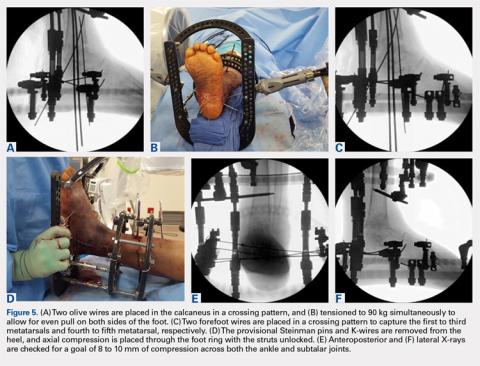
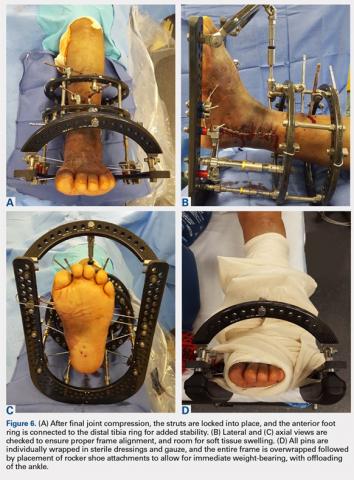
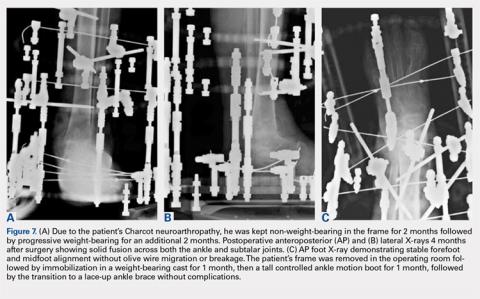
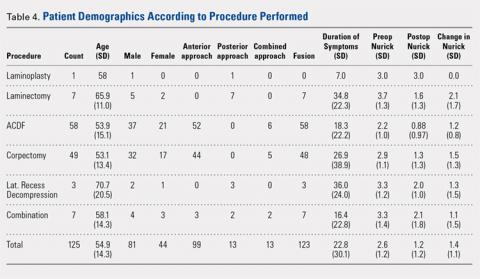
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Composite Fixation of Proximal Tibial Nonunions: A Technical Trick
ABSTRACT
Nonunion after a proximal tibia fracture is often associated with poor bone stock, (previous) infection, and compromised soft tissues. These conditions make revision internal fixation with double plating difficult. Combining a plate and contralateral 2-pin external fixator, coined composite fixation, can provide an alternative means of obtaining stability without further compromising soft tissues.
Three patients with a proximal tibia nonunion precluding standard internal fixation with double plating were treated with composite fixation. All 3 patients achieved union with deformity correction at a mean of 5.2 months (range, 5-5.5 months). The average range of motion (ROM) arc was 100° (range, 100°-115°) and postoperative ROM returned to pre-injury levels.
Composite fixation can be a helpful adjunct in the treatment of this challenging problem.
Continue to: Operative management of a proximal tibial nonunion...
Operative management of a proximal tibial nonunion is challenging, compromised by limited bone stock, pre-existing hardware, stiffness, poor soft tissue conditions, and infection. The goals of treatment include bone union, re-establishment of both joint stability and lower extremity alignment, restoration of an anatomic articular surface, and recovery of function.1 Currently, various treatment options such as plate fixation, bone grafting, intramedullary nailing, external fixation, functional bracing, or a combination of these are available.1-8 Rigid internal fixation is the gold standard for most nonunions. However, sometimes local soft tissues or bone quality preclude standard internal fixation. Bolhofner9 described the combination of a single plate and an external fixator on the contralateral side for the management of extra-articular proximal tibial fractures with compromised soft tissues, and the technique known as composite fixation was coined. The external fixator on 1 side and the plate on the other, generate a balanced, stable environment while limiting the use of foreign hardware, thereby avoiding both additional soft-tissue damage and periosteal stripping.9-11 In this technical article, we describe the indication, technique, and outcomes of 3 patients with proximal tibial nonunions, who were successfully treated with composite fixation.
MATERIALS AND METHODS
PATIENTS
Between January 2014 and July 2016, 3 patients each with a proximal tibial nonunion that developed after a bicondylar tibial plateau fracture (Schatzker type VI) were treated with composite fixation (Table). The 3 patients were female with an average age of 61 years (range, 60-62 years), and a body mass index of 23.7 kg/m2 (range, 19.0-31.9 kg/m2). All 3 patients had sustained a tibial plateau fracture that was primarily treated with open reduction and internal fixation. Two of them had a diagnosis of rheumatoid arthritis and were being treated with methotrexate and Humira (adalimumab) (case 1), and with methotrexate, prednisolone, and etanercept (case 3). The etanercept was discontinued after discussion with the treating rheumatologist when a deep infection developed. Two patients (cases 1 and 2) were referred to us because of their nonunions. All 3 patients developed extra-articular nonunions with compromised bone stock. Two patients had developed deep infections during treatment of their plateau fractures; 1 of these patients underwent a medial gastrocnemius flap for wound coverage (case 1). The second patient (case 3) with a deep infection underwent partial hardware removal, a Masquelet salvage procedure, and revision plate fixation. However, the infection recurred. The hardware was removed, and 2 débridements with conversion to a hybrid external fixator with thin wire fixation were done. Due to her longstanding rheumatoid arthritis, the patient had bilateral valgus knee malalignment causing the ring fixator to strike her contralateral knee when she walked. The period from the initial tibial plateau fracture to our composite fixation averaged 11.3 months (range, 11-12 months). Indications for the use of the composite fixation comprised previously infected soft tissue on the lateral side and inability to walk with a hybrid thin wire fixator because of valgus knees (case 3), a medial gastrocnemius flap (case 2), and poor bone quality (case 1). Follow-up consisted of clinical examination, Timed Up and Go (TUG) test that is a standardized test for mobility, and radiographic evaluation at routine appointments up to 1 year or until healed.12 At the last follow-up visit, patients filled out the International Knee Documentation Committee (IKDC) subjective knee form.13
SURGICAL TECHNIQUE
A fellowship-trained orthopedic trauma surgeon treated all patients. Patients were placed on a radiolucent operating table after general or regional anesthesia. Previous incisions were used. Two patients had a midline incision; the third had both a posteromedial and an anterolateral incision. Five deep tissue cultures were taken after which antibiotics were given intravenously. All unstable or failed hardware was removed. Aggressive débridement of the nonunion was performed. After débridement, multiple holes were drilled with a 2.0 mm drill bit until blood was seen to egress from both sides of the medullary canal. Malalignment of the proximal tibia was corrected and checked fluoroscopically. Fixation was done with an anatomic locking plate (LCP Proximal Tibia Plate 3.5; DePuy Synthes) with a mixture of locking and non-locking screws. In 2 patients, a tricortical graft from the posterior iliac crest was positioned in the defect. Additional autologous bone graft and demineralized bone matrix was added around the nonunion. Although locking screws were used, the fixation did not appear to be strong enough to resist the varus (cases 1 and 2), or the valgus (case 3) deforming forces. Additional fixation was thus needed. However, the contralateral soft tissues were compromised in case 2 (medial gastrocnemius flap), and case 3 (a previously infected area with very tenuous skin laterally), whereas the bone was considered to be of insufficient quality in case 1. The opposite side of the nonunion was stabilized using composite fixation with a 2-pin external fixator to circumvent the need for additional plate fixation. In 2 patients, the plate was placed laterally, and the external fixator medially. In the third patient, the plate was positioned medially, and the external fixator laterally. The plate was always placed first. The external fixator was placed last. Using fluoroscopy, we ensured that the fixator pins would not interfere with the screws. The pins were predrilled and positioned perpendicular to the tibia through small stab incisions. We prefer hydroxyapatite-coated pins (6-mm diameter, XCaliber Bone Screws; Pro-Motion Medical) to increase their holding power in the often osteopenic bone. Postoperative management consisted of toe-touch weight-bearing for 6 weeks and progressed to full weight-bearing at 3 months. Radiographs were taken on postoperative day 1, at 6 weeks, and at 12 weeks until healed. No continuous passive motion was used postoperatively. Antibiotics were continued until cultures were negative. No specific pin care was used. We advised patients to shower daily with the external fixator in place, once the wounds have healed.
Continue to: RESULTS...
RESULTS
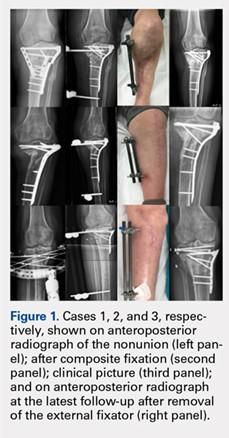
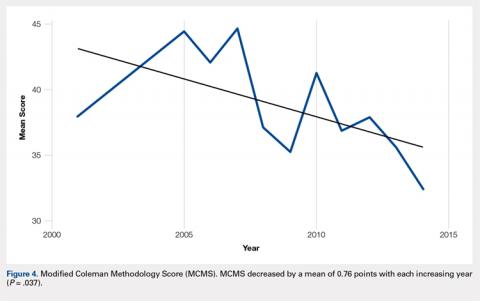
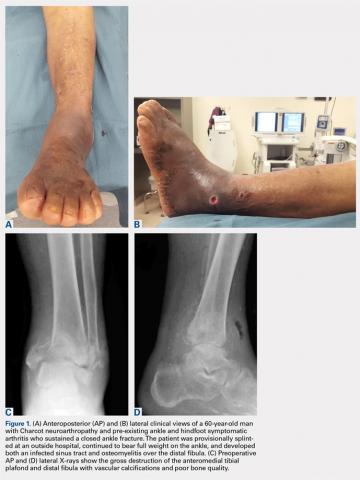
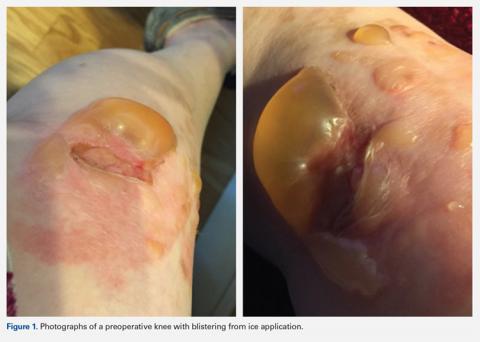
On average, patients were hospitalized for 5 days (range, 3-7 days). There were no postoperative complications. None of the patients developed a clinically significant pin site infection. There were no re-operations during follow-up. All patients achieved union at a mean of 5.2 months (range, 5-5.5 months) (Figure 1).
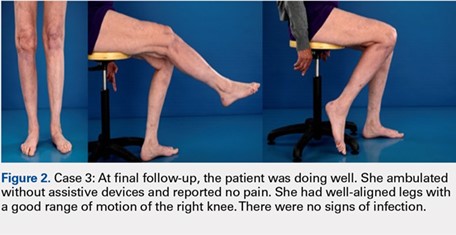
Deformity correction was achieved in all 3 patients. The average range of motion (ROM) arc was 100° (range, 100°-115°). None of the patients had an extension deficit. TUG test was <8 seconds in all patients. The IKDC knee score averaged 52 (range, 41-66). Of note is that 2 patients already had compromised knee function before the fracture because of rheumatoid arthritis. The Ahlbäck classification of osteoarthritis showed grade 1 in cases 1 and 3, and grade 2 in case 2.14 Postoperative ROM of the knee returned to pre-injury levels in all patients (Figure 2). The 2-pin external fixator was removed at 9 weeks on average (range, 6-12 weeks) postoperatively in the outpatient clinic. At the last follow-up appointment at an average of 10.3 months (range, 9-12 months), all wounds had healed without infection. All patients had a normal neurovascular examination.
DISCUSSION
Nonunion after a proximal tibial fracture is rare.4 In cases when nonunions do develop, they most often pertain to the extra-articular component with the plateau component healed. Surgical exposure for débridements, hardware removal, bone grafting, and revision of fixation carries the risk of wound breakdown, necrosis, and infection. The alternative strategy of composite fixation (a plate combined with a contralateral 2-pin external fixator) to limit additional soft tissue compromise was already described in proximal tibial fractures by Bolhofner.9 He treated 41 extra-articular proximal tibial fractures using this composite fixation technique and attained successful results with an average time to union of 12.1 weeks. There was only 1 malunion, 2 wound infections, and 3 delayed unions.
In our practice, we have extrapolated this idea to an extra-articular nonunion that developed after a tibial plateau fracture. With the use of an external fixator, we provided sufficient mechanical stability of the nonunion without unnecessarily compromising previously infected or tenuous soft tissues, a muscle flap, or further devascularizing poor bone. Limitations of this study include the retrospective data and small sample size prone to bias. However, all patients received the same treatment protocol from 1 orthopedic trauma surgeon, follow-up intervals were similar, and data were acquired consistently.
Meanwhile, we have used this technique in a fourth patient with a septic nonunion of a tibial plateau fracture. All 4 patients in whom we have used this method so far have healed successfully.
CONCLUSION
This technique respects both the demand for minimal soft tissue damage and a maximal stable environment without notable perioperative and postoperative complications. It also offers an alternative option for the treatment of a proximal tibial nonunion that is not amenable to invasive revision dual plate fixation. As such, it can be a useful addition to the existing armamentarium of the treating surgeon.
1. Wu CC. Salvage of proximal tibial malunion or nonunion with the use of angled blade plate. Arch Orthop Trauma Surg. 2006;126(2):82-87. doi:10.1007/s00402-006-0106-9.
2. Carpenter CA, Jupiter JB. Blade plate reconstruction of metaphyseal nonunion of the tibia. Clin Orthop Relat Res. 1996;332:23-28.
3. Gardner MJ, Toro-Arbelaez JB, Hansen M, Boraiah S, Lorich DG, Helfet DL. Surgical treatment and outcomes of extraarticular proximal tibial nonunions. Arch Orthop Trauma Surg. 2008;128(8):833-839. doi:10.1007/s00402-007-0383-y.
4. Toro-Arbelaez JB, Gardner MJ, Shindle MK, Cabas JM, Lorich DG, Helfet DL. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383. doi:10.1016/j.injury.2006.11.003.
5. Mechrefe AP, Koh EY, Trafton PG, DiGiovanni CW. Tibial nonunion. Foot Ankle Clin. 2006;11(1):1-18, vii. doi:10.1016/j.fcl.2005.12.003.
6. Chin KR, Nagarkatti DG, Miranda MA, Santoro VM, Baumgaertner MR, Jupiter JB. Salvage of distal tibia metaphyseal nonunions with the 90 degrees cannulated blade plate. Clin Orthop Relat Res. 2003;(409):241-249.
7. Devgan A, Kamboj P, Gupta V, Magu NK, Rohilla R. Pseudoarthrosis of medial tibial plateau fracture-role of alignment procedure. Chin J Traumatol. 2013;16(2):118-121. doi:10.3760/cma.j.issn.1008-1275.2013.02.011.
8. Helfet DL, Jupiter JB, Gasser S. Indirect reduction and tension-band plating of tibial non-union with deformity. J Bone Joint Surg Am. 1992;74(9):1286-1297.
9. Bolhofner BR. Indirect reduction and composite fixation of extraarticular proximal tibial fractures. Clin Orthop Relat Res. 1995;(315):75-83. doi:10.1097/00003086-199506000-00009.
10. Ries MD, Meinhard BP. Medial external fixation with lateral plate internal fixation in metaphyseal tibia fractures. A report of eight cases associated with severe soft-tissue injury. Clin Orthop Relat Res. 1988;(256):215-223.
11. Weiner LS, Kelley M, Yang E, et al. The use of combination internal fixation and hybrid external fixation in severe proximal tibia fractures. J Orthop Trauma. 1995;9(3):244-250.
12. Alghadir A, Anwer S, Brismee JM. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:174. doi:10.1186/s12891-015-0637-8.
13. Haverkamp D, Sierevelt IN, Breugem SJ, Lohuis K, Blankevoort L, van Dijk CN. Translation and validation of the Dutch version of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1680-1684. doi:10.1177/0363546506288854.
14. Ahlbäck S. Osteoartrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh). 1968;Suppl 277:7-72.
ABSTRACT
Nonunion after a proximal tibia fracture is often associated with poor bone stock, (previous) infection, and compromised soft tissues. These conditions make revision internal fixation with double plating difficult. Combining a plate and contralateral 2-pin external fixator, coined composite fixation, can provide an alternative means of obtaining stability without further compromising soft tissues.
Three patients with a proximal tibia nonunion precluding standard internal fixation with double plating were treated with composite fixation. All 3 patients achieved union with deformity correction at a mean of 5.2 months (range, 5-5.5 months). The average range of motion (ROM) arc was 100° (range, 100°-115°) and postoperative ROM returned to pre-injury levels.
Composite fixation can be a helpful adjunct in the treatment of this challenging problem.
Continue to: Operative management of a proximal tibial nonunion...
Operative management of a proximal tibial nonunion is challenging, compromised by limited bone stock, pre-existing hardware, stiffness, poor soft tissue conditions, and infection. The goals of treatment include bone union, re-establishment of both joint stability and lower extremity alignment, restoration of an anatomic articular surface, and recovery of function.1 Currently, various treatment options such as plate fixation, bone grafting, intramedullary nailing, external fixation, functional bracing, or a combination of these are available.1-8 Rigid internal fixation is the gold standard for most nonunions. However, sometimes local soft tissues or bone quality preclude standard internal fixation. Bolhofner9 described the combination of a single plate and an external fixator on the contralateral side for the management of extra-articular proximal tibial fractures with compromised soft tissues, and the technique known as composite fixation was coined. The external fixator on 1 side and the plate on the other, generate a balanced, stable environment while limiting the use of foreign hardware, thereby avoiding both additional soft-tissue damage and periosteal stripping.9-11 In this technical article, we describe the indication, technique, and outcomes of 3 patients with proximal tibial nonunions, who were successfully treated with composite fixation.
MATERIALS AND METHODS
PATIENTS
Between January 2014 and July 2016, 3 patients each with a proximal tibial nonunion that developed after a bicondylar tibial plateau fracture (Schatzker type VI) were treated with composite fixation (Table). The 3 patients were female with an average age of 61 years (range, 60-62 years), and a body mass index of 23.7 kg/m2 (range, 19.0-31.9 kg/m2). All 3 patients had sustained a tibial plateau fracture that was primarily treated with open reduction and internal fixation. Two of them had a diagnosis of rheumatoid arthritis and were being treated with methotrexate and Humira (adalimumab) (case 1), and with methotrexate, prednisolone, and etanercept (case 3). The etanercept was discontinued after discussion with the treating rheumatologist when a deep infection developed. Two patients (cases 1 and 2) were referred to us because of their nonunions. All 3 patients developed extra-articular nonunions with compromised bone stock. Two patients had developed deep infections during treatment of their plateau fractures; 1 of these patients underwent a medial gastrocnemius flap for wound coverage (case 1). The second patient (case 3) with a deep infection underwent partial hardware removal, a Masquelet salvage procedure, and revision plate fixation. However, the infection recurred. The hardware was removed, and 2 débridements with conversion to a hybrid external fixator with thin wire fixation were done. Due to her longstanding rheumatoid arthritis, the patient had bilateral valgus knee malalignment causing the ring fixator to strike her contralateral knee when she walked. The period from the initial tibial plateau fracture to our composite fixation averaged 11.3 months (range, 11-12 months). Indications for the use of the composite fixation comprised previously infected soft tissue on the lateral side and inability to walk with a hybrid thin wire fixator because of valgus knees (case 3), a medial gastrocnemius flap (case 2), and poor bone quality (case 1). Follow-up consisted of clinical examination, Timed Up and Go (TUG) test that is a standardized test for mobility, and radiographic evaluation at routine appointments up to 1 year or until healed.12 At the last follow-up visit, patients filled out the International Knee Documentation Committee (IKDC) subjective knee form.13

SURGICAL TECHNIQUE
A fellowship-trained orthopedic trauma surgeon treated all patients. Patients were placed on a radiolucent operating table after general or regional anesthesia. Previous incisions were used. Two patients had a midline incision; the third had both a posteromedial and an anterolateral incision. Five deep tissue cultures were taken after which antibiotics were given intravenously. All unstable or failed hardware was removed. Aggressive débridement of the nonunion was performed. After débridement, multiple holes were drilled with a 2.0 mm drill bit until blood was seen to egress from both sides of the medullary canal. Malalignment of the proximal tibia was corrected and checked fluoroscopically. Fixation was done with an anatomic locking plate (LCP Proximal Tibia Plate 3.5; DePuy Synthes) with a mixture of locking and non-locking screws. In 2 patients, a tricortical graft from the posterior iliac crest was positioned in the defect. Additional autologous bone graft and demineralized bone matrix was added around the nonunion. Although locking screws were used, the fixation did not appear to be strong enough to resist the varus (cases 1 and 2), or the valgus (case 3) deforming forces. Additional fixation was thus needed. However, the contralateral soft tissues were compromised in case 2 (medial gastrocnemius flap), and case 3 (a previously infected area with very tenuous skin laterally), whereas the bone was considered to be of insufficient quality in case 1. The opposite side of the nonunion was stabilized using composite fixation with a 2-pin external fixator to circumvent the need for additional plate fixation. In 2 patients, the plate was placed laterally, and the external fixator medially. In the third patient, the plate was positioned medially, and the external fixator laterally. The plate was always placed first. The external fixator was placed last. Using fluoroscopy, we ensured that the fixator pins would not interfere with the screws. The pins were predrilled and positioned perpendicular to the tibia through small stab incisions. We prefer hydroxyapatite-coated pins (6-mm diameter, XCaliber Bone Screws; Pro-Motion Medical) to increase their holding power in the often osteopenic bone. Postoperative management consisted of toe-touch weight-bearing for 6 weeks and progressed to full weight-bearing at 3 months. Radiographs were taken on postoperative day 1, at 6 weeks, and at 12 weeks until healed. No continuous passive motion was used postoperatively. Antibiotics were continued until cultures were negative. No specific pin care was used. We advised patients to shower daily with the external fixator in place, once the wounds have healed.
Continue to: RESULTS...
RESULTS
On average, patients were hospitalized for 5 days (range, 3-7 days). There were no postoperative complications. None of the patients developed a clinically significant pin site infection. There were no re-operations during follow-up. All patients achieved union at a mean of 5.2 months (range, 5-5.5 months) (Figure 1).
Deformity correction was achieved in all 3 patients. The average range of motion (ROM) arc was 100° (range, 100°-115°). None of the patients had an extension deficit. TUG test was <8 seconds in all patients. The IKDC knee score averaged 52 (range, 41-66). Of note is that 2 patients already had compromised knee function before the fracture because of rheumatoid arthritis. The Ahlbäck classification of osteoarthritis showed grade 1 in cases 1 and 3, and grade 2 in case 2.14 Postoperative ROM of the knee returned to pre-injury levels in all patients (Figure 2). The 2-pin external fixator was removed at 9 weeks on average (range, 6-12 weeks) postoperatively in the outpatient clinic. At the last follow-up appointment at an average of 10.3 months (range, 9-12 months), all wounds had healed without infection. All patients had a normal neurovascular examination.

DISCUSSION
Nonunion after a proximal tibial fracture is rare.4 In cases when nonunions do develop, they most often pertain to the extra-articular component with the plateau component healed. Surgical exposure for débridements, hardware removal, bone grafting, and revision of fixation carries the risk of wound breakdown, necrosis, and infection. The alternative strategy of composite fixation (a plate combined with a contralateral 2-pin external fixator) to limit additional soft tissue compromise was already described in proximal tibial fractures by Bolhofner.9 He treated 41 extra-articular proximal tibial fractures using this composite fixation technique and attained successful results with an average time to union of 12.1 weeks. There was only 1 malunion, 2 wound infections, and 3 delayed unions.
In our practice, we have extrapolated this idea to an extra-articular nonunion that developed after a tibial plateau fracture. With the use of an external fixator, we provided sufficient mechanical stability of the nonunion without unnecessarily compromising previously infected or tenuous soft tissues, a muscle flap, or further devascularizing poor bone. Limitations of this study include the retrospective data and small sample size prone to bias. However, all patients received the same treatment protocol from 1 orthopedic trauma surgeon, follow-up intervals were similar, and data were acquired consistently.
Meanwhile, we have used this technique in a fourth patient with a septic nonunion of a tibial plateau fracture. All 4 patients in whom we have used this method so far have healed successfully.
CONCLUSION
This technique respects both the demand for minimal soft tissue damage and a maximal stable environment without notable perioperative and postoperative complications. It also offers an alternative option for the treatment of a proximal tibial nonunion that is not amenable to invasive revision dual plate fixation. As such, it can be a useful addition to the existing armamentarium of the treating surgeon.
ABSTRACT
Nonunion after a proximal tibia fracture is often associated with poor bone stock, (previous) infection, and compromised soft tissues. These conditions make revision internal fixation with double plating difficult. Combining a plate and contralateral 2-pin external fixator, coined composite fixation, can provide an alternative means of obtaining stability without further compromising soft tissues.
Three patients with a proximal tibia nonunion precluding standard internal fixation with double plating were treated with composite fixation. All 3 patients achieved union with deformity correction at a mean of 5.2 months (range, 5-5.5 months). The average range of motion (ROM) arc was 100° (range, 100°-115°) and postoperative ROM returned to pre-injury levels.
Composite fixation can be a helpful adjunct in the treatment of this challenging problem.
Continue to: Operative management of a proximal tibial nonunion...
Operative management of a proximal tibial nonunion is challenging, compromised by limited bone stock, pre-existing hardware, stiffness, poor soft tissue conditions, and infection. The goals of treatment include bone union, re-establishment of both joint stability and lower extremity alignment, restoration of an anatomic articular surface, and recovery of function.1 Currently, various treatment options such as plate fixation, bone grafting, intramedullary nailing, external fixation, functional bracing, or a combination of these are available.1-8 Rigid internal fixation is the gold standard for most nonunions. However, sometimes local soft tissues or bone quality preclude standard internal fixation. Bolhofner9 described the combination of a single plate and an external fixator on the contralateral side for the management of extra-articular proximal tibial fractures with compromised soft tissues, and the technique known as composite fixation was coined. The external fixator on 1 side and the plate on the other, generate a balanced, stable environment while limiting the use of foreign hardware, thereby avoiding both additional soft-tissue damage and periosteal stripping.9-11 In this technical article, we describe the indication, technique, and outcomes of 3 patients with proximal tibial nonunions, who were successfully treated with composite fixation.
MATERIALS AND METHODS
PATIENTS
Between January 2014 and July 2016, 3 patients each with a proximal tibial nonunion that developed after a bicondylar tibial plateau fracture (Schatzker type VI) were treated with composite fixation (Table). The 3 patients were female with an average age of 61 years (range, 60-62 years), and a body mass index of 23.7 kg/m2 (range, 19.0-31.9 kg/m2). All 3 patients had sustained a tibial plateau fracture that was primarily treated with open reduction and internal fixation. Two of them had a diagnosis of rheumatoid arthritis and were being treated with methotrexate and Humira (adalimumab) (case 1), and with methotrexate, prednisolone, and etanercept (case 3). The etanercept was discontinued after discussion with the treating rheumatologist when a deep infection developed. Two patients (cases 1 and 2) were referred to us because of their nonunions. All 3 patients developed extra-articular nonunions with compromised bone stock. Two patients had developed deep infections during treatment of their plateau fractures; 1 of these patients underwent a medial gastrocnemius flap for wound coverage (case 1). The second patient (case 3) with a deep infection underwent partial hardware removal, a Masquelet salvage procedure, and revision plate fixation. However, the infection recurred. The hardware was removed, and 2 débridements with conversion to a hybrid external fixator with thin wire fixation were done. Due to her longstanding rheumatoid arthritis, the patient had bilateral valgus knee malalignment causing the ring fixator to strike her contralateral knee when she walked. The period from the initial tibial plateau fracture to our composite fixation averaged 11.3 months (range, 11-12 months). Indications for the use of the composite fixation comprised previously infected soft tissue on the lateral side and inability to walk with a hybrid thin wire fixator because of valgus knees (case 3), a medial gastrocnemius flap (case 2), and poor bone quality (case 1). Follow-up consisted of clinical examination, Timed Up and Go (TUG) test that is a standardized test for mobility, and radiographic evaluation at routine appointments up to 1 year or until healed.12 At the last follow-up visit, patients filled out the International Knee Documentation Committee (IKDC) subjective knee form.13

SURGICAL TECHNIQUE
A fellowship-trained orthopedic trauma surgeon treated all patients. Patients were placed on a radiolucent operating table after general or regional anesthesia. Previous incisions were used. Two patients had a midline incision; the third had both a posteromedial and an anterolateral incision. Five deep tissue cultures were taken after which antibiotics were given intravenously. All unstable or failed hardware was removed. Aggressive débridement of the nonunion was performed. After débridement, multiple holes were drilled with a 2.0 mm drill bit until blood was seen to egress from both sides of the medullary canal. Malalignment of the proximal tibia was corrected and checked fluoroscopically. Fixation was done with an anatomic locking plate (LCP Proximal Tibia Plate 3.5; DePuy Synthes) with a mixture of locking and non-locking screws. In 2 patients, a tricortical graft from the posterior iliac crest was positioned in the defect. Additional autologous bone graft and demineralized bone matrix was added around the nonunion. Although locking screws were used, the fixation did not appear to be strong enough to resist the varus (cases 1 and 2), or the valgus (case 3) deforming forces. Additional fixation was thus needed. However, the contralateral soft tissues were compromised in case 2 (medial gastrocnemius flap), and case 3 (a previously infected area with very tenuous skin laterally), whereas the bone was considered to be of insufficient quality in case 1. The opposite side of the nonunion was stabilized using composite fixation with a 2-pin external fixator to circumvent the need for additional plate fixation. In 2 patients, the plate was placed laterally, and the external fixator medially. In the third patient, the plate was positioned medially, and the external fixator laterally. The plate was always placed first. The external fixator was placed last. Using fluoroscopy, we ensured that the fixator pins would not interfere with the screws. The pins were predrilled and positioned perpendicular to the tibia through small stab incisions. We prefer hydroxyapatite-coated pins (6-mm diameter, XCaliber Bone Screws; Pro-Motion Medical) to increase their holding power in the often osteopenic bone. Postoperative management consisted of toe-touch weight-bearing for 6 weeks and progressed to full weight-bearing at 3 months. Radiographs were taken on postoperative day 1, at 6 weeks, and at 12 weeks until healed. No continuous passive motion was used postoperatively. Antibiotics were continued until cultures were negative. No specific pin care was used. We advised patients to shower daily with the external fixator in place, once the wounds have healed.
Continue to: RESULTS...
RESULTS
On average, patients were hospitalized for 5 days (range, 3-7 days). There were no postoperative complications. None of the patients developed a clinically significant pin site infection. There were no re-operations during follow-up. All patients achieved union at a mean of 5.2 months (range, 5-5.5 months) (Figure 1).
Deformity correction was achieved in all 3 patients. The average range of motion (ROM) arc was 100° (range, 100°-115°). None of the patients had an extension deficit. TUG test was <8 seconds in all patients. The IKDC knee score averaged 52 (range, 41-66). Of note is that 2 patients already had compromised knee function before the fracture because of rheumatoid arthritis. The Ahlbäck classification of osteoarthritis showed grade 1 in cases 1 and 3, and grade 2 in case 2.14 Postoperative ROM of the knee returned to pre-injury levels in all patients (Figure 2). The 2-pin external fixator was removed at 9 weeks on average (range, 6-12 weeks) postoperatively in the outpatient clinic. At the last follow-up appointment at an average of 10.3 months (range, 9-12 months), all wounds had healed without infection. All patients had a normal neurovascular examination.

