User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Orthopedics in the Age of Accountable Care Organizations and Population Health: From Profit-Center to Cost-Center
The way we are paid as doctors is changing. In some cases, the delivery of orthopedic care could change from healthcare institutions’ most significant financial asset to one of their most detrimental liabilities. These changes provide a chance to improve both the quality and efficiency of the care we deliver, but we are unlikely to capitalize on this opportunity unless we understand this shifting paradigm. This change requires us to first appreciate the recent history of our reimbursement environment.
Traditionally, healthcare has been a relatively lucrative field, especially for those providing surgical care: doctors are paid “physician fees” by insurance companies (including Medicare), and institutions where procedures are performed are paid “facility fees.” Profits are measured as revenue (ie, reimbursement) minus costs of providing care, and while there has always been the potential to make more money by lowering costs, providers have historically had much more to gain by increasing their revenue. This fact has been exacerbated by the “fee-for-service” (FFS) payment model, which unintentionally encourages physicians to provide high volumes of care by “paying more for doing more.” For example, rather than being paid a fixed sum to care for a patient’s knee arthritis, each provider involved in the patient’s care is paid for each intervention. Clearly, this system encourages providers to maximize their interventions (ie, earning revenue) rather than search for ways to cut costs.
The Centers for Medicare and Medicaid Services (CMS) partially addressed this issue during the 1980s by introducing the Diagnosis Related Group (DRG).1,2 Under this classification scheme, hospitals would be paid a pre-specified amount for a particular type of admission, often based on a specific procedure. For example, there is a DRG with a set payment for total knee arthroplasty (TKA).3 When reimbursement for the condition is set at a fixed amount, facilities are motivated to decrease their expenses since this is the only way to maximize the financial return for a given patient. This change, theoretically, encourages providers to cut their costs for providing a TKA as much as possible, potentially even to the point of sacrificing quality of care. As usual, when CMS makes a sweeping change, private insurers followed suit, and as a result, both government and corporate insurance is now structured around DRGs.
However, this was not a complete departure from FFS payment. We were still not paid to manage a patient’s knee arthritis as cheaply as possible; we were paid for each steroid injection, preoperative clinic visit, TKA (with numerous coding modifiers for complexity or comorbidities) as well as post-discharge admissions to skilled nursing and acute rehabilitation facilities. However, it was a start: for example, hospitals were no longer incentivized to keep TKA patients in house with a growing bill for each administered drug or therapy session. Yet, it is noteworthy that hospitals and physicians were still paid separately. This is important because doctors have historically made almost all treatment decisions and thereby determined the cost of care, yet hospitals have borne most of those costs, such as expensive implants or unplanned admissions, without a commensurate increase in reimbursement. As long as physicians are guaranteed their “fee,” they have little motivation to reduce those costs. Unsurprisingly, and as we well know, the advent of DRGs did not successfully curb our growing healthcare budget.
Recently, TKA and total hip arthroplasty reimbursement changed more dramatically. After experimenting with several pilots, CMS rolled out the Comprehensive Care for Joint Replacement (CJR) bundled payment program in 2015.1,4 Participation in CJR is mandatory for most arthroplasty providers in approximately half of all “metropolitan” areas. In this scheme, hospital and physician pay is intertwined. Specifically, hospitals are held accountable for costs, so if the total Medicare bill for a patient’s TKA exceeds the “target price,” the hospital faces a penalty. Conversely, a charge below the target can earn a bonus payment.4 The hospital and surgeons must decide how they will share the bonus (or penalty), which creates an incentive to work together to lower costs.
Continue to: While bundled payments like CJR shift some...
While bundled payments like CJR shift some of the risk for high costs to the hospital and surgeon, a much more extreme example of this type of shift is capitation (ie, paying a healthcare institution a set amount per patient to care for whatever maladies arise). Insurers have experimented with various forms of capitation in the past, which led to the expansion of health management organizations (HMOs) during the 1990s. In theory, capitation should encourage providers to invest in disease prevention to minimize the need for costly interventions. However, more nefarious incentives developed, resulting in “cherry picking” healthy patients, which restricts access to care for sicker patients, and even withholds care from patients in need. The most infamous example was arguably “drive-through deliveries,” where newborns and their mothers were prematurely discharged following birth.5 As a result, the “HMO backlash” occurred, and capitation temporarily fell out of favor. The heart of the problem was a strong incentive to reduce the cost of care without a counterbalancing incentive to maintain quality. CJR and other modern programs attempt to avoid similar adverse incentives by requiring participants to meet certain quality criteria.6
Since the passage of the Affordable Care Act in 2010, capitation has reemerged under a new name: Accountable Care Organizations (ACOs). Numerous forms of ACO’s exist with differing payment schemes7, but the most comprehensive version, named Next Generation (Next Gen), allows providers to choose full capitation.8 While early ACOs focused on individual patients, Next Gen ACOs are also focused on “population health.” That is, they must demonstrate outcomes for individuals and the patient population as a whole, while simultaneously assuming all financial risk via capitation. Specifically, these ACO’s are paid an “all-inclusive population-based payment” for each patient based on how much that type of patient’s care is expected to cost for the year.9 The ACO then provides all necessary treatment and, if the ACO cannot provide a necessary intervention, it is responsible for funding that care at another institution. Appropriately, there has been an increased focus on quality to avoid unintentional incentives to withhold care. Specifically, CMS has introduced mandatory quality metrics in the domains of patient experience, care coordination, preventive care, and management of at-risk populations.10 At present, unfortunately, these metrics are not nearly comprehensive enough nor adequately validated to assess the quality of care,11 especially for subspecialized fields like orthopedics where functional outcome scores are needed.
To date, very limited attention in the media or academic literature has been dedicated to subspecialty surgical care in the setting of ACOs even though implications for specialized surgeons could be immense. While ACOs bring numerous reporting requirements, the most essential first step for orthopedists in transition to this new reimbursement scheme will be a change in mindset. As explained above, orthopedics and other forms of specialized surgical care have traditionally been extremely profitable for healthcare institutions through relatively high revenue. However, within a capitated ACO all revenue has been paid upfront for each patient, and every orthopedic surgery performed represents a substantial cost to the institution rather than a large profit. For example, it has been reported that the average contribution margin earned by a hospital for an episode of care to provide a TKA (which includes postoperative care such as clinic visits, unplanned readmissions, and reoperations for complications) based on Medicare reimbursement is $11,726.12 This figure consists of reimbursement (median, $24,149) less variable costs (median, $10,190). Additionally, the surgeon currently receives $1400 in physician fees.13 These earnings represent a significant financial benefit for both the facility and doctor in the current FFS environment. However, a capitated ACO caring for a TKA patient would already have received full payment for his care for the year. As a result, providing a TKA would not afford any further financial benefit and would, instead, mean a loss of $10,190 (the aforementioned variable cost for the episode of care) directly from the bottom line. The orthopedic department within that ACO, along with other departments, can be expected to share that loss. This implies that upon becoming an ACO, an institution’s orthopedics department will change from a major profit-center to a major cost-center.
Continue to: CMS must establish adequate quality assurance...
CMS must establish adequate quality assurance measures to ensure that ACOs do not withhold cost-effective care, like TKAs,14,15 from their patients. Hopefully, for both professional and ethical reasons, providers will be active partners in this process. Groups like the International Consortium for Health Outcome Measurement, which has convened international expert panels to agree on comprehensive outcome sets for total joint arthroplasty and the management of low back pain, among other non-orthopedic conditions, may be useful examples in this process.16-18
At the provider level, surgeons will be more likely to be salaried employees, contracting directly with the ACO rather than primarily working to earn physician fees from insurance providers. Surgeons will likely be judged (and rewarded financially) on their ability to direct nonoperative care, to find non-surgical solutions to problems that may currently be treated operatively, and to reduce costs for patients that require surgery. Additionally, with an increased focus on quality assurance, there will likely be more pressure from ACOs and CMS to demonstrate results of both operative and nonoperative care, likely in the forms of patient-reported metrics and objective measures of physical function. Surgeons will have a strong incentive to be leaders in the process of collecting such data.
It is also worth considering the position of orthopedic practices that are not part of an ACO. Some ACOs will not have the capacity to provide all (or possibly any) of the orthopedic care their patients require. When necessary, they will contract with outside orthopedic practices. Compared with CMS, ACOs are much smaller purchasers and can be expected to be more sensitive to price, likely negotiating intensely between local orthopedic providers. As a result, even orthopedists outside of ACOs may feel the cost pressure created by this new reimbursement model and may be driven to implement cost-reduction measures such as standardized implant choices and discharge pathways.
ACOs are in an active growth phase,19,20 and recent updates to ACO policies make it clear that CMS intends for this trend to continue.8 Since ACOs are still a nascent reimbursement model, orthopedists will still do better financially, in almost all markets, by continuing to expend their energy and resources pursuing revenue, rather than cutting costs or demonstrating outcomes. However, as ACOs and population health gain traction, those orthopedists who recognize this shift and plan accordingly will have a definite strategic advantage, whether their practice is within an ACO, interacting with external ACOs, or both.
1. Carter Clement R, Bhat SB, Clement ME, Krieg JC. Medicare reimbursement and orthopedic surgery: past, present, and future. Curr Rev Musculoskelet Med. 2017;10(2):224-232. doi:10.1007/s12178-017-9406-7.
2. Centers for Medicare & Medicaid Services. Acute Inpatient PPS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html. Published August 2, 2017. Accessed September 8, 2018.
3. Centers for Medicare & Medicaid Services. Draft ICD-10-CM/PCS MS-DRGv28 Definitions Manual. https://www.cms.gov/icd10manual/fullcode_cms/P0185.html. Accessed September 8, 2018.
4. Centers for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/cjr. Accessed September 8, 2018.
5. Volpp KG, Bundorf MK. Consumer protection and the HMO backlash: are HMOs to blame for drive-through deliveries? Inquiry. 1999;36(1):101-109.
6. Centers for Medicare & Medicaid Services. Quality Measures and Performance Standards. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Quality_Measures_Standards.html. Published March 2, 2015. Accessed November 3, 2015.
7. Centers for Medicare & Medicaid Services. Accountable Care Organizations (ACOs): General Information. https://innovation.cms.gov/initiatives/aco/. Accessed September 8, 2018.
8. Centers for Medicare & Medicaid Services. Next Generation ACO Model. https://innovation.cms.gov/initiatives/Next-Generation-ACO-Model/. Accessed September 8, 2018.
9. Centers for Medicare & Medicaid Services. Next Generation Accountable Care Organization (ACO) Model: Frequently Asked Questions. https://innovation.cms.gov/Files/x/nextgenacofaq.pdf. Accessed September 8, 2018.
10. Centers for Medicare & Medicaid Services. Quality Measure Benchmarks for the 2018 and 2019 Reporting Years. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/2018-and-2019-quality-benchmarks-guidance.pdf. Published December 2017. Accessed September 8, 2018.
11. Toussaint J, Krueger D, Shortell SM, Milstein A, Cutler DM. ACO model should encourage efficient care delivery. Healthc (Amst). 2015;3(3):150-152. doi:10.1016/j.hjdsi.2015.06.003.
12. Clement RC, Kheir MM, Derman PB, et al. What are the economic consequences of unplanned readmissions after TKA? Clin Orthop Relat Res. 2014;472(10):3134-3141. doi:10.1007/s11999-014-3795-3.
13. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Results. http://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=0&T=0&HT=0&CT=0&H1=27447&M=1. Accessed June 4, 2015.
14. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122. doi:10.1001/archinternmed.2009.136.
15. Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
16. International Consortium for Health Outcomes Measurement. ICHOM web site. https://www.ichom.org/. Accessed November 3, 2015.
17. Rolfson O, Wissig S, van Maasakkers L, et al. Defining an international standard set of outcome measures for patients with hip or knee osteoarthritis: consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care Res (Hoboken). 2016;68(11):1631-1639. doi:10.1002/acr.22868.
18. Clement RC, Welander A, Stowell C, et al. A proposed set of metrics for standardized outcome reporting in the management of low back pain. Acta Orthop. 2015;86(5):523-533. doi:10.3109/17453674.2015.1036696.
19. Shortell SM, Colla CH, Lewis VA, Fisher E, Kessell E, Ramsay P. Accountable care organizations: the national landscape. J Health Polit Policy Law. 2015;40(4):647-668. doi:10.1215/03616878-3149976.
20. Centers for Medicare & Medicaid Services. CMS Proposes “Pathways to Success,” an Overhaul of Medicare’s ACO Program https://www.cms.gov/newsroom/press-releases/cms-proposes-pathways-success-overhaul-medicares-aco-program. Published August 9, 2018. Accessed September 10, 2018.
The way we are paid as doctors is changing. In some cases, the delivery of orthopedic care could change from healthcare institutions’ most significant financial asset to one of their most detrimental liabilities. These changes provide a chance to improve both the quality and efficiency of the care we deliver, but we are unlikely to capitalize on this opportunity unless we understand this shifting paradigm. This change requires us to first appreciate the recent history of our reimbursement environment.
