported no significant differences in operative time, intraoperative blood loss, or functional outcome. Regarding complications, the PFN group had none, and the LISS group had 3 (1 nonunion with locking screw breakage, 2 varus unions).
Haq and colleagues39 compared PFN and contralateral reverse distal femoral locking compression plate (reverse DFLCP) in the management of unstable intertrochanteric fractures with compromised lateral wall and reported better intraoperative variables, better functional outcomes, and lower failure rates in the PFN group than in the reverse DFLCP group.
Zha and colleagues22 followed up 110 patients with intertrochanteric and subtrochanteric fractures treated with PFLCP fixation and reported a 100% union rate at 1-year follow-up. Mean operative time was 35.5minutes, and mean bleeding amount was 150mL, which included operative blood loss and wound drainage. Mean radiologic exposure time was 5minutes, and mean incision length was 9cm. There was 1 case of implant breakage.
Strohm and colleagues40 reported good results in children with trochanteric fractures treated with conventional locking compression plate.
Brett and colleagues41 compared blade plate and PFLCP with and without a kickstand screw in a composite femur subtrochanteric fracture gap model. In their biomechanical study, the PFLCP with a kickstand screw provided higher axial but less torsional stiffness than the blade plate. The authors concluded that, though the devices are biomechanically equivalent, PFLCP may allow percutaneous insertion that avoids the potential morbidity associated with the blade plate’s extensile approach.
Our PFN group’s mean (SD) time to healing was 4.2 (1.3) months. In other studies, mean healing time for IMF-treated unstable trochanteric fractures was 3 to 4 months. Some authors have reported even longer healing times, up to 17 months,42 for PFN-treated trochanteric fractures. Many of the studies indicated that gradual weight-bearing was allowed around 6 weeks, when callus formation was adequate.43 Our treatment protocol differed in that its protected weight-bearing period was prolonged, and controlled weight-bearing was delayed until around 6 weeks, when callus formation was adequate.
The better PFLCP outcomes in our study, relative to most other studies, can be attributed to the relatively younger age of our PFN and PFLCP groups. In a study of 19 patients with trochanteric fractures treated with open reduction and internal fixation using PFLCP, Wirtz and colleagues44 reported 4 cases of secondary varus collapse, 2 cut-outs of the proximal fragment, and 1 implant failure caused by a broken proximal screw. Eight patients experienced persistent trochanteric pain, and 3 underwent hardware removal.
Streubel and colleagues45 retrospectively analyzed 29 patients with 30 OTA 31.A3 fractures treated with PFLCP and reported 11 failures (37%) at 20-month follow-up. The most frequent failure mode (5 cases) was varus collapse with screw cut-out. Presence of a kickstand screw and medial cortical reduction were not significantly different between cases that failed and those that did not.
Glassner and Tejwani46 retrospectively studied 10 patients with trochanteric fractures treated with open reduction and internal fixation with PFLCP. Failure modes were implant fracture (4 cases) and fixation loss (3 cases) resulting from varus collapse and implant cutout.
One of our PFN patients had a lower neck screw back out by 9-month follow-up. As the fracture had consolidated well, the patient underwent screw removal. Another PFN patient had a broken implant and fracture nonunion at 1-year follow-up. Various complications have been reported in the literature,13,14,47,48 but none occurred in our study. There were no implant-related complications in our PFLCP group, possibly because of the mechanical advantage of 3-dimensional and angular-stable fixation with PFLCP in unstable trochanteric fractures.
Gadegone and Salphale49 analyzed 100 cases of PFN-treated trochanteric fractures and reported femoral head cut-through (4.8%), intraoperative femoral shaft fracture (0.8%), implant breakage (0.8%), wound-healing impairment (9.7%), and false placement of osteosynthesis materials (0.8%). The 19% reoperation rate in their study mainly involved cephalic screw removal for lateral protrusion at the proximal thigh. Our PFN reoperation rate was 8.7%; none of our PFLCP patients required revision surgery.
Tyllianakis and colleagues50 analyzed 45 cases of PFN-treated unstable trochanteric fractures and concluded technical or mechanical complications were related more to fracture type, surgical technique, and time to weight-bearing than to the implant itself. Our postoperative wound complication rate was similar to that of other studies.14,47,51 Regarding functional outcomes, our groups’ HHSs were good and comparable at final follow-up, as were their PPM scores.
This study was limited in that it was a small prospective comparative single-center study with a small number of patients. Larger randomized controlled multicenter studies are needed to evaluate and compare both implants in displaced unstable trochanteric femur fractures.
This study found that both PFN and PFLCP were effective treatments for unstable trochanteric femur fractures. PFN is superior to PFLCP only in terms of shorter incisions and shorter time to full weight-bearing. Both devices can be used in unstable trochanteric fractures, and both have good functional outcomes and acceptable complication rates.
Am J Orthop. 2017;46(2):E116-E123. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Both PFN and PFLCP are effective treatments for unstable trochanteric femur fractures.