DISCUSSION
Nonunion after a proximal tibial fracture is rare.4 In cases when nonunions do develop, they most often pertain to the extra-articular component with the plateau component healed. Surgical exposure for débridements, hardware removal, bone grafting, and revision of fixation carries the risk of wound breakdown, necrosis, and infection. The alternative strategy of composite fixation (a plate combined with a contralateral 2-pin external fixator) to limit additional soft tissue compromise was already described in proximal tibial fractures by Bolhofner.9 He treated 41 extra-articular proximal tibial fractures using this composite fixation technique and attained successful results with an average time to union of 12.1 weeks. There was only 1 malunion, 2 wound infections, and 3 delayed unions.
In our practice, we have extrapolated this idea to an extra-articular nonunion that developed after a tibial plateau fracture. With the use of an external fixator, we provided sufficient mechanical stability of the nonunion without unnecessarily compromising previously infected or tenuous soft tissues, a muscle flap, or further devascularizing poor bone. Limitations of this study include the retrospective data and small sample size prone to bias. However, all patients received the same treatment protocol from 1 orthopedic trauma surgeon, follow-up intervals were similar, and data were acquired consistently.
Meanwhile, we have used this technique in a fourth patient with a septic nonunion of a tibial plateau fracture. All 4 patients in whom we have used this method so far have healed successfully.
CONCLUSION
This technique respects both the demand for minimal soft tissue damage and a maximal stable environment without notable perioperative and postoperative complications. It also offers an alternative option for the treatment of a proximal tibial nonunion that is not amenable to invasive revision dual plate fixation. As such, it can be a useful addition to the existing armamentarium of the treating surgeon.
1. Wu CC. Salvage of proximal tibial malunion or nonunion with the use of angled blade plate. Arch Orthop Trauma Surg. 2006;126(2):82-87. doi:10.1007/s00402-006-0106-9.
2. Carpenter CA, Jupiter JB. Blade plate reconstruction of metaphyseal nonunion of the tibia. Clin Orthop Relat Res. 1996;332:23-28.
3. Gardner MJ, Toro-Arbelaez JB, Hansen M, Boraiah S, Lorich DG, Helfet DL. Surgical treatment and outcomes of extraarticular proximal tibial nonunions. Arch Orthop Trauma Surg. 2008;128(8):833-839. doi:10.1007/s00402-007-0383-y.
4. Toro-Arbelaez JB, Gardner MJ, Shindle MK, Cabas JM, Lorich DG, Helfet DL. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383. doi:10.1016/j.injury.2006.11.003.
5. Mechrefe AP, Koh EY, Trafton PG, DiGiovanni CW. Tibial nonunion. Foot Ankle Clin. 2006;11(1):1-18, vii. doi:10.1016/j.fcl.2005.12.003.
6. Chin KR, Nagarkatti DG, Miranda MA, Santoro VM, Baumgaertner MR, Jupiter JB. Salvage of distal tibia metaphyseal nonunions with the 90 degrees cannulated blade plate. Clin Orthop Relat Res. 2003;(409):241-249.
7. Devgan A, Kamboj P, Gupta V, Magu NK, Rohilla R. Pseudoarthrosis of medial tibial plateau fracture-role of alignment procedure. Chin J Traumatol. 2013;16(2):118-121. doi:10.3760/cma.j.issn.1008-1275.2013.02.011.
8. Helfet DL, Jupiter JB, Gasser S. Indirect reduction and tension-band plating of tibial non-union with deformity. J Bone Joint Surg Am. 1992;74(9):1286-1297.
9. Bolhofner BR. Indirect reduction and composite fixation of extraarticular proximal tibial fractures. Clin Orthop Relat Res. 1995;(315):75-83. doi:10.1097/00003086-199506000-00009.
10. Ries MD, Meinhard BP. Medial external fixation with lateral plate internal fixation in metaphyseal tibia fractures. A report of eight cases associated with severe soft-tissue injury. Clin Orthop Relat Res. 1988;(256):215-223.
11. Weiner LS, Kelley M, Yang E, et al. The use of combination internal fixation and hybrid external fixation in severe proximal tibia fractures. J Orthop Trauma. 1995;9(3):244-250.
12. Alghadir A, Anwer S, Brismee JM. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:174. doi:10.1186/s12891-015-0637-8.
13. Haverkamp D, Sierevelt IN, Breugem SJ, Lohuis K, Blankevoort L, van Dijk CN. Translation and validation of the Dutch version of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1680-1684. doi:10.1177/0363546506288854.
14. Ahlbäck S. Osteoartrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh). 1968;Suppl 277:7-72.
1. Wu CC. Salvage of proximal tibial malunion or nonunion with the use of angled blade plate. Arch Orthop Trauma Surg. 2006;126(2):82-87. doi:10.1007/s00402-006-0106-9.
2. Carpenter CA, Jupiter JB. Blade plate reconstruction of metaphyseal nonunion of the tibia. Clin Orthop Relat Res. 1996;332:23-28.
3. Gardner MJ, Toro-Arbelaez JB, Hansen M, Boraiah S, Lorich DG, Helfet DL. Surgical treatment and outcomes of extraarticular proximal tibial nonunions. Arch Orthop Trauma Surg. 2008;128(8):833-839. doi:10.1007/s00402-007-0383-y.
4. Toro-Arbelaez JB, Gardner MJ, Shindle MK, Cabas JM, Lorich DG, Helfet DL. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383. doi:10.1016/j.injury.2006.11.003.
5. Mechrefe AP, Koh EY, Trafton PG, DiGiovanni CW. Tibial nonunion. Foot Ankle Clin. 2006;11(1):1-18, vii. doi:10.1016/j.fcl.2005.12.003.
6. Chin KR, Nagarkatti DG, Miranda MA, Santoro VM, Baumgaertner MR, Jupiter JB. Salvage of distal tibia metaphyseal nonunions with the 90 degrees cannulated blade plate. Clin Orthop Relat Res. 2003;(409):241-249.
7. Devgan A, Kamboj P, Gupta V, Magu NK, Rohilla R. Pseudoarthrosis of medial tibial plateau fracture-role of alignment procedure. Chin J Traumatol. 2013;16(2):118-121. doi:10.3760/cma.j.issn.1008-1275.2013.02.011.
8. Helfet DL, Jupiter JB, Gasser S. Indirect reduction and tension-band plating of tibial non-union with deformity. J Bone Joint Surg Am. 1992;74(9):1286-1297.
9. Bolhofner BR. Indirect reduction and composite fixation of extraarticular proximal tibial fractures. Clin Orthop Relat Res. 1995;(315):75-83. doi:10.1097/00003086-199506000-00009.
10. Ries MD, Meinhard BP. Medial external fixation with lateral plate internal fixation in metaphyseal tibia fractures. A report of eight cases associated with severe soft-tissue injury. Clin Orthop Relat Res. 1988;(256):215-223.
11. Weiner LS, Kelley M, Yang E, et al. The use of combination internal fixation and hybrid external fixation in severe proximal tibia fractures. J Orthop Trauma. 1995;9(3):244-250.
12. Alghadir A, Anwer S, Brismee JM. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:174. doi:10.1186/s12891-015-0637-8.
13. Haverkamp D, Sierevelt IN, Breugem SJ, Lohuis K, Blankevoort L, van Dijk CN. Translation and validation of the Dutch version of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1680-1684. doi:10.1177/0363546506288854.
14. Ahlbäck S. Osteoartrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh). 1968;Suppl 277:7-72.
TAKE-HOME POINTS
- Treatment goals for a nonunion are bone union, re-establishment of (joint) stability, extremity alignment, and recovery of function.
- A nonunion of a tibia plateau fracture is often associated with poor soft tissues from previous surgeries and/or infections.
- Ideally a combination of minimal soft tissue damage and maximal stable fixation is used for salvage.
- There is a high risk of complications when using dual plating in these cases.
- A combination of an external fixator with limited internal fixation can be a good alternative.
Analysis of Incidence and Outcome Predictors for Patients Admitted to US Hospitals with Acetabular Fractures from 1990 to 2010
ABSTRACT
The incidence of acetabular fractures and associated in-hospital complication rates in the United States are poorly defined. Studies evaluating predictors of outcome for isolated acetabular fractures are weakly generalizable due to small sample sizes or the inclusion of all types of pelvic fractures. This study sought to analyze trends in acetabular fractures and associated complications in the US using the largest and most recent national dataset available.
The National Hospital Discharge Survey was queried to identify all patients admitted to US hospitals with acetabular fractures between 1990 and 2010. A representative cohort of 497,389 patients was identified, and multivariable logistic regression was used to identify independent predictors of mortality, adverse events, requirement of blood transfusion, and operative treatment with open reduction and internal fixation (ORIF).
Between 1990 and 2010, the population-adjusted incidence of acetabular fractures increased from 7.8 to 9.5/100,000 capita (P < .001). Mortality declined from 5.9% to 0.4% (P < .001), paralleling an increase in the proportion of patients treated with ORIF (12.6%-20.4%, P < .001), which was the variable associated with the lowest odds of mortality. Surgical intervention was associated with higher odds of adverse events and a requirement for blood transfusion. The average in-hospital length of stay decreased from 17.0 days to 10.3 days (P < .001).
This study provides the largest and most comprehensive epidemiologic analysis of acetabular fractures in the US. Knowledge of the increasing incidence of acetabular fractures and prognostic factors associated with poor outcomes may improve outcomes.
Continue to: Acetabular fractures are major injuries...
Acetabular fractures are major injuries frequently associated with life-altering sequelae1 and a significant resulting cost to society.2 Acetabular fractures are most often the result of a high-energy trauma3-5 or fall from a height.5,6 Functional outcomes and the prevention of post-traumatic arthritis have been shown to depend upon the accuracy of operative reduction.7-9 However, literature on the epidemiology of acetabular fractures is largely limited to European countries,1,10 and their incidence in the United States is more poorly defined.11 Published mortality rates in the existing literature vary widely from 2% to 45%,12-14 and few studies have identified the risk factors associated with in-hospital complications.15 While age, gender, and high-velocity mechanisms have been linked to increased mortality and complications,14-16 the evidence for these associations is poorly generalizable due to the inclusion of all pelvic fractures in these studies. Some reports suggest that advances in surgical management have improved survival and functional outcome,15,17 but these are based upon small cohorts. Knowledge of the incidence and patterns of disease burden are crucial for the allocation of limited healthcare resources.
This study sought to describe the trends in incidence as well as the factors influencing mortality and the risk of complications for patients admitted to US hospitals with an acetabular fracture using the National Hospital Discharge Survey (NHDS), the most recently available Centers for Disease Control and Prevention data, which is also one of the largest inpatient databases in the US. Knowledge of the factors influencing outcomes for patients admitted with acetabular fractures may improve management and decrease complications.
METHODS
NATIONAL HOSPITAL DISCHARGE SURVEY
The NHDS, developed by the National Center for Healthcare Statistics division of the Centers for Disease Control and Prevention,18 was used to estimate the incidence of acetabular fractures and to evaluate the risk factors for ensuing mortality and inpatient complications. The NHDS is a publically available survey providing demographic and medical data for inpatients discharged from non-federal, short-stay hospitals in the US.19 The NHDS is the principal database used by the US government for monitoring hospital use and is considered the most comprehensive of all inpatient surgical databases in use today.19 The survey uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes20 to classify medical diagnoses and procedures. The NHDS uses a stratified, multistage probability design to collect demographic information (age, gender, race), expected source of payment (insurance status), medical information of up to 7 discharge diagnoses and up to 4 procedures, length of care, hospital size, US region, and inpatient outcomes including discharge destination.21 To ensure unbiased national sampling of inpatient records, the NHDS uses a complex, 3-stage probability design including inflation by reciprocals of the probabilities of sample selection, adjustment for no response, and population weighting ratio adjustments.19 This study did not require approval by the Institutional Review Board because the NHDS is a publically available database with no patient-identifying information.
Continue to: PATIENT SELECTION...
PATIENT SELECTION
All patients admitted to hospitals in the US with a fracture of the acetabulum between 1990 and 2010 were identified using ICD-9-CM codes. Discharges with a diagnosis code (ICD-9-CM) of closed fracture of the acetabulum (808.0) or open fracture of the acetabulum (808.1) were identified using previously described techniques.22 The database was subsequently queried to identify patients treated using open reduction and internal fixation (ORIF) (ICD-9-CM, 79.30/79.39), closed reduction and internal fixation (CRIF) (ICD-9-CM, 79.10/79.19), or external (ICD-9-CM, 78.10/78.19) or internal (ICD-9-CM, 78.50/78.59) fixation without reduction. Demographic variables were then collected, including age, sex, primary diagnosis, associated diagnoses, type of fracture (open vs closed), prevalence of comorbidities, length of stay, and discharge destination. The complication screening package23 was used to determine the incidence of complications. The variable adverse event was created on the basis of the variables postoperative bleeding (998.1), acute postoperative infection (998.5), acute postoperative anemia (285.1), acute renal failure (584), acute myocardial infarction (410), pulmonary embolism (415.1), induced mental disorder (293), pneumonia (480-486), pulmonary insufficiency (518.5), deep venous thrombosis (453.4), intubation (96.xx), and blood transfusion (99.x).
STATISTICAL ANALYSIS
Because of the large sample size, a normal distribution of the data was assumed. Differences between categorical variables were compared using the Pearson chi square test, while the independent-samples t test was used to compare differences between continuous variables. To determine independent predictors of in-hospital outcomes (death, adverse events, requirement for blood transfusion, or treatment with ORIF), all variables present in at least 2% of the population24 were included in a multivariable binary logistic regression model. For in-hospital adverse events, a 1% cutoff was used due to their lower rates of occurrence, as previously described.25The dichotomous variables were death, presence of adverse events, receipt of blood transfusion, and treatment with ORIF. A multivariable regression model allows for the control of potential confounders, isolating the effect of individual variables on inpatient outcomes. Covariates accounted for in the regression model included gender, age, region of the country, and preexisting comorbidities (diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation). To assess the association between individual variables and inpatient outcomes, odds ratios and confidence intervals were calculated. A P value of <.001 was used to define statistical significance, correcting for multiple comparisons, as previously described.25 US census data were used to obtain national population estimates for each year of the study from 1990 to 2010.26 Rates were presented as the number of acetabular fractures per 100,000 standard population. All data were analyzed using the software Statistical Package for the Social Sciences [SPSS] version 20.
RESULTS
INCIDENCE AND DEMOGRAPHICS
A cohort representative of 497,389 patients with a diagnosis of acetabular fracture was identified between 1990 and 2010 (Table 1). In 1990, 19,560 cases (7.84 per 100,000 capita) of acetabular fractures were recoded, while in 2010, the number of cases increased to 29,373 or 9.5 per 100,000 capita (P < .001) (Table 2). The mean age of patients with an acetabular fracture was 52.6 years (standard deviation [SD], 23.7) and 60.6% were male (Table 1). The most frequently associated diagnosis was closed fracture of the pelvis (29.8%) followed by fracture of the femur (13.1%) and closed fracture of the ilium (3.8%) (Table 1). Of the total cohort, 23.2% underwent ORIF (Table 1). In 1990, 12.6% of patients with a diagnosis of acetabular fracture underwent ORIF, whereas 20.4% of patients underwent ORIF in 2010 (P < .001) (Table 2). Average length of hospital stay was 8.3 days (SD, 17.9) overall (Table 1). In 1990 the average length of stay was 17.0 days (SD, 14.9), decreasing to 10.3 days (SD, 9.3) in 2010 (P < .001) (Table 2).
Table 1. Patient Characteristics for Patients with Acetabular Fractures in the United States from 1990 to 2007
Parameter | Total 1990-2010 |
Total Number | 497,389 |
Gender (%) |
|
Male | 60.6 |
Female | 39.4 |
Age, years (%) |
|
<20 | 6.7 |
20-40 | 31.5 |
41-60 | 22.3 |
61-85 | 30.4 |
>85 | 23.5 |
Race (%) |
|
White | 66.4 |
Black | 9.3 |
Asian | 1.7 |
Other | 2.4 |
Not stated | 20.2 |
Primary Diagnosis (%) |
|
Closed fracture of acetabulum (808.0) | 98.9 |
Open fracture of acetabulum (808.1) | 1.1 |
Associated diagnoses (%) |
|
Closed fracture of pubis (808.2) | 26.1 |
Open fracture of pubis (808.3) | 0.1 |
Closed fracture of ischium (808.42) | 1.7 |
Open fracture of ischium (808.52) | 0.0 |
Closed fracture of ilium (808.41) | 3.8 |
Open fracture of ilium (808.51) | 0.0 |
Closed fracture other part pelvis (808.49) | 0.7 |
Open fracture other part pelvis (808.59) | 0.0 |
Multiple closed pelvic fractures (808.43) | 0.5 |
Multiple open pelvic fractures (808.53) | 0.0 |
Any pelvic fracture from above | 29.8 |
Fracture of neck of femur (820) | 7.2 |
Fracture of any part of femur (820/821) | 13.1 |
Head trauma (959.01) | 0.7 |
Head/face trauma (959.0/959.01) | 0.7 |
Chest trauma (959.11) | 0.1 |
Chest/trunk trauma (959.1/959.11) | 0.1 |
Procedures (%) |
|
Open reduction internal fixation (79.30/79.39) | 23.2 |
Closed reduction internal fixation (79.10/79.19) | 1.3 |
External fixation (78.10/78.19) | 0.7 |
Internal fixation without reduction (78.50/78.59) | 0.4 |
Comorbidities (%) |
|
No | 72.9 |
Yes | 27.1 |
Adverse Events (%) |
|
No | 74.1 |
Yes | 25.9 |
Discharge Disposition (%) |
|
Routine/home (1) | 45.4 |
Left against medical advice (2) | 0.2 |
Short term fac (3) | 13.1 |
Long term fac (4) | 22.2 |
Alive, not stated (5) | 12 |
Dead (6) | 3.5 |
Not reported (9) | 3.6 |
Mortality (%) | 3.5 |
Age (y), mean (SD) | 52.6 (23.7) |
Days of Care, mean (SD) | 8.3 (17.9) |
Principal Source of Payment (%) |
|
Private insurance | 39 |
Medicare | 30.5 |
Medicaid | 7.7 |
Other government | 1.9 |
Self-pay | 7.9 |
Workmen’s comp | 4 |
Other | 4.7 |
Not stated | 4.4 |
Abbreviation: SD, standard deviation.
Table 2. Patient Characteristics in 1990, 1995, 1999, 2003, and 2007 Among Patients with Acetabular Fractures
Variable | 1990 | 1995 | 1999 | 2003 | 2007 | 2010 |
Total number | 19,560 | 17,506 | 22,767 | 27,133 | 34,027 | 29,373 |
Incidence per 100,000 capita | 7.84 | 6.57 | 8.16 | 9.35 | 11.30 | 9.5 |
Gender (%) |
| |||||
Male | 51.0 | 70.7 | 61.2 | 62.6 | 62.5 | 64.9 |
Female | 49.0 | 29.3 | 38.8 | 37.4 | 37.5 | 35.1 |
Fracture (%) |
| |||||
Open | 2.1 | 1.7 | 3.3 | 1.4 | 0.1 | 1.8 |
Closed | 97.9 | 98.3 | 96.7 | 98.6 | 99.9 | 98.2 |
Underwent ORIF (%) | 12.6 | 20.9 | 20.2 | 22.9 | 27.8 | 20.4 |
Adverse events (%) | 10.9 | 16.2 | 23.7 | 31 | 35.1 | 37.6 |
Transfusion (%) | 0.3 | 2.2 | 7.4 | 6.5 | 10.5 | 9.5 |
Discharge (%) |
| |||||
Routine | 58 | 65.6 | 35.6 | 45.9 | 40.2 | 41.6 |
Non-routine to inpatient facility | 26.8 | 23.1 | 46.4 | 33.8 | 40.8 | 34.6 |
Mortality (%) | 5.9 | 3.6 | 2 | 2.9 | 1.5 | 0.4 |
Mean Age (y) | 52.9 | 48.4 | 52.3 | 56.3 | 57 | 53.2 |
Mean DOC (days) | 17.0 | 13.4 | 8.7 | 10.8 | 8.5 | 10.3 |
Abbreviations: DOC, days of care; ORIF, open reduction internal fixation.
Continue to: MORTALITY...
MORTALITY
In-hospital mortality decreased from 5.9% in 1990 to 0.4% in 2010 (P < .001) (3.5% for the total cohort) (Tables 1 and 2). Multivariable logistic regression analysis demonstrated pulmonary insufficiency (odds ratio [OR], 9.07; 95% confidence interval [CI], 8.52-9.66; P < .01), pneumonia (OR, 3.22; 95% CI, 3.05-3.39; P < .01), and age >85 years (OR, 2.28; 95% CI, 2.16-2.40; P < .01) to be associated with the highest odds of inpatient mortality. CRIF (OR, 1.99; 95% CI, 1.78-2.23; P < .01), external fixator (OR, 1.82; 95% CI, 1.45-2.29; P < .01), and having received a blood transfusion (OR, 1.81; 95% CI, 1.71-1.91; P < .01) were also associated with increased odds of mortality. Treatment with ORIF (OR, 0.19; 95% CI, 0.17-0.20; P < .01) was independently associated with decreased odds of inpatient mortality, as was age <20 years (OR, 0.26; 95% CI, 0.23-0.30; P < .01) (model fit: for omnibus test of model coefficients, X = 25,966 P < .01; Nagelkerke, R2 = 0.20) (Table 3).
Table 3. Logistic Regression for Predictors of Mortality Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Pulmonary insufficiency | 9.07 (8.52–9.66) | < 0.01 |
Pneumonia | 3.22 (3.05–3.39) | < 0.01 |
Age >85 years | 2.28 (2.16–2.40) | < 0.01 |
Closed reduction internal fixation | 1.99 (1.78–2.23) | < 0.01 |
External Fixator | 1.82 (1.45–2.29) | < 0.01 |
Blood transfusion | 1.81 (1.71–1.91) | < 0.01 |
Gender (male) | 1.76 (1.70–1.83) | < 0.01 |
Associated femoral neck fracture | 1.23 (1.15–1.30) | < 0.01 |
Age 41-60 years | 1.19 (1.11–1.29) | < 0.01 |
Age 61-85 years | 1.17 (1.11–1.23) | < 0.01 |
Congestive heart failure | 1.14 (1.07–1.22) | < 0.01 |
Associated pelvic fracture | 1.13 (1.10–1.17) | < 0.01 |
Geographic region | 1.11 (1.09–1.12) | < 0.01 |
Source of payment | 1.02 (1.01–1.02) | < 0.01 |
Race | 0.99 (0.98–0.99) | < 0.01 |
DOC | 0.98 (0.98–0.98) | < 0.01 |
Hypertension | 0.67 (0.64–0.71) | < 0.01 |
Atrial fibrillation | 0.52 (0.48–0.57) | < 0.01 |
Diabetes mellitus | 0.35 (0.32–0.38) | < 0.01 |
Age 20-40 years | 0.32 (0.30–0.35) | < 0.01 |
Age <20 years | 0.26 (0.23–0.30) | < 0.01 |
Coronary artery disease | 0.21 (0.18–0.24) | < 0.01 |
Open reduction internal fixation | 0.19 (0.17–0.20) | < 0.01 |
Omnibus X 25,966, P < .01 | ||
Nagelkerke R2= 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
COMORBIDITIES AND ADVERSE EVENTS
The prevalence of comorbidities and adverse events is listed in Tables 4 and 5, respectively. Hypertensive disease was the most common comorbidity at 15.3%, followed by diabetes mellitus at 6.9%. Overall, 25.9% of patients experienced an in-hospital adverse event, with the most common being postoperative anemia (7.3%) and blood transfusion (8.1%) (Tables 1 and 5). The percentage of patients experiencing an adverse event increased from 10.9% in 1990 to 37.6% in 2010 (P < .01) (Table 2). Multivariable logistic regression analysis revealed CRIF (OR, 3.08; 95% CI, 2.91-3.26; P < .01), coronary artery disease (OR, 2.02; 95% CI, 1.91-2.15; P < .01), associated femoral neck fracture (OR, 1.53; 95% CI, 1.47-1.60; P < .01), and ORIF (OR, 1.22; 95% CI, 1.20-1.24; P < .01) to be associated with higher odds of inpatient adverse events (model fit: for omnibus test of model coefficients, X = 160,275, P < .01; Nagelkerke, R2 = 0.41) (Table 6).
Table 4. Prevalence of Comorbidities in Patients with Acetabular Fractures Between 1990 and 2007 (n = 403.927)
Parameter (ICD-9) | Percentage of Total |
Hypertensive disease (401–405) | 15.3% |
Diabetes mellitus (250) | 6.9% |
Atrial fibrillation (427.31) | 4.0% |
Congestive heart failure (428) | 3.9% |
Osteoporosis (733.0) | 2.1% |
Coronary artery disease (414.01) | 2.0% |
Obesity (278.00, 278.01) | 2.0% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 5. Prevalence of In-Hospital Adverse Events Among Patients with Acetabular Fractures Between 1990 and 2007 (n = 403,927)
Parameter (ICD-9) | Percentage of Total |
Transfusion of blood (99.0) | 8.1% |
Acute postoperative anemia (285.1) | 7.3% |
Intubation (96.x) | 4.9% |
Acute renal failure (584) | 3.4% |
Pneumonia (480-486) | 3.2% |
Pulmonary insufficiency (518.5) | 2.3% |
Pulmonary embolism (415.1) | 1.6% |
Deep venous thrombosis (453.4) | 1.0% |
Acute myocardial infarction (410) | 0.9% |
Postoperative bleeding (998.1) | 0.7% |
Acute postoperative infection (998.5) | 0.5% |
Induced mental disorder (293) | 0.4% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 6. Logistic Regression for Predictors of Adverse Events Among Patients Hospitalized for Acetabular Fracture (n = 403,927)
Variable | OR (95% CI) | P |
Closed reduction internal fixation | 3.08 (2.91-3.26) | < 0.01 |
Coronary artery disease | 2.02 (1.91-2.15) | < 0.01 |
Associated femoral neck fracture | 1.53 (1.47-1.60) | < 0.01 |
Open reduction internal fixation | 1.22 (1.20-1.24) | < 0.01 |
Gender (male) | 1.16 (1.14-1.18) | < 0.01 |
Associated fracture of any part of femur | 1.13 (1.10-1.17) | < 0.01 |
Age >85 years | 1.08 (1.05-1.12) | < 0.01 |
Geographic region | 1.07 (1.06-1.07) | < 0.01 |
DOC | 1.04 (1.04-1.04) | < 0.01 |
Race | 1.02 (1.02-1.03) | < 0.01 |
Source of payment | 1.01 (1.01-1.01) | < 0.01 |
Congestive heart failure | 1.01 (0.96-1.06) | 0.78 |
Atrial fibrillation | 0.88 (0.84-0.92) | < 0.01 |
Age 61-85 years | 0.68 (0.66-0.71) | < 0.01 |
Age <20 years | 0.67 (0.64-0.70) | < 0.01 |
Associated pelvis fracture | 0.64 (0.63-0.66) | < 0.01 |
Age 41-60 years | 0.58 (0.56-0.61) | < 0.01 |
Diabetes mellitus | 0.48 (0.46-0.50) | < 0.01 |
Age 20-40 years | 0.45 (0.43-0.47) | < 0.01 |
Hypertension | 0.44 (0.43-0.45) | < 0.01 |
External Fixator | 0.39 (0.35-0.44) | < 0.01 |
Omnibus X 160,275, P < .01 | ||
Nagelkerke R2 = 0.41 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
BLOOD TRANSFUSION
Overall, 7.3% of patients experienced acute postoperative anemia (Table 5). Between 1990 and 2010, the percentage of patients receiving blood transfusions increased from 0.3% to 9.5%, respectively (P < .01) (Table 2). In multivariable logistic regression analysis, patients treated with ORIF (OR, 8.13; 95% CI, 7.91-8.36; P < .01), those with congestive heart failure (OR, 4.23; 95% CI, 4.06-4.41; P < .01), those with an associated femur fracture (OR, 3.13; 95% CI, 2.99-3.27; P < .01), those with atrial fibrillation (OR, 1.96; 95% CI, 1.88-2.05; P < .01), and those treated with CRIF (OR, 1.42; 95% CI, 1.29-1.56; P < .01) were associated with significantly higher odds of blood transfusion (model fit: omnibus test of model coefficients, X = 42,653, P < .01; Nagelkerke, R2 = 0.19) (Table 7).
Table 7. Logistic Regression for Predictors of the Requirement for Blood Transfusion Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Open reduction internal fixation | 8.13 (7.91-8.36) | < 0.01 |
Congestive heart failure | 4.23 (4.06-4.41) | < 0.01 |
Associated fracture of any part of femur | 3.13 (2.99-3.27) | < 0.01 |
Atrial fibrillation | 1.96 (1.88-2.05) | < 0.01 |
Closed reduction internal fixation | 1.42 (1.29-1.56) | < 0.01 |
Geographic region | 1.38 (1.36-1.39) | < 0.01 |
Hypertension | 1.38 (1.34-1.42) | < 0.01 |
Associated pelvic fracture | 1.28 (1.25-1.31) | < 0.01 |
Age 61-85 years | 1.06 (1.02-1.11) | 0.01 |
Source of payment | 0.99 (0.98-0.99) | < 0.01 |
Race | 0.98 (0.97-0.98) | < 0.01 |
DOC | 0.96 (0.96-0.96) | < 0.01 |
Age >85 years | 0.74 (0.72-0.77) | < 0.01 |
External fixator | 0.69 (0.59-0.80) | < 0.01 |
Coronary artery disease | 0.62 (0.57-0.68) | < 0.01 |
Age 41-60 years | 0.57 (0.54-0.60) | < 0.01 |
Gender (male) | 0.54 (0.52-0.55) | < 0.01 |
Diabetes mellitus | 0.38 (0.36-0.41) | < 0.01 |
Age 20-40 years | 0.32 (0.30-0.34) | < 0.01 |
Associated femoral neck fracture | 0.29 (0.27-0.31) | < 0.01 |
Age <20 years | 0.24 (0.22-0.26) | < 0.01 |
Omnibus X = 42,653, P < .01 | ||
Nagelkerke R2 = 0.19 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
TREATMENT WITH ORIF
Over the 20-year study period, 23.2% of patients with acetabular fractures were treated with ORIF (Table 1). In 1990, 12.6% of patients underwent ORIF, while in 2010 this percentage increased to 20.4% (P < .001) (Table 2). Multivariable logistic regression analysis demonstrated that age between 41 and 60 years (OR, 1.88; 95% CI, 1.78-1.98; P < .01) was associated with the highest odds of undergoing ORIF. Age 20 to 40 years (OR, 1.86; 95% CI, 1.76-1.97; P < .01), age <20 years (OR, 1.82; 95% CI, 1.72-1.93; P < .01), and male gender (OR, 1.65; 95% CI, 1.63-1.68; P < .01) were also associated with being treated by ORIF. In contrast, coronary artery disease (OR, 0.27; 95% CI, 0.25-0.30; P < .01), age >85 years (OR, 0.46; 95% CI, 0.44-0.47; P < .01), and congestive heart failure (OR, 0.48; 95% CI, 0.46-0.51; P < .01) were associated with the lowest odds of undergoing ORIF (model fit: omnibus test of model coefficients, X = 71,118, P < .01; Nagelkerke, R2 = 0.20) (Table 8).
Table 8. Logistic Regression for Predictors of the Requirement for Discharge to Another Inpatient Facility Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Age 41-60 years | 1.88 (1.78-1.98) | < 0.01 |
Age 20-40 years | 1.86 (1.76-1.97) | < 0.01 |
Age <20 years | 1.82 (1.72-1.93) | < 0.01 |
Gender (male) | 1.65 (1.63-1.68) | < 0.01 |
Larger hospital bed size | 1.46 (1.45-1.47) | < 0.01 |
Hypertension | 1.35 (1.32-1.38) | < 0.01 |
Diabetes mellitus | 1.09 (1.05-1.13) | < 0.01 |
DOC | 1.02 (1.02-1.02) | < 0.01 |
Source of payment | 1.01 (1.01-1.02) | < 0.01 |
Race | 1.00 (0.99-1.00) | 0.17 |
Age 61-85 years | 0.94 (0.90-0.99) | 0.02 |
Region | 0.92 (0.91-0.93) | < 0.01 |
Atrial fibrillation | 0.83 (0.79-0.87) | < 0.01 |
Congestive heart failure | 0.48 (0.46-0.51) | < 0.01 |
Age >85 years | 0.46 (0.44-0.47) | < 0.01 |
Coronary artery disease | 0.27 (0.25-0.30) | < 0.01 |
Omnibus X 71,118, P < .01 | ||
Nagelkerke R2 = 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
Continue to: DISCUSSION...
DISCUSSION
This study evaluates the incidence of acetabular fractures in the US between 1990 and 2010, and identifies prognostic factors associated with complications and death. The study demonstrates an increase in the population-adjusted incidence of acetabular fractures between 1990 and 2010 (7.84 cases per 100,000 capita to 9.5 cases per 100,000 capita), in contrast to the decreasing trend reported by Mauffrey and colleagues.11 Some studies suggest that up to 80% of acetabular fractures are associated with motor vehicle collisions and motorcycle accidents.9,27 While the rate of motor vehicle accidents has remained stable over the study period, motorcycle ownership and deaths more than doubled between 2001 and 2008,28 primarily among individuals over 40 years of age. In this study, the mean age of patients with acetabular fractures ranged from 48 to 57 years. The dramatic increase in motorcycle ownership and deaths in these age groups may partially explain the rising incidence of acetabular fractures. The other possibility is that changes in automobile design and safety equipment may have altered the injury patterns observed in patients surviving motor vehicle crashes. Compared to the United Kingdom, in which studies report a fixed incidence of 3 per 100,000 capita1 between 1988 and 2003, the incidence of acetabular fractures in the US is greater. In contrast, the incidence of acetabular fractures reported in this study is less than the 20 per 100,000 reported in Sweden between 1976 and 1985,29 or the 37 per 100,000 reported in Rochester, Minnesota between 1968 and 1977,30 which may be due to increased seatbelt usage.31