Traditionally, healthcare has been a relatively lucrative field, especially for those providing surgical care: doctors are paid “physician fees” by insurance companies (including Medicare), and institutions where procedures are performed are paid “facility fees.” Profits are measured as revenue (ie, reimbursement) minus costs of providing care, and while there has always been the potential to make more money by lowering costs, providers have historically had much more to gain by increasing their revenue. This fact has been exacerbated by the “fee-for-service” (FFS) payment model, which unintentionally encourages physicians to provide high volumes of care by “paying more for doing more.” For example, rather than being paid a fixed sum to care for a patient’s knee arthritis, each provider involved in the patient’s care is paid for each intervention. Clearly, this system encourages providers to maximize their interventions (ie, earning revenue) rather than search for ways to cut costs.
The Centers for Medicare and Medicaid Services (CMS) partially addressed this issue during the 1980s by introducing the Diagnosis Related Group (DRG).1,2 Under this classification scheme, hospitals would be paid a pre-specified amount for a particular type of admission, often based on a specific procedure. For example, there is a DRG with a set payment for total knee arthroplasty (TKA).3 When reimbursement for the condition is set at a fixed amount, facilities are motivated to decrease their expenses since this is the only way to maximize the financial return for a given patient. This change, theoretically, encourages providers to cut their costs for providing a TKA as much as possible, potentially even to the point of sacrificing quality of care. As usual, when CMS makes a sweeping change, private insurers followed suit, and as a result, both government and corporate insurance is now structured around DRGs.
However, this was not a complete departure from FFS payment. We were still not paid to manage a patient’s knee arthritis as cheaply as possible; we were paid for each steroid injection, preoperative clinic visit, TKA (with numerous coding modifiers for complexity or comorbidities) as well as post-discharge admissions to skilled nursing and acute rehabilitation facilities. However, it was a start: for example, hospitals were no longer incentivized to keep TKA patients in house with a growing bill for each administered drug or therapy session. Yet, it is noteworthy that hospitals and physicians were still paid separately. This is important because doctors have historically made almost all treatment decisions and thereby determined the cost of care, yet hospitals have borne most of those costs, such as expensive implants or unplanned admissions, without a commensurate increase in reimbursement. As long as physicians are guaranteed their “fee,” they have little motivation to reduce those costs. Unsurprisingly, and as we well know, the advent of DRGs did not successfully curb our growing healthcare budget.
Recently, TKA and total hip arthroplasty reimbursement changed more dramatically. After experimenting with several pilots, CMS rolled out the Comprehensive Care for Joint Replacement (CJR) bundled payment program in 2015.1,4 Participation in CJR is mandatory for most arthroplasty providers in approximately half of all “metropolitan” areas. In this scheme, hospital and physician pay is intertwined. Specifically, hospitals are held accountable for costs, so if the total Medicare bill for a patient’s TKA exceeds the “target price,” the hospital faces a penalty. Conversely, a charge below the target can earn a bonus payment.4 The hospital and surgeons must decide how they will share the bonus (or penalty), which creates an incentive to work together to lower costs.
Continue to: While bundled payments like CJR shift some...
While bundled payments like CJR shift some of the risk for high costs to the hospital and surgeon, a much more extreme example of this type of shift is capitation (ie, paying a healthcare institution a set amount per patient to care for whatever maladies arise). Insurers have experimented with various forms of capitation in the past, which led to the expansion of health management organizations (HMOs) during the 1990s. In theory, capitation should encourage providers to invest in disease prevention to minimize the need for costly interventions. However, more nefarious incentives developed, resulting in “cherry picking” healthy patients, which restricts access to care for sicker patients, and even withholds care from patients in need. The most infamous example was arguably “drive-through deliveries,” where newborns and their mothers were prematurely discharged following birth.5 As a result, the “HMO backlash” occurred, and capitation temporarily fell out of favor. The heart of the problem was a strong incentive to reduce the cost of care without a counterbalancing incentive to maintain quality. CJR and other modern programs attempt to avoid similar adverse incentives by requiring participants to meet certain quality criteria.6
Since the passage of the Affordable Care Act in 2010, capitation has reemerged under a new name: Accountable Care Organizations (ACOs). Numerous forms of ACO’s exist with differing payment schemes7, but the most comprehensive version, named Next Generation (Next Gen), allows providers to choose full capitation.8 While early ACOs focused on individual patients, Next Gen ACOs are also focused on “population health.” That is, they must demonstrate outcomes for individuals and the patient population as a whole, while simultaneously assuming all financial risk via capitation. Specifically, these ACO’s are paid an “all-inclusive population-based payment” for each patient based on how much that type of patient’s care is expected to cost for the year.9 The ACO then provides all necessary treatment and, if the ACO cannot provide a necessary intervention, it is responsible for funding that care at another institution. Appropriately, there has been an increased focus on quality to avoid unintentional incentives to withhold care. Specifically, CMS has introduced mandatory quality metrics in the domains of patient experience, care coordination, preventive care, and management of at-risk populations.10 At present, unfortunately, these metrics are not nearly comprehensive enough nor adequately validated to assess the quality of care,11 especially for subspecialized fields like orthopedics where functional outcome scores are needed.
To date, very limited attention in the media or academic literature has been dedicated to subspecialty surgical care in the setting of ACOs even though implications for specialized surgeons could be immense. While ACOs bring numerous reporting requirements, the most essential first step for orthopedists in transition to this new reimbursement scheme will be a change in mindset. As explained above, orthopedics and other forms of specialized surgical care have traditionally been extremely profitable for healthcare institutions through relatively high revenue. However, within a capitated ACO all revenue has been paid upfront for each patient, and every orthopedic surgery performed represents a substantial cost to the institution rather than a large profit. For example, it has been reported that the average contribution margin earned by a hospital for an episode of care to provide a TKA (which includes postoperative care such as clinic visits, unplanned readmissions, and reoperations for complications) based on Medicare reimbursement is $11,726.12 This figure consists of reimbursement (median, $24,149) less variable costs (median, $10,190). Additionally, the surgeon currently receives $1400 in physician fees.13 These earnings represent a significant financial benefit for both the facility and doctor in the current FFS environment. However, a capitated ACO caring for a TKA patient would already have received full payment for his care for the year. As a result, providing a TKA would not afford any further financial benefit and would, instead, mean a loss of $10,190 (the aforementioned variable cost for the episode of care) directly from the bottom line. The orthopedic department within that ACO, along with other departments, can be expected to share that loss. This implies that upon becoming an ACO, an institution’s orthopedics department will change from a major profit-center to a major cost-center.
Continue to: CMS must establish adequate quality assurance...
CMS must establish adequate quality assurance measures to ensure that ACOs do not withhold cost-effective care, like TKAs,14,15 from their patients. Hopefully, for both professional and ethical reasons, providers will be active partners in this process. Groups like the International Consortium for Health Outcome Measurement, which has convened international expert panels to agree on comprehensive outcome sets for total joint arthroplasty and the management of low back pain, among other non-orthopedic conditions, may be useful examples in this process.16-18
At the provider level, surgeons will be more likely to be salaried employees, contracting directly with the ACO rather than primarily working to earn physician fees from insurance providers. Surgeons will likely be judged (and rewarded financially) on their ability to direct nonoperative care, to find non-surgical solutions to problems that may currently be treated operatively, and to reduce costs for patients that require surgery. Additionally, with an increased focus on quality assurance, there will likely be more pressure from ACOs and CMS to demonstrate results of both operative and nonoperative care, likely in the forms of patient-reported metrics and objective measures of physical function. Surgeons will have a strong incentive to be leaders in the process of collecting such data.
It is also worth considering the position of orthopedic practices that are not part of an ACO. Some ACOs will not have the capacity to provide all (or possibly any) of the orthopedic care their patients require. When necessary, they will contract with outside orthopedic practices. Compared with CMS, ACOs are much smaller purchasers and can be expected to be more sensitive to price, likely negotiating intensely between local orthopedic providers. As a result, even orthopedists outside of ACOs may feel the cost pressure created by this new reimbursement model and may be driven to implement cost-reduction measures such as standardized implant choices and discharge pathways.
ACOs are in an active growth phase,19,20 and recent updates to ACO policies make it clear that CMS intends for this trend to continue.8 Since ACOs are still a nascent reimbursement model, orthopedists will still do better financially, in almost all markets, by continuing to expend their energy and resources pursuing revenue, rather than cutting costs or demonstrating outcomes. However, as ACOs and population health gain traction, those orthopedists who recognize this shift and plan accordingly will have a definite strategic advantage, whether their practice is within an ACO, interacting with external ACOs, or both.
The way we are paid as doctors is changing. In some cases, the delivery of orthopedic care could change from healthcare institutions’ most significant financial asset to one of their most detrimental liabilities. These changes provide a chance to improve both the quality and efficiency of the care we deliver, but we are unlikely to capitalize on this opportunity unless we understand this shifting paradigm. This change requires us to first appreciate the recent history of our reimbursement environment.
Traditionally, healthcare has been a relatively lucrative field, especially for those providing surgical care: doctors are paid “physician fees” by insurance companies (including Medicare), and institutions where procedures are performed are paid “facility fees.” Profits are measured as revenue (ie, reimbursement) minus costs of providing care, and while there has always been the potential to make more money by lowering costs, providers have historically had much more to gain by increasing their revenue. This fact has been exacerbated by the “fee-for-service” (FFS) payment model, which unintentionally encourages physicians to provide high volumes of care by “paying more for doing more.” For example, rather than being paid a fixed sum to care for a patient’s knee arthritis, each provider involved in the patient’s care is paid for each intervention. Clearly, this system encourages providers to maximize their interventions (ie, earning revenue) rather than search for ways to cut costs.
The Centers for Medicare and Medicaid Services (CMS) partially addressed this issue during the 1980s by introducing the Diagnosis Related Group (DRG).1,2 Under this classification scheme, hospitals would be paid a pre-specified amount for a particular type of admission, often based on a specific procedure. For example, there is a DRG with a set payment for total knee arthroplasty (TKA).3 When reimbursement for the condition is set at a fixed amount, facilities are motivated to decrease their expenses since this is the only way to maximize the financial return for a given patient. This change, theoretically, encourages providers to cut their costs for providing a TKA as much as possible, potentially even to the point of sacrificing quality of care. As usual, when CMS makes a sweeping change, private insurers followed suit, and as a result, both government and corporate insurance is now structured around DRGs.
However, this was not a complete departure from FFS payment. We were still not paid to manage a patient’s knee arthritis as cheaply as possible; we were paid for each steroid injection, preoperative clinic visit, TKA (with numerous coding modifiers for complexity or comorbidities) as well as post-discharge admissions to skilled nursing and acute rehabilitation facilities. However, it was a start: for example, hospitals were no longer incentivized to keep TKA patients in house with a growing bill for each administered drug or therapy session. Yet, it is noteworthy that hospitals and physicians were still paid separately. This is important because doctors have historically made almost all treatment decisions and thereby determined the cost of care, yet hospitals have borne most of those costs, such as expensive implants or unplanned admissions, without a commensurate increase in reimbursement. As long as physicians are guaranteed their “fee,” they have little motivation to reduce those costs. Unsurprisingly, and as we well know, the advent of DRGs did not successfully curb our growing healthcare budget.
Recently, TKA and total hip arthroplasty reimbursement changed more dramatically. After experimenting with several pilots, CMS rolled out the Comprehensive Care for Joint Replacement (CJR) bundled payment program in 2015.1,4 Participation in CJR is mandatory for most arthroplasty providers in approximately half of all “metropolitan” areas. In this scheme, hospital and physician pay is intertwined. Specifically, hospitals are held accountable for costs, so if the total Medicare bill for a patient’s TKA exceeds the “target price,” the hospital faces a penalty. Conversely, a charge below the target can earn a bonus payment.4 The hospital and surgeons must decide how they will share the bonus (or penalty), which creates an incentive to work together to lower costs.
Continue to: While bundled payments like CJR shift some...
While bundled payments like CJR shift some of the risk for high costs to the hospital and surgeon, a much more extreme example of this type of shift is capitation (ie, paying a healthcare institution a set amount per patient to care for whatever maladies arise). Insurers have experimented with various forms of capitation in the past, which led to the expansion of health management organizations (HMOs) during the 1990s. In theory, capitation should encourage providers to invest in disease prevention to minimize the need for costly interventions. However, more nefarious incentives developed, resulting in “cherry picking” healthy patients, which restricts access to care for sicker patients, and even withholds care from patients in need. The most infamous example was arguably “drive-through deliveries,” where newborns and their mothers were prematurely discharged following birth.5 As a result, the “HMO backlash” occurred, and capitation temporarily fell out of favor. The heart of the problem was a strong incentive to reduce the cost of care without a counterbalancing incentive to maintain quality. CJR and other modern programs attempt to avoid similar adverse incentives by requiring participants to meet certain quality criteria.6