- PFN is superior to PFLCP only in terms of shorter incisions and shorter time to full weight-bearing.
- Both devices have good long-term functional outcomes.
- Complication rates in unstable trochanteric fractures treated with both implants are comparable.
- Larger randomized controlled multicenter studies are needed to further evaluate and compare both implants in displaced unstable trochanteric femur fractures.
Trochanteric fractures are among the most widely treated orthopedic injuries, occurring mainly as low-energy injuries in elderly patients and high-energy injuries in younger patients.1,2 About half of these injuries are unstable.3 According to the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) system, trochanteric fractures can be classified stable (AO/OTA 31.A1-1 to 31.A2-1) or unstable (AO/OTA 31.A2-2 to 31.A3.3).4,5 For surgical fixation of trochanteric femur fractures, various internal fixation devices have been used, either extramedullary (EM) or intramedullary (IM).6 The dynamic hip screw (DHS) is the implant of choice in the treatment of stable trochanteric femur fractures (AO/OTA 31-A1), as it provides secure fixation and controlled impaction.7 Mechanical and technical failures continue to occur in up to 6% to 18% of cases of unstable trochanteric fractures treated with DHS.8 Excessive sliding of the lag screw within the plate barrel results in limb shortening and distal fragment medialization, which are the main causes of these failures.9,10 Dissatisfaction with DHS use in unstable fractures led to the use of IM nails. The various IM devices available are condylocephalic (Ender) nails and cephalomedullary nails, such as gamma nails; IM hip screws; trochanteric antegrade nails; proximal femoral nails (PFNs); and trochanteric fixation nails.11,12 Unstable trochanteric fractures treated with these IM fixation devices have had good results.12-14 Because of their central location and shorter lever arm, IM nails decrease the tensile strain on the implant and thereby reduce the risk of implant failure and provide more efficient load transfer while maintaining the advantage of controlled fracture impaction, as in DHS.15,16 According to some authors, IM nail insertion theoretically requires less operative time and less soft-tissue dissection, potentially resulting in decreased overall morbidity.15,16 PFN is one of the most effective fixation methods used to treat unstable trochanteric femur fractures.17 However, it is associated with various technical problems and failures, such as anterior femoral cortex penetration (caused by mismatch of nail curvature and intact femur), lag screw prominence in the lateral thigh, creation of a large hole in the greater trochanter (leading to abductors weakness), and potential for the Z-effect.18,19 Studies have compared PFN with the Less Invasive Stabilization System-Distal Femur (LISS-DF) in the treatment of proximal femur fracture, and the clinical results are encouraging.20,21 Recently, the proximal femoral locking compression plate (PFLCP) was introduced as a new implant that allows for angular-stable plating in the treatment of complex comminuted and osteoporotic intertrochanteric fractures.22,23
To our knowledge, our study is the first to compare functional outcomes and complications of unstable trochanteric fractures treated with PFN and those treated with PFLCP. We hypothesized that both PFN and PFLCP would provide good functional outcomes with acceptable and comparable complications in the treatment of unstable trochanteric fractures.
Materials and Methods
The protocol for this prospective comparative study was approved by the Institutional Review Board at Mayo Institute of Medical Sciences. Informed consent was provided by all patients. A power analysis with power of 90% to detect a Harris Hip Score (HHS) difference of 10 as being significant at the 5% level, and with a 10% to 15% dropout rate, determined that a sample size of 50 patients was needed. Each group (PFN, PFLCP) required at least 25 participants. From April 2009 to June 2011, 74 patients with unilateral closed unstable trochanteric fractures were admitted to our hospital. Of these patients, 48 met our inclusion criteria and were included in the study. A sealed envelope method was used to randomly assign 24 of these patients to PFN treatment and the other 24 to PFLCP treatment. One patient died of causes unrelated to an implant during the study, and 2 were lost to follow-up (telephone numbers changed). The remaining 45 patients (23 PFN, 22 PFLCP) reached 2-year follow-up.
Inclusion criteria were unilateral, closed unstable trochanteric fractures, and age over 18 years. Exclusion criteria were bilateral fractures, polytrauma, pathologic fractures, open fractures (American Society of Anesthesiologists [ASA] grade 4 or 5),24 and associated hip osteoarthritis (Kellgren-Lawrence grade 3 or 4).25 We collected data on demographics, operative time, incision length, intraoperative blood loss (measured by gravimetric method), hospital length of stay (LOS), and time to full weight-bearing. Mean (SD) age was 58.3 (9.3) years for the PFN group (range, 19-82 years) and 60.5 (8.1) years for the PFLCP group (range, 20-84 years).
Before surgery, each patient’s standard plain radiographs (1 anteroposterior [AP], 1 lateral) were evaluated. Patients underwent surgery as soon as their general medical condition allowed. Surgery was performed through a lateral approach with the patient supine and in traction on a fracture table. PFN patients received 2 femoral neck screws (DePuy Synthes) (Figures A-D), and PFLCP patients received PFLCP (DePuy Synthes) in a fashion similar to that described in AO internal fixation manuals.