In addition to the national incidence, this study demonstrated that the proportion of patients with acetabular fractures treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010. This is substantially lower than the 77% reported by Ochs and colleagues32 in a German population. Concurrent with the increase in ORIF, there was a decrease in in-hospital mortality from 5.9% in 1990 to 0.4% in 2010. The initial mortality rates in this study are comparable to much earlier reports and some small studies,9,32-37 but the rates reported in the later years of this study show a substantial decrease that is likely a more accurate estimation of the current incidence. The improved survival rates may be due to advances in the operative treatment of acetabular fractures, in which mechanical stabilization allows for early patient mobilization and facilitation of optimal nursing care.38 With ORIF becoming the standard of care for displaced acetabular fractures,9 numerous reports have demonstrated an association between early definitive fixation and improved survival.17,39,40 This is similar to our study, which found ORIF to be associated with the lowest odds of mortality in multivariate logistic regression analysis. It is possible that advances in patient care by intensivists over this period have also contributed to the decrease in mortality, but the correlation with operative treatment in this study is very strong and agrees well with prior studies.16 Moreover, multiple studies have demonstrated decreased in-hospital mortality among patients undergoing various orthopedic surgical procedures during this period.41-43 The correlation with operative treatment in this study agrees well with prior studies.16
In contrast, higher odds of mortality were seen in patients over the age of 85 years with pulmonary insufficiency, congestive heart failure, pneumonia, or an associated femur or pelvic fracture. This is similar to prior reports in which patients with combined acetabulum and pelvic ring injuries fared worse than those with isolated injures,44,45 as did patients with associated non-musculoskeletal injuries.46 The finding that age over 85 years was associated with higher odds of mortality likely reflects the increased number of comorbidities and decreased physiologic reserve seen in this patient population. Finally, male gender was associated with higher odds of in-hospital mortality. There are 2 possible explanations for this: Either there is gender dimorphism in sex hormones and cytokine activity in response to hemorrhage and sepsis,38,47 or there is a greater tendency for males to be involved in higher energy accidents with more severe concomitant injuries.
The results of multivariable regression analysis demonstrated that patients were more likely to require blood transfusion if they were managed surgically or had atrial fibrillation, congestive heart failure, or associated femur fracture. Not surprisingly, concurrent pelvic fracture was also associated with higher odds of blood transfusion, as pelvic hemorrhage is reported to be the cause of death in up to half of patients who die following a pelvic fracture.46
Between 1990 and 2010, in-hospital days of care decreased from 17.0 days to 10.3 days. While a decreased length of stay has been demonstrated in other orthopedic conditions over the study period,41 it is possible the decrease in length of stay demonstrated in this study is due to improved surgical technique and the implementation of early surgical intervention.39,48-50 Plaisier and colleagues17 demonstrated superior functional outcomes, quicker return to baseline function, and decreased length of stay in patients treated with early ORIF of their acetabular fractures. Other studies have shown that the benefits of early surgery include improved reduction quality and ease of reduction,51 as well as control of bleeding, pain relief, and mobilization of the patient.39 Another possible explanation for the decreased length of stay is the increased rate of discharge to other inpatient facilities, such as rehabilitation facilities, which was demonstrated in this study.
Continue to: Interestingly, male gender and younger age...
Interestingly, male gender and younger age were associated with operative management of the acetabular fracture. In contrast, there was a decreased likelihood of operative treatment among elderly patients and those patients with cardiac comorbidities. It is possible that the relationship we found between the likelihood of ORIF and age relates to the bimodal distribution of fractures, with higher energy and potentially more displaced fractures occurring in younger patients3-5 and lower energy fractures in the elderly.
In contrast to decreasing in-hospital days of care, there was a rise in the number of adverse events between 1990 (10.9%) and 2010 (37.6%). This can be partially attributed to the increased rates of blood transfusion, which was received by 9.5% of patients with acetabular fractures in the final study year. Additionally, surgical intervention was associated with increased adverse events in this study, and surgical intervention increased over the study period. Other factors that may have contributed to an increase in adverse events include an aging population,52 as advanced age was independently associated with higher odds of adverse events in this study.
Despite the strengths of using large, national databases for epidemiological research,53 this study has several limitations. Like all large databases, the NHDS is subject to error in coding and data entry.54 Additionally, the database only allows for 7 diagnostic codes and 4 procedure codes per entry. As a result, the prevalence of comorbid conditions and adverse events may be underreported.25 Moreover, the severity of a comorbid disease cannot be appreciated when dichotomously classified.55 Another limitation is that the database only provides inpatient data, so complications that arise after discharge, as well as follow-up data, are unknown. Furthermore, the results of this study are limited to practice patterns in the US from 1990 to 2010. This database does not provide injury mechanisms, so we cannot distinguish between high-energy and low-energy injuries. Lastly, analysis of the different types of acetabular fractures was not performed since classification of acetabular fractures cannot be assessed with ICD-9 codes.
CONCLUSION
This study is the largest epidemiologic analysis of acetabular fractures in the US and also provides predictors of in-hospital mortality. The incidence of acetabular fractures in the US is increasing, while mortality is decreasing. Identifying risk factors associated with poor outcomes has the potential to change treatment strategies, resource allocation, in-hospital monitoring, and discharge planning for this patient population.
This paper will be judged for the Resident Writer’s Award.
1. Laird A, Keating JF. Acetabular fractures: a 16-year prospective epidemiological study. J Bone Joint Surg Br. 2005;87(7):969-973. doi:10.1302/0301-620X.87B7.16017.
2. Geoghegan JM, Longdon EJ, Hassan K, Calthorpe D. Acetabular fractures in the UK. What are the numbers? Injury. 2007;38(3):329-333. doi:10.1016/j.injury.2006.09.015.
3. Tavakoli Darestani R, Kazemian G, Emami Moghaddam M, Manafi Rasi A, Alipour Y, Bagherian Lemraski MM. An unusual combination of acetabular and pelvic fracture: is this a new subtype of acetabular fracture? Trauma Mon. 2013;18(1):37-40. doi:10.5812/traumamon.9613.
4. McDonnell M, Schachter AK, Phillips DP, Liporace FA. Acetabular fracture through the triradiate cartilage after low-energy trauma. J Orthop Trauma. 2007;21(7):495-498. doi:10.1097/BOT.0b013e31812f67ff.
5. Giannoudis PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875-883. doi:10.1097/01.ta.0000242259.67486.15.
6. Gänsslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27 Suppl 1:S-A13-A20. doi:10.1016/S0020-1383(96)90106-0.
7. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645. doi:10.2106/00004623-199611000-00002.
8. Wright R, Barrett K, Christie MJ, Johnson KD. Acetabular fractures: long-term follow-up of open reduction and internal fixation. J Orthop Trauma. 1994;8(5):397-403. doi:10.1097/00005131-199410000-00005.
9. Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2-9.
10. Davarinos N, Ellanti P, Morris S, Mc Elwain JP. Epidemiology of pelvic and acetabular trauma in a Dublin tertiary hospital: a 10-year experience. Ir J Med Sci. 2012;181(2):243-246. doi:10.1007/s11845-011-0791-4.
11. Mauffrey C, Hao J, Cuellar DO 3rd, et al. The epidemiology and injury patterns of acetabular fractures: are the USA and China comparable? Clin Orthop Relat Res. 2014;472(11):3332-3337. doi:10.1007/s11999-014-3462-8.
12. Dente CJ, Feliciano DV, Rozycki GS, et al. The outcome of open pelvic fractures in the modern era. Am J Surg. 2005;190(6):830-835. doi:10.1016/j.amjsurg.2005.05.050.
13. Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1-13. doi:10.1016/j.injury.2004.05.029.
14. Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985-991. doi:10.1016/j.injury.2011.06.003.
15. Arroyo W, Nelson KJ, Belmont PJ Jr, Bader JO, Schoenfeld AJ. Pelvic trauma: what are the predictors of mortality and cardiac, venous thrombo-embolic and infectious complications following injury? Injury. 2013;44(12):1745-1749. doi:10.1016/j.injury.2013.08.007.
16. Flint L, Cryer HG. Pelvic fracture: the last 50 years. J Trauma. 2010;69(3):483-488. doi:10.1097/TA.0b013e3181ef9ce1.
17. Plaisier BR, Meldon SW, Super DM, Malangoni MA. Improved outcome after early fixation of acetabular fractures. Injury. 2000;31(2):81-84. doi:10.1016/S0020-1383(99)00233-8.
18. Centers for Disease Control and Prevention: National Hospital. Discharge survey. http://www.cdc.gov/nchs/nhds.htm. Accessed August 22, 2013.
19. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;(39):1-42.
20. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed June 18, 2013.
21. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627. doi:10.1007/s11999-008-0402-5.
22. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157. doi:10.1016/j.psym.2012.08.009.
23. Iezzoni LI, Daley J, Heeren T, et al. Using administrative data to screen hospitals for high complication rates. Inquiry. 1994;31(1):40-55.
24. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478-2486.
25. Bot AG, Menendez ME, Neuhaus V, Ring D. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(4):519-527. doi:10.1016/j.jse.2013.12.006.
26. United States Census Bureau. Population. https://www.census.gov/topics/population.html. Accessed December 4, 2012.
27. Porter SE, Schroeder AC, Dzugan SS, Graves ML, Zhang L, Russell GV. Acetabular fracture patterns and their associated injuries. J Orthop Trauma. 2008;22(3):165-170. doi:10.1097/BOT.0b013e318165918b.
28. Centers for Disease Control and Prevention. Motorcycle Crash-Related Data. https://www.cdc.gov/motorvehiclesafety/mc/index.html Accessed September 23, 2018
29. Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300. doi:10.3109/17453679209154786.
30. Melton LJ 3rd, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;155(155):43-47. doi:10.1097/00003086-198103000-00008.
31. al-Qahtani S, O'Connor G. Acetabular fractures before and after the introduction of seatbelt legislation. Can J Surg. 1996;39(4):317-320.
32. Ochs BG, Marintschev I, Hoyer H, et al. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2010;41(8):839-851. doi:10.1016/j.injury.2010.04.010.
33. Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151(151):81-106. doi:10.1055/s-2007-980136.
34. de Ridder VA, de Lange S, Kingma L, Hogervorst M. Results of 75 consecutive patients with an acetabular fracture. Clin Orthop Relat Res. 1994;305(305):53-57. doi:10.1097/00003086-199408000-00008.
35. Aho AJ, Isberg UK, Katevuo VK. Acetabular posterior wall fracture. 38 Cases followed for 5 years. Acta Orthop Scand. 1986;57(2):101-105. doi:10.3109/17453678609000878.
36. Stöckle U, Hoffmann R, Südkamp NP, Reindl R, Haas NP. Treatment of complex acetabular fractures through a modified extended iliofemoral approach. J Orthop Trauma. 2002;16(4):220-230. doi:10.1097/00005131-200204000-00002.
37. Tibbs BM, Kopar P, Dente CJ. Acetabular and isolated pelvic ring fractures: a comparison of initial assessment and outcome. Am Surg. 2008;74(6):538-541; discussion 541.
38. Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012;470(8):2090-2097. doi:10.1007/s11999-012-2276-9.
39. Vallier HA, Cureton BA, Ekstein C, Oldenburg FP, Wilber JH. Early definitive stabilization of unstable pelvis and acetabulum fractures reduces morbidity. J Trauma. 2010;69(3):677-684. doi:10.1097/TA.0b013e3181e50914.
40. Enninghorst N, Toth L, King KL, McDougall D, Mackenzie S, Balogh ZJ. Acute definitive internal fixation of pelvic ring fractures in polytrauma patients: a feasible option. J Trauma. 2010;68(4):935-941. doi:10.1097/TA.0b013e3181d27b48.
41. Buller LT, Best MJ, Quinnan SM. A nationwide analysis of pelvic ring fractures: incidence and trends in treatment, length of stay, and mortality. Geriatr Orthop Surg Rehabil. 2016;7(1):9-17. doi:10.1177/2151458515616250.
42. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191. doi:10.2106/JBJS.M.01126.
43. Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214.
44. Halvorson JJ, Lamothe J, Martin CR, et al. Combined acetabulum and pelvic ring injuries. J Am Acad Orthop Surg. 2014;22(5):304-314. doi:10.5435/JAAOS-22-05-304.
45. Osgood GM, Manson TT, O'Toole RV, Turen CH. Combined pelvic ring disruption and acetabular fracture: associated injury patterns in 40 patients. J Orthop Trauma. 2013;27(5):243-247. doi:10.1097/BOT.0b013e31826c2751.
46. Poole GV, Ward EF, Muakkassa FF. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg. 1991;213(6):532-538; discussion 538.
47. Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi:10.1097/00000658-200201000-00014.
48. Goldstein A, Phillips T, Sclafani SJ, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26(4):325-333. doi:10.1097/00005373-198604000-00004.
49. Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28-31. doi:10.1097/00005373-199101000-00006.
50. Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35(5):671-675; discussion 676.
51. Madhu R, Kotnis R, Al-Mousawi A, et al. Outcome of surgery for reconstruction of fractures of the acetabulum. The time dependent effect of delay. J Bone Joint Surg Br. 2006;88(9):1197-1203. doi:10.1302/0301-620X.88B9.17588.
52. Centers for Disease Control and Prevention. The State of Aging & Health in America 2013. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Accessed December 5, 2013.
53. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
54. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449. [author reply:450-451]. doi:10.1097/ALN.0b013e3181adf739.
55. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706. doi:10.1007/s11999-013-2876-z.
ABSTRACT
The incidence of acetabular fractures and associated in-hospital complication rates in the United States are poorly defined. Studies evaluating predictors of outcome for isolated acetabular fractures are weakly generalizable due to small sample sizes or the inclusion of all types of pelvic fractures. This study sought to analyze trends in acetabular fractures and associated complications in the US using the largest and most recent national dataset available.
The National Hospital Discharge Survey was queried to identify all patients admitted to US hospitals with acetabular fractures between 1990 and 2010. A representative cohort of 497,389 patients was identified, and multivariable logistic regression was used to identify independent predictors of mortality, adverse events, requirement of blood transfusion, and operative treatment with open reduction and internal fixation (ORIF).
Between 1990 and 2010, the population-adjusted incidence of acetabular fractures increased from 7.8 to 9.5/100,000 capita (P < .001). Mortality declined from 5.9% to 0.4% (P < .001), paralleling an increase in the proportion of patients treated with ORIF (12.6%-20.4%, P < .001), which was the variable associated with the lowest odds of mortality. Surgical intervention was associated with higher odds of adverse events and a requirement for blood transfusion. The average in-hospital length of stay decreased from 17.0 days to 10.3 days (P < .001).
This study provides the largest and most comprehensive epidemiologic analysis of acetabular fractures in the US. Knowledge of the increasing incidence of acetabular fractures and prognostic factors associated with poor outcomes may improve outcomes.
Continue to: Acetabular fractures are major injuries...
Acetabular fractures are major injuries frequently associated with life-altering sequelae1 and a significant resulting cost to society.2 Acetabular fractures are most often the result of a high-energy trauma3-5 or fall from a height.5,6 Functional outcomes and the prevention of post-traumatic arthritis have been shown to depend upon the accuracy of operative reduction.7-9 However, literature on the epidemiology of acetabular fractures is largely limited to European countries,1,10 and their incidence in the United States is more poorly defined.11 Published mortality rates in the existing literature vary widely from 2% to 45%,12-14 and few studies have identified the risk factors associated with in-hospital complications.15 While age, gender, and high-velocity mechanisms have been linked to increased mortality and complications,14-16 the evidence for these associations is poorly generalizable due to the inclusion of all pelvic fractures in these studies. Some reports suggest that advances in surgical management have improved survival and functional outcome,15,17 but these are based upon small cohorts. Knowledge of the incidence and patterns of disease burden are crucial for the allocation of limited healthcare resources.
This study sought to describe the trends in incidence as well as the factors influencing mortality and the risk of complications for patients admitted to US hospitals with an acetabular fracture using the National Hospital Discharge Survey (NHDS), the most recently available Centers for Disease Control and Prevention data, which is also one of the largest inpatient databases in the US. Knowledge of the factors influencing outcomes for patients admitted with acetabular fractures may improve management and decrease complications.
METHODS
NATIONAL HOSPITAL DISCHARGE SURVEY
The NHDS, developed by the National Center for Healthcare Statistics division of the Centers for Disease Control and Prevention,18 was used to estimate the incidence of acetabular fractures and to evaluate the risk factors for ensuing mortality and inpatient complications. The NHDS is a publically available survey providing demographic and medical data for inpatients discharged from non-federal, short-stay hospitals in the US.19 The NHDS is the principal database used by the US government for monitoring hospital use and is considered the most comprehensive of all inpatient surgical databases in use today.19 The survey uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes20 to classify medical diagnoses and procedures. The NHDS uses a stratified, multistage probability design to collect demographic information (age, gender, race), expected source of payment (insurance status), medical information of up to 7 discharge diagnoses and up to 4 procedures, length of care, hospital size, US region, and inpatient outcomes including discharge destination.21 To ensure unbiased national sampling of inpatient records, the NHDS uses a complex, 3-stage probability design including inflation by reciprocals of the probabilities of sample selection, adjustment for no response, and population weighting ratio adjustments.19 This study did not require approval by the Institutional Review Board because the NHDS is a publically available database with no patient-identifying information.
Continue to: PATIENT SELECTION...
PATIENT SELECTION
All patients admitted to hospitals in the US with a fracture of the acetabulum between 1990 and 2010 were identified using ICD-9-CM codes. Discharges with a diagnosis code (ICD-9-CM) of closed fracture of the acetabulum (808.0) or open fracture of the acetabulum (808.1) were identified using previously described techniques.22 The database was subsequently queried to identify patients treated using open reduction and internal fixation (ORIF) (ICD-9-CM, 79.30/79.39), closed reduction and internal fixation (CRIF) (ICD-9-CM, 79.10/79.19), or external (ICD-9-CM, 78.10/78.19) or internal (ICD-9-CM, 78.50/78.59) fixation without reduction. Demographic variables were then collected, including age, sex, primary diagnosis, associated diagnoses, type of fracture (open vs closed), prevalence of comorbidities, length of stay, and discharge destination. The complication screening package23 was used to determine the incidence of complications. The variable adverse event was created on the basis of the variables postoperative bleeding (998.1), acute postoperative infection (998.5), acute postoperative anemia (285.1), acute renal failure (584), acute myocardial infarction (410), pulmonary embolism (415.1), induced mental disorder (293), pneumonia (480-486), pulmonary insufficiency (518.5), deep venous thrombosis (453.4), intubation (96.xx), and blood transfusion (99.x).
STATISTICAL ANALYSIS
Because of the large sample size, a normal distribution of the data was assumed. Differences between categorical variables were compared using the Pearson chi square test, while the independent-samples t test was used to compare differences between continuous variables. To determine independent predictors of in-hospital outcomes (death, adverse events, requirement for blood transfusion, or treatment with ORIF), all variables present in at least 2% of the population24 were included in a multivariable binary logistic regression model. For in-hospital adverse events, a 1% cutoff was used due to their lower rates of occurrence, as previously described.25The dichotomous variables were death, presence of adverse events, receipt of blood transfusion, and treatment with ORIF. A multivariable regression model allows for the control of potential confounders, isolating the effect of individual variables on inpatient outcomes. Covariates accounted for in the regression model included gender, age, region of the country, and preexisting comorbidities (diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation). To assess the association between individual variables and inpatient outcomes, odds ratios and confidence intervals were calculated. A P value of <.001 was used to define statistical significance, correcting for multiple comparisons, as previously described.25 US census data were used to obtain national population estimates for each year of the study from 1990 to 2010.26 Rates were presented as the number of acetabular fractures per 100,000 standard population. All data were analyzed using the software Statistical Package for the Social Sciences [SPSS] version 20.
RESULTS
INCIDENCE AND DEMOGRAPHICS
A cohort representative of 497,389 patients with a diagnosis of acetabular fracture was identified between 1990 and 2010 (Table 1). In 1990, 19,560 cases (7.84 per 100,000 capita) of acetabular fractures were recoded, while in 2010, the number of cases increased to 29,373 or 9.5 per 100,000 capita (P < .001) (Table 2). The mean age of patients with an acetabular fracture was 52.6 years (standard deviation [SD], 23.7) and 60.6% were male (Table 1). The most frequently associated diagnosis was closed fracture of the pelvis (29.8%) followed by fracture of the femur (13.1%) and closed fracture of the ilium (3.8%) (Table 1). Of the total cohort, 23.2% underwent ORIF (Table 1). In 1990, 12.6% of patients with a diagnosis of acetabular fracture underwent ORIF, whereas 20.4% of patients underwent ORIF in 2010 (P < .001) (Table 2). Average length of hospital stay was 8.3 days (SD, 17.9) overall (Table 1). In 1990 the average length of stay was 17.0 days (SD, 14.9), decreasing to 10.3 days (SD, 9.3) in 2010 (P < .001) (Table 2).
Table 1. Patient Characteristics for Patients with Acetabular Fractures in the United States from 1990 to 2007
Parameter | Total 1990-2010 |
Total Number | 497,389 |
Gender (%) |
|
Male | 60.6 |
Female | 39.4 |
Age, years (%) |
|
<20 | 6.7 |
20-40 | 31.5 |
41-60 | 22.3 |
61-85 | 30.4 |
>85 | 23.5 |
Race (%) |
|
White | 66.4 |
Black | 9.3 |
Asian | 1.7 |
Other | 2.4 |
Not stated | 20.2 |
Primary Diagnosis (%) |
|
Closed fracture of acetabulum (808.0) | 98.9 |
Open fracture of acetabulum (808.1) | 1.1 |
Associated diagnoses (%) |
|
Closed fracture of pubis (808.2) | 26.1 |
Open fracture of pubis (808.3) | 0.1 |
Closed fracture of ischium (808.42) | 1.7 |
Open fracture of ischium (808.52) | 0.0 |
Closed fracture of ilium (808.41) | 3.8 |
Open fracture of ilium (808.51) | 0.0 |
Closed fracture other part pelvis (808.49) | 0.7 |
Open fracture other part pelvis (808.59) | 0.0 |
Multiple closed pelvic fractures (808.43) | 0.5 |
Multiple open pelvic fractures (808.53) | 0.0 |
Any pelvic fracture from above | 29.8 |
Fracture of neck of femur (820) | 7.2 |
Fracture of any part of femur (820/821) | 13.1 |
Head trauma (959.01) | 0.7 |
Head/face trauma (959.0/959.01) | 0.7 |
Chest trauma (959.11) | 0.1 |
Chest/trunk trauma (959.1/959.11) | 0.1 |
Procedures (%) |
|
Open reduction internal fixation (79.30/79.39) | 23.2 |
Closed reduction internal fixation (79.10/79.19) | 1.3 |
External fixation (78.10/78.19) | 0.7 |
Internal fixation without reduction (78.50/78.59) | 0.4 |
Comorbidities (%) |
|
No | 72.9 |
Yes | 27.1 |
Adverse Events (%) |
|
No | 74.1 |
Yes | 25.9 |
Discharge Disposition (%) |
|
Routine/home (1) | 45.4 |
Left against medical advice (2) | 0.2 |
Short term fac (3) | 13.1 |
Long term fac (4) | 22.2 |
Alive, not stated (5) | 12 |
Dead (6) | 3.5 |
Not reported (9) | 3.6 |
Mortality (%) | 3.5 |
Age (y), mean (SD) | 52.6 (23.7) |
Days of Care, mean (SD) | 8.3 (17.9) |
Principal Source of Payment (%) |
|
Private insurance | 39 |
Medicare | 30.5 |
Medicaid | 7.7 |
Other government | 1.9 |
Self-pay | 7.9 |
Workmen’s comp | 4 |
Other | 4.7 |
Not stated | 4.4 |
Abbreviation: SD, standard deviation.
Table 2. Patient Characteristics in 1990, 1995, 1999, 2003, and 2007 Among Patients with Acetabular Fractures
Variable | 1990 | 1995 | 1999 | 2003 | 2007 | 2010 |
Total number | 19,560 | 17,506 | 22,767 | 27,133 | 34,027 | 29,373 |
Incidence per 100,000 capita | 7.84 | 6.57 | 8.16 | 9.35 | 11.30 | 9.5 |
Gender (%) |
| |||||
Male | 51.0 | 70.7 | 61.2 | 62.6 | 62.5 | 64.9 |
Female | 49.0 | 29.3 | 38.8 | 37.4 | 37.5 | 35.1 |
Fracture (%) |
| |||||
Open | 2.1 | 1.7 | 3.3 | 1.4 | 0.1 | 1.8 |
Closed | 97.9 | 98.3 | 96.7 | 98.6 | 99.9 | 98.2 |
Underwent ORIF (%) | 12.6 | 20.9 | 20.2 | 22.9 | 27.8 | 20.4 |
Adverse events (%) | 10.9 | 16.2 | 23.7 | 31 | 35.1 | 37.6 |
Transfusion (%) | 0.3 | 2.2 | 7.4 | 6.5 | 10.5 | 9.5 |
Discharge (%) |
| |||||
Routine | 58 | 65.6 | 35.6 | 45.9 | 40.2 | 41.6 |
Non-routine to inpatient facility | 26.8 | 23.1 | 46.4 | 33.8 | 40.8 | 34.6 |
Mortality (%) | 5.9 | 3.6 | 2 | 2.9 | 1.5 | 0.4 |
Mean Age (y) | 52.9 | 48.4 | 52.3 | 56.3 | 57 | 53.2 |
Mean DOC (days) | 17.0 | 13.4 | 8.7 | 10.8 | 8.5 | 10.3 |
Abbreviations: DOC, days of care; ORIF, open reduction internal fixation.
Continue to: MORTALITY...
MORTALITY
In-hospital mortality decreased from 5.9% in 1990 to 0.4% in 2010 (P < .001) (3.5% for the total cohort) (Tables 1 and 2). Multivariable logistic regression analysis demonstrated pulmonary insufficiency (odds ratio [OR], 9.07; 95% confidence interval [CI], 8.52-9.66; P < .01), pneumonia (OR, 3.22; 95% CI, 3.05-3.39; P < .01), and age >85 years (OR, 2.28; 95% CI, 2.16-2.40; P < .01) to be associated with the highest odds of inpatient mortality. CRIF (OR, 1.99; 95% CI, 1.78-2.23; P < .01), external fixator (OR, 1.82; 95% CI, 1.45-2.29; P < .01), and having received a blood transfusion (OR, 1.81; 95% CI, 1.71-1.91; P < .01) were also associated with increased odds of mortality. Treatment with ORIF (OR, 0.19; 95% CI, 0.17-0.20; P < .01) was independently associated with decreased odds of inpatient mortality, as was age <20 years (OR, 0.26; 95% CI, 0.23-0.30; P < .01) (model fit: for omnibus test of model coefficients, X = 25,966 P < .01; Nagelkerke, R2 = 0.20) (Table 3).
Table 3. Logistic Regression for Predictors of Mortality Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Pulmonary insufficiency | 9.07 (8.52–9.66) | < 0.01 |
Pneumonia | 3.22 (3.05–3.39) | < 0.01 |
Age >85 years | 2.28 (2.16–2.40) | < 0.01 |
Closed reduction internal fixation | 1.99 (1.78–2.23) | < 0.01 |
External Fixator | 1.82 (1.45–2.29) | < 0.01 |
Blood transfusion | 1.81 (1.71–1.91) | < 0.01 |
Gender (male) | 1.76 (1.70–1.83) | < 0.01 |
Associated femoral neck fracture | 1.23 (1.15–1.30) | < 0.01 |
Age 41-60 years | 1.19 (1.11–1.29) | < 0.01 |
Age 61-85 years | 1.17 (1.11–1.23) | < 0.01 |
Congestive heart failure | 1.14 (1.07–1.22) | < 0.01 |
Associated pelvic fracture | 1.13 (1.10–1.17) | < 0.01 |
Geographic region | 1.11 (1.09–1.12) | < 0.01 |
Source of payment | 1.02 (1.01–1.02) | < 0.01 |
Race | 0.99 (0.98–0.99) | < 0.01 |
DOC | 0.98 (0.98–0.98) | < 0.01 |
Hypertension | 0.67 (0.64–0.71) | < 0.01 |
Atrial fibrillation | 0.52 (0.48–0.57) | < 0.01 |
Diabetes mellitus | 0.35 (0.32–0.38) | < 0.01 |
Age 20-40 years | 0.32 (0.30–0.35) | < 0.01 |
Age <20 years | 0.26 (0.23–0.30) | < 0.01 |
Coronary artery disease | 0.21 (0.18–0.24) | < 0.01 |
Open reduction internal fixation | 0.19 (0.17–0.20) | < 0.01 |
Omnibus X 25,966, P < .01 | ||
Nagelkerke R2= 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
COMORBIDITIES AND ADVERSE EVENTS
The prevalence of comorbidities and adverse events is listed in Tables 4 and 5, respectively. Hypertensive disease was the most common comorbidity at 15.3%, followed by diabetes mellitus at 6.9%. Overall, 25.9% of patients experienced an in-hospital adverse event, with the most common being postoperative anemia (7.3%) and blood transfusion (8.1%) (Tables 1 and 5). The percentage of patients experiencing an adverse event increased from 10.9% in 1990 to 37.6% in 2010 (P < .01) (Table 2). Multivariable logistic regression analysis revealed CRIF (OR, 3.08; 95% CI, 2.91-3.26; P < .01), coronary artery disease (OR, 2.02; 95% CI, 1.91-2.15; P < .01), associated femoral neck fracture (OR, 1.53; 95% CI, 1.47-1.60; P < .01), and ORIF (OR, 1.22; 95% CI, 1.20-1.24; P < .01) to be associated with higher odds of inpatient adverse events (model fit: for omnibus test of model coefficients, X = 160,275, P < .01; Nagelkerke, R2 = 0.41) (Table 6).
Table 4. Prevalence of Comorbidities in Patients with Acetabular Fractures Between 1990 and 2007 (n = 403.927)
Parameter (ICD-9) | Percentage of Total |
Hypertensive disease (401–405) | 15.3% |
Diabetes mellitus (250) | 6.9% |
Atrial fibrillation (427.31) | 4.0% |
Congestive heart failure (428) | 3.9% |
Osteoporosis (733.0) | 2.1% |