Since the passage of the Affordable Care Act in 2010, capitation has reemerged under a new name: Accountable Care Organizations (ACOs). Numerous forms of ACO’s exist with differing payment schemes7, but the most comprehensive version, named Next Generation (Next Gen), allows providers to choose full capitation.8 While early ACOs focused on individual patients, Next Gen ACOs are also focused on “population health.” That is, they must demonstrate outcomes for individuals and the patient population as a whole, while simultaneously assuming all financial risk via capitation. Specifically, these ACO’s are paid an “all-inclusive population-based payment” for each patient based on how much that type of patient’s care is expected to cost for the year.9 The ACO then provides all necessary treatment and, if the ACO cannot provide a necessary intervention, it is responsible for funding that care at another institution. Appropriately, there has been an increased focus on quality to avoid unintentional incentives to withhold care. Specifically, CMS has introduced mandatory quality metrics in the domains of patient experience, care coordination, preventive care, and management of at-risk populations.10 At present, unfortunately, these metrics are not nearly comprehensive enough nor adequately validated to assess the quality of care,11 especially for subspecialized fields like orthopedics where functional outcome scores are needed.
To date, very limited attention in the media or academic literature has been dedicated to subspecialty surgical care in the setting of ACOs even though implications for specialized surgeons could be immense. While ACOs bring numerous reporting requirements, the most essential first step for orthopedists in transition to this new reimbursement scheme will be a change in mindset. As explained above, orthopedics and other forms of specialized surgical care have traditionally been extremely profitable for healthcare institutions through relatively high revenue. However, within a capitated ACO all revenue has been paid upfront for each patient, and every orthopedic surgery performed represents a substantial cost to the institution rather than a large profit. For example, it has been reported that the average contribution margin earned by a hospital for an episode of care to provide a TKA (which includes postoperative care such as clinic visits, unplanned readmissions, and reoperations for complications) based on Medicare reimbursement is $11,726.12 This figure consists of reimbursement (median, $24,149) less variable costs (median, $10,190). Additionally, the surgeon currently receives $1400 in physician fees.13 These earnings represent a significant financial benefit for both the facility and doctor in the current FFS environment. However, a capitated ACO caring for a TKA patient would already have received full payment for his care for the year. As a result, providing a TKA would not afford any further financial benefit and would, instead, mean a loss of $10,190 (the aforementioned variable cost for the episode of care) directly from the bottom line. The orthopedic department within that ACO, along with other departments, can be expected to share that loss. This implies that upon becoming an ACO, an institution’s orthopedics department will change from a major profit-center to a major cost-center.
Continue to: CMS must establish adequate quality assurance...
CMS must establish adequate quality assurance measures to ensure that ACOs do not withhold cost-effective care, like TKAs,14,15 from their patients. Hopefully, for both professional and ethical reasons, providers will be active partners in this process. Groups like the International Consortium for Health Outcome Measurement, which has convened international expert panels to agree on comprehensive outcome sets for total joint arthroplasty and the management of low back pain, among other non-orthopedic conditions, may be useful examples in this process.16-18
At the provider level, surgeons will be more likely to be salaried employees, contracting directly with the ACO rather than primarily working to earn physician fees from insurance providers. Surgeons will likely be judged (and rewarded financially) on their ability to direct nonoperative care, to find non-surgical solutions to problems that may currently be treated operatively, and to reduce costs for patients that require surgery. Additionally, with an increased focus on quality assurance, there will likely be more pressure from ACOs and CMS to demonstrate results of both operative and nonoperative care, likely in the forms of patient-reported metrics and objective measures of physical function. Surgeons will have a strong incentive to be leaders in the process of collecting such data.
It is also worth considering the position of orthopedic practices that are not part of an ACO. Some ACOs will not have the capacity to provide all (or possibly any) of the orthopedic care their patients require. When necessary, they will contract with outside orthopedic practices. Compared with CMS, ACOs are much smaller purchasers and can be expected to be more sensitive to price, likely negotiating intensely between local orthopedic providers. As a result, even orthopedists outside of ACOs may feel the cost pressure created by this new reimbursement model and may be driven to implement cost-reduction measures such as standardized implant choices and discharge pathways.
ACOs are in an active growth phase,19,20 and recent updates to ACO policies make it clear that CMS intends for this trend to continue.8 Since ACOs are still a nascent reimbursement model, orthopedists will still do better financially, in almost all markets, by continuing to expend their energy and resources pursuing revenue, rather than cutting costs or demonstrating outcomes. However, as ACOs and population health gain traction, those orthopedists who recognize this shift and plan accordingly will have a definite strategic advantage, whether their practice is within an ACO, interacting with external ACOs, or both.
1. Carter Clement R, Bhat SB, Clement ME, Krieg JC. Medicare reimbursement and orthopedic surgery: past, present, and future. Curr Rev Musculoskelet Med. 2017;10(2):224-232. doi:10.1007/s12178-017-9406-7.
2. Centers for Medicare & Medicaid Services. Acute Inpatient PPS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html. Published August 2, 2017. Accessed September 8, 2018.
3. Centers for Medicare & Medicaid Services. Draft ICD-10-CM/PCS MS-DRGv28 Definitions Manual. https://www.cms.gov/icd10manual/fullcode_cms/P0185.html. Accessed September 8, 2018.
4. Centers for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/cjr. Accessed September 8, 2018.
5. Volpp KG, Bundorf MK. Consumer protection and the HMO backlash: are HMOs to blame for drive-through deliveries? Inquiry. 1999;36(1):101-109.
6. Centers for Medicare & Medicaid Services. Quality Measures and Performance Standards. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Quality_Measures_Standards.html. Published March 2, 2015. Accessed November 3, 2015.
7. Centers for Medicare & Medicaid Services. Accountable Care Organizations (ACOs): General Information. https://innovation.cms.gov/initiatives/aco/. Accessed September 8, 2018.
8. Centers for Medicare & Medicaid Services. Next Generation ACO Model. https://innovation.cms.gov/initiatives/Next-Generation-ACO-Model/. Accessed September 8, 2018.
9. Centers for Medicare & Medicaid Services. Next Generation Accountable Care Organization (ACO) Model: Frequently Asked Questions. https://innovation.cms.gov/Files/x/nextgenacofaq.pdf. Accessed September 8, 2018.
10. Centers for Medicare & Medicaid Services. Quality Measure Benchmarks for the 2018 and 2019 Reporting Years. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/2018-and-2019-quality-benchmarks-guidance.pdf. Published December 2017. Accessed September 8, 2018.
11. Toussaint J, Krueger D, Shortell SM, Milstein A, Cutler DM. ACO model should encourage efficient care delivery. Healthc (Amst). 2015;3(3):150-152. doi:10.1016/j.hjdsi.2015.06.003.
12. Clement RC, Kheir MM, Derman PB, et al. What are the economic consequences of unplanned readmissions after TKA? Clin Orthop Relat Res. 2014;472(10):3134-3141. doi:10.1007/s11999-014-3795-3.
13. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Results. http://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=0&T=0&HT=0&CT=0&H1=27447&M=1. Accessed June 4, 2015.
14. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122. doi:10.1001/archinternmed.2009.136.
15. Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
16. International Consortium for Health Outcomes Measurement. ICHOM web site. https://www.ichom.org/. Accessed November 3, 2015.
17. Rolfson O, Wissig S, van Maasakkers L, et al. Defining an international standard set of outcome measures for patients with hip or knee osteoarthritis: consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care Res (Hoboken). 2016;68(11):1631-1639. doi:10.1002/acr.22868.
18. Clement RC, Welander A, Stowell C, et al. A proposed set of metrics for standardized outcome reporting in the management of low back pain. Acta Orthop. 2015;86(5):523-533. doi:10.3109/17453674.2015.1036696.
19. Shortell SM, Colla CH, Lewis VA, Fisher E, Kessell E, Ramsay P. Accountable care organizations: the national landscape. J Health Polit Policy Law. 2015;40(4):647-668. doi:10.1215/03616878-3149976.
20. Centers for Medicare & Medicaid Services. CMS Proposes “Pathways to Success,” an Overhaul of Medicare’s ACO Program https://www.cms.gov/newsroom/press-releases/cms-proposes-pathways-success-overhaul-medicares-aco-program. Published August 9, 2018. Accessed September 10, 2018.
1. Carter Clement R, Bhat SB, Clement ME, Krieg JC. Medicare reimbursement and orthopedic surgery: past, present, and future. Curr Rev Musculoskelet Med. 2017;10(2):224-232. doi:10.1007/s12178-017-9406-7.
2. Centers for Medicare & Medicaid Services. Acute Inpatient PPS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html. Published August 2, 2017. Accessed September 8, 2018.
3. Centers for Medicare & Medicaid Services. Draft ICD-10-CM/PCS MS-DRGv28 Definitions Manual. https://www.cms.gov/icd10manual/fullcode_cms/P0185.html. Accessed September 8, 2018.
4. Centers for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/cjr. Accessed September 8, 2018.
5. Volpp KG, Bundorf MK. Consumer protection and the HMO backlash: are HMOs to blame for drive-through deliveries? Inquiry. 1999;36(1):101-109.
6. Centers for Medicare & Medicaid Services. Quality Measures and Performance Standards. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Quality_Measures_Standards.html. Published March 2, 2015. Accessed November 3, 2015.
7. Centers for Medicare & Medicaid Services. Accountable Care Organizations (ACOs): General Information. https://innovation.cms.gov/initiatives/aco/. Accessed September 8, 2018.
8. Centers for Medicare & Medicaid Services. Next Generation ACO Model. https://innovation.cms.gov/initiatives/Next-Generation-ACO-Model/. Accessed September 8, 2018.
9. Centers for Medicare & Medicaid Services. Next Generation Accountable Care Organization (ACO) Model: Frequently Asked Questions. https://innovation.cms.gov/Files/x/nextgenacofaq.pdf. Accessed September 8, 2018.
10. Centers for Medicare & Medicaid Services. Quality Measure Benchmarks for the 2018 and 2019 Reporting Years. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/2018-and-2019-quality-benchmarks-guidance.pdf. Published December 2017. Accessed September 8, 2018.
11. Toussaint J, Krueger D, Shortell SM, Milstein A, Cutler DM. ACO model should encourage efficient care delivery. Healthc (Amst). 2015;3(3):150-152. doi:10.1016/j.hjdsi.2015.06.003.
12. Clement RC, Kheir MM, Derman PB, et al. What are the economic consequences of unplanned readmissions after TKA? Clin Orthop Relat Res. 2014;472(10):3134-3141. doi:10.1007/s11999-014-3795-3.
13. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Results. http://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=0&T=0&HT=0&CT=0&H1=27447&M=1. Accessed June 4, 2015.
14. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122. doi:10.1001/archinternmed.2009.136.
15. Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
16. International Consortium for Health Outcomes Measurement. ICHOM web site. https://www.ichom.org/. Accessed November 3, 2015.
17. Rolfson O, Wissig S, van Maasakkers L, et al. Defining an international standard set of outcome measures for patients with hip or knee osteoarthritis: consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care Res (Hoboken). 2016;68(11):1631-1639. doi:10.1002/acr.22868.
18. Clement RC, Welander A, Stowell C, et al. A proposed set of metrics for standardized outcome reporting in the management of low back pain. Acta Orthop. 2015;86(5):523-533. doi:10.3109/17453674.2015.1036696.
19. Shortell SM, Colla CH, Lewis VA, Fisher E, Kessell E, Ramsay P. Accountable care organizations: the national landscape. J Health Polit Policy Law. 2015;40(4):647-668. doi:10.1215/03616878-3149976.
20. Centers for Medicare & Medicaid Services. CMS Proposes “Pathways to Success,” an Overhaul of Medicare’s ACO Program https://www.cms.gov/newsroom/press-releases/cms-proposes-pathways-success-overhaul-medicares-aco-program. Published August 9, 2018. Accessed September 10, 2018.
Subcutaneous Ulnar Nerve Transposition Using Osborne’s Ligament as a Ligamentodermal or Ligamentofascial Sling
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
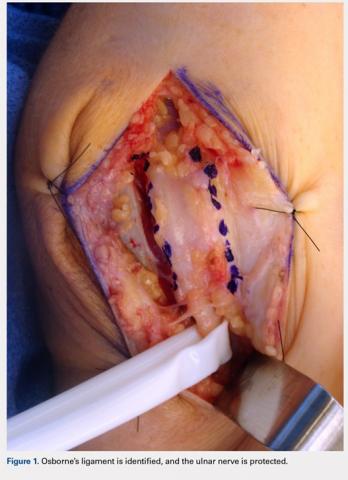
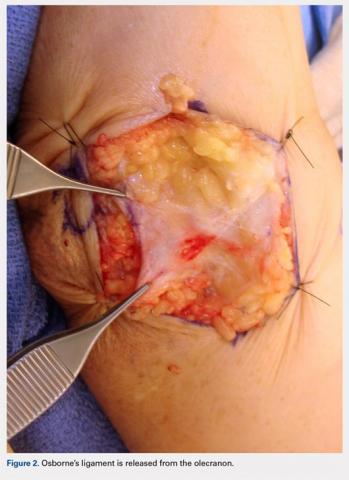
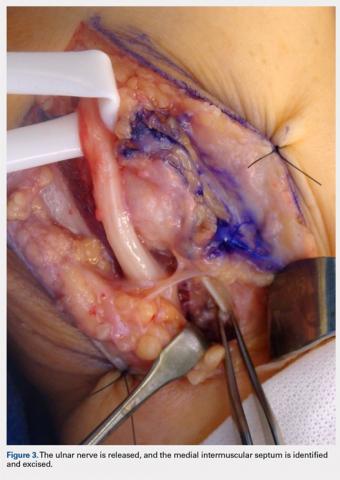
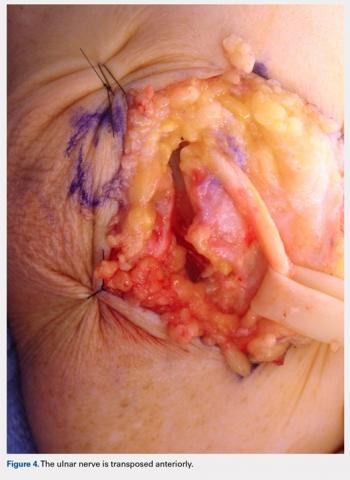
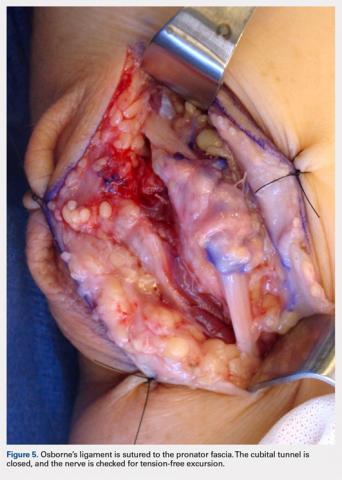
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
TAKE-HOME POINTS
- Optimal management of cubital tunnel syndrome is controversial.
- There are many different techniques for ulnar nerve transposition, each with their own set of pitfalls.
- Goal of any surgery for ulnar nerve compression is to eliminate all sites of compression and create a tension-free nerve that glides freely.
- Osborne’s ligament is a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel.
- Osborne’s ligament can be used in ulnar nerve transposition to create a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve.
A Three-View Radiographic Approach to Femoroacetabular Impingement
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40°may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140°may indicate coxa valga
<130°may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.
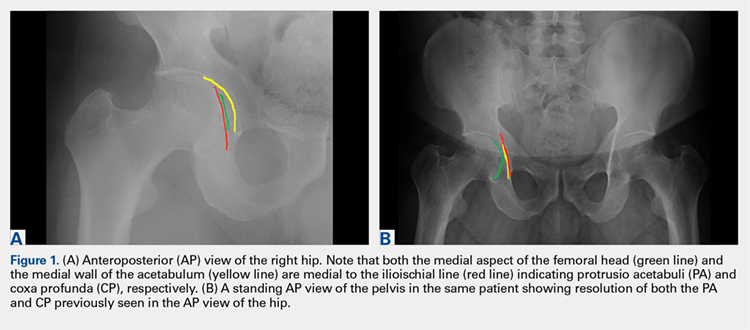
A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40°may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140°may indicate coxa valga
<130°may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40°may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140°may indicate coxa valga
<130°may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
TAKE-HOME POINTS
- FAI is a frequently unrecognized cause of hip pain in adolescents and young adults.
- Understanding the potential sites of impingement and the specific radiographs to visualize these sites can help avoid unnecessary imaging and delayed diagnosis.
- A simple radiographic approach consisting of a standing AP view of the pelvis, a cross-table lateral view, and a false profile view is often a sufficient screening tool.
- While we tend to classify FAI into cam and pincer osseous bumps, alterations in hip dynamics can result in functional impingement even in the absence of the osseous bumps.
- Advanced imaging is reserved for patients who have failed conservative management or are considering surgical intervention.
Time-to-Surgery for Definitive Fixation of Hip Fractures: A Look at Outcomes Based Upon Delay
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.
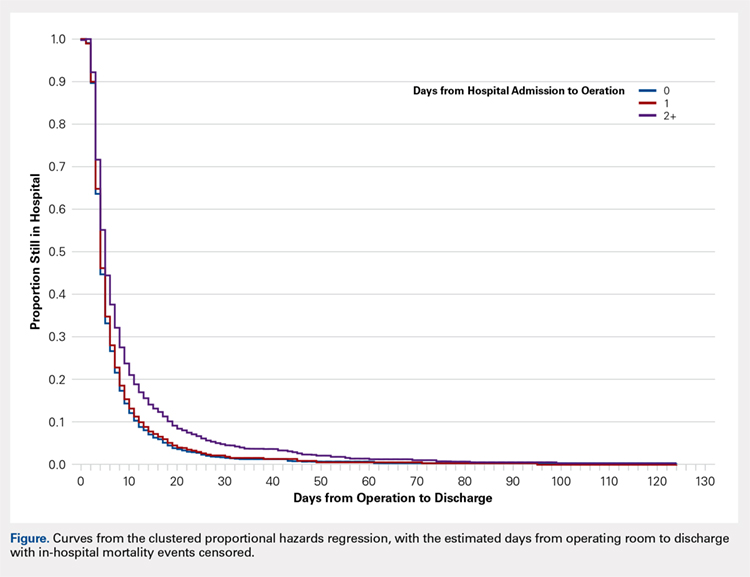
All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).
The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.