Absolute values of differences were used for statistical analysis. For categorical outcome variables (eg, reoperation reason and type), Pearson χ2 test was used; for continuous variables (eg, pain, HHS), Student t test was used. Statistical significance was set at P = .05 (2-sided).
Results
Intraoperative blood loss (P = .02) and incision length (P = .008) were significantly less in the PFN group than in the PFLCP group. No significant difference was found between the groups in terms of operative time (P = .08), reduction quality (P = .82), radiologic exposure time (P = .18), LOS (P = .32), union rate (P = .42), and time to union (P = .68).
Two PFN patients and 3 PFLCP patients developed a superficial infection (P = .36); all 5 infections were controlled with oral antibiotics. There was 1 nonunion in the PFN group but none in the PFLCP group (P = .28). The nonunion patient, who also had a broken implant without any history of fresh trauma, was treated with implant removal and bipolar hemiarthroplasty.
There was no significant difference between the groups in terms of functional outcome (HHS) at final follow-up (P = .48).
Discussion
The goal in managing proximal femoral fractures is to achieve near anatomical reduction with stable fracture fixation. Over the years, EM and IM devices have been used to treat trochanteric fractures; each has its merits and demerits.29,30 However, unstable trochanteric fractures treated with EM devices (eg, DHS, dynamic condylar screw) have high complication rates (6%-18%).8,31 Excessive sliding of the lag screw within the plate barrel may result in limb shortening and distal fragment medialization. EM devices cannot adequately prevent secondary limb shortening after weight-bearing, owing to medialization of the distal fragment.32,33 Varus collapse and implant failure (eg, cut-out of the femoral head screw) are also common.29 These complications led to the development of IM hip screw devices, such as PFN, which has several potential advantages, including a shorter lever arm (decreases tensile strain on implant) and efficient load transfer capacity. PFN has been found to have increased fracture stability, with no difference in operative time or intraoperative complication rates, but some studies have reported implant failure and other complications (3%-17%) in PFN-treated unstable trochanteric fractures.29,34,35
We conducted the present study to compare PFN and PFLCP, new treatment options for unstable and highly comminuted trochanteric fractures. The characteristics of the patients in this study are very different from those in most hip fracture studies. Our PFN and PFLCP groups’ mean ages were lower relative to other studies.14,15,36 In addition, time from injury to surgery was longer for both our groups than for groups in other studies, though some studies36 have reported comparable times. Moreover, our groups showed no statistically significant differences in operative time, radiologic exposure time, LOS, union rate, or time to union. Our PFN patients had significantly shorter incisions and less time to full weight-bearing.
Wang and colleagues37 compared the clinical outcomes of DHS, IM fixation (IMF), and PFLCP in the treatment of trochanteric fractures in elderly patients. Incision length and operative time were shorter for the IMF group than for DHS and PFLCP, but there were no significant differences between DHS and PFLCP. Intraoperative blood loss, rehabilitation, and time to healing were less for the IMF and PFLCP groups than for DHS, but there were no significant differences between IMF and PFLCP. Functional recovery was better for the IMF and PFLCP groups than for DHS, and there were significant differences among the 3 groups. There were fewer complications in the PFLCP group than in IMF and DHS.
Yao and colleagues38 compared reverse LISS and PFN treatment of intertrochanteric fractures and reported no significant differences in operative time, intraoperative blood loss, or functional outcome. Regarding complications, the PFN group had none, and the LISS group had 3 (1 nonunion with locking screw breakage, 2 varus unions).
Haq and colleagues39 compared PFN and contralateral reverse distal femoral locking compression plate (reverse DFLCP) in the management of unstable intertrochanteric fractures with compromised lateral wall and reported better intraoperative variables, better functional outcomes, and lower failure rates in the PFN group than in the reverse DFLCP group.
Zha and colleagues22 followed up 110 patients with intertrochanteric and subtrochanteric fractures treated with PFLCP fixation and reported a 100% union rate at 1-year follow-up. Mean operative time was 35.5minutes, and mean bleeding amount was 150mL, which included operative blood loss and wound drainage. Mean radiologic exposure time was 5minutes, and mean incision length was 9cm. There was 1 case of implant breakage.
Strohm and colleagues40 reported good results in children with trochanteric fractures treated with conventional locking compression plate.
Brett and colleagues41 compared blade plate and PFLCP with and without a kickstand screw in a composite femur subtrochanteric fracture gap model. In their biomechanical study, the PFLCP with a kickstand screw provided higher axial but less torsional stiffness than the blade plate. The authors concluded that, though the devices are biomechanically equivalent, PFLCP may allow percutaneous insertion that avoids the potential morbidity associated with the blade plate’s extensile approach.