Coronary artery disease (414.01) | 2.0% |
Obesity (278.00, 278.01) | 2.0% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 5. Prevalence of In-Hospital Adverse Events Among Patients with Acetabular Fractures Between 1990 and 2007 (n = 403,927)
Parameter (ICD-9) | Percentage of Total |
Transfusion of blood (99.0) | 8.1% |
Acute postoperative anemia (285.1) | 7.3% |
Intubation (96.x) | 4.9% |
Acute renal failure (584) | 3.4% |
Pneumonia (480-486) | 3.2% |
Pulmonary insufficiency (518.5) | 2.3% |
Pulmonary embolism (415.1) | 1.6% |
Deep venous thrombosis (453.4) | 1.0% |
Acute myocardial infarction (410) | 0.9% |
Postoperative bleeding (998.1) | 0.7% |
Acute postoperative infection (998.5) | 0.5% |
Induced mental disorder (293) | 0.4% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 6. Logistic Regression for Predictors of Adverse Events Among Patients Hospitalized for Acetabular Fracture (n = 403,927)
Variable | OR (95% CI) | P |
Closed reduction internal fixation | 3.08 (2.91-3.26) | < 0.01 |
Coronary artery disease | 2.02 (1.91-2.15) | < 0.01 |
Associated femoral neck fracture | 1.53 (1.47-1.60) | < 0.01 |
Open reduction internal fixation | 1.22 (1.20-1.24) | < 0.01 |
Gender (male) | 1.16 (1.14-1.18) | < 0.01 |
Associated fracture of any part of femur | 1.13 (1.10-1.17) | < 0.01 |
Age >85 years | 1.08 (1.05-1.12) | < 0.01 |
Geographic region | 1.07 (1.06-1.07) | < 0.01 |
DOC | 1.04 (1.04-1.04) | < 0.01 |
Race | 1.02 (1.02-1.03) | < 0.01 |
Source of payment | 1.01 (1.01-1.01) | < 0.01 |
Congestive heart failure | 1.01 (0.96-1.06) | 0.78 |
Atrial fibrillation | 0.88 (0.84-0.92) | < 0.01 |
Age 61-85 years | 0.68 (0.66-0.71) | < 0.01 |
Age <20 years | 0.67 (0.64-0.70) | < 0.01 |
Associated pelvis fracture | 0.64 (0.63-0.66) | < 0.01 |
Age 41-60 years | 0.58 (0.56-0.61) | < 0.01 |
Diabetes mellitus | 0.48 (0.46-0.50) | < 0.01 |
Age 20-40 years | 0.45 (0.43-0.47) | < 0.01 |
Hypertension | 0.44 (0.43-0.45) | < 0.01 |
External Fixator | 0.39 (0.35-0.44) | < 0.01 |
Omnibus X 160,275, P < .01 | ||
Nagelkerke R2 = 0.41 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
BLOOD TRANSFUSION
Overall, 7.3% of patients experienced acute postoperative anemia (Table 5). Between 1990 and 2010, the percentage of patients receiving blood transfusions increased from 0.3% to 9.5%, respectively (P < .01) (Table 2). In multivariable logistic regression analysis, patients treated with ORIF (OR, 8.13; 95% CI, 7.91-8.36; P < .01), those with congestive heart failure (OR, 4.23; 95% CI, 4.06-4.41; P < .01), those with an associated femur fracture (OR, 3.13; 95% CI, 2.99-3.27; P < .01), those with atrial fibrillation (OR, 1.96; 95% CI, 1.88-2.05; P < .01), and those treated with CRIF (OR, 1.42; 95% CI, 1.29-1.56; P < .01) were associated with significantly higher odds of blood transfusion (model fit: omnibus test of model coefficients, X = 42,653, P < .01; Nagelkerke, R2 = 0.19) (Table 7).
Table 7. Logistic Regression for Predictors of the Requirement for Blood Transfusion Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Open reduction internal fixation | 8.13 (7.91-8.36) | < 0.01 |
Congestive heart failure | 4.23 (4.06-4.41) | < 0.01 |
Associated fracture of any part of femur | 3.13 (2.99-3.27) | < 0.01 |
Atrial fibrillation | 1.96 (1.88-2.05) | < 0.01 |
Closed reduction internal fixation | 1.42 (1.29-1.56) | < 0.01 |
Geographic region | 1.38 (1.36-1.39) | < 0.01 |
Hypertension | 1.38 (1.34-1.42) | < 0.01 |
Associated pelvic fracture | 1.28 (1.25-1.31) | < 0.01 |
Age 61-85 years | 1.06 (1.02-1.11) | 0.01 |
Source of payment | 0.99 (0.98-0.99) | < 0.01 |
Race | 0.98 (0.97-0.98) | < 0.01 |
DOC | 0.96 (0.96-0.96) | < 0.01 |
Age >85 years | 0.74 (0.72-0.77) | < 0.01 |
External fixator | 0.69 (0.59-0.80) | < 0.01 |
Coronary artery disease | 0.62 (0.57-0.68) | < 0.01 |
Age 41-60 years | 0.57 (0.54-0.60) | < 0.01 |
Gender (male) | 0.54 (0.52-0.55) | < 0.01 |
Diabetes mellitus | 0.38 (0.36-0.41) | < 0.01 |
Age 20-40 years | 0.32 (0.30-0.34) | < 0.01 |
Associated femoral neck fracture | 0.29 (0.27-0.31) | < 0.01 |
Age <20 years | 0.24 (0.22-0.26) | < 0.01 |
Omnibus X = 42,653, P < .01 | ||
Nagelkerke R2 = 0.19 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
TREATMENT WITH ORIF
Over the 20-year study period, 23.2% of patients with acetabular fractures were treated with ORIF (Table 1). In 1990, 12.6% of patients underwent ORIF, while in 2010 this percentage increased to 20.4% (P < .001) (Table 2). Multivariable logistic regression analysis demonstrated that age between 41 and 60 years (OR, 1.88; 95% CI, 1.78-1.98; P < .01) was associated with the highest odds of undergoing ORIF. Age 20 to 40 years (OR, 1.86; 95% CI, 1.76-1.97; P < .01), age <20 years (OR, 1.82; 95% CI, 1.72-1.93; P < .01), and male gender (OR, 1.65; 95% CI, 1.63-1.68; P < .01) were also associated with being treated by ORIF. In contrast, coronary artery disease (OR, 0.27; 95% CI, 0.25-0.30; P < .01), age >85 years (OR, 0.46; 95% CI, 0.44-0.47; P < .01), and congestive heart failure (OR, 0.48; 95% CI, 0.46-0.51; P < .01) were associated with the lowest odds of undergoing ORIF (model fit: omnibus test of model coefficients, X = 71,118, P < .01; Nagelkerke, R2 = 0.20) (Table 8).
Table 8. Logistic Regression for Predictors of the Requirement for Discharge to Another Inpatient Facility Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Age 41-60 years | 1.88 (1.78-1.98) | < 0.01 |
Age 20-40 years | 1.86 (1.76-1.97) | < 0.01 |
Age <20 years | 1.82 (1.72-1.93) | < 0.01 |
Gender (male) | 1.65 (1.63-1.68) | < 0.01 |
Larger hospital bed size | 1.46 (1.45-1.47) | < 0.01 |
Hypertension | 1.35 (1.32-1.38) | < 0.01 |
Diabetes mellitus | 1.09 (1.05-1.13) | < 0.01 |
DOC | 1.02 (1.02-1.02) | < 0.01 |
Source of payment | 1.01 (1.01-1.02) | < 0.01 |
Race | 1.00 (0.99-1.00) | 0.17 |
Age 61-85 years | 0.94 (0.90-0.99) | 0.02 |
Region | 0.92 (0.91-0.93) | < 0.01 |
Atrial fibrillation | 0.83 (0.79-0.87) | < 0.01 |
Congestive heart failure | 0.48 (0.46-0.51) | < 0.01 |
Age >85 years | 0.46 (0.44-0.47) | < 0.01 |
Coronary artery disease | 0.27 (0.25-0.30) | < 0.01 |
Omnibus X 71,118, P < .01 | ||
Nagelkerke R2 = 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
Continue to: DISCUSSION...
DISCUSSION
This study evaluates the incidence of acetabular fractures in the US between 1990 and 2010, and identifies prognostic factors associated with complications and death. The study demonstrates an increase in the population-adjusted incidence of acetabular fractures between 1990 and 2010 (7.84 cases per 100,000 capita to 9.5 cases per 100,000 capita), in contrast to the decreasing trend reported by Mauffrey and colleagues.11 Some studies suggest that up to 80% of acetabular fractures are associated with motor vehicle collisions and motorcycle accidents.9,27 While the rate of motor vehicle accidents has remained stable over the study period, motorcycle ownership and deaths more than doubled between 2001 and 2008,28 primarily among individuals over 40 years of age. In this study, the mean age of patients with acetabular fractures ranged from 48 to 57 years. The dramatic increase in motorcycle ownership and deaths in these age groups may partially explain the rising incidence of acetabular fractures. The other possibility is that changes in automobile design and safety equipment may have altered the injury patterns observed in patients surviving motor vehicle crashes. Compared to the United Kingdom, in which studies report a fixed incidence of 3 per 100,000 capita1 between 1988 and 2003, the incidence of acetabular fractures in the US is greater. In contrast, the incidence of acetabular fractures reported in this study is less than the 20 per 100,000 reported in Sweden between 1976 and 1985,29 or the 37 per 100,000 reported in Rochester, Minnesota between 1968 and 1977,30 which may be due to increased seatbelt usage.31
In addition to the national incidence, this study demonstrated that the proportion of patients with acetabular fractures treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010. This is substantially lower than the 77% reported by Ochs and colleagues32 in a German population. Concurrent with the increase in ORIF, there was a decrease in in-hospital mortality from 5.9% in 1990 to 0.4% in 2010. The initial mortality rates in this study are comparable to much earlier reports and some small studies,9,32-37 but the rates reported in the later years of this study show a substantial decrease that is likely a more accurate estimation of the current incidence. The improved survival rates may be due to advances in the operative treatment of acetabular fractures, in which mechanical stabilization allows for early patient mobilization and facilitation of optimal nursing care.38 With ORIF becoming the standard of care for displaced acetabular fractures,9 numerous reports have demonstrated an association between early definitive fixation and improved survival.17,39,40 This is similar to our study, which found ORIF to be associated with the lowest odds of mortality in multivariate logistic regression analysis. It is possible that advances in patient care by intensivists over this period have also contributed to the decrease in mortality, but the correlation with operative treatment in this study is very strong and agrees well with prior studies.16 Moreover, multiple studies have demonstrated decreased in-hospital mortality among patients undergoing various orthopedic surgical procedures during this period.41-43 The correlation with operative treatment in this study agrees well with prior studies.16
In contrast, higher odds of mortality were seen in patients over the age of 85 years with pulmonary insufficiency, congestive heart failure, pneumonia, or an associated femur or pelvic fracture. This is similar to prior reports in which patients with combined acetabulum and pelvic ring injuries fared worse than those with isolated injures,44,45 as did patients with associated non-musculoskeletal injuries.46 The finding that age over 85 years was associated with higher odds of mortality likely reflects the increased number of comorbidities and decreased physiologic reserve seen in this patient population. Finally, male gender was associated with higher odds of in-hospital mortality. There are 2 possible explanations for this: Either there is gender dimorphism in sex hormones and cytokine activity in response to hemorrhage and sepsis,38,47 or there is a greater tendency for males to be involved in higher energy accidents with more severe concomitant injuries.
The results of multivariable regression analysis demonstrated that patients were more likely to require blood transfusion if they were managed surgically or had atrial fibrillation, congestive heart failure, or associated femur fracture. Not surprisingly, concurrent pelvic fracture was also associated with higher odds of blood transfusion, as pelvic hemorrhage is reported to be the cause of death in up to half of patients who die following a pelvic fracture.46
Between 1990 and 2010, in-hospital days of care decreased from 17.0 days to 10.3 days. While a decreased length of stay has been demonstrated in other orthopedic conditions over the study period,41 it is possible the decrease in length of stay demonstrated in this study is due to improved surgical technique and the implementation of early surgical intervention.39,48-50 Plaisier and colleagues17 demonstrated superior functional outcomes, quicker return to baseline function, and decreased length of stay in patients treated with early ORIF of their acetabular fractures. Other studies have shown that the benefits of early surgery include improved reduction quality and ease of reduction,51 as well as control of bleeding, pain relief, and mobilization of the patient.39 Another possible explanation for the decreased length of stay is the increased rate of discharge to other inpatient facilities, such as rehabilitation facilities, which was demonstrated in this study.
Continue to: Interestingly, male gender and younger age...
Interestingly, male gender and younger age were associated with operative management of the acetabular fracture. In contrast, there was a decreased likelihood of operative treatment among elderly patients and those patients with cardiac comorbidities. It is possible that the relationship we found between the likelihood of ORIF and age relates to the bimodal distribution of fractures, with higher energy and potentially more displaced fractures occurring in younger patients3-5 and lower energy fractures in the elderly.
In contrast to decreasing in-hospital days of care, there was a rise in the number of adverse events between 1990 (10.9%) and 2010 (37.6%). This can be partially attributed to the increased rates of blood transfusion, which was received by 9.5% of patients with acetabular fractures in the final study year. Additionally, surgical intervention was associated with increased adverse events in this study, and surgical intervention increased over the study period. Other factors that may have contributed to an increase in adverse events include an aging population,52 as advanced age was independently associated with higher odds of adverse events in this study.
Despite the strengths of using large, national databases for epidemiological research,53 this study has several limitations. Like all large databases, the NHDS is subject to error in coding and data entry.54 Additionally, the database only allows for 7 diagnostic codes and 4 procedure codes per entry. As a result, the prevalence of comorbid conditions and adverse events may be underreported.25 Moreover, the severity of a comorbid disease cannot be appreciated when dichotomously classified.55 Another limitation is that the database only provides inpatient data, so complications that arise after discharge, as well as follow-up data, are unknown. Furthermore, the results of this study are limited to practice patterns in the US from 1990 to 2010. This database does not provide injury mechanisms, so we cannot distinguish between high-energy and low-energy injuries. Lastly, analysis of the different types of acetabular fractures was not performed since classification of acetabular fractures cannot be assessed with ICD-9 codes.
CONCLUSION
This study is the largest epidemiologic analysis of acetabular fractures in the US and also provides predictors of in-hospital mortality. The incidence of acetabular fractures in the US is increasing, while mortality is decreasing. Identifying risk factors associated with poor outcomes has the potential to change treatment strategies, resource allocation, in-hospital monitoring, and discharge planning for this patient population.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The incidence of acetabular fractures and associated in-hospital complication rates in the United States are poorly defined. Studies evaluating predictors of outcome for isolated acetabular fractures are weakly generalizable due to small sample sizes or the inclusion of all types of pelvic fractures. This study sought to analyze trends in acetabular fractures and associated complications in the US using the largest and most recent national dataset available.
The National Hospital Discharge Survey was queried to identify all patients admitted to US hospitals with acetabular fractures between 1990 and 2010. A representative cohort of 497,389 patients was identified, and multivariable logistic regression was used to identify independent predictors of mortality, adverse events, requirement of blood transfusion, and operative treatment with open reduction and internal fixation (ORIF).
Between 1990 and 2010, the population-adjusted incidence of acetabular fractures increased from 7.8 to 9.5/100,000 capita (P < .001). Mortality declined from 5.9% to 0.4% (P < .001), paralleling an increase in the proportion of patients treated with ORIF (12.6%-20.4%, P < .001), which was the variable associated with the lowest odds of mortality. Surgical intervention was associated with higher odds of adverse events and a requirement for blood transfusion. The average in-hospital length of stay decreased from 17.0 days to 10.3 days (P < .001).
This study provides the largest and most comprehensive epidemiologic analysis of acetabular fractures in the US. Knowledge of the increasing incidence of acetabular fractures and prognostic factors associated with poor outcomes may improve outcomes.
Continue to: Acetabular fractures are major injuries...
Acetabular fractures are major injuries frequently associated with life-altering sequelae1 and a significant resulting cost to society.2 Acetabular fractures are most often the result of a high-energy trauma3-5 or fall from a height.5,6 Functional outcomes and the prevention of post-traumatic arthritis have been shown to depend upon the accuracy of operative reduction.7-9 However, literature on the epidemiology of acetabular fractures is largely limited to European countries,1,10 and their incidence in the United States is more poorly defined.11 Published mortality rates in the existing literature vary widely from 2% to 45%,12-14 and few studies have identified the risk factors associated with in-hospital complications.15 While age, gender, and high-velocity mechanisms have been linked to increased mortality and complications,14-16 the evidence for these associations is poorly generalizable due to the inclusion of all pelvic fractures in these studies. Some reports suggest that advances in surgical management have improved survival and functional outcome,15,17 but these are based upon small cohorts. Knowledge of the incidence and patterns of disease burden are crucial for the allocation of limited healthcare resources.
This study sought to describe the trends in incidence as well as the factors influencing mortality and the risk of complications for patients admitted to US hospitals with an acetabular fracture using the National Hospital Discharge Survey (NHDS), the most recently available Centers for Disease Control and Prevention data, which is also one of the largest inpatient databases in the US. Knowledge of the factors influencing outcomes for patients admitted with acetabular fractures may improve management and decrease complications.
METHODS
NATIONAL HOSPITAL DISCHARGE SURVEY
The NHDS, developed by the National Center for Healthcare Statistics division of the Centers for Disease Control and Prevention,18 was used to estimate the incidence of acetabular fractures and to evaluate the risk factors for ensuing mortality and inpatient complications. The NHDS is a publically available survey providing demographic and medical data for inpatients discharged from non-federal, short-stay hospitals in the US.19 The NHDS is the principal database used by the US government for monitoring hospital use and is considered the most comprehensive of all inpatient surgical databases in use today.19 The survey uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes20 to classify medical diagnoses and procedures. The NHDS uses a stratified, multistage probability design to collect demographic information (age, gender, race), expected source of payment (insurance status), medical information of up to 7 discharge diagnoses and up to 4 procedures, length of care, hospital size, US region, and inpatient outcomes including discharge destination.21 To ensure unbiased national sampling of inpatient records, the NHDS uses a complex, 3-stage probability design including inflation by reciprocals of the probabilities of sample selection, adjustment for no response, and population weighting ratio adjustments.19 This study did not require approval by the Institutional Review Board because the NHDS is a publically available database with no patient-identifying information.
Continue to: PATIENT SELECTION...
PATIENT SELECTION
All patients admitted to hospitals in the US with a fracture of the acetabulum between 1990 and 2010 were identified using ICD-9-CM codes. Discharges with a diagnosis code (ICD-9-CM) of closed fracture of the acetabulum (808.0) or open fracture of the acetabulum (808.1) were identified using previously described techniques.22 The database was subsequently queried to identify patients treated using open reduction and internal fixation (ORIF) (ICD-9-CM, 79.30/79.39), closed reduction and internal fixation (CRIF) (ICD-9-CM, 79.10/79.19), or external (ICD-9-CM, 78.10/78.19) or internal (ICD-9-CM, 78.50/78.59) fixation without reduction. Demographic variables were then collected, including age, sex, primary diagnosis, associated diagnoses, type of fracture (open vs closed), prevalence of comorbidities, length of stay, and discharge destination. The complication screening package23 was used to determine the incidence of complications. The variable adverse event was created on the basis of the variables postoperative bleeding (998.1), acute postoperative infection (998.5), acute postoperative anemia (285.1), acute renal failure (584), acute myocardial infarction (410), pulmonary embolism (415.1), induced mental disorder (293), pneumonia (480-486), pulmonary insufficiency (518.5), deep venous thrombosis (453.4), intubation (96.xx), and blood transfusion (99.x).
STATISTICAL ANALYSIS
Because of the large sample size, a normal distribution of the data was assumed. Differences between categorical variables were compared using the Pearson chi square test, while the independent-samples t test was used to compare differences between continuous variables. To determine independent predictors of in-hospital outcomes (death, adverse events, requirement for blood transfusion, or treatment with ORIF), all variables present in at least 2% of the population24 were included in a multivariable binary logistic regression model. For in-hospital adverse events, a 1% cutoff was used due to their lower rates of occurrence, as previously described.25The dichotomous variables were death, presence of adverse events, receipt of blood transfusion, and treatment with ORIF. A multivariable regression model allows for the control of potential confounders, isolating the effect of individual variables on inpatient outcomes. Covariates accounted for in the regression model included gender, age, region of the country, and preexisting comorbidities (diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation). To assess the association between individual variables and inpatient outcomes, odds ratios and confidence intervals were calculated. A P value of <.001 was used to define statistical significance, correcting for multiple comparisons, as previously described.25 US census data were used to obtain national population estimates for each year of the study from 1990 to 2010.26 Rates were presented as the number of acetabular fractures per 100,000 standard population. All data were analyzed using the software Statistical Package for the Social Sciences [SPSS] version 20.
RESULTS
INCIDENCE AND DEMOGRAPHICS
A cohort representative of 497,389 patients with a diagnosis of acetabular fracture was identified between 1990 and 2010 (Table 1). In 1990, 19,560 cases (7.84 per 100,000 capita) of acetabular fractures were recoded, while in 2010, the number of cases increased to 29,373 or 9.5 per 100,000 capita (P < .001) (Table 2). The mean age of patients with an acetabular fracture was 52.6 years (standard deviation [SD], 23.7) and 60.6% were male (Table 1). The most frequently associated diagnosis was closed fracture of the pelvis (29.8%) followed by fracture of the femur (13.1%) and closed fracture of the ilium (3.8%) (Table 1). Of the total cohort, 23.2% underwent ORIF (Table 1). In 1990, 12.6% of patients with a diagnosis of acetabular fracture underwent ORIF, whereas 20.4% of patients underwent ORIF in 2010 (P < .001) (Table 2). Average length of hospital stay was 8.3 days (SD, 17.9) overall (Table 1). In 1990 the average length of stay was 17.0 days (SD, 14.9), decreasing to 10.3 days (SD, 9.3) in 2010 (P < .001) (Table 2).
Table 1. Patient Characteristics for Patients with Acetabular Fractures in the United States from 1990 to 2007
Parameter | Total 1990-2010 |
Total Number | 497,389 |
Gender (%) |
|
Male | 60.6 |
Female | 39.4 |
Age, years (%) |
|
<20 | 6.7 |
20-40 | 31.5 |
41-60 | 22.3 |
61-85 | 30.4 |
>85 | 23.5 |
Race (%) |
|
White | 66.4 |
Black | 9.3 |
Asian | 1.7 |
Other | 2.4 |
Not stated | 20.2 |
Primary Diagnosis (%) |
|
Closed fracture of acetabulum (808.0) | 98.9 |
Open fracture of acetabulum (808.1) | 1.1 |
Associated diagnoses (%) |
|
Closed fracture of pubis (808.2) | 26.1 |
Open fracture of pubis (808.3) | 0.1 |
Closed fracture of ischium (808.42) | 1.7 |
Open fracture of ischium (808.52) | 0.0 |
Closed fracture of ilium (808.41) | 3.8 |
Open fracture of ilium (808.51) | 0.0 |
Closed fracture other part pelvis (808.49) | 0.7 |
Open fracture other part pelvis (808.59) | 0.0 |
Multiple closed pelvic fractures (808.43) | 0.5 |
Multiple open pelvic fractures (808.53) | 0.0 |
Any pelvic fracture from above | 29.8 |
Fracture of neck of femur (820) | 7.2 |
Fracture of any part of femur (820/821) | 13.1 |
Head trauma (959.01) | 0.7 |
Head/face trauma (959.0/959.01) | 0.7 |
Chest trauma (959.11) | 0.1 |
Chest/trunk trauma (959.1/959.11) | 0.1 |
Procedures (%) |
|
Open reduction internal fixation (79.30/79.39) | 23.2 |
Closed reduction internal fixation (79.10/79.19) | 1.3 |
External fixation (78.10/78.19) | 0.7 |
Internal fixation without reduction (78.50/78.59) | 0.4 |
Comorbidities (%) |
|
No | 72.9 |
Yes | 27.1 |
Adverse Events (%) |
|
No | 74.1 |
Yes | 25.9 |
Discharge Disposition (%) |
|
Routine/home (1) | 45.4 |
Left against medical advice (2) | 0.2 |
Short term fac (3) | 13.1 |
Long term fac (4) | 22.2 |
Alive, not stated (5) | 12 |
Dead (6) | 3.5 |
Not reported (9) | 3.6 |
Mortality (%) | 3.5 |
Age (y), mean (SD) | 52.6 (23.7) |
Days of Care, mean (SD) | 8.3 (17.9) |
Principal Source of Payment (%) |
|
Private insurance | 39 |
Medicare | 30.5 |
Medicaid | 7.7 |
Other government | 1.9 |
Self-pay | 7.9 |
Workmen’s comp | 4 |
Other | 4.7 |
Not stated | 4.4 |
Abbreviation: SD, standard deviation.
Table 2. Patient Characteristics in 1990, 1995, 1999, 2003, and 2007 Among Patients with Acetabular Fractures
Variable | 1990 | 1995 | 1999 | 2003 | 2007 | 2010 |
Total number | 19,560 | 17,506 | 22,767 | 27,133 | 34,027 | 29,373 |
Incidence per 100,000 capita | 7.84 | 6.57 | 8.16 | 9.35 | 11.30 | 9.5 |
Gender (%) |
| |||||
Male | 51.0 | 70.7 | 61.2 | 62.6 | 62.5 | 64.9 |
Female | 49.0 | 29.3 | 38.8 | 37.4 | 37.5 | 35.1 |
Fracture (%) |
| |||||
Open | 2.1 | 1.7 | 3.3 | 1.4 | 0.1 | 1.8 |
Closed | 97.9 | 98.3 | 96.7 | 98.6 | 99.9 | 98.2 |
Underwent ORIF (%) | 12.6 | 20.9 | 20.2 | 22.9 | 27.8 | 20.4 |
Adverse events (%) | 10.9 | 16.2 | 23.7 | 31 | 35.1 | 37.6 |
Transfusion (%) | 0.3 | 2.2 | 7.4 | 6.5 | 10.5 | 9.5 |
Discharge (%) |
| |||||
Routine | 58 | 65.6 | 35.6 | 45.9 | 40.2 | 41.6 |
Non-routine to inpatient facility | 26.8 | 23.1 | 46.4 | 33.8 | 40.8 | 34.6 |
Mortality (%) | 5.9 | 3.6 | 2 | 2.9 | 1.5 | 0.4 |
Mean Age (y) | 52.9 | 48.4 | 52.3 | 56.3 | 57 | 53.2 |
Mean DOC (days) | 17.0 | 13.4 | 8.7 | 10.8 | 8.5 | 10.3 |
Abbreviations: DOC, days of care; ORIF, open reduction internal fixation.
Continue to: MORTALITY...
MORTALITY
In-hospital mortality decreased from 5.9% in 1990 to 0.4% in 2010 (P < .001) (3.5% for the total cohort) (Tables 1 and 2). Multivariable logistic regression analysis demonstrated pulmonary insufficiency (odds ratio [OR], 9.07; 95% confidence interval [CI], 8.52-9.66; P < .01), pneumonia (OR, 3.22; 95% CI, 3.05-3.39; P < .01), and age >85 years (OR, 2.28; 95% CI, 2.16-2.40; P < .01) to be associated with the highest odds of inpatient mortality. CRIF (OR, 1.99; 95% CI, 1.78-2.23; P < .01), external fixator (OR, 1.82; 95% CI, 1.45-2.29; P < .01), and having received a blood transfusion (OR, 1.81; 95% CI, 1.71-1.91; P < .01) were also associated with increased odds of mortality. Treatment with ORIF (OR, 0.19; 95% CI, 0.17-0.20; P < .01) was independently associated with decreased odds of inpatient mortality, as was age <20 years (OR, 0.26; 95% CI, 0.23-0.30; P < .01) (model fit: for omnibus test of model coefficients, X = 25,966 P < .01; Nagelkerke, R2 = 0.20) (Table 3).
Table 3. Logistic Regression for Predictors of Mortality Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Pulmonary insufficiency | 9.07 (8.52–9.66) | < 0.01 |
Pneumonia | 3.22 (3.05–3.39) | < 0.01 |
Age >85 years | 2.28 (2.16–2.40) | < 0.01 |
Closed reduction internal fixation | 1.99 (1.78–2.23) | < 0.01 |
External Fixator | 1.82 (1.45–2.29) | < 0.01 |
Blood transfusion | 1.81 (1.71–1.91) | < 0.01 |
Gender (male) | 1.76 (1.70–1.83) | < 0.01 |
Associated femoral neck fracture | 1.23 (1.15–1.30) | < 0.01 |
Age 41-60 years | 1.19 (1.11–1.29) | < 0.01 |
Age 61-85 years | 1.17 (1.11–1.23) | < 0.01 |
Congestive heart failure | 1.14 (1.07–1.22) | < 0.01 |
Associated pelvic fracture | 1.13 (1.10–1.17) | < 0.01 |
Geographic region | 1.11 (1.09–1.12) | < 0.01 |
Source of payment | 1.02 (1.01–1.02) | < 0.01 |
Race | 0.99 (0.98–0.99) | < 0.01 |
DOC | 0.98 (0.98–0.98) | < 0.01 |
Hypertension | 0.67 (0.64–0.71) | < 0.01 |
Atrial fibrillation | 0.52 (0.48–0.57) | < 0.01 |
Diabetes mellitus | 0.35 (0.32–0.38) | < 0.01 |
Age 20-40 years | 0.32 (0.30–0.35) | < 0.01 |
Age <20 years | 0.26 (0.23–0.30) | < 0.01 |
Coronary artery disease | 0.21 (0.18–0.24) | < 0.01 |
Open reduction internal fixation | 0.19 (0.17–0.20) | < 0.01 |
Omnibus X 25,966, P < .01 | ||
Nagelkerke R2= 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
COMORBIDITIES AND ADVERSE EVENTS
The prevalence of comorbidities and adverse events is listed in Tables 4 and 5, respectively. Hypertensive disease was the most common comorbidity at 15.3%, followed by diabetes mellitus at 6.9%. Overall, 25.9% of patients experienced an in-hospital adverse event, with the most common being postoperative anemia (7.3%) and blood transfusion (8.1%) (Tables 1 and 5). The percentage of patients experiencing an adverse event increased from 10.9% in 1990 to 37.6% in 2010 (P < .01) (Table 2). Multivariable logistic regression analysis revealed CRIF (OR, 3.08; 95% CI, 2.91-3.26; P < .01), coronary artery disease (OR, 2.02; 95% CI, 1.91-2.15; P < .01), associated femoral neck fracture (OR, 1.53; 95% CI, 1.47-1.60; P < .01), and ORIF (OR, 1.22; 95% CI, 1.20-1.24; P < .01) to be associated with higher odds of inpatient adverse events (model fit: for omnibus test of model coefficients, X = 160,275, P < .01; Nagelkerke, R2 = 0.41) (Table 6).
Table 4. Prevalence of Comorbidities in Patients with Acetabular Fractures Between 1990 and 2007 (n = 403.927)
Parameter (ICD-9) | Percentage of Total |
Hypertensive disease (401–405) | 15.3% |
Diabetes mellitus (250) | 6.9% |
Atrial fibrillation (427.31) | 4.0% |
Congestive heart failure (428) | 3.9% |
Osteoporosis (733.0) | 2.1% |
Coronary artery disease (414.01) | 2.0% |
Obesity (278.00, 278.01) | 2.0% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 5. Prevalence of In-Hospital Adverse Events Among Patients with Acetabular Fractures Between 1990 and 2007 (n = 403,927)
Parameter (ICD-9) | Percentage of Total |
Transfusion of blood (99.0) | 8.1% |
Acute postoperative anemia (285.1) | 7.3% |
Intubation (96.x) | 4.9% |
Acute renal failure (584) | 3.4% |
Pneumonia (480-486) | 3.2% |