All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).

The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.

All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).

The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
TAKE-HOME POINTS
- Time-to-surgery for definitive fixation of hip fractures is a modifiable risk factor.
- This study fails to demonstrate a benefit in delaying surgery for medical optimization as there were no time-to-surgery related differences in complications (P = 1.43).
- Delay in definitive surgery results in an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001) without an improvement in overall complications, readmission or 30-day mortality rates.
- Despite numerous investigations, there are no consensus guidelines to decrease complications and mortality rates following hip fracture surgery.
- ACS-NSQIP database is a reliable and validated database.
5 Points on Meniscal Allograft Transplantation
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.
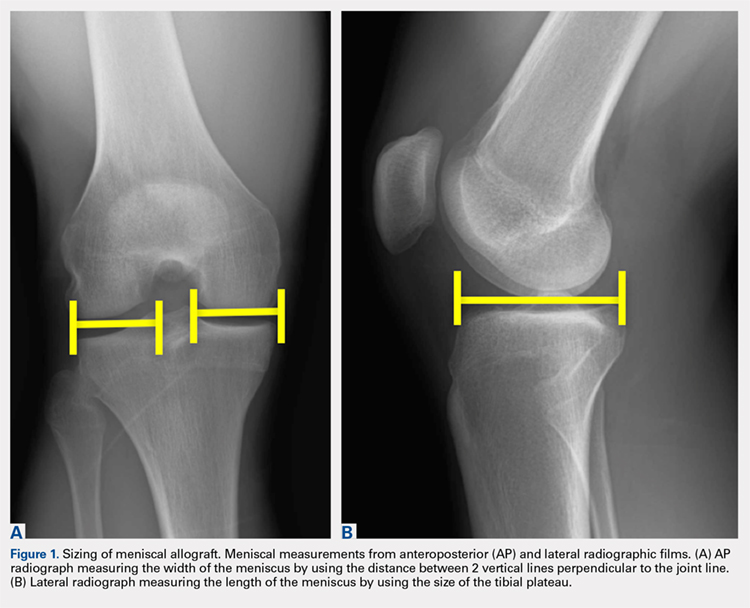
Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.
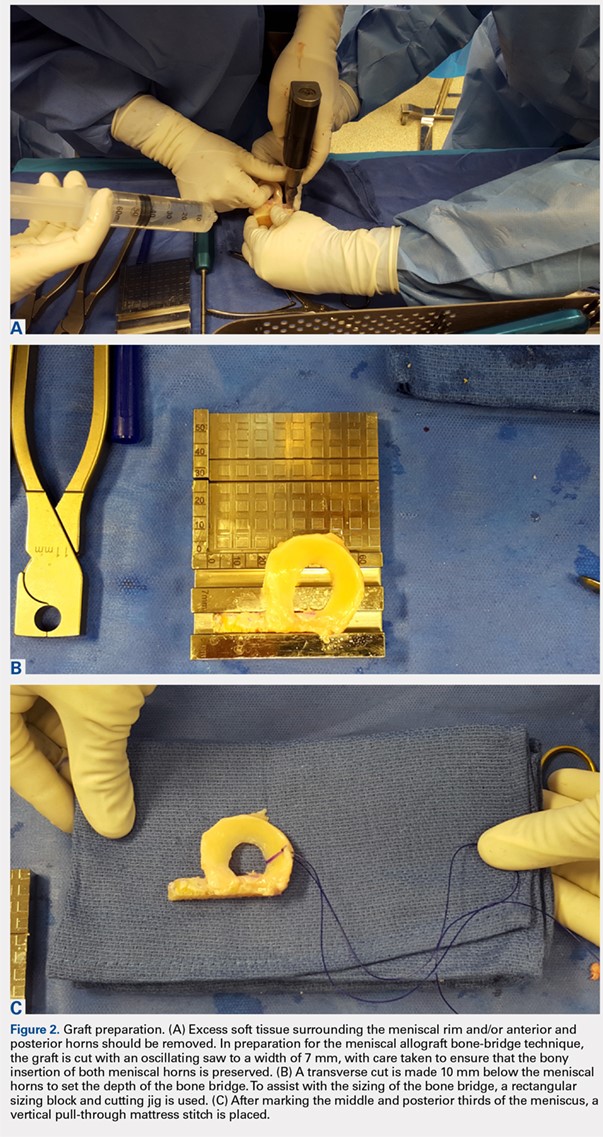
Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
TAKE-HOME POINTS
- Patient selection is critical for obtaining long-term functional outcome improvements and reduced pain, with the ideal MAT candidate being a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency.
- Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure.
- Accurate graft width and length measurements are vital, and the most common technique used today includes measuring the meniscus on anteroposterior and lateral radiographic images.
- When preparing the graft for the bone-bridge technique, the bone is fashioned to create a bone bridge 10 mm in depth by approximately 7 mm in width, incorporating the anterior and posterior horns of the meniscus.
- Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Case Series Evaluating the Operative and Nonoperative Treatment of Scapular Fractures
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.
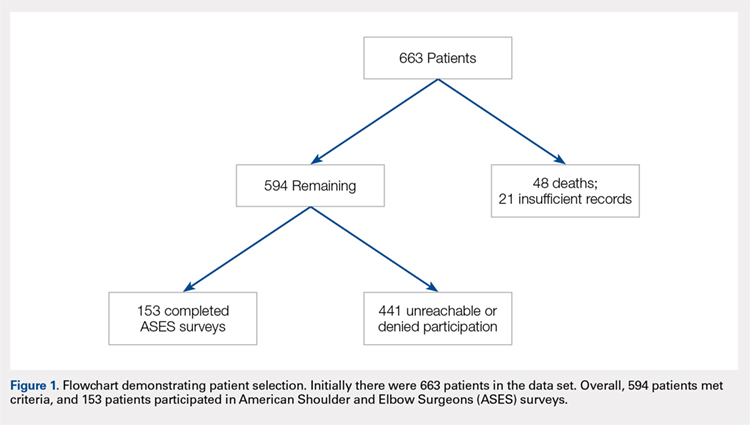
Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.
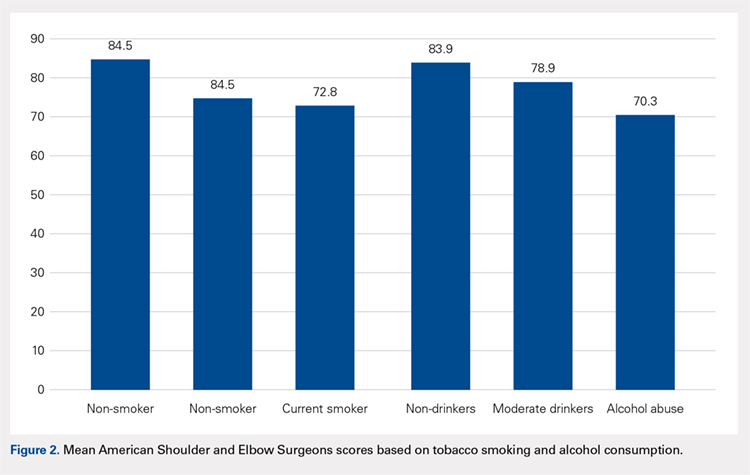
Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.

Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.

Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.

Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.

Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
TAKE-HOME POINTS
- The majority of patients with scapula fractures are multiply-injured.
- Despite being multiply-injured, most heal with minimal functional shoulder impairment.
- While concomitant injuries do not appear to affect shoulder function scores, tobacco use and alcohol abuse are associated with worse outcomes after scapula fractures.
- Most scapula fractures can be treated successfully without surgery.
- Although patients had higher average function scores after open reduction and internal fixation, further research should be done to define indications for fixation.
Epidemiology of Existing Extensor Mechanism Pathology in Primary Anterior Cruciate Ligament Ruptures in an Active-Duty Population
ABSTRACT
The purpose of this study is to determine the prevalence of potential graft-influencing pathologies of the extensor mechanism of the knee in patients presenting with a primary anterior cruciate ligament (ACL) rupture.
We performed a retrospective review of the plain radiographs and magnetic resonance imaging (MRI) of all active-duty patients presenting with a primary ACL rupture at our institution between July 2006 and February 2009. Imaging was reviewed to determine the presence of a multipartite patella, unresolved Osgood-Schlatter’s disease, and/or radiographic evidence suggestive of patella tendinopathy.
A total of 197 patients were reviewed, including 27 females and 170 males. One patient (0.5%) had a bipartite patella and 4 patients (2%) had free-floating ossicles about the tibial tuberosity consistent with unresolved Osgood-Schlatter’s disease. A total of 15 patients (7.6%) showed MRI evidence suggestive of patella tendinopathy.
This study revealed 20 patients out of 197 (10.1%) who presented with existing extensor mechanism pathologies in radiologic studies. While preoperative imaging is routinely used to confirm clinical suspicion of ACL rupture or identify associated injuries, this study shows that it can also identify existing extensor mechanism pathologies that could ultimately influence the use of an extensor mechanism graft.
Continue to: Anterior cruciate ligament (ACL) reconstruction...
Anterior cruciate ligament (ACL) reconstruction is an extremely common procedure; in fact, an estimated 60,000 to 175,000 ACL reconstructions are performed annually in the United States.1,2 One of the most widely debated aspects of ACL reconstruction is the choice of graft. Grafts are broadly categorized into allografts and autografts. The autograft selections for ACL reconstruction include patellar bone-tendon-bone (pBTB), combined semitendinosus and gracilis hamstrings (HS), free quadriceps tendon (QT)without accompanying bone block, and quadriceps tendon-bone (qTB). Allograft choices predominantly include pBTB and HS, as well as the tibialis anterior and Achilles tendons. The pBTB autograft is traditionally considered the reference standard for ACL reconstruction.3 Recent advances in allograft processing, along with improved fixation techniques and devices, have improved results following the use of soft-tissue autografts and both bony and soft tissue allografts.4 Thus, the optimal graft choice for ACL reconstruction has become controversial in light of several studies demonstrating no significant, long-term difference in clinical and/or functional outcomes based on graft selection.5-7
Given the lack of a clear gold standard in graft selection, multiple patient factors, such as age, activity demands, and patient preference, should be taken into account when considering the choice of graft. In addition, intrinsic factors that could potentially weaken an autograft should be considered. Several extensor mechanism pathological findings that are easily visualized on either plain radiographs or magnetic resonance imaging (MRI) could potentially affect graft selection. Findings such as a multipartite patella, free ossicles about the tibial tuberosity consistent with Osgood-Schlatter’s disease, and proximal patella tendon thickening suggestive of patellar tendinopathy are easily identifiable on preoperative imaging and could exert adverse effects on pBTB, QT, and qTB autografts. The purpose of this study is to identify the prevalence of these pre-existing conditions in active-duty military patients presenting with acute ACL tears.
METHODS
A retrospective review was conducted on all active-duty patients who underwent primary ACL reconstruction at our institution from July 2006 to February 2009. A systematic review of all plain radiographs and MRIs was performed on a calibrated picture archiving and communication system workstation. Imaging review was conducted by 2 of the authors. Pertinent findings included a multipartite patella, free ossicles within the patella tendon, and hypertrophy of the proximal aspect of the patella tendon. Assessment for multipartite patella and unresolved Osgood-Schlatter's disease was made using plain radiographs with MRI for confirmation. Measurements of the patella tendon were performed on the short tau inversion recovery and T2-weighted sagittal MRI images at the point of maximal tendon width. A width of ≥7 mm was considered suggestive of patella tendinopathy based on prior studies.8-10 The prevalence of each finding was then determined based on the total number of patients.
Continue to: RESULTS...
RESULTS
A total of 197 active-duty patients, including 27 females (13.7%) and 170 males (86.3%), underwent primary ACL reconstruction during the study time period. A total of 93 right knees and 104 left knees were evaluated. The average age at presentation was 29 years (range, 19-45 years).
Of the 197 patients, only 1 was found to have a multipartite patella (prevalence, 0.5%). This 37-year-old male patient showed a right bipartite patella located in the superior-lateral aspect (Figure 1).
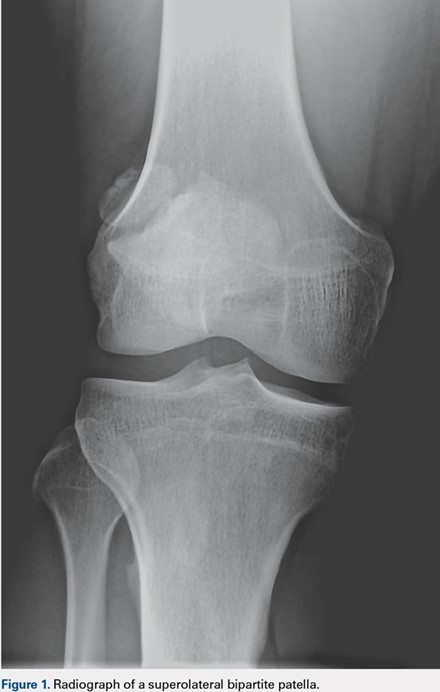
Four patients had free ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease (prevalence, 2.0%) (Figure 2). All 4 patients were male, which is consistent with the higher incidence of Osgood-Schlatter’s disease in males than in females. The average age of these patients was 27.5 years (range, 22-33 years).
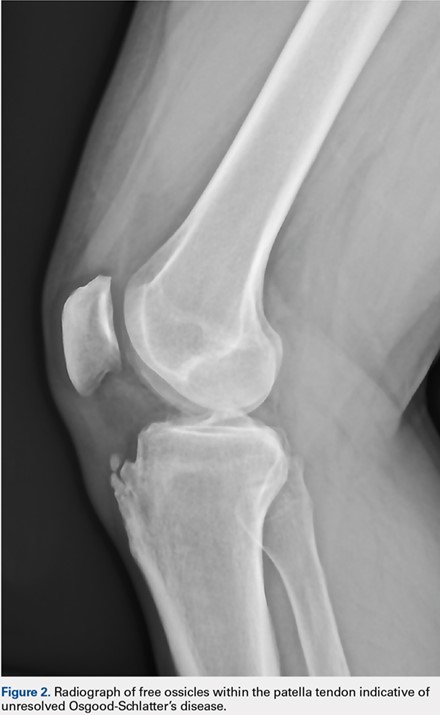
The most common extensor mechanism pathology present on preoperative imaging was proximal patella tendon thickening suggestive of patella tendinopathy. Thickening of the proximal portion of the patellar tendon was present in 15 of the 197 MRIs (prevalence, 7.6%) (Figure 3). The average width of this thickening was 8.49 mm (7.17-10.17 mm), and the average age of patients with radiographic evidence of patellar tendinopathy was 29.9 years (range, 20-43 years). Gender distribution was predominantly male (14 males, 1 female). Details of all extensor mechanism pathologies found are provided in the Table.
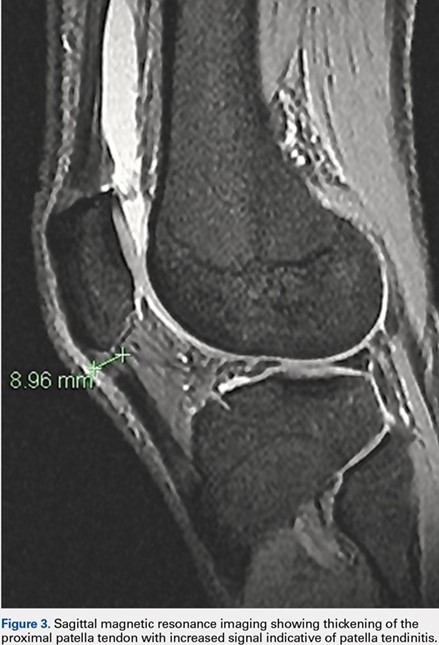
Table. Identified Extensor Mechanism Pathology
| Male | Female | Total |
Patients | 170 | 27 | 197 |
Multipartite Patella | 1 | 0 | 1 |
Osgood-Schlatter’s Disease | 4 | 0 | 4 |
Patella Tendinopathy | 14 | 1 | 15 |
|
| 20/97 (10.10%) |
|
DISCUSSION
When considering ACL reconstruction, determination of the graft type is one of the most important decisions to be made, perhaps second only to the decision to perform the surgery itself. Recent multiple, well-designed studies comparing differences among grafts have shown equivalent long-term results, leading to the lack of a universally accepted gold standard.5-7 Thus, both autograft and allograft ACL surgeries are routinely performed in the United States. Surgeons typically take into account factors such as patient age and physical demands, along with their own preferences and/or experience, when considering graft selection. A paucity of research concerning existing pathological conditions that could also influence preoperative decision-making has been observed; most reports consist only of expert opinion.11-13 Our goal is to determine the prevalence of several conditions that could potentially affect an autograft harvested from the extensor mechanism.
This study revealed an overall prevalence of 10.1% of existing extensor mechanism pathology in patients sustaining an acute ACL tear and presenting for ACL reconstruction. Only 1 (0.5%) showed evidence of a multipartite patella, which is below the reported prevalence of 0.2% to 6%.14 The presence of a multipartite patella could potentially have the most deleterious effect on a qTB autograft. Although not as commonly used as HS, QT, or pBTB autografts, some surgeons prefer a qTB autograft because of its increased surface area, bony fixation, and reported decreased donor site pain.15 A multipartite patella could complicate harvesting, disrupt the bone block, or lead to an unstable segment of the patella. These effects are of great concern since the most common location of a bipartite patella is superior-lateral and the quadriceps tendon has been shown to asymmetrically insert laterally.16 While these potential adverse effects have not been specifically studied, the availability of comparable options makes the use of a qTB autograft in the setting of a bipartite patella questionable.
Four patients (2%) revealed evidence of ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease. Osgood-Schlatter’s disease has been reported to occur in up to 21% of active adolescents and is historically considered a self-resolving process.17 Recent papers have reported persistent symptoms in up to 10% of patients, with a small percentage experiencing persistent free ossicles within their patella tendon on MRI.18,19 The presence of such ossicles raises concern about the integrity of the patellar tendon and questions its use as an autograft when present. This concern was published in a report with the surgeon opting to utilize an alternate graft due to the presence of unresolved Osgood-Schlatter’s disease.13
Fifteen patients (7.6%) demonstrated radiographic evidence suggestive of patella tendinopathy based on the thickness of the proximal patella tendon. Patella tendinopathy is the most common tendinopathy in skeletally mature athletes and one of the most common athletic injuries of the knee, with a reported career prevalence of 22%.20 It is described as an overuse injury due to the cumulative effect of micro trauma without an adequate healing interval. While it remains a clinical diagnosis, patellar tendinopathy often shows radiographic findings best assessed on sagittal MRIs. In general, the normal patella tendon appears as a homogenous low-intensity structure and is of uniform thickness. A tendon affected with tendinopathy typically demonstrates a focal increase in signal on T2-weighted sequences just distal to the tendon origin on the inferior pole of the patella. In addition, the patella tendon will usually demonstrate thickening, primarily in the proximal medial and posterior fibers. Patella marrow changes and indistinct tendon margins can also be present. The sensitivity and specificity of diagnosing patellar tendinopathy on MRI are 78% and 86%, respectively.20 We derived our criteria for MRI evidence suggestive of patella tendinopathy from studies by El-Khoury and colleagues,8 Johnson and colleagues,9 and Popp and colleagues.10 In a 1992 study, El-Khoury and colleagues8 compared MRI findings between a group of patients with a clinical diagnosis of patella tendonitis and a control group without knee complaints. The authors found that the average proximal patella tendon diameter in the control group was 3.7 mm while the average proximal patella tendon diameter in the patella tendinopathy group was 10.9 mm; no patella tendons in the control group were >7 mm.8 In a 1996 study, Johnson and colleagues9 determined that the most reliable MRI finding for patients with patellar tendonitis is significant thickening of the proximal patella tendon seen on the sagittal view. The average thickness in symptomatic patients was 8.5 mm (range, 5-15 mm). The average thickness in the control group was 5.5 mm. None of the control patients had a proximal tendon thickness >7 mm.9 Finally, Popp and colleagues10 reviewed the MRI of 11 knees of patients who underwent surgical débridement of chronic patellar tendonitis and reported an average proximal patella tendon thickness of 12 mm (range, 9-16 mm). We therefore used a proximal patella tendon thickness of >7 mm on the sagittal view as a radiographic finding suggestive of patella tendinopathy. No data regarding symptoms of anterior knee pain were available among our patients. Histological studies of patients with patella tendonitis have shown evidence of chronic inflammation, fibrinoid necrosis, mucoid degeneration, and synovial proliferation within the patella tendon insertion.21 Although no controlled data showing that patella tendons with a history of tendonitis are more prone to failure than those without such history when used as an autograft for ACL reconstruction, the idea of utilizing a diseased tendon for a graft is not ideal. Some surgeons question their patients regarding a history of anterior knee pain and will not use a pBTB autograft in a patient with a positive history.22
Continue to: The goal of this study is to obtain epidemiological evidence...
The goal of this study is to obtain epidemiological evidence of the prevalence of existing extensor mechanism pathologies in patients with acute ACL ruptures and determine how these pathologies may relate to the choice of graft. Out of 197 patients studied, over 10% presented with radiographic evidence of pathologies that could influence the choice of graft. This prevalence is certainly significant enough for surgeons to consider including a radiographic evaluation of the extensor mechanism in their standard ACL rupture work-up.
This study presents obvious limitations. While we report the prevalence of some extensor mechanism pathologies, no definitive evidence that recommends against the use of these autografts from these affected individuals has yet been published. In addition, our diagnosis of patella tendinopathy is based solely on MRI findings with no information regarding clinical symptoms. This limitation is a weakness as several additional studies have questioned the validity of a 7 mm proximal patella tendon thickness.23,24 Furthermore, no studies demonstrating the inferior strength of autografts with the co-existing findings described in our work have yet been performed.
CONCLUSION
We found that 10% of active-duty patients presenting for ACL reconstruction demonstrated radiographic evidence of an extensor mechanism pathology that could affect the harvesting of or integrity of select autografts. Given the recent trend of functionally equivocal results in ACL reconstructions utilizing a variety of grafts, this information could and should influence surgical recommendations for graft utilization to obtain optimal surgical results.
1. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321-2328. doi:10.2106/JBJS.H.00539.
2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135-2142. doi:10.1056/NEJMcp0804745.
3. Fu FH, Bennett CH, Lattermann CL, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27(6):821-830. doi:10.1177/03635465990270062501.
4. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft Versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(2):492-499. doi:10.1177/0363546513497566.
5. Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):214-220. doi:10.1177/03635465020300021201.
6. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: Allograft versus allograft. Arthroscopy. 2005;21(7):774-785. doi:10.1016/j.arthro.2005.04.112.
7. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):292-298. doi:10.1016/j.arthro.2007.08.029.
8. El-Khoury GY, Wira RL, Berbaum KS, Pope TL, Monu JUV. MR imaging of patellar tendinitis. Radiology. 1992;184(3):849-854. doi:10.1148/radiology.184.3.1509078.
9. Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Joint Surg Br. 1996;78(3):452-457. doi:10.1302/0301-620X.78B3.0780452.
10. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218-222. doi:10.1177/036354659702500214.
11. Provencher MT, Ryu JH, Gaston T, Dewing CB. Technique: bone-patellar tendon-bone autograft ACL reconstruction in the young, active patient. J Knee Surg. 2011;24(2):83-92. doi:10.1055/s-0031-1280875.
12. Fu F, Cohen S. Current Concepts in ACL Reconstruction. Thorofare: SLACK Incorporated; 2008.
13. Cosgarea AJ, Weng MS, Andrews M. Osgood Schlatter’s disease complicating anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(6):700-703. doi:10.1016/S0749-8063(05)80511-0.
14. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855. doi:10.1007/s11999-008-0367-4.
15. Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252-254. doi:10.1016/0749-8063(95)90078-0.
16. Scully WF, Wilson DJ, Arrington ED. “Central” quadriceps tendon harvest with patellar bone plug: surgical technique revisited. Arthrosc Tech. 2013;2(4):e427-e432.
17. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236-241. doi:10.1177/036354658501300404.
18. Pihlajamäki HK, Visuri TI. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 Pt 2):258-264. doi:10.2106/JBJS.J.00450.
19. Weiss JM, Jordan SS, Andersen JS, Lee BM, Kocher M. Surgical treatment of unresolved Osgood-Schlatter disease: ossicle resection with tibial tubercleplasty. J Pediatr Orthop. 2007;27(7):844-847. doi:10.1097/BPO.0b013e318155849b.
20. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee Among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561-567. doi:10.1177/0363546504270454.
21. O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725-739, vii. doi:10.1016/j.mric.2009.06.010.
22. Martens M, Wouters P, Burssens A, Mulier JC. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand. 1982;53(3):445-450. doi:10.3109/17453678208992239.
23. Shalaby M, Almekinders LC. Patellar tendinitis: the significance of magnetic resonance imaging findings. Am J Sports Med. 1999;27(3):345-349. doi:10.1177/03635465990270031301.
24. Reiff DB, Heenan SD, Heron CW. MRI appearances of the asymptomatic patellar tendon on gradient echo imaging. Skeletal Radiol. 1995;24(2):123-126. doi:10.1007/BF00198074.
ABSTRACT
The purpose of this study is to determine the prevalence of potential graft-influencing pathologies of the extensor mechanism of the knee in patients presenting with a primary anterior cruciate ligament (ACL) rupture.
We performed a retrospective review of the plain radiographs and magnetic resonance imaging (MRI) of all active-duty patients presenting with a primary ACL rupture at our institution between July 2006 and February 2009. Imaging was reviewed to determine the presence of a multipartite patella, unresolved Osgood-Schlatter’s disease, and/or radiographic evidence suggestive of patella tendinopathy.
A total of 197 patients were reviewed, including 27 females and 170 males. One patient (0.5%) had a bipartite patella and 4 patients (2%) had free-floating ossicles about the tibial tuberosity consistent with unresolved Osgood-Schlatter’s disease. A total of 15 patients (7.6%) showed MRI evidence suggestive of patella tendinopathy.
This study revealed 20 patients out of 197 (10.1%) who presented with existing extensor mechanism pathologies in radiologic studies. While preoperative imaging is routinely used to confirm clinical suspicion of ACL rupture or identify associated injuries, this study shows that it can also identify existing extensor mechanism pathologies that could ultimately influence the use of an extensor mechanism graft.
Continue to: Anterior cruciate ligament (ACL) reconstruction...
Anterior cruciate ligament (ACL) reconstruction is an extremely common procedure; in fact, an estimated 60,000 to 175,000 ACL reconstructions are performed annually in the United States.1,2 One of the most widely debated aspects of ACL reconstruction is the choice of graft. Grafts are broadly categorized into allografts and autografts. The autograft selections for ACL reconstruction include patellar bone-tendon-bone (pBTB), combined semitendinosus and gracilis hamstrings (HS), free quadriceps tendon (QT)without accompanying bone block, and quadriceps tendon-bone (qTB). Allograft choices predominantly include pBTB and HS, as well as the tibialis anterior and Achilles tendons. The pBTB autograft is traditionally considered the reference standard for ACL reconstruction.3 Recent advances in allograft processing, along with improved fixation techniques and devices, have improved results following the use of soft-tissue autografts and both bony and soft tissue allografts.4 Thus, the optimal graft choice for ACL reconstruction has become controversial in light of several studies demonstrating no significant, long-term difference in clinical and/or functional outcomes based on graft selection.5-7
Given the lack of a clear gold standard in graft selection, multiple patient factors, such as age, activity demands, and patient preference, should be taken into account when considering the choice of graft. In addition, intrinsic factors that could potentially weaken an autograft should be considered. Several extensor mechanism pathological findings that are easily visualized on either plain radiographs or magnetic resonance imaging (MRI) could potentially affect graft selection. Findings such as a multipartite patella, free ossicles about the tibial tuberosity consistent with Osgood-Schlatter’s disease, and proximal patella tendon thickening suggestive of patellar tendinopathy are easily identifiable on preoperative imaging and could exert adverse effects on pBTB, QT, and qTB autografts. The purpose of this study is to identify the prevalence of these pre-existing conditions in active-duty military patients presenting with acute ACL tears.
METHODS
A retrospective review was conducted on all active-duty patients who underwent primary ACL reconstruction at our institution from July 2006 to February 2009. A systematic review of all plain radiographs and MRIs was performed on a calibrated picture archiving and communication system workstation. Imaging review was conducted by 2 of the authors. Pertinent findings included a multipartite patella, free ossicles within the patella tendon, and hypertrophy of the proximal aspect of the patella tendon. Assessment for multipartite patella and unresolved Osgood-Schlatter's disease was made using plain radiographs with MRI for confirmation. Measurements of the patella tendon were performed on the short tau inversion recovery and T2-weighted sagittal MRI images at the point of maximal tendon width. A width of ≥7 mm was considered suggestive of patella tendinopathy based on prior studies.8-10 The prevalence of each finding was then determined based on the total number of patients.
Continue to: RESULTS...
RESULTS
A total of 197 active-duty patients, including 27 females (13.7%) and 170 males (86.3%), underwent primary ACL reconstruction during the study time period. A total of 93 right knees and 104 left knees were evaluated. The average age at presentation was 29 years (range, 19-45 years).
Of the 197 patients, only 1 was found to have a multipartite patella (prevalence, 0.5%). This 37-year-old male patient showed a right bipartite patella located in the superior-lateral aspect (Figure 1).