Our PFN group’s mean (SD) time to healing was 4.2 (1.3) months. In other studies, mean healing time for IMF-treated unstable trochanteric fractures was 3 to 4 months. Some authors have reported even longer healing times, up to 17 months,42 for PFN-treated trochanteric fractures. Many of the studies indicated that gradual weight-bearing was allowed around 6 weeks, when callus formation was adequate.43 Our treatment protocol differed in that its protected weight-bearing period was prolonged, and controlled weight-bearing was delayed until around 6 weeks, when callus formation was adequate.
The better PFLCP outcomes in our study, relative to most other studies, can be attributed to the relatively younger age of our PFN and PFLCP groups. In a study of 19 patients with trochanteric fractures treated with open reduction and internal fixation using PFLCP, Wirtz and colleagues44 reported 4 cases of secondary varus collapse, 2 cut-outs of the proximal fragment, and 1 implant failure caused by a broken proximal screw. Eight patients experienced persistent trochanteric pain, and 3 underwent hardware removal.
Streubel and colleagues45 retrospectively analyzed 29 patients with 30 OTA 31.A3 fractures treated with PFLCP and reported 11 failures (37%) at 20-month follow-up. The most frequent failure mode (5 cases) was varus collapse with screw cut-out. Presence of a kickstand screw and medial cortical reduction were not significantly different between cases that failed and those that did not.
Glassner and Tejwani46 retrospectively studied 10 patients with trochanteric fractures treated with open reduction and internal fixation with PFLCP. Failure modes were implant fracture (4 cases) and fixation loss (3 cases) resulting from varus collapse and implant cutout.
One of our PFN patients had a lower neck screw back out by 9-month follow-up. As the fracture had consolidated well, the patient underwent screw removal. Another PFN patient had a broken implant and fracture nonunion at 1-year follow-up. Various complications have been reported in the literature,13,14,47,48 but none occurred in our study. There were no implant-related complications in our PFLCP group, possibly because of the mechanical advantage of 3-dimensional and angular-stable fixation with PFLCP in unstable trochanteric fractures.
Gadegone and Salphale49 analyzed 100 cases of PFN-treated trochanteric fractures and reported femoral head cut-through (4.8%), intraoperative femoral shaft fracture (0.8%), implant breakage (0.8%), wound-healing impairment (9.7%), and false placement of osteosynthesis materials (0.8%). The 19% reoperation rate in their study mainly involved cephalic screw removal for lateral protrusion at the proximal thigh. Our PFN reoperation rate was 8.7%; none of our PFLCP patients required revision surgery.
Tyllianakis and colleagues50 analyzed 45 cases of PFN-treated unstable trochanteric fractures and concluded technical or mechanical complications were related more to fracture type, surgical technique, and time to weight-bearing than to the implant itself. Our postoperative wound complication rate was similar to that of other studies.14,47,51 Regarding functional outcomes, our groups’ HHSs were good and comparable at final follow-up, as were their PPM scores.
This study was limited in that it was a small prospective comparative single-center study with a small number of patients. Larger randomized controlled multicenter studies are needed to evaluate and compare both implants in displaced unstable trochanteric femur fractures.
This study found that both PFN and PFLCP were effective treatments for unstable trochanteric femur fractures. PFN is superior to PFLCP only in terms of shorter incisions and shorter time to full weight-bearing. Both devices can be used in unstable trochanteric fractures, and both have good functional outcomes and acceptable complication rates.
Am J Orthop. 2017;46(2):E116-E123. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;(252):163-166.
2. Kyle RF, Cabanela ME, Russell TA, et al. Fractures of the proximal part of the femur. Instr Course Lect. 1995;44:227-253.
3. Koval KJ, Aharonoff GB, Rokito AS, Lyon T, Zuckerman JD. Patients with femoral neck and intertrochanteric fractures. Are they the same? Clin Orthop Relat Res. 1996;(330):166-172.
4. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
5. Lindskog D, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
6. Kokoroghiannis C, Aktselis I, Deligeorgis A, Fragkomichalos E, Papadimas D, Pappadas I. Evolving concepts of stability and intramedullary fixation of intertrochanteric fractures—a review. Injury. 2012;43(6):686-693.
7. Larsson S, Friberg S, Hansson LI. Trochanteric fractures. Influence of reduction and implant position on impaction and complications. Clin Orthop Relat Res. 1990;(259):130-139.
8. Simpson AH, Varty K, Dodd CA. Sliding hip screws: modes of failure. Injury. 1989;20(4):227-231.
9. Rha JD, Kim YH, Yoon SI, Park TS, Lee MH. Factors affecting sliding of the lag screw in intertrochanteric fractures. Int Orthop. 1993;17(5):320-324.
10. Baixauli F, Vicent V, Baixauli E, et al. A reinforced rigid fixation device for unstable intertrochanteric fractures. Clin Orthop Relat Res. 1999;(361):205-215.
11. Harrington P, Nihal A, Singhania AK, Howell FR. Intramedullary hip screw versus sliding hip screw for unstable intertrochanteric femoral fractures in the elderly. Injury. 2002;33(1):23-28.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Pajarinen J, Lindahl J, Michelsson O, Savolainen V, Hirvensalo E. Pertrochanteric femoral fractures treated with a dynamic hip screw or a proximal femoral nail. A randomised study comparing postoperative rehabilitation. J Bone Joint Surg Br. 2005;87(1):76-81.