Pulmonary insufficiency (518.5) | 2.3% |
Pulmonary embolism (415.1) | 1.6% |
Deep venous thrombosis (453.4) | 1.0% |
Acute myocardial infarction (410) | 0.9% |
Postoperative bleeding (998.1) | 0.7% |
Acute postoperative infection (998.5) | 0.5% |
Induced mental disorder (293) | 0.4% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 6. Logistic Regression for Predictors of Adverse Events Among Patients Hospitalized for Acetabular Fracture (n = 403,927)
Variable | OR (95% CI) | P |
Closed reduction internal fixation | 3.08 (2.91-3.26) | < 0.01 |
Coronary artery disease | 2.02 (1.91-2.15) | < 0.01 |
Associated femoral neck fracture | 1.53 (1.47-1.60) | < 0.01 |
Open reduction internal fixation | 1.22 (1.20-1.24) | < 0.01 |
Gender (male) | 1.16 (1.14-1.18) | < 0.01 |
Associated fracture of any part of femur | 1.13 (1.10-1.17) | < 0.01 |
Age >85 years | 1.08 (1.05-1.12) | < 0.01 |
Geographic region | 1.07 (1.06-1.07) | < 0.01 |
DOC | 1.04 (1.04-1.04) | < 0.01 |
Race | 1.02 (1.02-1.03) | < 0.01 |
Source of payment | 1.01 (1.01-1.01) | < 0.01 |
Congestive heart failure | 1.01 (0.96-1.06) | 0.78 |
Atrial fibrillation | 0.88 (0.84-0.92) | < 0.01 |
Age 61-85 years | 0.68 (0.66-0.71) | < 0.01 |
Age <20 years | 0.67 (0.64-0.70) | < 0.01 |
Associated pelvis fracture | 0.64 (0.63-0.66) | < 0.01 |
Age 41-60 years | 0.58 (0.56-0.61) | < 0.01 |
Diabetes mellitus | 0.48 (0.46-0.50) | < 0.01 |
Age 20-40 years | 0.45 (0.43-0.47) | < 0.01 |
Hypertension | 0.44 (0.43-0.45) | < 0.01 |
External Fixator | 0.39 (0.35-0.44) | < 0.01 |
Omnibus X 160,275, P < .01 | ||
Nagelkerke R2 = 0.41 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
BLOOD TRANSFUSION
Overall, 7.3% of patients experienced acute postoperative anemia (Table 5). Between 1990 and 2010, the percentage of patients receiving blood transfusions increased from 0.3% to 9.5%, respectively (P < .01) (Table 2). In multivariable logistic regression analysis, patients treated with ORIF (OR, 8.13; 95% CI, 7.91-8.36; P < .01), those with congestive heart failure (OR, 4.23; 95% CI, 4.06-4.41; P < .01), those with an associated femur fracture (OR, 3.13; 95% CI, 2.99-3.27; P < .01), those with atrial fibrillation (OR, 1.96; 95% CI, 1.88-2.05; P < .01), and those treated with CRIF (OR, 1.42; 95% CI, 1.29-1.56; P < .01) were associated with significantly higher odds of blood transfusion (model fit: omnibus test of model coefficients, X = 42,653, P < .01; Nagelkerke, R2 = 0.19) (Table 7).
Table 7. Logistic Regression for Predictors of the Requirement for Blood Transfusion Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Open reduction internal fixation | 8.13 (7.91-8.36) | < 0.01 |
Congestive heart failure | 4.23 (4.06-4.41) | < 0.01 |
Associated fracture of any part of femur | 3.13 (2.99-3.27) | < 0.01 |
Atrial fibrillation | 1.96 (1.88-2.05) | < 0.01 |
Closed reduction internal fixation | 1.42 (1.29-1.56) | < 0.01 |
Geographic region | 1.38 (1.36-1.39) | < 0.01 |
Hypertension | 1.38 (1.34-1.42) | < 0.01 |
Associated pelvic fracture | 1.28 (1.25-1.31) | < 0.01 |
Age 61-85 years | 1.06 (1.02-1.11) | 0.01 |
Source of payment | 0.99 (0.98-0.99) | < 0.01 |
Race | 0.98 (0.97-0.98) | < 0.01 |
DOC | 0.96 (0.96-0.96) | < 0.01 |
Age >85 years | 0.74 (0.72-0.77) | < 0.01 |
External fixator | 0.69 (0.59-0.80) | < 0.01 |
Coronary artery disease | 0.62 (0.57-0.68) | < 0.01 |
Age 41-60 years | 0.57 (0.54-0.60) | < 0.01 |
Gender (male) | 0.54 (0.52-0.55) | < 0.01 |
Diabetes mellitus | 0.38 (0.36-0.41) | < 0.01 |
Age 20-40 years | 0.32 (0.30-0.34) | < 0.01 |
Associated femoral neck fracture | 0.29 (0.27-0.31) | < 0.01 |
Age <20 years | 0.24 (0.22-0.26) | < 0.01 |
Omnibus X = 42,653, P < .01 | ||
Nagelkerke R2 = 0.19 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
TREATMENT WITH ORIF
Over the 20-year study period, 23.2% of patients with acetabular fractures were treated with ORIF (Table 1). In 1990, 12.6% of patients underwent ORIF, while in 2010 this percentage increased to 20.4% (P < .001) (Table 2). Multivariable logistic regression analysis demonstrated that age between 41 and 60 years (OR, 1.88; 95% CI, 1.78-1.98; P < .01) was associated with the highest odds of undergoing ORIF. Age 20 to 40 years (OR, 1.86; 95% CI, 1.76-1.97; P < .01), age <20 years (OR, 1.82; 95% CI, 1.72-1.93; P < .01), and male gender (OR, 1.65; 95% CI, 1.63-1.68; P < .01) were also associated with being treated by ORIF. In contrast, coronary artery disease (OR, 0.27; 95% CI, 0.25-0.30; P < .01), age >85 years (OR, 0.46; 95% CI, 0.44-0.47; P < .01), and congestive heart failure (OR, 0.48; 95% CI, 0.46-0.51; P < .01) were associated with the lowest odds of undergoing ORIF (model fit: omnibus test of model coefficients, X = 71,118, P < .01; Nagelkerke, R2 = 0.20) (Table 8).
Table 8. Logistic Regression for Predictors of the Requirement for Discharge to Another Inpatient Facility Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Age 41-60 years | 1.88 (1.78-1.98) | < 0.01 |
Age 20-40 years | 1.86 (1.76-1.97) | < 0.01 |
Age <20 years | 1.82 (1.72-1.93) | < 0.01 |
Gender (male) | 1.65 (1.63-1.68) | < 0.01 |
Larger hospital bed size | 1.46 (1.45-1.47) | < 0.01 |
Hypertension | 1.35 (1.32-1.38) | < 0.01 |
Diabetes mellitus | 1.09 (1.05-1.13) | < 0.01 |
DOC | 1.02 (1.02-1.02) | < 0.01 |
Source of payment | 1.01 (1.01-1.02) | < 0.01 |
Race | 1.00 (0.99-1.00) | 0.17 |
Age 61-85 years | 0.94 (0.90-0.99) | 0.02 |
Region | 0.92 (0.91-0.93) | < 0.01 |
Atrial fibrillation | 0.83 (0.79-0.87) | < 0.01 |
Congestive heart failure | 0.48 (0.46-0.51) | < 0.01 |
Age >85 years | 0.46 (0.44-0.47) | < 0.01 |
Coronary artery disease | 0.27 (0.25-0.30) | < 0.01 |
Omnibus X 71,118, P < .01 | ||
Nagelkerke R2 = 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
Continue to: DISCUSSION...
DISCUSSION
This study evaluates the incidence of acetabular fractures in the US between 1990 and 2010, and identifies prognostic factors associated with complications and death. The study demonstrates an increase in the population-adjusted incidence of acetabular fractures between 1990 and 2010 (7.84 cases per 100,000 capita to 9.5 cases per 100,000 capita), in contrast to the decreasing trend reported by Mauffrey and colleagues.11 Some studies suggest that up to 80% of acetabular fractures are associated with motor vehicle collisions and motorcycle accidents.9,27 While the rate of motor vehicle accidents has remained stable over the study period, motorcycle ownership and deaths more than doubled between 2001 and 2008,28 primarily among individuals over 40 years of age. In this study, the mean age of patients with acetabular fractures ranged from 48 to 57 years. The dramatic increase in motorcycle ownership and deaths in these age groups may partially explain the rising incidence of acetabular fractures. The other possibility is that changes in automobile design and safety equipment may have altered the injury patterns observed in patients surviving motor vehicle crashes. Compared to the United Kingdom, in which studies report a fixed incidence of 3 per 100,000 capita1 between 1988 and 2003, the incidence of acetabular fractures in the US is greater. In contrast, the incidence of acetabular fractures reported in this study is less than the 20 per 100,000 reported in Sweden between 1976 and 1985,29 or the 37 per 100,000 reported in Rochester, Minnesota between 1968 and 1977,30 which may be due to increased seatbelt usage.31
In addition to the national incidence, this study demonstrated that the proportion of patients with acetabular fractures treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010. This is substantially lower than the 77% reported by Ochs and colleagues32 in a German population. Concurrent with the increase in ORIF, there was a decrease in in-hospital mortality from 5.9% in 1990 to 0.4% in 2010. The initial mortality rates in this study are comparable to much earlier reports and some small studies,9,32-37 but the rates reported in the later years of this study show a substantial decrease that is likely a more accurate estimation of the current incidence. The improved survival rates may be due to advances in the operative treatment of acetabular fractures, in which mechanical stabilization allows for early patient mobilization and facilitation of optimal nursing care.38 With ORIF becoming the standard of care for displaced acetabular fractures,9 numerous reports have demonstrated an association between early definitive fixation and improved survival.17,39,40 This is similar to our study, which found ORIF to be associated with the lowest odds of mortality in multivariate logistic regression analysis. It is possible that advances in patient care by intensivists over this period have also contributed to the decrease in mortality, but the correlation with operative treatment in this study is very strong and agrees well with prior studies.16 Moreover, multiple studies have demonstrated decreased in-hospital mortality among patients undergoing various orthopedic surgical procedures during this period.41-43 The correlation with operative treatment in this study agrees well with prior studies.16
In contrast, higher odds of mortality were seen in patients over the age of 85 years with pulmonary insufficiency, congestive heart failure, pneumonia, or an associated femur or pelvic fracture. This is similar to prior reports in which patients with combined acetabulum and pelvic ring injuries fared worse than those with isolated injures,44,45 as did patients with associated non-musculoskeletal injuries.46 The finding that age over 85 years was associated with higher odds of mortality likely reflects the increased number of comorbidities and decreased physiologic reserve seen in this patient population. Finally, male gender was associated with higher odds of in-hospital mortality. There are 2 possible explanations for this: Either there is gender dimorphism in sex hormones and cytokine activity in response to hemorrhage and sepsis,38,47 or there is a greater tendency for males to be involved in higher energy accidents with more severe concomitant injuries.
The results of multivariable regression analysis demonstrated that patients were more likely to require blood transfusion if they were managed surgically or had atrial fibrillation, congestive heart failure, or associated femur fracture. Not surprisingly, concurrent pelvic fracture was also associated with higher odds of blood transfusion, as pelvic hemorrhage is reported to be the cause of death in up to half of patients who die following a pelvic fracture.46
Between 1990 and 2010, in-hospital days of care decreased from 17.0 days to 10.3 days. While a decreased length of stay has been demonstrated in other orthopedic conditions over the study period,41 it is possible the decrease in length of stay demonstrated in this study is due to improved surgical technique and the implementation of early surgical intervention.39,48-50 Plaisier and colleagues17 demonstrated superior functional outcomes, quicker return to baseline function, and decreased length of stay in patients treated with early ORIF of their acetabular fractures. Other studies have shown that the benefits of early surgery include improved reduction quality and ease of reduction,51 as well as control of bleeding, pain relief, and mobilization of the patient.39 Another possible explanation for the decreased length of stay is the increased rate of discharge to other inpatient facilities, such as rehabilitation facilities, which was demonstrated in this study.
Continue to: Interestingly, male gender and younger age...
Interestingly, male gender and younger age were associated with operative management of the acetabular fracture. In contrast, there was a decreased likelihood of operative treatment among elderly patients and those patients with cardiac comorbidities. It is possible that the relationship we found between the likelihood of ORIF and age relates to the bimodal distribution of fractures, with higher energy and potentially more displaced fractures occurring in younger patients3-5 and lower energy fractures in the elderly.
In contrast to decreasing in-hospital days of care, there was a rise in the number of adverse events between 1990 (10.9%) and 2010 (37.6%). This can be partially attributed to the increased rates of blood transfusion, which was received by 9.5% of patients with acetabular fractures in the final study year. Additionally, surgical intervention was associated with increased adverse events in this study, and surgical intervention increased over the study period. Other factors that may have contributed to an increase in adverse events include an aging population,52 as advanced age was independently associated with higher odds of adverse events in this study.
Despite the strengths of using large, national databases for epidemiological research,53 this study has several limitations. Like all large databases, the NHDS is subject to error in coding and data entry.54 Additionally, the database only allows for 7 diagnostic codes and 4 procedure codes per entry. As a result, the prevalence of comorbid conditions and adverse events may be underreported.25 Moreover, the severity of a comorbid disease cannot be appreciated when dichotomously classified.55 Another limitation is that the database only provides inpatient data, so complications that arise after discharge, as well as follow-up data, are unknown. Furthermore, the results of this study are limited to practice patterns in the US from 1990 to 2010. This database does not provide injury mechanisms, so we cannot distinguish between high-energy and low-energy injuries. Lastly, analysis of the different types of acetabular fractures was not performed since classification of acetabular fractures cannot be assessed with ICD-9 codes.
CONCLUSION
This study is the largest epidemiologic analysis of acetabular fractures in the US and also provides predictors of in-hospital mortality. The incidence of acetabular fractures in the US is increasing, while mortality is decreasing. Identifying risk factors associated with poor outcomes has the potential to change treatment strategies, resource allocation, in-hospital monitoring, and discharge planning for this patient population.
This paper will be judged for the Resident Writer’s Award.
1. Laird A, Keating JF. Acetabular fractures: a 16-year prospective epidemiological study. J Bone Joint Surg Br. 2005;87(7):969-973. doi:10.1302/0301-620X.87B7.16017.
2. Geoghegan JM, Longdon EJ, Hassan K, Calthorpe D. Acetabular fractures in the UK. What are the numbers? Injury. 2007;38(3):329-333. doi:10.1016/j.injury.2006.09.015.
3. Tavakoli Darestani R, Kazemian G, Emami Moghaddam M, Manafi Rasi A, Alipour Y, Bagherian Lemraski MM. An unusual combination of acetabular and pelvic fracture: is this a new subtype of acetabular fracture? Trauma Mon. 2013;18(1):37-40. doi:10.5812/traumamon.9613.
4. McDonnell M, Schachter AK, Phillips DP, Liporace FA. Acetabular fracture through the triradiate cartilage after low-energy trauma. J Orthop Trauma. 2007;21(7):495-498. doi:10.1097/BOT.0b013e31812f67ff.
5. Giannoudis PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875-883. doi:10.1097/01.ta.0000242259.67486.15.
6. Gänsslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27 Suppl 1:S-A13-A20. doi:10.1016/S0020-1383(96)90106-0.
7. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645. doi:10.2106/00004623-199611000-00002.
8. Wright R, Barrett K, Christie MJ, Johnson KD. Acetabular fractures: long-term follow-up of open reduction and internal fixation. J Orthop Trauma. 1994;8(5):397-403. doi:10.1097/00005131-199410000-00005.
9. Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2-9.
10. Davarinos N, Ellanti P, Morris S, Mc Elwain JP. Epidemiology of pelvic and acetabular trauma in a Dublin tertiary hospital: a 10-year experience. Ir J Med Sci. 2012;181(2):243-246. doi:10.1007/s11845-011-0791-4.
11. Mauffrey C, Hao J, Cuellar DO 3rd, et al. The epidemiology and injury patterns of acetabular fractures: are the USA and China comparable? Clin Orthop Relat Res. 2014;472(11):3332-3337. doi:10.1007/s11999-014-3462-8.
12. Dente CJ, Feliciano DV, Rozycki GS, et al. The outcome of open pelvic fractures in the modern era. Am J Surg. 2005;190(6):830-835. doi:10.1016/j.amjsurg.2005.05.050.
13. Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1-13. doi:10.1016/j.injury.2004.05.029.
14. Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985-991. doi:10.1016/j.injury.2011.06.003.
15. Arroyo W, Nelson KJ, Belmont PJ Jr, Bader JO, Schoenfeld AJ. Pelvic trauma: what are the predictors of mortality and cardiac, venous thrombo-embolic and infectious complications following injury? Injury. 2013;44(12):1745-1749. doi:10.1016/j.injury.2013.08.007.
16. Flint L, Cryer HG. Pelvic fracture: the last 50 years. J Trauma. 2010;69(3):483-488. doi:10.1097/TA.0b013e3181ef9ce1.
17. Plaisier BR, Meldon SW, Super DM, Malangoni MA. Improved outcome after early fixation of acetabular fractures. Injury. 2000;31(2):81-84. doi:10.1016/S0020-1383(99)00233-8.
18. Centers for Disease Control and Prevention: National Hospital. Discharge survey. http://www.cdc.gov/nchs/nhds.htm. Accessed August 22, 2013.
19. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;(39):1-42.
20. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed June 18, 2013.
21. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627. doi:10.1007/s11999-008-0402-5.
22. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157. doi:10.1016/j.psym.2012.08.009.
23. Iezzoni LI, Daley J, Heeren T, et al. Using administrative data to screen hospitals for high complication rates. Inquiry. 1994;31(1):40-55.
24. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478-2486.
25. Bot AG, Menendez ME, Neuhaus V, Ring D. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(4):519-527. doi:10.1016/j.jse.2013.12.006.
26. United States Census Bureau. Population. https://www.census.gov/topics/population.html. Accessed December 4, 2012.
27. Porter SE, Schroeder AC, Dzugan SS, Graves ML, Zhang L, Russell GV. Acetabular fracture patterns and their associated injuries. J Orthop Trauma. 2008;22(3):165-170. doi:10.1097/BOT.0b013e318165918b.
28. Centers for Disease Control and Prevention. Motorcycle Crash-Related Data. https://www.cdc.gov/motorvehiclesafety/mc/index.html Accessed September 23, 2018
29. Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300. doi:10.3109/17453679209154786.
30. Melton LJ 3rd, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;155(155):43-47. doi:10.1097/00003086-198103000-00008.
31. al-Qahtani S, O'Connor G. Acetabular fractures before and after the introduction of seatbelt legislation. Can J Surg. 1996;39(4):317-320.
32. Ochs BG, Marintschev I, Hoyer H, et al. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2010;41(8):839-851. doi:10.1016/j.injury.2010.04.010.
33. Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151(151):81-106. doi:10.1055/s-2007-980136.
34. de Ridder VA, de Lange S, Kingma L, Hogervorst M. Results of 75 consecutive patients with an acetabular fracture. Clin Orthop Relat Res. 1994;305(305):53-57. doi:10.1097/00003086-199408000-00008.
35. Aho AJ, Isberg UK, Katevuo VK. Acetabular posterior wall fracture. 38 Cases followed for 5 years. Acta Orthop Scand. 1986;57(2):101-105. doi:10.3109/17453678609000878.
36. Stöckle U, Hoffmann R, Südkamp NP, Reindl R, Haas NP. Treatment of complex acetabular fractures through a modified extended iliofemoral approach. J Orthop Trauma. 2002;16(4):220-230. doi:10.1097/00005131-200204000-00002.
37. Tibbs BM, Kopar P, Dente CJ. Acetabular and isolated pelvic ring fractures: a comparison of initial assessment and outcome. Am Surg. 2008;74(6):538-541; discussion 541.
38. Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012;470(8):2090-2097. doi:10.1007/s11999-012-2276-9.
39. Vallier HA, Cureton BA, Ekstein C, Oldenburg FP, Wilber JH. Early definitive stabilization of unstable pelvis and acetabulum fractures reduces morbidity. J Trauma. 2010;69(3):677-684. doi:10.1097/TA.0b013e3181e50914.
40. Enninghorst N, Toth L, King KL, McDougall D, Mackenzie S, Balogh ZJ. Acute definitive internal fixation of pelvic ring fractures in polytrauma patients: a feasible option. J Trauma. 2010;68(4):935-941. doi:10.1097/TA.0b013e3181d27b48.
41. Buller LT, Best MJ, Quinnan SM. A nationwide analysis of pelvic ring fractures: incidence and trends in treatment, length of stay, and mortality. Geriatr Orthop Surg Rehabil. 2016;7(1):9-17. doi:10.1177/2151458515616250.
42. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191. doi:10.2106/JBJS.M.01126.
43. Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214.
44. Halvorson JJ, Lamothe J, Martin CR, et al. Combined acetabulum and pelvic ring injuries. J Am Acad Orthop Surg. 2014;22(5):304-314. doi:10.5435/JAAOS-22-05-304.
45. Osgood GM, Manson TT, O'Toole RV, Turen CH. Combined pelvic ring disruption and acetabular fracture: associated injury patterns in 40 patients. J Orthop Trauma. 2013;27(5):243-247. doi:10.1097/BOT.0b013e31826c2751.
46. Poole GV, Ward EF, Muakkassa FF. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg. 1991;213(6):532-538; discussion 538.
47. Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi:10.1097/00000658-200201000-00014.
48. Goldstein A, Phillips T, Sclafani SJ, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26(4):325-333. doi:10.1097/00005373-198604000-00004.
49. Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28-31. doi:10.1097/00005373-199101000-00006.
50. Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35(5):671-675; discussion 676.
51. Madhu R, Kotnis R, Al-Mousawi A, et al. Outcome of surgery for reconstruction of fractures of the acetabulum. The time dependent effect of delay. J Bone Joint Surg Br. 2006;88(9):1197-1203. doi:10.1302/0301-620X.88B9.17588.
52. Centers for Disease Control and Prevention. The State of Aging & Health in America 2013. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Accessed December 5, 2013.
53. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
54. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449. [author reply:450-451]. doi:10.1097/ALN.0b013e3181adf739.
55. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706. doi:10.1007/s11999-013-2876-z.
1. Laird A, Keating JF. Acetabular fractures: a 16-year prospective epidemiological study. J Bone Joint Surg Br. 2005;87(7):969-973. doi:10.1302/0301-620X.87B7.16017.
2. Geoghegan JM, Longdon EJ, Hassan K, Calthorpe D. Acetabular fractures in the UK. What are the numbers? Injury. 2007;38(3):329-333. doi:10.1016/j.injury.2006.09.015.
3. Tavakoli Darestani R, Kazemian G, Emami Moghaddam M, Manafi Rasi A, Alipour Y, Bagherian Lemraski MM. An unusual combination of acetabular and pelvic fracture: is this a new subtype of acetabular fracture? Trauma Mon. 2013;18(1):37-40. doi:10.5812/traumamon.9613.
4. McDonnell M, Schachter AK, Phillips DP, Liporace FA. Acetabular fracture through the triradiate cartilage after low-energy trauma. J Orthop Trauma. 2007;21(7):495-498. doi:10.1097/BOT.0b013e31812f67ff.
5. Giannoudis PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875-883. doi:10.1097/01.ta.0000242259.67486.15.
6. Gänsslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27 Suppl 1:S-A13-A20. doi:10.1016/S0020-1383(96)90106-0.
7. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645. doi:10.2106/00004623-199611000-00002.
8. Wright R, Barrett K, Christie MJ, Johnson KD. Acetabular fractures: long-term follow-up of open reduction and internal fixation. J Orthop Trauma. 1994;8(5):397-403. doi:10.1097/00005131-199410000-00005.
9. Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2-9.
10. Davarinos N, Ellanti P, Morris S, Mc Elwain JP. Epidemiology of pelvic and acetabular trauma in a Dublin tertiary hospital: a 10-year experience. Ir J Med Sci. 2012;181(2):243-246. doi:10.1007/s11845-011-0791-4.
11. Mauffrey C, Hao J, Cuellar DO 3rd, et al. The epidemiology and injury patterns of acetabular fractures: are the USA and China comparable? Clin Orthop Relat Res. 2014;472(11):3332-3337. doi:10.1007/s11999-014-3462-8.
12. Dente CJ, Feliciano DV, Rozycki GS, et al. The outcome of open pelvic fractures in the modern era. Am J Surg. 2005;190(6):830-835. doi:10.1016/j.amjsurg.2005.05.050.
13. Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1-13. doi:10.1016/j.injury.2004.05.029.
14. Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985-991. doi:10.1016/j.injury.2011.06.003.
15. Arroyo W, Nelson KJ, Belmont PJ Jr, Bader JO, Schoenfeld AJ. Pelvic trauma: what are the predictors of mortality and cardiac, venous thrombo-embolic and infectious complications following injury? Injury. 2013;44(12):1745-1749. doi:10.1016/j.injury.2013.08.007.
16. Flint L, Cryer HG. Pelvic fracture: the last 50 years. J Trauma. 2010;69(3):483-488. doi:10.1097/TA.0b013e3181ef9ce1.
17. Plaisier BR, Meldon SW, Super DM, Malangoni MA. Improved outcome after early fixation of acetabular fractures. Injury. 2000;31(2):81-84. doi:10.1016/S0020-1383(99)00233-8.
18. Centers for Disease Control and Prevention: National Hospital. Discharge survey. http://www.cdc.gov/nchs/nhds.htm. Accessed August 22, 2013.
19. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;(39):1-42.
20. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed June 18, 2013.
21. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627. doi:10.1007/s11999-008-0402-5.
22. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157. doi:10.1016/j.psym.2012.08.009.
23. Iezzoni LI, Daley J, Heeren T, et al. Using administrative data to screen hospitals for high complication rates. Inquiry. 1994;31(1):40-55.
24. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478-2486.
25. Bot AG, Menendez ME, Neuhaus V, Ring D. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(4):519-527. doi:10.1016/j.jse.2013.12.006.
26. United States Census Bureau. Population. https://www.census.gov/topics/population.html. Accessed December 4, 2012.
27. Porter SE, Schroeder AC, Dzugan SS, Graves ML, Zhang L, Russell GV. Acetabular fracture patterns and their associated injuries. J Orthop Trauma. 2008;22(3):165-170. doi:10.1097/BOT.0b013e318165918b.
28. Centers for Disease Control and Prevention. Motorcycle Crash-Related Data. https://www.cdc.gov/motorvehiclesafety/mc/index.html Accessed September 23, 2018
29. Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300. doi:10.3109/17453679209154786.
30. Melton LJ 3rd, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;155(155):43-47. doi:10.1097/00003086-198103000-00008.
31. al-Qahtani S, O'Connor G. Acetabular fractures before and after the introduction of seatbelt legislation. Can J Surg. 1996;39(4):317-320.
32. Ochs BG, Marintschev I, Hoyer H, et al. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2010;41(8):839-851. doi:10.1016/j.injury.2010.04.010.
33. Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151(151):81-106. doi:10.1055/s-2007-980136.
34. de Ridder VA, de Lange S, Kingma L, Hogervorst M. Results of 75 consecutive patients with an acetabular fracture. Clin Orthop Relat Res. 1994;305(305):53-57. doi:10.1097/00003086-199408000-00008.
35. Aho AJ, Isberg UK, Katevuo VK. Acetabular posterior wall fracture. 38 Cases followed for 5 years. Acta Orthop Scand. 1986;57(2):101-105. doi:10.3109/17453678609000878.
36. Stöckle U, Hoffmann R, Südkamp NP, Reindl R, Haas NP. Treatment of complex acetabular fractures through a modified extended iliofemoral approach. J Orthop Trauma. 2002;16(4):220-230. doi:10.1097/00005131-200204000-00002.
37. Tibbs BM, Kopar P, Dente CJ. Acetabular and isolated pelvic ring fractures: a comparison of initial assessment and outcome. Am Surg. 2008;74(6):538-541; discussion 541.
38. Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012;470(8):2090-2097. doi:10.1007/s11999-012-2276-9.
39. Vallier HA, Cureton BA, Ekstein C, Oldenburg FP, Wilber JH. Early definitive stabilization of unstable pelvis and acetabulum fractures reduces morbidity. J Trauma. 2010;69(3):677-684. doi:10.1097/TA.0b013e3181e50914.
40. Enninghorst N, Toth L, King KL, McDougall D, Mackenzie S, Balogh ZJ. Acute definitive internal fixation of pelvic ring fractures in polytrauma patients: a feasible option. J Trauma. 2010;68(4):935-941. doi:10.1097/TA.0b013e3181d27b48.
41. Buller LT, Best MJ, Quinnan SM. A nationwide analysis of pelvic ring fractures: incidence and trends in treatment, length of stay, and mortality. Geriatr Orthop Surg Rehabil. 2016;7(1):9-17. doi:10.1177/2151458515616250.
42. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191. doi:10.2106/JBJS.M.01126.
43. Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214.
44. Halvorson JJ, Lamothe J, Martin CR, et al. Combined acetabulum and pelvic ring injuries. J Am Acad Orthop Surg. 2014;22(5):304-314. doi:10.5435/JAAOS-22-05-304.
45. Osgood GM, Manson TT, O'Toole RV, Turen CH. Combined pelvic ring disruption and acetabular fracture: associated injury patterns in 40 patients. J Orthop Trauma. 2013;27(5):243-247. doi:10.1097/BOT.0b013e31826c2751.
46. Poole GV, Ward EF, Muakkassa FF. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg. 1991;213(6):532-538; discussion 538.
47. Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi:10.1097/00000658-200201000-00014.
48. Goldstein A, Phillips T, Sclafani SJ, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26(4):325-333. doi:10.1097/00005373-198604000-00004.
49. Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28-31. doi:10.1097/00005373-199101000-00006.
50. Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35(5):671-675; discussion 676.
51. Madhu R, Kotnis R, Al-Mousawi A, et al. Outcome of surgery for reconstruction of fractures of the acetabulum. The time dependent effect of delay. J Bone Joint Surg Br. 2006;88(9):1197-1203. doi:10.1302/0301-620X.88B9.17588.
52. Centers for Disease Control and Prevention. The State of Aging & Health in America 2013. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Accessed December 5, 2013.
53. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
54. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449. [author reply:450-451]. doi:10.1097/ALN.0b013e3181adf739.
55. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706. doi:10.1007/s11999-013-2876-z.
TAKE-HOME POINTS
- The population-adjusted incidence of acetabular fractures increased between 1990 and 2010. Mortality associated with acetabular fractures decreased from 5.9% to 0.4% between 1990 and 2010.
- The proportion of patients treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010.
- The average in-patient hospital length of stay following acetabular fracture decreased from 17.0 to 10.4 days between 1990 and 2010.
- ORIF is associated with the lowest odds of mortality following acetabular fracture.
Mycobacterium abscessus: A Rare Cause of Periprosthetic Knee Joint Infection
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).
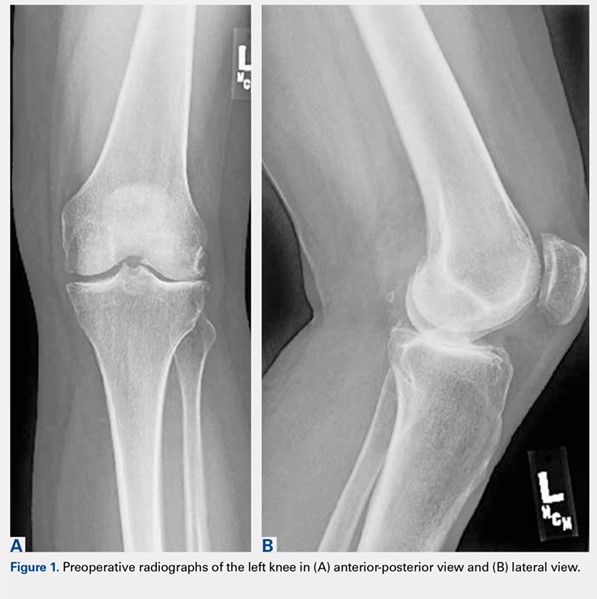
She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).
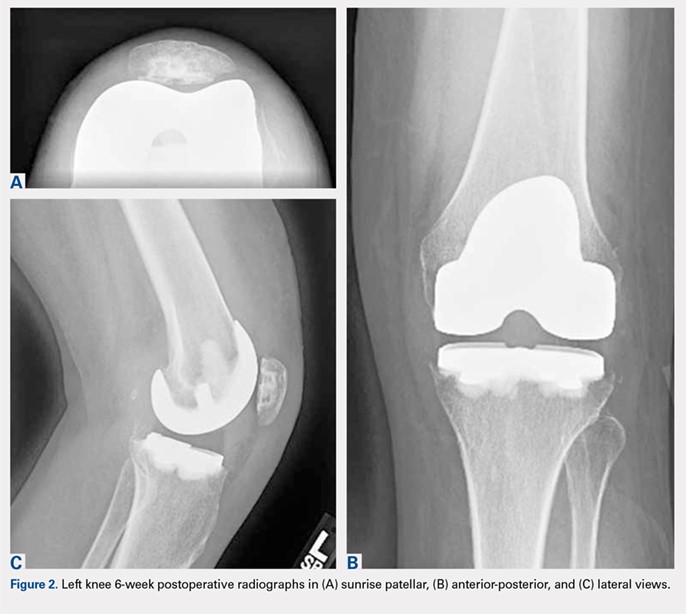
Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.
Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.
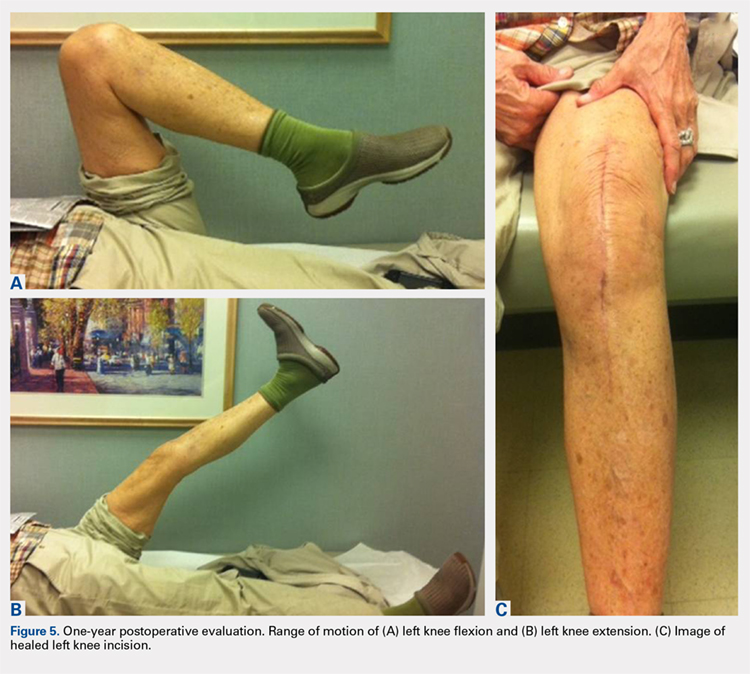
Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.
CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).