Four patients had free ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease (prevalence, 2.0%) (Figure 2). All 4 patients were male, which is consistent with the higher incidence of Osgood-Schlatter’s disease in males than in females. The average age of these patients was 27.5 years (range, 22-33 years).

The most common extensor mechanism pathology present on preoperative imaging was proximal patella tendon thickening suggestive of patella tendinopathy. Thickening of the proximal portion of the patellar tendon was present in 15 of the 197 MRIs (prevalence, 7.6%) (Figure 3). The average width of this thickening was 8.49 mm (7.17-10.17 mm), and the average age of patients with radiographic evidence of patellar tendinopathy was 29.9 years (range, 20-43 years). Gender distribution was predominantly male (14 males, 1 female). Details of all extensor mechanism pathologies found are provided in the Table.

Table. Identified Extensor Mechanism Pathology
| Male | Female | Total |
Patients | 170 | 27 | 197 |
Multipartite Patella | 1 | 0 | 1 |
Osgood-Schlatter’s Disease | 4 | 0 | 4 |
Patella Tendinopathy | 14 | 1 | 15 |
|
| 20/97 (10.10%) |
|
DISCUSSION
When considering ACL reconstruction, determination of the graft type is one of the most important decisions to be made, perhaps second only to the decision to perform the surgery itself. Recent multiple, well-designed studies comparing differences among grafts have shown equivalent long-term results, leading to the lack of a universally accepted gold standard.5-7 Thus, both autograft and allograft ACL surgeries are routinely performed in the United States. Surgeons typically take into account factors such as patient age and physical demands, along with their own preferences and/or experience, when considering graft selection. A paucity of research concerning existing pathological conditions that could also influence preoperative decision-making has been observed; most reports consist only of expert opinion.11-13 Our goal is to determine the prevalence of several conditions that could potentially affect an autograft harvested from the extensor mechanism.
This study revealed an overall prevalence of 10.1% of existing extensor mechanism pathology in patients sustaining an acute ACL tear and presenting for ACL reconstruction. Only 1 (0.5%) showed evidence of a multipartite patella, which is below the reported prevalence of 0.2% to 6%.14 The presence of a multipartite patella could potentially have the most deleterious effect on a qTB autograft. Although not as commonly used as HS, QT, or pBTB autografts, some surgeons prefer a qTB autograft because of its increased surface area, bony fixation, and reported decreased donor site pain.15 A multipartite patella could complicate harvesting, disrupt the bone block, or lead to an unstable segment of the patella. These effects are of great concern since the most common location of a bipartite patella is superior-lateral and the quadriceps tendon has been shown to asymmetrically insert laterally.16 While these potential adverse effects have not been specifically studied, the availability of comparable options makes the use of a qTB autograft in the setting of a bipartite patella questionable.
Four patients (2%) revealed evidence of ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease. Osgood-Schlatter’s disease has been reported to occur in up to 21% of active adolescents and is historically considered a self-resolving process.17 Recent papers have reported persistent symptoms in up to 10% of patients, with a small percentage experiencing persistent free ossicles within their patella tendon on MRI.18,19 The presence of such ossicles raises concern about the integrity of the patellar tendon and questions its use as an autograft when present. This concern was published in a report with the surgeon opting to utilize an alternate graft due to the presence of unresolved Osgood-Schlatter’s disease.13
Fifteen patients (7.6%) demonstrated radiographic evidence suggestive of patella tendinopathy based on the thickness of the proximal patella tendon. Patella tendinopathy is the most common tendinopathy in skeletally mature athletes and one of the most common athletic injuries of the knee, with a reported career prevalence of 22%.20 It is described as an overuse injury due to the cumulative effect of micro trauma without an adequate healing interval. While it remains a clinical diagnosis, patellar tendinopathy often shows radiographic findings best assessed on sagittal MRIs. In general, the normal patella tendon appears as a homogenous low-intensity structure and is of uniform thickness. A tendon affected with tendinopathy typically demonstrates a focal increase in signal on T2-weighted sequences just distal to the tendon origin on the inferior pole of the patella. In addition, the patella tendon will usually demonstrate thickening, primarily in the proximal medial and posterior fibers. Patella marrow changes and indistinct tendon margins can also be present. The sensitivity and specificity of diagnosing patellar tendinopathy on MRI are 78% and 86%, respectively.20 We derived our criteria for MRI evidence suggestive of patella tendinopathy from studies by El-Khoury and colleagues,8 Johnson and colleagues,9 and Popp and colleagues.10 In a 1992 study, El-Khoury and colleagues8 compared MRI findings between a group of patients with a clinical diagnosis of patella tendonitis and a control group without knee complaints. The authors found that the average proximal patella tendon diameter in the control group was 3.7 mm while the average proximal patella tendon diameter in the patella tendinopathy group was 10.9 mm; no patella tendons in the control group were >7 mm.8 In a 1996 study, Johnson and colleagues9 determined that the most reliable MRI finding for patients with patellar tendonitis is significant thickening of the proximal patella tendon seen on the sagittal view. The average thickness in symptomatic patients was 8.5 mm (range, 5-15 mm). The average thickness in the control group was 5.5 mm. None of the control patients had a proximal tendon thickness >7 mm.9 Finally, Popp and colleagues10 reviewed the MRI of 11 knees of patients who underwent surgical débridement of chronic patellar tendonitis and reported an average proximal patella tendon thickness of 12 mm (range, 9-16 mm). We therefore used a proximal patella tendon thickness of >7 mm on the sagittal view as a radiographic finding suggestive of patella tendinopathy. No data regarding symptoms of anterior knee pain were available among our patients. Histological studies of patients with patella tendonitis have shown evidence of chronic inflammation, fibrinoid necrosis, mucoid degeneration, and synovial proliferation within the patella tendon insertion.21 Although no controlled data showing that patella tendons with a history of tendonitis are more prone to failure than those without such history when used as an autograft for ACL reconstruction, the idea of utilizing a diseased tendon for a graft is not ideal. Some surgeons question their patients regarding a history of anterior knee pain and will not use a pBTB autograft in a patient with a positive history.22
Continue to: The goal of this study is to obtain epidemiological evidence...
The goal of this study is to obtain epidemiological evidence of the prevalence of existing extensor mechanism pathologies in patients with acute ACL ruptures and determine how these pathologies may relate to the choice of graft. Out of 197 patients studied, over 10% presented with radiographic evidence of pathologies that could influence the choice of graft. This prevalence is certainly significant enough for surgeons to consider including a radiographic evaluation of the extensor mechanism in their standard ACL rupture work-up.
This study presents obvious limitations. While we report the prevalence of some extensor mechanism pathologies, no definitive evidence that recommends against the use of these autografts from these affected individuals has yet been published. In addition, our diagnosis of patella tendinopathy is based solely on MRI findings with no information regarding clinical symptoms. This limitation is a weakness as several additional studies have questioned the validity of a 7 mm proximal patella tendon thickness.23,24 Furthermore, no studies demonstrating the inferior strength of autografts with the co-existing findings described in our work have yet been performed.
CONCLUSION
We found that 10% of active-duty patients presenting for ACL reconstruction demonstrated radiographic evidence of an extensor mechanism pathology that could affect the harvesting of or integrity of select autografts. Given the recent trend of functionally equivocal results in ACL reconstructions utilizing a variety of grafts, this information could and should influence surgical recommendations for graft utilization to obtain optimal surgical results.
ABSTRACT
The purpose of this study is to determine the prevalence of potential graft-influencing pathologies of the extensor mechanism of the knee in patients presenting with a primary anterior cruciate ligament (ACL) rupture.
We performed a retrospective review of the plain radiographs and magnetic resonance imaging (MRI) of all active-duty patients presenting with a primary ACL rupture at our institution between July 2006 and February 2009. Imaging was reviewed to determine the presence of a multipartite patella, unresolved Osgood-Schlatter’s disease, and/or radiographic evidence suggestive of patella tendinopathy.
A total of 197 patients were reviewed, including 27 females and 170 males. One patient (0.5%) had a bipartite patella and 4 patients (2%) had free-floating ossicles about the tibial tuberosity consistent with unresolved Osgood-Schlatter’s disease. A total of 15 patients (7.6%) showed MRI evidence suggestive of patella tendinopathy.
This study revealed 20 patients out of 197 (10.1%) who presented with existing extensor mechanism pathologies in radiologic studies. While preoperative imaging is routinely used to confirm clinical suspicion of ACL rupture or identify associated injuries, this study shows that it can also identify existing extensor mechanism pathologies that could ultimately influence the use of an extensor mechanism graft.
Continue to: Anterior cruciate ligament (ACL) reconstruction...
Anterior cruciate ligament (ACL) reconstruction is an extremely common procedure; in fact, an estimated 60,000 to 175,000 ACL reconstructions are performed annually in the United States.1,2 One of the most widely debated aspects of ACL reconstruction is the choice of graft. Grafts are broadly categorized into allografts and autografts. The autograft selections for ACL reconstruction include patellar bone-tendon-bone (pBTB), combined semitendinosus and gracilis hamstrings (HS), free quadriceps tendon (QT)without accompanying bone block, and quadriceps tendon-bone (qTB). Allograft choices predominantly include pBTB and HS, as well as the tibialis anterior and Achilles tendons. The pBTB autograft is traditionally considered the reference standard for ACL reconstruction.3 Recent advances in allograft processing, along with improved fixation techniques and devices, have improved results following the use of soft-tissue autografts and both bony and soft tissue allografts.4 Thus, the optimal graft choice for ACL reconstruction has become controversial in light of several studies demonstrating no significant, long-term difference in clinical and/or functional outcomes based on graft selection.5-7
Given the lack of a clear gold standard in graft selection, multiple patient factors, such as age, activity demands, and patient preference, should be taken into account when considering the choice of graft. In addition, intrinsic factors that could potentially weaken an autograft should be considered. Several extensor mechanism pathological findings that are easily visualized on either plain radiographs or magnetic resonance imaging (MRI) could potentially affect graft selection. Findings such as a multipartite patella, free ossicles about the tibial tuberosity consistent with Osgood-Schlatter’s disease, and proximal patella tendon thickening suggestive of patellar tendinopathy are easily identifiable on preoperative imaging and could exert adverse effects on pBTB, QT, and qTB autografts. The purpose of this study is to identify the prevalence of these pre-existing conditions in active-duty military patients presenting with acute ACL tears.
METHODS
A retrospective review was conducted on all active-duty patients who underwent primary ACL reconstruction at our institution from July 2006 to February 2009. A systematic review of all plain radiographs and MRIs was performed on a calibrated picture archiving and communication system workstation. Imaging review was conducted by 2 of the authors. Pertinent findings included a multipartite patella, free ossicles within the patella tendon, and hypertrophy of the proximal aspect of the patella tendon. Assessment for multipartite patella and unresolved Osgood-Schlatter's disease was made using plain radiographs with MRI for confirmation. Measurements of the patella tendon were performed on the short tau inversion recovery and T2-weighted sagittal MRI images at the point of maximal tendon width. A width of ≥7 mm was considered suggestive of patella tendinopathy based on prior studies.8-10 The prevalence of each finding was then determined based on the total number of patients.
Continue to: RESULTS...
RESULTS
A total of 197 active-duty patients, including 27 females (13.7%) and 170 males (86.3%), underwent primary ACL reconstruction during the study time period. A total of 93 right knees and 104 left knees were evaluated. The average age at presentation was 29 years (range, 19-45 years).
Of the 197 patients, only 1 was found to have a multipartite patella (prevalence, 0.5%). This 37-year-old male patient showed a right bipartite patella located in the superior-lateral aspect (Figure 1).