14. Papasimos S, Koutsojannis CM, Panagopoulos A, Megas P, Lambiris E. A randomised comparison of AMBI, TGN and PFN for treatment of unstable trochanteric fractures. Arch Orthop Trauma Surg. 2005;125(7):462-468.
15. Saudan M, Lübbeke A, Sadowski C, Riand N, Stern R, Hoffmeyer P. Pertrochanteric fractures: is there an advantage to an intramedullary nail? A randomized, prospective study of 206 patients comparing the dynamic hip screw and proximal femoral nail. J Orthop Trauma. 2002;16(6):386-393.
16. Schipper IB, Steyerberg EW, Castelein RM, et al. Treatment of unstable trochanteric fractures. Randomised comparison of the gamma nail and the proximal femoral nail. J Bone Joint Surg Br. 2004;86(1):86-94.
17. Gardenbroek TJ, Segers MJ, Simmermacher RK, Hammacher ER. The proximal femur nail antirotation: an identifiable improvement in the treatment of unstable pertrochanteric fractures? J Trauma. 2011;71(1):169-174.
18. Egol KA, Chang EY, Cvitkovic J, Kummer FJ, Koval KJ. Mismatch of current intramedullary nails with the anterior bow of the femur. J Orthop Trauma. 2004;18(7):410-415.
19. Werner-Tutschku W, Lajtai G, Schmiedhuber G, Lang T, Pirkl C, Orthner E. Intra- and perioperative complications in the stabilization of per- and subtrochanteric femoral fractures by means of PFN [in German]. Unfallchirurg. 2002;105(10):881-885.
20. Ma CH, Tu YK, Yu SW, Yen CY, Yeh JH, Wu CH. Reverse LISS plates for unstable proximal femoral fractures. Injury. 2010;41(8):827-833.
21. Pryce Lewis JR, Ashcroft GP. Reverse LISS plating for proximal segmental femoral fractures in the polytrauma patient: a case report. Injury. 2007;38(2):235-239.
22. Zha GC, Chen ZL, Qi XB, Sun JY. Treatment of pertrochanteric fractures with a proximal femur locking compression plate. Injury. 2011;42(11):1294-1299.
23. Oh CW, Kim JJ, Byun YS, et al. Minimally invasive plate osteosynthesis of subtrochanteric femur fractures with a locking plate: a prospective series of 20 fractures. Arch Orthop Trauma Surg. 2009;129(12):1659-1665.
24. American Society of Anesthesiologists new classification of physical status. Anesthesiology. 1963;24:111-114.
25. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
26. Vidyadhara S, Rao SK. One and two femoral neck screws with intramedullary nails for unstable trochanteric fractures of femur in the elderly—randomised clinical trial. Injury. 2007;38(7):806-814.
27. Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br. 1993;75(5):797-798.
28. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
29. Sadowski C, Lübbeke A, Saudan M, Riand N, Stern R, Hoffmeyer P. Treatment of reverse oblique and transverse intertrochanteric fractures with use of an intramedullary nail or a 95 degrees screw-plate: a prospective, randomized study. J Bone Joint Surg Am. 2002;84(3):372-381.
30. Suckel AA, Dietz K, Wuelker N, Helwig P. Evaluation of complications of three different types of proximal extra-articular femur fractures: differences in complications, age, sex and surviving rates. Int Orthop. 2007;31(5):689-695.
31. Nuber S, Schönweiss T, Rüter A. Stabilisation of unstable trochanteric femoral fractures. Dynamic hip screw (DHS) with trochanteric stabilisation plate vs. proximal femur nail (PFN) [in German]. Unfallchirurg. 2003;106(1):39-47.
32. Klinger HM, Baums MH, Eckert M, Neugebauer R. A comparative study of unstable per- and intertrochanteric femoral fractures treated with dynamic hip screw (DHS) and trochanteric butt-press plate vs. proximal femoral nail (PFN) [in German]. Zentralbl Chir. 2005;130(4):301-306.
33. Bridle SH, Patel AD, Bircher M, Calvert PT. Fixation of intertrochanteric fractures of the femur. A randomised prospective comparison of the gamma nail and the dynamic hip screw. J Bone Joint Surg Br. 1991;73(2):330-334.
34. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric gamma nail and compression hip screw for trochanteric fractures: a randomized, prospective, comparative study in 210 elderly patients with a new design of the gamma nail. J Orthop Trauma. 2005;19(4):229-233.
35. Lenich A, Mayr E, Rüter A, Möckl CH, Füchtmeier B. First results with the trochanter fixation nail (TFN): a report on 120 cases. Arch Orthop Trauma Surg. 2006;126(10):706-712.
36. Tao R, Lu Y, Xu H, Zhou ZY, Wang YH, Liu F. Internal fixation of intertrochanteric hip fractures: a clinical comparison of two implant designs. ScientificWorldJournal. 2013;2013:834825.