She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).

Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.

Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.


Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.

CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
ABSTRACT
A 61-year-old woman with a periprosthetic knee joint infection caused by Mycobacterium abscessus was successfully treated with surgical débridement, multidrug antimicrobial therapy, and staged reimplantation. To the authors’ knowledge, this represents the first report of successfully treating this organism after knee arthroplasty.
M. abscessus knee infections are rare, and there are no specific guidelines to inform treatment or successful treatment regimens for periprosthetic knee infections. Medical management alone was not successful in this case and hence cannot be recommended. Using a collaborative multidisciplinary approach, including surgical débridement, staged reimplantation, and multidrug antimicrobials, successful eradication of the periprosthetic joint infection caused by M. abscessus was achieved.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).

She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).

Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.

Continue to: One month postoperatively...
One month postoperatively, her constitutional symptoms, including fevers and night sweats, abated and inflammatory markers (ESR and CRP) had normalized. There were no clinical signs of infection. Amikacin was discontinued due to a 10-dB change on audiologic screening (4-6 kHz range), and tigecycline was substituted. Ultimately, she underwent 15 weeks of antimycobacterial therapy, 10 of which were after the explantation.
Eight weeks after cessation of her antibiotics, she underwent open biopsy. Multiple operative tissue samples showed negative results in pathology and culture tests.
Replantation was performed 14 weeks after stopping antimicrobials and 24 weeks after her explantation. The bone appeared healthy without evidence of osteomyelitis. A constrained reconstruction was secured with tobramycin-impregnated cement. One small island of necrotizing granuloma was observed within the bony cortex on histologic review; the granulomata appeared active with scattered neutrophils along with histiocytes and lymphocytes. AFB stains were negative. Intraoperative cultures, including mycobacterial cultures, were negative.
Based on the histologic evidence that infection may have persisted, and given the high stakes, antimicrobial treatment was reinitiated. Amikacin was again stopped after 3 weeks due to the development of tinnitus; tigecycline was substituted to complete the fourth and final week, at which point all antibiotics were discontinued. The patient was followed up uneventfully for 4 years (Figures 4A-4D and 5A-5C) with normal ESR and CRP. She continues to be ambulatory without assistive devices and walks an average of 30 miles per week without pain or constitutional symptoms.


Continue to: DISCUSSION...
DISCUSSION
Diagnosis of acute infection after TKA remains challenging, as some degree of pain, swelling, and even postoperative fevers may be common in noninfected TKA patients. Synovial white blood cell count and differential as well as alpha-defensin levels have been cited as predictive factors of infection.16,17 Deep tissue and synovial fluid cultures offer the advantage of both identification and antimicrobial sensitivity testing of the offending organism. In this case, culture of the knee joint fluid at the time of TKA led to the unexpected finding of M. abscessus infection.
Preventable outbreaks due to M. abscessus have been reported and attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, and improper skin preparation.11-13 Rarely, M. abscessus has been reported as the cause of PJI. When an unusual organism is encountered after native joint instrumentation, an investigation should be undertaken to identify the source of contamination, with the assistance of infection control practitioners and/or the US Food and Drug Administration reporting. Reporting and investigation was undertaken in this case, though no suspect source could be identified.
Although there were no signs of infection prior to the TKA, there is an ongoing debate as to whether intra-articular corticosteroid injections increase the risk of PJIs, and if so, what the optimal amount of time to wait between procedures is. Although several earlier studies have been underpowered to answer these questions,18 this patient underwent TKA 1 month following the corticosteroid injection. Recent meta-analyses have shown no definitive evidence to indicate that this increased her risk of PJI.19,20
Continue to: Treatments for mycobacterial infections...
Treatments for mycobacterial infections have been described with variable efficacy,21,22 and only 2 cases of successfully treated PJIs have been reported after infection with M. abscessus. Both these cases were described in total hip arthroplasties,23,24 and to the authors’ knowledge, this report represents the first described successfully treated case after TKA. Staged reconstruction remains a standard treatment for invasive organisms chronically infecting prosthetic joint implants, with reimplantation pending joint sterility and improvement in inflammatory markers.3 Previous successful reports of treating M. abscessus describe either resection arthroplasty21 or staged reconstruction.23,24 The authors reported variable multidrug antimicrobial regimens, as summarized in Table 2, as guidelines for the treatment of mycobacterial PJI are currently not available.