Four patients had free ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease (prevalence, 2.0%) (Figure 2). All 4 patients were male, which is consistent with the higher incidence of Osgood-Schlatter’s disease in males than in females. The average age of these patients was 27.5 years (range, 22-33 years).

The most common extensor mechanism pathology present on preoperative imaging was proximal patella tendon thickening suggestive of patella tendinopathy. Thickening of the proximal portion of the patellar tendon was present in 15 of the 197 MRIs (prevalence, 7.6%) (Figure 3). The average width of this thickening was 8.49 mm (7.17-10.17 mm), and the average age of patients with radiographic evidence of patellar tendinopathy was 29.9 years (range, 20-43 years). Gender distribution was predominantly male (14 males, 1 female). Details of all extensor mechanism pathologies found are provided in the Table.

Table. Identified Extensor Mechanism Pathology
| Male | Female | Total |
Patients | 170 | 27 | 197 |
Multipartite Patella | 1 | 0 | 1 |
Osgood-Schlatter’s Disease | 4 | 0 | 4 |
Patella Tendinopathy | 14 | 1 | 15 |
|
| 20/97 (10.10%) |
|
DISCUSSION
When considering ACL reconstruction, determination of the graft type is one of the most important decisions to be made, perhaps second only to the decision to perform the surgery itself. Recent multiple, well-designed studies comparing differences among grafts have shown equivalent long-term results, leading to the lack of a universally accepted gold standard.5-7 Thus, both autograft and allograft ACL surgeries are routinely performed in the United States. Surgeons typically take into account factors such as patient age and physical demands, along with their own preferences and/or experience, when considering graft selection. A paucity of research concerning existing pathological conditions that could also influence preoperative decision-making has been observed; most reports consist only of expert opinion.11-13 Our goal is to determine the prevalence of several conditions that could potentially affect an autograft harvested from the extensor mechanism.
This study revealed an overall prevalence of 10.1% of existing extensor mechanism pathology in patients sustaining an acute ACL tear and presenting for ACL reconstruction. Only 1 (0.5%) showed evidence of a multipartite patella, which is below the reported prevalence of 0.2% to 6%.14 The presence of a multipartite patella could potentially have the most deleterious effect on a qTB autograft. Although not as commonly used as HS, QT, or pBTB autografts, some surgeons prefer a qTB autograft because of its increased surface area, bony fixation, and reported decreased donor site pain.15 A multipartite patella could complicate harvesting, disrupt the bone block, or lead to an unstable segment of the patella. These effects are of great concern since the most common location of a bipartite patella is superior-lateral and the quadriceps tendon has been shown to asymmetrically insert laterally.16 While these potential adverse effects have not been specifically studied, the availability of comparable options makes the use of a qTB autograft in the setting of a bipartite patella questionable.
Four patients (2%) revealed evidence of ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease. Osgood-Schlatter’s disease has been reported to occur in up to 21% of active adolescents and is historically considered a self-resolving process.17 Recent papers have reported persistent symptoms in up to 10% of patients, with a small percentage experiencing persistent free ossicles within their patella tendon on MRI.18,19 The presence of such ossicles raises concern about the integrity of the patellar tendon and questions its use as an autograft when present. This concern was published in a report with the surgeon opting to utilize an alternate graft due to the presence of unresolved Osgood-Schlatter’s disease.13
Fifteen patients (7.6%) demonstrated radiographic evidence suggestive of patella tendinopathy based on the thickness of the proximal patella tendon. Patella tendinopathy is the most common tendinopathy in skeletally mature athletes and one of the most common athletic injuries of the knee, with a reported career prevalence of 22%.20 It is described as an overuse injury due to the cumulative effect of micro trauma without an adequate healing interval. While it remains a clinical diagnosis, patellar tendinopathy often shows radiographic findings best assessed on sagittal MRIs. In general, the normal patella tendon appears as a homogenous low-intensity structure and is of uniform thickness. A tendon affected with tendinopathy typically demonstrates a focal increase in signal on T2-weighted sequences just distal to the tendon origin on the inferior pole of the patella. In addition, the patella tendon will usually demonstrate thickening, primarily in the proximal medial and posterior fibers. Patella marrow changes and indistinct tendon margins can also be present. The sensitivity and specificity of diagnosing patellar tendinopathy on MRI are 78% and 86%, respectively.20 We derived our criteria for MRI evidence suggestive of patella tendinopathy from studies by El-Khoury and colleagues,8 Johnson and colleagues,9 and Popp and colleagues.10 In a 1992 study, El-Khoury and colleagues8 compared MRI findings between a group of patients with a clinical diagnosis of patella tendonitis and a control group without knee complaints. The authors found that the average proximal patella tendon diameter in the control group was 3.7 mm while the average proximal patella tendon diameter in the patella tendinopathy group was 10.9 mm; no patella tendons in the control group were >7 mm.8 In a 1996 study, Johnson and colleagues9 determined that the most reliable MRI finding for patients with patellar tendonitis is significant thickening of the proximal patella tendon seen on the sagittal view. The average thickness in symptomatic patients was 8.5 mm (range, 5-15 mm). The average thickness in the control group was 5.5 mm. None of the control patients had a proximal tendon thickness >7 mm.9 Finally, Popp and colleagues10 reviewed the MRI of 11 knees of patients who underwent surgical débridement of chronic patellar tendonitis and reported an average proximal patella tendon thickness of 12 mm (range, 9-16 mm). We therefore used a proximal patella tendon thickness of >7 mm on the sagittal view as a radiographic finding suggestive of patella tendinopathy. No data regarding symptoms of anterior knee pain were available among our patients. Histological studies of patients with patella tendonitis have shown evidence of chronic inflammation, fibrinoid necrosis, mucoid degeneration, and synovial proliferation within the patella tendon insertion.21 Although no controlled data showing that patella tendons with a history of tendonitis are more prone to failure than those without such history when used as an autograft for ACL reconstruction, the idea of utilizing a diseased tendon for a graft is not ideal. Some surgeons question their patients regarding a history of anterior knee pain and will not use a pBTB autograft in a patient with a positive history.22
Continue to: The goal of this study is to obtain epidemiological evidence...
The goal of this study is to obtain epidemiological evidence of the prevalence of existing extensor mechanism pathologies in patients with acute ACL ruptures and determine how these pathologies may relate to the choice of graft. Out of 197 patients studied, over 10% presented with radiographic evidence of pathologies that could influence the choice of graft. This prevalence is certainly significant enough for surgeons to consider including a radiographic evaluation of the extensor mechanism in their standard ACL rupture work-up.
This study presents obvious limitations. While we report the prevalence of some extensor mechanism pathologies, no definitive evidence that recommends against the use of these autografts from these affected individuals has yet been published. In addition, our diagnosis of patella tendinopathy is based solely on MRI findings with no information regarding clinical symptoms. This limitation is a weakness as several additional studies have questioned the validity of a 7 mm proximal patella tendon thickness.23,24 Furthermore, no studies demonstrating the inferior strength of autografts with the co-existing findings described in our work have yet been performed.
CONCLUSION
We found that 10% of active-duty patients presenting for ACL reconstruction demonstrated radiographic evidence of an extensor mechanism pathology that could affect the harvesting of or integrity of select autografts. Given the recent trend of functionally equivocal results in ACL reconstructions utilizing a variety of grafts, this information could and should influence surgical recommendations for graft utilization to obtain optimal surgical results.
1. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321-2328. doi:10.2106/JBJS.H.00539.
2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135-2142. doi:10.1056/NEJMcp0804745.
3. Fu FH, Bennett CH, Lattermann CL, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27(6):821-830. doi:10.1177/03635465990270062501.
4. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft Versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(2):492-499. doi:10.1177/0363546513497566.
5. Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):214-220. doi:10.1177/03635465020300021201.
6. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: Allograft versus allograft. Arthroscopy. 2005;21(7):774-785. doi:10.1016/j.arthro.2005.04.112.
7. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):292-298. doi:10.1016/j.arthro.2007.08.029.
8. El-Khoury GY, Wira RL, Berbaum KS, Pope TL, Monu JUV. MR imaging of patellar tendinitis. Radiology. 1992;184(3):849-854. doi:10.1148/radiology.184.3.1509078.
9. Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Joint Surg Br. 1996;78(3):452-457. doi:10.1302/0301-620X.78B3.0780452.
10. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218-222. doi:10.1177/036354659702500214.
11. Provencher MT, Ryu JH, Gaston T, Dewing CB. Technique: bone-patellar tendon-bone autograft ACL reconstruction in the young, active patient. J Knee Surg. 2011;24(2):83-92. doi:10.1055/s-0031-1280875.
12. Fu F, Cohen S. Current Concepts in ACL Reconstruction. Thorofare: SLACK Incorporated; 2008.
13. Cosgarea AJ, Weng MS, Andrews M. Osgood Schlatter’s disease complicating anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(6):700-703. doi:10.1016/S0749-8063(05)80511-0.
14. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855. doi:10.1007/s11999-008-0367-4.
15. Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252-254. doi:10.1016/0749-8063(95)90078-0.
16. Scully WF, Wilson DJ, Arrington ED. “Central” quadriceps tendon harvest with patellar bone plug: surgical technique revisited. Arthrosc Tech. 2013;2(4):e427-e432.
17. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236-241. doi:10.1177/036354658501300404.
18. Pihlajamäki HK, Visuri TI. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 Pt 2):258-264. doi:10.2106/JBJS.J.00450.
19. Weiss JM, Jordan SS, Andersen JS, Lee BM, Kocher M. Surgical treatment of unresolved Osgood-Schlatter disease: ossicle resection with tibial tubercleplasty. J Pediatr Orthop. 2007;27(7):844-847. doi:10.1097/BPO.0b013e318155849b.
20. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee Among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561-567. doi:10.1177/0363546504270454.
21. O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725-739, vii. doi:10.1016/j.mric.2009.06.010.
22. Martens M, Wouters P, Burssens A, Mulier JC. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand. 1982;53(3):445-450. doi:10.3109/17453678208992239.
23. Shalaby M, Almekinders LC. Patellar tendinitis: the significance of magnetic resonance imaging findings. Am J Sports Med. 1999;27(3):345-349. doi:10.1177/03635465990270031301.
24. Reiff DB, Heenan SD, Heron CW. MRI appearances of the asymptomatic patellar tendon on gradient echo imaging. Skeletal Radiol. 1995;24(2):123-126. doi:10.1007/BF00198074.
1. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321-2328. doi:10.2106/JBJS.H.00539.
2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135-2142. doi:10.1056/NEJMcp0804745.
3. Fu FH, Bennett CH, Lattermann CL, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27(6):821-830. doi:10.1177/03635465990270062501.
4. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft Versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(2):492-499. doi:10.1177/0363546513497566.
5. Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):214-220. doi:10.1177/03635465020300021201.
6. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: Allograft versus allograft. Arthroscopy. 2005;21(7):774-785. doi:10.1016/j.arthro.2005.04.112.
7. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):292-298. doi:10.1016/j.arthro.2007.08.029.
8. El-Khoury GY, Wira RL, Berbaum KS, Pope TL, Monu JUV. MR imaging of patellar tendinitis. Radiology. 1992;184(3):849-854. doi:10.1148/radiology.184.3.1509078.
9. Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Joint Surg Br. 1996;78(3):452-457. doi:10.1302/0301-620X.78B3.0780452.
10. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218-222. doi:10.1177/036354659702500214.
11. Provencher MT, Ryu JH, Gaston T, Dewing CB. Technique: bone-patellar tendon-bone autograft ACL reconstruction in the young, active patient. J Knee Surg. 2011;24(2):83-92. doi:10.1055/s-0031-1280875.
12. Fu F, Cohen S. Current Concepts in ACL Reconstruction. Thorofare: SLACK Incorporated; 2008.
13. Cosgarea AJ, Weng MS, Andrews M. Osgood Schlatter’s disease complicating anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(6):700-703. doi:10.1016/S0749-8063(05)80511-0.
14. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855. doi:10.1007/s11999-008-0367-4.
15. Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252-254. doi:10.1016/0749-8063(95)90078-0.
16. Scully WF, Wilson DJ, Arrington ED. “Central” quadriceps tendon harvest with patellar bone plug: surgical technique revisited. Arthrosc Tech. 2013;2(4):e427-e432.
17. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236-241. doi:10.1177/036354658501300404.
18. Pihlajamäki HK, Visuri TI. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 Pt 2):258-264. doi:10.2106/JBJS.J.00450.
19. Weiss JM, Jordan SS, Andersen JS, Lee BM, Kocher M. Surgical treatment of unresolved Osgood-Schlatter disease: ossicle resection with tibial tubercleplasty. J Pediatr Orthop. 2007;27(7):844-847. doi:10.1097/BPO.0b013e318155849b.
20. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee Among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561-567. doi:10.1177/0363546504270454.
21. O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725-739, vii. doi:10.1016/j.mric.2009.06.010.
22. Martens M, Wouters P, Burssens A, Mulier JC. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand. 1982;53(3):445-450. doi:10.3109/17453678208992239.
23. Shalaby M, Almekinders LC. Patellar tendinitis: the significance of magnetic resonance imaging findings. Am J Sports Med. 1999;27(3):345-349. doi:10.1177/03635465990270031301.
24. Reiff DB, Heenan SD, Heron CW. MRI appearances of the asymptomatic patellar tendon on gradient echo imaging. Skeletal Radiol. 1995;24(2):123-126. doi:10.1007/BF00198074.
TAKE-HOME POINTS
- Extensor mechanism pathology is a common finding in patients with ACL injuries.
- Extensor mechanism pathology such as a multipartite patella, unresolved Osgood-Schlatter’s disease, and patella tendinopathy are easily identifiable on standard imaging.
- It is unknown what type of effect, if any, these pathologies may have on graft strength.
- The bone-patella tendon-bone and quadriceps autograft are the most likely to be affected.
- Surgeons should take into account existing extensor mechanism pathology when considering individual patient graft selection for ACL reconstruction.
Headless Compression Screw Fixation of Vertical Medial Malleolus Fractures is Superior to Unicortical Screw Fixation
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.
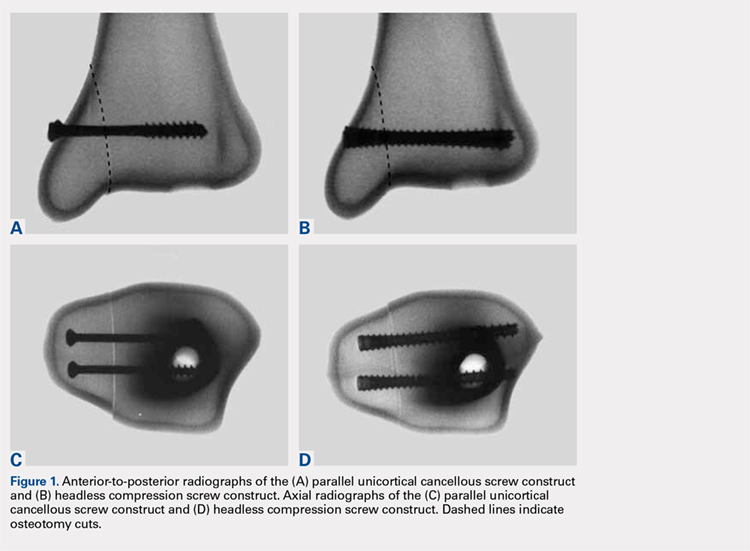
Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.
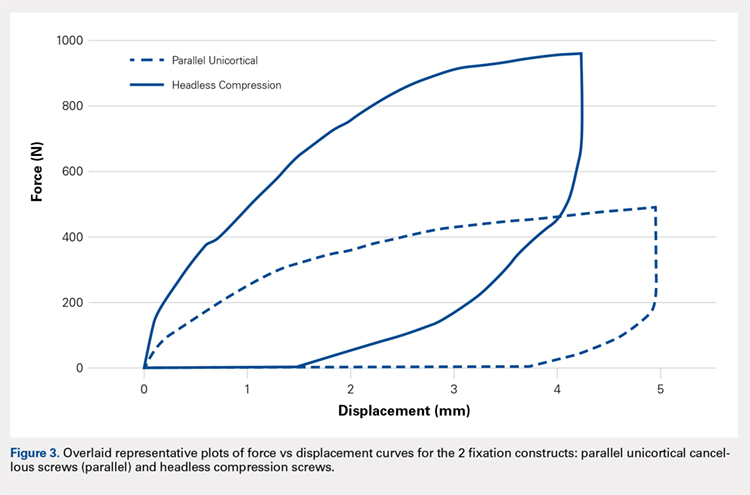
RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.
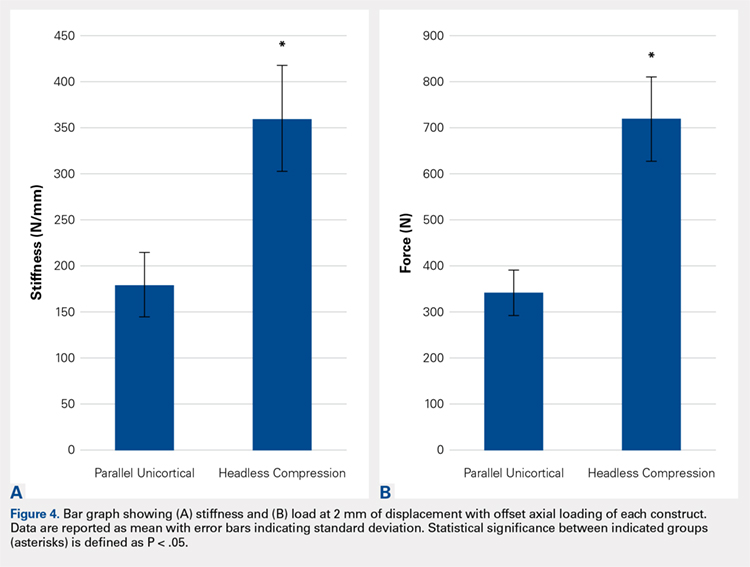
Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).
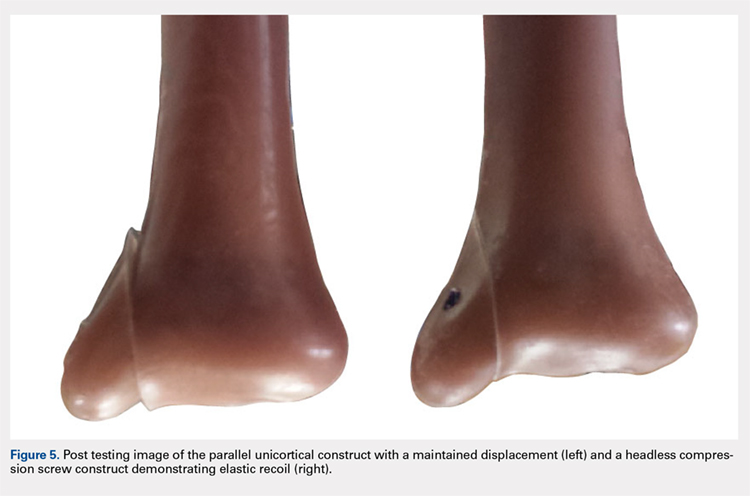
Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.

Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.

RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.

Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).

Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.

Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.

RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.

Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).

Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
TAKE-HOME POINTS
- Optimal fixation of vertical sheer ankle fractures is unknown.
- Headless compression screws are stiffer than cancellous screws in offset axial load.
- Headless compression screws have a higher load to failure than cancellous screws.
- Headless compression screws may offer a soft tissue friendly fixation of method for vertical sheer ankle fractures.
- These findings may not apply when subject to cyclic loads or in osteoporotic bone.
High Body Mass Index is Related to Increased Perioperative Complications After Periacetabular Osteotomy
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1
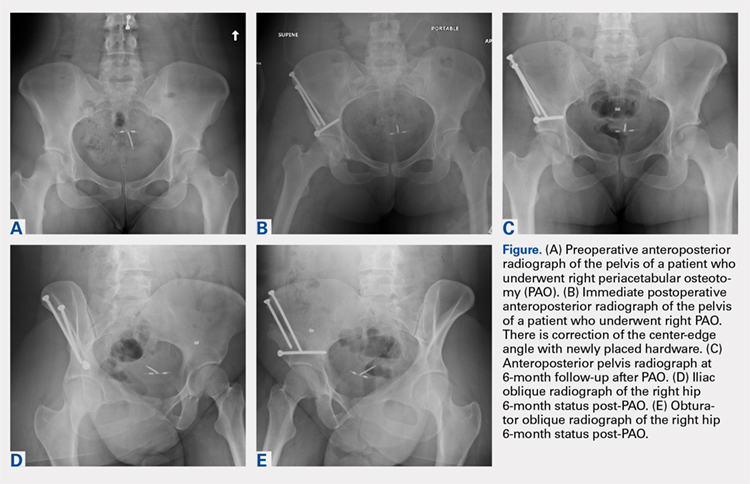
There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1

There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1

There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
TAKE-HOME POINTS
- PAO is an effective procedure to treat symptomatic hip dysplasia in patients without degenerative changes.
- The postoperative correction of dysplasia as measured by center-edge angles were similar in low and high BMI groups.
- Patients with obesity (BMI >30) have a higher incidence of postoperative complications following PAO.
- There were too few patients with diabetes or smoking to determine a significantly increased rate of complications. However, we believe based on the literature these patient populations are at higher risk for complications in the early postoperative period.
- Patients with BMI >30 can have a successful outcome with a PAO procedure. However, this patient population should have counseling about their increased risk of complications, and be given opportunity to lose weight when possible preoperatively.
Screw Fixation Without Bone Grafting for Delayed Unions and Nonunions of Minimally Displaced Scaphoids
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.
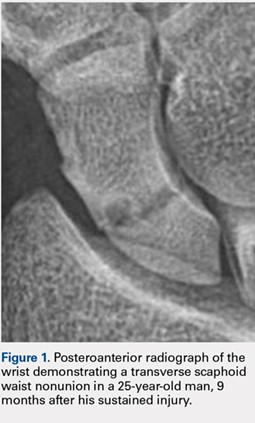
Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.
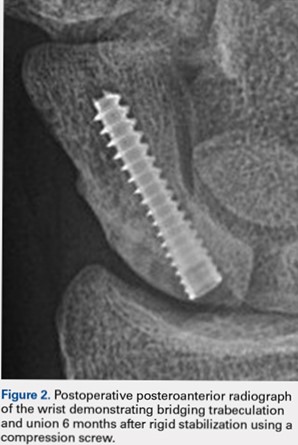
DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.

Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.

DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.

Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.

DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
TAKE-HOME POINTS
- Scaphoid nonunions can occur in minimally displaced fractures.
- If there is no deformity of the scaphoid delayed or nonunion, then a percutaneous screw fixation without bone grafting can reliably lead to bony union.
- Not all scaphoid delayed unions and nonunions require bone grafting.