37. Wang Y, Yang YY, Yu ZH, Li CQ, Wu YS, Zheng XX. Comparative study of intertrochanteric fractures treated with proximal femur locking compress plate in aged [in Chinese]. Zhongguo Gu Shang. 2011;24(5):370-373.
38. Yao C, Zhang CQ, Jin DX, Chen YF. Early results of reverse less invasive stabilization system plating in treating elderly intertrochanteric fractures: a prospective study compared to proximal femoral nail. Chin Med J (Engl). 2011;124(14):2150-2157.
39. Haq RU, Manhas V, Pankaj A, Srivastava A, Dhammi IK, Jain AK. Proximal femoral nails compared with reverse distal femoral locking plates in intertrochanteric fractures with a compromised lateral wall; a randomised controlled trial. Int Orthop. 2014;38(7):1443-1449.
40. Strohm PC, Schmal H, Kuminack K, Reising K, Südkamp NP. Intertrochanteric femoral fractures in children [in German]. Unfallchirurg. 2006;109(5):425-430.
41. Brett CD, Lee MA, Khalafi AK, Hazelwood SJ. A comparison of percutaneous versus traditional open plate fixation in a subtrochanteric fracture gap model. In: Proceedings of the Annual Meeting of the Orthopaedic Trauma Association (OTA); October 5-7, 2006; Phoenix, AZ. Basic science poster 71 (abstract).
42. Park SY, Yang KH, Yoo JH, Yoon HK, Park HW. The treatment of reverse obliquity intertrochanteric fractures with the intramedullary hip nail. J Trauma. 2008;65(4):852-857.
43. Habernek H, Wallner T, Aschauer E, Schmid L. Comparison of Ender nails, dynamic hip screws, and gamma nails in the treatment of peritrochanteric femoral fractures. Orthopedics. 2000;23(2):121-127.
44. Wirtz C, Abbassi F, Evangelopoulos DS, Kohl S, Siebenrock KA, Krüger A. High failure rate of trochanteric fracture osteosynthesis with proximal femoral locking compression plate. Injury. 2013;44(6):751-756.
45. Streubel PN, Moustoukas MJ, Obremskey WT. Mechanical failure after locking plate fixation of unstable intertrochanteric femur fractures. J Orthop Trauma. 2013;27(1):22-28.
46. Glassner PJ, Tejwani NC. Failure of proximal femoral locking compression plate: a case series. J Orthop Trauma. 2011;25(2):76-83.
47. Ekström W, Karlsson-Thur C, Larsson S, Ragnarsson B, Alberts KA. Functional outcome in treatment of unstable trochanteric and subtrochanteric fractures with the proximal femoral nail and the Medoff sliding plate. J Orthop Trauma. 2007;21(1):18-25.
48. Boldin C, Seibert FJ, Fankhauser F, Peicha G, Grechenig W, Szyszkowitz R. The proximal femoral nail (PFN)—a minimal invasive treatment of unstable proximal femoral fractures: a prospective study of 55 patients with a follow-up of 15 months. Acta Orthop Scand. 2003;74(1):53-58.
49. Gadegone WM, Salphale YS. Proximal femoral nail—an analysis of 100 cases of proximal femoral fractures with an average follow up of 1 year. Int Orthop. 2007;31(3):403-408.
50. Tyllianakis M, Panagopoulos A, Papadopoulos A, Papasimos S, Mousafiris K. Treatment of extracapsular hip fractures with the proximal femoral nail (PFN): long term results in 45 patients. Acta Orthop Belg. 2004;70(5):444-454.
51. Morihara T, Arai Y, Tokugawa S, Fujita S, Chatani K, Kubo T. Proximal femoral nail for treatment of trochanteric femoral fractures. J Orthop Surg (Hong Kong). 2007;15(3):273-277.
1. Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;(252):163-166.
2. Kyle RF, Cabanela ME, Russell TA, et al. Fractures of the proximal part of the femur. Instr Course Lect. 1995;44:227-253.
3. Koval KJ, Aharonoff GB, Rokito AS, Lyon T, Zuckerman JD. Patients with femoral neck and intertrochanteric fractures. Are they the same? Clin Orthop Relat Res. 1996;(330):166-172.
4. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
5. Lindskog D, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
6. Kokoroghiannis C, Aktselis I, Deligeorgis A, Fragkomichalos E, Papadimas D, Pappadas I. Evolving concepts of stability and intramedullary fixation of intertrochanteric fractures—a review. Injury. 2012;43(6):686-693.
7. Larsson S, Friberg S, Hansson LI. Trochanteric fractures. Influence of reduction and implant position on impaction and complications. Clin Orthop Relat Res. 1990;(259):130-139.
8. Simpson AH, Varty K, Dodd CA. Sliding hip screws: modes of failure. Injury. 1989;20(4):227-231.
9. Rha JD, Kim YH, Yoon SI, Park TS, Lee MH. Factors affecting sliding of the lag screw in intertrochanteric fractures. Int Orthop. 1993;17(5):320-324.
10. Baixauli F, Vicent V, Baixauli E, et al. A reinforced rigid fixation device for unstable intertrochanteric fractures. Clin Orthop Relat Res. 1999;(361):205-215.
11. Harrington P, Nihal A, Singhania AK, Howell FR. Intramedullary hip screw versus sliding hip screw for unstable intertrochanteric femoral fractures in the elderly. Injury. 2002;33(1):23-28.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Pajarinen J, Lindahl J, Michelsson O, Savolainen V, Hirvensalo E. Pertrochanteric femoral fractures treated with a dynamic hip screw or a proximal femoral nail. A randomised study comparing postoperative rehabilitation. J Bone Joint Surg Br. 2005;87(1):76-81.
14. Papasimos S, Koutsojannis CM, Panagopoulos A, Megas P, Lambiris E. A randomised comparison of AMBI, TGN and PFN for treatment of unstable trochanteric fractures. Arch Orthop Trauma Surg. 2005;125(7):462-468.
15. Saudan M, Lübbeke A, Sadowski C, Riand N, Stern R, Hoffmeyer P. Pertrochanteric fractures: is there an advantage to an intramedullary nail? A randomized, prospective study of 206 patients comparing the dynamic hip screw and proximal femoral nail. J Orthop Trauma. 2002;16(6):386-393.
16. Schipper IB, Steyerberg EW, Castelein RM, et al. Treatment of unstable trochanteric fractures. Randomised comparison of the gamma nail and the proximal femoral nail. J Bone Joint Surg Br. 2004;86(1):86-94.
17. Gardenbroek TJ, Segers MJ, Simmermacher RK, Hammacher ER. The proximal femur nail antirotation: an identifiable improvement in the treatment of unstable pertrochanteric fractures? J Trauma. 2011;71(1):169-174.
18. Egol KA, Chang EY, Cvitkovic J, Kummer FJ, Koval KJ. Mismatch of current intramedullary nails with the anterior bow of the femur. J Orthop Trauma. 2004;18(7):410-415.
19. Werner-Tutschku W, Lajtai G, Schmiedhuber G, Lang T, Pirkl C, Orthner E. Intra- and perioperative complications in the stabilization of per- and subtrochanteric femoral fractures by means of PFN [in German]. Unfallchirurg. 2002;105(10):881-885.
20. Ma CH, Tu YK, Yu SW, Yen CY, Yeh JH, Wu CH. Reverse LISS plates for unstable proximal femoral fractures. Injury. 2010;41(8):827-833.
21. Pryce Lewis JR, Ashcroft GP. Reverse LISS plating for proximal segmental femoral fractures in the polytrauma patient: a case report. Injury. 2007;38(2):235-239.
22. Zha GC, Chen ZL, Qi XB, Sun JY. Treatment of pertrochanteric fractures with a proximal femur locking compression plate. Injury. 2011;42(11):1294-1299.
23. Oh CW, Kim JJ, Byun YS, et al. Minimally invasive plate osteosynthesis of subtrochanteric femur fractures with a locking plate: a prospective series of 20 fractures. Arch Orthop Trauma Surg. 2009;129(12):1659-1665.
24. American Society of Anesthesiologists new classification of physical status. Anesthesiology. 1963;24:111-114.
25. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
26. Vidyadhara S, Rao SK. One and two femoral neck screws with intramedullary nails for unstable trochanteric fractures of femur in the elderly—randomised clinical trial. Injury. 2007;38(7):806-814.
27. Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br. 1993;75(5):797-798.
28. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
29. Sadowski C, Lübbeke A, Saudan M, Riand N, Stern R, Hoffmeyer P. Treatment of reverse oblique and transverse intertrochanteric fractures with use of an intramedullary nail or a 95 degrees screw-plate: a prospective, randomized study. J Bone Joint Surg Am. 2002;84(3):372-381.
30. Suckel AA, Dietz K, Wuelker N, Helwig P. Evaluation of complications of three different types of proximal extra-articular femur fractures: differences in complications, age, sex and surviving rates. Int Orthop. 2007;31(5):689-695.
31. Nuber S, Schönweiss T, Rüter A. Stabilisation of unstable trochanteric femoral fractures. Dynamic hip screw (DHS) with trochanteric stabilisation plate vs. proximal femur nail (PFN) [in German]. Unfallchirurg. 2003;106(1):39-47.
32. Klinger HM, Baums MH, Eckert M, Neugebauer R. A comparative study of unstable per- and intertrochanteric femoral fractures treated with dynamic hip screw (DHS) and trochanteric butt-press plate vs. proximal femoral nail (PFN) [in German]. Zentralbl Chir. 2005;130(4):301-306.
33. Bridle SH, Patel AD, Bircher M, Calvert PT. Fixation of intertrochanteric fractures of the femur. A randomised prospective comparison of the gamma nail and the dynamic hip screw. J Bone Joint Surg Br. 1991;73(2):330-334.
34. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric gamma nail and compression hip screw for trochanteric fractures: a randomized, prospective, comparative study in 210 elderly patients with a new design of the gamma nail. J Orthop Trauma. 2005;19(4):229-233.
35. Lenich A, Mayr E, Rüter A, Möckl CH, Füchtmeier B. First results with the trochanter fixation nail (TFN): a report on 120 cases. Arch Orthop Trauma Surg. 2006;126(10):706-712.
36. Tao R, Lu Y, Xu H, Zhou ZY, Wang YH, Liu F. Internal fixation of intertrochanteric hip fractures: a clinical comparison of two implant designs. ScientificWorldJournal. 2013;2013:834825.
37. Wang Y, Yang YY, Yu ZH, Li CQ, Wu YS, Zheng XX. Comparative study of intertrochanteric fractures treated with proximal femur locking compress plate in aged [in Chinese]. Zhongguo Gu Shang. 2011;24(5):370-373.
38. Yao C, Zhang CQ, Jin DX, Chen YF. Early results of reverse less invasive stabilization system plating in treating elderly intertrochanteric fractures: a prospective study compared to proximal femoral nail. Chin Med J (Engl). 2011;124(14):2150-2157.
39. Haq RU, Manhas V, Pankaj A, Srivastava A, Dhammi IK, Jain AK. Proximal femoral nails compared with reverse distal femoral locking plates in intertrochanteric fractures with a compromised lateral wall; a randomised controlled trial. Int Orthop. 2014;38(7):1443-1449.
40. Strohm PC, Schmal H, Kuminack K, Reising K, Südkamp NP. Intertrochanteric femoral fractures in children [in German]. Unfallchirurg. 2006;109(5):425-430.
41. Brett CD, Lee MA, Khalafi AK, Hazelwood SJ. A comparison of percutaneous versus traditional open plate fixation in a subtrochanteric fracture gap model. In: Proceedings of the Annual Meeting of the Orthopaedic Trauma Association (OTA); October 5-7, 2006; Phoenix, AZ. Basic science poster 71 (abstract).
42. Park SY, Yang KH, Yoo JH, Yoon HK, Park HW. The treatment of reverse obliquity intertrochanteric fractures with the intramedullary hip nail. J Trauma. 2008;65(4):852-857.
43. Habernek H, Wallner T, Aschauer E, Schmid L. Comparison of Ender nails, dynamic hip screws, and gamma nails in the treatment of peritrochanteric femoral fractures. Orthopedics. 2000;23(2):121-127.
44. Wirtz C, Abbassi F, Evangelopoulos DS, Kohl S, Siebenrock KA, Krüger A. High failure rate of trochanteric fracture osteosynthesis with proximal femoral locking compression plate. Injury. 2013;44(6):751-756.
45. Streubel PN, Moustoukas MJ, Obremskey WT. Mechanical failure after locking plate fixation of unstable intertrochanteric femur fractures. J Orthop Trauma. 2013;27(1):22-28.
46. Glassner PJ, Tejwani NC. Failure of proximal femoral locking compression plate: a case series. J Orthop Trauma. 2011;25(2):76-83.
47. Ekström W, Karlsson-Thur C, Larsson S, Ragnarsson B, Alberts KA. Functional outcome in treatment of unstable trochanteric and subtrochanteric fractures with the proximal femoral nail and the Medoff sliding plate. J Orthop Trauma. 2007;21(1):18-25.
48. Boldin C, Seibert FJ, Fankhauser F, Peicha G, Grechenig W, Szyszkowitz R. The proximal femoral nail (PFN)—a minimal invasive treatment of unstable proximal femoral fractures: a prospective study of 55 patients with a follow-up of 15 months. Acta Orthop Scand. 2003;74(1):53-58.
49. Gadegone WM, Salphale YS. Proximal femoral nail—an analysis of 100 cases of proximal femoral fractures with an average follow up of 1 year. Int Orthop. 2007;31(3):403-408.
50. Tyllianakis M, Panagopoulos A, Papadopoulos A, Papasimos S, Mousafiris K. Treatment of extracapsular hip fractures with the proximal femoral nail (PFN): long term results in 45 patients. Acta Orthop Belg. 2004;70(5):444-454.
51. Morihara T, Arai Y, Tokugawa S, Fujita S, Chatani K, Kubo T. Proximal femoral nail for treatment of trochanteric femoral fractures. J Orthop Surg (Hong Kong). 2007;15(3):273-277.
Systemic Hypothermia as Treatment for an Acute Cervical Spinal Cord Injury in a Professional Football Player: 9-Year Follow-Up
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Superior Mesenteric Artery Syndrome as a Complication of Scoliosis Surgery
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Subscapularis Tenotomy Versus Lesser Tuberosity Osteotomy for Total Shoulder Arthroplasty: A Systematic Review
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Removal of the Distal Aspect of a Broken Tibial Nail
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.