CONCLUSION
This case report represents an episode of iatrogenic septic arthritis caused by Mycobacteria of the native knee after previous history of instrumentation, corticosteroid, and hyaluronic acid injections, with an overall indolent clinical course until subsequent arthroplasty. There were several important lessons learned, which are as follows: 1) Multidrug combination with antimicrobial therapy combined with aggressive surgical débridement and staged reimplantation permitted successful eradication of TKA PJI caused by M. abscessus in this patient. 2) Initial medical management alone was not successful and cannot be recommended for the treatment of M. abscessus in the setting of PJI. 3) Delaying the surgical débridement and the reconstructive course for a trial of medical management contributed to the ultimate requirement of a tibial tubercle osteotomy for an ankylosed knee at replantation. In this case, we initially had a low index of suspicion for deep infection, contributing to delayed surgical débridement. Ideally, a high degree of clinical suspicion should be maintained for joint infection in the presence of positive culture isolates of M. abscessus, as it may have a delayed clinical presentation of the typical features of PJI (fevers, swelling, erythema, etc). In such cases, the authors recommend consideration of early surgical débridement. 4) Medical management of TKA PJI is not without risks. Careful monitoring of patient side effects during antimicrobial administration remains paramount, as this patient did sustain a degree of hearing loss associated with prolonged medical therapy. 5) In complicated PJIs involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach, including specialists in orthopedic surgery, infectious disease, microbiology, pharmacy, and pathology, may be the preferred path to clinical cure.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Cobo J, Del Pozo JL. Prosthetic joint infection: diagnosis and management. Expert Rev Anti Infect Ther. 2011;9(9):787-802. doi:10.1586/eri.11.95.
3. Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88(2):149-155. doi:10.1302/0301-620X.88B2.17058.
4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803.
5. Restrepo C, Schmitt S, Backstein D, et al. Antibiotic treatment and timing of reimplantation. J Orthop Res. 2014;32 Suppl 1:S136-S140. doi:10.1002/jor.22557.
6. De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42(12):1756-1763. doi:10.1086/504381.
7. Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), Confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367-1376. doi:10.1128/AAC.01275-08.
8. Furuya EY, Paez A, Srinivasan A, et al. Outbreak of Mycobacterium abscessus wound infections among "lipotourists" from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181-1188. doi:10.1086/529191.
9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52(5):565-571. doi:10.1093/cid/ciq237.
10. Mueller PS, Edson RS. Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review. Diagn Microbiol Infect Dis. 2001;39(1):33-37. doi:10.1016/S0732-8893(00)00211-X.
11. Mushatt DM, Witzig RS. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin Infect Dis. 1995;20(5):1441-1442. doi:10.1093/clinids/20.5.1441.
12. Tiwari TS, Ray B, Jost KC Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36(8):954-962. doi:10.1086/368192.
13. Villanueva A, Calderon RV, Vargas BA, et al. Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24(6):1147-1153. doi:10.1086/513656.
14. Gale DW, Harding ML. Total knee arthroplasty in the presence of active tuberculosis. J Bone Joint Surg Br. 1991;73(6):1006-1007. doi:10.1302/0301-620X.73B6.1955424.
15. Kim YH. Total knee arthroplasty for tuberculous arthritis. J Bone Joint Surg Am. 1988;70(9):1322-1330. doi:10.2106/00004623-198870090-00008.
16. Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34-40. doi:10.1007/s11999-010-1433-2.
17. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009. doi:10.1007/s11999-014-3900-7.
18. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21(1):6-11. doi:10.1016/j.knee.2013.07.003.
19. Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplast. 2014;29(11):2175-2180. doi:10.1016/j.arth.2014.07.013.
20. Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi:10.1186/s13018-014-0107-2.
21. Eid AJ, Berbari EF, Sia IG, Wengenack NL, Osmon DR, Razonable RR. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687-694. doi:10.1086/520982.
22. Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res. 1987;216(216):183-186. doi:10.1097/00003086-198703000-00029.
23. Petrosoniak A, Kim P, Desjardins M, Lee BC. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20(3):e94-e96.
24. Yinkey LM, Halsey ES, Lloyd BA. Successful tigecycline combination therapy for Mycobacterium abscessus infection of a total hip arthroplasty. Infect Dis Clin Practice. 2010;18(4):269-270. doi:10.1097/IPC.0b013e3181d04a09.
25. AAOS Guidelines: the diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors; June 18th, 2010. AAOS Publication: 2010.
26. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcomittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
TAKE-HOME POINTS:
- Periprosthetic joint infections due to Mycobacterium abscess have been rarely reported, and no specific guidlines exist to inform treatment.
- Medical management alone was not successful in our clinical case and cannot be recommended.
- Combination medical and surgical management may provide the best opportunity for clincal cure of periprosthetic infections.
- In complicated periprosthetic joint infections involving rare and intrinsically resistant organisms, a collaborative multidisciplinary approach likley represents the preferred path to clinical cure.
- Successful erradiation of periprosthetic infection with M. abscessus may not preclude acceptable outcomes after revision TKA.
Reverse Total Shoulder Arthroplasty: Indications and Techniques Across the World
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Reverse total shoulder arthroplasty (RTSA) is a common treatment for rotator cuff tear arthropathy. We performed a systematic review of all the RTSA literature to answer if we are treating the same patients with RTSA, across the world.
A systematic review was registered with PROSPERO, the international prospective register of systematic reviews, and performed with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines using 3 publicly available free databases. Therapeutic clinical outcome investigations reporting RTSA outcomes with levels of evidence I to IV were eligible for inclusion. All study, subject, and surgical technique demographics were analyzed and compared between continents. Statistical comparisons were conducted using linear regression, analysis of variance (ANOVA), Fisher's exact test, and Pearson's chi-square test.
There were 103 studies included in the analysis (8973 patients; 62% female; mean age, 70.9 ± 6.7 years; mean length of follow-up, 34.3 ± 19.3 months) that had a low Modified Coleman Methodology Score (MCMS) (mean, 36.9 ± 8.7: poor). Most patients (60.8%) underwent RTSA for a diagnosis of rotator cuff arthropathy, whereas 1% underwent RTSA for fracture; indications varied by continent. There were no consistent reports of preopeartive or postoperative scores from studies in any region. Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° ± 11.3°) (P = .004) compared with studies from Europe. North America had the greatest total number of publications followed by Europe. The total yearly number of publications increased each year (P < .001), whereas the MCMS decreased each year (P = .037).
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent, although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
Continue to: Reverse total shoulder arthroplasty...
Reverse total shoulder arthroplasty (RTSA) is a common procedure with indications including rotator cuff tear arthropathy, proximal humerus fractures, and others.1,2 Studies have shown excellent, reliable, short- and mid-term outcomes in patients treated with RTSA for various indications.3-5 Al-Hadithy and colleagues6 reviewed 41 patients who underwent RTSA for pseudoparalysis secondary to rotator cuff tear arthropathy and, at a mean follow-up of 5 years, found significant improvements in range of motion (ROM) as well as age-adjusted Constant and Oxford Outcome scores. Similarly, Ross and colleagues7 evaluated outcomes of RTSA in 28 patients in whom RTSA was performed for 3- or 4-part proximal humerus fractures, and found both good clinical and radiographic outcomes with no revision surgeries at a mean follow-up of 54.9 months. RTSA is performed across the world, with specific implant designs, specifically humeral head inclination, but is more common in some areas when compared with others.3,8,9
The number of RTSAs performed has steadily increased over the past 20 years, with recent estimates of approximately 20,000 RTSAs performed in the United States in 2011.10,11 However, there is little information about the similarities and differences between those patients undergoing RTSA in various parts of the world regarding surgical indications, patient demographics, and outcomes. The purpose of this study is to perform a systematic review and meta-analysis of the RTSA body of literature to both identify and compare characteristics of studies published (level of evidence, whether a conflict of interest existed), patients analyzed (age, gender), and surgical indications performed across both continents and countries. Essentially, the study aims to answer the question, "Across the world, are we treating the same patients?" The authors hypothesized that there would be no significant differences in RTSA publications, subjects, and indications based on both the continent and country of publication.
METHODS
A systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines using a PRISMA checklist.12 A systematic review registration was performed using PROSPERO, the international prospective register of systematic reviews (registration number CRD42014010578).13Two reviewers independently conducted the search on March 25, 2014, using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I to IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine14) clinical studies were eligible. Medical conference abstracts were ineligible for inclusion. All references within included studies were cross-referenced for inclusion if missed by the initial search with any additionally located studies screened for inclusion. Duplicate subject publications within separate unique studies were not reported twice, but rather the study with longer duration follow-up or, if follow-up was equal, the study with the greater number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical and cadaver studies, total shoulder arthroplasty (TSA) papers, arthroscopic shoulder surgery papers, imaging, surgical techniques, and classification studies were excluded.
A total of 255 studies were identified, and, after implementation of the exclusion criteria, 103 studies were included in the final analysis (Figure 1). Subjects of interest in this systematic review underwent RTSA for one of many indications including rotator cuff tear arthropathy, osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, instability, revision from a previous RTSA for instability, infection, acute proximal humerus fracture, revision from a prior proximal humerus fracture, revision from a prior hemiarthroplasty, revision from a prior TSA, osteonecrosis, pseudoparalysis, tumor, and a locked shoulder dislocation. There was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, body mass index, diagnoses treated, and surgical positioning. Clinical outcome scores sought were the DASH (Disability of the Arm, Shoulder, and Hand), SPADI (Shoulder Pain And Disability Index), Absolute Constant, ASES (American Shoulder and Elbow Score), KSS (Korean Shoulder Score), SST-12 (Simple Shoulder Test), SF-12 (12-item Short Form), SF-36 (36-item Short Form), SSV (Subjective Shoulder Value), EQ-5D (EuroQol-5 Dimension), SANE (Single Assessment Numeric Evaluation), Rowe Score for Instability, Oxford Instability Score, UCLA (University of California, Los Angeles) activity score, Penn Shoulder Score, and VAS (visual analog scale). In addition, ROM (forward elevation, abduction, external rotation, internal rotation) was analyzed. Radiographs and magnetic resonance imaging data were extracted when available. The methodological quality of the study was evaluated using the MCMS (Modified Coleman Methodology Score).15
STATISTICAL ANALYSIS
First, the number of publications per year, level of evidence, and Modified Coleman Methodology Score were tested for association with the calendar year using linear regression. Second, demographic data were tested for association with the continent using Pearson’s chi-square test or ANOVA. Third, indications were tested for association with the continent using Fisher’s exact test. Finally, clinical outcome scores and ROM were tested for association with the continent using ANOVA. Statistical significance was extracted from studies when available. Statistical significance was defined as P < .05.
Continue to: RESULTS...
RESULTS
There were 103 studies included in the analysis (Figure 1). A total of 8973 patients were included, 62% of whom were female with a mean age of 70.9 ± 6.7 years (Table 1). The average follow-up was 34.3 ± 19.3 months. North America had the overall greatest total number of publications on RTSA, followed by Europe (Figure 2). The total yearly number of publications increased by a mean of 1.95 publications each year (P < .001). There was no association between the mean level of evidence with the year of publication (P = .296) (Figure 3). Overall, the rating of studies was poor for the MCMS (mean 36.9 ± 8.7). The MCMS decreased each year by a mean of 0.76 points (P = .037) (Figure 4).
Table 1. Demographic Data by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Number of studies | 52 | 43 | 4 | 4 | 103 | - |
Number of subjects | 6158 | 2609 | 51 | 155 | 8973 | - |
Level of evidence |
|
|
|
|
| 0.693 |
II | 5 (10%) | 3 (7%) | 0 (0%) | 0 (0%) | 8 (8%) |
|
III | 10 (19%) | 4 (9%) | 0 (0%) | 1 (25%) | 15 (15%) |
|
IV | 37 (71%) | 36 (84%) | 4 (100%) | 3 (75%) | 80 (78%) |
|
Mean MCMS | 34.6 ± 8.4 | 40.2 ± 8.0 | 32.5 12.4 | 34.5 ± 6.6 | 36.9 ± 8.7 | 0.010 |
Institutional collaboration |
|
|
|
|
| 1.000 |
Multi-center | 7 (14%) | 6 (14%) | 0 (0%) | 0 (0%) | 13 (13%) |
|
Single-center | 45 (86%) | 37 (86%) | 4 (100%) | 4 (100%) | 90 (87%) |
|
Financial conflict of interest |
|
|
|
|
| 0.005 |
Present | 28 (54%) | 15 (35%) | 0 (0%) | 0 (0%) | 43 (42%) |
|
Not present | 19 (37%) | 16 (37%) | 4 (100%) | 4 (100%) | 43 (42%) |
|
Not reported | 5 (10%) | 12 (28%) | 0 (0%) | 0 (0%) | 17 (17%) |
|
Sex |
|
|
|
|
| N/A |
Male | 2157 (38%) | 1026 (39%) | 13 (25%) | 61 (39%) | 3257 (38%) |
|
Female | 3520 (62%) | 1622 (61%) | 38 (75%) | 94 (61%) | 5274 (62%) |
|
Mean age (years) | 71.3 ± 5.6 | 70.1 ± 7.9 | 68.1 ± 5.3 | 76.9 ± 3.0 | 70.9 ± 6.7 | 0.191 |
Minimum age (mean across studies) | 56.9 ± 12.8 | 52.8 ± 15.7 | 62.8 ± 6.2 | 68.0 ± 12.1 | 55.6 ± 14.3 | 0.160 |
Maximum age (mean across studies) | 82.1 ± 8.6 | 83.0 ± 5.5 | 73.0 ± 9.4 | 85.0 ± 7.9 | 82.2 ± 7.6 | 0.079 |
Mean length of follow-up (months) | 26.5 ± 13.7 | 43.1 ± 21.7 | 29.4 ± 7.9 | 34.2 ± 16.6 | 34.3 ± 19.3 | <0.001 |
Prosthesis type |
|
|
|
|
| N/A |
Cemented | 988 (89%) | 969 (72%) | 0 (0%) | 8 (16%) | 1965 (78%) |
|
Press fit | 120 (11%) | 379 (28%) | 0 (0%) | 41 (84%) | 540 (22%) |
|
Abbreviations: MCMS, Modified Coleman Methodology Score; N/A, not available.
In studies that reported press-fit vs cemented prostheses, the highest percentage of press-fit prostheses compared with cemented prostheses was seen in Australia (84% press-fit), whereas the highest percentage of cemented prostheses was seen in North America (89% cemented). A higher percentage of studies from North America had a financial conflict of interest (COI) than did those from other countries (54% had a COI).
Continue to: Rotator cuff tear arthropathy...
Rotator cuff tear arthropathy was the most common indication for RTSA overall in 5459 patients, followed by pseudoparalysis in 1352 patients (Tables 2 and 3). While studies in North America reported rotator cuff tear arthropathy as the indication for RTSA in 4418 (75.8%) patients, and pseudoparalysis as the next most common indication in 535 (9.2%) patients, studies from Europe reported rotator cuff tear arthropathy as the indication in 895 (33.5%) patients, and pseudoparalysis as the indication in 795 (29.7%) patients. Studies from Asia also had a relatively even split between rotator cuff tear arthropathy and pseudoparalysis (45.3% vs 37.8%), whereas those from Australia were mostly rotator cuff tear arthropathy (77.7%).
Table 2. Number (Percent) of Studies With Each Indication by Continent
| North America | Europe | Asia | Australia | Total | P-value |
Rotator cuff arthropathy | 29 (56%) | 19 (44%) | 3 (75%) | 3 (75%) | 54 (52%) | 0.390 |
Osteoarthritis | 4 (8%) | 10 (23%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.072 |
Rheumatoid arthritis | 9 (17%) | 10 (23%) | 0 (0%) | 2 (50%) | 21 (20%) | 0.278 |
Post-traumatic arthritis | 3 (6%) | 5 (12%) | 0 (0%) | 1 (25%) | 9 (9%) | 0.358 |
Instability | 6 (12%) | 3 (7%) | 0 (0%) | 1 (25%) | 10 (10%) | 0.450 |
Revision of previous RTSA for instability | 5 (10%) | 1 (2%) | 0 (0%) | 1 (25%) | 7 (7%) | 0.192 |
Infection | 4 (8%) | 1 (2%) | 1 (25%) | 0 (0%) | 6 (6%) | 0.207 |
Unclassified acute proximal humerus fracture | 9 (17%) | 5 (12%) | 1 (25%) | 1 (25%) | 16 (16%) | 0.443 |
Acute 2-part proximal humerus fracture | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Acute 3-part proximal humerus fracture | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.574 |
Acute 4-part proximal humerus fracture | 5 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 0.183 |
Acute 3- or 4-part proximal humerus fracture | 6 (12%) | 2 (5%) | 0 (0%) | 0 (0%) | 8 (8%) | 0.635 |
Revised from previous nonop proximal humerus fracture | 7 (13%) | 3 (7%) | 0 (0%) | 0 (0%) | 10 (10%) | 0.787 |
Revised from ORIF | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 1.000 |
Revised from CRPP | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.495 |
Revised from hemi | 8 (15%) | 4 (9%) | 0 (0%) | 1 (25%) | 13 (13%) | 0.528 |
Revised from TSA | 15 (29%) | 11 (26%) | 0 (0%) | 2 (50%) | 28 (27%) | 0.492 |
Osteonecrosis | 4 (8%) | 2 (5%) | 1 (25%) | 0 (0%) | 7 (7%) | 0.401 |
Pseudoparalysis irreparable tear without arthritis | 20 (38%) | 18 (42%) | 2 (50%) | 1 (25%) | 41 (40%) | 0.919 |
Bone tumors | 0 (0%) | 4 (9.3%) | 0 (0%) | 0 (0%) | 4 (4%) | 0.120 |
Locked shoulder dislocation | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 1 (1%) | 0.078 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 3. Number of Patients With Each Indication as Reported by Individual Studies by Continent
| North America | Europe | Asia | Australia | Total |
Rotator cuff arthropathy | 4418 | 895 | 24 | 122 | 5459 |
Osteoarthritis | 90 | 251 | 1 | 14 | 356 |
Rheumatoid arthritis | 59 | 87 | 0 | 2 | 148 |
Post-traumatic arthritis | 62 | 136 | 0 | 1 | 199 |
Instability | 23 | 15 | 0 | 1 | 39 |
Revision of previous RTSA for instability | 29 | 2 | 0 | 1 | 32 |
Infection | 28 | 11 | 2 | 0 | 41 |
Unclassified acute proximal humerus fracture | 42 | 30 | 4 | 8 | 84 |
Acute 3-part proximal humerus fracture | 60 | 0 | 0 | 0 | 6 |
Acute 4-part proximal humerus fracture | 42 | 0 | 0 | 0 | 42 |
Acute 3- or 4-part proximal humerus fracture | 92 | 46 | 0 | 0 | 138 |
Revised from previous nonop proximal humerus fracture | 43 | 53 | 0 | 0 | 96 |
Revised from ORIF | 3 | 9 | 0 | 0 | 12 |
Revised from CRPP | 0 | 3 | 0 | 0 | 3 |
Revised from hemi | 105 | 51 | 0 | 1 | 157 |
Revised from TSA | 192 | 246 | 0 | 5 | 443 |
Osteonecrosis | 9 | 6 | 1 | 0 | 16 |
Pseudoparalysis irreparable tear without arthritis | 535 | 795 | 20 | 2 | 1352 |
Bone tumors | 0 | 38 | 0 | 0 | 38 |
Locked shoulder dislocation | 0 | 0 | 1 | 0 | 1 |
Abbreviations: CRPP, closed reduction and percutaneous pinning; ORIF, open reduction internal fixation; RTSA, reverse total shoulder arthroplasty; TSA, total shoulder arthroplasty.
The ASES, SST-12, and VAS scores were the most frequently reported outcome scores in studies from North America, whereas the Absolute Constant score was the most common score reported in studies from Europe (Table 4). Studies from North America reported significantly higher postoperative external rotation (34.1° ± 13.3° vs 19.3° ± 8.9°) (P < .001) and a greater change in flexion (69.0° ± 24.5° vs 56.3° +/- 11.3°) (P = .004) compared with studies from Europe (Table 5).
Table 4. Outcomes by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
DASH | 1 | 2 | 0 | 0 |
|
Preoperative | 54.0 | 62.0 ± 8.5 | - | - | 0.582 |
Postoperative | 24.0 | 32.0 ± 2.8 | - | - | 0.260 |
Change | -30.0 | -30.0 ± 11.3 | - | - | 1.000 |
SPADI | 2 | 0 | 0 | 0 |
|
Preoperative | 80.0 ± 4.2 | - | - | - | N/A |
Postoperative | 34.8 ± 1.1 | - | - | - | N/A |
Change | -45.3 ± 3.2 | - | - | - | N/A |
Absolute constant | 2 | 27 | 0 | 1 |
|
Preopeartive | 33.0 ± 0.0 | 28.2 ± 7.1 | - | 20.0 | 0.329 |
Postoperative | 54.5 ± 7.8 | 62.9 ± 9.0 | - | 65.0 | 0.432 |
Change | +21.5 ± 7.8 | +34.7 ± 8.0 | - | +45.0 | 0.044 |
ASES | 13 | 0 | 2 | 0 |
|
Preoperative | 33.2 ± 5.4 | - | 32.5 ± 3.5 | - | 0.867 |
Postoperative | 73.9 ± 6.8 | - | 75.7 ± 10.8 | - | 0.752 |
Change | +40.7 ± 6.5 | - | +43.2 ± 14.4 | - | 0.670 |
UCLA | 3 | 2 | 1 | 0 |
|
Preoperative | 10.1 ± 3.4 | 11.2 ± 5.7 | 12.0 | - | 0.925 |
Postoperative | 24.5 ± 3.1 | 24.3 ± 3.7 | 24.0 | - | 0.991 |
Change | +14.4 ± 1.6 | +13.1 ± 2.0 | +12.0 | - | 0.524 |
KSS | 0 | 0 | 2 | 0 |
|
Preopeartive | - | - | 38.2 ± 1.1 | - | N/A |
Postoperative | - | - | 72.3 ± 6.0 | - | N/A |
Change | - | - | +34.1 ± 7.1 | - | N/A |
SST-12 | 12 | 1 | 0 | 0 |
|
Preoperative | 1.9 ± 0.8 | 1.2 | - | - | N/A |
Postoperative | 7.1 ± 1.5 | 5.6 | - | - | N/A |
Change | +5.3 ± 1.2 | +4.4 | - | - | N/A |
SF-12 | 1 | 0 | 0 | 0 |
|
Preoperative | 34.5 | - | - | - | N/A |
Postoperative | 38.5 | - | - | - | N/A |
Change | +4.0 | - | - | - | N/A |
SSV | 0 | 5 | 0 | 0 |
|
Preopeartive | - | 22.0 ± 7.4 | - | - | N/A |
Postoperative | - | 63.4 ± 7.9 | - | - | N/A |
Change | - | +41.4 ± 2.1 | - | - | N/A |
EQ-5D | 0 | 2 | 0 | 0 |
|
Preoperative | - | 0.5 ± 0.2 | - | - | N/A |
Postoperative | - | 0.8 ± 0.1 | - | - | N/A |
Change | - | +0.3 ± 0.1 | - | - | N/A |
OOS | 1 | 0 | 0 | 0 |
|
Preoperative | 24.7 | - | - | - | N/A |
Postoperative | 14.9 | - | - | - | N/A |
Change | -9.9 | - | - | - | N/A |
Rowe | 0 | 1 | 0 | 0 |
|
Preoperative | - | 50.2 | - | - | N/A |
Postoperative | - | 82.1 | - | - | N/A |
Change | - | 31.9 | - | - | N/A |
Oxford | 0 | 2 | 0 | 0 |
|
Preoperative | - | 119.9 ± 138.8 | - | - | N/A |
Postoperative | - | 39.9 ± 3.3 | - | - | N/A |
Change | - | -80.6 ± 142.2 | - | - | N/A |
Penn | 1 | 0 | 0 | 0 |
|
Preoperative | 24.9 | - | - | - | N/A |
Postoperative | 66.4 | - | - | - | N/A |
Change | +41.5 | - | - | - | N/A |
VAS | 10 | 1 | 1 | 1 |
|
Preoperative | 6.6 ± 0.8 | 7.0 | 8.4 | 7.0 | N/A |
Postoperative | 2.0 ± 0.7 | 1.0 | 0.8 | 0.8 | N/A |
Change | -4.6 ± 0.8 | -6.0 | -7.6 | -6.2 | N/A |
SF-36 physical | 2 | 0 | 0 | 0 |
|
Preoperative | 32.7 ± 1.2 | - | - | - | N/A |
Postoperative | 39.6 ± 4.0 | - | - | - | N/A |
Change | +7.0 ± 2.8 | - | - | - | N/A |
SF-36 mental | 2 | 0 | 0 | 0 |
|
Preoperative | 43.6 ± 2.8 | - | - | - | N/A |
Postoperative | 48.1 ± 1.0 | - | - | - | N/A |
Change | +4.5 ± 1.8 | - | - | - | N/A |
Abbreviations: ASES, American Shoulder and Elbow Surgeon score; DASH, Disability of the Arm, Shoulder, and Hand; EQ-5D, EuroQol-5 Dimension; KSS, Korean Shoulder Scoring system; N/A, not available; OOS, Orthopaedic Outcome Score; SF, short form; SPADI, Shoulder Pain and Disability Index; SST, Simple Shoulder Test; SSV, Subjective Shoulder Value; UCLA, University of California, Los Angeles; VAS, visual analog scale.
Table 5. Shoulder Range of Motion, by Continent
Metric (number of studies) | North America | Europe | Asia | Australia | P-value |
Flexion | 18 | 22 | 1 | 1 |
|
Preoperative | 57.6 ± 17.9 | 65.5 ± 17.2 | 91.0 | 30.0 | 0.060 |
Postoperative | 126.6 ± 14.4 | 121.8 ± 19.0 | 133.0 | 150.0 | 0.360 |
Change | +69.0 ± 24.5 | +56.3 ± 11.3 | +42.0 | 120.0 | 0.004 |
Abduction | 11 | 12 | 1 | 0 |
|
Preoperative | 53.7 ± 25.0 | 52.0 ± 19.0 | 88.0 | - | 0.311 |
Postoperative | 109.3 ± 15.1 | 105.4 ± 19.8 | 131.0 | - | 0.386 |
Change | 55.5 ± 25.5 | 53.3 ± 8.3 | 43.0 | - | 0.804 |
External rotation | 17 | 19 | 0 | 0 |
|
Preoperative | 19.4 ± 9.9 | 11.2 ± 6.1 | - | - | 0.005 |
Postoperative | 34.1 ± 13.3 | 19.3 ± 8.9 | - | - | <0.001 |
Change | +14.7 ± 13.2 | +8.1 ± 8.5 | - | - | 0.079 |
Continue to: DISCUSSION...
DISCUSSION
RTSA is a common procedure performed in many different areas of the world for a variety of indications. The study hypotheses were partially confirmed, as there were no significant differences seen in the characteristics of the studies published and patients analyzed; although, the majority of studies from North America reported rotator cuff tear arthropathy as the primary indication for RTSA, whereas studies from Europe were split between rotator cuff tear arthropathy and pseudoparalysis as the primary indication. Hence, based on the current literature the study proved that we are treating the same patients. Despite this finding, we may be treating them for different reasons with an RTSA.
RTSA has become a standard procedure in the United States, with >20,000 RTSAs performed in 2011.10 This number will continue to increase as it has over the past 20 years given the aging population in the United States, as well as the expanding indications for RTSA.11 Indications of RTSA have become broad, although the main indication remains as rotator cuff tear arthropathy (>60% of all patients included in this study), and pseudoparalysis (>15% of all patients included in this study). Results for RTSA for rotator cuff tear arthropathy and pseudoparalysis have been encouraging.16,17 Frankle and colleagues16 evaluated 60 patients who underwent RTSA for rotator cuff tear arthropathy at a minimum of 2 years follow-up (average, 33 months). The authors found significant improvements in all measured clinical outcome variables (P < .0001) (ASES, mean function score, mean pain score, and VAS) as well as ROM, specifically forward flexion increased from 55° to 105.1°, and abduction increased from 41.4° to 101.8°. Similarly, Werner and colleagues17 evaluated 58 consecutive patients who underwent RTSA for pseudoparalysis secondary to irreparable rotator cuff dysfunction at a mean follow-up of 38 months. Overall, significant improvements (P < .0001) were seen in the SSV score, relative Constant score, and Constant score for pain, active anterior elevation (42° to 100° following RTSA), and active abduction (43° to 90° following RTSA).
It is essential to understand the similarities and differences between patients undergoing RTSA in different parts of the world so the literature from various countries can be compared between regions, and conclusions extrapolated to the correct patients. For example, an interesting finding in this study is that the majority of patients in North America have their prosthesis cemented whereas the majority of patients in Australia have their prosthesis press-fit. While the patients each continent is treating are not significantly different (mostly older women), the difference in surgical technique could have implications in long- or short-term functional outcomes. Prior studies have shown no difference in axial micromotion between cemented and press-fit humeral components, but the clinical implications surrounding this are not well defined.18 Small series comparing cementless to cemented humeral prosthesis in RTSA have found no significant differences in clinical outcomes or postoperative ROM, but larger series are necessary to validate these outcomes.19 However, studies have shown lower rates of postoperative infections in patients who receive antibiotic-loaded cement compared with those who receive plain bone cement following RTSA.20
Similarly, as the vast majority of patients in North America had an RTSA for rotator cuff arthropathy (75.8%) whereas those from Europe had RTSA almost equally for rotator cuff arthropathy (33.5%) and pseudoparalysis (29.7%), one must ensure similar patient populations before attempting to extrapolate results of a study from a different country to patients in other areas. Fortunately, the clinical results following RTSA for either indication have been good.6,21,22
One final point to consider is the cost effectiveness of the implant. Recent evidence has shown that RTSA is associated with a higher risk for in-hospital death, multiple perioperative complications, prolonged hospital stay, and increased hospital cost when compared with TSA.23 This data may be biased as the patient selection for RTSA varies from that of TSA, but it is a point that must be considered. Other studies have shown that an RTSA is a cost-effective treatment option for treating patients with rotator cuff tear arthropathy, and is a more cost-effective option in treating rotator cuff tear arthropathy than hemiarthroplasty.24,25 Similarly, RTSA offers a more cost-effective treatment option with better outcomes for patients with acute proximal humerus fractures when compared with open reduction internal fixation and hemiarthroplasty.26 However, TSA is a more cost-effective treatment option than RTSA for patients with glenohumeral osteoarthritis.27 With changing reimbursement in healthcare, surgeons must scrutinize not only anticipated outcomes with specific implants but the cost effectiveness of these implants as well. Further cost analysis studies are necessary to determine the ideal candidate for an RTSA.
LIMITATIONS
Despite its extensive review of the literature, this study had several limitations. While 2 independent authors searched for studies, it is possible that some studies were missed during the search process, introducing possible selection bias. No abstracts or unpublished works were included which could have introduced publication bias. Several studies did not report all variables the authors examined, and this could have skewed some of the results since the reporting of additional variables could have altered the data to show significant differences in some measured variables. As outcome measures for various pathologies were not compared, conclusions cannot be drawn on the best treatment option for various indications. As case reports were included, this could have lowered both the MCMS as well as the average in studies reporting outcomes. Furthermore, given the overall poor quality of the underlying data available for this study, the validity/generalizability of the results could be limited as the level of evidence of this systematic review is only as high as the studies it includes. There are subtle differences between rotator cuff arthropathy and pseudoparalysis, and some studies may have classified patients differently than others, causing differences in indications. Finally, as the primary goal of this study was to report on demographics, no evaluation of concomitant pathology at the time of surgery or rehabilitation protocols was performed.
CONCLUSION
The quantity, but not the quality of RTSA studies is increasing. Indications for RTSA varied by continent although most patients underwent RTSA for rotator cuff arthropathy. The majority of patients undergoing RTSA are female over the age of 60 years for a diagnosis of rotator cuff arthropathy with pseudoparalysis.
This paper will be judged for the Resident Writer’s Award.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
1. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567. doi:10.1007/s11999-011-1775-4.
2. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures-a systematic review of 92 studies including 4,500 patients. J Orthop Trauma. 2014;29(1):54-59.
3. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97. doi:10.1016/j.otsr.2013.12.005.
4. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41. doi:10.1016/j.jse.2011.04.009.
5. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(suppl 1):S27-SS34. doi:10.1007/s12306-012-0193-4.
6. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662-1668. doi:10.1016/j.jse.2014.03.001.
7. Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215-222. doi:10.1016/j.jse.2014.05.022.
8. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556. doi:10.2106/JBJS.I.00912.
9. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993. doi:10.1016/j.jse.2015.01.001.
10. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
11. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi:10.1016/j.jclinepi.2009.06.006.
13. University of York Centre for Reviews and Dissemination, National Institute for Health Research. PROSPERO International prospective register of systematic reviews. University of York Web site. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 1, 2016.
14. Oxford Centre for Evidence-based Medicine – Levels of evidence (March 2009). University of Oxford Web site: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed November 1, 2016.
15. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699. doi:10.2106/JBJS.F.00858.
16. Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):178-190. doi:10.2106/JBJS.F.00123.
17. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486. doi:10.2106/JBJS.D.02342.
18. Peppers TA, Jobe CM, Dai QG, Williams PA, Libanati C. Fixation of humeral prostheses and axial micromotion. J Shoulder Elbow Surg. 1998;7(4):414-418. doi:10.1016/S1058-2746(98)90034-9.
19. Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208-1214. doi:10.1016/j.jse.2013.11.032.
20. Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324-328. doi:10.1016/j.jse.2011.08.072.
21. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469-2475. doi:10.1007/s11999-011-1833-y.
22. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61. doi:10.1302/0301-620X.93B1.24218.
23. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467. doi:10.1016/j.jse.2014.08.016.
24. Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21(10):1278-1288. doi:10.1016/j.jse.2011.10.010.
25. Renfree KJ, Hattrup SJ, Chang YH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):1656-1661. doi:10.1016/j.jse.2013.08.002.
26. Chalmers PN, Slikker W, 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204. doi:10.1016/j.jse.2013.07.044.
27. Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg. 2015;24(9):1433-1441. doi:10.1016/j.jse.2015.01.005.
TAKE-HOME POINTS
- RTSA is an effective treatment for rotator cuff tear arthropathy (the most common reason patients undergo RTSA).
- While there has been a plethora of literature surrounding outcomes of RTSA over the past several years, the methodological quality of this literature has been limited.
- Similarly, this study found the number of publications surrounding RTSA is increasing each year while the average methodological quality of these studies is decreasing.
- Females undergo RTSA more commonly than males, and the average age of patients undergoing RTSA is 71 years.
- Interestingly, patients’ postoperative external rotation was higher in studies out of North America compared to other continents. Further research into this area is needed to understand more about this finding.
Arthroscopically-Guided, Cannulated, Headless Compression Screw Fixation of the Symptomatic Os Acromiale
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
ABSTRACT
Os acromiale is a failure of fusion between 1 or more ossification centers of the scapula and the acromion process. Pain can be caused by motion and impingement of the unfused segment. Several methods for the management of os acromiale have been described. Internal fixation is the most common surgical technique, followed by excision and acromioplasty. We present a novel technique for treatment of symptomatic os acromiale using arthroscopically-guided headless compression screws. This is a viable technique in the management of symptomatic os acromiale due to preservation of the periosteal blood supply and less concern for symptomatic hardware.
Continue to: Os acromiale results from a failure of...
Os acromiale results from a failure of fusion between 1 or more ossification centers and the acromion process.1 The acromion consists of 4 different ossification centers, which appear by 14 years of age and fuse by age 25 years. The 4 ossification centers are the basi-acromion, meta-acromion, mesoacromion, and pre-acromion (Figure 1). Formation of an os acromiale occurs most often due to failure of fusion between the meta-acromion and mesoacromion. Os acromiale appears to occur in approximately 8% of the population, according to cadaveric studies.2 This anatomic variant occurs more commonly in African-Americans than Caucasians, and shows a preponderance for males over females.3
Plain radiographs are usually adequate for diagnosis. Axillary views are most sensitive for detection, which can be difficult to see on anteroposterior radiographs.4 In os acromiale, the unfused segment is connected to the acromioclavicular joint and the coracoid, which can lead to motion of the segment and impingement of the rotator cuff.2-4 Patients frequently experience localized tenderness and symptomatic pain with signs and symptoms of impingement. Rotator cuff tears may occur secondary to chronic impingement.5
Various forms of repair have been described. A recent meta-analysis showed that internal fixation (60%) was the most common surgical technique reported, followed by excision (27%) and acromioplasty (13%).6 Rotator cuff repair is a common concurrent surgical procedure.7-11 The available literature favors internal fixation through an open technique with or without bone grafting.5,7,8,12-15 Various forms of fixation have been presented in the literature, including Kirschner wire fixation, cannulated screw fixation alone, cannulated screw fixation with FiberWire Suture (Arthrex), and cannulated screw fixation with a stainless steel wire tension band technique. Based on the results of the meta-analysis, surgical fixation with cannulated screws has been shown to lead to a significantly greater rate of radiographic healing (23/24 patients) compared to Kirschner wire fixation (31/49 patients).6 Further, radiographic healing is significantly associated with improved clinical outcomes.12 Removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation cases (88%; 43/49) compared to cannulated screw fixation cases (38%; 9/24). However, hardware issues may also be encountered with screw fixation, with 1 case series reporting a 25% rate of hardware complication.16 The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
The patient is a 19-year-old right-hand-dominant woman who injured her right shoulder while diving into the bleachers during a volleyball game 4 years prior to presentation. She suffered a direct blow to her shoulder and immediately became symptomatic. She underwent a long period of nonoperative management, which included physical therapy, strengthening, nonsteroidal anti-inflammatory drug (NSAID) therapy, and narcotic pain medications. Her primary complaints upon presentation were pain with lifting, as well as mechanical symptoms. On examination, the patient had moderate tenderness directly over the acromion. She also had evidence of mild impingement symptoms. Plain radiographs revealed a mesoacromial-type os acromiale clearly seen on the axillary lateral film (Figure 2). She underwent magnetic resonance imaging, which suggested rotator cuff tendinosis and evidence of edema at the os acromiale site. She underwent a diagnostic injection directly into the site of maximal tenderness at the os, which provided complete transient relief of her pain. Despite the transient pain relief, the patient continued to be symptomatic after the local anesthetic effect wore off. Surgical options were then discussed with the patient.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
A standard diagnostic shoulder arthroscopy was performed using anterior, posterior and direct lateral portals. The rotator cuff was evaluated, and no evidence of a tear was found. The undersurface of the acromion was exposed, and the os acromiale was identified arthroscopically (Figure 3). This was found to be unstable under direct digital pressure.
We then elected to repair the unstable fibrous os acromiale (Figures 4A-4D). The fibrous nonunion was first debrided to bleeding bone with a 4.0-mm round burr aligned with the os using the direct lateral portal (Smith & Nephew Endoscopy). Through the anterior portal, two AcutrakTM guide wires (Acumed) were placed under arthroscopic visualization from the anterior margin of the acromion, across the os site, and into the posterior acromion. A 1-cm counter incision was made at the level of the posterior acromion to allow confirmation of the guide wire position and to permit placement of a large, pointed reduction clamp, used to reduce the mesoacromial fragment to the stable portion of the acromion. The calibrated, cannulated drill bit was passed over each guide wire to a depth of 34 mm, according to standard technique, and viewed arthroscopically from the subacromial space. Two 34-mm AcutrakTM cannulated headless compression screws (Acumed) were then placed across the defect. Direct arthroscopic visualization confirmed reduction and complete intraosseous placement of the screws (Figure 5). Screw position was also assessed with image intensification. Fluoroscopic views showed the repair to be stable when the shoulder was taken through range of motion. The os site was never exposed directly through an incision. The surgery was performed on an outpatient basis.
POSTOPERATIVE COURSE
The patient was maintained in a sling and small abduction pillow (Ultrasling IIITM, DonJoy). She was kept non-weight-bearing but was permitted unrestricted motion through the elbow, wrist, and hand for the first 6 weeks. She was permitted supine passive external rotation of the shoulder to 30° and forward flexion to 45° for the first 2 weeks, and 90° through 6 weeks. At her initial postoperative visit 2 weeks later, she noted minimal pain in the shoulder, much improved from her preoperative pain. She was no longer taking any pain medicine, including NSAIDs. Radiographs showed no change in fixation.
At her second visit (6 weeks), she was completely pain free. Clinical examination showed no tenderness at the acromion, healed incisions, and pain-free passive ROM. Radiographs demonstrated early evidence of consolidation and no sign of fixation failure (Figures 6-8). Her Single Assessment Numeric Evaluation (SANE) score was 85%, and her Simple Shoulder Test (SST) score was 3/12. She was permitted to discontinue the sling, to begin using the arm actively at the side, and progress with unloaded use above shoulder height over the next 6 weeks.
She was seen in follow-up at 4 months, where she was found to have no pain but had not yet returned to sports. At her 6-month follow-up, she showed continued improvement with no limitation of activity. At 1-year follow-up, her SANE score improved from 85% at 6 weeks postoperatively to 100%, and her SST improved from 3/12 at 6 weeks to 12/12. She demonstrated full function of her shoulder with no evidence of hardware loosening. At that time, her os acromiale had completely fused radiographically.
Continue to: DISCUSSION...
DISCUSSION
A variety of methods for the management of os acromiale have been described in the literature. Internal fixation is reported as the most common surgical technique, followed by excision and acromioplasty.6 Surgical fixation with cannulated screws is effective at achieving radiographic union.5,9,12,13,15
Excision is also an option in cases where there is a symptomatic pre-acromion with a relatively small fragment. In the case of a larger fragment, techniques that preserve the vascularity of the os acromiale appear more likely to be successful than excision.17 While excision can be performed arthroscopically to preserve the blood supply, a recent report showed that 35% of patients still had residual pain.18 Another study suggests that protecting the vascular supply with an arthroscopic technique would be a better option to promote healing to union.19
Given that removal of symptomatic internal fixation hardware is significantly more common after Kirschner wire fixation (88%; 43/49) than after cannulated screw fixation (38%; 9/24),6 and given that significant hardware complications can arise from screw tips,16 we chose headless, cannulated Acutrak compression screws for arthroscopic-assisted fixation. Performing the operation arthroscopically minimized soft-tissue violation, allowing us to directly visualize the reduction and also allowing confirmation that the screws were not at risk for impingement of the rotator cuff. The tapered nature of the Acutrak screws allowed for excellent compression at the reduction site without a prominent screw head.
CONCLUSION
Arthroscopic management of the symptomatic os acromiale has been documented in the literature. Cannulated screw fixation has shown to lead to a higher rate of radiographic union than Kirschner wire fixation. Arthroscopically guided placement of headless, cannulated compression screw fixation may be a viable repair alternative in the management of the symptomatic os acromiale with less concern for symptomatic hardware.6,20-27
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
1. Barbier O, Block D, Dezaly C, Sirveaux F, Mole D. Os acromiale, a cause of shoulder pain, not to be overlooked. Orthop Traumatol Surg Res. 2013;99(4):465-472. doi: 10.1016/j.otsr.2012.10.020.
2. Swain RA, Wilson FD, Harsha DM. The os acromiale: another cause of impingement. Med Sci Sports Exerc. 1996;28(12):1459-1462. doi:10.1097/00005768-199612000-00003.
3. Kurtz CA, Humble BJ, Rodosky MW, Sekiya JK. Symptomatic os acromiale. J Am Acad Orthop Surg. 2006;14(1):12-19. doi:10.5435/00124635-200601000-00004.
4. Buss DD, Freehill MQ, Marra G. Typical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfalls. Instr Course Lect. 2009;58:447-457.
5. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80(9):1320-1326. doi:10.2106/00004623-199809000-00011.
6. Harris JD, Griesser MJ, Jones GL. Systematic review of the surgical treatment for symptomatic os acromiale. Int J Shoulder Surg. 2011;5(1):9-16. doi:10.4103/0973-6042.80461.
7. Abboud JA, Silverberg D, Pepe M, et al. Surgical treatment of os acromiale with and without associated rotator cuff tears. J Shoulder Elbow Surg. 2006;15(3):265-270. doi:10.1016/j.jse.2005.08.024.
8. Boehm TD, Matzer M, Brazda D, Gohlke FE. Os acromiale associated with tear of the rotator cuff treated operatively Review of 33 patients. J Bone Joint Surg Br. 2003;85(4):545-549. doi:10.1302/0301-620X.85B4.13634.
9. Boehm TD, Rolf O, Martetschlaeger F, Kenn W, Gohlke F. Rotator cuff tears associated with os acromiale. Acta Orthop. 2005;76(2):241-244. doi:10.1080/00016470510030643.
10. Barbiera F, Bellissima G, Iovane A, De Maria M. OS acromiale producing rotator cuff impingement and rupture. A case report. Radiol Med. 2002;104(4):359-362.
11. Neer CS 2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
12. Hertel R, Windisch W, Schuster A, Ballmer FT. Transacromial approach to obtain fusion of unstable os acromiale. J Shoulder Elbow Surg. 1998;7(6):606-609. doi:10.1016/S1058-2746(98)90008-8.
13. Ozbaydar MU, Keriş I, Altun M, Yalaman O. Results of the surgical treatment for symptomatic mesoacromion. Acta Orthop Traumatol Turc. 2006;40(2):123-129.
14. Satterlee CC. Successful osteosynthesis of an unstable mesoacromion in 6 shoulders: a new technique. J Shoulder Elbow Surg. 1999;8(2):125-129. doi:10.1016/S1058-2746(99)90004-6.
15. Ryu RK, Fan RS, Dunbar WHt. The treatment of symptomatic os acromiale. Orthopedics. 1999;22(3):325-328.
16. Atoun E, van Tongel A, Narvani A, Rath E, Sforza G, Levy O. Arthroscopically assisted internal fixation of the symptomatic unstable os acromiale with absorbable screws. J Shoulder Elbow Surg. 2012;21(12):1740-1745. doi:10.1016/j.jse.2011.12.011.
17. Johnston PS, Paxton ES, Gordon V, Kraeutler MJ, Abboud JA, Williams GR. Os acromiale: a review and an introduction of a new surgical technique for management. Orthop Clin North Am. 2013;44(4):635-644. doi:10.1016/j.ocl.2013.06.015.
18. Campbell PT, Nizlan NM, Skirving AP. Arthroscopic excision of os acromiale: effects on deltoid function and strength. Orthopedics. 2012;35(11):e1601-e1605. doi:10.3928/01477447-20121023-16.
19. Yepes H, Al-Hibshi A, Tang M, Morris SF, Stanish WD. Vascular anatomy of the subacromial space: a map of bleeding points for the arthroscopic surgeon. Arthroscopy. 2007;23(9):978-984. doi:10.1016/j.arthro.2007.03.093.
20. Kummer FJ, Van Gelderen J, Meislin RJ. Two-screw, arthroscopic fixation of os acromiale compared to a similar, open procedure incorporating a tension band: a laboratory study. Shoulder Elbow. 2011;3(2):85-87. doi:10.1111/j.1758-5740.2011.00115.x.
21. Wright RW, Heller MA, Quick DC, Buss DD. Arthroscopic decompression for impingement syndrome secondary to an unstable os acromiale. Arthroscopy. 2000;16(6):595-599. doi:10.1053/jars.2000.9239.
22. Edelson JG, Zuckerman J, Hershkovitz I. Os acromiale: anatomy and surgical implications. J Bone Joint Surg Br. 1993;75(4):551-555. doi:10.1302/0301-620X.75B4.8331108.
23. Fery A, Sommelet J. Os acromiale: significance--diagnosis--pathology Apropos of 28 cases including 2 with fracture separation. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(2):160-172.
24. Lee DH. The double-density sign: a radiographic finding suggestive of an os acromiale. J Bone Joint Surg Am. 2004;86-A(12):2666-2670. doi:10.2106/00004623-200412000-00012.
25. Ortiguera CJ, Buss DD. Surgical management of the symptomatic os acromiale. J Shoulder Elbow Surg. 2002;11(5):521-528. doi:10.1067/mse.2002.122227.
26. Peckett WR, Gunther SB, Harper GD, Hughes JS, Sonnabend DH. Internal fixation of symptomatic os acromiale: a series of twenty-six cases. J Shoulder Elbow Surg. 2004;13(4):381-385. doi:10.1016/S1058274604000400.
27. Sahajpal D, Strauss EJ, Ishak C, Keyes JM, Joseph G, Jazrawi LM. Surgical management of os acromiale: a case report and review of the literature. Bull NYU Hosp Jt Dis. 2007;65(4):312-316.
TAKE-HOME POINTS
- Os acromiale is a failure of acromial ossification centers to fuse, and occurs in 8% of the population.
- Symptomatic os acromiale can be treated with repair, or sometimes excision or acromioplasty.
- Repair preserves the anterior deltoid origin and can result in less pain than excision of the fragment.
- Repair of larger fragments can be completed with cannulated screws to reliably achieve union.
- The arthroscope-assisted repair technique described in this article preserves vascularity and can reduce the risk of hardware-related complaints.
Complex Ankle and Hindfoot Arthrodesis Using Circular External Fixation
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
TAKE-HOME POINTS
- Ankle and hindfoot fusion using circular external fixation is a useful surgical technique in patients with diabetes, Charcot, osteomyelitis, deformity, and/or bone and soft tissue compromise in order to obtain solid bony fusion, stable limb alignment, and eradication of infection in cases of complex pathology.
- Deformity correction with osteotomies and meticulous joint preparation is required in order to obtain broad, cancellous bony surfaces for fusion with neutral alignment. Autograft from the distal fibula and/or medial malleolus can be combined with bone allograft to assist with joint fusion.
- The ankle and hindfoot are provisionally pinned into neutral coronal and sagittal alignment through the plantar surface of the foot using large K-wires prior to placement of the lower leg in the center of a circular 3-ring compression frame. Typically, 2 to 3 points of fixation are used per ring with a combination of half-pins and smooth wires.
- Ring attachments are built up or down to the level of the half-pins and wires in order to prevent pins and wires from bending, breaking, or causing iatrogenic deformity during tensioning. Crossing olive wires are used in the midfoot and calcaneus with dual tensioning devices to ensure an even pull on both sides of the foot.
- Dynamic or static compression struts are used to obtain 8 to 10 mm of compression across the ankle and hindfoot, followed by addition of an anterior foot ring to increase construct rigidity. Daily pin care is started 3 to 4 days after surgery and patients are kept non-weight-bearing for approximately 2 months in the frame with a total frame period of 3 to 8 months depending on bony healing on X-ray.
The Effect of Age on the Benefits of Early Decompression for Cervical Spondylotic Myelopathy
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
TAKE-HOME POINTS
- Decompression of cervical myelopathy within 24 months of symptom onset results in greater functional improvement compared to delayed decompression.
- The improvement with respect to time is more significant for patients older than 65 years compared to younger patients.
- Duration of symptoms does not seem to influence the severity of the preoperative Nurick score.
- Preoperative severity of symptoms is related to postoperative outcomes.
- Other significant predictors of worse outcomes include tobacco use, diabetes, and number of levels fused.
The Flint Lock: A Novel Technique in Total Knee Arthroplasty Closure
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.
The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.
When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).
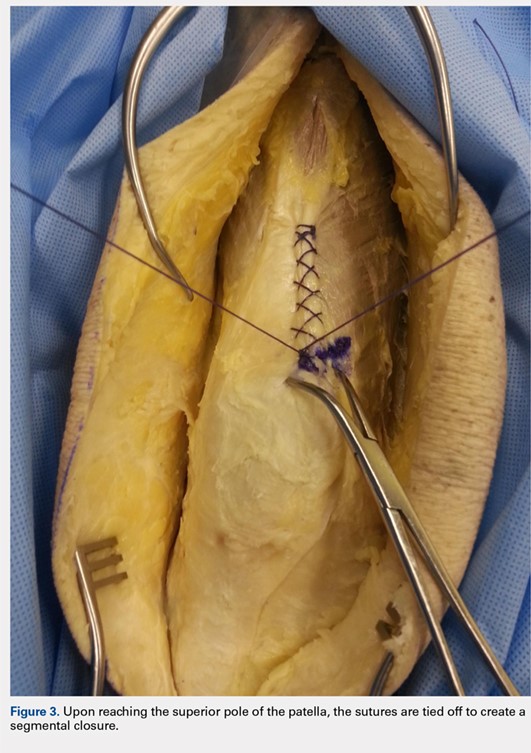
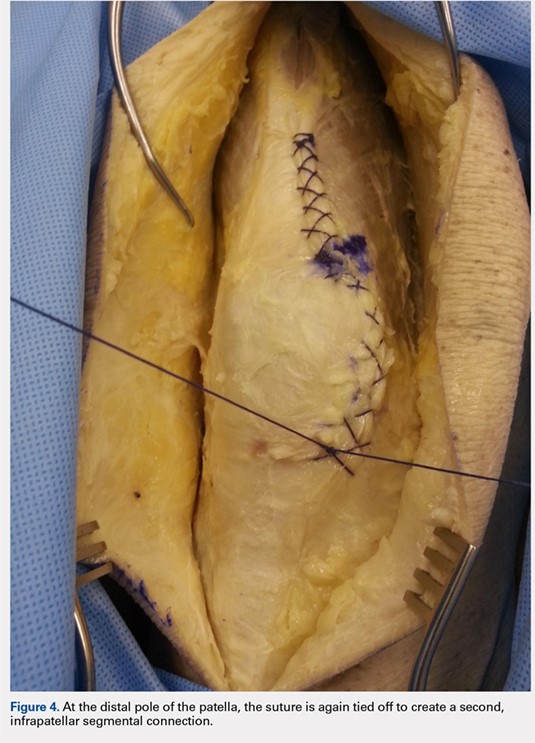
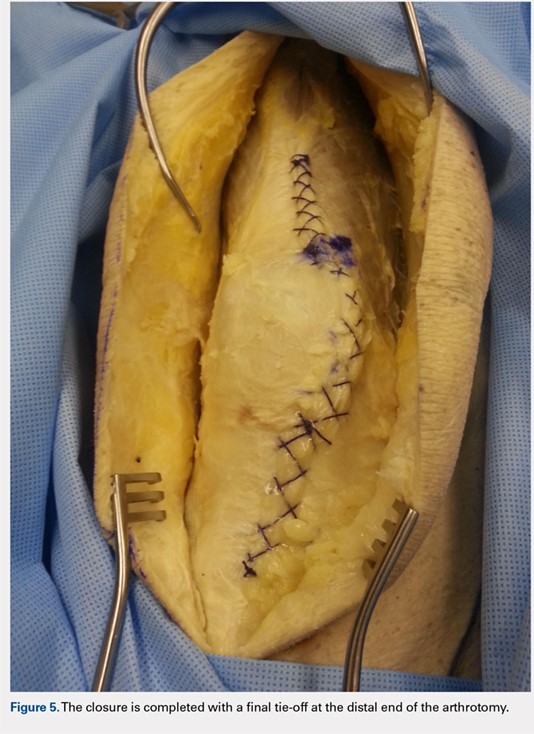
Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.

The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.

When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).



Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.

The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.

When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).



Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
TAKE-HOME POINTS
- The Flint Lock is a novel technique in TKA closure.
- Its continuous nature provides a tight seal with extensor mechanism closure.
- The utilization of a segmental closure with double suture provides a safeguard for suture failure.
- The suture used in the technique is less expensive than barbed suture.
- Future investigation is warranted to further validate the use of the Flint Lock.
The Effect of Insurance Type on Patient Access to Ankle Fracture Care Under the Affordable Care Act
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
TAKE-HOME POINTS
- One method in which the PPACA increased the number of individuals with health insurance coverage was by expanding Medicaid eligibility requirements.
- Despite this, Medicaid patients confronted more barriers to accessing care.
- The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross. Patients with Medicaid also confronted longer appointment wait times.
- The disparity in access for this operative trauma scenario suggests that patients with Medicaid are likely to be excluded from the practice of their choice and may need to make considerably more effort to secure an appointment.
- Ultimately, Medicaid patients may have access to care through federally funded community health centers and public and non-profit safety net hospitals, which generally care for more uninsured and Medicaid patient populations.
The Cold, Hard Facts of Cryotherapy in Orthopedics
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
TAKE-HOME POINTS
- Cryotherapy is often used in postoperative orthopedic care but there is limited literature demonstrating its efficacy.
- Postoperative cryotherapy has been used to reduce visual analog scale pain scores, analgesic consumption, and to increase range of motion.
- There is no consensus on the advantages of postoperative cryotherapy vs traditional ice application.
- Adverse outcomes from postoperative cryotherapy use include frostbite/skin loss, compartment syndrome, and perniosis.
- Future studies, including a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery.